Summary
Purpose
In temporal lobe epilepsy (TLE) with evidence of hippocampal sclerosis (TLE-MTS) volumetric gray (GM) and white (WM) matter abnormalities are not restricted to the hippocampus but also are found in extrahippocampal structures. Less is known about extrahippocampal volumetric abnormalities in TLE without hippocampal sclerosis (TLE-no). In this study, we used optimized voxel-based morphometry (VBM) with and without modulation with the following aims: (a) to identify WM and GM abnormalities beyond the hippocampus in TLE-MTS and TLE-no; and (b) to determine whether extratemporal WM and GM abnormalities differ between TLE-MTS and TLE-no.
Methods
Optimized VBM of GM and WM with and without modulation was performed in 26 TLE-MTS (mean age, 35.6 ± 9.7 years), 17 TLE-no (mean age, 35.6 ± 11.1 years), and 30 healthy controls (mean age, 30.3 ± 11.1 years).
Results
In TLE-MTS, GM/WM volume and concentration reductions were found in the ipsilateral limbic system, ipsi- and contralateral neocortical regions, thalamus, cerebellum, internal capsule, and brainstem when compared with controls. In contrast, no differences of GM/WM volumes/concentrations were found between TLE-no and controls or between TLE-no and TLE-MTS.
Conclusions
In TLE-MTS, optimized VBM showed extensive GM and WM volume reductions in the ipsilateral hippocampus and in ipsi- and contralateral extrahippocampal regions. In contrast, no GM/WM volume or concentration reductions were found in TLE-no. This further supports the hypothesis that TLE-no is a distinct clinicopathologic entity from TLE-MTS and probably heterogeneous in itself.
Keywords: TLE, Extratemporal, Voxel-based morphometry, Mesiotemporal sclerosis, Normal MRI
Mesial temporal lobe epilepsy (mTLE) is one of most frequent forms of partial epilepsy in adults. Based on neuroimaging and histologic characteristics, two main subtypes of mTLE can be distinguished: (a) TLE with hippocampal sclerosis (TLE-MTS), found in ~60–70% of mTLE patients, which is characterized by an increased hippocampal T2 signal and/or atrophied hippocampal formation on the MRI and significant neuronal loss in one or more hippocampal subfields in the histologic examination (1); and (b) MTLE without structural abnormalities on MRI (TLE-no) and only very mild or no neuronal loss in the hippocampus, which is found in ~20–30% of mTLE patients. In both types of mTLE, seizures are not restricted to the medial temporal lobe but involve other brain areas during seizure spread. Accordingly, several studies using volume of interest analyses (VOI) describe volumetric abnormalities beyond the hippocampal formation in TLE-MTS [e.g., in amygdala and parahippocampal region (2), temporopolar region (3,4), temporal lobe (5) thalamic and striatal nuclei (6,7), cerebellum (8), and diffuse whole-brain volume loss (9)]. In contrast, the number of studies concentrating on volumetric abnormalities in TLE-no is relatively small, and except for diffuse whole-brain volume loss in a subgroup of TLE-no (9), structural abnormalities were mostly found in the entorhinal cortex (10) and in the amygdala (11). Voxel-based morphometry (VBM) has been developed to assess differences of brain tissue concentrations between subject groups (12). Because it is minimally operator dependent and allows the assessment of regional volumetric effects without an a priori hypothesis about their localization in the brain, VBM is less prone to investigator bias than are VOI techniques. Early attempts to use this method to detect volumetric abnormalities in mTLE were not encouraging, as the technique was not able to identify hippocampal sclerosis in TLE-MTS (13). However, recent technical improvements of VBM, particularly the introduction of the so-called optimized VBM with modulation, allowed not only the detection of hippocampal sclerosis but also extrahippocampal abnormalities similar to those described in VOI analyses (14–17). Therefore the aims of this study were the following: (a) to identify white matter (WM) and gray matter (GM) reductions beyond the hippocampus in TLE-MTS. In particular, we expected to find GM and WM reductions in extrahippocampal brain regions receiving direct hippocampal afferents, (e.g., ipsilateral limbic structures, temporal neocortical structures, and ipsilateral thalamus) but also in brain structures not directly connected to the hippocampus but secondarily involved in seizure spread; (b) to identify WM and GM reductions beyond the hippocampus in TLE-no. In this group, we expected to find GM and WM reductions in regions previously described by VOI analyses (e.g., amygdala and entorhinal cortex and brain regions connected to them) and also in brain regions secondarily involved in seizure spread; and (c) to determine whether extratemporal WM and GM reductions differ between TLE-MTS and TLE-no.
METHODS
Study population
The committee of human research at the University of California, San Francisco (UCSF), approved the study, and written informed consent was obtained from each subject according to the Declaration of Helsinki. Forty-five consecutive patients with drug-resistant TLE were recruited from the Northern California Comprehensive Epilepsy Center, UCSF, and the Pacific Epilepsy Program, California Pacific Medical Center, where they underwent evaluation for epilepsy surgery. Twenty-six patients (mean age, 35.6 ± 9.7 years; left TLE/right TLE, 14:12; female/male patients, 10:16) had evidence for mesial temporal lobe sclerosis on MRI [i.e., atrophy of the hippocampal formation and/or hippocampal signal hyperintensity on T2-weighted or images obtained with a fluid attenuated inversion recovery sequence (FLAIR)] (TLE-MTS), and 17 patients (mean age, 35.6 ± 11.1 years; left TLE/right TLE, 10:7; female/male patients, 9:8) had normal MRI examinations (TLE-no). Age at onset of epilepsy was different between the two groups (TLE-MTS, 9.3 ± 7.8 years; TLE-no, 17 ± 12.2 years; p = 0.026), whereas the duration of epilepsy was not different (TLE-MTS, 26.24 ± 13.0 years; TLE-no, 19.7 ± 12.1 years). The identification of the epileptogenic focus was based on seizure semiology and prolonged ictal and interictal video-EEG telemetry (VET) in all patients. All patients had been seizure free for ≥24 h before the MRI. The control population consisted of 30 healthy volunteers (mean age, 30.3 ± 7.9 years; female/male subjects, 15:15).
T1-weighted images (3D volumetric magnetization-prepared rapid gradient echo (MPRAGE) sequence with TR/TE/TI, 13.5/7/300 ms timing; 15-degree flip angle; 1.0 × 1.0 mm2 in-plane resolution, and slice thickness, 1.5 mm; 144 coronal images; acquisition time, 12 min) were obtained for all subjects using a 1.5 T VISION MR system (Siemens Inc., Iselin, NJ, U.S.A.). This examination was part of a 90-min research protocol consisting of several different imaging and spectroscopy sequences.
Postprocessing and voxel-based morphometry
Data were analyzed by using SPM2 (Wellcome Department of Cognitive Neurology, www.fil.ion.ucl.ac.uk) running MATLAB 6.1 (The MathWorks, Natick, MA, U.S.A.) on a Windows XP desktop computer. The method used for optimized VBM without and with modulation, as described by Good et al. (18), was slightly modified for this study. To allow a combination of right and left TLE in the analysis, a symmetrical customized template and customized, symmetrical priors were generated because previous VBM studies found significant left–right asymmetries in GM in healthy brains (19). To create this template, the original (30 images) and left–right flipped images (30 images) of the control group were normalized to the standard SPM template. The normalized data were then smoothed with an 8 mm full-width-at-half-maximum (FWHM) isotropic gaussian kernel and averaged. To create symmetrical priors, the normalized original and left–right flipped images of the control group were segmented into GM, WM, and CSF by using the SPM standard segmentation procedure and standard priors (12). After smoothing of the segmented images with an 8-mm FWHM isotropic gaussian kernel, they were averaged to create customized symmetrical GM, WM, and CSF priors. All images (original and left–right flipped control images, original right TLE images and left–right flipped left TLE images) were then segmented in native space into GM and WM images, followed by a series of fully automated morphologic operations for removing unconnected nonbrain voxels from the segmented images. GM images were then spatially normalized to the customized symmetrical GM template, and these deformation parameters applied to the whole-brain structural image. The normalized whole-brain structural images were then segmented into GM, WM, and CSF, and the brain-extraction step was repeated. To correct for volume changes during spatial normalization, the voxel values in the segmented image were multiplied by the Jacobian determinants derived from the spatial normalization step (modulation). The analysis of the modulated GM and WM images can detect regional differences in the volume of GM (GMV) or WM (WMV), whereas the analysis of the unmodulated GM and WM images allows the detection of regional differences of GM (GMC) and WM (WMC) concentrations. Unmodulated and modulated images were used in this study. Modulated and unmodulated segmented GM and WM images were smoothed with a 4-mm FWHM isotropic gaussian kernel (20). Based on the results of previous VOI studies (10,11), we expected the changes in TLE-no to be subtle and to affect small regions (e.g. amydgala), and thus the smallest smoothing kernel possible to guarantee a high sensitivity for the detection of small volume changes without rendering the analysis invalid due to nonnormality (20) was chosen for this analysis. However, analyses using larger smoothing kernels (12 mm, 10 mm) also were performed and showed essentially the same results, except that they did not allow a distinction of the affected structures and gyri within the temporal lobe (data not shown).
Statistical analysis
The normalized, smoothed, segmented, modulated and unmodulated GM and WM images were analyzed by using statistical parametric mapping (SPM2). The following analyses were performed: (a) comparison of GMC, WMC, GMV, and WMV of TLE-MTS with GMC, WMC, GMV, and WMV in controls; (b) comparison of GMC, WMC, GMV, and WMV of TLE-no with GMC, WMC, GMV, and WMV in controls; and (c) comparison of GMC, WMC, GMV, and WMV of TLE-MTS with GMC, WMC, GMV, and WMV in TLE-no. Normalization for global differences in voxel intensity across scans was effected by inclusion of the global mean voxel value (GM globals for GM analyses and WM globals for WM analyses) as a confounding covariate in an analysis of covariance. The analysis included grand mean scaling and absolute threshold masking (0.05). Age and gender were included as nuisance variables for GMC and WMC comparisons; age, gender, and total intracranial volume were included as nuisance variables for GMV and WMV comparisons. Contrasts were defined to detect whether each voxel of tissue had a lower probability of being GM or WM between TLE-MTS and controls, TLE-no and controls, and TLE-MTS and TLE-no. Resultant t statistic maps were thresholded at a p value of <0.05 by using False Discovery Rate to correct for multiple comparisons.
RESULTS
GM concentrations/volume changes
Table 1 displays the results of GM changes. Compared with healthy controls, TLE-MTS had regions with significantly reduced GMC in the ipsilateral hippocampus, parahippocampus, and in the ipsi- and contralateral cerebellum. Regions with significantly reduced GMV were larger and more extensive and involved, in addition to the ipsilateral hippocampus, several ipsilateral extrahippocampal brain regions directly connected to the hippocampus (i.e., parahippocampal gyrus, entorhinal cortex, posterior cingulate), and circumscribed neocortical regions in the ipsilateral temporal, occipital, and parietal lobes (cf Fig. 1). Two clusters of significant GMV reductions were found in the ipsilateral thalamus, one in the medial and one in the lateral thalamus. The localization of the medial cluster corresponded to the region of the anterior, lateral dorsal, and medial nucleus (i.e., nuclei receiving direct afferents from the hippocampus and amygdala) and the pulvinar, which mainly projects to association areas of the frontal, parietal, and occipital lobes. The lateral cluster was in the region of the reticular nucleus, which has no direct hippocampal connections but receives collaterals from both thalamocortical and corticothalamic fibers (21). In addition to this, several smaller regions of reduced GMV were noted in the contralateral frontal, parietal, and occipital lobes and medial thalamus and large regions of reduced GMV in both cerebral hemispheres. TLE-no had no significant GMC or GMV reductions compared with controls, and no difference was seen when TLE-no and TLE-MTS were compared with each other.
TABLE 1.
Gray matter: regions with reduced GM concentration/volume in TLE-MTS compared to controls
| Comparison | Region | Cluster | p corrected | Coordinates | ||
|---|---|---|---|---|---|---|
| GM unmodulated Controls>TLE-MTS | ||||||
| Ipsi hippocampal head | 1178 | 0.000 | 28 | −14 | −21 | |
| Ipsi parahippocampal gyrus | 59 | 0.003 | 34 | −36 | −17 | |
| Ipsi cerebellar hemisphere | 88 | 0.000 | 28 | −73 | −52 | |
| 104 | 0.005 | 26 | −34 | −31 | ||
| Contra cerebellar hemisphere | 174 | 0.000 | −35 | −67 | −55 | |
| GM modulated Controls>TLE-MTS | ||||||
| Ipsi hippocampal head, body, fornix | 7650 | 0.000 | 30 | −13 | −20 | |
| Ipsi medial thalamus | ||||||
| Ipsi entorhinal cortex | 117 | 0.009 | 19 | −3 | −34 | |
| Ipsi parahippocampal gyrus | 594 | 0.000 | 35 | −37 | −17 | |
| Ipsi inferior temproal gyrus | 72 | 0.008 | 52 | 3 | −40 | |
| Ipsi middle temproal gyrus | 80 | 0.019 | 60 | −2 | −32 | |
| Ipsi temporal pol | 50 | 0.030 | 23 | 8 | −38 | |
| Ipsi medial occipitotemporal gyrus | 76 | 0.006 | 42 | −18 | −35 | |
| Ipsi occipital pol | 221 | 0.001 | 18 | −96 | −12 | |
| 183 | 0.012 | 20 | −100 | −3 | ||
| 41 | 0.024 | 28 | −92 | 3 | ||
| 61 | 0.014 | 10 | −86 | −2 | ||
| Ipsi insula | 136 | 0.006 | 40 | 0 | −18 | |
| Ipsi lateral thalamus | 132 | 0.010 | 20 | −21 | 2 | |
| 36 | 0.022 | 17 | −15 | 3 | ||
| Ipsi posterior cingulate | 370 | 0.000 | 3 | −37 | 25 | |
| Ipsi superior parietal lobule | 70 | 0.010 | 19 | −63 | 67 | |
| Ipsi cerebellar hemisphere | 393 | 0.004 | 26 | −34 | −32 | |
| 60 | 0.025 | 9 | −74 | −28 | ||
| 39 | 0.025 | 33 | −83 | −29 | ||
| Contra medial thalamus | 1170 | 0.003 | −14 | −28 | 12 | |
| Contra frontal pol | 38 | 0.009 | −24 | 63 | 26 | |
| Contra postcentral gyrus | 51 | 0.012 | −34 | −26 | 52 | |
| Contra occipital pol | 61 | 0.012 | −7 | −97 | 0 | |
| 52 | 0.025 | −3 | −87 | −4 | ||
| 40 | 0.018 | −10 | −100 | 5 | ||
| Contra cerebellar hemisphere | 20051 | 0.000 | −31 | −47 | −50 | |
| 184 | 0.029 | −23 | −83 | −36 | ||
| 41 | 0.021 | −7 | −72 | −28 | ||
FIG. 1.
Gray matter (GM) abnormalities in TLE-MTS: Left side: Reductions of GM concentration (GMC) compared to controls. GM reductions are restricted to the hippocampus, parahippocampal gyrus and cerebellum. Right side: Reductions of GM volumes (GMV) compared to controls. GM volume losses are more widespread and also involve extrahippocampal and extralimbic regions (cf. Table 1).
WM concentration/volume changes
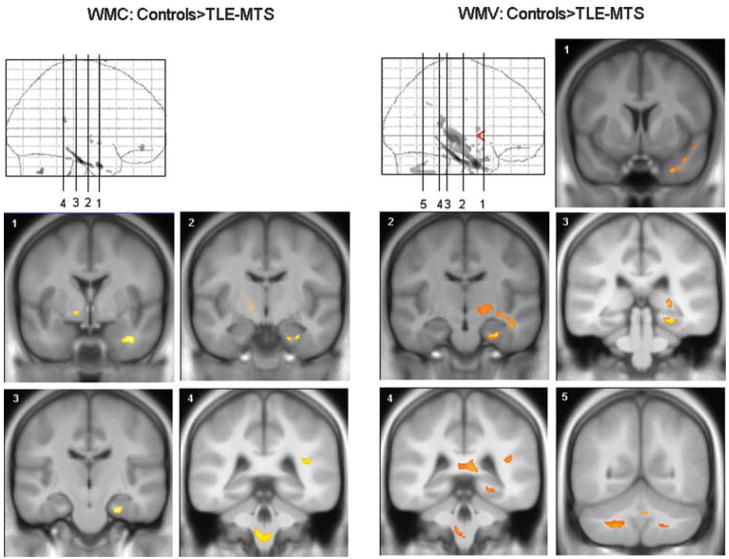
Table 2 displays the results of the WM changes. Compared with controls, TLE-MTS had significantly reduced WMC in WM tracts of the ipsilateral parahippocampal gyrus, temporal, frontal, and parietal lobes, and in the contralateral internal capsule and cerebellum and brainstem. As for GM, WMV reductions affected similar regions as WMC reductions but were more extensive and affected additional regions (i.e., WM regions in ipsilateral internal capsule and cerebellum and in the splenium of the corpus callosum) (Fig. 2). Again, TLE-no had no regions of significant WMC or WMV reductions when compared with controls or with TLE-MTS.
TABLE 2.
White matter: regions with reduced WM concentration/volume in TLE-MTS compared to controls
| Comparison | Region | Cluster | p Corrected | Coordinates | ||
|---|---|---|---|---|---|---|
| WM unmodulated Controls>TLE-MTS | ||||||
| Ipsi parahippocampal gyrus | 872 | 0.000 | 26 | −20 | −26 | |
| Ipsi temporal lobe | 321 | 0.000 | 37 | 0 | −30 | |
| 39 | 0.020 | 30 | 8 | −36 | ||
| 38 | 0.014 | 37 | −6 | −42 | ||
| Ipsi frontal lobe | 110 | 0.008 | 20 | 45 | −12 | |
| Ipsi parietal lobe | 72 | 0.006 | 36 | −38 | 18 | |
| Contra internal capsula | 51 | 0.008 | −13 | −8 | −5 | |
| 46 | 0.005 | −13 | −1 | −7 | ||
| Contra cerebellum (corpus medullare) | 124 | 0.001 | −18 | −63 | −37 | |
| Brainstem (tegmentum) | 687 | 0.001 | −3 | −36 | −50 | |
| WM modulated Controls> TEL-MTS | ||||||
| Ipsi parahippocampal gyrus | 8207 | 0.000 | 26 | −21 | −25 | |
| Ipsi temporal lobe | 49 | 0.018 | 50 | 6 | −15 | |
| Ipsi inferior temporal gyrus | 39 | 0.009 | 39 | −34 | −23 | |
| Ipsi internal capsula (crus posterius) | 80 | 0.015 | 10 | 0 | 7 | |
| Ipsi frontal lobe | 53 | 0.013 | 21 | 45 | −12 | |
| Ipsi parietal lobe | 178 | 0.009 | 42 | −37 | 18 | |
| 38 | 0.027 | 41 | −52 | 34 | ||
| Ipsi cerebellum (corpus medullare) | 890 | 0.003 | 4 | −57 | −31 | |
| Splenium corpus callosi | 440 | 0.002 | 10 | −39 | 10 | |
| Contra cerebellum (corpus medullare) | 1813 | 0.001 | −22 | −49 | −45 | |
| Brainstem (tegmentum) | 166 | 0.013 | −6 | −38 | −49 | |
FIG. 2.
White matter (WM) abnormalities in TLE-MTS: Left side: Reductions of white matter concentration (WMC) compared to controls. WM reductions are restricted to the parahippocampal gyrus, temporal white matter and capsula interna. Right side: Reductions of WM volumes (WMV) compared to controls. WM volume losses are more extensive, involve larger parts of the temporal lobe but also extrahippocampal regions, e.g., splenium, posterior limb of capsula interna, cerebellum (cf. Table 2).
DISCUSSION
The major findings in this study were the following:
TLE-MTS had significant extrahippocampal GMV reductions in several neocortical, limbic and subcortical brain regions directly connected to the epileptogenic hippocampus but also in ipsi- and contralateral brain regions not directly connected to it (e.g., contralateral neocortical regions, and ipsi- and contralateral subcortical and cerebellar regions). GMC reductions were restricted to the ipsilateral hippocampus and small regions in the ipsilateral limbic system and in the cerebellar hemispheres. Furthermore, regions of WMC/WMV reductions were found in the ipsilateral limbic system; temporal, frontal, and parietal lobes; internal capsule; and ipsi- and contralateral cerebral hemispheres and brainstem (i.e., in WM regions connecting GM regions with extrahippocampal volume reductions).
TLE-no as a group had no significant GM or WM reductions when compared with controls or with TLE-MTS.
The first major finding was that TLE-MTS had significant GMV reductions in several extrahippocampal brain regions. These were most prominent ipsilaterally and affected limbic (entorhinal cortex, parahippocampal cortex, posterior cingulate), neocortical (insula, temporal, parietal, occipital lobe), subcortical (thalamus), and cerebellar brain regions. However, contralateral subcortical, cerebellar, and neocortical structures also were affected. These findings are in good agreement with previous studies using optimized VBM in TLE-MTS (14–17,22). These GM volume reductions were associated with WM volume reductions in the ipsilateral limbic system (parahippocampal gyrus); temporal, frontal, and parietal lobe; and splenium of the corpus callosum. Again, these findings are in good agreement with extrahippocampal WM reductions in TLE-MTS described by other VBM studies (16,23) and also with WM abnormalities described by DTI and relaxometry studies (24–26). However, in addition to this, we found WM reductions in the ipsi- and contralateral cerebellum, brainstem (tegmentum), and internal capsule (posterior limb). The nature of the volume loss in extrahippocampal structures in TLE-MTS is unknown. Several possible mechanisms could account for it:
The loss of efferent neurons in the epileptogenic hippocampus could lead to deafferentation and thus volume loss in synaptically connected extrahippocampal brain regions. This mechanism is consistent with the fact that the most pronounced GM/WM volume losses were found in ipsilateral brain regions receiving direct afferent input from the epileptogenic hippocampus. However, as ipsilateral structures not receiving direct input from the hippocampus and contralateral structures were also affected, deafferentation cannot be solely responsible for GM volume loss in TLE-MTS.
Epileptic discharges are often not confined to the epileptogenic focus but propagate to other brain regions (27–30). Therefore local excitotoxic effects of the spreading epileptogenic activity could lead to neuronal loss not only in regions receiving direct input from the hippocampus but also in distant brain regions, secondarily involved in seizure spread (e.g., contralateral thalamic and neocortical brain regions). This hypothesis is supported by the fact that a good agreement exists between ipsi- and contralateral regions with GM loss found in this study and regions showing hyperperfusion in early and late ictal SPECT studies (31–33).
The first two mechanisms assume that extrahippocampal volume losses in TLE-MTS are entirely acquired (i.e., a consequence of ongoing seizure activity). However, it cannot be excluded that at least part of the extrahippocampal volume loss results from a subtle developmental abnormality (34), which not only predisposes for the development of epilepsy later in life but also results in an abnormal connectivity (35) and thus in structural abnormalities in other brain regions (36).
Finally, it is very possible that not one mechanism is responsible for the extrahippocampal volume losses in TLE-MTS but a combination of several of them.
The second major finding was the absence of WM and GM reductions in TLE-no in comparison to controls but also in comparison to TLE-MTS. Whereas the absence of volumetric changes in the hippocampus was expected, the complete absence of volume losses in extrahippocampal brain regions was not. Two possible explanations exist for this finding.
No extrahippocampal structural abnormalities exist in TLE-no. However, in this case, it would be expected that a comparison of TLE-MTS with TLE-no would show a similar atrophy pattern as does the comparison of TLE-MTS with controls. This was not the case.
Structural abnormalities exist in TLE-no, but they are not detected because of limitations of the VBM technique. For example, VBM might fail, if the volume reductions are subtle or confined to small brain regions prone to imaging artifacts or both. Studies using VOI analyses found structural abnormalities in the ipsilateral amygdala and entorhinal cortex (10,11). Even though the VBM technique used in this study was able to detect atrophic changes in the ipsilateral entorhinal cortex in TLE-MTS, entorhinal volume reductions in TLE-no are subtler than those in TLE-MTS [TLE-no, about −20%; TLE-MTS, about −30 to −40% volume loss compared with controls (10,37)] and thus might be missed by VBM. Furthermore, VBM is based on group comparisons and thus only detects atrophic abnormalities, which are homogeneous within a patient group. Therefore we conclude that the absence of extrahippocampal atrophic changes detected by VBM in TLE-no does not necessarily indicate that no structural abnormalities are present but rather that they are subtle and less homogeneous than those in TLE-MTS. The assumption that TLE-no is associated with extrahippocampal abnormalities is supported by findings of PET and MR spectroscopy studies. These studies found metabolic abnormalities in TLE-no in the same brain regions as found in TLE-MTS. However, in TLE-no, these abnormalities tended to be subtler and regionally less well defined than those in TLE-MTS (38,39). The fact that extrahippocampal metabolic and most probably structural abnormalities are less severe in TLE-no suggests that the mechanisms causing these abnormalities in TLE-MTS are absent or mitigated in TLE-no. The reason for this can only be speculated on, but it further supports the notion that, despite their similar seizure semiology and presumably identical seizure origin, these two forms of mTLE are two different clinicopathologic entities.
This study has limitations.
Because we used a symmetrical template and priors, we did no test for differences of GM and WM reductions between right-sided TLE-MTS and left-sided TLE-MTS, as was done in other studies. Because the findings in TLE-MTS were in good agreement with previous VBM studies, we do not believe that the side-flipping left TLE and the use of a symmetric template and priors influenced our results in this group. However, we cannot completely exclude that this approach prevented us from detecting subtle abnormalities in TLE-no.
The number of TLE-no patients was relatively small. Thus it cannot be completely excluded that the statistical power might have been too small to detect the subtle changes suspected in this group.
We did not try to include a measure of epilepsy severity (e.g., lifetime seizure burden) in our analysis, as this information could often not be obtained reliably.
All patients in this study were referred from a tertiary care center where they were evaluated for epilepsy surgery and thus might not be representative of a more general TLE population.
In conclusion, TLE-MTS is associated with GM and WM abnormalities that are not restricted to the focus and brain regions directly receiving neuronal input from the focus but also affect more distant brain regions: the nature of these abnormalities is not clear and most probably is due to a combination of several mechanisms (deafferentation, seizure spread, developmental abnormality). In contrast, TLE-no is not associated with extrahippocampal abnormalities that can be detected by VBM. This further supports the hypothesis that TLE-no is a distinct clinicopathologic entity from TLE-MTS and probably heterogeneous in itself.
Acknowledgments
The study was supported by a NIH grant (ROI-NS31966) to Dr. Laxer.
References
- 1.De Lanerolle NC, Kim JH, Williamson A, et al. A retrospective analysis of hippocampal pathology in human temporal lobe epilepsy: evidence for distinctive patient subcategories. Epilepsia. 2003;44:677–687. doi: 10.1046/j.1528-1157.2003.32701.x. [DOI] [PubMed] [Google Scholar]
- 2.Bernasconi N, Bernasconi A, Caramanos Z, et al. Mesial temporal damage in temporal lobe epilepsy: a volumetric MRI study of the hippocampus, amygdala and parahippocampal region. Brain. 2003;126:462–469. doi: 10.1093/brain/awg034. [DOI] [PubMed] [Google Scholar]
- 3.Jutila L, Ylinen A, Partanen K, et al. MR volumetry of the entorhinal, perirhinal and temporopolar cortices in drug refractory temporal lobe epilepsy. AMNR Am J Neuroradiol. 2001;22:1490–1501. [PMC free article] [PubMed] [Google Scholar]
- 4.Coste S, Ryvlin P, Hermier M, et al. Temporopolar changes in temporal lobe epilepsy: a quantitative MRI-based study. Neurology. 2002;59:855–861. doi: 10.1212/wnl.59.6.855. [DOI] [PubMed] [Google Scholar]
- 5.Moran NE, Lemieux L, Kitchen ND, et al. Extrahippocampal temporal lobe atrophy in temporal lobe epilepsy and mesial temporal sclerosis. Brain. 2001;124:167–175. doi: 10.1093/brain/124.1.167. [DOI] [PubMed] [Google Scholar]
- 6.Desay NP, Jaroz JM, Elwes RCD, et al. Thalamic changes with mesial temporal sclerosis: MRI. Neuroradiology. 2000;42:346–351. doi: 10.1007/s002340050896. [DOI] [PubMed] [Google Scholar]
- 7.Dreifuss S, Vingerhoets FJG, Lazeyras F, et al. Volumetric measurements of subcortical nuclei in patients with temporal lobe epilepsy. Neurology. 2001;57:1636–1641. doi: 10.1212/wnl.57.9.1636. [DOI] [PubMed] [Google Scholar]
- 8.Hagemann G, Lemieux L, Free SL, et al. Cerebellar volumes in newly diagnosed and chronic epilepsy. J Neurol. 2002;249:1651–1658. doi: 10.1007/s00415-002-0843-9. [DOI] [PubMed] [Google Scholar]
- 9.Liu RSN, Lemieux L, Bell GS, et al. Progressive neocortical damage in epilepsy. Ann Neurol. 2003;53:312–324. doi: 10.1002/ana.10463. [DOI] [PubMed] [Google Scholar]
- 10.Bernasconi N, Bernasconi A, Caramanos Z, et al. Entorhinal cortex atrophy in epilepsy patients exhibiting normal hippocampal volumes. Neurology. 2001;56:1335–1339. doi: 10.1212/wnl.56.10.1335. [DOI] [PubMed] [Google Scholar]
- 11.Bowers SPC, Vogrin SJ, Morris K, et al. Amygdala volumetry in “imaging-negative” temporal lobe epilepsy. J Neurol Neurosurg Psychiatry. 2003;74:1245–1249. doi: 10.1136/jnnp.74.9.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ashburner J, Friston KJ. Voxel-based morphometry: the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 13.Woermann FG, Free SL, Koepp MJ, et al. Voxel-by-voxel comparison of automatically segmented cerebral gray matter: a rater independent comparison of structural MRI in patients with epilepsy. Neuroimage. 1999;10:373–384. doi: 10.1006/nimg.1999.0481. [DOI] [PubMed] [Google Scholar]
- 14.Keller SS, Wieshmann UC, Mackay CE, et al. Voxel based morphometry of grey matter abnormalities in patients with medically intractable temporal lobe epilepsy: effects of side of seizure onset and epilepsy duration. J Neurol Neurosurg Psychiatry. 2002;73:648–656. doi: 10.1136/jnnp.73.6.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keller SS, Wilke M, Wieshmann UC, et al. Comparison of standard and optimized voxel-based morphometry for analysis of brain changes associated with temporal lobe epilepsy. Neuroimage. 2004;23:860–868. doi: 10.1016/j.neuroimage.2004.07.030. [DOI] [PubMed] [Google Scholar]
- 16.McMillan AB, Herman BP, Johnson SC, et al. Voxel-based morphometry of unilateral temporal lobe epilepsy reveals abnormalities in cerebral white matter. Neuroimage. 2004;23:167–174. doi: 10.1016/j.neuroimage.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 17.Bonilha L, Rorden C, Castellano G, et al. Voxel-based morphometry reveals gray matter network atrophy in refractory medial temporal lobe epilepsy. Arch Neurol. 2004;61:1379–1384. doi: 10.1001/archneur.61.9.1379. [DOI] [PubMed] [Google Scholar]
- 18.Good CD, Johnsrude IS, Ashburner J, et al. A voxel-based morphometric study of aging in 465 normal adult human brains. Neuroimage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- 19.Good CD, Johnsrude IS, Ashburner J, et al. Cerebral asymmetry and the effects of sex and handedness on brain structure: a voxel-based morphometric analysis of 465 normal adult human brains. Neuroimage. 2001;14:685–700. doi: 10.1006/nimg.2001.0857. [DOI] [PubMed] [Google Scholar]
- 20.Salmond CH, Ashburner J, Vargha-Khadem F, et al. Distributional assumptions in voxel-based morphometry. Neuroimage. 2002;17:1027–1030. [PubMed] [Google Scholar]
- 21.Nieuwenhuys R, Voogd J, van Huijzen C. The Human Central Nervous System: A Synposis and Atlas. 3. New York: Springer Verlag; 1996. [Google Scholar]
- 22.Bonilha L, Rorden C, Castellano G, et al. Voxel-based morphometry of the thalamus in patients with refractory medial temporal lobe epilepsy. Neuroimage. 2005;25:1016–1021. doi: 10.1016/j.neuroimage.2004.11.050. [DOI] [PubMed] [Google Scholar]
- 23.Bernasconi N, Duchesne S, Janke A, et al. Whole-brain voxel-based statistical analysis of gray matter and white matter in temporal lobe epilepsy. Neuroimage. 2004;23:717–723. doi: 10.1016/j.neuroimage.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 24.Arafanakis K, Herman BP, Rogers BP, et al. Diffusion tensor MRI in temporal lobe epilepsy. Magn Reson Imag. 2002;20:511–519. doi: 10.1016/s0730-725x(02)00509-x. [DOI] [PubMed] [Google Scholar]
- 25.Townsend TN, Bernasconi N, Pike GB, et al. Quantitative analysis of temporal lobe white matter T2 relaxation time in temporal lobe epilepsy. Neuroimage. 2004;23:318–324. doi: 10.1016/j.neuroimage.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 26.Pell GS, Briellmann RS, Waites AB, et al. Voxel-based relaxometry: a new approach for analysis of T2 relaxometry changes in epilepsy. Neuroimage. 2004;21:707–713. doi: 10.1016/j.neuroimage.2003.09.059. [DOI] [PubMed] [Google Scholar]
- 27.Lieb JP, Dasheiff RM, Engel J., Jr Role of frontal lobes in the propagation of mesial temporal lobe seizures. Epilepsia. 1991;32:822–837. doi: 10.1111/j.1528-1157.1991.tb05539.x. [DOI] [PubMed] [Google Scholar]
- 28.Adam C, Saint-Hilaire JM, Richer F. Temporal and spatial characteristics of intracerebral seizure propagation: predictive value in surgery for temporal lobe epilepsy. Epilepsia. 1994;35:1065–1072. doi: 10.1111/j.1528-1157.1994.tb02556.x. [DOI] [PubMed] [Google Scholar]
- 29.Kramer U, Carmant L, Mikati MA. Electroencephalographic discharges of temporal lobe seizures in children and young adults. Electroencephalogr Clin Neurophysiol. 1998;107:353–360. doi: 10.1016/s0013-4694(98)00091-1. [DOI] [PubMed] [Google Scholar]
- 30.Isnard J, Guénot M, Ostrowsky K, et al. The role of the insular cortex in temporal lobe epilepsy. Ann Neurol. 2000;48:614–623. [PubMed] [Google Scholar]
- 31.Van Paesschen W, Dupont P, Van Driel H, et al. SPECT perfusion changes during complex partial seizures in patients with hippocampal sclerosis. Brain. 2003;126:1103–1111. doi: 10.1093/brain/awg108. [DOI] [PubMed] [Google Scholar]
- 32.Blumenfeld H, McNally KA, Vaderhill SD, et al. Positive and negative network correlations in temporal lobe epilepsy. Cereb Cortex. 2004;14:892–902. doi: 10.1093/cercor/bhh048. [DOI] [PubMed] [Google Scholar]
- 33.Tae WS, Joo EY, Kim JH, et al. Cerebral perfusion changes in mesial temporal lobe epilepsy: SPM analysis of ictal and interictal SPECT. Neuroimage. 2005;24:101–110. doi: 10.1016/j.neuroimage.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 34.Kaspar BS, Stefan H, Paulus W. Microdysgenesis in mesial temporal lobe epilepsy: a clinicopathological study. Ann Neurol. 2003;54:501–506. doi: 10.1002/ana.10694. [DOI] [PubMed] [Google Scholar]
- 35.Chevassus-au-Louis N, Represa A. The right neuron at the wrong place: biology of heterotopic neurons in cortical neuronal migration disorders with special reference to associated pathologies. Cell Mol Life Sci. 1999;55:1206–1215. doi: 10.1007/s000180050367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sisodiya SM, Free SL. Disproportion of cerebral surface areas and volumes in cerebral dysgenesis: MRI-based evidence for connectional abnormalities. Brain. 1997;120:271–281. doi: 10.1093/brain/120.2.271. [DOI] [PubMed] [Google Scholar]
- 37.Bernasconi N, Bernasconi A, Andermann F, et al. Entorhinal cortex in temporal lobe epilepsy: a quantitative study. Neurology. 1999;52:1870–1876. doi: 10.1212/wnl.52.9.1870. [DOI] [PubMed] [Google Scholar]
- 38.Carne RP, O’Brien TJ, Kilpatrick CJ, et al. MRI-negative PET-positive temporal lobe epilepsy: a distinct surgically remediable syndrome. Brain. 2004;127:2276–2285. doi: 10.1093/brain/awh257. [DOI] [PubMed] [Google Scholar]
- 39.Mueller SG, Laxer KD, Cashdollar N, et al. Identification of abnormal neuronal metabolism outside the seizure focus in temporal lobe epilepsy. Epilepsia. 2004;45:355–366. doi: 10.1111/j.0013-9580.2004.27603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]