Abstract
PURPOSE
To study the feasibility and clinical potential of visual inspection of hydrogen 1 magnetic resonance (MR) spectroscopic metabolite images for the lateralization of unilateral nonlesional temporal lobe epilepsy (TLE).
MATERIALS AND METHODS
MR imaging and 1H MR spectroscopic imaging were performed of the temporal lobes in 50 patients with TLE and 23 age-matched healthy volunteers. N-acetylaspartate (NAA) and creatine plus choline metabolite images were read by two neuroradiologists who determined lateralization according to the side of lower NAA signal intensity. Quantitative estimates of NAA were calculated by using an automated fitting program.
RESULTS
Agreement in lateralization between readers was significant with a κ score of 0.53 for all patients with TLE and 0.63 for patients displaying mild or marked NAA asymmetry. Among the 50 patients with TLE, lateralization was determined correctly by reader 1 in 38 (76%) patients and by reader 2 in 31 (62%) patients. If limited to patients with mild or marked NAA asymmetry, correct lateralization improved to 30 (77%) of 39 and 16 (80%) of 20 patients, respectively. Combined qualitative reading and quantitative spectral fitting enabled lateralization in 34 (85%) of 40 patients with TLE for reader 1 and 30 (77%) of 39 for reader 2, including nine of 14 patients with TLE with negative MR images.
CONCLUSION
Reading of metabolite images is a feasible and fast means for noninvasive evaluation of patients with TLE who are candidates for surgery and enables lateralization in some patients with negative MR images.
Keywords: Brain, MR, 134.121411, 134.121412, 134.121413; Epilepsy; Magnetic resonance (MR), comparative studies; Magnetic resonance (MR), spectroscopy, 134.12145
Temporal lobe epilepsy (TLE) is the most prevalent cause of both focal and refractory seizures (1). Surgical removal of the focus eliminates or greatly reduces seizures in about 90% of patients with TLE who have concordant hippocampal atrophy (2). Mesial temporal sclerosis is the sole pathologic finding in 65% of temporal lobectomy specimens from adult patients with TLE (3). On magnetic resonance (MR) images, mesial temporal sclerosis displays hippocampal atrophy, prolonged T2, and structural distortion (4). These changes may be assessed both qualitatively or by means of hippocampal volumetry and T2 relaxometry, which increase MR imaging sensitivity (5). Even after quantitative studies, some 20% of patients with TLE have negative MR images (6). Patients with negative MR images have poorer seizure control after hippocampectomy (7).
Hydrogen 1 MR spectroscopic imaging depicts the anatomic distribution of metabolite signals from N-acetylaspartate (NAA), which is a putative neuronal marker (8,9), and creatine (Cr) and choline (Cho)-containing compounds. Previous studies (10-13) have shown that interictal NAA is reduced in the ipsilateral mesial temporal lobe, assisting in the lateralization of TLE even in cases with negative MR images. 1H MR spectroscopic imaging data usually undergo quantitative analysis, which requires time-consuming postprocessing, including voxel selection and spectral fitting. A simpler approach more suitable in the clinical setting is to display the metabolite signals as an image for qualitative assessment. Constantinidis et al (14) previously reported that visual reading of NAA images provides lateralization information in patients with TLE.
Therefore, we tested the feasibility and clinical value of reading 1H MR spectroscopic images in patients with TLE. The first aim of this study was to test the lateralization achieved with 1H MR spectroscopic image readings against that achieved with the electroencephalogram. The second aim was to compare the lateralization from metabolite images with that based on MR images and quantitative spectral analysis, and to explore if lateralization could be determined according to the metabolite images in patients with negative MR images. The third aim was to test if the combination of quantitative spectral analysis and metabolite image reading improved lateralization compared with that of each method alone.
MATERIALS AND METHODS
Subjects
All subjects who entered into this study gave their informed consent according to the guidelines of the University of California San Francisco Committee on Human Research. Fifty patients (24 women, 26 men; mean age, 35.8 years ± 10.6 [SD]) with the clinical and electrophysiologic diagnosis of nonlesional unilateral TLE were examined. Criteria for diagnosis were reported previously (10). In summary, diagnosis of nonlesional unilateral TLE was based on the following criteria: (a) no lesion on diagnostic MR images other than mesial temporal sclerosis, (b) focal ictal temporal lobe patterns recorded with scalpsphenoidal electroencephalography or mesial temporal seizure onset in patients requiring subdural or depth electrode recordings, (c) electroencephalographic recording of at least three spontaneous seizures and no independent contralateral seizure onsets, and (d) clinical features consistent with seizures of temporal lobe origin. Epileptogenic foci were lateralized to the left in 34 patients and to the right in 16 patients. A group of 23 healthy volunteers (12 women, 11 men; mean age, 33.7 years ± 8.5) served as the control population.
Clinical MR Imaging
All patients underwent diagnostic MR imaging independent of the research MR studies. MR imaging was performed with a 1.5-T Signa magnet (GE Medical Systems, Milwaukee, Wis). The protocol included a three-dimensional spoiled gradient-recalled acquisition in the steady state (spoiled GRASS; GE Medical Systems) sequence (repetition time of 36 msec with a minimal echo time, 35° flip angle, 22 × 18-cm field of view, 256 × 192 matrix, 1.5-mm section thickness, no gap), coronal fluid-attenuated inversion-recovery sequence (10,000/120 [repetition time msec/echo time msec], 22 × 18-cm field of view, 256 × 192 matrix, 3-mm section thickness, no gap), coronal T2*-weighted gradient-echo (500/15–34, 20° flip angle), and a high-spatial-resolution coronal T2-weighted fast spin-echo data set (512 × 256 matrix) in the temporal lobes. In-plane resolution for both the spoiled gradient-recalled and fluid-attenuated inversion-recovery sequences used to evaluate the hippocampi was 0.86 × 0.94 mm with a section thickness of 1.5 and 3 mm, respectively.
Diagnostic MR images were assessed by the radiologist on call in a blinded fashion. The reader was informed only that the patient had seizures without mention of the side or the lobe of origin as inferred from clinical and/or electroencephalographic data.
1H MR Spectroscopic Imaging
All research MR acquisitions were performed with a 1.5-T Magnetom Vision unit (Siemens, Erlangen, Germany) with use of a standard circularly polarized head coil. Localization imaging was performed with two-dimensional fast low-angle shot sequences in the sagittal, coronal, and oblique transverse planes. T1-weighted transverse images (500/14, 70° flip angle) were acquired parallel to the long axis of the hippocampus.
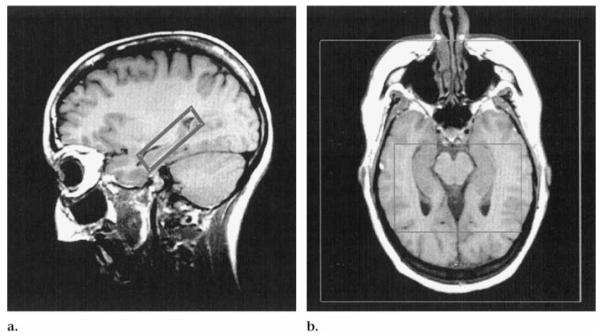
After localized shimming, a two-dimensional MR spectroscopic imaging sequence (1,800/135) with point-resolved spectroscopy (PRESS) (90°, 180°, 180°) volume selection was performed. The PRESS box was positioned parallel to the transverse images, covering both hippocampi and adjacent mesial temporal lobes (Fig 1). The length and width of the volume of interest were adjusted for each individual patient depending on head size, with typical values of 115 × 60 mm. Section thickness was set to 15 mm, which resulted in the inclusion of cerebrospinal fluid and parahippocampal white and gray matter other than the hippocampus, particularly at the level of the hippocampal tail. Posterior parahippocampal NAA reduction in TLE was consistently more pronounced than in more anterior regions (12), so the use of a 15-mm-thick section would result in a good signal-to-noise ratio without loss of information relevant to lateralization. The field of view was 210 × 210 mm. Circular k-space sampling (15) equivalent to a maximum of 24 × 24 phase-encoding steps was applied, with a nominal voxel size of 8.75 × 8.75 × 15 mm3. Water suppression was achieved with chemical shift selective, or CHESS, pulses (16). Measurement time for the MR spectroscopic imaging sequence was 13 minutes 30 seconds.
Figure 1.
PRESS volume of interest positioned in the (a) sagittal and (b) oblique transverse planes on T1-weighted fast low-angle shot images (500/14, 70° flip angle). Outer box in b defines the field of view.
The 90° pulse of the PRESS sequence was applied perpendicular to the sagittal orientation to exclude the influence of an imperfect 180° pulse profile on right-left comparisons of signal intensity. Because of the chemical shift difference between NAA and the Cr-Cho ratio (about 1 ppm), the application of a section selective pulse causes the Cr and Cho signals and the NAA signal to arise from slightly different section locations and might thus produce artifacts. However, we used a gradient strength of 3 mT/m applied with the section selective pulse, which at a field strength of 1.5 T results in a difference in the section position for NAA and the Cr-Cho ratio of approximately 0.5 mm. With a section thickness of 15 mm, we considered this difference too small to have a substantial effect on the results.
Data Processing
Postprocessing of the MR spectroscopic imaging data was performed by using software developed in our laboratory (17,18). Zero filling from 512 to 1,024 time domain data points, water removal, and 4-Hz Gaussian multiplication were applied before Fourier transformation. Spatial domain data were zero filled to 32 × 32 k-space points and apodized, which resulted in an effective voxel size of 2.4 cm3. The areas under the peaks of NAA, Cr, and Cho were calculated by using a least-squares fitting procedure. This procedure included automatic phasing, baseline-correction, and spectral fitting to obtain the peak integrals.
For quantitative spectral analysis, hippocampal voxels were selected on a coregistered MR image. A single radiologist (A.A.C.) performed all voxel selection in the hippocampus. For time comparison purposes with qualitative reading, it required about 10 minutes per case to select and save good-quality spectra from both mesial temporal lobes.
The following metabolite images of the medial temporal lobes were created: (a) raw NAA, by integrating the signal between 1.85 and 2.15 ppm without baseline correction; (b) raw Cr and Cho, by integrating the signal between 2.9 and 3.4 ppm without baseline correction; and (c) fitted NAA images, which resulted from the integration of the NAA area in the baseline-corrected spectrum. The fitted image was thus a closer reflection of the quantitative data than were the raw images. On the other hand, it was also susceptible to errors in baseline determination (eg, due to inadequate water or lipid suppression), which resulted in dark or bright spots. The inclusion of raw and fitted images was decided on when we observed that cases concordant on both images were more robustly lateralized than those lateralized on a single type of image. On display of the metabolite images, an additional linear interpolation to a matrix size of 128 × 128 was performed. To help the reader identify the anatomic position of the MR spectroscopic imaging measurement, the MR image outline of the head was superimposed on images of type a and b and the PRESS box outline on images of type c.
Image Display and Reading
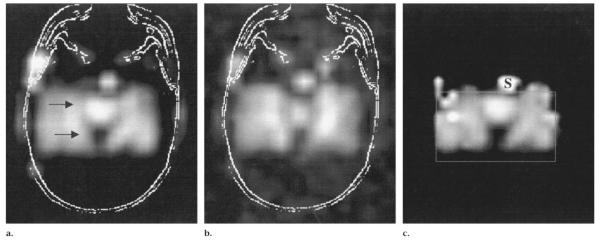
Three metabolite images per case were shown to two neuroradiologists (D.N., H.R.) blinded to the subjects’ diagnosis by means of a program designed ad hoc. The program allowed for windowing of the metabolite images. Figure 2 shows the three metabolite images corresponding to a healthy control subject. The anatomic scout MR images were excluded from the reading to avoid biasing from hippocampal atrophy. The readers freely optimized image windowing in each case. They first went through a training session in which they reviewed a subset of 21 representative cases that illustrated the rationale for interpretation and the features of images in healthy control subjects and in those with TLE. The readers required less than 2 hours to review 73 cases, each of which consisted of three images, including time for optimizing the window level. Therefore, they required a mean of about 90 seconds per case.
Figure 2.
PRESS (1,800/135) two-dimensional MR spectroscopic metabolite images in the transverse plane in a healthy volunteer. (a) Raw NAA image with MR image outline at the same plane as in Figure 1b. The arrows point to the anterior and posterior limits of the hippocampus. (b) Cr and Cho image. (c) Fitted NAA image. S = sphenoid sinus.
For the reading sessions, metabolite images from patients with left and right TLE and from control subjects were randomly intermixed. Each examiner completed two readings of the images; readings were separated by a minimum of 2 weeks to evaluate intrarater and interrater reliability. Lateralization from the second reading is reported herein.
The criterion for lateralization was based on the right-left asymmetry in NAA signal intensity in anterior and mesial temporal lobe regions by using both the raw and fitted NAA images. The Cho and Cr images were checked for signal hypointensities spatially concordant to NAA signal intensity loss. Concordant signal intensity loss on all metabolite images might be indicative of susceptibility artifacts at that position (nonlateralizing) or due to atrophy (lateralizing). In the latter case, a recognizable anatomic pattern was expected. Conversely, if the Cho and Cr image showed hyperintensity in the area with lower NAA signal intensity, it was taken as indication of neuronal loss and gliosis (lateralizing).
The readers assigned a side of decreased relative NAA signal intensity for all the subjects (forced call). The rationale for this forced-call approach was that the study was limited to patients who after extensive clinical evaluation displayed onset of unilateral mesial temporal lobe seizures; thus, the goal was to identify the side of the seizure focus.
From our previous experience, we expected that the NAA asymmetry of many patients with TLE would be in the same range as that of the healthy control subjects. Asymmetry was categorized into three increasing degrees: 1, asymmetry in the range of that of the control group as shown in the training session; 2, mild asymmetry larger than that seen in the control group; and 3, marked asymmetry present on both raw and fitted NAA images. If NAA signal intensity asymmetry was discordant between the raw and fitted images (which happened only in a few cases with degree 1 of asymmetry), then lateralization was decided on the image with the clearer asymmetry in the reader’s opinion.
Most TLE and control metabolite images available in our database were entered into this study; we excluded only those with grossly poor quality due to motion or other artifacts. In addition, readers were asked to indicate which images in their opinion were of poor quality.
Lateralization Based on Quantitative Spectral Data
NAA signal intensity calculated from voxel-selected spectra in the hippocampus was averaged on each side. Only symmetric voxel positions across the midline (same anterior-posterior coordinates) were selected in the right and left sides. A right-left asymmetry index of NAA signal intensity was calculated as follows: AI = 100(RNAA - LNAA)/[(RNAA + LNAA)/2], where AI = asymmetry index, RNAA = right NAA signal intensity, and LNAA = left NAA signal intensity.
All cases with a positive asymmetry index (left side lower than right) were lateralized as left, whereas all cases with a negative asymmetry index (right side lower than left) were lateralized to the right. Lateralization was based on the sign of the asymmetry index regardless of its actual value (forced call).
Statistical Analysis
Intra- and interrater agreement for lateralization was measured by means of the κ statistic, as is standard for such purposes. We used recently devised (19) methods for CI estimation based on profile variances that have superior statistical properties to conventional approaches. κ scores were interpreted as follows: greater than 0.75 indicated excellent agreement; between 0.4 and 0.75, fair or good agreement; and less than 0.4, poor agreement (20).
RESULTS
Seizure Duration
The mean duration of seizures for the subgroup of patients with mesial temporal sclerosis on MR images was 24.5 years ± 11.8, whereas for patients with negative MR images it was 14.5 years ± 11.42. This difference in symptoms duration was statistically significant (P < .01, two-tailed Student t test).
Diagnostic MR Images
The clinical MR images in 32 (64%) of the 50 patients with TLE showed hippocampal atrophy with or without hippocampal hyperintensity on T2-weighted images. In 18 (36%) patients, the clinical MR images were within normal limits. Hippocampal atrophy, when present, was concordant with electroencephalographic lateralization in all cases.
Metabolite Image Reading
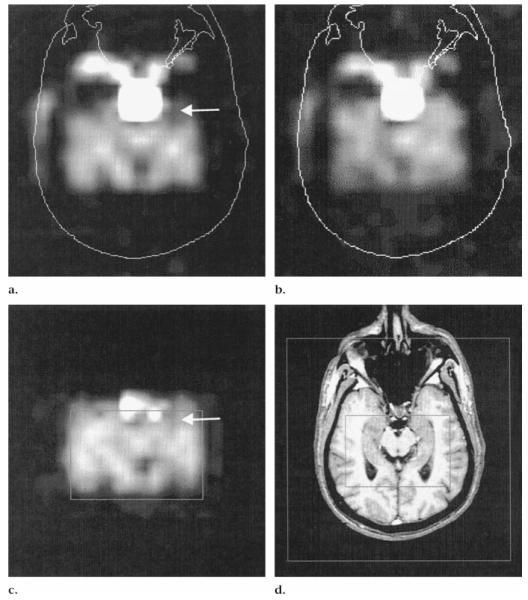
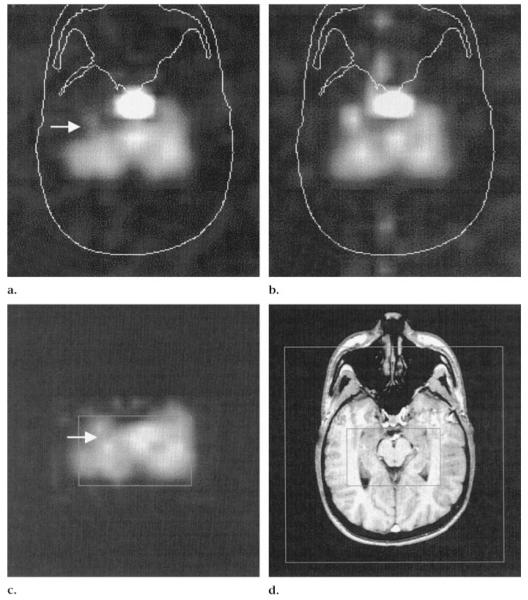
Figures 3 and 4 show examples of metabolite images from patients with TLE with negative MR images. The images clearly demonstrated ipsilateral NAA reduction that could be assessed qualitatively. Figure 5 shows an MR image and MR spectra in a patient with TLE.
Figure 3.
Left TLE in a patient with negative MR images. (a— c) PRESS (1,800/135) two-dimensional MR spectroscopic images in the transverse plane. (a) Raw NAA image shows loss of signal intensity in left anterior temporal lobe (arrow) not detected on (b) the Cr and Cho image. (c) Fitted NAA image displays a similar area of signal intensity loss (arrow) as in a. (d) T1-weighted fast low-angle shot MR image (500/14, 70° flip angle). Inner box corresponds to the box in c; outer box defines the field of view.
Figure 4.
Right TLE in a patient with negative MR images. (a— c) PRESS (1,800/135) two-dimensional MR spectroscopic images in the transverse plane. (a) NAA image shows signal intensity loss (arrow) in the anterior right temporal lobe. (b) Cr and Cho image shows lower signal intensity on the right than on the left. (c) Fitted NAA image displays lower signal intensity on right side (arrow) compared to that on the left. (d) T1-weighted fast low-angle shot MR image (500/14, 70° flip angle). Inner box corresponds to the box in c; outer box defines the field of view.
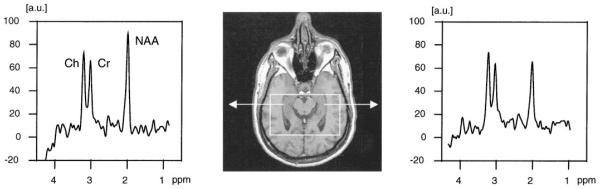
Figure 5.
Scout transverse MR image and spectra from right and left hippocampi in a patient with a left TLE. NAA signal intensity is clearly reduced with respect to Cr and Cho on the abnormal side. a.u. = arbitrary units.
Image quality
Reader 1 marked seven TLE images as having fair or poor quality, whereas reader 2 did so for eight images. They agreed that images in four cases had poor quality.
Poor-quality images were mostly the result of susceptibility artifact due to suboptimal shimming. On the fitted images, inadequate baseline correction produced either bright or dark spots. Images rated as poor quality by the readers were not excluded from the study, but lateralization calculated for each reader after these images were removed is reported later.
Reliability
Intrarater agreement was as follows: The κ score between the first and second readings for lateralization by reader 1 was 0.56; for reader 2, κ was 0.42. By limiting the analysis to cases assigned asymmetry degrees 2 and 3, intrarater agreement rose for reader 1 to a κ of 0.85 and for reader 2 to 0.75.
Interrater agreement was as follows: Consistency in lateralization between the two readers had a κ score of 0.53 for all patients with TLE for the second reading. For cases assigned an asymmetry degree of 2 or 3 by both readers, the interrater κ score was 0.63. These κ scores were statistically significant at the 95% CI (19), which meant that the measured agreement was not coincidental.
Lateralization in Patients with TLE
Results of lateralization are summarized in the Table. Reader 1 correctly determined lateralization in 38 (76%) of 50 patients regardless of the assigned degree of asymmetry and in 33 (77%) of 43 patients after poor-quality images were excluded. When we considered only the 39 cases with asymmetry degrees of 2 or 3 (ie, greater than normal range) assigned by reader 1, correct lateralization was determined by reader 1 in 30 patients (77%). Reader 2 identified the correct side in 31 (62%) of 50 patients and in 27 (64%) of 42 patients after images rated with poor quality were excluded.
TLE Lateralization Summary
| Patients | Reading of Spectroscopic Images |
Spectral Quantitation | Reading and Quantitation |
||
|---|---|---|---|---|---|
| Reader 1 | Reader 2 | Reader 1 | Reader 2 | ||
| All with TLE | 38 of 50 (76) | 31 of 50 (62) | 31 of 50 (62) | 34 of 40 (85) | 30 of 39 (77) |
| With degree 2–3 TLE | 30 of 39 (77) | 16 of 20 (80) | 40 of 50 (80) | 27 of 32 (84) | 16 of 18 (89) |
| All with TLE and negative MR images | 10 of 18 (56) | 10 of 18 (56) | 12 of 18 (67) | 9 of 14 (64) | 9 of 14 (64) |
| All with degree 2–3 TLE and negative MR images | 8 of 14 (57) | 6 of 9 (67) | 12 of 18 (67) | 7 of 11 (64) | 8 of 8 (100) |
Note.—Data in parentheses are the percentage of lateralization.
When restricted to the 20 cases with asymmetry degrees of 2 or 3 assigned by reader 2, correct lateralization was achieved by reader 2 in 16 (80%) patients. Lateralization of TLE in patients (n = 32) with abnormal MR images and those (n = 18) with negative MR images was correct with reader 1 in 28 (88%) and 10 patients (56%), respectively, and correct with reader 2 in 21 (66%) and 10 patients (56%), respectively.
Control subjects
We evaluated how well patients with TLE could be separated from control subjects solely on the basis of reading metabolite images. For this purpose, control subjects were redefined as cases having an asymmetry index of 1, and patients were defined as those having an asymmetry index of 2 or 3; the standard was the clinical classification. Reader 1 correctly discriminated between patients and control subjects in 47 (64%) of 73 cases, and reader 2 discriminated between patients and control subjects in 36 (49%) cases.
Quantitative Spectral Analysis
Correct lateralization based on the asymmetry index of NAA spectral signal intensity was achieved in 40 (80%) of 50 patients; the remaining were incorrectly lateralized. Therefore, quantitative spectral analysis achieved a higher percentage of lateralization than did qualitative reading of metabolite images for both readers (76% for reader 1 and 62% for reader 2 vs 80% for spectral quantitation) when all patients were analyzed. If only cases with marked asymmetry were included, lateralization with qualitative image reading was close to that of quantitative spectral analysis (77% for reader 1 and 80% for reader 2 vs 80% for quantitative analysis) (Table).
The same trend was true when the analysis was focused on patients with negative MR images; thus, quantitative analysis did better than image reading for the complete set of patients with negative MR images (67% vs 55%), but both methods had similar results (57% for reader 1 and 67% for reader 2 vs 67% for quantitative lateralization) when only patients who displayed marked NAA asymmetry were considered.
Combined Spectral Analysis and Metabolite Image Reading
Concordant lateralization with both the quantitative asymmetric index and metabolites image reading was achieved in 40 (80%) of 50 patients with reader 1 and in 39 (78%) patients with reader 2. When we considered only these patients in whom both the quantitative and qualitative lateralization were concordant, reader 1 correctly determined lateralization in 34 (85%) of 40 patients; reader 2, in 30 (77%) of 39 cases. Each reader, in agreement with the quantitative asymmetry index, correctly determined lateralization in nine of 14 patients with negative MR images in the subgroup of patients with TLE with concordant quantitative and qualitative lateralization.
Furthermore, the combination of quantitative asymmetry index and qualitative reading restricted to asymmetry degrees 2 and 3 yielded the following lateralization: Reader 1 correctly determined lateralization in 27 (84%) of 32 patients with TLE with concordant quantitative and reading lateralization and in seven of 11 patients with TLE with negative MR images in this subgroup. Reader 2 correctly determined lateralization in 16 (89%) of 18 patients with TLE with concordant quantitative and qualitative measures and in all eight patients with negative MR images who were in this category.
DISCUSSION
The main findings of this study are the following: (a) Metabolite image reading is feasible and reliable in terms of intrarater and interrater agreement. However, considerable training in reading metabolic images is required. (b) In patients with TLE, including a fraction of those with negative MR images, lateralization can be determined on the basis of NAA image signal intensity asymmetry in agreement with electroencephalographic results. (c) The combination of metabolite image reading and quantitative spectral analysis improved the lateralization compared with each that of method alone.
A display of metabolite images is a fast way to evaluate MR spectroscopic imaging data. When right-left asymmetries are assessed, one side can be compared with the other, eliminating the need for an absolute reference. This is the case in unilateral TLE, where the identification of the abnormal temporal lobe can be achieved in reference to the contralateral side. NAA is concentrated exclusively in neurons in the mature human brain (9), and it is reduced ipsilaterally in TLE due to neuron loss and/or metabolic impairment (11-13,21).
Intrarater reliability for lateralization of TLE between first and second readings had a κ score of 0.85 for reader 1 and 0.75 for reader 2 when the analysis was limited to patients with degree of asymmetry 2 or 3. Interrater reliability had a κ score of 0.53, which is comparable to that of the study by Constantinidis et al (14), who reported a κ score of 0.502. When we considered only patients with mild or marked NAA asymmetry, the κ score increased to 0.63. All of the preceding κ values were statistically significant at the 95% CI (19).
There was a consistent difference between readers in the assignment of the degree of asymmetry, with reader 2 scoring fewer cases with degrees 2 or 3. In consequence, reader 2 attained better lateralization for cases with higher asymmetry than did reader 1, but with less sensitivity (Table). Furthermore, reader 1 achieved higher intrarater reliability than that of reader 2. Optimization of training for readers is expected to improve performance over the present results.
Distinction of patients with TLE from healthy control subjects solely on the basis of NAA asymmetry was poor, possibly due to the presence of NAA asymmetry between temporal lobes in both the healthy control subjects and those with TLE. Additionally, it may reflect the fact that some patients with TLE have contralateral NAA reduction (11), which would reduce the signal intensity asymmetry between ipsilateral and contralateral sides. However, the clinically relevant question to be addressed was if the abnormal temporal lobe in TLE could be identified by using qualitative (and a combination of qualitative and quantitative) estimates of NAA asymmetry. Percentages for TLE lateralization in this study were comparable with the 70% reported by Constantinidis et al (14) in a series of 20 patients. The percentage of correctly lateralized TLE increased with increasing asymmetry (degrees 2 and 3) and approached 80% for both readers.
Qualitative reading resulted in a lower lateralization than did quantitative analysis when images of all patients were analyzed, but both methods had similar results when only those with marked asymmetry were included. The same trend was true when the analysis was focused on patients with negative MR images. We conclude that quantitative spectral lateralization is superior to metabolite image reading in patients displaying subtle NAA signal intensity asymmetry, whereas both methods are equivalent when a strong asymmetry is present.
Furthermore, the combination of quantitative NAA signal intensity asymmetry with metabolite image reading in patients with mild or marked asymmetry yielded a lateralization of 84% and 89% for each reader, which was better than that achieved with either method alone. From this we conclude that in clinical practice, if the NAA asymmetry is not strong, quantitative spectral analysis should be performed.
Thirty-six percent of the patients in this study had negative clinical MR images, whereas the rest displayed hippocampal atrophy with or without hyperintensity on T2-weighted images. The high percentage of patients with TLE and negative MR images included in this study represents a bias in the recruitment of subjects for our MR spectroscopic imaging studies caused by our interest in the evaluation of TLE without evidence of mesial temporal sclerosis on MR images.
Reading of metabolite images attained a better lateralization for both readers in patients with TLE and hippocampal atrophy than in patients with negative MR images. Reduced NAA ipsilateral to hippocampal atrophy can be ascribed to neuronal loss or metabolic impairment (13) in the focus and surrounding tissue including damage secondary to seizure activity. According to the results of prior quantitative spectroscopic studies in patients with TLE and negative MR images (11,22), we hypothesized that NAA asymmetry could help in the detection of some of the abnormal temporal lobes in the group of patients with negative MR images. Figures 3 and 4 show example images of patients with TLE with negative MR images who had evidence of ipsilateral NAA reduction that could be assessed qualitatively.
Each reader correctly determined lateralization at a rate just better than chance (55%) in patients with negative MR images. However, if cases with mild or marked asymmetry were analyzed with the quantitative asymmetry index, lateralization in patients with negative MR images increased to 64% for reader 1 and 100% for reader 2. This finding supports the fact that metabolite image reading may have a role in the evaluation of TLE when MR imaging fails to depict hippocampal changes.
The metabolite images covered an extensive portion of the mesial and anterior temporal lobes, sampling areas not typically included in hippocampal voxel-selected analysis. This broad brain coverage allowed more thorough evaluation of temporal lobe abnormalities in TLE that extend outside the mesial temporal structures. Interictal 2-[fluorine 18]fluoro-2-deoxy-D-glucose positron emission tomography in patients with TLE has depicted extensive ipsilateral hypometabolism (23,24). The combination of voxel-selected spectra from the hippocampus with metabolite images from the temporal lobes yielded an increase in correct lateralization when the hippocampal asymmetry index score and metabolite images were concordant.
The present study has a number of limitations. The forced call approach used for image lateralization could have caused overestimation of NAA asymmetries in the normal range, leading to wrong lateralization. Even after selection of cases with higher asymmetry and concordant quantitative lateralization, there were a number of patients in whom lateralization was incorrectly determined who might still benefit from epilepsy surgery. This fact emphasizes the need for multidisciplinary evaluation of TLE. The role of MR spectroscopic images in such work-ups might be to increase the robustness of lateralization in patients with hippocampal atrophy and to identify the abnormal temporal lobe in patients with negative MR images. Finally, electroencephalography should be complemented with measures of outcome after surgery to obtain a more reliable criterion standard in the lateralization of TLE.
In summary, reading of MR spectroscopic metabolite images in patients with TLE is a feasible means of assessing NAA signal intensity asymmetry between temporal lobes and enables lateralization of the epileptogenic side in agreement with electroencephalographic results in a substantial number of patients with TLE, including those with negative MR images. Improvement in validity and lateralization requires appropriate training of the readers. Quantitative and qualitative TLE lateralization are equivalent in patients displaying strong NAA asymmetry. We conclude that the combination of metabolite image reading with quantitative spectral analysis yields better lateralization than that of each measure alone presumably because of the complementary information provided by each method. However, in patients for which reading the metabolic images does not show a strong asymmetry, quantitative spectral analysis must be performed.
Acknowledgments
K.D.L. supported by NIH grant RO1-NS31966. A.A.M. supported by NIH grant 12119.
Abbreviations
- Cho
choline
- Cr
creatine
- NAA
N-acetylaspartate
- PRESS
point-resolved spectroscopy
- TLE
temporal lobe epilepsy
Footnotes
From the Magnetic Resonance Spectroscopy Unit (114M), Dept of Veterans Affairs Medical Center, 4150 Clement St, San Francisco, CA 94121 (A.A.C., P.V., G.R.E., G.B.M., A.A.M., M.W.W.); and Depts of Radiology (A.A.C., D.N., H.R., M.W.W.), Neurology (K.D.L., H.R., M.W.W.), Medicine (M.W.W.), Psychiatry (M.W.W.), and Div of Biostatistics (M.R.S.), University of California, San Francisco.
References
- 1.Engel J., Jr Surgery for seizures. N Engl J Med. 1996;334:647–652. doi: 10.1056/NEJM199603073341008. [DOI] [PubMed] [Google Scholar]
- 2.Garcia PA, Laxer KD, Barbaro NM, Dillon WP. The prognostic value of qualitative MRI hippocampal abnormalities in patients undergoing temporal lobectomy for medically refractory seizures. Epilepsia. 1994;35:520–524. doi: 10.1111/j.1528-1157.1994.tb02471.x. [DOI] [PubMed] [Google Scholar]
- 3.Babb TL, Brown WJ. Pathological findings in epilepsy. In: Engel J Jr, editor. Surgical treatment of the epilepsies. Raven; New York, NY: 1987. pp. 511–540. [Google Scholar]
- 4.Berkovic SF, Andermann F, Oliver A, et al. Hippocampal sclerosis in temporal lobe epilepsy demonstrated by magnetic resonance imaging. Ann Neurol. 1991;29:175–182. doi: 10.1002/ana.410290210. [DOI] [PubMed] [Google Scholar]
- 5.Van Paesschen W, Connelly A, King MD, et al. The spectrum of hippocampal sclerosis: a quantitative magnetic resonance imaging study. Ann Neurol. 1997;41:41–51. doi: 10.1002/ana.410410109. [DOI] [PubMed] [Google Scholar]
- 6.Van Paesschen W, Connelly A, Johnson CL, Duncan JS. The amygdala and intractable temporal lobe epilepsy: a quantitative magnetic resonance imaging study. Neurology. 1996;47:1021–1031. doi: 10.1212/wnl.47.4.1021. [DOI] [PubMed] [Google Scholar]
- 7.Berkovic SF, McIntosh AM, Kalnins RM, et al. Preoperative MRI predicts outcome of temporal lobectomy: an actuarial analysis. Neurology. 1995;45:1358–1363. doi: 10.1212/wnl.45.7.1358. [DOI] [PubMed] [Google Scholar]
- 8.Urenjak J, Williams SR, Gadian DG, Noble M. Specific expression of N-acetylaspartate in neurons, oligodendrocyte-type-2 astrocyte progenitors, and immature oligodendrocytes in vitro. J Neurochem. 1992;59:55–61. doi: 10.1111/j.1471-4159.1992.tb08875.x. [DOI] [PubMed] [Google Scholar]
- 9.Birken DL, Oldendorf WH. N-acetyl-L-aspartic acid: a literature review of a compound prominent in 1H-NMR spectroscopic studies of brain. Neurosci Biobehav Rev. 1989;13:23–31. doi: 10.1016/s0149-7634(89)80048-x. [DOI] [PubMed] [Google Scholar]
- 10.Knowlton RC, Laxer KD, Ende G, et al. Presurgical multimodality neuroimaging in electroencephalographic lateralized temporal lobe epilepsy. Ann Neurol. 1997;42:829–837. doi: 10.1002/ana.410420603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ende GR, Laxer KD, Knowlton RC, et al. Temporal lobe epilepsy: bilateral hippocampal metabolite changes revealed at proton MR spectroscopic imaging. Radiology. 1997;202:809–817. doi: 10.1148/radiology.202.3.9051038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cendes F, Caramanos Z, Andermann F, Dubeau F, Arnold DL. Proton magnetic resonance spectroscopic imaging and magnetic resonance imaging volumetry in the lateralization of temporal lobe epilepsy: a series of 100 patients. Ann Neurol. 1997;42:737–746. doi: 10.1002/ana.410420510. [DOI] [PubMed] [Google Scholar]
- 13.Connelly A, Jackson GD, Duncan JS, King MD, Gadian DG. Magnetic resonance spectroscopy in temporal lobe epilepsy. Neurology. 1994;44:1411–1417. doi: 10.1212/wnl.44.8.1411. [DOI] [PubMed] [Google Scholar]
- 14.Constantinidis I, Malko JA, Peterman SB, et al. Evaluation of 1H magnetic resonance spectroscopic imaging as a diagnostic tool for the lateralization of epileptogenic seizure foci. Br J Radiol. 1996;69:15–24. doi: 10.1259/0007-1285-69-817-15. [DOI] [PubMed] [Google Scholar]
- 15.Maudsley AA, Matson GB, Hugg JW, Weiner MW. Reduced phase encoding in spectroscopic imaging. Magn Reson Med. 1994;31:645–651. doi: 10.1002/mrm.1910310610. [DOI] [PubMed] [Google Scholar]
- 16.Haase A, Frahm J, Hanicke W, Matthaei D. 1H NMR chemical shift selective (CHESS) imaging. Phys Med Biol. 1985;30:341–344. doi: 10.1088/0031-9155/30/4/008. [DOI] [PubMed] [Google Scholar]
- 17.Maudsley AA, Lin E, Weiner MW. Spectroscopic imaging display and analysis. Magn Reson Imaging. 1992;10:471–485. doi: 10.1016/0730-725x(92)90520-a. [DOI] [PubMed] [Google Scholar]
- 18.Soher BJ, Young K, Govindaraju V, Maudsley AA. Automated spectral analysis III: application to in vivo proton MR spectroscopy and spectroscopic imaging. Magn Reson Med. 1998;40:822–831. doi: 10.1002/mrm.1910400607. [DOI] [PubMed] [Google Scholar]
- 19.Lee JJ, Tu ZN. A better confidence interval for kappa on measuring agreement between two raters with binary outcomes. J Comput Graph Stat. 1994;3:301–321. [Google Scholar]
- 20.Fleiss JL. Statistical methods for rates and proportions. Wiley; New York, NY: 1981. [Google Scholar]
- 21.Cendes F, Andermann F, Dubeau F, Matthews PM, Arnold DL. Normalization of neuronal metabolic dysfunction after surgery for temporal lobe epilepsy: evidence from proton MR spectroscopic imaging. Neurology. 1997;49:1525–1533. doi: 10.1212/wnl.49.6.1525. [DOI] [PubMed] [Google Scholar]
- 22.Connelly A, Van Paesschen W, Porter DA, Johnson CL, Duncan JS, Gadian DG. Proton magnetic resonance spectroscopy in MRI-negative temporal lobe epilepsy. Neurology. 1998;51:61–66. doi: 10.1212/wnl.51.1.61. [DOI] [PubMed] [Google Scholar]
- 23.Henry TR, Mazziota JC, Engel J., Jr Interictal metabolic anatomy of mesial temporal lobe epilepsy. Arch Neurol. 1993;50:582–589. doi: 10.1001/archneur.1993.00540060022011. [DOI] [PubMed] [Google Scholar]
- 24.Sackellares JC, Siegel GJ, Abou-Khalil BW, et al. Differences between lateral and mesial temporal metabolism interictally in epilepsy of mesial temporal origin. Neurology. 1990;40:1420–1426. doi: 10.1212/wnl.40.9.1420. [DOI] [PubMed] [Google Scholar]