Abstract
Previous studies have demonstrated a positive correlation between glucocorticoid levels and circadian reentrainment time following a shift in the light:dark (LD) cycle. We conducted a series of experiments to examine the circadian dependence of the corticosterone (CORT) response to light. Exp. 1 measured CORT release in rats exposed to light at six timepoints. Light presented during the subjective night increased CORT (p < 0.05), while light presented during the subjective day did not. In Exp. 2, we documented the time course of the CORT response to light in entrained animals. Rats exposed to light at zeitgeber time (ZT) 18 had a maximal increase in CORT levels following 60min of stimulus presentation (p < 0.05). There was also an increase in adrenocorticotropic hormone following 15min of light at ZT18 (p < 0.05). In an effort to elucidate the effect of changes in the LD cycle on the circadian profile of CORT, Exp. 3 followed the CORT rhythm (in cerebrospinal fluid) of rats prior to and following a shift in the LD cycle. The CORT nadir was elevated following a 6h photic advance (p < 0.05), as was the mean CORT concentration during the peak phase (p < 0.05). Most components of the circadian CORT rhythm, however, failed to show an immediate shift towards the change in the light cycle. Together, these data support the hypothesis that a photic phase-shift results in elevated CORT levels, while the rhythm of CORT secretion is robust against changes in the photic environment.
Keywords: circadian, corticosterone, adrenocorticotropic hormone, stress, light, rat, jetlag, phase-shift
Introduction
Abrupt changes in the light cycle result in desynchrony of internal circadian rhythms, which may last for days or even weeks. In humans, this type of chronic disruption can manifest in the form of ulcers, depression, and emotional distress [1, 2]. These symptoms are similar to those associated with stress and are commonly correlated with an increase in glucocorticoid secretion [3, 4].
Glucocorticoids, typically under the control of the hypothalamic-pituitary-adrenal (HPA) axis, also play an important role in allowing an organism to rapidly adapt to changes in the environment and in maintaining general homeostasis. The HPA axis and circadian system interact at many levels, with HPA hormones following a circadian pattern of release [5-7] and the magnitude of a stress response being dependent upon time of day [8-10]. Furthermore, glucocorticoids are thought to play a role in maintaining circadian entrainment in peripheral tissues [11-14].
In addition to the relationship between glucocorticoids and circadian rhythms under entrained conditions, there is increasing evidence that stress responsive systems can influence reentrainment following a phase-shift of the light cycle. We have previously demonstrated that altering the stress state of an animal can modify the rate of reentrainment of circadian activity rhythms following a shift of the light:dark (LD) cycle [15, 16]. Restraint stress presented at the time of a photic phase-shift delays recovery of normal circadian entrainment [15], while blocking the glucocorticoid response accelerates reentrainment [16, 17]. The magnitude of the corticosterone (CORT) response of rats to restraint stress under normal, entrained conditions is positively correlated with the time it takes those animals to reentrain following a shift in the LD cycle [18]. This raises the possibility that glucocorticoid activation, during or around the time of a phase-shift, contributes to the interindividual variability seen in reentrainment rate.
There is some support for the hypothesis that the adrenal is activated in response to a photic phase-shift. A rise in glucocorticoid levels has been observed in rodents and humans following a shift in the LD cycle or other circadian challenge [16, 19-22]. It remains unclear, however, to what extent the glucocorticoid rise is a result of the photic stimulus/phase-shift itself and to what extent it is the result of other confounding variables.
The current study sought to quantify the CORT response to light exposure. We tested the circadian nature of light exposure on the CORT response as well as the immediate and longer-lasting effects of a change in light on the circadian rhythm of CORT release.
In the first experiment, male rats were released into constant darkness and then exposed to 1h of light at six timepoints over a 24h period. CORT levels were measured following the full hour of light exposure. It was hypothesized that light would only elicit a CORT response during the subjective night, a time of day when light is not normally seen.
In a second study, we hypothesized that a light pulse would initiate a rapid HPA response. We examined the temporal profile of CORT and adrenocorticotropic hormone (ACTH) activation in response to light presented during the middle of the dark period. Animals in this study were presented with 1h of light exposure at zeitgeber time (ZT) 18, on the background of an entraining LD cycle. This is a time of light onset consistent with the phase-shifts imposed in our previous studies [15, 16].
Another aim of this study was to document the circadian rhythm of CORT secretion in response to a photic phase-shift. We hypothesized that a phase-shift results in both an immediate and a long-lasting effect on CORT levels, but not an immediate shift of the CORT rhythm. In the third experiment, we used in vivo microdialysis to observe the profile of CORT concentrations in the lateral ventricle before and after a shift of the LD cycle. This method has been established as valid for measurement of CORT secretion in rodents [7, 23], and allows animals to remain undisturbed during sampling.
Methods
Experiment 1: Effect of Light on CORT
Subjects
Male Sprague-Dawley rats (n = 60), approximately 60 days of age, were obtained from Charles River Laboratories (Wilmington, MA). Rats were housed singly in 26.7 × 20.3cm Nalgene cages. Rodent chow (Purina 5001) and water were available ad libitum. Prior to entering the experiment, all animals were maintained in a 12:12 LD cycle for at least two weeks to ensure that the animals were entrained to the light cycle. All procedures were approved by the University Committee for the Care and Use of Animals at the University of Michigan.
Experimental Procedure
Animals were released into constant darkness (DD). Following 24h in DD, animals were exposed to 1h of either control (no light) or light-exposed conditions at one of the following circadian timepoints (n = 10 animals/timepoint): CT1-CT2, CT5-CT6, CT9-CT10, CT13-CT14, CT17-CT18, or CT21-CT22 (where CT0 is subjective dawn and CT12 is subjective dusk). Light exposure consisted of 1h of white light of an intensity equal to that present in the vivarium under LD conditions (∼250 lux). Control conditions consisted of 1h in continued darkness, with sampling done under dim red light. Following 1h of treatment, blood samples were taken via a tail clip procedure (see General Methods).
After animals were sampled, they were placed back onto a 12:12 LD cycle. Following thirteen days in LD, animals were again released into DD and the experimental procedures above were replicated. Animals previously assigned to the control condition were now exposed to light at the same CT as their previous treatment. Animals previously exposed to light were now treated with control conditions, resulting in a cross-over design. Animals were sampled as described above and all samples were assayed for CORT (see General Methods).
Experiment 2: Temporal Profile of Hormone Release in Response to Light at ZT18
Subjects
Male Sprague-Dawley rats (n = 46), approximately 60 days of age, were obtained from Charles River Laboratories (Wilmington, MA) and housed as described above.
Experimental Procedure
Thirty-four of the animals were assigned to one of five experimental conditions, with blood samples taken at the following durations following the onset of light exposure: 0min (control), 15min, 30min, 60min, and 180min. The control group contained 10 animals, a larger N than the groups experiencing light, because during preliminary trials it proved difficult to obtain assayable amounts of blood under the dim red light conditions. The four treatment groups contained 6 animals each. The 15min, 30min, 60min, and 180min groups were exposed to light beginning at ZT18 and continuing, in the case of the 60 and 180min groups, until ZT19. Control animals were sampled, using the blood sampling procedure described below, under dim red light at ZT18. Fifteen minutes after the onset of light exposure (ZT18.25), the 15min group was sampled. The 30min, 60min, and 180min groups were sampled at ZT18.5, ZT19, and ZT21 respectively. Thus, the 180min group was exposed to only 60min of light, followed by 120min of darkness prior to sampling. The samples were processed as described below (see General Methods) and stored for later CORT analysis.
The remaining 12 rats were randomly assigned to a control or 15min light exposure group (n = 6 animals/group) to obtain blood samples for ACTH assay. Time of blood draw was chosen based on preliminary studies demonstrating a maximal ACTH response to light following 15min of exposure. Additionally, the rise in ACTH happens rapidly following stressor exposure and declines more quickly than CORT [24]. The control animals were sampled, as described below, under dim red light at ZT18. The 15min group was sampled at ZT18.25, following a 15min light exposure. The samples were processed as described below and stored for later ACTH analysis.
Experiment 3: Profile of CORT Rhythm following a 6h photic phase advance
Subjects
Male Sprague-Dawley rats (n = 5), approximately 60 days of age, were obtained from Charles River Laboratories (Wilmington, MA). The rats were maintained in a 12:12 LD cycle (lights on 1200h to 2400h) for at least 3 weeks after arrival to ensure that the animals were entrained to the local LD cycle. Rats were fed approximately 25g of rodent chow (Purina 5001) per day to maintain a stable weight and prevent the animals from getting so large as to interfere with the experimental chamber. Animals rarely consumed all 25g of food before the next feeding (feeding time varied from day to day). Water was made available ad libitum. Animals were housed in pairs in 26.7 × 20.3cm Nalgene cages until surgery was performed, after which they were housed singly.
Surgery
A guide cannula was implanted into the lateral ventricle of each animal, which would later house a microdialysis probe. Rats were anesthetized with 5% isoflurane gas (Aerrane, Baxter Pharmaceutical, Deefield, IL) at a rate of 5 liters/min. Following initial anesthesis, the isoflurane rate was reduced to 3 liters/min and 0.1mg/kg butorphenol tartrate was injected intraperitoneally. A guide cannula (CMA12, CMA/MD, North Chelmsford, MA) was implanted unilaterally into the lateral ventricle (stereotaxic coordinates: 0.8mm anterior and 1.4mm lateral to Bregma, at a depth of 3.8mm). The cannula was held in place using four small anchor screws (Plastics One, Roanoke, VA) and dental acrylic (Fastray™, Bosworth, Skokie, IL). Postoperatively, 0.05mg/kg butorphenol tartrate, 0.2mg/kg dexamethasone, 20mg/kg choloramphenicol, and 3cc sterile saline were injected subcutaneously. All rats were allowed an undisturbed recovery period of two weeks.
Microdialysis Apparatus
Following the recovery period, rats were moved to the microdialysis housing apparatus. The apparatus consisted of a cylindrical cage (diameter = 9.5cm, height = 44.5cm) with a counter-balance arm (Instech, Boston, MA) affixed to the side. Rats were tethered to the counterbalance arm from their guide cannulae, allowing for a full range of movement within the cage. Animals were allowed at least one week to adjust to the microdialysis housing apparatus and tether before further experimental manipulation occurred.
Probe Implantation
Rats were briefly anesthetized with isoflurane gas. A microdialysis probe (membrane length 4.0mm, 0.5mm OD, membrane molecular weight cutoff of 20,000 daltons; CMA 12, CMA/MD) was lowered into the guide cannula of each animal. Plastic glue was used to affix the microdialysis probe to the cannula. Artificial cerebrospinal fluid [CSF, a modified ringer's solution, 145.0mM NaCl, 2.7mM KCl, 1.2mM CaCl2 ; based on a protocol from (25)] was continuously infused through PEEK tubing (0.65mm OD, 0.12 ID; Bioanalytical Systems, West Lafayette, ID) at a flow rate of 1μl/min. To prevent the tubing from tangling, rats were connected to the counterbalance arm via a dual-channel swivel (Instech, Boston, MA). Dialysate samples were pooled into a fraction collector (CF-1, Spectrum Chromatography, Houston, TX) in 90min intervals. There was no inter-probe variability in dialysate recovery rate.
Procedure
Samples were not collected for 1 day following implantation in order to allow the animals time to recover from any stress response elicited by the probe implantation procedure. Following the recovery period, baseline samples of product dialysate were obtained for a period of 3 days. The LD cycle was then phase advanced 6h, with the new lights-on at 0600h. Dialysate sampling continued at 90min intervals for 2 days post-shift. Dialysate samples were stored at -20°C for later CORT analysis (see General Methods).
General Methods
Blood Sampling Procedure
Blood (approximately 300μl) was collected, via a tail clip procedure [similar to that described in (26)], into microcentrifuge tubes containing 10μL of 0.25M EDTA. All samples were collected within 60s of the experimenter approaching the home cage (to avoid any effect of handling stress on sampled ACTH or CORT levels). Samples were centrifuged at 2500-3000 rpm for 15 minutes at 4°C. Plasma was then separated and stored at -20°C for later analyses.
Radioimmunoassay
Plasma ACTH concentrations were measured using a prepared radioiummunoassay kit (ACTH 125I, MPBiomedicals, Irvine, CA). The minimum detection threshold for ACTH was 10.0pg/ml; the reported average coefficient of variance is 5.5%. CORT concentrations were measured using a prepared radioimmunoassay kit (Cortisosterone 125I, MPBiomedicals, Irvine, CA). The minimum detection threshold was 0.77μg/dl. The manufacturer reports an average intra-assay coefficient of variance of 7.3%. Plasma samples from were assayed for CORT in duplicate. For Exp. 2, undiluted dialysate (30μl/sample) samples from each individual animal were run with a single radioimmunoassay kit.
Statistical Analyses
CORT data in Exp. 1 were analyzed using a repeated measures analysis of variance (ANOVA) with post-hoc t-test comparisons. CORT data in Exp. 2 were analyzed by ANOVA with post-hoc Bonferroni comparisons. ACTH data in Exp. 2 were analyzed using a two-groups t-test.
Repeated measures ANOVA was used to compare measured endpoints from Exp. 3 across baseline days 1, 2, and 3. One animal required more than 1 day to recover from the implantation procedure; data from that animal are therefore excluded from analyses for the first pre-shift day. There was not a significant difference between the 24h CORT rhythms across baseline days, so data from these days were pooled as “pre-shift” for the purposes of further analyses. Mean daily CORT was calculated for each animal on each day, with values above the mean defined as “peak phase” and values below the daily mean as “nadir phase”. Pre- and post-shift values were calculated for daily mean, mean during the peak phase, mean during the nadir phase, hours spent in peak phase (duration of peak phase), hours spent in nadir phase (duration of nadir phase), and timing of onset and offset of peak (acrophase of rhythm) and nadir (trough of rhythm). In addition, daily amplitude, nadir value from 1030 to 1800h (time of pre-shift nadir), and peak value from 2100 to 0300h (time of pre-shift peak) were calculated. ANOVA and t-test analyses were used to compare pre- and post-shift data.
Results
Experiment 1: Effect of Light on CORT
There was a significant main effect of condition on CORT (F(1, 54) = 16.353, p < 0.001), with CORT higher in the light-exposed condition as compared to control (no light). There was also a significant effect of CT on CORT (F(5, 54) = 12.483, p < 0.001). There was an interaction between condition and CT (F(5, 54) = 2.689, p < 0.05; Fig. 1), with light-exposure resulting in significantly greater CORT than control conditions only during the subjective night. Post-hoc comparisons revealed a trend towards light-exposure increasing CORT at CT14 (p = 0.052) and a significant increase in CORT at CT18 and CT22 following light-exposure (p = 0.008 and 0.017 respectively). The order of treatment exposure (light vs. control) did not affect overall CORT levels in either the control or light-exposed conditions (p > 0.05 for both conditions).
Figure 1.
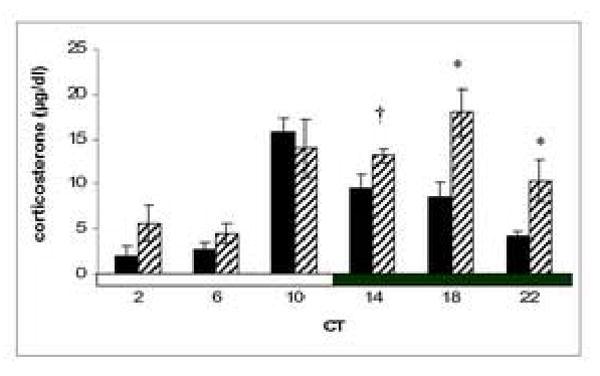
Mean ± SEM CORT for animals under the control (solid bars) or light-exposed (hatched bars) conditions in Exp. 1. The time of blood collection (in circadian time, where CT0 is subjective dawn) is represented on the x-axis. † denotes trend towards a difference from control (p = 0.052); * denotes significant difference from control (p < 0.05).
Experiment 2: Temporal Profile of Hormone Release in Response to Light at ZT18
There was a significant effect of condition on CORT (F(4, 23) = 3.287, p < 0.05), with CORT higher in the 60min group as compared to controls (p < 0.05). Means ± SEM (standard error of the mean) are in Table 1. There was a significant effect of light exposure on ACTH (Student's t(7.1) = -2.594, p < 0.05; Table 1).
Table 1.
Experiment 2: Mean ± SEM CORT and ACTH
| Mean CORT | n | Mean ACTH (pg/ml) | n | |
|---|---|---|---|---|
| Control | 3.5 ± 0.7 | 7 | 48.1 ± 5.9 | 5 |
| 15min | 7.9 ± 2.5 | 4 | 83.6 ± 12.3 * | 6 |
| 30min | 6.4 ± 1.5 | 6 | ----- | |
| 60min | 9.7 ± 1.6* | 6 | ----- | |
| 180min | 4.0 ± 1.3 | 5 | ----- | |
denotes significantly higher than control animals, p < 0.05. Two control animals exhibited atypical behavior during the blood collection procedure and were therefore excluded from CORT analyses.
Experiment 3: Profile of CORT Rhythm following a 6h photic phase advance
Across days 1-3 (pre-shift), there were no significant differences between or within subjects (p > 0.05). The rhythm of CORT cycled with a period of 24h, within the margin of error for 90min samples. There was a trend toward elevated CORT during the peak phase post-shift (F(1, 12) = 3.774, p = 0.070, see Table 2). CORT was significantly increased post-shift between 1030-1800h, the time of the pre-shift nadir (F(1, 14) = 5.679, p = 0.032). There was also a trend towards an effect of the phase-shift on number of hours spent in the peak phase (F(1, 12) = 3.952, p = 0.070). There was a statistically significant difference between the hour at which the peak began post-shift as compared to pre-shift (F(1, 15) = 5.042, p = 0.040). The time of day that the nadir ended was also significantly altered post-shift (F(1, 16) = 7.163, p = 0.017). The post-shift amplitude of the CORT rhythm was not statistically different from the pre-shift amplitude (F(1, 10) = 0.093, p = 0.767). See Fig. 2.
Table 2.
Experiment 3: Mean ± SEM CORT and ACTH
| Pre-shift | Post-shift | ||
|---|---|---|---|
| Duration of peak phase | 8.00 ± 0.53 hours | 5.70 ± 1.29 hours | † |
| Duration of nadir phase | 7.83 ± 0.49 hours | 5.67 ± 0.98 hours | |
| Mean of peak phase (above daily mean) | 23.97 ± 1.37 μg/dl | 30.40 ± 4.54 μg/dl | † |
| Mean of nadir phase (below daily mean) | 4.85 ± 0.90 μg/dl | 6.21 ± 0.73 μg/dl | |
| Mean from 2100-0300h (pre-shift peak) | 25.46 ± 1.25 μg/dl | 34.46 ± 6.19 μg/dl | |
| Mean from 1030-1800h (pre-shift nadir) | 4.24 ± 0.75 μg/dl | 10.52 ± 2.01 μg/dl | * |
| Time of peak onset | 2135h ± 13min | 0204h ± 173min | * |
| Time of nadir offset | 1808h ± 13min | 1350h ± 92min | * |
denotes significantly different from pre-shift values, p < 0.05.
denotes a trend towards change from pre-shift values, 0.10> p > 0.05. Time of peak onset and Time of nadir offset are in clock time (pre-shift lights-off occurred at 2400h).
Figure 2.
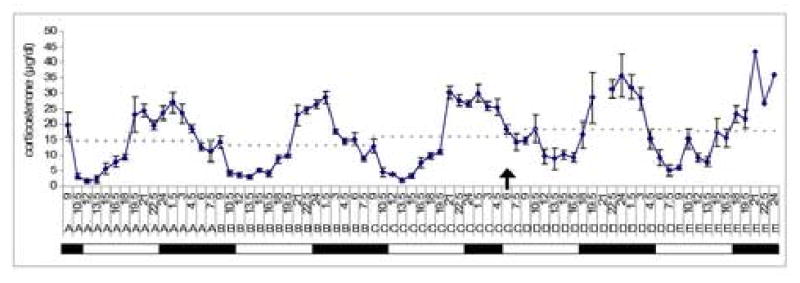
CORT concentration in dialysate for rats on pre-shift days (days A, B, and C) and post-shift days (D and E). The solid line is the mean ± SEM CORT concentration at each timepoint. The dashed line represents the average daily mean (across a 24h period). Clock time is on the x-axis where 12 = 1200h (pre-shift lights-on) and 24 = 2400 (pre-shift lights-off). The bars represent light and dark periods. The time of the 6h photic phase advance is indicated by the arrow (0600h on day C).
Discussion
We investigated the effects of light exposure on ACTH and CORT release. The results of these studies demonstrate that light is capable of eliciting a rapid CORT response in rats, similar to previous findings in humans [21] and mice [20]. This response, however, is limited to the subjective night, suggesting that only light exposures capable of eliciting a robust phase-shift result in a CORT increase. There was an acute ACTH rise following light exposure in the middle of the entrained dark period (Exp. 2). Thus, it appears that the observed CORT increase is, at least in part, HPA-mediated. A shift of the LD cycle immediately altered the level of CORT release, with higher levels of CORT seen at the nadir. The rhythm of CORT release, however, did not immediately adjust its phase to the new LD cycle. We conclude that a phase advance (or light exposure during the dark period) activates glucocorticoid release in a manner consistent with a stress response and that the rhythm of CORT release is largely resistant to changes in the environment.
One hour of light exposure during the subjective night resulted in increased CORT when compared to CORT under control conditions (no light). This rise in CORT is likely due to a direct effect of light on HPA and/or sympathoadrenal function. It is unlikely that the rise in CORT was due to an effect of the treatment stimulus on general activity level, as light suppresses locomotor behavior in nocturnal animals [27]. We observed a rise in CORT following light exposure at CT18 and 22, with a trend towards increased CORT as a result of light at CT14. Light exposure during the subjective day, however, did not result in increased CORT. These data suggest that light occurring at an unexpected time of day results in a stress response. The largest magnitude of CORT increase was observed at CT18, which is the very middle of the subjective night. We therefore conclude that there is a circadian influence on the CORT response to light exposure. This conclusion corroborates similar suggestions made in the existing literature [21, 28]. In addition, light fails to elicit a CORT response in mice with lesions of their suprachiasmatic nuclei (SCN) [20], providing further support for the conclusion that circadian phase is an important factor in light-induced adrenal stimulation.
We examined the immediate effect of light exposure at ZT18 on CORT and ACTH levels. CORT was increasing (as compared to controls) following as little as 15min of light exposure, with this increase in CORT reaching significance at the 60min timepoint. Following 60min of light, CORT levels returned to baseline by 180min. This finding, combined with the stimulating effect of light on ACTH at 15min, demonstrates that light can activate the HPA axis. The continual rise of CORT, however, may also be the result of sympathetic contributions, a hypothesis supported by data from the mouse [20]. It should be noted that in the mouse, light failed to elicit an ACTH response [20]. The reason for the discrepancy between this previous study and our data is unclear, but could be the result of interspecies differences. Ishida et al. used C57/BL mice, a strain with decreased behavioral and physiological expression of anxiety [29]. In addition, this strain often displays a blunted ACTH and CORT response to stressors known to be mediated via the HPA axis [30, 31].
Examination of the 24h CORT rhythm across multiple days further elucidated the effect of a change in the light cycle on CORT. CORT levels were declining at the time of the initial 6h phase advance (when lights came on at 0600h for the first time). The phase-shift prevented the normal CORT decline, with CORT levels remaining elevated above the pre-shift nadir. In addition to the rise in nadir, the peak levels of CORT were elevated in dialysate following the photic phase-shift.
The overall stability of the CORT rhythm following the photic phase advance is noteworthy. The circadian rhythm of CORT does not immediately adjust to the change in the light cycle, suggesting that this rhythm is slow to synchronize to the environmental change. While the end of the nadir exhibited a phase advance of approximately 4h, the onset of the peak actually delayed by approximately 2.5h. This pattern is similar to that seen for melatonin. In response to a photic phase-shift, one component of the melatonin rhythm responds immediately, while a second component shifts more gradually. This is followed by gradual reentrainment of the full profile of melatonin secretion (Liu and Borjigin, 2005). As both melatonin and CORT receive circadian input from the SCN by way of the paraventricular nucleus of the hypothalamus (PVN), the similarity between the responses of these two endocrine profiles is not surprising. It would be informative to measure the CORT and melatonin profiles from the same animals across the period of recovery following a photic phase-shift. In addition, further investigation of the role of the PVN in relaying circadian signals from the SCN to the adrenal may provide further insight into the mechanism by which light increases CORT levels.
While we found a stimulating effect of light on CORT in this study, there is also evidence that light can suppress adrenal function [28]. The opposing effects of light on CORT may be a function of the duration of light exposure. We presented a longer period of light exposure (60min) and sampled animals for a longer time (from 15-180min after the onset of light) than did the previous study, which stopped sampling at 15min following the onset of light. Both the longer light exposure and the additional, later sampling times in the current study may explain the difference in our findings as compared to those of Buijs et al (1999). Buijs et al (1999) found a decrease in CORT only when animals were sampled 5min after light exposure (and only at ZT14); their data show a trend towards a rise in CORT following 15min of light exposure at ZT20. Our data corroborate those obtained in a study of the mouse [20], which reported a maximal CORT response to light 60min after light onset. Ishida et al. (2005) used a methodology similar to ours, with extended light exposure and later sampling times following the onset of the stimulus.
Taken together, our data provide a new perspective with which to view the existing literature on circadian entrainment. Previous studies by our laboratory and others suggest that the length of time it takes to recover normal circadian entrainment, following a shift in the LD cycle, may be influenced by the magnitude of CORT activation [16, 18]. In rodents, suppressing the CORT response at the time of a phase-shift results in accelerated reentrainment [16, 17]. CORT reactivity correlates positively with time to reentrain [18] and exposing animals to additional stress at the time of an LD shift delays reentrainment [15]. The ACTH data presented here suggest that this CORT activation is HPA-mediated in the rat. It is unknown whether CORT or another messenger in the HPA axis (such as arginine vasopressin) or sympathoadrenal system is the factor (or factors) causing the alteration in reentrainment time.
The implications of research on interactions between glucocorticoid release and circadian inputs are varied and far-reaching. Humans experiencing circadian desynchrony suffer from a variety of symptoms [1, 2] which are commonly correlated with glucocorticoid activation [3, 4]. The change in the LD cycle itself could be at least partly responsible for the change in glucocorticoid levels and resulting symptoms, a hypothesis supported by the current study. Keeping CORT levels constant following an LD shift may result in faster recovery of normal circadian entrainment for humans, as it does in rodents [16, 17]. Also, as most elements of the CORT rhythm failed to shift immediately in response to the change in the light cycle, it is tempting to speculate that this prolonged period of desynchrony between CORT release and the environment may contribute to symptoms resulting from jetlag and nightshift work. It has been suggested that CORT acts as an endogenous synchronizer of circadian rhythms in the periphery [11, 12]. The failure of the CORT rhythm we observed to immediately adjust its phase to the new LD cycle may result in a delayed response of peripheral clocks to changes in the phase of the environment and SCN. Pharmacological manipulation of cortisol may prevent this internal desynchrony and prove useful in the treatment of shift-workers and transmeridian travelers. Further research in this area will also have implications for investigation of the interaction between circadian malfunction and depression and aging.
Acknowledgments
The authors would like to thank Kathy Gimson, Julie Stewlow, and Jim Donner for their assistance in the care of animal subjects. Thanks also to Marc Bradshaw and Drs. Jimo Borjigin and Tiecheng Liu for help with the development of the microdialysis apparatus and procedures. Also, thanks to Shawn Brickner, Megan Hagenauer, Rebecca Lane, Bethany Nestor, Jamie Perryman, Eila Roberts, and Blair Sutton for their help with data collection and to C. Vining and Dr. Seema Bhatnagar for their assistance with the ACTH assay in Exp. 2. This research was funded by NIH R03MH069518 (T.M.L.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Winget CM, DeRoshia CW, Markley CL, Holley DC. A review of human physiological and performance changes associated with desynchronosis of biological rhythms. Aviat Space Environ Med. 1984;55:1085–96. [PubMed] [Google Scholar]
- 2.Katz G, Durst R, Zislin Y, Barel Y, Knobler HY. Psychiatric aspects of jet lag: review and hypothesis. Med Hypotheses. 2001;56:20–3. doi: 10.1054/mehy.2000.1094. [DOI] [PubMed] [Google Scholar]
- 3.Munck A, Guyre PM, Holbrook NJ. Physiological functions of glucocorticoids in stress and their relation to pharmacological actions. Endocr Rev. 1984;5:25–44. doi: 10.1210/edrv-5-1-25. [DOI] [PubMed] [Google Scholar]
- 4.Stephens DB. Stress and its measurement in domestic animals: a review of behavioral and physiological studies under field and laboratory situations. Adv Vet Sci Comp Med. 1980;24:179–210. [PubMed] [Google Scholar]
- 5.Bailey SL, Heitkemper MM. Circadian rhythmicity of cortisol and body temperature: morningness-eveningness effects. Chronobiol Int. 2001;18:249–61. doi: 10.1081/cbi-100103189. [DOI] [PubMed] [Google Scholar]
- 6.Ixart G, Szafarczyk A, Belugou JL, Assenmacher I. Temporal relationships between the diurnal rhythm of hypothalamic corticotrophin releasing factor, pituitary corticotrophin and plasma corticosterone in the rat. J Endocrinol. 1977;72:113–20. doi: 10.1677/joe.0.0720113. [DOI] [PubMed] [Google Scholar]
- 7.Verhagen LA, Pevet P, Saboureau M, Sicard B, Nesme B, Claustrat B, Buijs RM, Kalsbeek A. Temporal organization of the 24-h corticosterone rhythm in the diurnal murid rodent Arvicanthis ansorgei Thomas 1910. Brain Res. 2004;995:197–204. doi: 10.1016/j.brainres.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 8.Bradbury MJ, Cascio CS, Scribner KA, Dallman MF. Stress-induced adrenocorticotropin secretion: diurnal responses and decreases during stress in the evening are not dependent on corticosterone. Endocrinology. 1991;128:680–8. doi: 10.1210/endo-128-2-680. [DOI] [PubMed] [Google Scholar]
- 9.Dunn J, Scheving L, Millet P. Circadian variation in stress-evoked increases in plasma corticosterone. Am J Physiol. 1972;223:402–6. doi: 10.1152/ajplegacy.1972.223.2.402. [DOI] [PubMed] [Google Scholar]
- 10.Kant GJ, Mougey EH, Meyerhoff JL. Diurnal variation in neuroendocrine response to stress in rats: plasma ACTH, beta-endorphin, beta-LPH, corticosterone, prolactin and pituitary cyclic AMP responses. Neuroendocrinology. 1986;43:383–90. doi: 10.1159/000124553. [DOI] [PubMed] [Google Scholar]
- 11.Balsalobre A, Brown SA, Marcacci L, Tronche F, Kellendonk C, Reichardt HM, Schutz G, Schibler U. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science. 2000;289:2344–7. doi: 10.1126/science.289.5488.2344. [DOI] [PubMed] [Google Scholar]
- 12.Le Minh N, Damiola F, Tronche F, Schutz G, Schibler U. Glucocorticoid hormones inhibit food-induced phase-shifting of peripheral circadian oscillators. Embo J. 2001;20:7128–36. doi: 10.1093/emboj/20.24.7128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horseman ND, Ehret CF. Glucocorticosteroid injection is a circadian zeitgeber in the laboratory rat. Am J Physiol. 1982;243:R373–8. doi: 10.1152/ajpregu.1982.243.3.R373. [DOI] [PubMed] [Google Scholar]
- 14.Moore-ede MC, Schmelzer WS, Kass DA, Herd JA. Cortisol-mediated synchrinization of circadian rhythm in urinary potassium excretion. Am J Physiol. 1977;233:R230–8. doi: 10.1152/ajpregu.1977.233.5.R230. [DOI] [PubMed] [Google Scholar]
- 15.Mohawk JA, Lee TM. Restraint stress delays reentrainment in male and female diurnal and nocturnal rodents. J Biol Rhythms. 2005;20:245–56. doi: 10.1177/0748730405276323. [DOI] [PubMed] [Google Scholar]
- 16.Mohawk JA, Cashen K, Lee TM. Inhibiting cortisol response accelerates recovery from a photic phase shift. Am J Physiol Regul Integr Comp Physiol. 2005;288:R221–8. doi: 10.1152/ajpregu.00272.2004. [DOI] [PubMed] [Google Scholar]
- 17.Sage D, Ganem J, Guillaumond F, Laforge-Anglade G, Francois-Bellan AM, Bosler O, Becquet D. Influence of the corticosterone rhythm on photic entrainment of locomotor activity in rats. J Biol Rhythms. 2004;19:144–56. doi: 10.1177/0748730403261894. [DOI] [PubMed] [Google Scholar]
- 18.Weibel L, Maccari S, Van Reeth O. Circadian clock functioning is linked to acute stress reactivity in rats. J Biol Rhythms. 2002;17:438–46. doi: 10.1177/074873002237138. [DOI] [PubMed] [Google Scholar]
- 19.Cho K, Ennaceur A, Cole JC, Suh CK. Chronic jet lag produces cognitive deficits. J Neurosci. 2000;20:RC66. doi: 10.1523/JNEUROSCI.20-06-j0005.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ishida A, Mutoh T, Ueyama T, Bando H, Masubuchi S, Nakahara D, Tsujimoto G, Okamura H. Light activates the adrenal gland: timing of gene expression and glucocorticoid release. Cell Metab. 2005;2:297–307. doi: 10.1016/j.cmet.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 21.Leproult R, Colecchia EF, L'Hermite-Baleriaux M, Van Cauter E. Transition from dim to bright light in the morning induces an immediate elevation of cortisol levels. J Clin Endocrinol Metab. 2001;86:151–7. doi: 10.1210/jcem.86.1.7102. [DOI] [PubMed] [Google Scholar]
- 22.Sei H, Fujihara H, Ueta Y, Morita K, Kitahama K, Morita Y. Single eight-hour shift of light-dark cycle increases brain-derived neurotrophic factor protein levels in the rat hippocampus. Life Sciences. 2003;73:53–59. doi: 10.1016/s0024-3205(03)00251-0. [DOI] [PubMed] [Google Scholar]
- 23.Linthorst AC, Flachskamm C, Holsboer F, Reul JM. Local administration of recombinant human interleukin-1 beta in the rat hippocampus increases serotonergic neurotransmission, hypothalamic-pituitary-adrenocortical axis activity, and body temperature. Endocrinology. 1994;135:520–32. doi: 10.1210/endo.135.2.7518383. [DOI] [PubMed] [Google Scholar]
- 24.Sage D, Maurel D, Bosler O. Involvement of the suprachiasmatic nucleus in diurnal ACTH and corticosterone responsiveness to stress. Am J Physiol Endocrinol Metab. 2001;280:E260–9. doi: 10.1152/ajpendo.2001.280.2.E260. [DOI] [PubMed] [Google Scholar]
- 25.Becker JB, Adams F, Robinson TE. Intraventricular microdialysis: a new method for determining monoamine metabolite concentrations in the cerebrospinal fluid of freely moving rats. J Neurosci Methods. 1988;24:259–69. doi: 10.1016/0165-0270(88)90171-9. [DOI] [PubMed] [Google Scholar]
- 26.Vahl TP, Ulrich-Lai YM, Ostrander MM, Dolgas CM, Elfers EE, Seeley RJ, D'Alessio DA, Herman JP. Comparative analysis of ACTH and corticosterone sampling methods in rats. Am J Physiol Endocrinol Metab. 2005;289:E823–8. doi: 10.1152/ajpendo.00122.2005. [DOI] [PubMed] [Google Scholar]
- 27.Mrosovsky N. Masking: history, definitions, and measurement. Chronobiol Int. 1999;16:415–29. doi: 10.3109/07420529908998717. [DOI] [PubMed] [Google Scholar]
- 28.Buijs RM, Wortel J, Van Heerikhuize JJ, Feenstra MG, Ter Horst GJ, Romijn HJ, Kalsbeek A. Anatomical and functional demonstration of a multisynaptic suprachiasmatic nucleus adrenal (cortex) pathway. Eur J Neurosci. 1999;11:1535–44. doi: 10.1046/j.1460-9568.1999.00575.x. [DOI] [PubMed] [Google Scholar]
- 29.Bouwknecht JA, Paylor R. Behavioral and physiological mouse assays for anxiety: a survey in nine mouse strains. Behav Brain Res. 2002;136:489–501. doi: 10.1016/s0166-4328(02)00200-0. [DOI] [PubMed] [Google Scholar]
- 30.Anisman H, Lacosta S, Kent P, McIntyre DC, Merali Z. Stressor-induced corticotropin-releasing hormone, bombesin, ACTH and corticosterone variations in strains of mice differentially responsive to stressors. Stress. 1998;2:209–20. doi: 10.3109/10253899809167284. [DOI] [PubMed] [Google Scholar]
- 31.Lu ZW, Song C, Ravindran AV, Merali Z, Anisman H. Influence of a psychogenic and a neurogenic stressor on several indices of immune functioning in different strains of mice. Brain Behav Immun. 1998;12:7–22. doi: 10.1006/brbi.1997.0510. [DOI] [PubMed] [Google Scholar]


