Abstract
Rationale
Central administration of corticotropin-releasing factor (CRF) elicits a specific pattern of behavioral responses resembling a stress-like state, and is anxiogenic in rodent models of anxiety.
Objectives
Specific behaviors evoked by the administration of CRF were measured. The roles of CRF receptor subtypes and that of serotonergic and noradrenergic systems in mediating these responses were studied.
Methods
Burying, grooming and head shakes were quantified in rats following intracerebroventricular administration of CRF and urocortin II and after pretreatment with antagonists. The role of forebrain norepinephrine in the behavioral responses to CRF (0.3 μg) was examined following pretreatment with the neurotoxin DSP-4 and that of serotonin after depletion using systemic administration of para-chlorophenylalanine (p-CPA).
Results
CRF at 0.3 and 3.0 μg caused robust increases in burying, grooming and head shakes, but urocortin II was ineffective. Pretreatment with either antalarmin or propranolol significantly attenuated the CRF-evoked behaviors. Destruction of forebrain NE pathways blocked spontaneous burying behavior elicited by CRF and conditioned burying directed towards an electrified shock probe. In contrast, depletion of 5-HT selectively attenuated CRF-evoked grooming.
Conclusions
Overt behavioral responses produced by CRF, burying, grooming, and head shakes, appeared to be mediated through the CRF1 receptor. Spontaneous burying behavior evoked by CRF or conditioned burying directed towards a shock probe were disrupted by lesion of the dorsal noradrenergic bundle and may represent anxiety-like behavior caused by CRF activation of the LC. In contrast, CRF-evoked increases in grooming were dependent on serotonin.
Keywords: CRF, anxiety, burying, grooming, head shakes, norepinephrine, LC, serotonin
Introduction
Corticotropin releasing factor (CRF), a 41-amino acid neuropeptide, is the primary regulator of adrenocorticotrophic hormone (ACTH) release from the anterior pituitary in response to stress (Vale et al. 1981). In addition to this role, CRF also acts as an extra-hypothalamic neuromodulator and neurotransmitter, mediating adaptive behavioral responses to stress, including increased arousal, suppression of appetitive and reproductive behaviors and an increase in defensive responding and avoidance behaviors (Brown and Fisher 1985; Dunn and Berridge 1990; Owens and Nemeroff 1993; Spina et al. 1996; Spina et al. 2000). These behavioral activating effects have been shown to be independent of HPA axis activation, and thus represent extra-hypothalamic actions of CRF in the CNS (Britton et al. 1986; Eaves et al. 1985).
Given the multiple roles of CRF in the mediation of the stress response, it has been hypothesized that stress-related disorders may result from the exacerbated stimulation of one or more CRF-regulated pathways (Koob and Heinrichs 1999; Owens and Nemeroff 1993; Risbrough and Stein 2006). In rats, intracerebroventricular (i.c.v.) and site-specific administration of CRF mimics behavioral responses to stressful stimuli (Campbell et al. 2004; Liang et al. 1992) and produces anxiogenic effects in a number of behavioral tests of anxiety (Dunn and File 1987; Zorrilla et al. 2002). Furthermore, CRF receptor antagonists are effective in blocking many of the behavioral effects of CRF, and reverse the suppression and activation of behaviors observed in response to stress (Basso et al. 1999; Heinrichs et al. 1992; Korte et al. 1994; Takahashi 2001; Zorrilla et al. 2002). Clinical findings have also implicated increased central CRF drive in the etiology of anxiety and affective disorders and suggest significant potential for CRF receptor antagonists in the treatment of anxiety disorders (Nemeroff 2004; Zobel et al. 2000).
Serotonin (5-HT) and norepinephrine (NE) are neurotransmitter systems that are implicated in affective and anxiety responses and represent potential substrates on which CRF could act to elicit stress-related behaviors and to mediate stress-related psychopathology (Valentino et al. 1983; Aston-Jones et al. 1996; Graeff 1997). CRF administration, i.c.v. and site-specifically, alters the firing of NE and 5-HT neurons (Kirby et al. 2000; Price et al. 1998; Valentino and Foote 1987), and modulates neurotransmitter release at their respective terminal fields (Curtis et al. 1997; de Groote et al. 2005; Price and Lucki 2001). Evocation of anxiety responses by CRF infusion is accompanied by concomitant changes in forebrain 5-HT (Kagamiishi et al. 2003) and NE (Matsuzaki et al. 1989) transmission. Moreover, stress-induced alterations in extracellular 5-HT and NE are blocked by pretreatment with CRF receptor antagonists (Isogawa et al. 2000; Price et al. 1998). Taken together, these findings suggest that CRF can play a critical role in affective and anxiety disorders through its actions on 5-HT and NE systems associated with anxiety and depression.
The purpose of these studies was to characterize behaviors evoked by central administration of CRF to rats that appear to represent an increase of defensive and avoidance responses associated with a stress-like state. CRF administration in rodents has been shown to evoke a specific pattern of behavioral activation, characterized by increased locomotor activity, rearing and grooming (Dunn and Berridge 1990; Koob et al. 1984; Sherman and Kalin 1987), and head shakes or wet dog shakes (Gargiulo and Donoso 1996; Price et al. 1998). The present study focused on three prominent behavioral responses that were observed after intraventricular injections of CRF in rats, burying behavior, grooming and head shakes. In addition to characterizing and quantifying the CRF-evoked behaviors, the role of CRF receptor subtypes in mediating these specific behavioral responses was examined. Given the evidence for a neuromodulatory role for CRF on NE and 5-HT function, the roles of these neurotransmitters in the elicitation of specific CRF-evoked behaviors were then investigated. Because CRF-evoked spontaneous burying and conditioned burying of an electrified prod may have common mechanisms, the contribution of NE as a common substrate for the two behaviors was evaluated. Spontaneous burying behavior evoked by CRF and conditioned burying were found to be associated with the NE pathways originating from the LC, whereas grooming behavior evoked by CRF was found to be associated with 5-HT transmission.
Materials and methods
Animals
Adult male Sprague Dawley rats (250-300 g; Charles River Laboratories, Wilmington, MA) were initially housed two per cage in a temperature-controlled (22°C) colony room. The colony was illuminated under a 12-hour alternating light/dark schedule with light onset at 07:00. Standard rat chow and water were freely available. The care and use of animals was in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Surgery
Animals were anesthetized by intraperitoneal (i.p.) injection of sodium pentobarbital (40 mg/kg, i.p.). Guide cannulae aimed at the lateral ventricle were implanted surgically to facilitate the i.c.v. injection of CRF. Cranioplastic cement and stainless steel anchor screws were used to fix the guide cannula to the skull. A stainless steel obdurator was inserted into each guide cannula to help prevent occlusions. Following surgery, animals were housed individually for one week to allow for recovery prior to behavioral testing. Rats were handled 3 times prior to testing to minimize potential disruptive behavioral effects of handling during the experiment.
Behavioral Testing
CRF-evoked behaviors
At 09:00 on the morning of testing, animals were placed in a clear polycarbonate cylindrical chamber (37.5 cm high, 30 cm in diameter) containing bedding (0.5 cm, Bed o’Cobs, The Andersons, Maumee, OH) spread 7.5 cm in depth evenly across the chamber floor. Three hours were allowed for habituation to the testing chamber, after which animals received an injection of vehicle or drug. Following injection, animals were videotaped over a two-hour period for detailed behavioral analysis. The most prominent behavioral effects produced by vehicle or CRF administration were subsequently quantified by an experimenter blind to the treatment. The behaviors scored were: 1) time spent burying; 2) time spent grooming; 3) total number of head shakes. Burying was defined as rapidly repeated forward-and-backward movements of either a single forepaw or rapidly alternating bilateral forward-and-backward movements of both forepaws, which pushed or sprayed bedding forward, creating a mound of bedding in front of the animal. This was accompanied at times by hind limb movements which pushed bedding back behind the rat as it progressed forwards. This pattern of behavior has been previously described for increases of spontaneous burying behavior induced by muscimol injected into the nucleus accumbens (Reynolds and Berridge 2001). Grooming was defined as licking of the forelimbs and body and/or paw strokes of the face and body.
Defensive Burying
On the morning of testing, animals were transported to a room adjacent to the testing room to habituate for 3 hours. Light levels in both rooms were maintained at 125 Lux. The shock-probe defensive burying test, adapted from Pinel and Treit (1978), was conducted in a clear polycarbonate cage, 25 × 45 × 20 cm, identical to the home cage with a 1 cm diameter hole located 7 cm from the base at one end to accommodate the shock-probe. Fresh bedding (1/8 inch, Bed o’Cobs, The Andersons, Maumee, OH) lined the cage to a depth of 5 cm. The shock probe was a 1.0 cm diameter glass rod wrapped with two alternating, non-touching 18 ga copper wires, spaced 5 loops/cm. The probe extended 6 cm into one end of the cage, 2 cm above the surface of the bedding. The probe was attached to a shock generator (SGS-004, BRS-LVE, Laurel, MD) set to deliver 5.5 mA DC current when the probe was touched. Animals were placed in the cage at the end opposite the shock probe, facing away from the probe. Rats typically approached the probe to investigate within 10–15 seconds, making contact with their paw or snout. Upon contacting the probe a 15-min test period began. Test sessions were videotaped for subsequent analysis. After each test, the cage was replaced with an identical clean testing chamber, containing fresh bedding. Behaviors were evaluated by an experimenter blind to the condition of the test animal using a method adapted from De Boer and Koolhaas (2003). In addition to the total time spent burying, total immobility time and latency to contact the probe were scored as indices of potential changes in locomotion or exploratory activity. Burying consisted of burrowing into the bedding with the snout and upper body, then pushing or flicking the bedding toward the probe using the forepaws. Each rat was also scored subjectively for reactivity to the shock on a 4-point scale (Treit and Pesold 1990).
Histology
After behavioral testing, animals were sacrificed with a lethal dose of pentobarbital (100 mg/kg, i.p.) and immediately microinjected with 3 μl of Fast Green dye (Sigma, St Louis). The brains were removed, and cannula placement verified by presence of dye in the ventricular system. Only animals with accurate cannula placement were included in statistical analyses.
Tissue analysis of norepinephrine and serotonin
Tissue content of NE and 5-HT was measured in the frontal cortex and hippocampus to provide representative evidence for the depletion of 5-HT and NE by p-CPA and DSP-4. In order to offset potential acute effects of CRF infusion on neurotransmitter levels, monoamine levels were also measured 24 hours after behavioral testing. Animals were sacrificed with an overdose of pentobarbital, and their brains removed. The frontal cortex and hippocampus were dissected in a Petri dish over ice, quickly frozen on dry ice and stored at -80°C until preparation for analysis.
Frozen tissue samples were thawed and homogenized in 0.1 N perchloric acid with 100 μM EDTA (15 μl/mg of tissue) using a tissuemizer (Tekmar, Cleveland, OH, USA). Samples were centri fuged at 15,000 rpm (23,143 × g) for 15 min at 2–8°C. The supernatants were filtered using Costar Spin-X™ centrifugal filters (Fisher Scientific, Pittsburgh, PA, USA) and then split into two aliquots. Samples (12 μl) were analyzed in separate assays for 5-HT and 5-HIAA and for the catecholamines NE and DA using high performance liquid chromatography (HPLC) coupled with electrochemical detection, as previously described (Mayorga et al., 2001). Standard concentrations of 5-HT and 5-HIAA or NE and DA were prepared before injection of tissue samples. Tissue concentrations of monoamines were determined using a linear regression analysis of the peak heights obtained from a range of standards.
Drugs and injections
Ovine CRF or urocortin II (Dr. Jean Rivier, The Salk Institute, La Jolla, CA) were dissolved in artificial cerebrospinal fluid (aCSF), which also acted as the vehicle. I.c.v. injections were made using a 28 gauge stainless steel injector which extended 1 mm beyond the tip of the guide cannula. Without handling the animal, the obdurator was removed, and the injector was inserted into the guide cannula. CRF or vehicle (3 μl injections) was infused over a 3-min period using a syringe pump (Instech, Plymouth Meeting, PA). After allowing 1 min for diffusion of the drug, the obdurator was replaced.
In each of the combination blocker experiments, antagonists (or vehicle) were administered systemically 30 min prior to CRF injection (0.3 μg i.c.v.) and the cumulative response to CRF was measured for 2 hours. Antalarmin (Kenner C. Rice, Laboratory of Medicinal Chemistry, NIH/NIDDK, Bethesda, MD) was suspended in a solution containing 24% cremaphor and 6% ethanol and injected i.p. (20 mg/kg) in a volume of 1 ml/kg. Propranolol hydrochloride (Sigma, St Louis, MO) was dissolved in 0.9% physiological saline and administered i.p. (5 and 10 mg/kg) in a volume of 2 ml/kg.
Yohimbine (Sigma, St Louis, MO), was dissolved in deionized water and administered i.p. in a volume of 2 ml/kg. The tryptophan hydroxylase inhibitor para-chlorophenylalanine (p-CPA) methylester HCl (Sigma, St Louis, MO) was used for whole-brain serotonin depletion. p-CPA was dissolved in deionized water and injected i.p. in two 150 mg/kg doses, 3 and 2 days prior to behavioral testing (Koe and Weissman, 1966). The noradrenergic neurotoxin N-(2-Chloroethyl)-N-ethyl-2-bromobenzylamine hydrochloride or DSP-4 (Sigma, St Louis, MO) was dissolved in deionized water and injected i.p. at 50 mg/kg 8 days prior to behavioral testing. All rats were pretreated with the selective serotonin reuptake inhibitor paroxetine (10 mg/kg, i.p.) 30 min prior to DSP-4 administration, in order to protect serotonergic nerve terminals (Jonsson et al. 1981). Drug doses were determined by the base weight.
Statistical analyses
In the CRF, urocortin II and yohimbine experiments, effects on each behavior were analyzed using one-way analysis of variance (ANOVA). In the antagonist experiments (antalarmin, propranolol), effects on each behavior were analyzed using a two-way ANOVA with pretreatment (drug/vehicle) and i.c.v. infusion (CRF/vehicle) as the main factors. All statistics were performed using Statview software (Adept Scientific Inc, Acton, MA). In all experiments, a significant main effect or interaction was required for further examination using Fisher’s PLSD test. Significance was established at p < 0.05.
Results
CRF-evoked behaviors
As shown in Figure 1, CRF administration produced dose-dependent increases in burying (a and d), grooming (b and e) and head shakes (c and f). Both 0.3 μg and 3.0 μg CRF evoked a similar duration of burying during the first hour post-infusion. However, the lower dose showed a gradual reduction of burying during the second hour, while the higher dose displayed steadily increased burying responses over the two hours. The cumulative duration of burying during the study period shows that CRF infusion significantly increased burying behavior (F3, 28 = 3.75, p < 0.05).
Figure 1.
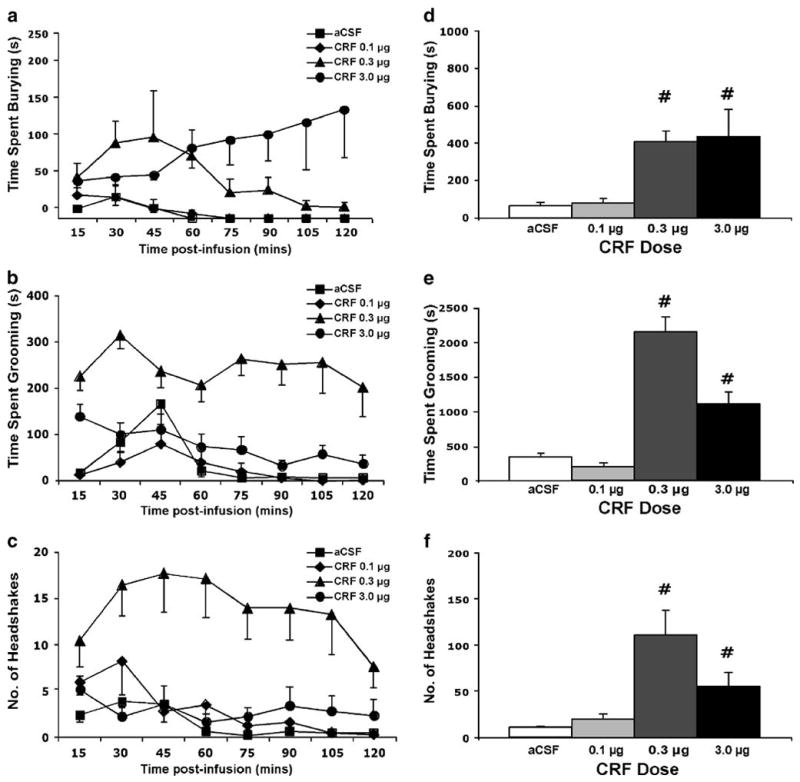
Time-course of CRF-evoked (a) burying, (b) grooming and (c) head shakes over a two-hour period following i.c.v. CRF infusion are shown in panels a and b, respectively. Points represent mean values with error bars indicating SEM. aCSF n = 9, CRF 0.1 μg n = 6, CRF 0.3 μg n = 6, CRF 3.0 μg n = 10. Cumulative behavioral responses for 2 hours following CRF are shown in panels (d), (e) and (f) illustrating burying and grooming, respectively. Bars represent mean values with error bars indicating SEM. aCSF n = 9, CRF 0.1 μg n = 6, CRF 0.3 μg n = 6, CRF 3.0 μg n = 10. Pound sign indicates group that differed significantly from the Vehicle (aCSF) group, p < 0.05.
A steady level of grooming was produced by 0.3 μg CRF over the two-hour period, while the 3.0 μg CRF dose evoked increased grooming levels only during the first hour (Figure 1b). The cumulative duration of grooming during the study period (Figure 1e) showed a significant increase in grooming behavior by CRF (F3, 23 = 17.81, p < 0.001). The increase in grooming behavior at 0.3 μg was significantly greater than that at 3.0 μg (p < 0.05).
Head shakes (Figure 1c) were also greatly elevated by the 0.3 μg dose of CRF over the duration of the observation period, whereas they were only modestly increased by the higher dose and this effect diminished over the two-hour test period. Figure 1f shows that CRF infusion significantly increased the cumulative total head shakes during the study period (F3, 21 = 5.81, p < 0.005).
In contrast to ovine CRF, which binds preferentially to the CRF1 receptor, administration of the selective CRF2 receptor agonist urocortin II (0.3 and 3.0 μg) caused no significant changes in burying, grooming or head shakes as compared to aCSF infusion (data not shown, p > 0.05). Taken together, these findings indicate that the behavioral changes elicited by CRF infusion were likely mediated by activation of the CRF1, but not the CRF2 receptor.
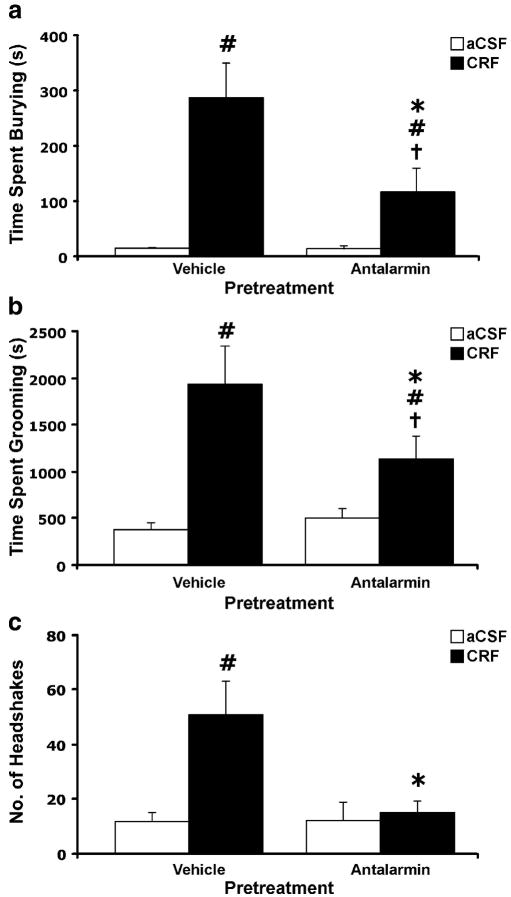
To further examine the role of the CRF1 receptor in the observed behaviors, the effects of the CRF1 receptor antagonist antalarmin on CRF-evoked behaviors were investigated (Figure 2). Pretreatment with antalarmin reduced the frequency of CRF-evoked increases in burying (Figure 2a), grooming (Figure 2b) and headshakes (Figure 2c). However, blockade of the burying and grooming behaviors was incomplete, as demonstrated by the significant difference between the antalarmin/CRF groups and their respective control groups.
Figure 2.
Effect of pretreatment with the CRF receptor antagonist antalarmin (20 mg/kg i.p.) given 30 min prior to injection of CRF (0.3 μg, i.c.v.). Behaviors evoked by CRF were: (a) burying, (b) grooming and (c) head shakes. V/aCSF n = 4, V/CRF n = 4, Antalarmin/aCSF n = 5, Antalarmin/CRF n = 6. ANOVA revealed a significant interaction between antalarmin and CRF for burying (F1, 15 = 6.64, p < 0.05), grooming (F1, 15 = 5.76, p < 0.05) and head shakes (F1, 15 = 7.99, p < 0.05). Bars represent mean cumulative values for 2 hours with error bars indicating SEM. Pound sign indicates group that differed significantly from Vehicle/aCSF group, p < 0.05; Asterisk indicates group that differed significantly from Vehicle/CRF group p < 0.05; † indicates group that differed significantly from relative control group, p < 0.05.
Behavioral effects of yohimbine
The effects of systemic administration of yohimbine, an α2 adrenergic antagonist and a known anxiogenic compound, were measured on the same behaviors evoked by CRF administration (Figure 3). Yohimbine produced a significant increase in spontaneous burying behavior (F2, 23 = 4.02, p < 0.05). In contrast, yohimbine produced no significant changes in grooming behavior (Figure 3b) or head shakes (Figure 3c).
Figure 3.
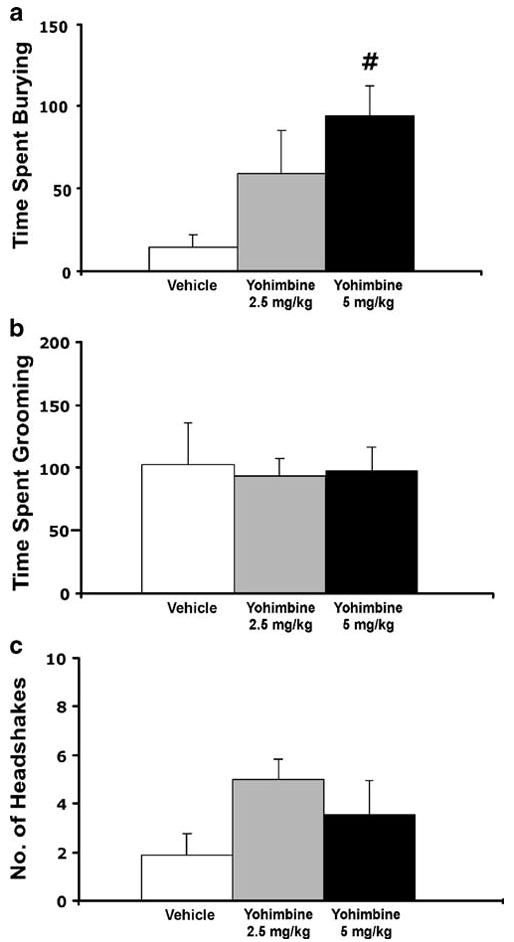
Effects of yohimbine on (a) burying, (b) grooming and (c) head shakes. Doses were administered to separate groups of animals: Vehicle (V) n = 8, Yohimbine 2.5 mg/kg n = 10, Yohimbine 5 mg/kg n = 10. Bars represent mean cumulative values for 2 hours with error bars indicating SEM. Pound sign indicates group that differed significantly from the Vehicle group, p < 0.05.
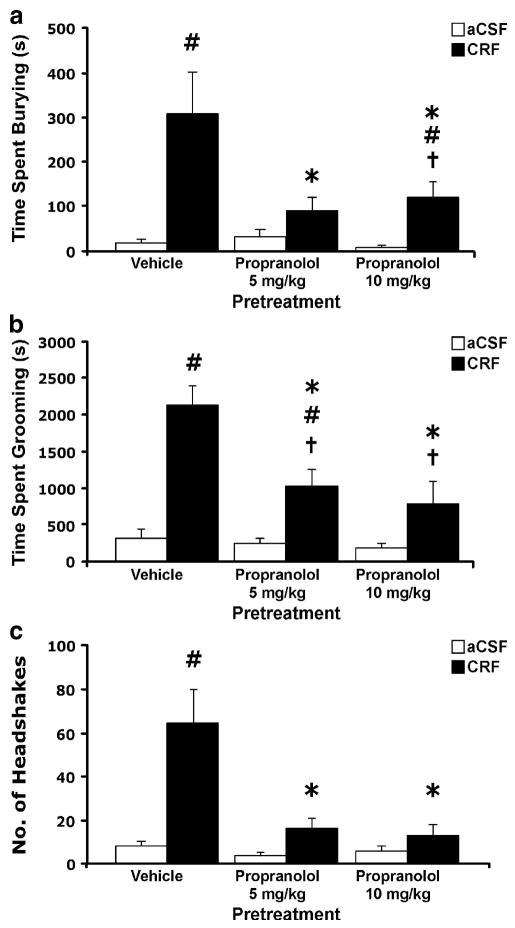
Effects of propranolol pretreatment on CRF-evoked behaviors
To further investigate the role of NE in CRF-evoked behaviors, the effects of pretreatment with the ß-adrenergic antagonist propranolol were assessed (Figure 4). Propranolol pretreatment significantly attenuated the frequency of CRF-evoked increases in burying (Figure 4a), grooming (Figure 4b) and headshakes (Figure 4c). However, the blockade of burying and grooming was incomplete, as demonstrated by the significant difference between the propranolol/CRF groups and their respective control groups.
Figure 4.
Effect of pretreatment with propranolol on behaviors produced by injection of CRF (0.3 μg, i.c.v.): (a) burying, (b) grooming and (c) head shakes. Propranolol was administered 30 min prior to CRF. V/aCSF n = 7, V/CRF n = 6, Prop 5 mg/kg/aCSF n = 7, Prop 5 mg/kg/CRF n = 6, Prop 10 mg/kg/aCSF n = 6, Prop 10 mg/kg/CRF n = 7. Bars represent mean cumulative values for 2 hours with error bars indicating SEM. ANOVA revealed a significant interaction between propranolol and CRF for burying (F2, 32 = 5.25, p < 0.05), grooming (F2, 32 = 5.52, p < 0.01) and head shakes (F2, 32 = 9.79, p < 0.001). Pound sign indicates group that differed significantly from Vehicle/aCSF group, p < 0.05; Asterisk indicates group that differed significantly from Vehicle/CRF group p < 0.05; † indicates group that differed significantly from their corresponding control group, p < 0.05.
Effects of lesion of forebrain NE on CRF-evoked behaviors
The role of the dorsal noradrenergic bundle in mediating CRF-evoked behaviors was examined using the selective noradrenergic neurotoxin DSP-4 (50 mg/kg, i.p.) given 8 days prior to CRF administration (0.3 μg i.c.v.). The depletion of NE content in forebrain structures targeted by LC projections was confirmed by HPLC analysis of tissue homogenates from the frontal cortex and hippocampus (Table 1). In the frontal cortex, DSP-4 produced a 78% and 79% depletion of NE in the DSP-4/aCSF and DSP-4/CRF groups (F1, 24 = 50.67, p < 0.001). In the hippocampus, DSP-4 produced an 84% and 86% decrease in NE in the DSP-4/aCSF and DSP-4/CRF groups, (F1, 16 = 57.22, p < 0.001). No significant changes in the concentrations of 5-HT, 5-HIAA and dopamine were measured in the frontal cortex or hippocampus after DSP-4.
Table 1.
Changes in norepinephrine, serotonin (5-HT), 5-hydroxyindoleacetic acid (5-HIAA) and dopamine content in the frontal cortex and hippocampus measured 9 days following administration of DSP-4 (50 mg/kg).
| Norepinephrine | 5-HT | 5-HIAA | Dopamine | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Depletion Treatment |
Infusion | Frontal Cortex |
Hippocampus | Frontal Cortex |
Hippocampus | Frontal Cortex |
Hippocampus | Frontal Cortex |
Hippocampus |
| Vehicle | aCSF | 164 ± 30 | 119 ± 22 | 191 ± 16 | 86 ± 18 | 230 ± 182 | 266 ± 12 | 33 ± 6 | 18 ± 3 |
| DSP-4 | aCSF | 33 ± 12 | 20 ± 9 | 160 ± 39 | 89 ± 37 | 174 ± 15 | 248 ± 31 | 35 ± 7 | 19 ± 4 |
| % Change | ↓ 79% * | ↓ 85% * | ↓ 17% | ↑ 3% | ↓ 25% | ↓ 7% | ↑ 7% | ↑ 5% | |
| Vehicle | CRF | 213 ± 28 | 142 ± 23 | 182 ± 28 | 76 ± 15 | 226 ± 28 | 216 ± 12 | 50 ± 11 | 17 ± 5 |
| DSP-4 | CRF | 46 ± 10 | 21 ± 13 | 189 ± 36 | 66 ± 16 | 198 ± 17 | 220 ± 11 | 34 ± 10 | 19 ± 5 |
| % Change | ↓ 78% * | ↓ 86% * | ↑4% | ↓ 14% | ↓ 13 % | ↑ 2% | ↓ 31% | ↑ 11% | |
Tissue content is expressed as mean values (pg/mg tissue) ± 1 SEM. % change denotes content change in the DSP-4-treated groups as compared to respective controls. Frontal Cortex: n = 7 rats per group. Hippocampus: n = 7 rats per group. Asterisk denotes significant main effect of DSP-4 pretreatment p < 0.001.
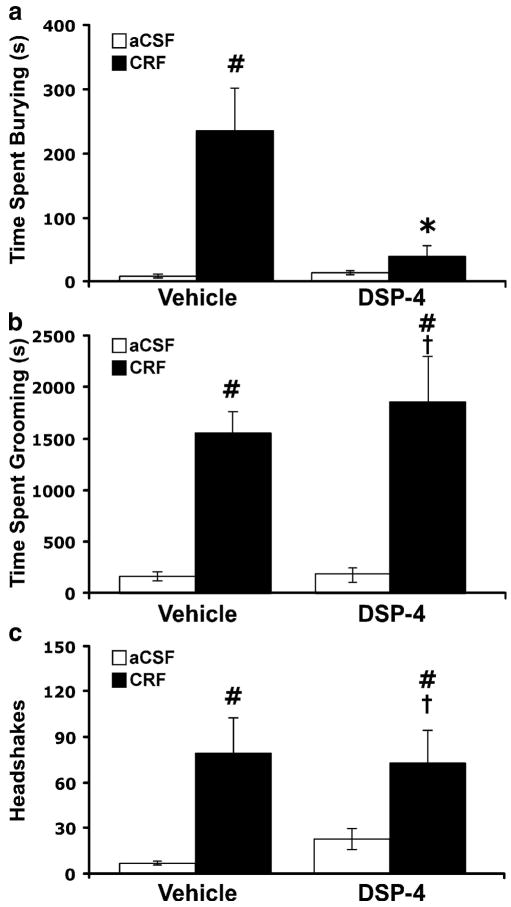
The effects of DSP-4 pretreatment on CRF-evoked behaviors are shown in Figure 5. CRF-evoked burying (Figure 5a) was completely blocked by DSP-4 pretreatment (DSP-4 × CRF interaction, F1, 22 = 13.81, p < 0.05). In contrast, CRF-evoked grooming and head shakes were unaffected by DSP-4 pretreatment, as shown in Figure 5b and 5c, respectively. These results indicate that an intact dorsal noradrenergic bundle is necessary for the evocation of CRF-evoked burying, but not grooming or head shakes.
Figure 5.
Effects of pretreatment with DSP-4 on behaviors elicited by injection of CRF (0.3 μg, i.c.v.): (a) burying, (b) grooming and (c) head shakes. DSP-4 (50 mg/kg) was given 8 days prior to testing to deplete forebrain NE. V/aCSF n = 7, V/CRF n = 5, DSP-4/aCSF n = 7, DSP-4/CRF n = 7. Bars represent mean cumulative values for 2 hours with error bars indicating SEM. ANOVA revealed a significant interaction between DSP-4 and CRF for only burying (F1, 22 = 13.81, p < 0.05). Pound sign indicates group that differed significantly from Vehicle/aCSF group, p < 0.05; Asterisk indicates group that differed significantly from Vehicle/CRF group p < 0.05; † indicates group that differed significantly from corresponding control group, p < 0.05.
Effects of 5-HT depletion on CRF-evoked behaviors
To investigate the role of 5-HT in mediating the behavioral effects of CRF, whole-brain 5-HT was depleted using the tryptophan hydroxylase inhibitor p-CPA (300 mg/kg, i.p.) prior to administration of CRF (0.3 μg i.c.v.). In the frontal cortex (Table 2), p-CPA produced a 94 % and 95 % decrease in 5-HT in both p-CPA/aCSF and p-CPA /CRF groups, respectively, as compared to relative controls (F1, 24 = 373.48, p < 0.001). In the hippocampus (Table 2), p-CPA produced a 92.7% and 93.2% decrease of 5-HT in p-CPA/aCSF and p-CPA /CRF groups, respectively, as compared to relative controls (F 1, 24 = 45.24, p < 0.001). p-CPA pretreatment elicited similar decreases in 5-HIAA concentrations in both brain regions. Frontal cortex concentrations were decreased by 94% and 96% in p-CPA/aCSF and p-CPA /CRF groups, respectively, as compared to relative controls (F1, 24 = 755.51, p < 0.001). Hippocampal concentrations of 5-HIAA were similarly decreased by 94% and 96% in p-CPA/aCSF and p-CPA /CRF groups, respectively, as compared to relative controls (F1, 24 = 787.68, p < 0.001). No significant changes in dopamine concentrations were detected in either the frontal cortex or the hippocampus following p-CPA pretreatment.
Table 2.
Changes in serotonin (5-HT), 5-hydroxyindoleacetic acid (5-HIAA) and dopamine content in the frontal cortex and hippocampus measured 96 hours after the initiation of p-CPA administration (150 mg/kg × 2 days).
| 5-HT | 5-HIAA | Dopamine | |||||
|---|---|---|---|---|---|---|---|
| Depletion Treatment | Infusion | Frontal Cortex | Hippocampus | Frontal Cortex | Hippocampus | Frontal Cortex | Hippocampus |
| Vehicle | aCSF | 330 ± 21 | 192 ± 14 | 413 ± 16 | 547 ± 27 | 72 ± 11 | 84 ± 20 |
| p-CPA | aCSF | 21 ± 2 | 14 ± 1 | 26 ± 4 | 34 ± 4 | 56 ± 8 | 57 ± 7 |
| % Change | ↓ 94% * | ↓ 93% * | ↓ 94% * | ↓ 94 % * | ↓ 22 % | ↓ 33 % | |
| Vehicle | CRF | 326 ± 23 | 185 ± 12 | 405 ± 23 | 545 ± 25 | 79 ± 7 | 63 ± 17 |
| p-CPA | CRF | 18 ± 1 | 13 ± 1 | 18 ± 2 | 21 ± 3 | 50 ± 4 | 39 ± 12 |
| % Change | ↓ 95 % * | ↓ 89% * | ↓ 96% * | ↓ 96 % * | ↓ 37 % | ↓ 39% | |
Tissue content is expressed as mean values (pg/mg tissue) ± 1 SEM. % change denotes content change in the p-CPA treated groups as compared to respective controls. Frontal Cortex: n = 7 rats per group. Hippocampus: n = 7 rats per group. Asterisk denotes significant main effect of p-CPA pretreatment p < 0.001.
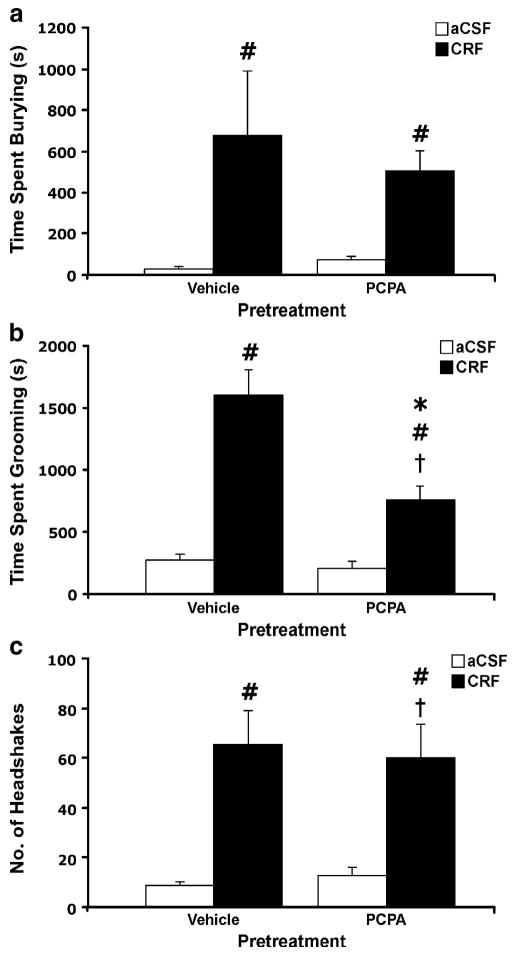
The effects of p-CPA on CRF-evoked behaviors are depicted in Figure 6. There was no overall effect of p–CPA pretreatment on the duration of burying (Figure 6a) or the frequency of head shakes (Figure 6c). In contrast, the duration of grooming following CRF (Figure 6b) was significantly attenuated by p–CPA pretreatment (p-CPA × CRF interaction, F1, 51 = 7.46, p < 0.01). For all behaviors measured, no significant differences were found between aCSF-infused groups, regardless of pretreatment (vehicle or p-CPA). Together, these results indicate that CRF-evoked grooming, but not burying or head shakes, is dependent on serotonergic transmission.
Figure 6.
Effect of p-CPA pretreatment on behaviors evoked by administration of CRF (0.3 μg, i.c.v.): (a) burying, (b) grooming and (c) head shakes. p-CPA (150 mg/kg × 2 days) was given 72 and 48 h prior to testing to deplete 5-HT. V/aCSF n = 11, V/CRF n = 13, p-CPA/aCSF n = 10, p-CPA/CRF n = 15. Bars represent mean values with error bars indicating SEM. Bars represent mean cumulative values for 2 hours with error bars indicating SEM. ANOVA revealed a significant interaction between p-CPA and CRF for only grooming (F1, 51 = 7.46, p < 0.01). Pound sign indicates group that differed significantly from Vehicle/aCSF group, p < 0.05; Asterisk indicates group that differed significantly from Vehicle/CRF group p < 0.05; † indicates group that differed significantly from corresponding control group, p < 0.05.
Effects of DSP-4 lesions on conditioned shock-probe burying
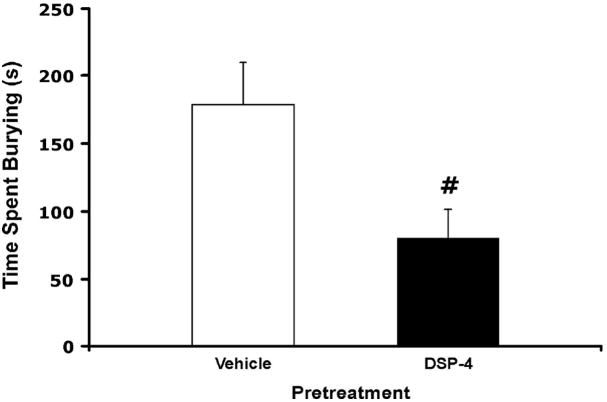
Given the role of the dorsal noradrenergic bundle in the effects of CRF infusion on spontaneous burying, the effects of DSP-4 lesions were also assessed in the conditioned shock-probe defensive burying paradigm. This commonly-used behavioral test of anxiety measures burying behavior in rats that is directed towards an electrified probe (Treit et al. 1981). As shown in Figure 7, DSP-4 pretreatment produced a significant decrease in time spent burying the shock-probe (t(17) = 2.77, p = 0.01). Although the latency (s ± 1 SEM) to initiate burying for rats treated with DSP-4 (313 ± 132) was about 70% longer than for vehicle (186 ± 36), the difference between groups was not significant. The latency to approach the probe (Vehicle: 18.0 ± 5.0; DSP-4: 19.2 ± 6.1) and the number of approaches (Vehicle: 4.6 ± 0.6; DSP-4: 4.6 ± 1.0) did not differ significantly between groups, indicating that the reduced burying time was not caused by a lack of exploration or probe contact. These findings implicate LC-NE pathways in the mediation of burying responses evoked both by CRF infusion and shock-probe exposure.
Figure 7.
Effect of pretreatment with DSP-4 (50 mg/kg) on time spent burying the probe in the conditioned burying test. Vehicle, n = 12, DSP-4, n = 8. Bars represent mean cumulative values for 15 min after probe contact with error bars indicating SEM. Pound sign indicates significant difference from Vehicle group, p < 0.05.
Discussion
Characterization of CRF-evoked behaviors
The robust increases in burying, grooming and head shakes produced by CRF are consistent with the role of CRF in facilitating the emission of defensive behaviors. Rodent burying behavior has long been described as a defensive coping strategy in natural habitats (Calhoun 1962; MacClintock 1970; Owings and Coss 1977). Furthermore, in the context of defensive avoidance, this proactive form of stress management provides an index of anxiety when directed at an electrified probe (Bondi et al. 2007; De Boer et al. 1990; Korte et al. 1992; Treit et al. 1981), and is enhanced and attenuated by anxiogenic and anxiolytic drugs, respectively (De Boer and Koolhaas 2003; Diamant et al. 1992). To the best of our knowledge, the present studies are the first to specifically characterize and quantify the burying response to i.c.v. CRF administration, though this response has been previously reported in mice (Litvin et al. 2007) and rats (Korte et al. 1993; Price et al. 1998; Wiersma et al. 1995) and could only be seen if bedding material were present. Although the burying response evoked by CRF appeared almost identical to behaviors directed towards the electrified probe in the defensive burying response, the occurrence of burying in the absence of a specific aversive stimulus made the behavior appear spontaneous as reported following muscimol injected into the nucleus accumbens (Reynolds and Berridge, 2001). Bedding was pushed or sprayed in front of the rat by vigorous and repetitive movement of the forelimbs, and the formation of multiple elevated mounds of bedding distinguished the behavior from nest building or burrowing. In accord with previous findings (Abrams et al. 2005), burying was also evoked by systemic administration of yohimbine, an adrenergic acting compound with anxiogenic effects both in humans (Charney et al. 1983) and in rodent models (Graeff et al. 1998). Together, these findings provide support for the interpretation of burying as a potential index of anxiety-like behavior.
Doses of CRF which evoked burying behavior also produced robust increases in grooming, in line with previous reports (Diamant et al. 1992; Dunn et al. 1987). Grooming behavior is highly sensitive to stressful stimuli and has been previously used as an indicator of a stress-like state in rodents (Choleris et al. 2001; Dunn et al. 1987; Kalueff and Tuohimaa 2005; To et al. 1999). The attenuation of both stress- and CRF-induced grooming by anxiolytic drugs supports the hypothesis of grooming as an anxiety-associated behavior (Lazosky and Britton 1991; Spruijt et al. 1992).
The evocation of burying, grooming and head shakes to i.c.v. CRF appeared to reflect specific activation of the CRF1 receptor. The ovine CRF used in this study binds preferentially to the CRF1 receptor, with a considerably lower affinity for the CRF2 receptor (Dautzenberg et al. 2001). The attenuation of all behaviors by blockade of the CRF1 receptor using antalarmin further supported mediation by the CRF1 receptor. Moreover, no effect of CRF2 receptor stimulation with the selective agonist urocortin II was observed on any of the behaviors, with doses that are reported to affect feeding behvior (Zorilla et al., 2004). The role of CRF1 receptors in grooming is supported by evidence from previous investigations (Bressers et al. 1995; de Groote et al. 2005; Drago et al. 1999; Dunn et al. 1987; Lumley et al. 2001; Ohata and Shibasaki 2004). Taken together, these findings are consistent with the proposed role of CRF1 receptors in mediating stress-like behavioral responses and anxiogenic effects of CRF (Arborelius et al. 2000; Gehlert et al. 2005; Griebel et al. 2002; Zorrilla et al. 2002). The attenuation of behaviors following antalarmin pretreatment was only observed in CRF-infused animals and not in aCSF-infused controls indicating that they are likely not due to non-specific effects on locomotion or exploratory behavior. Previous studies have reported anxiolytic effects of CRF1 receptor antagonists only in pre-stressed animals, or in those tested under stressful conditions, pointing to a possible necessity of a high endogenous CRF tone for the efficacy of CRF receptor antagonists (Dunn and File 1987; Liebsch et al. 1998; Menzaghi et al. 1994). The findings reported here may describe such an effect, with the anxiolytic efficacy of antalarmin evident on behaviors caused by exogenous CRF administration.
Burying behavior is mediated by forebrain NE
The present findings support an interaction between CRF and noradrenergic systems in the mediation of CRF-evoked burying. First, burying behavior was evoked by yohimbine, which blocks α2 autoreceptors to increase NE cell firing and release (Aghajanian and VanderMaelen 1982; Abercrombie et al. 1988). Second, administration of the non-selective ß-adrenergic antagonist propranolol significantly attenuated CRF-evoked burying, as well as grooming and head shakes, implicating the ß-adrenergic receptor in these behavioral responses. Propranolol has been shown to attenuate the anxiogenic effects of CRF in the defensive withdrawal and conditioned fear paradigms (Cole and Koob 1988; Gorman and Dunn 1993; Yang and Dunn 1990). Third, CRF-evoked burying, but not grooming or head shakes, was attenuated by pretreatment with DSP-4, which selectively lesions the dorsal noradrenergic bundle arising from nucleus locus ceruleus, thereby implicating this specific NE circuit in that behavior. None of the noradrenergic interventions had any effect on baseline behavioral activity in aCSF-infused animals. While considerable differences were noted between control groups in the yohimbine and CRF-evoked behavioral studies, this is likely accounted for by the different routes of drug administration involved, (i.c.v. versus i.p.)
These findings are consistent with a broader literature that have implicated CRF, and activation of noradrenergic neurons at the LC in particular, in the mediation of responses to stress and anxiety (Carrasco and Van de Kar 2003; Koob 1999; Matsuzaki et al. 1989; Melia and Duman 1991; Reyes et al. 2006; Weiss et al. 1994). The LC is a major target of CRF neurotransmission in the brain (Van Bockstaele et al. 1996) and is positioned to influence the functioning of the entire nervous system though modulation of noradrenergic activity (Abercrombie and Jacobs 1988; Curtis et al. 1997; Valentino et al. 1993). Intraventricular or intra-LC administration of CRF increases LC neuronal activity (Valentino and Foote 1987; Valentino et al. 1983), forebrain NE release, and activates forebrain electroencephalographic activity (Curtis et al. 1997). Moreover, intra-LC CRF administration produces anxiogenic behavioral effects in the open field activity and defensive withdrawal paradigms, thereby linking CRF-evoked anxiogenesis and LC activation (Butler et al. 1990; Matsuzaki et al. 1989).
In agreement with its attenuation of burying responses to CRF administration, DSP-4 pretreatment also decreased the duration of probe burying in the conditioned defensive burying test, which is considered a validated rat test of anxiety (Treit et al. 1981). No differences in the latency to contact the probe were observed between DSP-4-treated and control animals, indicating that the attenuation of burying was not due to an overall decrease in locomotion or exploratory activity. CRF administration has been shown to increase probe burying, while this behavior was blocked by CRF antagonists (Basso et al. 1999; Diamant et al. 1992; Korte et al. 1994). Furthermore, shock-probe burying is accompanied by increases in NE transmission (Bondi et al. 2007; Korte et al. 1992) and attenuated by pretreatment with noradrenergic antagonists (Morilak et al. 2005). Taken together, these data suggest that burying by rodents may be a model response for stress associated with the activation of NE pathways from the LC.
CRF-evoked grooming behavior and head shakes
CRF-evoked grooming was attenuated following the depletion of 5-HT by p-CPA, but not after NE depletion produced by DSP4. Thus the ability of CRF to increase grooming behavior appears to be mediated at least in part by serotonergic transmission, and occurred independently of activation of the dorsal noradrenergic bundle. CRF-evoked grooming was also attenuated by propranolol but this could not distinguish between a role for NE and 5-HT due to the nonselective effects of this antagonist. Studies of intraventricular and intra-raphe CRF administration provide substantial evidence for CRF-mediated modulation of serotonergic transmission (de Groote et al. 2005; Kirby et al. 2000; Price et al. 1998; Price and Lucki 2001). The anxiogenic effects of CRF, including increased grooming behavior, have been linked with its activation of serotonergic transmission (Kagamiishi et al. 2003; Lazosky and Britton 1991). An extensive literature on grooming behavior proposes the involvement of multiple neuromediators and brain regions in this behavior (Bressers et al. 1995; Drago et al. 1999; Lumley et al. 2001). The specific circuitry that could underlie the involvement of 5-HT in grooming behavior has not been elucidated.
Head shakes are elicited by a variety of neuromediators and have been suggested as useful quantitative indices of neurotransmitter system interactions and receptor function (Handley and Singh 1986). While both noradrenergic and serotonergic transmission have been implicated in the evocation of head shakes (Tricklebank 1985; Vetulani et al. 1980), neither the DSP-4 nor p-CPA treatments altered CRF-evoked head shakes in the present study. Propranolol attenuated CRF-evoked head shakes, suggesting a potential contribution of noradrenergic pathways outside the dorsal bundle. However, multiple neurotransmitter systems have been implicated in the evocation of head shakes (Singh et al. 1986; Turski et al. 1981), and may do so through independent mechanisms (Drust and Connor 1983). As such, the neurochemical substrate(s) underlying CRF-evoked head shakes remains unclear.
In summary, these studies characterized three behaviors prominently emitted following the i.c.v. administration of CRF in rats that are mediated by the activation of CRF1 receptors. The robust burying behavior evoked by CRF was likely caused by activation of the LC and dorsal noradrenergic bundle, whereas the increases in grooming behavior appeared to involve modulation of serotonergic transmission. These quantitative measures of CRF-evoked responses will be useful for examining the role of CRF in specific neural circuitry associated with anxiety and in studying the regulation of the effects of CRF.
Acknowledgments
This research was supported by USPHS grants MH40008 and MH58250. The authors thank Dr. Candace Hoffmann and Dr. Brian Hoshaw for their development of the conditioned burying test.
References
- Abercrombie ED, Jacobs BL. Systemic naloxone administration potentiates locus coeruleus noradrenergic neuronal activity under stressful but not non-stressful conditions. Brain Res. 1988;441:362–366. doi: 10.1016/0006-8993(88)91415-1. [DOI] [PubMed] [Google Scholar]
- Abercrombie ED, Keller RW, Jr, Zigmond MJ. Characterization of hippocampal norepinephrine release as measured by microdialysis perfusion: pharmacological and behavioral studies. Neuroscience. 1988;27:897–904. doi: 10.1016/0306-4522(88)90192-3. [DOI] [PubMed] [Google Scholar]
- Abrams JK, Johnson PL, Hay-Schmidt A, Mikkelsen JD, Shekhar A, Lowry CA. Serotonergic systems associated with arousal and vigilance behaviors following administration of anxiogenic drugs. Neuroscience. 2005;133:983–997. doi: 10.1016/j.neuroscience.2005.03.025. [DOI] [PubMed] [Google Scholar]
- Aghajanian GK, VanderMaelen CP. Alpha 2-adrenoceptor-mediated hyperpolarization of locus coeruleus neurons: intracellular studies in vivo. Science. 1982;215:1394–1396. doi: 10.1126/science.6278591. [DOI] [PubMed] [Google Scholar]
- Arborelius L, Skelton KH, Thrivikraman KV, Plotsky PM, Schulz DW, Owens MJ. Chronic administration of the selective corticotropin-releasing factor 1 receptor antagonist CP-154,526: behavioral, endocrine and neurochemical effects in the rat. J Pharmacol Exp Ther. 2000;294:588–597. [PubMed] [Google Scholar]
- Aston-Jones G, Rajkowski J, Kubiak P, Valentino RJ, Shipley MT. Role of the locus coeruleus in emotional activation. Prog Brain Res. 1996;107:379–402. doi: 10.1016/s0079-6123(08)61877-4. [DOI] [PubMed] [Google Scholar]
- Basso AM, Spina M, Rivier J, Vale W, Koob GF. Corticotropin-releasing factor antagonist attenuates the “anxiogenic-like” effect in the defensive burying paradigm but not in the elevated plus-maze following chronic cocaine in rats. Psychopharmacology (Berl) 1999;145:21–30. doi: 10.1007/s002130051028. [DOI] [PubMed] [Google Scholar]
- Bondi CO, Barrera G, Lapiz MD, Bedard T, Mahan A, Morilak DA. Noradrenergic facilitation of shock-probe defensive burying in lateral septum of rats, and modulation by chronic treatment with desipramine. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:482–495. doi: 10.1016/j.pnpbp.2006.11.015. [DOI] [PubMed] [Google Scholar]
- Bressers WM, Kruk MR, Van Erp AM, Willekens-Bramer DC, Haccou P, Meelis E. Time structure of self-grooming in the rat: self-facilitation and effects of hypothalamic stimulation and neuropeptides. Behav Neurosci. 1995;109:955–964. doi: 10.1037//0735-7044.109.5.955. [DOI] [PubMed] [Google Scholar]
- Britton DR, Varela M, Garcia A, Rosenthal M. Dexamethasone suppresses pituitary-adrenal but not behavioral effects of centrally administered CRF. Life Sci. 1986;38:211–216. doi: 10.1016/0024-3205(86)90305-x. [DOI] [PubMed] [Google Scholar]
- Brown MR, Fisher LA. Corticotropin-releasing factor: effects on the autonomic nervous system and visceral systems. Fed Proc. 1985;44:243–248. [PubMed] [Google Scholar]
- Butler PD, Weiss JM, Stout JC, Nemeroff CB. Corticotropin-releasing factor produces fear-enhancing and behavioral activating effects following infusion into the locus coeruleus. J Neurosci. 1990;10:176–183. doi: 10.1523/JNEUROSCI.10-01-00176.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun J. The Ecology and Sociology of the Norway Rat. US Dept Health, Education and Welfare. 1962:1–287. [Google Scholar]
- Campbell BM, Morrison JL, Walker EL, Merchant KM. Differential regulation of behavioral, genomic, and neuroendocrine responses by CRF infusions in rats. Pharmacol Biochem Behav. 2004;77:447–455. doi: 10.1016/j.pbb.2003.12.010. [DOI] [PubMed] [Google Scholar]
- Carrasco GA, Van de Kar LD. Neuroendocrine pharmacology of stress. Eur J Pharmacol. 2003;463:235–272. doi: 10.1016/s0014-2999(03)01285-8. [DOI] [PubMed] [Google Scholar]
- Charney DS, Heninger GR, Redmond DE., Jr Yohimbine induced anxiety and increased noradrenergic function in humans: effects of diazepam and clonidine. Life Sci. 1983;33:19–29. doi: 10.1016/0024-3205(83)90707-5. [DOI] [PubMed] [Google Scholar]
- Choleris E, Thomas AW, Kavaliers M, Prato FS. A detailed ethological analysis of the mouse open field test: effects of diazepam, chlordiazepoxide and an extremely low frequency pulsed magnetic field. Neurosci Biobehav Rev. 2001;25:235–260. doi: 10.1016/s0149-7634(01)00011-2. [DOI] [PubMed] [Google Scholar]
- Cole BJ, Koob GF. Propranolol antagonizes the enhanced conditioned fear produced by corticotropin releasing factor. J Pharmacol Exp Ther. 1988;247:902–910. [PubMed] [Google Scholar]
- Curtis AL, Lechner SM, Pavcovich LA, Valentino RJ. Activation of the locus coeruleus noradrenergic system by intracoerulear microinfusion of corticotropin-releasing factor: effects on discharge rate, cortical norepinephrine levels and cortical electroencephalographic activity. J Pharmacol Exp Ther. 1997;281:163–172. [PubMed] [Google Scholar]
- Dautzenberg FM, Py-Lang G, Higelin J, Fischer C, Wright MB, Huber G. Different binding modes of amphibian and human corticotropin-releasing factor type 1 and type 2 receptors: evidence for evolutionary differences. J Pharmacol Exp Ther. 2001;296:113–120. [PubMed] [Google Scholar]
- De Boer SF, Koolhaas JM. Defensive burying in rodents: ethology, neurobiology and psychopharmacology. Eur J Pharmacol. 2003;463:145–161. doi: 10.1016/s0014-2999(03)01278-0. [DOI] [PubMed] [Google Scholar]
- De Boer SF, Slangen JL, Van der Gugten J. Plasma catecholamine and corticosterone levels during active and passive shock-prod avoidance behavior in rats: effects of chlordiazepoxide. Physiol Behav. 1990;47:1089–1098. doi: 10.1016/0031-9384(90)90357-a. [DOI] [PubMed] [Google Scholar]
- de Groote L, Penalva RG, Flachskamm C, Reul JM, Linthorst AC. Differential monoaminergic, neuroendocrine and behavioural responses after central administration of corticotropin-releasing factor receptor type 1 and type 2 agonists. J Neurochem. 2005;94:45–56. doi: 10.1111/j.1471-4159.2005.03164.x. [DOI] [PubMed] [Google Scholar]
- Diamant M, Croiset G, de Wied D. The effect of corticotropin-releasing factor (CRF) on autonomic and behavioral responses during shock-prod burying test in rats. Peptides. 1992;13:1149–1158. doi: 10.1016/0196-9781(92)90022-u. [DOI] [PubMed] [Google Scholar]
- Drago F, Contarino A, Busa L. The expression of neuropeptide-induced excessive grooming behavior in dopamine D1 and D2 receptor-deficient mice. Eur J Pharmacol. 1999;365:125–131. doi: 10.1016/s0014-2999(98)00877-2. [DOI] [PubMed] [Google Scholar]
- Drust EG, Connor JD. Pharmacological analysis of shaking behavior induced by enkephalins, thyrotropin-releasing hormone or serotonin in rats: evidence for different mechanisms. J Pharmacol Exp Ther. 1983;224:148–154. [PubMed] [Google Scholar]
- Dunn AJ, Berridge CW. Physiological and behavioral responses to corticotropin-releasing factor administration: is CRF a mediator of anxiety or stress responses? Brain Res Brain Res Rev. 1990;15:71–100. doi: 10.1016/0165-0173(90)90012-d. [DOI] [PubMed] [Google Scholar]
- Dunn AJ, Berridge CW, Lai YI, Yachabach TL. CRF-induced excessive grooming behavior in rats and mice. Peptides. 1987;8:841–844. doi: 10.1016/0196-9781(87)90069-6. [DOI] [PubMed] [Google Scholar]
- Dunn AJ, File SE. Corticotropin-releasing factor has an anxiogenic action in the social interaction test. Horm Behav. 1987;21:193–202. doi: 10.1016/0018-506x(87)90044-4. [DOI] [PubMed] [Google Scholar]
- Eaves M, Thatcher-Britton K, Rivier J, Vale W, Koob GF. Effects of corticotropin releasing factor on locomotor activity in hypophysectomized rats. Peptides. 1985;6:923–926. doi: 10.1016/0196-9781(85)90323-7. [DOI] [PubMed] [Google Scholar]
- Gargiulo PA, Donoso AO. Distinct grooming patterns induced by intracerebroventricular injection of CRH, TRH and LHRH in male rats. Braz J Med Biol Res. 1996;29:375–379. [PubMed] [Google Scholar]
- Gehlert DR, Shekhar A, Morin SM, Hipskind PA, Zink C, Gackenheimer SL, Shaw J, Fitz SD, Sajdyk TJ. Stress and central Urocortin increase anxiety-like behavior in the social interaction test via the CRF1 receptor. Eur J Pharmacol. 2005;509:145–153. doi: 10.1016/j.ejphar.2004.12.030. [DOI] [PubMed] [Google Scholar]
- Gorman AL, Dunn AJ. Beta-adrenergic receptors are involved in stress-related behavioral changes. Pharmacol Biochem Behav. 1993;45:1–7. doi: 10.1016/0091-3057(93)90078-8. [DOI] [PubMed] [Google Scholar]
- Graeff FG. Serotonergic systems. Psychiatr Clin North Am. 1997;20:723–739. doi: 10.1016/s0193-953x(05)70342-7. [DOI] [PubMed] [Google Scholar]
- Graeff FG, Netto CF, Zangrossi H., Jr The elevated T-maze as an experimental model of anxiety. Neurosci Biobehav Rev. 1998;23:237–246. doi: 10.1016/s0149-7634(98)00024-4. [DOI] [PubMed] [Google Scholar]
- Griebel G, Simiand J, Steinberg R, Jung M, Gully D, Roger P, Geslin M, Scatton B, Maffrand JP, Soubrie P. 4-(2-Chloro-4-methoxy-5-methylphenyl)-N-[(1S)-2-cyclopropyl-1-(3-fluoro-4- methylphenyl)ethyl]5-methyl-N-(2-propynyl)-1, 3-thiazol-2-amine hydrochloride (SSR125543A), a potent and selective corticotrophin-releasing factor(1) receptor antagonist. II. Characterization in rodent models of stress-related disorders. J Pharmacol Exp Ther. 2002;301:333–345. doi: 10.1124/jpet.301.1.333. [DOI] [PubMed] [Google Scholar]
- Handley SL, Singh L. Neurotranmsitters and shaking behaviour - more than a ‘gut bath’ for the brain? Trends in Pharmacological Sciences. 1986;7:324–328. [Google Scholar]
- Heinrichs SC, Pich EM, Miczek KA, Britton KT, Koob GF. Corticotropin-releasing factor antagonist reduces emotionality in socially defeated rats via direct neurotropic action. Brain Res. 1992;581:190–197. doi: 10.1016/0006-8993(92)90708-h. [DOI] [PubMed] [Google Scholar]
- Isogawa K, Akiyoshi J, Hikichi T, Yamamoto Y, Tsutsumi T, Nagayama H. Effect of corticotropin releasing factor receptor 1 antagonist on extracellular norepinephrine, dopamine and serotonin in hippocampus and prefrontal cortex of rats in vivo. Neuropeptides. 2000;34:234–239. doi: 10.1054/npep.2000.0806. [DOI] [PubMed] [Google Scholar]
- Kagamiishi Y, Yamamoto T, Watanabe S. Hippocampal serotonergic system is involved in anxiety-like behavior induced by corticotropin-releasing factor. Brain Res. 2003;991:212–221. doi: 10.1016/j.brainres.2003.08.021. [DOI] [PubMed] [Google Scholar]
- Kalueff AV, Tuohimaa P. The grooming analysis algorithm discriminates between different levels of anxiety in rats: potential utility for neurobehavioural stress research. J Neurosci Methods. 2005;143:169–177. doi: 10.1016/j.jneumeth.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Kirby LG, Rice KC, Valentino RJ. Effects of corticotropin-releasing factor on neuronal activity in the serotonergic dorsal raphe nucleus. Neuropsychopharmacology. 2000;22:148–162. doi: 10.1016/S0893-133X(99)00093-7. [DOI] [PubMed] [Google Scholar]
- Koe BK, Weissman A. p-Chlorophenylalanine: a specific depletor of brain serotonin. J Pharmacol Exp Ther. 1966;154:499–516. [PubMed] [Google Scholar]
- Koob GF. Corticotropin-releasing factor, norepinephrine, and stress. Biol Psychiatry. 1999;46:1167–1180. doi: 10.1016/s0006-3223(99)00164-x. [DOI] [PubMed] [Google Scholar]
- Koob GF, Heinrichs SC. A role for corticotropin releasing factor and urocortin in behavioral responses to stressors. Brain Res. 1999;848:141–152. doi: 10.1016/s0006-8993(99)01991-5. [DOI] [PubMed] [Google Scholar]
- Koob GF, Swerdlow N, Seeligson M, Eaves M, Sutton R, Rivier J, Vale W. Effects of alpha-flupenthixol and naloxone on CRF-induced locomotor activation. Neuroendocrinology. 1984;39:459–464. doi: 10.1159/000124021. [DOI] [PubMed] [Google Scholar]
- Korte SM, Bouws GA, Bohus B. Central actions of corticotropin-releasing hormone (CRH) on behavioral, neuroendocrine, and cardiovascular regulation: brain corticoid receptor involvement. Horm Behav. 1993;27:167–183. doi: 10.1006/hbeh.1993.1013. [DOI] [PubMed] [Google Scholar]
- Korte SM, Bouws GA, Koolhaas JM, Bohus B. Neuroendocrine and behavioral responses during conditioned active and passive behavior in the defensive burying/probe avoidance paradigm: effects of ipsapirone. Physiol Behav. 1992;52:355–361. doi: 10.1016/0031-9384(92)90284-9. [DOI] [PubMed] [Google Scholar]
- Korte SM, Korte-Bouws GA, Bohus B, Koob GF. Effect of corticotropin-releasing factor antagonist on behavioral and neuroendocrine responses during exposure to defensive burying paradigm in rats. Physiol Behav. 1994;56:115–120. doi: 10.1016/0031-9384(94)90268-2. [DOI] [PubMed] [Google Scholar]
- Lazosky AJ, Britton DR. Effects of 5-HT-1A receptor agonists on CRF-induced behavior. Psychopharmacology (Berl) 1991;104:132–136. doi: 10.1007/BF02244567. [DOI] [PubMed] [Google Scholar]
- Liang KC, Melia KR, Miserendino MJ, Falls WA, Campeau S, Davis M. Corticotropin-releasing factor: long-lasting facilitation of the acoustic startle reflex. J Neurosci. 1992;12:2303–2312. doi: 10.1523/JNEUROSCI.12-06-02303.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebsch G, Montkowski A, Holsboer F, Landgraf R. Behavioural profiles of two Wistar rat lines selectively bred for high or low anxiety-related behaviour. Behav Brain Res. 1998;94:301–310. doi: 10.1016/s0166-4328(97)00198-8. [DOI] [PubMed] [Google Scholar]
- Litvin Y, Pentkowski NS, Blanchard DC, Blanchard RJ. CRF type 1 receptors in the dorsal periaqueductal gray modulate anxiety-induced defensive behaviors. Horm Behav. 2007;52:244–251. doi: 10.1016/j.yhbeh.2007.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumley LA, Robison CL, Chen WK, Mark B, Meyerhoff JL. Vasopressin into the preoptic area increases grooming behavior in mice. Physiol Behav. 2001;73:451–455. doi: 10.1016/s0031-9384(01)00501-7. [DOI] [PubMed] [Google Scholar]
- MacClintock D. Squirrels of North America. 1970:28–51. [Google Scholar]
- Matsuzaki I, Takamatsu Y, Moroji T. The effects of intracerebroventricularly injected corticotropin-releasing factor (CRF) on the central nervous system: behavioural and biochemical studies. Neuropeptides. 1989;13:147–155. doi: 10.1016/0143-4179(89)90085-1. [DOI] [PubMed] [Google Scholar]
- Mayorga AJ, Dalvi A, Page ME, Zimov-Levinson S, Hen R, Lucki I. Antidepressant-like behavioral effects in 5-hydroxytryptamine1A and 5- hydroxytryptamine1B receptor mutant mice. J Pharmacol Exp Ther. 2001;298:1101–1107. [PubMed] [Google Scholar]
- Melia KR, Duman RS. Involvement of corticotropin-releasing factor in chronic stress regulation of the brain noradrenergic system. Proc Natl Acad Sci U S A. 1991;88:8382–8386. doi: 10.1073/pnas.88.19.8382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzaghi F, Howard RL, Heinrichs SC, Vale W, Rivier J, Koob GF. Characterization of a novel and potent corticotropin-releasing factor antagonist in rats. J Pharmacol Exp Ther. 1994;269:564–572. [PubMed] [Google Scholar]
- Morilak DA, Barrera G, Echevarria DJ, Garcia AS, Hernandez A, Ma S, Petre CO. Role of brain norepinephrine in the behavioral response to stress. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1214–1224. doi: 10.1016/j.pnpbp.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB. Early-Life Adversity, CRF Dysregulation, and Vulnerability to Mood and Anxiety Disorders. Psychopharmacol Bull. 2004;38(Suppl 1):14–20. [PubMed] [Google Scholar]
- Ohata H, Shibasaki T. Effects of urocortin 2 and 3 on motor activity and food intake in rats. Peptides. 2004;25:1703–1709. doi: 10.1016/j.peptides.2004.05.023. [DOI] [PubMed] [Google Scholar]
- Owens MJ, Nemeroff CB. The role of corticotropin-releasing factor in the pathophysiology of affective and anxiety disorders: laboratory and clinical studies. Ciba Found Symp. 1993;172:296–308. doi: 10.1002/9780470514368.ch15. discussion 308-316. [DOI] [PubMed] [Google Scholar]
- Owings DH, Coss RG. Snake mobbing by California ground squirrels: Adaptive variation and ontogeny. Behaviour. 1977;62:50–69. [Google Scholar]
- Price ML, Curtis AL, Kirby LG, Valentino RJ, Lucki I. Effects of corticotropin-releasing factor on brain serotonergic activity. Neuropsychopharmacology. 1998;18:492–502. doi: 10.1016/S0893-133X(97)00197-8. [DOI] [PubMed] [Google Scholar]
- Price ML, Lucki I. Regulation of serotonin release in the lateral septum and striatum by corticotropin-releasing factor. J Neurosci. 2001;21:2833–2841. doi: 10.1523/JNEUROSCI.21-08-02833.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes BA, Fox K, Valentino RJ, Van Bockstaele EJ. Agonist-induced internalization of corticotropin-releasing factor receptors in noradrenergic neurons of the rat locus coeruleus. Eur J Neurosci. 2006;23:2991–2998. doi: 10.1111/j.1460-9568.2006.04820.x. [DOI] [PubMed] [Google Scholar]
- Reynolds SM, Berridge CW. Fear and feeding in the nucleus accumbens shell: rostrocaudal segregation of GABA-elicited defensive behavior versus eating behavior. J Neurosci. 2001;21:3261–3270. doi: 10.1523/JNEUROSCI.21-09-03261.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risbrough VB, Stein MB. Role of corticotropin releasing factor in anxiety disorders: a translational research perspective. Horm Behav. 2006;50:550–561. doi: 10.1016/j.yhbeh.2006.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman JE, Kalin NH. The effects of ICV-CRH on novelty-induced behavior. Pharmacol Biochem Behav. 1987;26:699–703. doi: 10.1016/0091-3057(87)90599-5. [DOI] [PubMed] [Google Scholar]
- Singh L, Heaton JC, Rea PJ, Handley SL. Involvement of noradrenaline in potentiation of the head-twitch response by GABA-related drugs. Psychopharmacology (Berl) 1986;88:315–319. doi: 10.1007/BF00180831. [DOI] [PubMed] [Google Scholar]
- Spina M, Merlo-Pich E, Chan RK, Basso AM, Rivier J, Vale W, Koob GF. Appetite-suppressing effects of urocortin, a CRF-related neuropeptide. Science. 1996;273:1561–1564. doi: 10.1126/science.273.5281.1561. [DOI] [PubMed] [Google Scholar]
- Spina MG, Basso AM, Zorrilla EP, Heyser CJ, Rivier J, Vale W, Merlo-Pich E, Koob GF. Behavioral effects of central administration of the novel CRF antagonist astressin in rats. Neuropsychopharmacology. 2000;22:230–239. doi: 10.1016/S0893-133X(99)00108-6. [DOI] [PubMed] [Google Scholar]
- Spruijt BM, van Hooff JA, Gispen WH. Ethology and neurobiology of grooming behavior. Physiol Rev. 1992;72:825–852. doi: 10.1152/physrev.1992.72.3.825. [DOI] [PubMed] [Google Scholar]
- Takahashi LK. Role of CRF(1) and CRF(2) receptors in fear and anxiety. Neurosci Biobehav Rev. 2001;25:627–636. doi: 10.1016/s0149-7634(01)00046-x. [DOI] [PubMed] [Google Scholar]
- To CT, Anheuer ZE, Bagdy G. Effects of acute and chronic fluoxetine treatment of CRH-induced anxiety. Neuroreport. 1999;10:553–555. doi: 10.1097/00001756-199902250-00020. [DOI] [PubMed] [Google Scholar]
- Treit D, Pesold C. Septal lesions inhibit fear reactions in two animal models of anxiolytic drug action. Physiol Behav. 1990;47:365–371. doi: 10.1016/0031-9384(90)90155-w. [DOI] [PubMed] [Google Scholar]
- Treit D, Pinel JP, Fibiger HC. Conditioned defensive burying: a new paradigm for the study of anxiolytic agents. Pharmacol Biochem Behav. 1981;15:619–626. doi: 10.1016/0091-3057(81)90219-7. [DOI] [PubMed] [Google Scholar]
- Tricklebank MD. The behavioural response to 5-HT receptor agonists and subtypes of the central 5-HT receptor. Trends in Pharmacological Sciences. 1985;6:403–407. [Google Scholar]
- Turski L, Turski W, Czuczwar SJ, Kleinrok Z. Evidence against the involvement of serotonergic mechanisms in wet dog shake behavior induced by carbachol chloride in rats. Psychopharmacology (Berl) 1981;73:376–380. doi: 10.1007/BF00426469. [DOI] [PubMed] [Google Scholar]
- Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Foote SL. Corticotropin-releasing factor disrupts sensory responses of brain noradrenergic neurons. Neuroendocrinology. 1987;45:28–36. doi: 10.1159/000124700. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Foote SL, Aston-Jones G. Corticotropin-releasing factor activates noradrenergic neurons of the locus coeruleus. Brain Res. 1983;270:363–367. doi: 10.1016/0006-8993(83)90615-7. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Foote SL, Page ME. The locus coeruleus as a site for integrating corticotropin-releasing factor and noradrenergic mediation of stress responses. Ann N Y Acad Sci. 1993;697:173–188. doi: 10.1111/j.1749-6632.1993.tb49931.x. [DOI] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Colago EE, Valentino RJ. Corticotropin-releasing factor-containing axon terminals synapse onto catecholamine dendrites and may presynaptically modulate other afferents in the rostral pole of the nucleus locus coeruleus in the rat brain. J Comp Neurol. 1996;364:523–534. doi: 10.1002/(SICI)1096-9861(19960115)364:3<523::AID-CNE10>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Vetulani J, Bednarczyk B, Reichenberg K, Rokosz A. Head twitches induced by LSD and quipazine: similarities and differences. Neuropharmacology. 1980;19:155–158. doi: 10.1016/0028-3908(80)90131-8. [DOI] [PubMed] [Google Scholar]
- Weiss JM, Stout JC, Aaron MF, Quan N, Owens MJ, Butler PD, Nemeroff CB. Depression and anxiety: role of the locus coeruleus and corticotropin-releasing factor. Brain Res Bull. 1994;35:561–572. doi: 10.1016/0361-9230(94)90170-8. [DOI] [PubMed] [Google Scholar]
- Wiersma A, Baauw AD, Bohus B, Koolhaas JM. Behavioural activation produced by CRH but not alpha-helical CRH (CRH-receptor antagonist) when microinfused into the central nucleus of the amygdala under stress-free conditions. Psychoneuroendocrinology. 1995;20:423–432. doi: 10.1016/0306-4530(94)00074-3. [DOI] [PubMed] [Google Scholar]
- Yang XM, Dunn AJ. Central beta 1-adrenergic receptors are involved in CRF-induced defensive withdrawal. Pharmacol Biochem Behav. 1990;36:847–851. doi: 10.1016/0091-3057(90)90088-y. [DOI] [PubMed] [Google Scholar]
- Zobel AW, Nickel T, Kunzel HE, Ackl N, Sonntag A, Ising M, Holsboer F. Effects of the high-affinity corticotropin-releasing hormone receptor 1 antagonist R121919 in major depression: the first 20 patients treated. J Psychiatr Res. 2000;34:171–181. doi: 10.1016/s0022-3956(00)00016-9. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Valdez GR, Nozulak J, Koob GF, Markou A. Effects of antalarmin, a CRF type 1 receptor antagonist, on anxiety-like behavior and motor activation in the rat. Brain Res. 2002;952:188–199. doi: 10.1016/s0006-8993(02)03189-x. [DOI] [PubMed] [Google Scholar]
- Zorilla EP, Reinhardt LE, Valdez GR, Inoue K, Rivier JE, Vale WW, Koob GF. Human urocortin 2, a corticotropin-releasing factor (CRF)2 agonist, and ovine CRF, a CRF1 agonist, differentially alter feeding and motor activity. J Pharmacol Exp Ther. 2004;310:1027–1034. doi: 10.1124/jpet.104.068676. [DOI] [PubMed] [Google Scholar]