Abstract
Schinzel–Giedion syndrome (SGS) is a rare disorder characterized by midface retraction, hypertrichosis, and multiple skeletal anomalies with severe mental retardation. Various skeletal manifestations of the disease have been previously described. We present the first case of SGS developing scoliosis. The patient presented with scoliosis at the age of 8 years which rapidly progressed to severe thoraco-lumbar scoliosis. Survival beyond 2 years is rare in this syndrome. The objective of this report is to describe the possibility of development of scoliosis in SGS due to the neuromuscular nature of the syndrome, especially in long survivors.
Keywords: Schinzel–Giedion syndrome, scoliosis
Introduction
Schinzel and Giedion first described a syndrome characterized by facial dysmorphism, hypertrichosis, multiple skeletal abnormalities, cardiac and renal anomalies, and severe growth and mental retardation [1]. An autosomal recessive inheritance has been speculated [2–4]. Less than 50 cases of Schinzel–Giedion syndrome (SGS) have been reported [2, 5, 6]. There is no ethnic or geographic predilection for this syndrome reported in the literature. Survival beyond 2 years is not common [5]. Phenotypic features vary between patients affected with SGS [3, 6, 7].
Multiple skeletal abnormalities have been described including abnormality of the small bones of the hand, thick cortex of the long bones, broad ribs, and bowed tibia and fibula [1–3, 6–11]. There is no report of scoliosis in a case of SGS. We present the first case of SGS with scoliosis.
Case report
This patient is the second child of nonconsanguineous, healthy parents, born at 38 weeks of gestation. Prenatal history was unremarkable. The patient’s sibling is a 5-year-old healthy boy. Family history was negative for scoliosis or any genetic syndrome. Apgar scores were 1, 5, and 5 at 1, 3, and 5 min, respectively. The birth weight was 2,740 g, length 45 cm, and head circumference was 34 cm. At birth, the patient had coarse facial features with midface retraction and hypertrichosis, especially in the upper part of the torso. She had prominent forehead, abnormally bulging anterior and posterior fontanelles, and bitemporal narrowing. Deep sulci were present under both eyes along with hypertelorism and proptosis. The nose was small with upturned nares and depressed nasal bridge. Ears were small and low set. The palate was high arched with macroglossia. A simian crease was present in bilateral hands with short digits. Finger and toe nails were small and hyperconvex. No organomegaly was noted on abdominal palpation. The clitoris was hypertrophied with a deep sulcus between labia minora and majora.
X-ray evaluation showed a steep and sclerotic base of the skull, hypoplastic terminal phalanges of the hands and feet, and broad ribs. No spine abnormality was noted at birth. Ultrasound of the abdomen revealed bilateral hydronephrosis with mild dilatation of both pevicalyceal systems, which is a characteristic feature of the syndrome [2]. There was no heart murmur, and echocardiogram of the heart was normal. Blood and urinary marker for storage or metabolic disease were normal. Genetic studies failed to reveal presence of any known disorder with normal karyotype. A diagnosis of SGS was made due to presence of typical facial and skeletal features with renal anomaly.
At the age of 5 years, the patient underwent bilateral subureteral injection for severe reflux and repeated episodes of urinary tract infections. Cystoscopy with left ureteral stenting was done 3 years ago because of severe subureteral scarring. A vesicocystoureterogram done 2 years ago showed presence of vesico-ureteral reflux on the left with no reflux on the right. She currently voids with vesicotomy. The patient had an intractable seizure disorder during childhood which was partially controlled with anti-epileptic drugs. She exhibited severe physical and mental retardation throughout her growth and development. Head control and sitting balance was achieved at the age of 1.5 and 4 years, respectively. A gastric tube was placed for nutrition at the age of 5 years. Since birth, the patient had multiple admissions to the hospital for pulmonary, neurological, and renal problems.
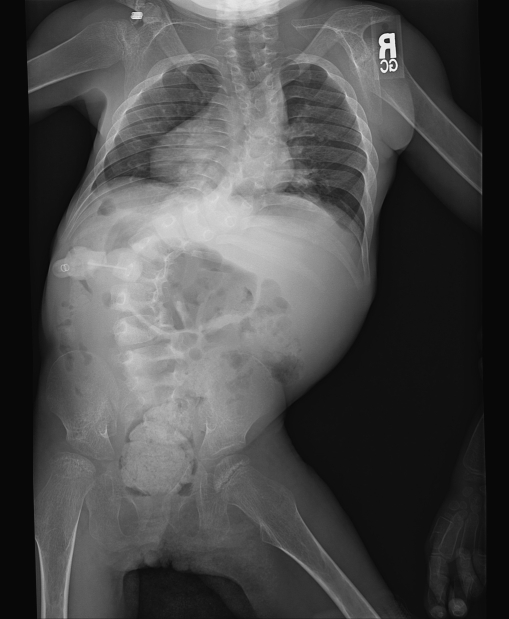
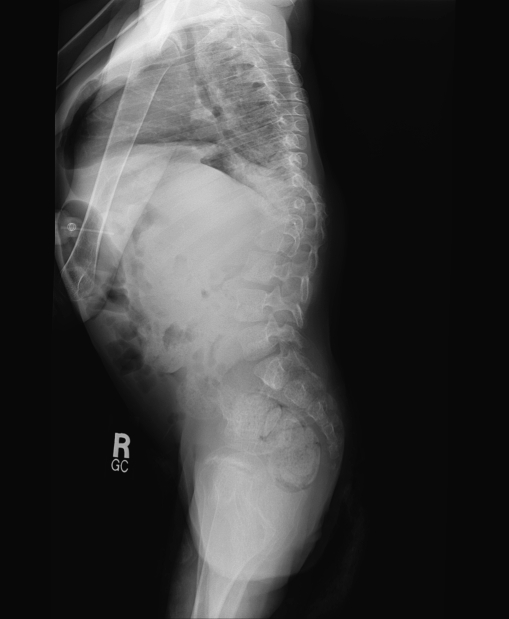
She presented to our clinic at age 10 years for evaluation of spinal deformity. Her body weight was 10.4 kg and head circumference 45 cm. She had diffuse spasticity with hyperreflexia in her limbs. She was nonverbal, non-ambulatory, and wheelchair bound with severe mental retardation. She had been maintained on multiple anti-epileptic drugs. Facial dysmorphism was still present with midface retraction and a figure of “8” shaped face. The nose was small and upturned with depressed nasal bridge. The head was dolicocephalic with a prominent forehead and accentuated bitemporal narrowing. Hypertrichosis was persistent. She had poor dentition. The deformity in the back was first noticed 2 years before referral to our service; however, no treatment was taken. At the time of our evaluation, the deformity of the back was severe with an S-shaped scoliotic curve in the thoracic and thoraco-lumbar regions. The thoracic curve was convex to the right, and the thoraco-lumbar curve was convex to the left with a prominent right rib hump and prominent left lumbar paraspinal muscles. Radiographic examination showed a right convex thoracic curve between T-3 and T-11 of 72° and left convex lumbar curve between T-11 and L-4 of 80° (Figs. 1 and 2). There was mild thoraco-lumbar kyphosis evident on the lateral view X-ray of the spine. There were no vertebral anomalies noted suggestive of any congenital etiology of the scoliosis. Ribs were broad. Magnetic resonance imaging (MRI) of the spine failed to reveal any intraspinal pathology. The patient had good sitting balance with minor wheelchair modifications. Bracing was considered ineffective considering the magnitude of the curves and probable neuromuscular etiology of the scoliosis. A decision was taken not to perform surgery because of the poor medical condition of the patient.
Fig. 1.
Antero-posterior radiograph of the spine of the patient showing the scoliosis
Fig. 2.
Lateral radiograph of the spine demonstrating spinal deformity with thoraco-lumbar kyphosis
Discussion
SGS is a rare syndrome characterized by various phenotypic features with no known genetic or biochemical abnormality [1–14]. Typical phenotypic abnormalities together with the skeletal and renal imaging and absence of metabolic and genetic abnormalities were the basis of diagnosis of SGS in our patient.
There is controversy regarding persistence of morphological features of the disease with advancing age. Some of the features like facial dysmorphism, hypertrichosis, and frontal bossing have been found to be less conspicuous with advancing age [4, 11]. However, lack of a large number of patients, especially long survivors, makes any assumption inaccurate. In our patient, all these features were still present at 10 years of age.
In most of the previously cited reports, there was no description regarding the spine, or the spine was devoid of any abnormality [2, 8]. Joss et al. [6] have mentioned presence of excessive lumbar lordosis and thoracic kyphosis in their patient with SGS. The presence of scoliosis has not yet been reported with SGS. Though this patient was devoid of any spine deformity at birth, during the course of her life, she developed scoliosis which was rapidly progressive based on reports from the family, and sequential clinical examination and radiographic examinations. Absence of bony or spinal cord pathology on X-ray and MRI ruled out the possibility of congenital scoliosis. The etiology of the scoliosis is most likely syndromic in this patient; however, as the patients with SGS have severe motor retardation and muscle tone abnormality, a possibility of neuromuscular component cannot be ruled out. Most patients with SGS die before 2 years of age [1, 3, 9, 13] and usually do not have sufficient time to develop scoliosis, explaining the rare association of scoliosis with this condition. In the present case, the patient was 8 years old when the scoliosis was first diagnosed. Poor muscle balance with non-ambulatory, wheelchair-bound status was presumably a contributing factor in the development and rapid progression of the deformity. We propose yearly spine examinations in long-term survivors of SGS to diagnose spine deformity at an early stage. Presence of multiple medical disorders makes surgical intervention and correction of deformity difficult with ensuing problems in sitting balance and care of the patient.
Footnotes
Each author certifies that he or she has no commercial associations (e.g., consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest in connection with the submitted article.
No financial support in any form was received for this study from any source.
Each author certifies that his or her institution has approved the reporting of this case, that all investigations were conducted in conformity with ethical principles of research
Study performed at Children’s Hospital, New Orleans, LA
References
- 1.Schinzel A, Giedion A (1978) A syndrome of severe midface retraction, multiple skull anomalies, clubfeet, and cardiac and renal malformations in sibs. Am J Med Genet 1(4):361–375 [DOI] [PubMed]
- 2.Albano LM, Sakae PP, Mataloun MM, Leone CR, Bertola DR, Kim CA (2004) Hydronephrosis in Schinzel–Giedion syndrome: an important clue for the diagnosis. Rev Hosp Clin Fac Med Sao Paulo 59(2):89–92 [DOI] [PubMed]
- 3.Labrune P, Lyonnet S, Zupan V, Imbert MC, Goutieres F, Hubert P, Le Merrer M (1994) Three new cases of the Schinzel–Giedion syndrome and review of the literature. Am J Med Genet 50(1):90–93 [DOI] [PubMed]
- 4.Ozkinay FF, Akisü M, Kültürsay N, Oral R, Tansug N, Sapmaz G (1996) Agenesis of the corpus callosum in Schinzel–Giedion syndrome associated with 47,XXY karyotype. Clin Genet 50(3):145–148 [DOI] [PubMed]
- 5.Beschorner R, Wehrmann M, Ernemann U, Bonin M, Horber V, Oehl-Jaschkowitz B, Meyermann R, Dufke A (2007) Extradural ependymal tumor with myxopapillary and ependymoblastic differentiation in a case of Schinzel–Giedion syndrome. Acta Neuropathol 113(3):339–346 [DOI] [PubMed]
- 6.Joss S, Dean JC (2002) A Schinzel–Giedion-like syndrome—a milder version or a separate condition. Clin Dysmorphol 11(4):271–275 [DOI] [PubMed]
- 7.Alavi S, Kher A, Bharucha BA (1994) Schinzel–Giedion syndrome. Indian Pediatr 31(9):1111–1114 [PubMed]
- 8.Alembik Y, Christmann D, de Saint Martin A, Eliot M, Dollfus H, Pauly F, Stoll C (1999) Schinzel–Giedion syndrome with severe deafness and neurodegenerative process. Ann Genet 42(4):225–230 [PubMed]
- 9.Culi V, Resic B, Oorthuys JW, Overweg-Plandsoen WC, Hennekam RC (1996) A Croatian case of the Schinzel–Giedion syndrome. Genet Couns 7(1):21–25 [PubMed]
- 10.Robin NH, Grace K, DeSouza TG, McDonald-McGinn D, Zackai EH (1993) New finding of Schinzel–Giedion syndrome: a case with a malignant sacrococcygeal teratoma. Am J Med Genet 47(6):852–856 [DOI] [PubMed]
- 11.Verloes A, Moës D, Palumbo L, Elmer C, François A, Bricteux G (1993) Schinzel–Giedion syndrome. Eur J Pediatr 152(5):421–423 [DOI] [PubMed]
- 12.Kondoh T, Kamimura N, Tsuru A, Matsumoto T, Matsuzaka T, Moriuchi H (2001) A case of Schinzel–Giedion syndrome complicated with progressive severe gingival hyperplasia and progressive brain atrophy. Pediatr Int 43(2):181–184 [DOI] [PubMed]
- 13.Matsumoto F, Tohda A, Shimada K, Okamoto N (2005) Malignant retroperitoneal tumor arising in a multicystic dysplastic kidney of a girl with Schinzel–Giedion syndrome. Int J Urol 12(12):1061–1062 [DOI] [PubMed]
- 14.Santos H, Cordeiro I, Medeira A, Mendonça E, Antunes NL, Rosa FC (1994) Schinzel–Giedion syndrome. A patient with hypothyroidism and diabetes insipidus. Genet Couns 5(2):187–189 [PubMed]