Abstract
Giant cell tumors are neoplasms of mesenchymal stromal cells with varied manifestations. There is no uniform accepted treatment protocol for these tumors. Curettage, although an accepted method of treatment, carries a high local recurrence rate. Adjuvant therapies including high-speed burr debridement, cryotherapy, and phenol treatment have been advocated to reduce local recurrence. We have used these adjuvants to determine if improved cure rate with improved outcomes could be attained with regard to local tumor control and functional outcome. Twenty-eight cases of proven giant cell tumors of the distal femur and proximal tibia were included in this prospective case series. The lesions were at the upper tibia in 14 cases and the lower femur in 14 patients. The patients were evaluated clinically, radiologically, and by histological examination. Companacci grading and Enneking staging were determined. The treatment was done in the following steps: Curettage and further debridement with a high-speed burr, cryotherapy, impaction of the cavity with subchondral iliac crest bone graft, and, finally, cementation with or without internal fixation. Functional evaluation was done by Enneking’s system. The follow-up time was between 24–40 months with a mean of 34 months. The functional results of the procedure were rated as good to excellent with a mean of 93.9%. This technique has the advantages of joint preservation, excellent functional outcome, and low recurrence rate when compared with other treatment modalities. For these reasons, it is recommended as an adjuvant to curettage for most giant cell tumors of bone.
Keywords: giant cell tumor, cryosurgery, bone graft
Introduction
Giant cell tumor of bone first was described in 1818 by Cooper and Travers [1]. Its local aggressiveness was described by Nelaton [2] and its malignant potential by Virchow [3].
Giant cell tumor represents approximately 5% of all primary bone tumors. Seventy percent of these lesions occur in the third or fourth decades of life [4–7]. The tumor is thought to arise in the metaphyseoepiphyseal junction [8]. Large tumors may extend into the metaphysis and, more rarely, into the diaphysis. The primary areas of involvement are the femoral condyles, tibial plateau, proximal humerus, and distal radius [6, 7].
Giant cell tumor (GCT) of the bone has an unpredictable behavior not always related to radiographic or histological appearance [9]. This makes the treatment of the disease a subject of constant debate. The best treatment should ensure local control of disease and maintain limb function. Curettage has been the preferred treatment for most cases of GCT. Many earlier studies had shown very high (25–50%) local recurrence rates after simple curettage and bone grafting [4, 9, 10].
The use of modern imaging techniques and extended curettage through the use of power burrs and local adjuvants have improved outcome with reduced recurrence rates (10–20%). Phenol, liquid nitrogen, bone cement, hydrogen peroxide, zinc chloride, and, more recently, argon beam cauterization have been employed as local adjuvants. Chemical or physical agents work by inducing an additional circumferential area of necrosis to “extend” the curettage [11]. During the 1970s, Marcove et al. [12] pioneered the development of cryotherapy in the treatment of giant cell tumor of the bone and described the effectiveness of a direct pour method in freezing the walls of a curetted cavity. This technique used wide incision, thorough curettage, and repetitive exposure of the curetted area to temperatures below −20°C by liquid nitrogen instillation [13, 14]. They advocated this method as a physical adjuvant in the hope of decreasing the high rates of local recurrence after curettage [14].
We present the outcome of treatment of GCT of the bone around the knee with curettage, cryosurgery, with impaction subchondral iliac crest bone graft followed by cementation of the remaining cavity with or without internal fixation. The aim of this study was to evaluate this technique as regard to local tumor control (recurrence) and assess functional outcome in the treated limbs. Finally, we wished to review the complications which developed as well.
Patients and methods
Twenty-eight patients with giant cell tumor of the bone at the proximal tibia and distal femur were treated between June 2005 and October 2008 at our institution. All participating surgeons used the same technique of curettage, cryotherapy, and reconstruction.
There were ten male and 18 female patients. Ages ranged from 25 to 45 years (average, 36.3 years). The average follow-up was 34 months (range, 24–40 months). All patients underwent staging studies that included plain radiography, computed tomography (CT), magnetic resonance imaging (MRI), and chest CT.
Campanacci’s [15] staging system for giant cell tumor of bone was used for cortical breach. Grade I tumors had a well-marginated border of a thin rim of mature bone and the cortex was intact or slightly thinned but not deformed. Grade II tumors had relatively well-defined margins but no radio-opaque rim. Grade III tumors had fuzzy borders. According to this system, ten tumors were classified as stage I, 14 tumors as stage II, and four tumors as stage III (Table 1).
Table 1.
The characteristics of the patients, the follow-up period, the use of internal fixation, the grading, complications, and functional results
| Cases | Age | Sex | Site | Follow-up (months) | Internal Fixation | Campanacci’s Grade | Comations | Functional score (%) |
|---|---|---|---|---|---|---|---|---|
| Case 1 | 42 | M | Tibia | 40 | No | Grade I | None | 96 |
| Case 2 | 35 | F | Femur | 40 | Yes | Grade II | None | 96 |
| Case 3 | 25 | F | Tibia | 35 | Yes | Grade II | None | 96 |
| Case 4 | 28 | F | Tibia | 35 | No | Grade I | None | 96 |
| Case 5 | 34 | M | Femur | 38 | Yes | Grade II | Local Recurrence | 90 |
| Case 6 | 33 | F | Tibia | 36 | Yes | Grade III | None | 93 |
| Case 7 | 39 | M | Tibia | 37 | No | Grade I | None | 93 |
| Case 8 | 45 | F | Tibia | 38 | No | Grade I | Fracure | 93 |
| Case 9 | 44 | M | Femur | 39 | Yes | Grade II | Partial skin necrosis | 90 |
| Case 10 | 34 | M | Tibia | 36 | Yes | Grade III | None | 93 |
| Case 11 | 29 | F | Femur | 39 | Yes | Grade II | None | 93 |
| Case 12 | 43 | F | Femur | 38 | Yes | Grade III | None | 93 |
| Case 13 | 43 | F | Tibia | 29 | Yes | Grade II | None | 96 |
| Case 14 | 29 | M | Femur | 38 | Yes | Grade II | None | 96 |
| Case 15 | 30 | F | Femur | 32 | No | Grade I | None | 93 |
| Case 16 | 29 | M | Femur | 25 | No | Grade I | Fracture | 90 |
| Case 17 | 42 | M | Femur | 30 | Yes | Grade II | None | 93 |
| Case 18 | 45 | F | Femur | 32 | No | Grade I | None | 96 |
| Case 19 | 33 | M | Tibia | 24 | Yes | Grade II | None | 96 |
| Case 20 | 38 | M | Tibia | 27 | No | Grade I | None | 96 |
| Case 21 | 36 | F | Tibia | 29 | Yes | Grade II | None | 96 |
| Case 22 | 38 | F | Femur | 28 | Yes | Grade III | Superficial infection | 93 |
| Case 23 | 43 | F | Tibia | 34 | Yes | Grade II | None | 96 |
| Case 24 | 42 | F | Femur | 36 | No | Grade I | None | 90 |
| Case 25 | 27 | F | Femur | 33 | No | Grade I | None | 96 |
| Case 26 | 44 | F | Tibia | 37 | Yes | Grade II | None | 96 |
| Case 27 | 36 | F | Tibia | 38 | Yes | Grade II | None | 90 |
| Case 28 | 30 | F | Femur | 39 | Yes | Grade II | None | 96 |
Selection of the patients was decided based on CT scan findings. All cases which on CT scan showed a break in the cortex confined to one surface and a cortical break less than one third of the circumference were included in the study. As a result, only Campanacci’s grades 1, 2 or grade 3 tumors which fulfilled the above criteria were included. We excluded any tumors demonstrating cortical breach of more than one third of the bone diameter, those with pathological fractures, cases with soft tissue extension, and cases with intraarticular extension. These more advanced tumors were all subjected to wide resection and reconstruction.
If the clinical presentation and the imaging studies were compatible with diagnosis of a classic benign giant cell tumor of bone, the biopsy (frozen section) and surgery were performed during the same session. In case of atypical clinical or radiologic presentation, either CT-guided core needle or open incisional biopsy was performed and surgery was delayed until histopathologic evaluation had been completed.
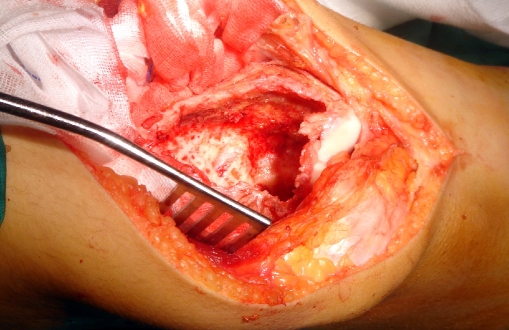
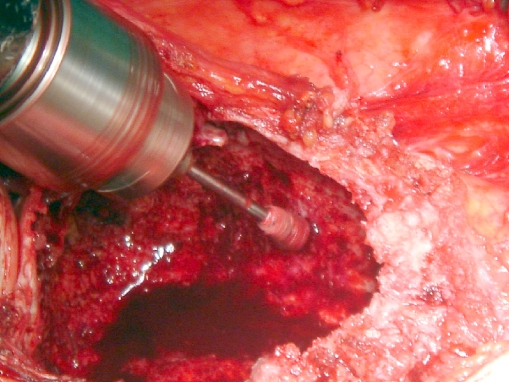
For the curettage, a pneumatic tourniquet was applied to decrease bleeding and to prevent blood from acting as a thermal barrier when cryosurgery was performed. After exposure of the involved bone and soft tissues, a cortical window the size of the longest longitudinal dimension of the tumor is made, ensuring that it is large enough to expose the entire tumor. It has to be elliptical with its axis parallel to the long axis of the bone to reduce the stress riser effect. All gross tumors were removed with hand curettes (Fig. 1). High-speed burr drilling (resectional curettage) was used for the removal of the tumor of the inner reactive shell (Fig. 2).
Fig. 1.
Intraoperative photo showing a case of giant cell tumor of the distal femur after removing all tumor tissues
Fig. 2.
Intraoperative photo showing he use of high speed burr in case of GCT of the proximal tibia
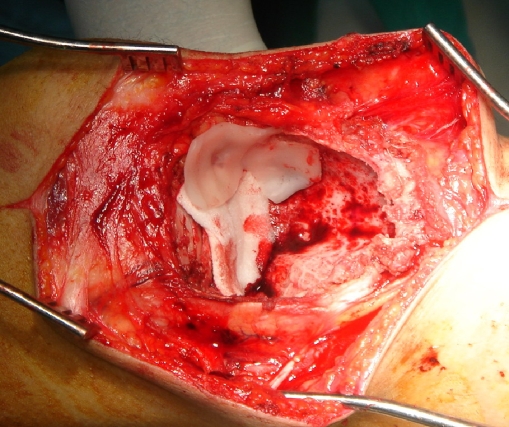
For cryotherapy before introduction of liquid nitrogen, bony perforations were identified and sealed using gel foam. The surrounding skin, soft tissues, and neurovascular bundles were protected and shielded using gelfoam and gauze soaked with saline to prevent extravasations of liquid nitrogen and thus protect the adjacent neurovascular structures and the skin. Large skin flaps were retracted to protect them from any possible spillage of the liquid nitrogen (Fig. 3). The direct pour technique as described by Marcove et al. [12] was performed in our cases. Liquid nitrogen (−196°C) was poured through a stainless steel funnel into the tumor cavity (Fig. 4). The surrounding soft tissues were irrigated with warm normal saline to avoid thermal injury. Two freeze and thaw cycles were administered, each cycle lasting 1 to 2 min, and spontaneous thaw was allowed to occur for 3 to 5 min. After evaporation, the cavity was irrigated with normal saline and good hemostasis was ensured.
Fig. 3.
Intraoperative photo showing that the bony perforations were identified and sealed using gelfoam
Fig. 4.
Intraoperative photo showing the use of liquid nitrogen by the direct pour technique
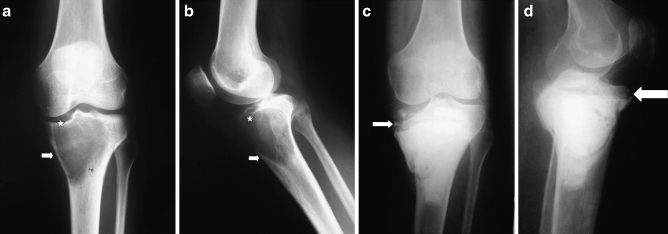
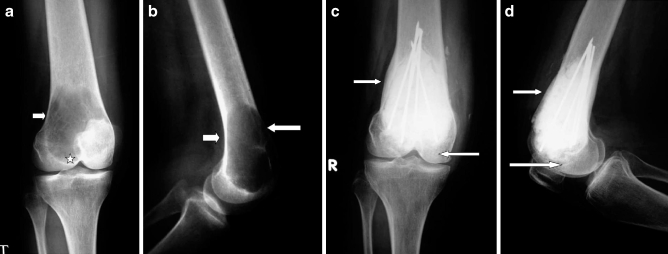
Reconstruction of the bone defect remaining after the curettage and cryotherapy were performed according to the methods described by Malawar et al. [16]. This approach depends upon the site and size of the cavity. Small cavities of less than 2 cm in non-weight-bearing areas (type 1) require no reconstruction; usually, for type 2, polymethylmethacrylate plus or minus bone graft before the routine use of internal fixation and type 3, polymethylmethacrylate plus or minus bone graft plus internal fixation with intramedullary hardware. In our cases, we used type 2 reconstruction in ten cases (all were Campanacci’s grade I; Fig. 5). Type 3 reconstruction was used in 18 patients (Campanacci’s grades II and III; Fig. 6).
Fig. 5.
a, b Preoperative X-rays in case of GCT of the proximal tibia (case 4) showing cortical thinning (arrow) and resorption of the subchondral bone (star). c, d, 35 months follow-up in a follow-up X-ray shows good remodeling of the subchondral bone graft (arrow)
Fig. 6.
a, b Preoperative X-rays of a case of GCT of the distal femur (case 2) showing cortical thinning (arrow) and subchondral extension (star). c, d Follow-up X-rays showing internal fixation with intramedullary wires with 40 month post-reconstruction showing cortical and subchondral thickening (arrows)
Postoperatively, patients were followed up at weekly intervals in the first month, every 2 weeks for the next 2 months, and monthly thereafter. X-rays were taken at every visit after the 8 weeks and then every 6 weeks and were examined by at least two of the operating surgeons in the bone tumors clinic. The aim of the early follow-up is to detect local recurrence. Following curettage and cementation, an osteolytic zone caused by thermal injury measuring 2 mm surrounds the cement. This radiolucent zone is bordered by a thin outer sclerotic rim for about 6 months [17, 18]. Recurrence was considered to be present when there was progressive lysis of more than 5 mm at cement–bone or graft–host interface or if there was an absence of a sclerotic rim at the above said interface [19]. Peripheral calcification around a soft tissue mass of uniform density was the criteria for recurrence in soft tissues [19]. MRI was performed to confirm the recurrence.
Functional evaluation of the treated limbs was performed according to Enneking’s system [20]. This system is applicable in evaluating limb salvage surgeries. This evaluates pain, function, and emotional acceptance, besides dexterity as a measure of upper limb functions and walking ability and gait as a measure of lower limb functions. It was done 6 months post-surgery and in the last follow-up.
Results
At the end of the follow-up period, all the patients were alive and free from the diseases. Using the standard system of the musculoskeletal tumor society, the functional score at the final follow-up ranges from 90% to 96%, with an average of 93.9%.
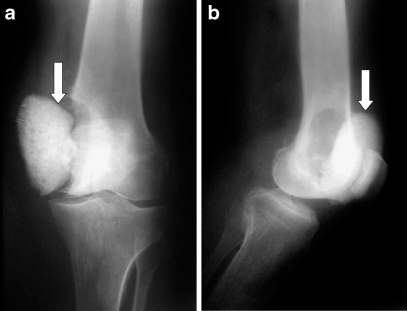
Local recurrence occurred in one case at the distal femur after 2 years and was treated by wide resection and endoprosthetic reconstruction (Fig. 7).
Fig. 7.
a, b Local recurrence resulting in cement extrusion (arrow) occurred in one case at the distal femur (case 5) after 2 years and was treated by wide resection and endoprosthetic reconstruction
Complications associated with this treatment occurred in four patients. One patient sustained partial skin necrosis. This damage resulted from contact with leaking liquid nitrogen and was managed satisfactorily by nonsurgical treatment. Postoperative fracture occurred in two patients, none of whom had undergone internal fixation. No fractures had occurred in the patients who had internal fixation. All fractures occurred during the first 2 years after the operation and often after minor trauma to the extremity. All fractures eventually united after conservative treatment by means of closed reduction and external immobilization with cast or braces for an average of 10 months. Superficial wound infection was reported in one case and had been controlled by the routine use of postoperative antibiotics.
Discussion
In our prospective clinical study, 28 patients with giant cell tumor of bone at distal femur and proximal tibia were treated using extended curettage, cryotherapy, bone cement, and subchondral bone graft with or without internal fixation. At a mean follow-up of 34 months, all the patients were alive and free from any signs of local or systemic recurrence, with good functional outcome. Our results are comparable with the published results of these techniques.
Campanacci et al. [4] reported that their recurrence rate was 27% after intralesional excision, 7% after marginal excision, and zero after wide excision. The rate of local recurrence in our study was 2.5% (one case), and it was managed with wide resection and endoprosthetic reconstruction.
Postoperative fracture is the most common and serious complication associated with cryosurgery [21]. Fracture is an inherent risk after reconstruction of any large bone defect and especially after cryosurgery near a weight-bearing joint. After cryosurgery, bone necrosis and disruption of osteoid extend the period through which reossification occurs and delay bone healing. Vigorous freezing increases the likelihood of cure at the cost of higher rate of pathologic fractures, whereas inadequate freezing of bone surrounding the tumor may predispose to local recurrence [22]. We encountered two cases with postoperative fracture that was treated by conservative mean and yielded good results at the final follow-up [12].
Joint function, evaluated by the American Musculoskeletal Tumor Society system, was well preserved (excellent function) in all of the patients in the current series. This rate is similar to the rate reported by Jacobs and Clemency [21] who reported preservation of joint function in 10 of 12 patients treated by cryosurgery.
When the tumor is less than 1 cm from the articular surface, the incidence of degenerative changes in articular cartilage after the use of cement alone is more than 2.5 times greater than that when the tumor is more than 1 cm away. Interposing a centimeter or two of bone graft between the cartilage and cement may reduce heat damage and the resultant early degenerative changes. Studies have shown that cement constructs are less rigid than normal subchondral bone or successful bone graft [23].
Cryosurgery is recommended as a physical adjuvant to curettage in the treatment of giant cell tumor of the bone. It extends the margin of a simple curettage or resection curettage and makes it biologically equivalent to that of a wide resection. Compared with other techniques, cryosurgery with composite fixation not only preserves joint function but also significantly decreases the rate of local tumor recurrence. The routine use of internal fixation with polymethylmethacrylate and bone graft is recommended.
We have routinely used curettage, cryosurgery, and cementation with subchondral bone graft in the treatment of giant cell tumors. Our goals have been to preserve native bone stock and articular integrity, eliminate local tumor recurrence, and secure immediate, stable fixation for early motion and rehabilitation. We report the oncologic and functional results and complications associated with curettage, cryosurgery, and cementation.
Several steps are crucial for obtaining an adequate curettage, ensuring the entire tumor cavity is frozen with liquid nitrogen, and minimizing complications. A long incision should be made to allow wide retraction of skin flaps, including the neurovascular bundle. The cortical window should be the length of the tumor and the width of the bone
Footnotes
None of the authors received financial support for this study.
The Research Ethics Committee (REC, FWA 0000644) of the Faculty of Medicine Ain Shams University has approved this study from the scientific and ethical point of view (FMASU94\2009). This study meets the ethical standards and comply with the national as well as the local standards set within the department.
References
- 1.Cooper A, Travers B. Surgical Essays. 3 ed. London: Cox and Son 195;1818
- 2.Nelaton E. D’une Nouvelle Espece de Tumerus Benignes des Os, ou Tumerus a Myeloplaxes. Paris: Adrien Delahaye; 1860:65–72
- 3.Virchow R. Die Krankhaften Geschwulste. Vol 2. Berlin: Hirschwald; 1846:90–97
- 4.Campanacci M, Baldini N, Boriani S, Sudanese A, Giant cell tumor of bone. J. Bone Joint Surg. 1987; 69A: 106–114 [PubMed]
- 5.Dorfman HD, Czerniak B. Giant cell lesions. In: Dorfman HD, Czerniak B, eds. Bone Tumors. St Louis: CV Mosby; 1998:559–598
- 6.Enneking WF. Lesions of uncertain origin originating in bone. Giant-Cell Tumor. In: Ennecking WF, ed. Musculoskeletal Tumor Surgery. Vol 2. New York: Churchill Livingstone; 1983:1435–1476
- 7.Hutter RVP, Worcester Jr JN, Francis KC, et al, Benign and malignant giant-cell tumor of bone. A clinicopathological analysis of the natural history of the disease. Cancer. 1962; 15: 653–690 [DOI] [PubMed]
- 8.Picci P, Manfrini M, Zucchi V, et al, Giant cell tumor in skeletally immature patients. J. Bone Joint Surg. 1983; 65A: 468–490 [PubMed]
- 9.Sung HW, Kuo DP, Shu WP, Chai YB, Liu CC, Li SM, Giant cell tumor of bone: Analysis of two hundred and eight cases in Chinese patients. J. Bone Joint Surg. Am. 1982; 64: 755–761 [PubMed]
- 10.Campanacci M. Giant cell tumor. In: Gaggi A, ed. Bone and Soft-Tissue Tumors. Bologna, Italy: Springer-Verlag; 1990: 17–53
- 11.Turcotte RE. Giant cell tumor of bone. Orthop. Clin. North Am. 2006; 37: 35–51 [DOI] [PubMed]
- 12.Marcove RC, Weis LD, Vaghaiwalla MR, et al, Cryosurgery in the treatment of giant cell tumor of bone. A report of 52 consecutive cases. Cancer. 1978; 41: 957–969 [DOI] [PubMed]
- 13.Marcove RC, Sheth DS, Brien EW, Huvos AG, Healey JH, Conservative surgery for giant cell tumors of the sacrum. The role of cryosurgery as a supplement to curettage and partial excision. Cancer. 1994; 74: 1253–1260 [DOI] [PubMed]
- 14.Malawer MM, Bickels J, Meller I, Buch RG, Henshaw RM, Kollender Y. Cryosurgery in the treatment of giant cell tumor. A long-term followup study. Clin. Orthop. Relat. Res. 1999; 359: 176–188 [DOI] [PubMed]
- 15.Campanacci M. Giant cell tumor and chondrosarcoma. Grading, treatment and results. Cancer Res. 1976; 54: 257–261 [DOI] [PubMed]
- 16.Malawer M, Bickels J, Meller I, et al, Cryosurgery in the treatment of giant cell tumor a long term followup study. Clin. Orthop. Relat. Res. 1999; 359: 176–188 [DOI] [PubMed]
- 17.Mjoberg BH, Petersson R, Rosenqvist A. Dynamics of the radiolucent zone around bone cement. Acta Orthop. Scand. 1984; 55: 597–600 [DOI] [PubMed]
- 18.Pettersson H, Rydholm, Persson B, Early radiologic detection of local recurrence after curettage and acrylic cementation of giant cell tumours. Eur. J. Radiol. 1986; 6: 1–4 [PubMed]
- 19.Turcotte RE, Giant cell tumor of bone. Orthop. Clin. North Am. 2006; 37: 35–51 [DOI] [PubMed]
- 20.Enneking, Dunham W, Gebhardt MC, Malawar M, Pritchard DJ, A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system. Clin. Orthop. Relat. Res. 1993; 241–246 [PubMed]
- 21.Jacobs PA, Clemency RE, The closed cryosurgical treatment of giant cell tumor. Clin. Orthop. 1989; 192: 149–158 [PubMed]
- 22.Malawer MM, Marks MR, McChesney D, Piasio M, Gunther SF, Shmookler BM. The effect of cryosurgery and polymethylmethacrylate in dogs with experimental bone defects comparable to tumor defects. Clin. Orthop. 1988; 226: 299–310 [PubMed]
- 23.Campanacci M, Capanna R, Fabbri N, Bettelli G. Curettage of giant cell tumor of bone. Reconstruction with subchondral grafts and cement. Chir. Organi. Mov. 1990; 75: 212–213 [PubMed]