SUMMARY
More than 85% of the global population requires repair or replacement of a craniofacial structure. These defects range from simple tooth decay to radical oncologic craniofacial resection. Regeneration of oral and craniofacial tissues presents a formidable challenge that requires synthesis of basic science, clinical science and engineering technology. Identification of appropriate scaffolds, cell sources and spatial and temporal signals (the tissue engineering triad) is necessary to optimize development of a single tissue, hybrid organ or interface. Furthermore, combining the understanding of the interactions between molecules of the extracellular matrix and attached cells with an understanding of the gene expression needed to induce differentiation and tissue growth will provide the design basis for translating basic science into rationally developed components of this tissue engineering triad. Dental tissue engineers are interested in regeneration of teeth, oral mucosa, salivary glands, bone and periodontium. Many of these oral structures are hybrid tissues. For example, engineering the periodontium requires growth of alveolar bone, cementum and the periodontal ligament. Recapitulation of biological development of hybrid tissues and interfaces presents a challenge that exceeds that of engineering just a single tissue. Advances made in dental interface engineering will allow these tissues to serve as model systems for engineering other tissues or organs of the body. This review will begin by covering basic tissue engineering principles and strategic design of functional biomaterials. We will then explore the impact of biomaterials design on the status of craniofacial tissue engineering and current challenges and opportunities in dental tissue engineering.
Keywords: biomaterials, design, cell recognition, teeth, oral mucosa, salivary gland, bone, periodontium
I. Introduction: clinical need for tissue engineering
Defects in oral and craniofacial tissues, resulting from trauma, congenital abnormalities, oncological resection or progressive deforming diseases, present a formidable challenge and restoration of these tissues is a subject of clinical, basic science and engineering concern (1, 2). In addition to leaving patients with aesthetic deformities, oral and craniofacial defects may be uncomfortable to the patient and affect function. Thus, structure, function, aesthetics and pain must all be managed effectively resulting in treatment challenges that are often more complex than in other parts of the body. In addition to problems associated with cranial and facial tissues, 15% of the US population has periodontal disease severe enough to warrant surgery (1). It is further estimated that 9–15 million people in the US experience temporomandibular joint (TMJ) disorders (3), over 30 000 per year have undergone craniofacial resective surgery (4), 2–4 million suffer from salivary gland hypofunction (5) and 85% require replacement or repair of one or more teeth (6). Expansion of these figures to include worldwide dental and craniofacial needs provides staggering support for the need to engineer dental and craniofacial tissues.
In many cases, tissues of the craniofacial complex need to be repaired because of structural deficiencies. There is, however, inadequate guidance regarding patient-selection criteria for many procedures, such as TMJ reconstruction (4, 7). Surgical treatment of TMJ, periodontal and other craniofacial defects is therefore not predictable and does not fully restore function to the tissues in many cases (1). Collectively, therefore, defects associated with orofacial tissues may result in aesthetic deformity, pain and reduced function and represent a substantial clinical problem in need of new solutions.
Techniques to repair orofacial defects parallel accepted therapies for restoring tissue structure and/or function elsewhere in the body, and include synthetic materials, autografts and allografts (8–10). Each of these reconstructive strategies has limitations and lacks clinical predictability. Only a minimal amount of tissue can be harvested for autografts, the harvesting procedure may lead to donor site discomfort and morbidity and it may be difficult to form this tissue into desired shapes (8–10), a problem that is particularly important in the craniofacial region. Autografting, the current ‘gold standard’ for bone regeneration, has failure rates as high as 30% (11). Allografts have the potential of transferring pathogens (12). Freeze-drying, demineralization and irradiation to reduce immunogenic potential can also reduce structural integrity, leading to graft fracture (12). Other complications with autografts and allografts include unreliable incorporation, resorption and non-union of the graft/host tissue interface (13, 14). Induction of new tissue by growth factors requires large amounts of recombinant material, which may not be realistic in cases of massive defects (15). Additionally, successful use of growth factors relies on the presence of a sufficient population of undifferentiated progenitor cells capable of responding to the inductive cues provided by the growth factor (16). Such a population may not be available in aged or compromised patients.
Synthetic materials are primarily designed to be permanently implanted. Long-term complications include stress shielding, loosening and mechanical or chemical breakdown of the material itself (8, 9, 17). Demographics on total joint replacements, such as TMJ replacements, indicate that 25% of the procedures performed each year are revisions (18). Many TMJ patients have had multiple surgeries and the greater the number of surgical procedures, the lower the chance for functional improvement. Of particular importance with the use of synthetic materials is that most problems manifest themselves at the biomaterial/tissue interface, in part because the tissue has the ability to adapt functionally, whereas the synthetic material does not. More biologically interactive biomaterials could potentially solve the problem of implant/tissue interface failure and improve the clinical treatment of craniofacial defects. The desire to create more biological alternatives to the permanent implantation of static synthetic materials has inspired the field of tissue engineering. The basic premise of tissue engineering is that controlled manipulation (engineering) of the extracellular microenvironment can lead to control over the ability of cells to organize, grow, differentiate, form a functional extracellular matrix (ECM) and, ultimately, new functional tissue. Such control is a complex process that requires autocrine, paracrine and endocrine signals, positional cues, cell-matrix interactions, mechanical forces and cell-cell contacts to mediate the formation of 3D tissue architecture and function. In the last decade, many advances in oral and craniofacial tissue engineering have been made, including the in vitro and in vivo engineering of craniofacial bone (19, 20), cranial sutures (21), periodontium (16, 22–24), oral mucosa (25, 26), tooth-associated structures (27–29) and the TMJ (30–33) from combinations of biomaterials, stem cells and/or recombinant growth factors.
This review will examine advances in tissue engineering of craniofacial structures. Following a summary of the principles of tissue engineering, advances in biomaterials used to engineer tissue structure and function will be reviewed. Focus is placed on second generation biomaterials for tissue engineering, which are more biologically interactive and mimic some of the regulatory aspects of the ECM. Building upon the principles of tissue engineering and material design strategies presented, specific dental and craniofacial applications, including engineering of teeth, periodontium, the TMJ, skin, oral mucosa and salivary glands will be discussed with emphasis on application of cells, scaffolds and signalling strategies. Lastly, issues unique to engineering oral and craniofacial tissues will be discussed, along with the difficulties in engineering craniofacial tissues versus tissues elsewhere in the body. The potential for oral tissues to serve as model tissue engineering systems is also discussed.
II. Tissue engineering principles
To engineer functional tissues, cells (host and/or donor) must be provided with appropriate spatial and temporal cues to enable growth, differentiation and synthesis of an ECM of sufficient volume and functional integrity. Explicit in the definition of tissue engineering (34) is the need to understand structure-function relations in normal and diseased tissues and to use these insights as design criteria for engineering new tissues. Upon understanding how tissues develop in vivo and what constituents are most critical for eliciting function, such information can be used in design strategies to recapitulate aspects of developmental biology. Structure-function relations and resultant design strategies are needed at multiple levels of dimensional scale and challenges include deciphering what ‘instructions’ cells need to organize into tissues, which cells should be targeted and what level of hierarchy is most critical to control. Tissue engineering goes beyond regenerative medicine and incorporates the unique qualities of engineering design and use of the engineering method (35) as bases for developing the approaches used to control biological systems.
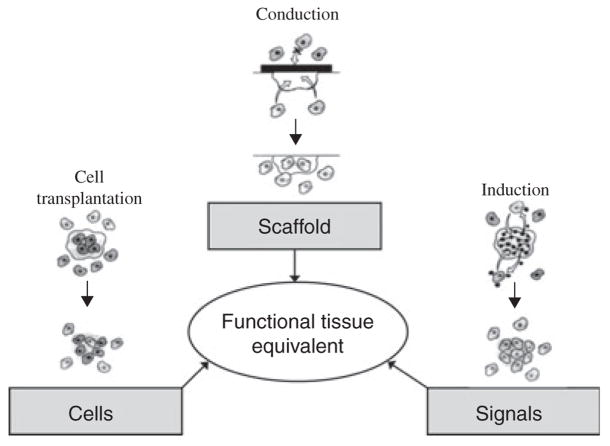
Many tissue engineering approaches are based on the tissue engineering triad, which was derived from the three major components of tissues: cells, their ECM and a signalling system (36) (Fig. 1). A functional tissue can be developed via the use of one or more of these components. Combining the understanding of the interactions between molecules of the ECM and attached cells with an understanding of the gene expression needed to induce differentiation and tissue specific growth provides the design basis for translating basic science into rationally developed components of the tissue engineering triad.
Fig. 1.
The Tissue Engineering Triad (228). The three main design components in tissue engineering are based on the three main components of tissues: cells, their extracellular matrix (scaffolds) and a signalling system. Each of these components represents a design strategy, cell transplantation, conduction and induction, respectively, which can be used individually or in combination to optimize regeneration and engineering of a functional tissue [Figure reprinted with permission from (228)]. In this review, several specific design considerations for each component have been highlighted. Throughout the text, these considerations and their implications for oral tissue engineering will be explored.
Biomaterials are clearly central to the advancement of tissue engineering and a variety of biomaterial ‘scaffolds’ have been developed as ECM analogues capable of supporting cell attachment (e.g. conduction) and, in some cases, providing the cues necessary for controlled spatial and temporal development (e.g. induction). In addition to material-based means of controlling cell fate, soluble or insoluble instructional molecules may be used to provide guidance to cells. Materials and signals can be used to provide instruction to host and/or donor cells, with control of cell growth and differentiation manipulated via exogeneous (e.g. engineering the extracellular microenvironment) or endogeneous (e.g. genetic engineering) means.
Each component of the tissue engineering triad may be implemented in a variety of ways. Some of the challenges in tissue engineering involve identifying the most appropriate form of each constituent of the tissue engineering triad for a specific application. For example, the material, as well as its form (gel, foam or fibre) can significantly affect biological response (37, 38). Likewise, identification of appropriate cell sources for a desired application [autogenous versus allogenic cells; primary cells, cell lines, genetically modified cells versus stem cells; adult versus embryonic cells; mesenchymal versus pulpal versus adipose versus periodontal ligament stem cells (PLSCs)] is a core challenge in tissue engineering (39–41) that will be discussed later in this review in the context of specific dental tissues. Identification of spatial and temporal signals (e.g. growth factors, cytokines, chemokines) for tissue-specific differentiation and morphogenesis and the approach to deliver these signals (soluble versus insoluble; temporal and spatial control) represent design choices along the third axis of the tissue engineering triad (16, 42).
One common strategy is to create a composite graft in which cells from any of the sources mentioned above are seeded into a degradable biomaterial (scaffold) that can serve as an ECM analogue and support cell adhesion, proliferation, differentiation and secretion of a natural ECM. Following cell-seeding, cell/scaffold constructs may be immediately implanted or cultured further and then implanted. In the latter case, the cells proliferate and secrete new ECM and factors necessary for tissue growth in vitro and the biomaterial/tissue construct is then implanted as a graft. Once implanted, the scaffold is also populated by cells from surrounding host tissue. Ideally, secretion of an ECM and subsequent tissue growth occur concurrently with scaffold degradation. In the long-term, a functional ECM and tissue are regenerated and are devoid of any residual scaffold.
Utilizing the principles of tissue engineering in a rational manner offers promise to regenerate or develop de-novo oral and craniofacial tissues. As discussed later in this review, dentistry can both capitalize on advances made in the engineering of non-dental tissues and organs, as well serve as a paradigm for the engineering of non-dental tissues.
III. Design of materials for engineering tissue structure and function
Historically, the biomaterials used in dentistry and medicine had their origins in other fields. For example, the acrylics used in dentures were developed in the paint industry and base metals have their origin in the aerospace industry. Although many biomaterials have had a lengthy history of clinical success, very few interact with their surrounding host environment or promote integration with host tissue in an intelligent, proactive fashion. The desire for more biological approaches to biomaterials design that could yield materials that are more instructive to cells has led to an expansion and paradigm shift in the field of biomaterials. The discipline of biomaterials now extends beyond the field of materials science and incorporates cell and molecular biology, genetics, biochemistry and other engineering disciplines.
An ideal tissue substitute should possess the biological advantages of an autograft and supply advantages of an allograft, while alleviating the complications of these grafts (43, 44). Such a construct should also satisfy the following design requirements (8): (i) biocompatibility, (ii) conductivity for attachment and proliferation of committed cells or their progenitors and production of new ECM, (iii) ability to incorporate inductive factors to direct and enhance new tissue growth, (iv) support of vascular ingrowth for oxygen and biomolecule transport, (v) mechanical integrity to support loads at the implant site, (vi) controlled, predictable, reproducible rate of degradation into non-toxic species that are easily metabolized or excreted and (vii) easy and cost-effective processing into irregular 3D shapes of sufficient size to fill clinically relevant defects. Particularly difficult is the integration of criteria (iv) and (v) into a single material design, as transport is typically maximized by maximizing porosity, while mechanical properties are frequently maximized by minimizing porosity. Integration of criteria (ii) and (iii) also presents materials design challenges that require more biomimetic complexity than many of the current simplified ECM mimics can provide.
First generation synthetic and natural materials that mimic structural and/or functional aspects of natural ECMs and satisfy at least some of the design requirements listed above include both organic and inorganic biomaterials: co-polymers of polylactic-glycolic acid (45, 46), collagen (20), polyphosphazenes (47), polyurethanes (48), polycaprolactone (49), polyethylene glycol (PEG) (50), poly (propylene fumarate) (51), starch-based materials (52), alginate (53), silk (54), bioactive glasses and glass ceramics (55, 56), calciumphosphate ceramics (15, 57), calcium-phosphate and collagen blends (20) and synthetic polymer/apatite composites (58–60). Varying parameters of the biomaterial, such as composition, topology and crystallinity, even subtly, can lead to a significant variation in cell attachment and proliferation, protein synthesis and RNA transcription in vitro (61–63). The parameters of the scaffold can also significantly affect progenitor cell differentiation, amount and rate of tissue formation, and intensity or duration of any transient or sustained inflammatory response in vivo (8, 37, 57, 64).
To extend performance beyond the capabilities of these first generation tissue engineering scaffolds and incorporate more of the above design criteria into a single material, better control of biofunctionality is needed. In particular, the specifics of the microenvironment that the material will interact with must be taken into consideration in the design process, such that remodeling and functionality can be maintained in the long term. The complexity of this design process is exemplified by the dynamic states of cells and tissues, which are regulated by the spatial and temporal coordination of multiple cell processes, each of which in turn is regulated by multiple reciprocal interactions between cells and their extracellular microenvironment.
Biomaterial modification can take on different levels of complexity, resulting in increasing levels of physiological ‘mimicry’ and functionality. In addition to using natural ECMs, surface and bulk chemical modifications of synthetic materials can enhance integration. Material modifications include changes in hydrophilicity, surface functionalization with charged groups, incorporation of insoluble ligands and peptide cell recognition sequences, attachment of larger proteins, supramolecular self-assembly and development of materials that bind and release soluble factors (42, 65). Strategies based on physical cues include the reproduction of nanoscale topology, superposition of mechanical cues and control of degradation. Designing biological recognition into a biomaterial may also obviate the need for therapies based on delivery of cells or recombinant growth factors, which are subject to regulatory constraints. More detail on these strategies is presented in section IV, within the context of specific dental and craniofacial tissue engineering applications.
IV. Dental and craniofacial tissue engineering applications
A. Tooth and periodontium
Advances in engineering and dentistry have led to the overwhelming success of dental implants. However, many patients continue to inquire about the regrowth or regeneration of their natural teeth. The goal of tooth regeneration is complicated by the nature of the tooth itself. An intact tooth is composed of four distinct tissues; mesenchymal derived pulp, dentin, cementum and epithelial derived enamel. The tooth root is then supported by a proprioceptive periodontal ligament (PDL) and encased in alveolar bone. This diversity in tissue types coupled with the need for the tooth to withstand forces of mastication makes tissue engineering of the dentition quite complex. Regeneration of the tooth or its supporting structures has been the focus of much effort in the last two decades (66). While some groups focus on regenerating one or two of the subtissues for targeted repair, others have moved towards regrowing an intact tooth and alveolus simultaneously for total replacement (67–69).
Unlike the many options available for bone tissue engineering, whole tooth engineering maintains a complete reliance on autologous stem cells due to our lack of understanding of the complex signalling required for shape specification, tissue interface and eruption. Complete tooth regeneration is further complicated by the fact that regrowth of a tooth is desired at a site where a tooth no longer exists. Thus, unlike engineering bone and mucosa, where scaffolds and signals can draw on cues and cells present in the surrounding host tissue, an ideal engineered tooth implant must be self-reliant. Non-stem cell based strategies have been developed for regeneration and repair of single dental tissues such as pulp (70), dentin (70, 71), cementum (29) and enamel (72) and may later inform efforts to generate multi-tissue organs and functional interfaces between dental tissues. This section will begin by outlining the stem cell sources available for tooth repair and regeneration, continue with a discussion of more advanced scaffold technologies for craniofacial and periodontal bone tissue engineering and end with a look at current clinical prospects for these emerging technologies. Although the details of many cellular, scaffold and signalling strategies have been confined to this section, it is important to recognize that similar methods are highly relevant to engineering of TMJ, mucosa and salivary gland.
A.1. Cells
Enamel is the most highly mineralized tissue in the biological world and when fully mature contains <4% organic material by weight (73). The basic structural unit of enamel is the enamel rod. These rods are secreted by epithelial derived ameloblasts. Ameloblasts undergo apoptosis as they elaborate the enamel matrix leaving erupted teeth without an enamel progenitor population.
Unlike enamel, the inner surface of dentin is lined by matrix secreting odontoblasts that provide a low basal level of tertiary or reactionary dentin formation throughout life. These odontoblasts are derived from dental pulp stem cells (DPSCs). Dental pulp stem cells are a unique mesenchymal stem cell (MSC) population that is present in the cell rich zone and core of the pulp (74). Dental pulp stem cells have the ability to differentiate into odontoblast-like cells, pulpal fibroblasts, adipocytes and neural-like cells (74). Primary human DPSCs maintain their stem-ness and continue to express the stem cell surface marker Stro-1 even after cryopreservation and extensive cell culture (75). The transition of DPSC to odontoblast is accompanied by deposition and mineralization of collagenous matrix (76). In vivo transplantation of human DPSCs on a hydroxyapatite (HA)/tri-calcium phosphate scaffold subcutaneously in SCID mice results in generation of a dentin/pulp-like complex (77). This complex contains vascularized pulp tissue with well-defined functional odontoblasts lining mineralized primary dentin tissue. While DPSCs have potential for dentin regeneration and tooth repair, limited understanding of the molecular regulation of DPSCs impacts our ability to use them for clinical tissue engineering. As caries progresses towards the pulp, the lining odontoblasts capable of dentin regeneration may be lost. With the discovery of this DPSC population, it may be possible to regenerate new odontoblasts from an injured pulp that are capable of repairing the carious dentin. Control of odontoblast differentiation has been shown to be regulated by bone morphogenic proteins (78), Wnt glycoproteins (79) and Notch signalling (80). Further understanding of these molecular regulators of differentiation and mineralization will allow for co-ordinated dentin engineering and aid tooth regeneration and repair efforts.
A second tooth-associated MSC population has been isolated from the surrounding PDL, termed periodontal ligament stem cells (PLSCs) (81). Since the 1980s, evidence for the existence of this population and the fact that it resides in a perivascular niche has been steadily accumulating (82, 83). Periodontal ligament stem cells and DPSCs maintain higher growth potential than bone marrow stromal cells (BMSCs) when cultured in vitro and proliferating colonies can undergo over 100 population doublings before reaching quiescence (84). A correlation exists between this phenomenon and increased expression of cell cycle mediators cyclin-dependent kinase-6 and insulin-like growth factor (IGF)-2 (85–87). Periodontal ligament stem cells are capable of regeneration of wounded periodontium in rats and even surpass embryonic stem cells in their repair capacity (88). These findings provide positive support for those interested in cell-based therapies for periodontitis, an inflammatory disease that results in progressive loss of periodontal attachment and alveolar bone.
Before eruption of a new tooth, a third population of primitive stem cells in the tooth bud is capable of providing the instructions necessary for growth of the entire tooth. It is therefore reasonable to assume that these cells could also regrow an entire tooth in vivo. One of the first demonstrations of this phenomenon occurred when a 2 mm square of foil was inserted into the centre of a rat tooth bud in vivo. This resulted in two teeth forming, one on each side of the foil, where normally only one would be present (89). Anatomically correct tooth crown formation was also achieved when cells were isolated from rat or pig tooth bud, cultured for up to 6 days and reimplanted into the omentum of live rats (90, 91). After 12–30 weeks, implanted cell/scaffold combinations demonstrated formation of distinct pulp, predentin, dentin and enamel layers (73).
Advances in engineering whole teeth are limited by the poor availability of human primitive tooth bud stem cells and limitations in the ability to isolate and purify them. Advances in purification include sorting for STRO-1 positive (92) and Hoescht dye negative cells (93) to enrich for primitive stem cells. Formation of tooth crowns in vivo using primitive stem cells has been accompanied by poor tooth root formation (67, 73). It appears that the transplanted cells lack the ability or instructions necessary to form root dentin and cementum that would be necessary to guide eruption. It is possible that these signals are derived from the supporting PDL or alveolar bone during development. Indeed, when tissue engineered pig tooth and alveolar bone are grown simultaneously in rat omentum for 8 weeks, the development of a primitive cementum layer and PDL is observed (67). Further characterization of the molecular interactions between these mixed mesenchymal and epithelial populations and development of interface-supporting biomaterials will allow increased control over tooth regeneration in vivo.
A.2. Scaffolds
Development of biomaterials capable of supporting regrowth of the individual mineralized tissues of the tooth and periodontium (bone, dentin, cementum and enamel) and functionally graded interfaces between these tissues is an active area of research. Most current studies have used known osteo-conductive materials to guide tooth and periodontal engineering efforts and these materials parallel those used in engineering other mineralized tissues. Scaffolds including collagen (94–96), polyglycolic acid (PGA) (94), self-assembling peptides (97), gelatin-chondroitin- hyaluronan tri-copolymer (27) and silk (54) have been used for tooth and periodontal regeneration. The success of in vivo tooth regeneration currently hovers around 20–50% with implanted cell/scaffold combinations. The definition of success varies, but generally includes the production of at least three histologically intact structures including enamel, dentin, pulp, cementum and PDL. Two novel strategies that may improve the success of tooth regeneration and other areas of mineralized tissue engineering include self-assembling peptides and nanoscale biomaterials.
Self-assembling peptides or peptide amphiphiles are based on principles of protein-protein interactions and protein folding. All biomolecules self-assemble to form well-defined structures that impart a specific function. Nature has used proteins and peptides to synthesize an array of materials whose hierarchy and function far exceed those of man-made materials. By understanding how supra-molecular structures are assembled in nature, these processes can be exploited in the synthesis of synthetic materials (98). Such approaches use non-covalent intermolecular interactions to synthesize higher order structures via the self-assembly of biological (nucleotide, oligomeric, peptide) and non-biological amphiphilic building blocks. Self-assembling peptides can support cell encapsulation, promote enamel remineralization by providing a bio-mimetic scaffold capable of HA nucleation, promote neural differentiation and maintain the functions of differentiated chondrocytes (42, 97, 99, 100). Generation of an injectible selfassembling peptide gel that could be applied to small carious lesions to promote enamel remineralization is one possible application of this scaffold technology (97).
Cell behaviour is also regulated by surface topology. A variety of cell functions, such as adhesion and intracellular signalling pathways, are sensitive to micro- and nano-scale topology on the orders of 10–100 000 nm (101, 102). Creation of nano-fibrous materials from ECM constituents or blends of synthetic and natural polymers can provide a material with both the physical scale necessary to influence biological function and a biochemical composition that is similar to the ECM environment that cells interact with in vivo (42, 102, 103). The combination of physical and biochemical cues can enhance cell adhesion, proliferation and tissue-specific differentiation, as well as promote tissue integration in vivo. A key to translating nano-technology into an implant that has clinical relevance is to integrate the nanoscale features needed to control cell function with a larger 3D implant that has the dimensions and bulk properties required to fulfill a desired dental application. One design approach to achieve such an integration of dimensional scales in a single material is to use bulk poly-crystalline materials with grain sizes in the sub-micron range. Such materials exhibit enhanced biological responses, as well as improved physical properties (104, 105). Reproduction of nanoscale features exhibited by natural ECMs in a tissue engineering scaffold has been achieved down to the scale of 10 nm via the creation of nanofibrous scaffolds. These scaffolds can be synthesized via well-established materials synthesis approaches, including electrospinning and thermally induced phase separation, as well as protein self-assembly (98, 106). Use of nanoscale surface topology to enhance cell adhesion and osseointegration has already established itself in the context of dental implant processing and coating (107) and will probably be expanded to larger scaffolds designed to support osseous fill of periodontal and other craniofacial defects (108).
In addition to soft materials, advances in nanoscale inorganic biomimetic materials may impact future engineering of the tooth and periodontium. Compared with synthetic materials, natural biominerals reflect a remarkable level of control in their composition, size, shape and organization (109). A biomimetic mineral surface could therefore promote preferential absorption of biological molecules that regulate cell function, serving to promote events leading to cell-mediated biomineralization (8). Bioactive ceramics bond to bone through a layer of bone-like apatite, which forms on the surfaces of these materials in vivo and is characterized by a carbonate-containing apatite structure with small crystallites (110, 111). A bone-like apatite layer can be formed in vitro at standard temperature and pressure (112–114), providing a way to control the in vivo response to a biomaterial. Synthesis of bone-like mineral in a biomimetic fashion is based on the principles of biomineralization, in which organisms use macromolecules to control mineral nucleation and growth (109). Macromolecules usually contain functional groups that are negatively charged at the crystallization pH (109), enabling them to chelate ions present in the surrounding media, which stimulate mineral crystal nucleation (115). The self-assembly of nanoscale mineral within the pores of a polymer scaffold enhances cell adhesion, proliferation and osteogenic differentiation and modulates cytoskeletal organization and cell motility in vitro (116, 117). When osteoblast progenitor cells are transplanted on these materials, a larger and more spatially uniform volume of bone is regenerated, compared with non-mineralized templates (117). The success of bone-like apatites in bone tissue engineering is an encouraging sign for the impact and use of biomimetic materials in tooth and periodontal engineering.
A.3. Signals
In addition to its structural role the ECM provides adhesive ligands such as fibronectin, vitronectin and laminin that direct cell function. Reproduction of these and other signals on engineered scaffolds can allow a more precise regulation of cell function and tissue formation. Incorporation of peptides to provide specific instructive cues to cells, delivery of inductive factors and control of cell-cell communication are three approaches to optimize regeneration of craniofacial structures. These approaches have clear applicability to tooth and periodontal engineering and may expand strategies to engineer whole teeth beyond just stem cells.
Most biomaterials, especially polymers, will non-specifically adsorb proteins through weak interactions at the protein-water and biomaterial-water interfaces. Incorporation of proteins or their sub-sequences into the backbone of a polymer can control cell processes such as differentiation and matrix degradation. Proteins, growth factors and peptides have been ionically or covalently attached to biomaterial surfaces to increase cell adhesion and ultimately, the amount of tissue regenerated. While several proteins enhance cell adhesion, proteins are challenging to isolate and prone to degradation (118, 119). Proteins can also change conformation or orientation because they possess sections with varying hydrophobicities that address cellular functions other than adhesion. On the other hand, peptides can mimic the same response as a protein while being smaller, cheaper and less susceptible to degradation. Peptides may therefore have a greater potential for controlling initial biological activity because they can contain specific target amino acid sequences and can permit control of hydrophilic properties through sequence design (119, 120).
The identification of peptide sequences within ECM proteins that are responsible for cell adhesion led to the development of peptide-functionalized biomaterials (121). Incorporation of peptide motifs containing sequences that are recognized by integrin receptors, such as arginine-glycine-aspartic acid (RGD)-based sequences PRGDSGYRGDS and DGRGDSVAYG, are now a common strategy to enhance biological functionality as well as proliferation and differentiation of a variety of cells, including osteoblasts, odontoblasts, fibroblasts and DPSCs (42, 119, 122–124). Materials with RGD-containing sequences enhance cell adhesion and direct differentiation into bone (125, 126), cartilage (127, 128), neural (129) and endothelial tissue (4, 130) and are therefore applicable to engineering tooth, bone, cartilage and oral mucosa. Cell adhesion, migration and lineage direction of cell phenotype are dependent on ligand specificity, surface density, gradient, conformation and binding affinity (131). Using recombinant DNA technology, synthetic proteins can be designed to mimic specific ECM constituents. In addition to the ubiquitous RGD sequence derived from the cell binding domain of fibronectin and vitronectin, sequences derived from the heparin binding domain, such as FHRRIKA and KRSR, improve osteoblast adhesion and mineralization (131–133) and RGD peptides derived from dentin phosphophoryn and dentin matrix protein 1 promote selective attachment and migration of dental pulp cells (124, 134). Peptide sequences that mimic sections of collagen (135) and non-collagenous proteins, including laminin (136), bone sialoprotein (137), osteopontin (138), statherin (138), elastin (139) and osteonectin (140) have also improved cell adhesion, proliferation and differentiation of osteoblast-like cells and may therefore promote function of other cells that can form mineralized tissues of the oral cavity.
In addition to using recombinant technologies to synthesize sequences within proteins known to promote a specific biological function, domains within a protein can be deleted to investigate the effect of targeted sequence deletions on the function of the protein. Subsequently, sequences deemed to control a specific function can be synthesized for use in a tissue engineering application. Another discovery technique is phage display, a high throughput approach in which a bacterial phage library expressing combinations of linear or cyclic peptide inserts is used to identify amino acid sequences that have high affinity to a substrate or cell type. Phage displays have identified sequences with high affinity to cell lines cultured in vitro (141, 142), cell/organ targets in vivo (143), enzymes and their inhibitors and specific tissues (120, 144, 145). Phage display technologies have also been used to isolate peptide sequences attracted to inorganic or organic materials (146–148). Most germane to craniofacial tissue engineering is the use of phage display to identify amino acid sequences with preferential affinity to the bioactive materials HA and bone-like mineral (149).
The ECM has the ability to bind and release soluble factors in a spatially and temporally controlled manner. Inductive properties can be integrated into a material using methods to immobilize proteins, such as adsorption, entrapment, cross-linking or covalent binding, each of which results in different loading efficiencies and levels of protein retention (150). The method of integrating organic factors into a biomaterial can influence the resultant release profile and therefore influence the response of surrounding cells. The controlled release of a growth factor can be achieved by incorporating the factor into the bulk of a scaffold or hydrogel during polymerization and designing a release profile based on drug diffusion and/or material degradation. Many such systems have been developed for drug delivery and several have been adapted to enable macro-porous tissue engineering scaffolds to be used as vehicles for delivery of bioactive factors (151–154). Spheres or pellets with growth factor incorporated may also be bound together to form a 3D construct. Adsorbing or covalently binding a drug to each layer of a material created by layer-by-layer assembly can provide temporal control over its delivery (155). This technology is most widely recognized in dentistry for its use in delivery of microsphere encapsulated antibiotics such as Minocycline hydrochloride to aid repair of periodontal pockets (156).
Advances in the understanding of biomineralization have resulted in the synthesis of mineral-organic hybrids consisting of bone like apatites combined with inductive factors to control cell proliferation, differentiation and bone formation (154, 157). Organic/inorganic hybrids show promise in combining the osteoconductive properties provided by the apatite with the osteoinductive potential provided by growth factors, DNA or peptides. Biomolecules can be incorporated at different stages of calcium phosphate nucleation and growth (157) enabling spatial localization of the biomolecule through the apatite thickness and allowing for its controlled release. An advantage of this approach is its ability to produce calcium phosphate coatings at a physiological temperature, minimizing conditions that would compromise the biological activity of the factors. Co-precipitation of mineral and inductive molecules results in an increased protein loading capacity and more controlled release in comparison with adsorption (157). Techniques used to incorporate growth factors into bone-like mineral can also be used to incorporate genetic material. The mineral increases substrate stiffness (113), which also enhances cellular uptake of plasmid DNA (158) providing an added advantage to such hybrid systems.
Most cell functions are dependent on multiple signals, so delivery of multiple factors such as platelet derived growth factor (PDGF), vascular endothelial growth factor and bone morphogenetic proteins (BMP) will probably result in greater advances in periodontal tissue regeneration than delivery of a single factor (159). As protein release from bone-like mineral/organic hybrid systems is proportional to apatite dissolution, temporally controlling the release profile as well as developing multi-factor delivery systems is possible because of the ability to localize spatially the protein within the biomimetically nucleated mineral (157).
Gap junction intercellular communication (GJIC) also plays a prominent role in the differentiation and function of cells and their response to stimuli. As such, it is possible to design materials or present signals to cells that enhance GJIC (160). One example of the potential for the controlled use of gap junctions in tissue engineering involves a cell transplantation approach, in which BMSCs are transduced with a Cx43 lentivirus (160). Overexpression of Cx43 in BMSCs leads to significant increases in GJIC and elevated expression of alkaline phosphatase and osteocalcin in vitro, indicative of enhanced osteogenic differentiation (160). Transplantation of cells transduced with a Cx43 lentivirus also shows that overexpression of Cx43 significantly increases the volume fraction of regenerated bone relative to the amount of bone regenerated from transplantation of control BMSCs (160, 161). These in vitro and in vivo results suggest that increasing GJIC can be used as a strategy to enhance periodontal bone tissue engineering. The ubiquitous nature of GJIC makes such an approach also applicable to other oral tissues.
A.4. Clinical prospects
Success of a tissue engineering strategy in a small animal model does not necessarily translate into humans or even larger animals. Filling of defects in a rodent is more readily achieved because of the well-controlled geometry, smaller size and higher remodeling rate. As the size of a defect gets larger, the ability to engineer a vascular supply becomes more difficult as cells must be within 100 μm of an oxygen source to survive (162). To date, direct growth factor delivery and blank or growth factor containing scaffolds (i.e. conductive and inductive materials, respectively) are the only strategies discussed above for periodontal regeneration that have been used for human clinical trials (16, 118). This is yet to be expanded to include a specific autologous cell population.
Delivery of growth factors such as recombinant BMP-2 and BMP-7, approved by the United States Food and Drug Administration for clinical use in 2004 (163), PDGF, IGF-1 and fibroblast growth factor (FGF) significantly enhance the repair of periodontal alveolar bone defects when delivered locally. Specifically, rhBMP-2 increases alveolar defect bone height repair by 2.4-fold and total bone area by 7.8-fold in a canine model, results equivalent to or better than autografting (164–166). In humans, application of rhBMP-2 lyophilized to xenogenic bone substitute Bio-Oss®(Luitpold Pharmaceuticals Inc., Shirley, NY, USA) to alveolar ridge defects demonstrated a statistically significant enhancement of vertical defect reduction when compared with Bio-Oss® alone (167). The first human clinical trial for periodontal disease used rhPDGF/rhIGF-1 in a methylcellulose vehicle and revealed 43.5% osseous defect fill in the treated group compared to only 18.5% osseous defect fill in the vehicle or surgery alone group (16). Additional clinical trials are currently planned or in progress for PDGF treatment of post-extraction sockets (U Alabama*) and healing of periodontal defects (Virchow Group), rhBMP-2 treatment of vertical and horizontal alveolar defects associated with implants† and FGF treatment for periodontal tissue regeneration‡ (168). Given these preliminary clinical successes using a basic scaffold and single or dual growth factors for periodontal defect regeneration, it is logically anticipated that significant improvement can be expected in the near future through application of optimized scaffold, cell, and signalling combinations as discussed in the previous sections. Furthermore, the combinations discovered for periodontal bone tissue engineering will serve as a fundamental starting point for in vivo engineering of other mineralized tissues such as dentin and cementum.
B. Temporomandibular joint
The articulating joint is a complex system that is regularly subjected to trauma, metabolic and inflammatory processes. Over 30 million Americans and countless more worldwide suffer from some debilitation of the joints and thus development of interventive and regenerative cures is a global priority. Conventional joint treatment methods such as Pridie’s perforations, microfractures or subchondral abrasion lead to less than adequate results in about 50% of cases in joints such as the knee. These techniques often lead to the formation of fibrocartilaginous scar tissue whose biomechanical properties are significantly inferior to those of hyaline cartilage. Most of the cell, scaffold and signalling strategies discussed in section IV.A.1–A.3 can be applied to engineering of functional cartilage and underlying bone. TMJ engineering requires optimization of these combinations for shape specification and bone/cartilage interface formation.
B.1. Cells and scaffolds
To overcome the drawbacks inherent in traditional surgical methods of TMJ treatment, alternative methods have been developed such as osteochondral or chondrocyte allografts and autografts [e.g. Carticel® (Genzyme Corporation, Cambridge, MA, USA), ChondroSelect® (TiGenix, Leuven, Belgium)] and progress in regenerative medical approaches for both bone and cartilage is promising. Chondrocytes seeded on materials like PGA (169–171) and collagen (172, 173) develop cartilage-like structures that express markers of chondrocyte differentiation and have compositions similar to normal articular cartilage. A number of joint repair studies suggest that these strategies have merit (32, 33, 174–176). For a review of scaffold technologies applicable to TMJ regeneration, please see sections III and IV.A.2 of this article.
B.2. Signals
In many ways, cartilage repair is more complicated than bone regeneration. Mesenchymal stem cells can be differentiated into chondrocytes in cartilage defects, but the regenerated tissue rarely matches the normal structure and function of mature endogenous cartilage (177). Sustained delivery of appropriate growth factors such as basic FGF (bFGF), transforming growth factor (TGF)-β and Sox9 is necessary for cartilage regeneration. Basic FGF is one of the most potent substances for chondrocyte proliferation and differentiation because it can trigger a cascade of events in the cartilage repair process (178). Both in vivo injection and ex vivo delivery of virus encoding bFGF by chondrocytes into rabbit knee joints can enhance articular cartilage repair (179, 180). The TGF-β superfamily, including the BMPs and TGF-β1, has been shown to promote chondrogenesis by regulating differentiation of certain precursor cells (181). TGF-β1 can stimulate proteoglycan and collagen synthesis (182) and MSCs transduced by virus expressing TGF-β1 can enhance cartilage repair of osteochondral defects in athymic rats (183). Sox9 is also a potential regulator of cartilage regeneration because it is one of the earliest transcription factors required for differentiation of MSCs towards a chondrogenic lineage (184). Viral-mediated Sox9 overexpression in chondrocytes derived from human osteoarthritic articular cartilage allows restoration of major ECM components proteoglycan and type II collagen to levels similar to those of healthy articular cartilage (185). These gene therapy strategies can be widely applied to joint repair and will enhance regeneration of a functional TMJ in concert with appropriate delivery vehicles.
B.3. Clinical prospects
To date, there is no ideal solution to engineer a functional TMJ replacement. Allografts are associated with donor site morbidity and are poorly shaped for placement into defects and alloplastics do not respond to normal biochemical or mechanical signals (31, 186). Engineering a functional osteochondral graft will require the production of both bone and cartilage with a defined interface. Strides have been made in engineering of these tissues separately (187–189) and initial experiments have demonstrated simultaneous formation of bone and cartilage with a mineralized interface in vivo in mice (30, 190). In this investigation, image-based design followed by solid free-form fabrication to control scaffold size and shape was used to generate a biphasic poly-L-lactic acid (PLLA)/HA composite scaffold (30, 190) (Fig. 2a and b). Differentiated pig chondrocytes and Ad.BMP7 transduced human gingival fibroblasts were seeded onto the polymer and HA, respectively, and implanted subcutaneously into N:Nih-bg-nu-xid immunocompromised mice (30, 190). After 4 weeks, marrow-containing vascularized bone, mature cartilage and a defined mineralized interface were formed (30, 190) (Fig. 2c). This pioneering study provides proof of principle evidence for the fabrication of a physiological osteochondral graft that may be further developed for clinical use.
Fig. 2.

Tissue Engineering in Practice – Temporomandibular Joint (30). (a) Image-based design of a theoretical site-specific implant for temporomandibular joint engineering using solid free-form fabrication. (b) A composite scaffold consisting of PLLA was seeded with differentiated porcine chondrocytes and hydroxyapatite (HA) seeded with Ad.BMP7 transduced gingival fibroblasts was implanted subcutaneously into immunocompromised mice. (c) Four weeks post-transplant harvested implants were sectioned and analysed for presence of osteo-chondral structures. Cartilage (arrows) and bone (*) were observed separated by a defined interface (dotted line) [Adapted with permission from (30)].
C. Skin and oral mucosa
Engineering of both skin and mucosal equivalents is essential for the aesthetic reconstruction of individuals disfigured by trauma, resective surgery or severe burns. Skin is composed of layered dermis and epidermis in a configuration that must be preserved for optimum regeneration. The first description of skin grafting occurred over 2500 years ago by the Hindu Tilemaker Caste, in which skin grafting was used to reconstruct noses that were amputated as a means of judicial punishment (191). However, the first attempts to repair damaged skin and mucosa with an engineered graft did not occur until the 1980s. Investigators derived cultured epithelial sheets from a small biopsy and reintroduced them to the patient for treatment of burns (192) and for intra-oral grafting (193). Indeed, skin with both dermal and epidermal components was the first FDA approved tissue engineered construct that has been put into clinical practice.
C.1. Cells
All of the skin regeneration products approved by the FDA rely on cells derived from neonatal foreskin (194). Derivation of fibroblasts from a single source such as foreskin controls for factors such as cell age, gender and anatomic location. The cells of one foreskin have proliferative potential capable of providing starting cells for over 80 000 m of final tissue-engineered product (194). Tissue engineered skin grafts provide a glimpse of the reproducibility, expandability and immune tolerance capabilities of a cell population. Derivation of new or modification of existing populations of cells with these characteristics is essential for successful engineering of all tissues.
Though the oral mucosa comprises <5% of the total surface of the human body it is a highly specialized tissue. The oral mucosa, like the skin, is made up of stratified squamous epithelium overlying a supportive lamina propria. However, unlike the skin, mucosa may be non-keratinized or keratinized, does not contain hair follicles and may be further specialized to convey sensations such as taste. Ideal engineering of oral mucosa would allow reproduction of a physiologically correct ‘full-thickness’ tissue. This mucosa should have three distinct layers: the lamina propria, basement membrane, and stratified squamous epithelium. In vivo the lamina propria consists of an abundant ECM network of collagen and elastin fibers that support a dense fibroblast population. The lamina propria is also responsible for support of vascular components, lymphatic vessels, nerves and salivary gland ducts. Early efforts to engineer monolayer or multilayer epithelial sheets neglected to generate this supportive lamina propria (195). Above the lamina propria, stratified squamous epithelium rests on a continuous basement membrane. The epithelial layer is made up of densely packed keratinocytes that differentiate as they migrate to the surface. This results in the generation of four distinct layers of cells: the basal layer, spinous layer, granular layer and keratinized layer. Although difficult, it is possible to mimic this layered differentiation in vitro by culturing the keratinocytes at an air-liquid interface in defined medium containing keratinocyte growth factors (196, 197). Multilayer culture of gingival keratinocytes has met with some clinical success and is useful for in vitro biocompatibility testing and oral biology research. Commercially available products include SkinEthic’s gingival epithelium and keratinized stratified squamous epithelial products EpiOral™§ and EpiGigival™§
C.2. Scaffolds and signals
To move beyond current gingival epithelium products, a full-thickness engineered mucosa with intact lamina propria is needed. This requires engineered scaffolds capable of supporting fibroblast infiltration with minimum resulting shrinkage and controlled biodegradation (198). Scaffolds used for mucosa and skin reconstruction include natural derivatives such as acellular dermis, ECM protein-based scaffolds, synthetic materials and hybrid scaffolds of both natural and synthetic matrices. Extensive reviews of these materials and their use for skin and mucosa engineering have been published elsewhere (198).
C.3. Clinical prospects
Mucosal and gingival grafts are desired to augment intraoral reconstructive surgery, periodontal surgical procedures and to repair defects left by gingival recession. In the past decade, research for development of an engineered oral mucosa focused on introducing new dermal scaffolds and improving epithelial cell culture methods (198). The ability to produce a supportive dermis with a functional epithelial layer is limited by current dermal matrices and poor differentiation of multilayered keratinocyte constructs. To address these issues, investigators are working to optimize the cell source, culture conditions and choice of scaffold. Since 1996, many combinations of cell type, culture condition and scaffold have been tested both in vitro and in vivo. For example, cells derived from oral tissues have been used successfully for ocular reconstruction in rabbits (rabbit oral mucosa cells/human amniotic membrane scaffold) (199), intra-oral grafting in humans (human oral fibroblast cells/AlloDerm™ scaffold) (25) and burn treatment in humans [human oral fibroblast cells/AlloDerm™ scaffold (LifeCell Corporation, Branchburg, NJ, USA)] (26). Future directions include expansion of these applications with a commercially available engineered oral mucosa product similar to skin substitutes Dermagraft™ (200; Advanced Biohealing Inc., Westport, CT, USA) and Apligraf™ (Organogenesis Inc., Canton, MA, USA) used for coverage of burns and acute wounds (201). It should, however, be noted that even without a commercial full-thickness mucosa, clinical success using the skin substitute Dermagraft™ has been reported (200) for preprosthetic intraoral vestibular extension (202) (Fig. 3).
Fig. 3.

Tissue Engineering in Practice – Oral Mucosa (202). Tissue engineered dermal replacement DermaGraft™ was used in place of autologous tissue for vestibuloplasty post-squamous cell carcinoma removal. (a) Post-surgical scars limited patient closure. (b) Intraoral view shows insufficient vestibular depth with extensive fibrous and muscular insertion. (c) Mucogingival junction and periosteal dissection was followed by implantation of Dermagraft™. (d) Patient demonstrating improved vestibular depth after 3 months. [Reprinted with permission from (202)].
D. Salivary gland
Loss of salivary gland function can result as a pharmacological side-effect, from radiation therapy or as a consequence of autoimmune diseases such as Sjogren’s syndrome. Saliva is a complex hypotonic solution that carries water, electrolytes, bioactive proteins and peptides into the oral cavity (203). Loss of salivary flow, referred to as xerostomia, significantly impacts quality of life and predisposes affected individuals to caries, dysphagia, dysgeusia and mucosal infection. Although most pharmacological loss-of-function is reversible, there remain 2–4 million people in the US with irreversible destruction of salivary gland tissue (5). Tissue engineering of a salivary gland substitute or replacement is one way to treat these patients (5). To accomplish this complex task, three objectives must be satisfied: identification of a cell population capable of appropriate differentiation and fluid movement, optimization of scaffold material properties and definition of ideal culture conditions and ECM components. This section will focus on current salivary gland tissue engineering strategies, which combine cell transplantation and gene transfer with engineered scaffolds to generate an artificial salivary gland substitute. Signals that could potentially be used to enhance salivary gland function are also discussed.
D.1. Cells
There are many varieties of human salivary glands. Besides the paired major salivary glands, the oral cavity contains up to one thousand additional minor salivary glands embedded in the lamina propria of the oral mucosa. Each of the three types of paired major glands (parotid, submandibular and sublingual) secretes a unique fluid composed of mixed mucous and serous secretions depending on the cellular content of the gland. The major salivary gland most important to tissue engineers is the parotid due its location and size, serous or ‘watery’ saliva secretion profile and tendency to be damaged by radiation or autoimmune disease. The parotid is the largest of the salivary glands and is located in the subcutaneous tissue of the face, over the mandibular ramus and anterior to the ear. The parotid is the main producer of serous saliva in the oral cavity and loss of its function is severely detrimental to the patient. All major salivary glands consist of a mesenchymal scaffold, which is host to four distinct epithelial cell types: acinar, duct, myoepithelial and basal cells. Saliva is secreted by the mucous and serous acinar cells, modified by the duct cells and transported to the oral cavity with the support of myoepithelial and basal cells. Recapitulation of physiological salivary gland structure and function is a challenging issue that researchers, including tissue engineers, are just beginning to understand.
Natural salivary glands are made up of highly specialized cells capable of fluid secretion, modification and directional movement. The challenge of culturing and implanting cells capable of all of these functions is the primary focus of salivary gland tissue engineers. Efforts with a human ductal epithelial salivary gland cell line (HSG) were promising when the cells were found to respond to ECM in culture (204), form monolayers on PLLA scaffolds (205) and have the systems necessary to generate osmotic gradients for saliva formation (206). Unfortunately, it was later found that HSG cells lack the ability to form tight junctions and thus are not capable of supporting unidirectional fluid movement (207). To circumvent these issues, primary salivary gland epithelial cells have been isolated from mouse (208) and non-human primates (209). Primary parotid cultures from rhesus monkey are duct-like, can form polarized monolayers and are able to mediate fluid movement after application of an external osmotic gradient (209).
An additional cellular strategy includes identification and use of an autologous stem cell population. Salivary gland epithelial progenitor cells may be identified by their expression of alpha6-beta1 integrin receptors (210) and have been recently noted as a novel autologous cell population for use in salivary gland tissue engineering (211). Rat BMSCs may also have the ability to trans-differentiate into alpha-amylase expressing acinar cells (212). This implies that stem cells external to the salivary gland may be of utility. With the number of cellular options increasing, a deeper understanding of molecular control and coordination of fluid and protein secretion is necessary. Application can then be facilitated by appropriately designed scaffolds.
D.2. Scaffolds
Device design for an ideal artificial salivary gland consists of a blind end tube made from a porous, biodegradable material (5). This scaffold is envisioned to be coated with matrix components on the inner surface of the tube to promote formation of polarized epithelial cell ‘ducts’ capable of unidirectional fluid movement. Such a device could then be implanted into the buccal mucosa with an exit into the oral cavity, similar to a natural parotid gland duct. This device would then allow secretion of fluids and salts from the body into the oral cavity, mimicking a natural salivary gland. Scaffold selection for salivary gland replacement is taking cues from materials used to engineer replacements for other tubular structures such as intestine, vasculature, ureter and the trachea (5). To this end, tissue compatibility and in vitro analysis of scaffolds of PLLA (205), poly-glycolic acid coated with PLLA (205), PEG-terephthalate/poly(butylene terephthalate) (PEGTPBT) (213), chitosan (214) and collagen/matrigel (215) have been tested. Results show acceptable levels of local inflammation around PLLA and PGA-PLLA when implanted adjacent to the buccal mucosa in mice (216) as well as in vitro formation of polarized monolayers on PEG-TPBT scaffolds with successful maintenance of acinar cells (213).
D.3. Signals
Unlike acinar cells, traditional ductal epithelial cells are incapable of fluid secretion. As isolation and expansion of acinar cells in vitro is currently not possible, identification and localization of membrane proteins required for ionic gradient formation and fluid flow in acinar cells will inform efforts to modify ductal cell populations using gene transfer. Acinar cells require four membrane proteins to generate an osmotic gradient for unidirectional fluid movement: (i) the N+K+-ATPase used to maintain membrane potential, (ii) a Ca2+ activated K+ channel, (iii) the secretory isoform of the Na+/K+/2Cl− cotransporter and (iv) the apical membrane bound Ca2+activated Cl− channel (5, 217). Salivation occurs in response to agonists that generate an increase in intracellular Ca2+ concentration and is facilitated by osmotic gradient directed fluid movement through water channels in the apical membrane known as aquaporins (AQP) (217). It is now recognized that isolated ductal epithelial cells lack expression of AQP and as such cannot mediate fluid movement (209). Re-introduction of transient AQP expression using adenoviral transduction has been successful in rhesus monkey parotid duct cells in vitro (218), rat and mini-pig salivary gland tissue in vivo (219) and is the subject of an ongoing clinical trial (168).
In addition to re-engineering of ductal epithelial cells, modification of potential salivary gland scaffolds to optimize monolayer culture is necessary. Purified matrix proteins already examined for their ability to support in vitro HSG cell culture include fibronectin, laminin, collagen I, collagen IV and gelatin (205). In the absence of pre-adsorbed proteins, HSG cells did not attach to PLLA or PGA-PLLA (205). However, on matrix protein-coated biomaterials, HSG cells were able to form a uniform monolayer, which was dependent on time and protein concentration (205).
D.4. Clinical prospects
Current treatment of salivary gland hypofunction includes pharmacological stimulation of remaining acinar tissue and palliative care with mucosal lubricating agents (220). To date, regenerative efforts have been focused on both repair and replacement. Indeed, attempts to restore salivary flow by in vivo transduction of adenovirus encoding AQP1 into remaining glandular tissue of patients treated with radiation for head and neck cancer is the first human craniofacial repair gene therapy clinical trial and is currently ongoing (168, 219). Replacement strategies include salivary gland transplantation and re-engineering of new glandular tissue as discussed above (221, 222). Although there have not yet been any reports of salivary gland prototype experiments in vivo, the work carried out to date supports the idea that an engineered gland may be a future reality.
V. Unique challenges and opportunities in oral and craniofacial tissue engineering
The biomimetic approaches discussed above, along with all other strategies to reproduce the design rules of biological systems, do not completely mimic nature. However, if the selected biomimicry is rationally designed into a biomaterial, then the biological system will be able to respond in a more controlled, predictable and efficient manner, providing an exciting new arena for biomaterials research and development. Regeneration of oral and craniofacial tissues requires synthesis of engineering, clinical and basic science. The tissues of this region are complex and engineering these tissues presents many unique design challenges. In the craniofacial region, maintaining or restoring aesthetics in addition to restoring structure and function result in a more complex design problem than in other regions of the body. In oral tissue engineering, it is also necessary to consider the microbial environment and the potentially altered host immune response (39).
Many oral structures are hybrid tissues. For example, engineering the periodontium requires growth of alveolar bone, cementum and the PDL. Engineering a tooth requires the development of dentin and enamel in the exquisite organic/inorganic organization that provides mechanical function to these tissues. Engineering of a TMJ requires the creation of functional bone and cartilage. For each of these hybrid tissue systems, it is also necessary to recreate the functionally graded structures that result from normal development. Simply creating dentin and enamel without a graded dentin-enamel junction, or bone and cartilage without the appropriate transition zone between the two tissues may not be sufficient to impart functionality. While there are significant challenges in engineering hybrid oral and craniofacial tissues, there are also significant opportunities for these tissues to serve as model systems for engineering other tissues and organs of the body.
Developmental differences between the craniofacial and appendicular skeletons must be considered when orchestrating craniofacial tissue engineering strategies (39). The appendicular skeleton is derived from the mesoderm and bone forms via endochondral ossification. On the other hand, the cranial skeleton is derived from the cranial neural crest and paraxial mesoderm and its bones are formed via both intramembranous and endochondral ossification (39, 223). Given the different developmental processes and the goal of recapitulating development in tissue engineering, it is reasonable to suggest that engineering of craniofacial bones might necessitate approaches different from those used to engineer long bones. This notion is supported by differences in osteoblast response to mechanical and molecular signals, depending on the origin of the osteoblast (39, 224–226).
The accessibility of the oral environment and ability to create minimally invasive models of hybrid tissues renders oral tissues convenient platforms for testing tissue engineered prototypes (39, 227, 228). In this regard, oral and craniofacial tissue engineering can have a two-fold impact that extends beyond dentistry. First, advances initially made in dentistry because of the relatively simple surgical models can be translated into other organ systems. Second, technologies developed outside of dentistry can be tested in oral models. For example, one of the most frequently used screening tools for bone tissue engineering is the critical size calvarial defect model (229). As a second example, the hybrid tissue systems of the periodontium, TMJ, cranial suture and mineralized tissues of the tooth can provide insight into how to design a composite tissue consisting of multiple cell types (190). As a third example, oral and craniofacial-derived stem cells from the dental pulp, PDL and cranial sutures have utility in non-dental and noncraniofacial applications (77). The impact of the bidirectional synergy between engineering oral tissues and engineering tissues elsewhere in the body is that oral and craniofacial tissue engineering is an integral component of the larger field of tissue engineering and should not be viewed as dental researchers solving dental problems in isolation.
Acknowledgments
Parts of the authors’ research discussed in this Chapter were supported by NIH/NIDCR Tissue Engineering and Regeneration, T32 DE07057 (PHK), R01 DE 13835 (PHK), R01 DE 013380 (DHK) and R01 DE015411 (DHK).
Footnotes
Biohorizons Implant Systems (Birmingham, AL, USA).
Nobel Biocare (Goteborg, Sweden).
Kaken Pharmaceutical (New York, NY, USA).
MatTek Corp., Ashland, MA, USA.
References
- 1.Laurell L, Gottlow J, Zybutz M, Persson R. Treatment of intrabony defects by different surgical procedures. A literature review. J Periodontol. 1998;69:303–313. doi: 10.1902/jop.1998.69.3.303. [DOI] [PubMed] [Google Scholar]
- 2.Phillips JH, Forrest CR, Gruss JS. Current concepts in the use of bone grafts in facial fractures. Basic science considerations. Clin Plast Surg. 1992;19:41–58. [PubMed] [Google Scholar]
- 3.Kashi A, Saha S, Christensen RW. Temporomandibular joint disorders: artificial joint replacements and future research needs. J Long Term Eff Med Implants. 2006;16:459–474. doi: 10.1615/jlongtermeffmedimplants.v16.i6.60. [DOI] [PubMed] [Google Scholar]
- 4.Garcia-Godoy F, Murray PE. Status and potential commercial impact of stem cell-based treatments on dental and craniofacial regeneration. Stem Cells Dev. 2006;15:881–887. doi: 10.1089/scd.2006.15.881. [DOI] [PubMed] [Google Scholar]
- 5.Aframian DJ, Palmon A. Current status of the development of an artificial salivary gland. Tissue Eng. 2008;14:187–198. doi: 10.1089/ten.teb.2008.0044. [DOI] [PubMed] [Google Scholar]
- 6.CDC. National oral health surveillance survey. Atlanta, GA: United States Government, Centers for Disease Control and Prevention; 1999. [Google Scholar]
- 7.National Institutes of Health Technology Assessment Conference Statement. Management of temporomandibular disorders. J Am Dent Assoc. 1996;127:1595–1606. [PubMed] [Google Scholar]
- 8.Kohn DH. Bioceramics. In: Kutz M, editor. Biomedical Engineering and Design Handbook. I. New York: McGraw-Hill; 2009. [Google Scholar]
- 9.Damien CJ, Parsons JR. Bone graft and bone graft substitutes: a review of current technology and applications. J Appl Biomater. 1991;2:187–208. doi: 10.1002/jab.770020307. [DOI] [PubMed] [Google Scholar]
- 10.Goldberg VM, Stevenson S. Natural history of autografts and allografts. Clin Orthop Relat Res. 1987;225:7–16. [PubMed] [Google Scholar]
- 11.Jackson IT, Helden G, Marx R. Skull bone grafts in maxillofacial and craniofacial surgery. J Oral Maxillofac Surg. 1986;44:949–955. doi: 10.1016/s0278-2391(86)80048-9. [DOI] [PubMed] [Google Scholar]
- 12.Friedlaender GE. Bone grafts. The basic science rationale for clinical applications. J Bone Joint Surg. 1987;69:786–790. [PubMed] [Google Scholar]
- 13.Tong L, Buchman SR. Facial bone grafts: contemporary science and thought. J Craniomaxillofac Trauma. 2000;6:31–41. (discussion 2) [PubMed] [Google Scholar]
- 14.Oklund SA, Prolo DJ, Gutierrez RV, King SE. Quantitative comparisons of healing in cranial fresh autografts, frozen autografts and processed autografts, and allografts in canine skull defects. Clin Orthop Relat Res. 1986;205:269–291. [PubMed] [Google Scholar]
- 15.Yoshikawa T, Ohgushi H, Tamai S. Immediate bone forming capability of prefabricated osteogenic hydroxyapatite. J Biomed Mater Res. 1996;32:481–492. doi: 10.1002/(SICI)1097-4636(199611)32:3<481::AID-JBM23>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 16.Giannobile WV. Periodontal tissue engineering by growth factors. Bone. 1996;19:23S–37S. doi: 10.1016/s8756-3282(96)00127-5. [DOI] [PubMed] [Google Scholar]
- 17.Yaszemski MJ, Payne RG, Hayes WC, Langer R, Mikos AG. Evolution of bone transplantation: molecular, cellular and tissue strategies to engineer human bone. Biomaterials. 1996;17:175–185. doi: 10.1016/0142-9612(96)85762-0. [DOI] [PubMed] [Google Scholar]
- 18.Kurtz S, Mowat F, Ong K, Chan N, Lau E, Halpern M. Prevalence of primary and revision total hip and knee arthroplasty in the United States from 1990 through 2002. J Bone Joint Surg. 2005;87:1487–1497. doi: 10.2106/JBJS.D.02441. [DOI] [PubMed] [Google Scholar]
- 19.Huang YC, Kaigler D, Rice KG, Krebsbach PH, Mooney DJ. Combined angiogenic and osteogenic factor delivery enhances bone marrow stromal cell-driven bone regeneration. J Bone Miner Res. 2005;20:848–857. doi: 10.1359/JBMR.041226. [DOI] [PubMed] [Google Scholar]
- 20.Krebsbach PH, Kuznetsov SA, Satomura K, Emmons RV, Rowe DW, Robey PG. Bone formation in vivo: comparison of osteogenesis by transplanted mouse and human marrow stromal fibroblasts. Transplantation. 1997;63:1059–1069. doi: 10.1097/00007890-199704270-00003. [DOI] [PubMed] [Google Scholar]
- 21.Hong L, Mao JJ. Tissue-engineered rabbit cranial suture from autologous fibroblasts and BMP2. J Dent Res. 2004;83:751–756. doi: 10.1177/154405910408301003. [DOI] [PubMed] [Google Scholar]
- 22.Jin Q, Anusaksathien O, Webb SA, Printz MA, Giannobile WV. Engineering of tooth-supporting structures by delivery of PDGF gene therapy vectors. Mol Ther. 2004;9:519–526. doi: 10.1016/j.ymthe.2004.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dogan A, Ozdemir A, Kubar A, Oygur T. Healing of artificial fenestration defects by seeding of fibroblast-like cells derived from regenerated periodontal ligament in a dog: a preliminary study. Tissue Eng. 2003;9:1189–1196. doi: 10.1089/10763270360728099. [DOI] [PubMed] [Google Scholar]
- 24.Nakahara T, Nakamura T, Kobayashi E, Kuremoto K, Matsuno T, Tabata Y, et al. In situ tissue engineering of periodontal tissues by seeding with periodontal ligament-derived cells. Tissue Eng. 2004;10:537–544. doi: 10.1089/107632704323061898. [DOI] [PubMed] [Google Scholar]
- 25.Izumi K, Feinberg SE, Iida A, Yoshizawa M. Intraoral grafting of an ex vivo produced oral mucosa equivalent: a preliminary report. Int J Oral Maxillofac Surg. 2003;32:188–197. doi: 10.1054/ijom.2002.0365. [DOI] [PubMed] [Google Scholar]
- 26.Iida T, Takami Y, Yamaguchi R, Shimazaki S, Harii K. Development of a tissue-engineered human oral mucosa equivalent based on an acellular allogeneic dermal matrix: a preliminary report of clinical application to burn wounds. Scand J Plast Reconstr Surg Hand Surg. 2005;39:138–146. doi: 10.1080/0284431051006376. [DOI] [PubMed] [Google Scholar]
- 27.Kuo TF, Huang AT, Chang HH, Lin FH, Chen ST, Chen RS, et al. Regeneration of dentin-pulp complex with cementum and periodontal ligament formation using dental bud cells in gelatin-chondroitin-hyaluronan tri-copolymer scaffold in swine. J Biomed Mater Res. 2008;86:1062–1068. doi: 10.1002/jbm.a.31746. [DOI] [PubMed] [Google Scholar]
- 28.Yu J, Deng Z, Shi J, Zhai H, Nie X, Zhuang H, et al. Differentiation of dental pulp stem cells into regular-shaped dentin-pulp complex induced by tooth germ cell conditioned medium. Tissue Eng. 2006;12:3097–3105. doi: 10.1089/ten.2006.12.3097. [DOI] [PubMed] [Google Scholar]
- 29.Jin QM, Zhao M, Webb SA, Berry JE, Somerman MJ, Giannobile WV. Cementum engineering with three-dimensional polymer scaffolds. J Biomed Mater Res. 2003;67:54–60. doi: 10.1002/jbm.a.10058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schek RM, Taboas JM, Hollister SJ, Krebsbach PH. Tissue engineering osteochondral implants for temporomandibular joint repair. Orthod Craniofac Res. 2005;8:313–319. doi: 10.1111/j.1601-6343.2005.00354.x. [DOI] [PubMed] [Google Scholar]
- 31.Feinberg SE, Hollister SJ, Halloran JW, Chu TM, Krebsbach PH. Image-based biomimetic approach to reconstruction of the temporomandibular joint. Cells Tissues Organs. 2001;169:309–321. doi: 10.1159/000047896. [DOI] [PubMed] [Google Scholar]
- 32.Alhadlaq A, Mao JJ. Tissue-engineered neogenesis of human-shaped mandibular condyle from rat mesenchymal stem cells. J Dent Res. 2003;82:951–956. doi: 10.1177/154405910308201203. [DOI] [PubMed] [Google Scholar]
- 33.Alhadlaq A, Mao JJ. Tissue-engineered osteochondral constructs in the shape of an articular condyle. J Bone Joint Surg Am. 2005;87:936–944. doi: 10.2106/JBJS.D.02104. [DOI] [PubMed] [Google Scholar]
- 34.Skalak R, Fox CF, editors. Tissue engineering. Proceedings for a Workshop held at Granlibakken; Lake Tahoe, California. February 26–29; New York: Alan Liss; 1988. [Google Scholar]
- 35.The Engineers’ Council for Professional Development. Science. 1941;94:456. doi: 10.1126/science.94.2446.456. [DOI] [PubMed] [Google Scholar]
- 36.Nerem R. The challenge of imitating nature. In: Lanza RP, Langer R, Vacanti JP, editors. Principles of tissue engineering. 2. San Diego: Academic Press; 2000. pp. 9–16. [Google Scholar]
- 37.James K, Levene H, Parsons JR, Kohn J. Small changes in polymer chemistry have a large effect on the bone-implant interface: evaluation of a series of degradable tyrosine-derived polycarbonates in bone defects. Biomaterials. 1999;20:2203–2212. doi: 10.1016/s0142-9612(99)00151-9. [DOI] [PubMed] [Google Scholar]
- 38.Yeung T, Georges PC, Flanagan LA, Marg B, Ortiz M, Funaki M, et al. Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil Cytoskeleton. 2005;60:24–34. doi: 10.1002/cm.20041. [DOI] [PubMed] [Google Scholar]
- 39.Mao JJ, Giannobile WV, Helms JA, Hollister SJ, Krebsbach PH, Longaker MT, et al. Craniofacial tissue engineering by stem cells. J Dent Res. 2006;85:966–979. doi: 10.1177/154405910608501101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9:641–650. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 41.Bianco P, Robey PG. Stem cells in tissue engineering. Nature. 2001;414:118–121. doi: 10.1038/35102181. [DOI] [PubMed] [Google Scholar]
- 42.Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol. 2005;23:47–55. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 43.Rajan GP, Fornaro J, Trentz O, Zellweger R. Cancellous allograft versus autologous bone grafting for repair of comminuted distal radius fractures: a prospective, randomized trial. J Trauma. 2006;60:1322–1329. doi: 10.1097/01.ta.0000195977.18035.40. [DOI] [PubMed] [Google Scholar]
- 44.Glidear J. Bone grafts: a review of the literature. J Foot Surg. 1977;16:146–148. [PubMed] [Google Scholar]
- 45.Vacanti CA, Kim W, Upton J, Vacanti MP, Mooney D, Schloo B, et al. Tissue-engineered growth of bone and cartilage. Transplant Proc. 1993;25:1019–1021. [PubMed] [Google Scholar]
- 46.Ishaug-Riley SL, Crane GM, Gurlek A, Miller MJ, Yasko AW, Yaszemski MJ, et al. Ectopic bone formation by marrow stromal osteoblast transplantation using poly(DL-lactic-co-glycolic acid) foams implanted into the rat mesentery. J Biomed Mater Res. 1997;36:1–8. doi: 10.1002/(sici)1097-4636(199707)36:1<1::aid-jbm1>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 47.Laurencin CT, El-Amin SF, Ibim SE, Willoughby DA, Attawia M, Allcock HR, et al. A highly porous 3-dimensional polyphosphazene polymer matrix for skeletal tissue regeneration. J Biomed Mater Res. 1996;30:133–138. doi: 10.1002/(SICI)1097-4636(199602)30:2<133::AID-JBM1>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 48.Gorna K, Gogolewski S. Preparation, degradation, and calcification of biodegradable polyurethane foams for bone graft substitutes. J Biomed Mater Res. 2003;67:813–827. doi: 10.1002/jbm.a.10148. [DOI] [PubMed] [Google Scholar]
- 49.Li WJ, Tuli R, Huang X, Laquerriere P, Tuan RS. Multilineage differentiation of human mesenchymal stem cells in a three-dimensional nanofibrous scaffold. Biomaterials. 2005;26:5158–5166. doi: 10.1016/j.biomaterials.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 50.Nuttelman CR, Tripodi MC, Anseth KS. In vitro osteogenic differentiation of human mesenchymal stem cells photo-encapsulated in PEG hydrogels. J Biomed Mater Res. 2004;68:773–782. doi: 10.1002/jbm.a.20112. [DOI] [PubMed] [Google Scholar]
- 51.Payne RG, McGonigle JS, Yaszemski MJ, Yasko AW, Mikos AG. Development of an injectable, in situ crosslinkable, degradable polymeric carrier for osteogenic cell populations. Part 3. Proliferation and differentiation of encapsulated marrow stromal osteoblasts cultured on crosslinking poly(propylene fumarate) Biomaterials. 2002;23:4381–4387. doi: 10.1016/s0142-9612(02)00186-2. [DOI] [PubMed] [Google Scholar]
- 52.Marques AP, Cruz HR, Coutinho OP, Reis RL. Effect of starch-based biomaterials on the in vitro proliferation and viability of osteoblast-like cells. J Mater Sci Mater Med. 2005;16:833–842. doi: 10.1007/s10856-005-3580-7. [DOI] [PubMed] [Google Scholar]
- 53.Alsberg E, Anderson KW, Albeiruti A, Franceschi RT, Mooney DJ. Cell-interactive alginate hydrogels for bone tissue engineering. J Dent Res. 2001;80:2025–2029. doi: 10.1177/00220345010800111501. [DOI] [PubMed] [Google Scholar]
- 54.Xu WP, Zhang W, Asrican R, Kim HJ, Kaplan DL, Yelick PC. Accurately shaped tooth bud cell-derived mineralized tissue formation on silk scaffolds. Tissue Eng Part A. 2008;14:549–557. doi: 10.1089/tea.2007.0227. [DOI] [PubMed] [Google Scholar]
- 55.Ducheyne P, el-Ghannam A, Shapiro I. Effect of bioactive glass templates on osteoblast proliferation and in vitro synthesis of bone-like tissue. J Cell Biochem. 1994;56:162–167. doi: 10.1002/jcb.240560207. [DOI] [PubMed] [Google Scholar]
- 56.Reilly GC, Radin S, Chen AT, Ducheyne P. Differential alkaline phosphatase responses of rat and human bone marrow derived mesenchymal stem cells to 45S5 bioactive glass. Biomaterials. 2007;28:4091–4097. doi: 10.1016/j.biomaterials.2007.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ohgushi H, Okumura M, Tamai S, Shors EC, Caplan AI. Marrow cell induced osteogenesis in porous hydroxyapatite and tricalcium phosphate: a comparative histomorphometric study of ectopic bone formation. J Biomed Mater Res. 1990;24:1563–1570. doi: 10.1002/jbm.820241202. [DOI] [PubMed] [Google Scholar]
- 58.Thomson RC, Yaszemski MJ, Powers JM, Mikos AG. Hydroxyapatite fiber reinforced poly(alpha-hydroxy ester) foams for bone regeneration. Biomaterials. 1998;19:1935–1943. doi: 10.1016/s0142-9612(98)00097-0. [DOI] [PubMed] [Google Scholar]
- 59.Kretlow JD, Mikos AG. Review: mineralization of synthetic polymer scaffolds for bone tissue engineering. Tissue Eng. 2007;13:927–938. doi: 10.1089/ten.2006.0394. [DOI] [PubMed] [Google Scholar]
- 60.Segvich S, Smith HC, Luong LN, Kohn DH. Uniform deposition of protein incorporated mineral layer on three-dimensional porous polymer scaffolds. J Biomed Mater Res B Appl Biomater. 2008;84:340–349. doi: 10.1002/jbm.b.30877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chou YF, Huang W, Dunn JC, Miller TA, Wu BM. The effect of biomimetic apatite structure on osteoblast viability, proliferation, and gene expression. Biomaterials. 2005;26:285–295. doi: 10.1016/j.biomaterials.2004.02.030. [DOI] [PubMed] [Google Scholar]
- 62.Puleo DA, Holleran LA, Doremus RH, Bizios R. Osteoblast responses to orthopedic implant materials in vitro. J Biomed Mater Res. 1991;25:711–723. doi: 10.1002/jbm.820250603. [DOI] [PubMed] [Google Scholar]
- 63.Zreiqat H, Evans P, Howlett CR. Effect of surface chemical modification of bioceramic on phenotype of human bone-derived cells. J Biomed Mater Res. 1999;44:389–396. doi: 10.1002/(sici)1097-4636(19990315)44:4<389::aid-jbm4>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 64.Hartman EH, Vehof JW, Spauwen PH, Jansen JA. Ectopic bone formation in rats: the importance of the carrier. Biomaterials. 2005;26:1829–1835. doi: 10.1016/j.biomaterials.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 65.Stevens MM. Biomaterials for bone tissue engineering. Mater Today. 2008;11:18–25. [Google Scholar]
- 66.Ferreira CF, Magini RS, Sharpe PT. Biological tooth replacement and repair. J Oral Rehabil. 2007;34:933–939. doi: 10.1111/j.1365-2842.2007.01785.x. [DOI] [PubMed] [Google Scholar]
- 67.Young CS, Abukawa H, Asrican R, Ravens M, Troulis MJ, Kaban LB, et al. Tissue-engineered hybrid tooth and bone. Tissue Eng. 2005;11:1599–1610. doi: 10.1089/ten.2005.11.1599. [DOI] [PubMed] [Google Scholar]
- 68.Hu B, Nadiri A, Kuchler-Bopp S, Perrin-Schmitt F, Peters H, Lesot H. Tissue engineering of tooth crown, root, and periodontium. Tissue Eng. 2006;12:2069–2075. doi: 10.1089/ten.2006.12.2069. [DOI] [PubMed] [Google Scholar]
- 69.Nakao K, Morita R, Saji Y, Ishida K, Tomita Y, Ogawa M, et al. The development of a bioengineered organ germ method. Nat Methods. 2007;4:227–230. doi: 10.1038/nmeth1012. [DOI] [PubMed] [Google Scholar]
- 70.Nakashima M, Akamine A. The application of tissue engineering to regeneration of pulp and dentin in endodontics. J Endod. 2005;31:711–718. doi: 10.1097/01.don.0000164138.49923.e5. [DOI] [PubMed] [Google Scholar]
- 71.Dard M, Sewing A, Meyer J, Verrier S, Roessler S, Scharnweber D. Tools for tissue engineering of mineralized oral structures. Clin Oral Invest. 2000;4:126–129. doi: 10.1007/s007840050128. [DOI] [PubMed] [Google Scholar]
- 72.Yamazaki H, Litman A, Margolis HC. Effect of fluoride on artificial caries lesion progression and repair in human enamel: regulation of mineral deposition and dissolution under in vivo-like conditions. Arch Oral Biol. 2007;52:110–120. doi: 10.1016/j.archoralbio.2006.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Duailibi SE, Duailibi MT, Vacanti JP, Yelick PC. Prospects for tooth regeneration. Periodontology 2000. 2006;41:177–187. doi: 10.1111/j.1600-0757.2006.00165.x. [DOI] [PubMed] [Google Scholar]
- 74.Gronthos S, Brahim J, Li W, Fisher LW, Cherman N, Boyde A, et al. Stem cell properties of human dental pulp stem cells. J Dent Res. 2002;81:531–535. doi: 10.1177/154405910208100806. [DOI] [PubMed] [Google Scholar]
- 75.Zhang W, Walboomers XF, Shi S, Fan M, Jansen JA. Multilineage differentiation potential of stem cells derived from human dental pulp after cryopreservation. Tissue Eng. 2006;12:2813–2823. doi: 10.1089/ten.2006.12.2813. [DOI] [PubMed] [Google Scholar]
- 76.Liu J, Jin T, Ritchie HH, Smith AJ, Clarkson BH. In vitro differentiation and mineralization of human dental pulp cells induced by dentin extract. In Vitro Cell Dev Biol. 2005;41:232–238. doi: 10.1290/0502014.1. [DOI] [PubMed] [Google Scholar]
- 77.Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci USA. 2000;97:13625–13630. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nakashima M, Iohara K, Zheng L. Gene therapy for dentin regeneration with bone morphogenetic proteins. Curr Gene Ther. 2006;6:551–560. doi: 10.2174/156652306778520665. [DOI] [PubMed] [Google Scholar]
- 79.Scheller EL, Chang J, Wang CY. Wnt/beta-catenin inhibits dental pulp stem cell differentiation. J Dent Res. 2008;87:126–130. doi: 10.1177/154405910808700206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang C, Chang J, Sonoyama W, Shi S, Wang CY. Inhibition of human dental pulp stem cell differentiation by Notch signaling. J Dent Res. 2008;87:250–255. doi: 10.1177/154405910808700312. [DOI] [PubMed] [Google Scholar]
- 81.Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364:149–155. doi: 10.1016/S0140-6736(04)16627-0. [DOI] [PubMed] [Google Scholar]
- 82.McCulloch CA. Progenitor cell populations in the periodontal ligament of mice. Anat Rec. 1985;211:258–262. doi: 10.1002/ar.1092110305. [DOI] [PubMed] [Google Scholar]
- 83.Gould TR, Melcher AH, Brunette DM. Migration and division of progenitor cell populations in periodontal ligament after wounding. J Periodontal Res. 1980;15:20–42. doi: 10.1111/j.1600-0765.1980.tb00258.x. [DOI] [PubMed] [Google Scholar]
- 84.Shi S, Bartold PM, Miura M, Seo BM, Robey PG, Gronthos S. The efficacy of mesenchymal stem cells to regenerate and repair dental structures. Orthod Craniofac Res. 2005;8:191–199. doi: 10.1111/j.1601-6343.2005.00331.x. [DOI] [PubMed] [Google Scholar]
- 85.Shi S, Robey PG, Gronthos S. Comparison of human dental pulp and bone marrow stromal stem cells by cDNA microarray analysis. Bone. 2001;29:532–539. doi: 10.1016/s8756-3282(01)00612-3. [DOI] [PubMed] [Google Scholar]
- 86.Ekholm SV, Reed SI. Regulation of G(1) cyclin-dependent kinases in the mammalian cell cycle. Curr Opin Cell Biol. 2000;12:676–684. doi: 10.1016/s0955-0674(00)00151-4. [DOI] [PubMed] [Google Scholar]
- 87.Grossel MJ, Baker GL, Hinds PW. cdk6 can shorten G(1) phase dependent upon the N-terminal INK4 interaction domain. J Biol Chem. 1999;274:29960–29967. doi: 10.1074/jbc.274.42.29960. [DOI] [PubMed] [Google Scholar]
- 88.Nayak BN, Wiltshire WA, Ganss B, Tenenbaum H, McCulloch CA, Lekic C. Healing of periodontal tissues following transplantation of cells in a rat orthodontic tooth movement model. Angle Orthod. 2008;78:826–831. doi: 10.2319/082807-396.1. [DOI] [PubMed] [Google Scholar]
- 89.Zajicek G, Michaeli Y. On the potential of the adult rat incisor odontogenic organ to differentiate into two intact teeth. J Biol Buccale. 1978;6:339–342. [PubMed] [Google Scholar]
- 90.Duailibi MT, Duailibi SE, Young CS, Bartlett JD, Vacanti JP, Yelick PC. Bioengineered teeth from cultured rat tooth bud cells. J Dent Res. 2004;83:523–528. doi: 10.1177/154405910408300703. [DOI] [PubMed] [Google Scholar]
- 91.Honda MJ, Shinohara Y, Hata KI, Ueda M. Subcultured odontogenic epithelial cells in combination with dental mesenchymal cells produce enamel-dentin-like complex structures. Cell Transplant. 2007;16:833–847. doi: 10.3727/000000007783465208. [DOI] [PubMed] [Google Scholar]
- 92.Simmons PJ, Torok-Storb B. Identification of stromal cell precursors in human bone marrow by a novel monoclonal antibody, STRO-1. Blood. 1991;78:55–62. [PubMed] [Google Scholar]
- 93.Goodell MA, Rosenzweig M, Kim H, Marks DF, DeMaria M, Paradis G, et al. Dye efflux studies suggest that hematopoietic stem cells expressing low or undetectable levels of CD34 antigen exist in multiple species. Nat Med. 1997;3:1337–1345. doi: 10.1038/nm1297-1337. [DOI] [PubMed] [Google Scholar]
- 94.Sumita Y, Honda MJ, Ohara T, Tsuchiya S, Sagara H, Kagami H, et al. Performance of collagen sponge as a 3-D scaffold for tooth-tissue engineering. Biomaterials. 2006;27:3238–3248. doi: 10.1016/j.biomaterials.2006.01.055. [DOI] [PubMed] [Google Scholar]
- 95.Honda MJ, Tsuchiya S, Sumita Y, Sagara H, Ueda M. The sequential seeding of epithelial and mesenchymal cells for tissue-engineered tooth regeneration. Biomaterials. 2007;28:680–689. doi: 10.1016/j.biomaterials.2006.09.039. [DOI] [PubMed] [Google Scholar]
- 96.Taira M, Toyosawa S, Ijyuin N, Takahashi J, Araki Y. Studies on osteogenic differentiation of rat bone marrow stromal cells cultured in type I collagen gel by RT-PCR analysis. J Oral Rehabil. 2003;30:802–807. doi: 10.1046/j.1365-2842.2003.01170.x. [DOI] [PubMed] [Google Scholar]
- 97.Kirkham J, Firth A, Vernals D, Boden N, Robinson C, Shore RC, et al. Self-assembling peptide scaffolds promote enamel remineralization. J Dent Res. 2007;86:426–430. doi: 10.1177/154405910708600507. [DOI] [PubMed] [Google Scholar]
- 98.Zhang S. Fabrication of novel biomaterials through molecular self-assembly. Nat Biotechnol. 2003;21:1171–1178. doi: 10.1038/nbt874. [DOI] [PubMed] [Google Scholar]
- 99.Menger FM. Supramolecular chemistry and self-assembly. Proc Natl Acad Sci USA. 2002;99:4818–4822. doi: 10.1073/pnas.062524299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kisiday J, Jin M, Kurz B, Hung H, Semino C, Zhang S, et al. Self-assembling peptide hydrogel fosters chondrocyte extracellular matrix production and cell division: implications for cartilage tissue repair. Proc Natl Acad Sci USA. 2002;99:9996–10001. doi: 10.1073/pnas.142309999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Curtis A, Wilkinson C. New depths in cell behaviour: reactions of cells to nanotopography. Biochem Soc Symp. 1999;65:15–26. [PubMed] [Google Scholar]
- 102.Stevens MM, George JH. Exploring and engineering the cell surface interface. Science. 2005;310:1135–1138. doi: 10.1126/science.1106587. [DOI] [PubMed] [Google Scholar]
- 103.Buttafoco L, Kolkman NG, Engbers-Buijtenhuijs P, Poot AA, Dijkstra PJ, Vermes I, et al. Electrospinning of collagen and elastin for tissue engineering applications. Biomaterials. 2006;27:724–734. doi: 10.1016/j.biomaterials.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 104.Webster TJ, Ergun C, Doremus RH, Siegel RW, Bizios R. Enhanced osteoclast-like cell functions on nanophase ceramics. Biomaterials. 2001;22:1327–1333. doi: 10.1016/s0142-9612(00)00285-4. [DOI] [PubMed] [Google Scholar]
- 105.Balasundaram G, Sato M, Webster TJ. Using hydroxyapatite nanoparticles and decreased crystallinity to promote osteoblast adhesion similar to functionalizing with RGD. Biomaterials. 2006;27:2798–2805. doi: 10.1016/j.biomaterials.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 106.Silva GA, Czeisler C, Niece KL, Beniash E, Harrington DA, Kessler JA, et al. Selective differentiation of neural progenitor cells by high-epitope density nanofibers. Science. 2004;303:1352–1355. doi: 10.1126/science.1093783. [DOI] [PubMed] [Google Scholar]
- 107.Mendonca G, Mendonca DB, Aragao FJ, Cooper LF. Advancing dental implant surface technology - From micron-to nanotopography. Biomaterials. 2008;29:3822–3835. doi: 10.1016/j.biomaterials.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 108.Dalby MJ, Gadegaard N, Tare R, Andar A, Riehle MO, Herzyk P, et al. The control of human mesenchymal cell differentiation using nanoscale symmetry and disorder. Nat Mater. 2007;6:997–1003. doi: 10.1038/nmat2013. [DOI] [PubMed] [Google Scholar]
- 109.Weiner S. Organization of extracellularly mineralized tissues: a comparative study of biological crystal growth. CRC Crit Rev Biochem. 1986;20:365–408. doi: 10.3109/10409238609081998. [DOI] [PubMed] [Google Scholar]
- 110.Ducheyne P. Bioceramics: material characteristics versus in vivo behavior. J Biomed Mater Res. 1987;21:219–236. [PubMed] [Google Scholar]
- 111.Nakamura T, Yamamuro T, Higashi S, Kokubo T, Itoo S. Anew glass-ceramic for bone replacement: evaluation of its bonding to bone tissue. J Biomed Mater Res. 1985;19:685–698. doi: 10.1002/jbm.820190608. [DOI] [PubMed] [Google Scholar]
- 112.Shin K, Jayasuriya AC, Kohn DH. Effect of ionic activity products on the structure and composition of mineral self assembled on three-dimensional poly(lactide-co-glycolide) scaffolds. J Biomed Mater Res. 2007;83:1076–1086. doi: 10.1002/jbm.a.31437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Murphy WL, Kohn DH, Mooney DJ. Growth of continuous bonelike mineral within porous poly(lactide-co-glycolide) scaffolds in vitro. J Biomed Mater Res. 2000;50:50–58. doi: 10.1002/(sici)1097-4636(200004)50:1<50::aid-jbm8>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 114.Kokubo T, Kushitani H, Sakka S, Kitsugi T, Yamamuro T. Solutions able to reproduce in vivo surface-structure changes in bioactive glass-ceramic A-W. J Biomed Mater Res. 1990;24:721–734. doi: 10.1002/jbm.820240607. [DOI] [PubMed] [Google Scholar]
- 115.Bunker BC, Rieke PC, Tarasevich BJ, Campbell AA, Fryxell GE, Graff GL, et al. Ceramic thin-film formation on functionalized interfaces through biomimetic processing. Science. 1994;264:48–55. doi: 10.1126/science.264.5155.48. [DOI] [PubMed] [Google Scholar]
- 116.Leonova EV, Pennington KE, Krebsbach PH, Kohn DH. Substrate mineralization stimulates focal adhesion contact redistribution and cell motility of bone marrow stromal cells. J Biomed Mater Res. 2006;79:263–270. doi: 10.1002/jbm.a.30786. [DOI] [PubMed] [Google Scholar]
- 117.Kohn DH, Shin K, Hong SI, Jayasuriya AC, Leonova EV, Rossello RA. Self-assembled mineral scaffolds as model systems for biomineralization and tissue engineering. Proc 8th International Conference on the Chemistry and Biology of Mineralized Tissues; Toronto: University of Toronto Press; 2005. pp. 216–219. [Google Scholar]
- 118.Kaigler D, Cirelli JA, Giannobile WV. Growth factor delivery for oral and periodontal tissue engineering. Expert Opin Drug Deliv. 2006;3:647–662. doi: 10.1517/17425247.3.5.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hersel U, Dahmen C, Kessler H. RGD modified polymers: biomaterials for stimulated cell adhesion and beyond. Biomaterials. 2003;24:4385–4415. doi: 10.1016/s0142-9612(03)00343-0. [DOI] [PubMed] [Google Scholar]
- 120.Ladner RC, Sato AK, Gorzelany J, de Souza M. Phage display-derived peptides as therapeutic alternatives to antibodies. Drug Discov Today. 2004;9:525–529. doi: 10.1016/S1359-6446(04)03104-6. [DOI] [PubMed] [Google Scholar]
- 121.Massia SP, Hubbell JA. Covalent surface immobilization of Arg-Gly-Asp- and Tyr-Ile-Gly-Ser-Arg-containing peptides to obtain well-defined cell-adhesive substrates. Anal Biochem. 1990;187:292–301. doi: 10.1016/0003-2697(90)90459-m. [DOI] [PubMed] [Google Scholar]
- 122.Horii A, Wang X, Gelain F, Zhang S. Biological designer self-assembling Peptide nanofiber scaffolds significantly enhance osteoblast proliferation, differentiation and 3-D migration. PLoS ONE. 2007;2:e190. doi: 10.1371/journal.pone.0000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Garcia AJ. Get a grip: integrins in cell-biomaterial interactions. Biomaterials. 2005;26:7525–7529. doi: 10.1016/j.biomaterials.2005.05.029. [DOI] [PubMed] [Google Scholar]
- 124.Yasuda Y, Izumikawa M, Okamoto K, Tsukuba T, Saito T. Dentin phosphophoryn promotes cellular migration of human dental pulp cells. J Endod. 2008;34:575–578. doi: 10.1016/j.joen.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 125.Rezania A, Healy KE. The effect of peptide surface density on mineralization of a matrix deposited by osteogenic cells. J Biomed Mater Res. 2000;52:595–600. doi: 10.1002/1097-4636(20001215)52:4<595::aid-jbm3>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 126.Itoh D, Yoneda S, Kuroda S, Kondo H, Umezawa A, Ohya K, et al. Enhancement of osteogenesis on hydroxyapatite surface coated with synthetic peptide (EEEEEEEPRGDT) in vitro. J Biomed Mater Res. 2002;62:292–298. doi: 10.1002/jbm.10338. [DOI] [PubMed] [Google Scholar]
- 127.Hwang NS, Varghese S, Zhang Z, Elisseeff J. Chondrogenic differentiation of human embryonic stem cell-derived cells in arginine-glycine-aspartate-modified hydrogels. Tissue Eng. 2006;12:2695–2706. doi: 10.1089/ten.2006.12.2695. [DOI] [PubMed] [Google Scholar]
- 128.Salinas CN, Cole BB, Kasko AM, Anseth KS. Chondrogenic differentiation potential of human mesenchymal stem cells photoencapsulated within poly(ethylene glycol)-arginine-glycine- aspartic acid-serine thiol-methacrylate mixed-mode networks. Tissue Eng. 2007;13:1025–1034. doi: 10.1089/ten.2006.0126. [DOI] [PubMed] [Google Scholar]
- 129.Gunn JW, Turner SD, Mann BK. Adhesive and mechanical properties of hydrogels influence neurite extension. J Biomed Mater Res. 2005;72:91–97. doi: 10.1002/jbm.a.30203. [DOI] [PubMed] [Google Scholar]
- 130.Patel S, Tsang J, Harbers GM, Healy KE, Li S. Regulation of endothelial cell function by GRGDSP peptide grafted on interpenetrating polymers. J Biomed Mater Res. 2007;83:423–433. doi: 10.1002/jbm.a.31320. [DOI] [PubMed] [Google Scholar]
- 131.Harbers GM, Healy KE. The effect of ligand type and density on osteoblast adhesion, proliferation, and matrix mineralization. J Biomed Mater Res. 2005;75:855–869. doi: 10.1002/jbm.a.30482. [DOI] [PubMed] [Google Scholar]
- 132.Dee KC, Andersen TT, Bizios R. Design and function of novel osteoblast-adhesive peptides for chemical modification of biomaterials. J Biomed Mater Res. 1998;40:371–377. doi: 10.1002/(sici)1097-4636(19980605)40:3<371::aid-jbm5>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 133.Rezania A, Healy KE. Biomimetic peptide surfaces that regulate adhesion, spreading, cytoskeletal organization, and mineralization of the matrix deposited by osteoblast-like cells. Biotechnol Prog. 1999;15:19–32. doi: 10.1021/bp980083b. [DOI] [PubMed] [Google Scholar]
- 134.Kulkarni GV, Chen B, Malone JP, Narayanan AS, George A. Promotion of selective cell attachment by the RGD sequence in dentine matrix protein 1. Arch Oral Biol. 2000;45:475–484. doi: 10.1016/s0003-9969(00)00010-8. [DOI] [PubMed] [Google Scholar]
- 135.Reyes CD, Petrie TA, Burns KL, Schwartz Z, Garcia AJ. Biomolecular surface coating to enhance orthopaedic tissue healing and integration. Biomaterials. 2007;28:3228–3235. doi: 10.1016/j.biomaterials.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Schense JC, Hubbell JA. Three-dimensional migration of neurites is mediated by adhesion site density and affinity. J Biol Chem. 2000;275:6813–6818. doi: 10.1074/jbc.275.10.6813. [DOI] [PubMed] [Google Scholar]
- 137.Fujisawa R, Mizuno M, Nodasaka Y, Kuboki Y. Attachment of osteoblastic cells to hydroxyapatite crystals by a synthetic peptide (Glu7-Pro-Arg-Gly-Asp-Thr) containing two functional sequences of bone sialoprotein. Matrix Biol. 1997;16:21–28. doi: 10.1016/s0945-053x(97)90113-x. [DOI] [PubMed] [Google Scholar]
- 138.Gilbert M, Shaw WJ, Long JR, Nelson K, Drobny GP, Giachelli CM, et al. Chimeric peptides of statherin and osteopontin that bind hydroxyapatite and mediate cell adhesion. J Biol Chem. 2000;275:16213–16218. doi: 10.1074/jbc.M001773200. [DOI] [PubMed] [Google Scholar]
- 139.Simionescu A, Philips K, Vyavahare N. Elastin-derived peptides and TGF-beta1 induce osteogenic responses in smooth muscle cells. Biochem Biophys Res Commun. 2005;334:524–532. doi: 10.1016/j.bbrc.2005.06.119. [DOI] [PubMed] [Google Scholar]
- 140.Fujisawa R, Wada Y, Nodasaka Y, Kuboki Y. Acidic amino acid-rich sequences as binding sites of osteonectin to hydroxyapatite crystals. Biochim Biophys Acta. 1996;1292:53–60. doi: 10.1016/0167-4838(95)00190-5. [DOI] [PubMed] [Google Scholar]
- 141.Samoylova TI, Ahmed BY, Vodyanoy V, Morrison NE, Samoylov AM, Globa LP, et al. Targeting peptides for microglia identified via phage display. J Neuroimmunol. 2002;127:13–21. doi: 10.1016/s0165-5728(02)00071-1. [DOI] [PubMed] [Google Scholar]
- 142.Zhang J, Spring H, Schwab M. Neuroblastoma tumor cell-binding peptides identified through random peptide phage display. Cancer Lett. 2001;171:153–164. doi: 10.1016/s0304-3835(01)00575-4. [DOI] [PubMed] [Google Scholar]
- 143.Pasqualini R, Koivunen E, Ruoslahti E. A peptide isolated from phage display libraries is a structural and functional mimic of an RGD-binding site on integrins. J Cell Biol. 1995;130:1189–1196. doi: 10.1083/jcb.130.5.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Uchiyama F, Tanaka Y, Minari Y, Tokui N. Designing scaffolds of peptides for phage display libraries. J Biosci Bioeng. 2005;99:448–456. doi: 10.1263/jbb.99.448. [DOI] [PubMed] [Google Scholar]
- 145.Marks C, Marks JD. Phage libraries–a new route to clinically useful antibodies. N Engl J Med. 1996;335:730–733. doi: 10.1056/NEJM199609053351008. [DOI] [PubMed] [Google Scholar]
- 146.Adey NB, Mataragnon AH, Rider JE, Carter JM, Kay BK. Characterization of phage that bind plastic from phagedisplayed random peptide libraries. Gene. 1995;156:27–31. doi: 10.1016/0378-1119(95)00058-e. [DOI] [PubMed] [Google Scholar]
- 147.Whaley SR, English DS, Hu EL, Barbara PF, Belcher AM. Selection of peptides with semiconductor binding specificity for directed nanocrystal assembly. Nature. 2000;405:665–668. doi: 10.1038/35015043. [DOI] [PubMed] [Google Scholar]
- 148.Kriplani U, Kay BK. Selecting peptides for use in nanoscale materials using phage-displayed combinatorial peptide libraries. Curr Opin Biotechnol. 2005;16:470–475. doi: 10.1016/j.copbio.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 149.Segvich S, Biswas S, Becker U, Kohn DH. Identification of peptides with targeted adhesion to bone-like mineral via phage display and computational modeling. Cells Tissues Organs. 2009;189:245–251. doi: 10.1159/000151380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hoffman A. Biologically functional materials. In: Ratner BD, Hoffman AS, Schoen FJ, Lemons JE, editors. Biomaterials science: an introduction to materials in medicine. San Diego: Academic Press; 1996. pp. 124–130. [Google Scholar]
- 151.Whang K, Tsai DC, Nam EK, Aitken M, Sprague SM, Patel PK, et al. Ectopic bone formation via rhBMP-2 delivery from porous bioabsorbable polymer scaffolds. J Biomed Mater Res. 1998;42:491–499. doi: 10.1002/(sici)1097-4636(19981215)42:4<491::aid-jbm3>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 152.Shea LD, Smiley E, Bonadio J, Mooney DJ. DNA delivery from polymer matrices for tissue engineering. Nat Biotechnol. 1999;17:551–554. doi: 10.1038/9853. [DOI] [PubMed] [Google Scholar]
- 153.Sheridan MH, Shea LD, Peters MC, Mooney DJ. Bioabsorbable polymer scaffolds for tissue engineering capable of sustained growth factor delivery. J Control Release. 2000;64:91–102. doi: 10.1016/s0168-3659(99)00138-8. [DOI] [PubMed] [Google Scholar]
- 154.Murphy WL, Peters MC, Kohn DH, Mooney DJ. Sustained release of vascular endothelial growth factor from mineralized poly(lactide-co-glycolide) scaffolds for tissue engineering. Biomaterials. 2000;21:2521–2527. doi: 10.1016/s0142-9612(00)00120-4. [DOI] [PubMed] [Google Scholar]
- 155.Campbell AA, Song L, Li XS, Nelson BJ, Bottoni C, Brooks DE, et al. Development, characterization, and anti-microbial efficacy of hydroxyapatite-chlorhexidine coatings produced by surface-induced mineralization. J Biomed Mater Res. 2000;53:400–407. doi: 10.1002/1097-4636(2000)53:4<400::aid-jbm14>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 156.Van Dyke TE, Offenbacher S, Braswell L, Lessem J. Enhancing the value of scaling and root-planing: Arestin clinical trial results. J Int Acad Periodontol. 2002;4:72–76. [PubMed] [Google Scholar]
- 157.Luong LN, Hong SI, Patel RJ, Outslay ME, Kohn DH. Spatial control of protein within biomimetically nucleated mineral. Biomaterials. 2006;27:1175–1186. doi: 10.1016/j.biomaterials.2005.07.043. [DOI] [PubMed] [Google Scholar]
- 158.Kong HJ, Liu J, Riddle K, Matsumoto T, Leach K, Mooney DJ. Non-viral gene delivery regulated by stiffness of cell adhesion substrates. Nat Mater. 2005;4:460–464. doi: 10.1038/nmat1392. [DOI] [PubMed] [Google Scholar]
- 159.Richardson TP, Peters MC, Ennett AB, Mooney DJ. Polymeric system for dual growth factor delivery. Nat Biotechnol. 2001;19:1029–1034. doi: 10.1038/nbt1101-1029. [DOI] [PubMed] [Google Scholar]
- 160.Rossello RA, Kohn DH. Gap junction intercellular communication: a review of a potential platform to modulate craniofacial tissue engineering. J Biomed Mater Res B Appl Biomater. 2009;88:509–518. doi: 10.1002/jbm.b.31127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Rossello RA. PhD Dissertation. Ann Arbor: University of Michigan; 2007. Cell-to-cell communication as a strategy to regenerate three-dimensional tissue. [Google Scholar]
- 162.Lightfoot E. The roles of mass transport in tissue function. In: Bronzino JD, editor. CRC handbook of biomedical engineering. Boca Raton, FL: CRC Press; 1995. pp. 1656–1670. [Google Scholar]
- 163.Bessa PC, Casal M, Reis RL. Bone morphogenetic proteins in tissue engineering: the road from laboratory to clinic, part II (BMP delivery) J Tissue Eng Regen Med. 2008;2:81–96. doi: 10.1002/term.74. [DOI] [PubMed] [Google Scholar]
- 164.Wikesjo UM, Xiropaidis AV, Thomson RC, Cook AD, Selvig KA, Hardwick WR. Periodontal repair in dogs: rhBMP-2 significantly enhances bone formation under provisions for guided tissue regeneration. J Clin Periodontol. 2003;30:705–714. doi: 10.1034/j.1600-051x.2003.00363.x. [DOI] [PubMed] [Google Scholar]
- 165.Mayer M, Hollinger J, Ron E, Wozney J. Maxillary alveolar cleft repair in dogs using recombinant human bone morphogenetic protein-2 and a polymer carrier. Plast Reconstr Surg. 1996;98:247–259. doi: 10.1097/00006534-199608000-00006. [DOI] [PubMed] [Google Scholar]
- 166.Kawakatsu N, Oda S, Kinoshita A, Kikuchi S, Tsuchioka H, Akizuki T, et al. Effect of rhBMP-2 with PLGA/gelatin sponge type (PGS) carrier on alveolar ridge augmentation in dogs. J Oral Rehabil. 2008;35:647–655. doi: 10.1111/j.1365-2842.2008.01850.x. [DOI] [PubMed] [Google Scholar]
- 167.Jung RE, Glauser R, Scharer P, Hammerle CH, Sailer HF, Weber FE. Effect of rhBMP-2 on guided bone regeneration in humans. Clin Oral Implant Res. 2003;14:556–568. doi: 10.1034/j.1600-0501.2003.00921.x. [DOI] [PubMed] [Google Scholar]
- 168.NIH. ClinicalTrials.gov. Bethesda (MD): US National Institutes of Health; 2008. [Google Scholar]
- 169.Chu CR, Coutts RD, Yoshioka M, Harwood FL, Monosov AZ, Amiel D. Articular cartilage repair using allogeneic perichondrocyte- seeded biodegradable porous polylactic acid (PLA): a tissue-engineering study. J Biomed Mater Res. 1995;29:1147–1154. doi: 10.1002/jbm.820290915. [DOI] [PubMed] [Google Scholar]
- 170.Ma PX, Schloo B, Mooney D, Langer R. Development of biomechanical properties and morphogenesis of in vitro tissue engineered cartilage. J Biomed Mater Res. 1995;29:1587–1595. doi: 10.1002/jbm.820291215. [DOI] [PubMed] [Google Scholar]
- 171.Sherwood JK, Riley SL, Palazzolo R, Brown SC, Monkhouse DC, Coates M, et al. A three-dimensional osteochondral composite scaffold for articular cartilage repair. Biomaterials. 2002;23:4739–4751. doi: 10.1016/s0142-9612(02)00223-5. [DOI] [PubMed] [Google Scholar]
- 172.Liao E, Yaszemski M, Krebsbach P, Hollister S. Tissue-engineered cartilage constructs using composite hyaluronic acid/collagen I hydrogels and designed poly(propylene fumarate) scaffolds. Tissue Eng. 2007;13:537–550. doi: 10.1089/ten.2006.0117. [DOI] [PubMed] [Google Scholar]
- 173.Willers C, Chen J, Wood D, Xu J, Zheng MH. Autologous chondrocyte implantation with collagen bioscaffold for the treatment of osteochondral defects in rabbits. Tissue Eng. 2005;11:1065–1076. doi: 10.1089/ten.2005.11.1065. [DOI] [PubMed] [Google Scholar]
- 174.Cucchiarini M, Madry H, Ma C, Thurn T, Zurakowski D, Menger MD, et al. Improved tissue repair in articular cartilage defects in vivo by rAAV-mediated overexpression of human fibroblast growth factor 2. Mol Ther. 2005;12:229–238. doi: 10.1016/j.ymthe.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 175.Nagura I, Fujioka H, Kokubu T, Makino T, Sumi Y, Kurosaka M. Repair of osteochondral defects with a new porous synthetic polymer scaffold. J Bone Joint Surg Br. 2007;89:258–264. doi: 10.1302/0301-620X.89B2.17754. [DOI] [PubMed] [Google Scholar]
- 176.Schreiber RE, Ilten-Kirby BM, Dunkelman NS, Symons KT, Rekettye LM, Willoughby J, et al. Repair of osteochondral defects with allogeneic tissue engineered cartilage implants. Clin Orthop Relat Res. 1999;367:S382–S395. doi: 10.1097/00003086-199910001-00037. [DOI] [PubMed] [Google Scholar]
- 177.Buckwalter JA, Mankin HJ. Articular cartilage repair and transplantation. Arthritis Rheum. 1998;41:1331–1342. doi: 10.1002/1529-0131(199808)41:8<1331::AID-ART2>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 178.Cuevas P, Burgos J, Baird A. Basic fibroblast growth factor (FGF) promotes cartilage repair in vivo. Biochem Biophys Res Commun. 1988;156:611–618. doi: 10.1016/s0006-291x(88)80887-8. [DOI] [PubMed] [Google Scholar]
- 179.Hiraide A, Yokoo N, Xin KQ, Okuda K, Mizukami H, Ozawa K, et al. Repair of articular cartilage defect by intraarticular administration of basic fibroblast growth factor gene, using adeno-associated virus vector. Hum Gene Ther. 2005;16:1413–1421. doi: 10.1089/hum.2005.16.1413. [DOI] [PubMed] [Google Scholar]
- 180.Yokoo N, Saito T, Uesugi M, Kobayashi N, Xin KQ, Okuda K, et al. Repair of articular cartilage defect by autologous transplantation of basic fibroblast growth factor gene-transduced chondrocytes with adeno-associated virus vector. Arthritis Rheum. 2005;52:164–170. doi: 10.1002/art.20739. [DOI] [PubMed] [Google Scholar]
- 181.Gelse K, von der Mark K, Aigner T, Park J, Schneider H. Articular cartilage repair by gene therapy using growth factor-producing mesenchymal cells. Arthritis Rheum. 2003;48:430–441. doi: 10.1002/art.10759. [DOI] [PubMed] [Google Scholar]
- 182.Redini F, Galera P, Mauviel A, Loyau G, Pujol JP. Transforming growth factor beta stimulates collagen and glycos-aminoglycan biosynthesis in cultured rabbit articular chondrocytes. FEBS Lett. 1988;234:172–176. doi: 10.1016/0014-5793(88)81327-9. [DOI] [PubMed] [Google Scholar]
- 183.Pagnotto MR, Wang Z, Karpie JC, Ferretti M, Xiao X, Chu CR. Adeno-associated viral gene transfer of transforming growth factor-beta1 to human mesenchymal stem cells improves cartilage repair. Gene Ther. 2007;14:804–813. doi: 10.1038/sj.gt.3302938. [DOI] [PubMed] [Google Scholar]
- 184.Akiyama H, Chaboissier MC, Martin JF, Schedl A, de Crombrugghe B. The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6. Genes Dev. 2002;16:2813–2828. doi: 10.1101/gad.1017802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Cucchiarini M, Thurn T, Weimer A, Kohn D, Terwilliger EF, Madry H. Restoration of the extracellular matrix in human osteoarthritic articular cartilage by overexpression of the transcription factor SOX9. Arthritis Rheum. 2007;56:158–167. doi: 10.1002/art.22299. [DOI] [PubMed] [Google Scholar]
- 186.Rubin JP, Yaremchuk MJ. Complications and toxicities of implantable biomaterials used in facial reconstructive and aesthetic surgery: a comprehensive review of the literature. Plast Reconstr Surg. 1997;100:1336–1353. doi: 10.1097/00006534-199710000-00043. [DOI] [PubMed] [Google Scholar]
- 187.Alsberg E, Anderson KW, Albeiruti A, Rowley JA, Mooney DJ. Engineering growing tissues. Proc Natl Acad Sci USA. 2002;99:12025–12030. doi: 10.1073/pnas.192291499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Solchaga LA, Goldberg VM, Caplan AI. Cartilage regeneration using principles of tissue engineering. Clin Orthop Relat Res. 2001;391:S161–S170. doi: 10.1097/00003086-200110001-00016. [DOI] [PubMed] [Google Scholar]
- 189.Tognana E, Padera RF, Chen F, Vunjak-Novakovic G, Freed LE. Development and remodeling of engineered cartilage-explant composites in vitro and in vivo. Osteoarthritis Cartilage. 2005;13:896–905. doi: 10.1016/j.joca.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 190.Schek RM, Taboas JM, Segvich SJ, Hollister SJ, Krebsbach PH. Engineered osteochondral grafts using biphasic composite solid free-form fabricated scaffolds. Tissue Eng. 2004;10:1376–1385. doi: 10.1089/ten.2004.10.1376. [DOI] [PubMed] [Google Scholar]
- 191.Hauben DJ, Baruchin A, Mahler A. On the history of the free skin graft. Ann Plast Surg. 1982;9:242–245. doi: 10.1097/00000637-198209000-00009. [DOI] [PubMed] [Google Scholar]
- 192.Madden MR, Finkelstein JL, Staiano-Coico L, Goodwin CW, Shires GT, Nolan EE, et al. Grafting of cultured allogeneic epidermis on second- and third-degree burn wounds on 26 patients. J Trauma. 1986;26:955–962. doi: 10.1097/00005373-198611000-00001. [DOI] [PubMed] [Google Scholar]
- 193.Lauer G, Schimming R. Tissue-engineered mucosa graft for reconstruction of the intraoral lining after freeing of the tongue: a clinical and immunohistologic study. J Oral Maxillofac Surg. 2001;59:169–175. doi: 10.1053/joms.2001.20489. (discussion 75–7) [DOI] [PubMed] [Google Scholar]
- 194.Naughton GK. From lab bench to market: critical issues in tissue engineering. Ann NY Acad Sci. 2002;961:372–385. doi: 10.1111/j.1749-6632.2002.tb03127.x. [DOI] [PubMed] [Google Scholar]
- 195.Rheinwald JG, Green H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell. 1975;6:331–343. doi: 10.1016/s0092-8674(75)80001-8. [DOI] [PubMed] [Google Scholar]
- 196.Izumi K, Terashi H, Marcelo CL, Feinberg SE. Development and characterization of a tissue-engineered human oral mucosa equivalent produced in a serum-free culture system. J Dent Res. 2000;79:798–805. doi: 10.1177/00220345000790030301. [DOI] [PubMed] [Google Scholar]
- 197.Ophof R, van Rheden RE, Von den HJ, Schalkwijk J, Kuijpers-Jagtman AM. Oral keratinocytes cultured on dermal matrices form a mucosa-like tissue. Biomaterials. 2002;23:3741–3748. doi: 10.1016/s0142-9612(02)00108-4. [DOI] [PubMed] [Google Scholar]
- 198.Moharamzadeh K, Brook IM, Van Noort R, Scutt AM, Thornhill MH. Tissue-engineered oral mucosa: a review of the scientific literature. J Dent Res. 2007;86:115–124. doi: 10.1177/154405910708600203. [DOI] [PubMed] [Google Scholar]
- 199.Nakamura T, Kinoshita S. Ocular surface reconstruction using cultivated mucosal epithelial stem cells. Cornea. 2003;22:S75–S80. doi: 10.1097/00003226-200310001-00011. [DOI] [PubMed] [Google Scholar]
- 200.Purdue GF, Hunt JL, Still JM, Jr, Law EJ, Herndon DN, Goldfarb IW, et al. A multicenter clinical trial of a biosynthetic skin replacement, Dermagraft-TC, compared with cryopreserved human cadaver skin for temporary coverage of excised burn wounds. J Burn Care Rehabil. 1997;18:52–57. doi: 10.1097/00004630-199701000-00009. [DOI] [PubMed] [Google Scholar]
- 201.Eaglstein WH, Iriondo M, Laszlo K. A composite skin substitute (graftskin) for surgical wounds. A clinical experience. Dermatol Surg. 1995;21:839–843. doi: 10.1111/j.1524-4725.1995.tb00709.x. [DOI] [PubMed] [Google Scholar]
- 202.Raguse JD, Gath HJ. A metabolically active dermal replacement (Dermagraft) for vestibuloplasty. J Oral Rehabil. 2005;32:337–340. doi: 10.1111/j.1365-2842.2004.01430.x. [DOI] [PubMed] [Google Scholar]
- 203.Xie HT, Wang GJ, Sun JG, Tucker I, Zhao XC, Xie YY, et al. High performance liquid chromatographic-mass spectrometric determination of ginsenoside Rg3 and its metabolites in rat plasma using solid-phase extraction for pharmacokinetic studies. J Chromatogr. 2005;818:167–173. doi: 10.1016/j.jchromb.2004.12.028. [DOI] [PubMed] [Google Scholar]
- 204.Vag J, Byrne EM, Hughes DH, Hoffman M, Ambudkar I, Maguire P, et al. Morphological and functional differentiation of HSG cells: role of extracellular matrix and trpc 1. J Cell Physiol. 2007;212:416–423. doi: 10.1002/jcp.21035. [DOI] [PubMed] [Google Scholar]
- 205.Aframian DJ, Cukierman E, Nikolovski J, Mooney DJ, Yamada KM, Baum BJ. The growth and morphological behavior of salivary epithelial cells on matrix protein-coated biodegradable substrata. Tissue Eng. 2000;6:209–216. doi: 10.1089/10763270050044380. [DOI] [PubMed] [Google Scholar]
- 206.Hoffman MP, Kibbey MC, Letterio JJ, Kleinman HK. Role of laminin-1 and TGF-beta 3 in acinar differentiation of a human submandibular gland cell line (HSG) J Cell Sci. 1996;109:2013–2021. doi: 10.1242/jcs.109.8.2013. [DOI] [PubMed] [Google Scholar]
- 207.Aframian DJ, Tran SD, Cukierman E, Yamada KM, Baum BJ. Absence of tight junction formation in an allogeneic graft cell line used for developing an engineered artificial salivary gland. Tissue Eng. 2002;8:871–878. doi: 10.1089/10763270260424231. [DOI] [PubMed] [Google Scholar]
- 208.Aframian DJ, David R, Ben-Bassat H, Shai E, Deutsch D, Baum BJ, et al. Characterization of murine autologous salivary gland graft cells: a model for use with an artificial salivary gland. Tissue Eng. 2004;10:914–920. doi: 10.1089/1076327041348518. [DOI] [PubMed] [Google Scholar]
- 209.Tran SD, Sugito T, Dipasquale G, Cotrim AP, Bandyopadhyay BC, Riddle K, et al. Re-engineering primary epithelial cells from rhesus monkey parotid glands for use in developing an artificial salivary gland. Tissue Eng. 2006;12:2939–2948. doi: 10.1089/ten.2006.12.2939. [DOI] [PubMed] [Google Scholar]
- 210.David R, Shai E, Aframian DJ, Palmon A. Isolation and cultivation of integrin alpha6beta1-expressing salivary gland graft cells: a model for use with an artificial salivary gland. Tissue Eng. 2007 doi: 10.1089/tea.2007.0122. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 211.Sato A, Okumura K, Matsumoto S, Hattori K, Hattori S, Shinohara M, et al. Isolation, tissue localization, and cellular characterization of progenitors derived from adult human salivary glands. Cloning Stem Cells. 2007;9:191–205. doi: 10.1089/clo.2006.0054. [DOI] [PubMed] [Google Scholar]
- 212.Lin CY, Lee BS, Liao CC, Cheng WJ, Chang FM, Chen MH. Transdifferentiation of bone marrow stem cells into acinar cells using a double chamber system. J Formos Med Assoc. 2007;106:1–7. doi: 10.1016/S0929-6646(09)60209-6. [DOI] [PubMed] [Google Scholar]
- 213.Sun T, Zhu J, Yang X, Wang S. Growth of miniature pig parotid cellsonbiomaterials in vitro. ArchOralBiol. 2006;51:351–358. doi: 10.1016/j.archoralbio.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 214.Yang TL, Young TH. J Cell Mol Med. 2008. Chitosan cooperates with mesenchyme-derived factors in regulating salivary gland epithelial morphogenesis; p. 18627424. PMID. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Joraku A, Sullivan CA, Yoo J, Atala A. In-vitro reconstitution of three-dimensional human salivary gland tissue structures. Differentiation. 2007;75:318–324. doi: 10.1111/j.1432-0436.2006.00138.x. [DOI] [PubMed] [Google Scholar]
- 216.Aframian DJ, Redman RS, Yamano S, Nikolovski J, Cukierman E, Yamada KM, et al. Tissue compatibility of two biodegradable tubular scaffolds implanted adjacent to skin or buccal mucosa in mice. Tissue Eng. 2002;8:649–659. doi: 10.1089/107632702760240562. [DOI] [PubMed] [Google Scholar]
- 217.Melvin JE, Yule D, Shuttleworth T, Begenisich T. Regulation of fluid and electrolyte secretion in salivary gland acinar cells. Annu Rev Physiol. 2005;67:445–469. doi: 10.1146/annurev.physiol.67.041703.084745. [DOI] [PubMed] [Google Scholar]
- 218.Tran SD, Wang J, Bandyopadhyay BC, Redman RS, Dutra A, Pak E, et al. Primary culture of polarized human salivary epithelial cells for use in developing an artificial salivary gland. Tissue Eng. 2005;11:172–181. doi: 10.1089/ten.2005.11.172. [DOI] [PubMed] [Google Scholar]
- 219.Baum BJ, Zheng C, Cotrim AP, Goldsmith CM, Atkinson JC, Brahim JS, et al. Transfer of the AQP1 cDNA for the correction of radiation-induced salivary hypofunction. Biochim Biophys Acta. 2006;1758:1071–1077. doi: 10.1016/j.bbamem.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 220.Brosky ME. The role of saliva in oral health: strategies for prevention and management of xerostomia. J Support Oncol. 2007;5:215–225. [PubMed] [Google Scholar]
- 221.Tanaka Isomura E, Yoshitomi K, Hamaguchi M, Yamamoto YE, Kogo M. Transplantation of vascularized submandibular gland in dogs. J Oral Maxillofac Surg. 2006;64:1561–1565. doi: 10.1016/j.joms.2005.10.040. [DOI] [PubMed] [Google Scholar]
- 222.Seikaly H, Jha N, Harris JR, Barnaby P, Liu R, Williams D, et al. Long-term outcomes of submandibular gland transfer for prevention of postradiation xerostomia. Arch Otolaryngol Head Neck Surg. 2004;130:956–961. doi: 10.1001/archotol.130.8.956. [DOI] [PubMed] [Google Scholar]
- 223.Jiang X, Iseki S, Maxson RE, Sucov HM, Morriss-Kay GM. Tissue origins and interactions in the mammalian skull vault. Dev Biol. 2002;241:106–116. doi: 10.1006/dbio.2001.0487. [DOI] [PubMed] [Google Scholar]
- 224.Uddstromer L, Ritsila V. Healing of membranous and long bone defects. An experimental study in growing rabbits. Scand J Plast Reconstr Surg. 1979;13:281–287. doi: 10.3109/02844317909013071. [DOI] [PubMed] [Google Scholar]
- 225.Barry FP, Murphy JM. Mesenchymal stem cells: clinical applications and biological characterization. Int J Biochem Cell Biol. 2004;36:568–584. doi: 10.1016/j.biocel.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 226.Allen MR, Hock JM, Burr DB. Periosteum: biology, regulation, and response to osteoporosis therapies. Bone. 2004;35:1003–1012. doi: 10.1016/j.bone.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 227.Hollinger JO, Winn SR. Tissue engineering of bone in the craniofacial complex. Ann NY Acad Sci. 1999;875:379–385. doi: 10.1111/j.1749-6632.1999.tb08520.x. [DOI] [PubMed] [Google Scholar]
- 228.Alsberg E, Hill EE, Mooney DJ. Craniofacial tissue engineering. Crit Rev Oral Biol Med. 2001;12:64–75. doi: 10.1177/10454411010120010501. [DOI] [PubMed] [Google Scholar]
- 229.Krebsbach PH, Kuznetsov SA, Bianco P, Robey PG. Bone marrow stromal cells: characterization and clinical application. Crit Rev Oral Biol Med. 1999;10:165–181. doi: 10.1177/10454411990100020401. [DOI] [PubMed] [Google Scholar]