Abstract
Activation of apoptosis is one of the most ancient mechanisms to eliminate intracellular infections; the capacity to subvert this programmed cell death provides an adaptive advantage to pathogens that persist in an intracellular environment. Leishmania species are obligate intracellular parasites that primarily reside within host macrophages. We demonstrate here that Leishmania infection protects macrophages from cycloheximide induced apoptosis in a species and strain specific manner. Our data further reveal that Leishmania phosphoglycans and direct contact between parasites and host cells are required for the inhibitory phenotype.
Keywords: Leishmania, apoptosis, macrophage
Introduction
Apoptosis, or programmed cell death is a mechanism that cells utilize to activate intracellular pathways for terminating themselves in response to a wide range of stimuli (Kerr, et al., 1972). Traditionally apoptosis has been described as a method of maintaining homeostasis, leading to elimination of potentially harmful cells (Raff, 1998), be it auto-reactive lymphocytes or cells with potentially cancerous alterations. More recently, apoptosis has been demonstrated to aid in clearance of viruses (Dragovich, et al., 1998) and has been shown to be induced by the bacterial pathogen, Legionella pneumophila (Lee and Esteban, 1994). However, when considering host cell infection with an intracellular pathogen, it would be to the advantage of the invading organism to subvert the apoptotic machinery, hence not destroying its niche before egression. Several different pathogens do undermine the apoptotic progression; including Chlamydia trachomatis (Xiao, et al., 2005), Escherichia coli (Sukumaran, et al., 2004), Mycobacterium tuberculosis (Park, et al., 2006), Toxoplasma gondii (Nash, et al., 1998), Plasmodium berghei (van de Sand, et al., 2005) and Leishmania spp. (Moore and Matlashewski, 1994). One potential mechanism behind this inhibition has been through the activation of several different signaling cascades. Leishmania infection stimulates the NF-κB and the PI-3 Kinase/Akt pathways (Ruhland, et al., 2007). Both of these pathways previously have been demonstrated to not only regulate inflammatory events, such as the production of cytokines, the also induce anti-apoptotic proteins, such as Bcl-XL (Chen, et al., 2000, Song, et al., 2005).
Leishmaniasis is endemic in 88 countries with approximately 12 million infected and 350 million people at risk (Alvar, et al., 2006). A variety of disease manifestations are associated with Leishmania infection, primarily dictated by the infecting species. In the old world, infection with L. major results in a cutaneous, self-healing lesion and infection with L. donovani leads to potentially fatal visceral infection. Leishmania species are transmitted by the bites of infected female Phlebotomine sand flies. During the course of feeding, sand flies deposit the highly infective metacyclic promatigote form. These parasites are quickly taken up by resident antigen presenting cells (APCs), primarily langerhans cells (dendritic cells of the skin) and macrophages.
Leishmania parasites are covered by a thick surface coat, composed primarily of lipid-containing molecules, lipophosphoglycan (LPG) and glycoinositol phospholipds (GIPLs). LPG has been attributed with many different biological functions, ranging from guarding the parasite from the degradative environment of the sand fly midgut (Sacks, et al., 2000), to protection from complement-mediated lysis (McConville, et al., 1992), to inhibition of phagosome maturation (Desjardins and Descoteaux, 1997). Interestingly, treatment of monocyte-like cell line, U-937, with LPG isolated from L. infantum inhibits actinomycin D-induced apoptosis (Lisi, et al., 2005).
LPG is polymorphic among different species and life cycle stages of Leishmania (Ilg, et al., 1992, McConville, et al., 1995, McConville, et al., 1990, Turco, et al., 1987). All Leishmania species express an LPG with a conserved region, consisting of a lipid anchor and glycan core, along with two polymorphic domains, a polysaccharide backbone and an oligosaccharide cap. LPG polymorphisms are defined by modifications made at the three position of the galactose residue on the polysaccharide backbone. L. donovani contain no branching sugars to a few glucose substitutions, depending on the strain (Turco, et al., 1987), L. mexicana contains few sugar substitutions (Ilg, et al., 1992), while L. major (McConville, et al., 1990) and L. tropica (McConville, et al., 1995) contain a variety of sugar substitutions of various lengths and compositions. The repeating phosphoglycan (PG) characteristic of LPG also is found in other molecules of the Leishmania glycocalyx including proteophosphoglycans (Ilg, et al., 1994, Ilg, et al., 1996, Ilg, et al., 1994), secreted PG and acid phosphatase (Shakarian and Dwyer, 2000).
As LPG plays an important role in protecting Leishmania from the host’s immune response, LPG structural polymorphisms could explain some noted differences in host response to different Leishmania species. LPG is implicated in modulating host cell signaling, resulting in a lack of interleukin-12 (IL-12) synthesis in murine macrophages (Descoteaux, et al., 1992, Descoteaux, et al., 1991, Proudfoot, et al., 1996); LPG also has been shown to induce IL-12 in the same cells (de Veer, et al., 2003), a paradox possibly attributed to species and strain differences in LPG structure. In addition, LPG is implicated in modulating CD40L-dependent IL-12 synthesis in human dendritic cells, with many L. major subspecies eliciting bioactive IL-12 upon infection, whereas L. donovani fails to induce IL-12 production (McDowell, et al., 2002). It is intriguing to speculate that polymorphisms in LPG structure, and thus possible function, could contribute to some of the noted variations in disease manifestation caused by different Leishmania species.
Here we investigated the impact of Leishmania infection on murine macrophage apoptosis. We demonstrate that not only does Leishmania infection protect murine macrophages from cycloheximide induced apoptosis, it does so in a species and strain specific manner. Some Leishmania strains completely abrogate cycloheximide-induced apoptosis and complete apoptosis inhibition requires PGs.
Materials and Methods
Mammalian Cells
RAW 264.7 macrophage cell line was grown in RPMI-1640 media supplemented with 2mM L-glutamine, 100μg/mL Penicillin/Streptomycin, and 10% fetal bovine serum, with passage every two days.
Parasites and Infection
Leishmania major strains
NIH Friedlin V1 Strain (MHOM/IL/80/FN) and V1 LPG mutant, Spock (kind gift of David Sacks, National Institutes of Health), IR173 (MHOM/IR/-173), LV39 (MRHO/SU/59/P), and NIH S strain (MHOM/SN/74/Seidman). Leishmania donovani strains: 9515 (MHOM/IN/95/9515), 1S (MHOM/SD/62/1S), L. donovani LPG mutants R2D2 and C3PO (mutants of L. donovani 1S) (the kind gifts of Salvatore Turco, University of Kentucky), Mongi (MHOM/IN/83/Mongi-142) and Leishmania tropica KK27 (MHOM/AF/88/KK27), were cultured in M199 supplemented with 20% FBS, 1μg/ml Penicillin/Streptomycin, 2mM L-glutamine and incubated at 26°C. Infectious, stationary phase metacyclics were used for all infections. Metacyclic promastigotes were isolated via Ficoll (ICN, Aurora, OH) gradients (Spath and Beverley, 2001) and were complement opsonized with 5% murine serum before infecting at a concentration of 10 parasites:1 macrophage. At 16h post-infection, cells were harvested and approximately 1×104 cells were used for cytospins to determine infection rates. After adherence to slides, cells were methanol fixed, stained with Diff-Quick (Biochemical Sciences Inc., Swedesboro, NJ), and visualized with light microscopy. Heat killed parasites were heated for 1 hour at 65°C, and were monitored for lack of motility prior to infection. Paraformaldehyde fixed parasites were fixed in 2% paraformaldehyde for 30 minutes on ice, and subsequently washed 5 times in 0.1% BSA in PBS. Parasites were counted and used for infection. Transwell experiments were carried out using 0.4 μM pore size cell culture inserts (BD Falcon, San Jose, CA) with 2.5×106 macrophages per mL.
Apoptosis Assays
RAW 264.7 were fixed with 2% paraformaldehyde, washed in PBS and left for 24h at −20°C in 70% ethanol. Cells were stained using the Apo BrdU Kit (BD Pharmingen San Jose, CA) according to manufacturer’s instructions. Following ethanol permeabilization, cells were washed and incubated with terminal deoxynucleotidyl transferase enzyme in the presence of BrdU and subsequently stained with a FITC-conjugated anti-BrdU antibody, and incubated in a propidium iodide/RNase solution. Cells were analyzed on an MPL500 flow cytometer (Beckman Coulter, Fullerton, CA).
Western Blot Analysis
RAW 264.7 cells were treated for 20 min with ice-cold lysis buffer (150 mM NaCl, 1 mM PMSF, 1 μg/ml aprotinin, 1 μg/ml leupeptin, 1 μg/ml pepstatin, 1 mM pervanadate, 1 mM EDTA, 1% Igepal, 0.25% deoxycholic acid, 1 mM NaF, and 50 mM Tris-HCl (pH 7.4)). Lystates were collected and stored at −80°C until use. Samples were loaded according to cell equivalents (4×105 = ~100μg of protein as determined by Bradford Assay), separated by SDS-PAGE gels, and transferred onto polyvinylidene difluoride membrane (Millipore, Billerica, MA). Membranes were blocked in TBST (Tris-buffered saline with 0.1% Tween 20) supplemented with 5% powdered milk and then incubated with a 1:500 dilution of primary antibodies against Bcl-XL (Cell Signaling, Danvers, MA) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (Biogenesis, Poole, England), followed by 1:2500 dilution of either HRP-conjugated anti-mouse or anti-rabbit Ig (BD Biosciences San Jose, CA). Bound antibodies were detected using SuperSignal West Pico and Fempto ECL reagents (Pierce, Rockland, IL) followed by exposure to X-ray film. Relative band intensities were determined using Adobe Photoshop CS3 (Adobe, San Jose, CA); for each experiment Bcl-XL bands were first normalized to GAPDH and then expressed as a fold change over the uninfected sample.
Statistical Analysis
A Student’s t test was used for comparisons of % apoptosis and infection rates. One-way ANOVA followed by Tukey’s multiple comparison test was utilized to etect differences of Bcl-XL expression. In all cases, a P value of less than 0.05 was considered statistically significant.
Results and Discussion
Leishmania major V1 inhibits cycloheximide-induced apoptosis
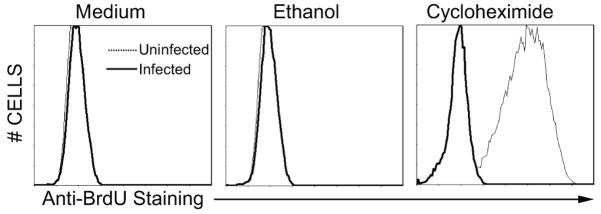
Leishmania infection has previously been demonstrated to inhibit apoptosis in mammalian cells induced with a variety of compounds, including actinomycin D (Lisi, et al., 2005, Ruhland, et al., 2007), campothecin (Ruhland, et al., 2007), and staurosporine (Akarid, et al., 2004), all inducers of the intrinsic apoptotic pathway. Here, we utilized the potent intrinsic apoptosis inducer cycloheximide (CHX); this protein synthesis inhibitor functions by halting translational elongation (Martin, et al., 1995). To assess the impact of L. major V1 infection on CHX-induced apoptosis, RAW 264.7 macrophages were infected with L. major V1 4 hours prior to treatment with ethanol (ETOH) alone (CHX carrier) or CHX dissolved in ethanol and allowed to incubate for an additional 16 hours. Apoptosis was assessed using flow cytometric based BdrU incorporation and staining (Li, et al., 1995). Numbers of apoptosis positive cells were comparable in media and ETOH treated cells in both uninfected and L. major infected macrophages (Fig. 1). Uninfected, CHX treated cells exhibit drastically more apoptosis; however, L. major-infected CHX-treated macrophages exhibit virtually no apoptosis as do non-CHX treated cells (Fig. 1), demonstrating that L. major V1 infection inhibits CHX-induced apoptosis.
Figure 1.
L. major infection of RAW 264.7 macrophages inhibits CHX-induced apoptosis. RAW 264.7 macrophages were infected with L. major V1 4 hrs prior to ETOH (carrier control), CHX, or no additional (Medium) treatment for 16 hrs. Apoptosis was determined using a flow cytometry based BrdU staining. Uninfected (---) and L. major V1 infected (—) infected histograms were overlaid using CXP software (Beckman Coulter). One of 3 independent experiments is presented.
Leishmania induced apoptosis is both species and strain dependent
Previous studies demonstrate that Leishmania species are able to inhibit apoptosis stimulated by a variety of compounds (Akarid, et al., 2004, Moore and Matlashewski, 1994, Ruhland, et al., 2007). Although different methods of detecting apoptosis including detection of DNA fragmentation (Moore and Matlashewski, 1994), Caspase-3 assays (Ruhland, et al., 2007), and assessing mitochodria ion potential (Akarid, et al., 2004) have been utilized, none have directly compared the ability of different species and strains to inhibit apoptosis. In addition to different Leishmania species-specific disease manifestations, intra-species (or strain) variations also can induce different clinical states; for example some L. major strains cause severe disease, rather than spontaneously resolving lesions (Neva, et al., 1979).
To assess the strain specificity of L. major-infection induced inhibition of apoptosis, a total of five L. major strains were utilized: V1, Spock, IR173, LV39, and NIH S (Fig. 2a). Three strains, V1, Spock, and IR173 completely inhibit apoptosis; however, infection of macrophages with the other two strains (LV39 and NIH S) significantly reduced apoptotic staining compared to uninfected CHX-treated macrophages, but did so to a much more limited extent (Fig 2a). The strains that completely inhibited apoptosis (V1, Spock, and IR173) upon infection of macrophages resulted in positive apoptotic staining in approximately 1% of the population, whereas infection with the other three strains (LV39 and NIH S) resulted in approximately 75% of the macrophage population staining positive for apoptosis.
Figure 2.
Inhibition of apoptosis is species and strain dependent. RAW 264.7 macrophages were infected with L. major strains (V1, Spock, IR173, LV39, and NIH S) (a) or L. donovani strains (1S, 9515, and Mongi) (c) 4 hrs prior to ETOH (■), CHX ( ), or no additional (□) treatment for 16 hrs. Apoptosis was determined using a flow cytometry based BrdU staining. Mean ± SEM of % apoptosis from 3 independent experiments is presented. *p ≤ 0.05 compared to uninfected CHX treatment unless noted. (b & d) Infections were quantified to ensure equal parasite infection and presented as number of parasites per 100 cells. No statistically significant differences of infection rates were detected.
), or no additional (□) treatment for 16 hrs. Apoptosis was determined using a flow cytometry based BrdU staining. Mean ± SEM of % apoptosis from 3 independent experiments is presented. *p ≤ 0.05 compared to uninfected CHX treatment unless noted. (b & d) Infections were quantified to ensure equal parasite infection and presented as number of parasites per 100 cells. No statistically significant differences of infection rates were detected.
Interestingly, these same strains have been shown to group in a similar fashion in different model systems. In a human monocyte derived dendritic cells, L. major V1 and IR173 induce CD40L-dependent IL-12p70, while neither LV39 and NIH S induce IL-12p70 secretion (McDowell, et al., 2002). Furthermore, infection of IL-4 deficient Balb/c mice with L. major V1 and IR173 leads to healing, however infection of this mouse strain with L. major LV39 results in a more severe, non-healing disease, similar to wild-type Balb/c mice (Noben-Trauth, et al., 2003, Noben-Trauth, et al., 1999). Clearly, these L. major strains utilize different evasion mechanisms to subvert host immune responses of both human and murine origin.
L. donovani subspecies also display a strain-dependent inhibition of CHX-induced apoptosis phenotype (Fig. 2b). L. donovani strain 1S completely inhibits CHX-induced apoptosis in RAW 264.7 macrophages, while two other strains, 9515 and Mongi, significantly reduce apoptosis, but to a far lesser degree. Similar to L. major strains, parasites that result in nearly complete apoptosis inhibition (1S) stain for a much lower incidence of apoptosis (1%) compared to 9515 and Mongi (70%). Interestingly, infection of macrophages with L. tropica, results in no significant inhibition of CHX-mediated apoptosis (data not shown). No significant differences in infection rates were detected (Fig. 2b and d).
L. donovani 1S, originally isolated from a patient with visceral lieshmaniasis in the Sudan differs in geographic locale compared to 9515 and Mongi, which were both isolated from visceral leishmaniasis patients in India. Interestingly, the LPG from Mongi and 1S differs (the LPG structure from 9515 has yet to be resolved); 1S displays an LPG backbone with no branching sugars attached (Thomas, et al., 1992), however Mongi has 1–2 glucose substitutions every 4–5 backbone repeat units (Mahoney, et al., 1999). Although L. major Spock also lacks branching carbohydrate substitutions (Butcher, et al., 1996) and completely inhibits apoptosis, this phenotype can not fully explain the absolute inhibition, as L. major NIH S also lacks additional sugar residues (Sacks and da Silva, 1987) and only inhibits apoptosis by 25%. Furthermore, L. major V1 also completely abrogates CHX-induced apoptosis and the PGs of this strain are decorated with galactose residues terminating in arabinose (McConville, et al., 1990). Nonetheless, these intra-species LPG or other PG polymorphisms potentially may contribute to differences in apoptosis inhibition.
Leishmania induced apoptosis is dependent on phosphoglycans
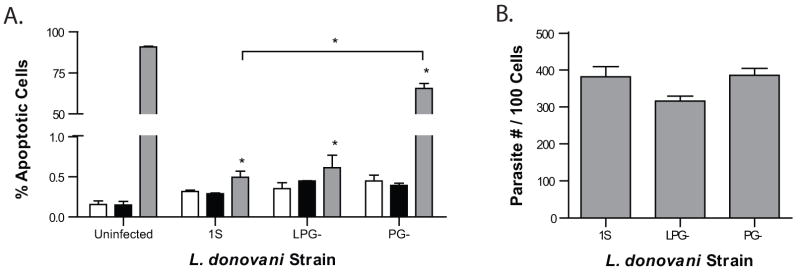
To address the potential role of LPG and total PGs in Leishmania mediated apoptosis inhibition, two mutants of L. donovani 1S were utilized; LPG-parasites (strain R2D2) lack only LPG and PG- parasites (strain C3PO) lack all phosphoglycans (King and Turco, 1988). L. donovani LPG- infection of macrophages results in nearly complete inhibition of CHX-mediated apoptosis, similar to wild type L. donovani 1S (Fig. 3a). Interestingly, infection of macrophages with L. donovani PG- results in significant inhibition of apoptosis (66%), however not nearly to the same extent as L. donovani 1S and LPG-(0.45% and 0.66%, respectively) (Fig. 3a). Once again, infection rates did not differ between infection groups (Fig. 3b).
Figure 3.
Non-LPG PGs are vital for inhibition of CHX-induced apoptosis. (a) RAW 264.7 macrophages were infected with L. donovani strains (1S, LPG-, and PG-) 4 hrs prior to ETOH (■), CHX ( ), or no additional (□) treatment for 16 hrs. Apoptosis was determined using a flow cytometry based BrdU staining. Mean ± SEM of % apoptosis from 3 independent experiments is presented. *p ≤ 0.05 compared to uninfected CHX treatment unless noted. (b) Infections were quantified to ensure equal parasite infection and presented as number of parasites per 100 cells. No statistically significant differences of infection rates were detected.
), or no additional (□) treatment for 16 hrs. Apoptosis was determined using a flow cytometry based BrdU staining. Mean ± SEM of % apoptosis from 3 independent experiments is presented. *p ≤ 0.05 compared to uninfected CHX treatment unless noted. (b) Infections were quantified to ensure equal parasite infection and presented as number of parasites per 100 cells. No statistically significant differences of infection rates were detected.
While much of the current focus on virulence factors of Leishmania has centered on LPG, few studies have illustrated the importance of total PGs during Leishmania infection. Some of these studies have used the equivalent of LPG-and PG- parasites in L. major. In vivo, both Balb/c and SCID mice infected with PG- L. major (lpg2-) fail to develop any disease, however, infection with LPG- L. major (lpg1-) results in severe disease manifestation, similar to infection with wild type L. major (Spath, et al., 2003, Uzonna, et al., 2004). Similarly, using the same L. donovani PG and LPG deficient parasites utilized here, infection with wild type L. donovani and LPG- parasites results in higher nitric oxide production relative to PG- parasites in siRNA MyD88-depleted, interferon-primed, RAW 264.7 macrophages (Flandin, et al., 2006). Likely, PGs also play a vital role in establishing Leishmania and preventing CHX-mediated apoptosis.
Leishmania induces Bcl XL protein expression
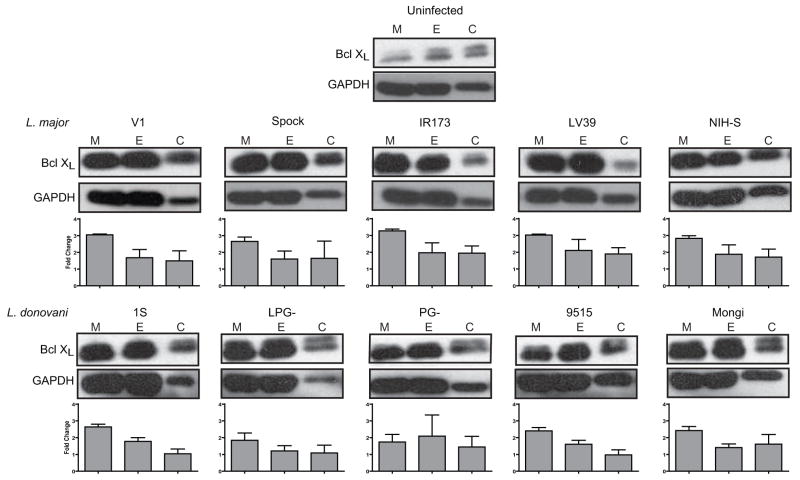
L. major infection is able to circumvent the apoptotic machinery in macrophages induced by both staurosporine treatment and MCSF deprivation by preventing Cytochrome C (Cyt C) release from the mitochondria (Akarid, et al., 2004). Several inhibitors of Cyt C release, including Bcl-2 and Bcl-XL are transcribed as a result of NF-κB activation (Chen, et al., 2000, Song, et al., 2005), and NF-κB is activated as a result of Leishmania infection (Ruhland, et al., 2007). To address the potential that species- and strain-dependent differences in inhibition of CHX-mediated apoptosis could be mediated by Bcl XL, infections with all of the strains and species detailed in Figure 2 were repeated and protein levels of Bcl XL were assessed. Infection of macrophages with all the Leishmania strains tested (Fig. 4) results in up regulation of Bcl XL in untreated macrophages. As expected CHX treatment lowered overall protein levels in all cells as determined by GAPDH expression. Unexpectedly, the Bcl-XL/GAPDH expression ratios were lower in the EtOH and CHX treated groups compared to the medium control for most groups.
Figure 4.
Leishmania infection induces Bcl-XL protein expression, but is not the factor contributing to strain dependent inhibition of apoptosis. RAW 264.7 macrophages were infected with L. major strains (V1, Spock, IR173, LV39, and NIH S) and L. donovani strains (1S, LPG-, PG-, 9515, and Mongi) 4 hrs prior to ETOH (E), CHX (C), or no additional (M) treatment for 16 hrs. Lysates were separated via SDS-PAGE by loading 4×105 cell equivalents and assessed by western blotting with anti-Bcl-XL and GAPDH antibodies. Blots from 1 of 3 independent experiments are presented. Relative band intensities were determined for each experiment, normalized to GAPDH, and expressed as fold change over the uninfected sample. Mean fold change ± SEM is presented.
While Bcl-XL is strongly up-regulated at the protein level in response to Leishmania infection, it does not explain the dependence on strain specificity (Fig. 4). No significant difference in Bcl-XL expression between any of the infections in response to EtOH (carrier control) or CHX were detected. While there are statistically significant differences between Leishmania strains in the untreated samples (L. donovani PG- and LPG- samples significantly lower than L major V1, SPOCK, IR173, LV39 and L. donvani 9515 significantly lower than L. major IR173, LV39) is there is no detectable pattern in Bcl-XL levels between strains that completely inhibit CHX-induced apoptosis (L. major V1, SPOCK, IR173, and L donovani 1S, LPG-) and strains that only reduce apoptosis levels (L. major LV39, NIH-S, and L donovani PG-, 9515, Mongi). Previous studies have demonstrated that Leishmania infection leads to Akt-dependent phosphorylation of Bad, another anti-apoptotic factor that prevents Cyt C release from the mitochondria (Ruhland, et al., 2007). Clearly, Leishmania infection induces the activation of more than one anti-apoptotic protein.
Leishmania infection induced inhibition of apoptosis requires contact between host and parasite
Inhibition of CHX-mediated apoptosis in L. major V1 infected cells is nearly total and does not significantly differ from non-apoptosis induced macrophages (Fig. 2a). While infection rates for L. major V1 are high (80%), not every cell is infected, however very few of the macrophages stain positive for apoptosis (about 1%), suggesting a possible paracrine mechanism. To test if a macrophage secreted factor was responsible for the observed apoptosis inhibition, a transwell system was utilized. Macrophages were separated into two distinct populations; one in a lower well and one in an upper insert, separated by a 0.4 μM membrane (Fig. 5). Macrophages in the lower chamber were harvested to dissect the importance of direct parasite contact with macrophages in inhibition of CHX-induced apoptosis. L. major V1 parasites added directly to the lower well resulted in little apoptosis positive staining similar to levels previously observed (Figs. 1 and 2a). However, L. major V1 infection in the insert did not result in inhibition of CHX-mediated apoptosis in macrophages in the lower well (Fig. 5), indicating that paracrine signaling is not sufficient to inhibit CHX-induced apoptosis.
Figure 5.

Inhibition of apoptosis requires direct contact between Leishmania and macrophage. RAW 264.7 macrophages were plated both in a lower chamber and a 0.4μM cell culture insert in a 6 well plate and either the macrophages in the well or insert were infected with L. major V1 4 hrs prior to ETOH (■), CHX ( ), or no additional (□) treatment for 16 hrs. Apoptosis was determined using a flow cytometry based BrdU staining. Mean ± SEM of % apoptosis from 3 independent experiments is presented. *p ≤ 0.05 compared to uninfected CHX treatment. No statistically significant differences of infection rates were detected.
), or no additional (□) treatment for 16 hrs. Apoptosis was determined using a flow cytometry based BrdU staining. Mean ± SEM of % apoptosis from 3 independent experiments is presented. *p ≤ 0.05 compared to uninfected CHX treatment. No statistically significant differences of infection rates were detected.
Metabolically active parasites are required for complete inhibition of CHX-mediated apoptosis
Leishmania products, especially LPG have powerful properties, effects that can be observed in the absence of live parasites. For example, the addition of LPG alone is able to inhibit actinomycin-D induced apoptosis in U-937 cells (Lisi, et al., 2005). In order to understand the role that parasite antigen compared to live infection might have on CHX-mediated apoptosis, both heat-killed and paraformaldehyde-fixed (PFA) L. major V1 parasites were utilized to treat macrophages before ETOH or CHX treatment. Infection with non-metabolically active L. major V1 (both heat-killed and PFA fixed) significantly inhibited CHX-mediated apoptosis, however, not nearly to the same extent as macrophage infection with live L. major (Fig. 6) (1% vs. 77% Heat killed vs. 55% PFA fixed). Interestingly, there were fewer apoptotic positive cells in the PFA fixed group compared to heat-killed condition.
Figure 6.
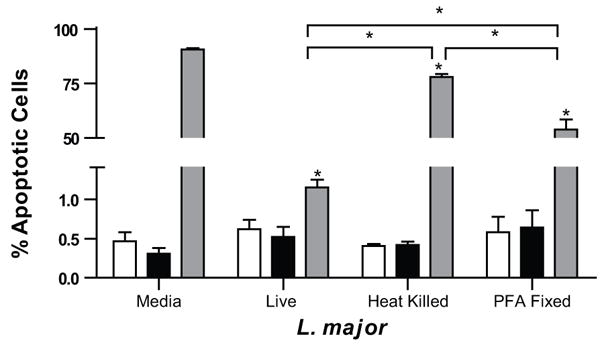
Inhibition of apoptosis requires infection with metabolically active Leishmania. RAW 264.7 macrophages were infected with one of three different conditions, live L. major V1, heat killed L. major V1 (heated at 65°C for 1 hour) or paraformaldehyde fixed L. major V1 4 hrs prior to ETOH (■), CHX ( ), or no additional (□) treatment for 16 hrs. Apoptosis was determined using a flow cytometry based BrdU staining. Mean ± SEM of % apoptosis from 3 independent experiments is presented. *p ≤ 0.05 compared to uninfected CHX treatment unless noted. No statistically significant differences of infection rates were detected.
), or no additional (□) treatment for 16 hrs. Apoptosis was determined using a flow cytometry based BrdU staining. Mean ± SEM of % apoptosis from 3 independent experiments is presented. *p ≤ 0.05 compared to uninfected CHX treatment unless noted. No statistically significant differences of infection rates were detected.
In order to fully inactivate parasites via exposure to heat (as monitored visually), an hour incubation is necessary; in this time heat labile molecules potentially necessary for efficient inhibition of apoptosis could be denatured, whereas fixation would not necessarily cause alteration of these molecules. One such molecule, LDAA-12, a L. donovani antigen, known to induce colony stimulation factors, is heat labile (Singal and Singh, 2005). Also, gp63 (leishmanolysin), another major surface glycoprotein, is heat labile; after only 15 min at raised temperatures, it loses its inhibitory effect on monocyte chemotaxis (Russell and Wilhelm, 1986).
Recently, a model has been proposed which posits that Mycobacteria-infected macrophages undergo apoptosis and the subsequent blebs are taken up by surrounding dendritic cells. This allows for cross-presentation of Mycobacteria antigen to CD8+ T cells as well as lipid presentation using CD1b (Schaible, et al., 2003). Subversion of this pathway during a Leishmania infection could potentially have a profound effect. Leishmania, like Mycobacteria, have several lipid antigens, including glycoinositol phospholipids (GIPLs) and LPG. Leishmania infection down-regulates CD1 expression in human dendritic cells (Amprey, et al., 2004, Donovan, et al., 2007). Potentially, Leishmania could be targeting both apoptosis and lipid antigen presentation to avoid immune surveillance.
It is intriguing to speculate that Leishmania mediated inhibition of CHX-induced apoptosis may be a two tiered process, with actual contact with the host cell surface being the initial step. Contact between parasites and macrophages is absolutely required for protection (Fig. 5) and even metabolically inactive parasites are able to significantly reduce apoptosis compared to uninfected macrophages treated with CHX (Fig. 6). This initial interaction likely leads to the significant reduction evident in macrophages infected with all of the L. major and L. donovani strains. The early inhibition of apoptosis is independent of either PGs or LPG, as infection of macrophages with PG- parasites (which also lack LPG) significantly reduce CHX-induced apoptosis (Fig 3a). The second tier of apoptosis inhibition is likely what arises as a result of strain specificity. This step clearly is not dependent on LPG; once promastigotes are phagocytosed they halt maturation of the endosome in an LPG-dependent manner (Desjardins and Descoteaux, 1997), and transform into amastigotes. In the amastigote form, a second, unknown as of yet, signal is likely to account for the remainder of the apoptosis inhibition. This signal could potentially be a PG (or due to a PG-host interaction) that is polymorphic among different strains.
Our report demonstrates that infection of RAW 264.7 macrophages with Leishmania species inhibits CHX-induced apoptosis. Strain dependent immune modulation (and in turn variations in inhibition of apoptosis) are supported by our data, with similar patterns of host modulation observed in other studies (McDowell, et al., 2002, Noben-Trauth, 2000, Noben-Trauth, et al., 1999). This inhibition is due in part to PGs, independent of LPG, and requires parasite contact with the macrophage. Overall, this work demonstrates yet another manner in which Leishmania is able to subvert immune surveillance in a strain-dependent manner.
Acknowledgments
We are grateful to Dr. Michelle Whaley for her support on the initiation of this project and thank undergraduate students, Rachel Colden, Kathryn Kemnetz, Jonathon Weyerbacher, Eamon Malone, and Emily Swanson for their assistance. We thank Dr. Salvatore Turco for providing us with the L. donovani mutant parasites. This work was supported by National Institutes of Health grant AI056242 and American Heart Association grant 043533Z (MAM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Akarid K, Arnoult D, Micic-Polianski J, Sif J, Estaquier J, Ameisen JC. Leishmania major-mediated prevention of programmed cell death induction in infected macrophages is associated with the repression of mitochondrial release of cytochrome c. Journal of Leukocyte Biology. 2004;76:95–103. doi: 10.1189/jlb.1001877. [DOI] [PubMed] [Google Scholar]
- 2.Alvar J, Yactayo S, Bern C. Leishmaniasis and poverty. Trends in Parasitology. 2006;22:552–557. doi: 10.1016/j.pt.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 3.Amprey JL, Spath GF, Porcelli SA. Inhibition of CD1 expression in human dendritic cells during intracellular infection with Leishmania donovani. Infection and Immunity. 2004;72:589–592. doi: 10.1128/IAI.72.1.589-592.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Butcher BA, Turco SJ, Hilty BA, Pimenta PF, Panunzio M, Sacks DL. Deficiency in beta1,3-galactosyltransferase of a Leishmania major lipophosphoglycan mutant adversely influences the Leishmania-sand fly interaction. Journal of Biological Chemistry. 1996;271:20573–20579. doi: 10.1074/jbc.271.34.20573. [DOI] [PubMed] [Google Scholar]
- 5.Chen C, Edelstein LC, Gelinas C. The Rel/NF-kappaB family directly activates expression of the apoptosis inhibitor Bcl-x(L) Molecular and Cellular Biology. 2000;20:2687–2695. doi: 10.1128/mcb.20.8.2687-2695.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Veer MJ, Curtis JM, Baldwin TM, DiDonato JA, Sexton A, McConville MJ, Handman E, Schofield L. MyD88 is essential for clearance of Leishmania major: possible role for lipophosphoglycan and Toll-like receptor 2 signaling. European Journal of Immunology. 2003;33:2822–2831. doi: 10.1002/eji.200324128. [DOI] [PubMed] [Google Scholar]
- 7.Descoteaux A, Matlashewski G, Turco SJ. Inhibition of macrophage protein kinase C-mediated protein phosphorylation by Leishmania donovani lipophosphoglycan. Journal of Immunology. 1992;149:3008–3015. [PubMed] [Google Scholar]
- 8.Descoteaux A, Turco SJ, Sacks DL, Matlashewski G. Leishmania donovani lipophosphoglycan selectively inhibits signal transduction in macrophages. Journal of Immunology. 1991;146:2747–2753. [PubMed] [Google Scholar]
- 9.Desjardins M, Descoteaux A. Inhibition of phagolysosomal biogenesis by the Leishmania lipophosphoglycan. Journal of Experimental Medicine. 1997;185:2061–2068. doi: 10.1084/jem.185.12.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Donovan MJ, Jayakumar A, McDowell MA. Inhibition of groups 1 and 2 CD1 molecules on human dendritic cells by Leishmania species. Parasite Immunology. 2007;29:515–524. doi: 10.1111/j.1365-3024.2007.00970.x. [DOI] [PubMed] [Google Scholar]
- 11.Dragovich T, Rudin CM, Thompson CB. Signal transduction pathways that regulate cell survival and cell death. Oncogene. 1998;17:3207–3213. doi: 10.1038/sj.onc.1202587. [DOI] [PubMed] [Google Scholar]
- 12.Flandin JF, Chano F, Descoteaux A. RNA interference reveals a role for TLR2 and TLR3 in the recognition of Leishmania donovani promastigotes by interferon-gamma-primed macrophages. European Journal of Immunology. 2006;36:411–420. doi: 10.1002/eji.200535079. [DOI] [PubMed] [Google Scholar]
- 13.Ilg T, Etges R, Overath P, McConville MJ, Thomas-Oates J, Thomas J, Homans SW, Ferguson MA. Structure of Leishmania mexicana lipophosphoglycan. Journal of Biological Chemistry. 1992;267:6834–6840. [PubMed] [Google Scholar]
- 14.Ilg T, Overath P, Ferguson MA, Rutherford T, Campbell DG, McConville MJ. O- and N-glycosylation of the Leishmania mexicana-secreted acid phosphatase. Characterization of a new class of phosphoserine-linked glycans. Journal of Biological Chemistry. 1994;269:24073–24081. [PubMed] [Google Scholar]
- 15.Ilg T, Stierhof YD, Craik D, Simpson R, Handman E, Bacic A. Purification and structural characterization of a filamentous, mucin-like proteophosphoglycan secreted by Leishmania parasites. Journal of Biological Chemistry. 1996;271:21583–21596. doi: 10.1074/jbc.271.35.21583. [DOI] [PubMed] [Google Scholar]
- 16.Ilg T, Stierhof YD, Wiese M, McConville MJ, Overath P. Characterization of phosphoglycan-containing secretory products of Leishmania. Parasitology. 1994;108:S63–71. doi: 10.1017/s0031182000075739. [DOI] [PubMed] [Google Scholar]
- 17.Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. British Journal of Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.King DL, Turco SJ. A ricin agglutinin-resistant clone of Leishmania donovani deficient in lipophosphoglycan. Molecular and Biochemical Parasitology. 1988;28:285–293. doi: 10.1016/0166-6851(88)90013-8. [DOI] [PubMed] [Google Scholar]
- 19.Lee SB, Esteban M. The interferon-induced double-stranded RNA-activated protein kinase induces apoptosis. Virology. 1994;199:491–496. doi: 10.1006/viro.1994.1151. [DOI] [PubMed] [Google Scholar]
- 20.Li X, Traganos F, Melamed MR, Darzykiewics Z. Single-step procedure for labeling DNA strand breaks with fluorescein- or BODIPY-conjugated deoxynucleotides: detection of apoptosis and bromodeoxyuridine incorporation. Cytometry. 1995;20:172–180. doi: 10.1002/cyto.990200210. [DOI] [PubMed] [Google Scholar]
- 21.Lisi S, Sisto M, Acquafredda A, Spinelli R, Schiavone M, Mitolo V, Brandonisio O, Panaro M. Infection with Leishmania infantum Inhibits actinomycin D-induced apoptosis of human monocytic cell line U-937. Journal of Eukaryotic Microbiology. 2005;52:211–217. doi: 10.1111/j.1550-7408.2005.00026.x. [DOI] [PubMed] [Google Scholar]
- 22.Mahoney AB, Sacks DL, Saraiva E, Modi G, Turco SJ. Intra-species and stage-specific polymorphisms in lipophosphoglycan structure control Leishmania donovani-sand fly interactions. Biochemistry. 1999;38:9813–9823. doi: 10.1021/bi990741g. [DOI] [PubMed] [Google Scholar]
- 23.Martin SJ, Reutelingsperger CP, McGahon AJ, Rader JA, van Schie RC, LaFace DM, Green DR. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. Journal of Experimental Medicine. 1995;182:1545–1556. doi: 10.1084/jem.182.5.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McConville MJ, Schnur LF, Jaffe C, Schneider P. Structure of Leishmania lipophosphoglycan: inter- and intra-specific polymorphism in Old World species. Biochemistry Journal. 1995;310:807–818. doi: 10.1042/bj3100807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McConville MJ, Thomas-Oates JE, Ferguson MA, Homans SW. Structure of the lipophosphoglycan from Leishmania major. Journal of Biological Chemistry. 1990;265:19611–19623. [PubMed] [Google Scholar]
- 26.McConville MJ, Turco SJ, Ferguson MA, Sacks DL. Developmental modification of lipophosphoglycan during the differentiation of Leishmania major promastigotes to an infectious stage. EMBO Journal. 1992;11:3593–3600. doi: 10.1002/j.1460-2075.1992.tb05443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McDowell MA, Marovich M, Lira R, Braun M, Sacks D. Leishmania priming of human dendritic cells for CD40 Ligand-induced interleukin-12p70 secretion is strain and species dependent. Infection and Immunity. 2002;70:3994–4001. doi: 10.1128/IAI.70.8.3994-4001.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moore KJ, Matlashewski G. Intracellular infection by Leishmania donovani inhibits macrophage apoptosis. Journal of Immunology. 1994;152:2930–2937. [PubMed] [Google Scholar]
- 29.Nash PB, Purner MB, Leon RP, Clarke P, Duke RC, Curiel TJ. Toxoplasma gondii-infected cells are resistant to multiple inducers of apoptosis. Journal of Immunology. 1998;160:1824–1830. [PubMed] [Google Scholar]
- 30.Neva FA, Wyler D, Nash T. Cutaneous leishmaniasis--a case with persistent organisms after treatment in presence of normal immune response. American Journal of Tropical Medicine and Hygiene. 1979;28:467–471. [PubMed] [Google Scholar]
- 31.Noben-Trauth N. Susceptibility to Leishmania major infection in the absence of IL-4. Immunology Letters. 2000;75:41–44. doi: 10.1016/s0165-2478(00)00280-7. [DOI] [PubMed] [Google Scholar]
- 32.Noben-Trauth N, Lira R, Nagase H, Paul WE, Sacks DL. The relative contribution of IL-4 receptor signaling and IL-10 to susceptibility to Leishmania major. Journal of Immunology. 2003;170:5152–5158. doi: 10.4049/jimmunol.170.10.5152. [DOI] [PubMed] [Google Scholar]
- 33.Noben-Trauth N, Paul WE, Sacks DL. IL-4- and IL-4 receptor-deficient BALB/c mice reveal differences in susceptibility to Leishmania major parasite substrains. Journal of Immunology. 1999;162:6132–6140. [PubMed] [Google Scholar]
- 34.Noben-Trauth N, Paul WE, Sacks DL. IL-4- and IL-4 receptor-deficient BALB/c mice reveal differences in susceptibility to Leishmania major parasite substrains. Journal of Immunology. 1999;162:6132–6140. [PubMed] [Google Scholar]
- 35.Park JS, Tamayo MH, Gonzalez-Juarrero M, Orme IM, Ordway DJ. Virulent clinical isolates of Mycobacterium tuberculosis grow rapidly and induce cellular necrosis but minimal apoptosis in murine macrophages. Journal of Leukocyte Biology. 2006;79:80–86. doi: 10.1189/jlb.0505250. [DOI] [PubMed] [Google Scholar]
- 36.Proudfoot L, Nikolaev AV, Feng GJ, Wei WQ, Ferguson MA, Brimacombe JS, Liew FY. Regulation of the expression of nitric oxide synthase and leishmanicidal activity by glycoconjugates of Leishmania lipophosphoglycan in murine macrophages. Proceedings of the National Academy of Science U S A. 1996;93:10984–10989. doi: 10.1073/pnas.93.20.10984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Raff M. Cell suicide for beginners. Nature. 1998;396:119–122. doi: 10.1038/24055. [DOI] [PubMed] [Google Scholar]
- 38.Ruhland A, Leal N, Kima PE. Leishmania promastigotes activate PI3K/Akt signalling to confer host cell resistance to apoptosis. Cellular Microbiology. 2007;9:84–96. doi: 10.1111/j.1462-5822.2006.00769.x. [DOI] [PubMed] [Google Scholar]
- 39.Russell DG, Wilhelm H. The involvement of the major surface glycoprotein (gp63) of Leishmania promastigotes in attachment to macrophages. Journal of Immunology. 1986;136:2613–2620. [PubMed] [Google Scholar]
- 40.Sacks DL, da Silva RP. The generation of infective stage Leishmania major promastigotes is associated with the cell-surface expression and release of a developmentally regulated glycolipid. Journal of Immunology. 1987;139:3099–3106. [PubMed] [Google Scholar]
- 41.Sacks DL, Modi G, Rowton E, Spath G, Epstein L, Turco SJ, Beverley SM. The role of phosphoglycans in Leishmania-sand fly interactions. Proceedings of the National Academy of Science U S A. 2000;97:406–411. doi: 10.1073/pnas.97.1.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schaible UE, Winau F, Sieling PA, Fischer K, Collins HL, Hagens K, Modlin RL, Brinkmann V, Kaufmann SH. Apoptosis facilitates antigen presentation to T lymphocytes through MHC-I and CD1 in tuberculosis. Nature Medicine. 2003;9:1039–1046. doi: 10.1038/nm906. [DOI] [PubMed] [Google Scholar]
- 43.Shakarian AM, Dwyer DM. Structurally conserved soluble acid phosphatases are synthesized and released by Leishmania major promastigotes. Experimental Parasitology. 2000;95:79–84. doi: 10.1006/expr.2000.4511. [DOI] [PubMed] [Google Scholar]
- 44.Singal P, Singh PP. Leishmania donovani amastigote components-induced colony-stimulating factors production. Parasitology International. 2005;54:9–20. doi: 10.1016/j.parint.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 45.Song G, Ouyang G, Bao S. The activation of Akt/PKB signaling pathway and cell survival. Journal of Cellular and Molecular Medicine. 2005;9:59–71. doi: 10.1111/j.1582-4934.2005.tb00337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Spath GF, Beverley SM. A lipophosphoglycan-independent method for isolation of infective Leishmania metacyclic promastigotes by density gradient centrifugation. Experimental Parasitology. 2001;99:97–103. doi: 10.1006/expr.2001.4656. [DOI] [PubMed] [Google Scholar]
- 47.Spath GF, Lye LF, Segawa H, Sacks DL, Turco SJ, Beverley SM. Persistence without pathology in phosphoglycan-deficient Leishmania major. Science. 2003;301:1241–1243. doi: 10.1126/science.1087499. [DOI] [PubMed] [Google Scholar]
- 48.Sukumaran SK, Selvaraj SK, Prasadarao NV. Inhibition of apoptosis by Escherichia coli K1 is accompanied by increased expression of BclXL and blockade of mitochondrial cytochrome c release in macrophages. Infection and Immunity. 2004;72:6012–6022. doi: 10.1128/IAI.72.10.6012-6022.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thomas JR, McConville MJ, Thomas-Oates JE, Homans SW, Ferguson MA, Gorin PA, Greis KD, Turco SJ. Refined structure of the lipophosphoglycan of Leishmania donovani. Journal of Biological Chemistry. 1992;267:6829–6833. [PubMed] [Google Scholar]
- 50.Turco SJ, Hull SR, Orlandi PA, Jr, Shepherd SD, Homans SW, Dwek RA, Rademacher TW. Structure of the major carbohydrate fragment of the Leishmania donovani lipophosphoglycan. Biochemistry. 1987;26:6233–6238. doi: 10.1021/bi00393a042. [DOI] [PubMed] [Google Scholar]
- 51.Uzonna JE, Spath GF, Beverley SM, Scott P. Vaccination with phosphoglycan-deficient Leishmania major protects highly susceptible mice from virulent challenge without inducing a strong Th1 response. Journal of Immunology. 2004;172:3793–3797. doi: 10.4049/jimmunol.172.6.3793. [DOI] [PubMed] [Google Scholar]
- 52.van de Sand C, Horstmann S, Schmidt A, Sturm A, Bolte S, Krueger A, Lutgehetmann M, Pollok JM, Libert C, Heussler VT. The liver stage of Plasmodium berghei inhibits host cell apoptosis. Molecular Microbiology. 2005;58:731–742. doi: 10.1111/j.1365-2958.2005.04888.x. [DOI] [PubMed] [Google Scholar]
- 53.Xiao Y, Zhong Y, Su H, Zhou Z, Chiao P, Zhong G. NF-kappa B activation is not required for Chlamydia trachomatis inhibition of host epithelial cell apoptosis. Journal of Immunology. 2005;174:1701–1708. doi: 10.4049/jimmunol.174.3.1701. [DOI] [PubMed] [Google Scholar]