Abstract
Therapeutic vaccines for B-cell non-Hodgkin lymphoma using the clonal tumor immunoglobulin idiotype, have been under development for more than three decades. A major obstacle for rapid progress in the field has been that the idiotype vaccine is patient-specific and required the generation of a custom-made product. The manufacturing issues were recently overcome by advances in hybridoma and recombinant DNA technology and facilitated the completion of several Phase I and II clinical trials. The strong immunogenicity and apparent clinical benefit observed on the early phase studies led to the initiation of three randomized Phase III clinical trials that are also nearing completion. This review will focus on the development of idiotype vaccines before and after the introduction of rituximab for the treatment of B-cell non-Hodgkin lymphomas and also discuss potential strategies to enhance the efficacy of active immunotherapy in the future.
Keywords: Lymphoma, vaccine, idiotype, immunotherapy, non-Hodgkin lymphoma
Introduction
Evidence in the literature suggests that non-Hodgkin lymphomas of B-cell origin may be especially sensitive to immunotherapy. First, spontaneous remissions lasting longer than 1 year have been observed in up to 23% of patients with follicular lymphoma (Horning, et al 1984). Second, the survival of patients with follicular lymphoma appeared to correlate with the gene expression signatures of infiltrating nonmalignant immune cells in the tumor (Dave, et al 2004). Third, graft versus lymphoma effect has been demonstrated in a number of different lymphomas following allogeneic stem cell transplantation (Thomson, et al 2006). Lastly, administration of rituximab, an anti-CD20 monoclonal antibody, either as a single agent or in combination with chemotherapy, results in clinical remission in a significant proportion of patients with B-cell non-Hodgkin lymphoma (Colombat, et al 2001, Witzig, et al 2005). As opposed to passive immunotherapy with monoclonal antibodies such as rituximab, active immunotherapy with a therapeutic vaccine may induce an antitumor antibody response, as well as anti-tumor CD4+ and CD8+ T-cell responses. In addition, immune responses induced by a vaccine are likely to be polyclonal, directed against multiple epitopes of a candidate tumor antigen, and have immunological memory. These advantages of active immunotherapy over monoclonal antibodies support the development of therapeutic vaccination strategies for the treatment of lymphomas, since a long-lasting polyclonal immune response directed against multiple epitopes may limit the emergence of tumor escape mutants and diminish the risk of relapse.
Components of therapeutic cancer vaccines
Most therapeutic cancer vaccines that are being tested in clinical trials have at least three components, a tumor-specific or tumor-associated antigen, a carrier, and an adjuvant. The tumor antigen is usually a protein or peptide derived from the tumor that is either uniquely expressed or is hyperexpressed in the tumor as compared with normal tissues. The unique or hyperexpression of the tumor antigen is necessary to prevent the induction of an unwanted autoimmune response against normal tissues following vaccination. The second component of a cancer vaccine, the carrier, is necessary for delivery of the tumor antigen to antigen-presenting cells, such as dendritic cells, in order to induce the immune response against the tumor antigen. A carrier can be a foreign protein, such as keyhole-limpet haemocyanin (KLH), or an inert vehicle, such as liposomes. KLH is an oxygen-carrying respiratory protein obtained from a marine mollusc, Megathura crenulata, a native of the Pacific Coast of California and Mexico (Harris, et al 2000). It is highly immunogenic and has been tested as a nonspecific stimulant of the immune system to decrease the risk of relapse in various human cancers (Harris, et al 2000). Liposomes can also be very effective carriers of tumor antigens. They can produce a depot effect at the site of injection and cause a slow release of antigens over a prolonged period of time (Wassef, et al 1994; Van Slooten, et al 2001). Liposomes traffic preferentially via the lymphatic system to local lymph nodes that are the sites for induction of immune responses (Kaledin, et al 1982; Oussoren, et al 2001). Reports in the literature also suggest that liposomes deliver the encapsulated antigens to both the endosomal and cytosolic compartments of antigen processing, thereby generating both CD4+ and CD8+ T-cell responses (Harding, et al 1991; Rao, et al 2000; Van Slooten, et al 2001). The third component of a cancer vaccine, the adjuvant, is usually a cytokine, such as granulocyte-macrophage colony stimulating factor (GM-CSF) or interleukin-2 (IL-2), to facilitate an enhanced immune response against the tumor antigen. GM-CSF likely acts by recruiting and promoting maturation of professional antigen-presenting cells, such as dendritic cells, which may in turn activate pathways of antigen processing that allow exogenous proteins to be presented by class I molecules (Eager, et al 2005). IL-2 may act as an adjuvant by augmenting the proliferation of activated T cells induced by the tumor antigen-carrier complex. However, IL-2 may potentially induce proliferation of regulatory T cells as well, and therefore, needs to be evaluated carefully in clinical trials for its adjuvant effects.
Idiotype is a model tumor antigen
An ideal tumor antigen is one that is selectively expressed in the tumor, universally present in all cancer patients, is essential for tumor cell survival, and should induce a polyclonal humoral and cellular immune response. The idiotype (Id), the most commonly used tumor antigen in therapeutic cancer vaccination studies in B-cell non-Hodgkin lymphomas has many of the desirable characteristics of an ideal tumor antigen. The idiotype refers to the unique amino acid sequences within the complementarity determining regions (CDR) of the variable regions of the heavy and light chains of the surface immunoglobulin expressed on B-cell malignancies (Fig 1). Since malignancies of B-cell origin are clonal, the Id of the tumor immunoglobulin is distinct from the immunoglobulins expressed on the surface of normal B cells. Therefore, the idiotype can be considered as a tumor-specific antigen; an immune response directed against the Id is expected to affect the tumor, but not normal B cells. Since the variable region of the tumor immunoglobulin is different from patient to patient, the Id is considered patient-specific and is not a universal tumor antigen. For this reason, the use of idiotype as a therapeutic lymphoma vaccine would require the generation of a custom-made product for each patient. The idiotype, however, appears to be essential for tumor cell survival, since immunoglobulin loss variants have rarely been described in certain B-cell non-Hodgkin lymphomas, such as follicular lymphoma (Kaleem, et al 2000, Li, et al 2002). This may be because of the fact that the surface immunoglobulin serves as the B-cell receptor and may transmit prosurvival and antiapoptotic signals necessary for the tumor growth (Kuppers, et al 2005). Furthermore, idiotype vaccination was associated with induction of polyclonal antibody and T-cell responses that may minimize the emergence of immune escape variants (Bendandi, et al 1999; Baskar, et al 2004).
Figure 1. Idiotype.
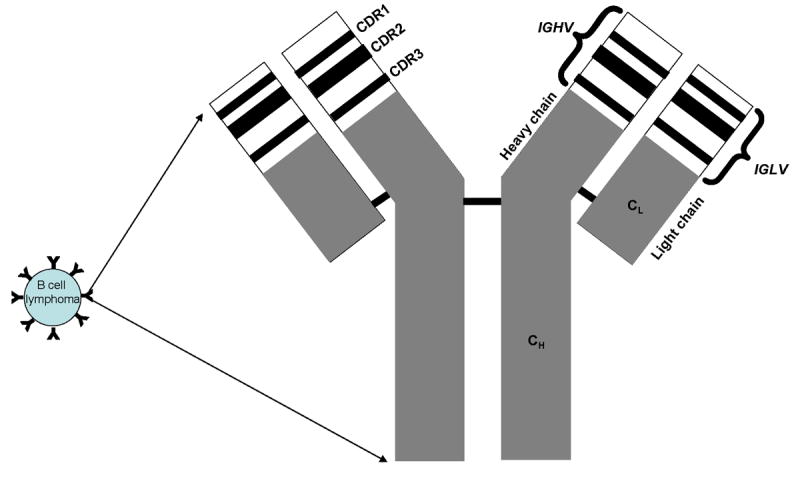
The idiotype refers to the unique amino acid sequences within the complementarity determining regions (CDR) of the variable regions of the heavy and light chains of the surface immunoglobulin expressed on B-cell malignancies. IGHV – variable region of heavy chain; IGLV – variable region of light chain; CH – constant region of heavy chain; CL – constant region of light chain.
Methods of idiotype vaccine generation
Traditionally, the idiotype protein was produced by a rescue hybridization technique developed by Ronald Levy's laboratory at Stanford University (Carroll, et al 1986). In this method, the lymphoma tumor cells obtained from a lymph node biopsy are fused to hypoxanthine-aminopterin-thymidine-sensitive heterohybridoma K6H6/B5 cells to produce hybridomas that secrete the tumor immunoglobulin (Fig 2). The hybridomas secreting the immunoglobulins with idiotype of interest are identified by comparing the immunoglobulin heavy chain CDR3 sequences of the fusions with the patient's tumor. The selected hybridoma clones are expanded and the Id protein is purified from the culture supernatant by affinity chromatography and formulated into a vaccine with a carrier and an adjuvant (Lee, et al 2007). Although this technique is successful, it is time-consuming and laborious requiring up to 3 to 6 months to make a vaccine for each patient.
Figure 2. Schema for idiotype vaccine generation.
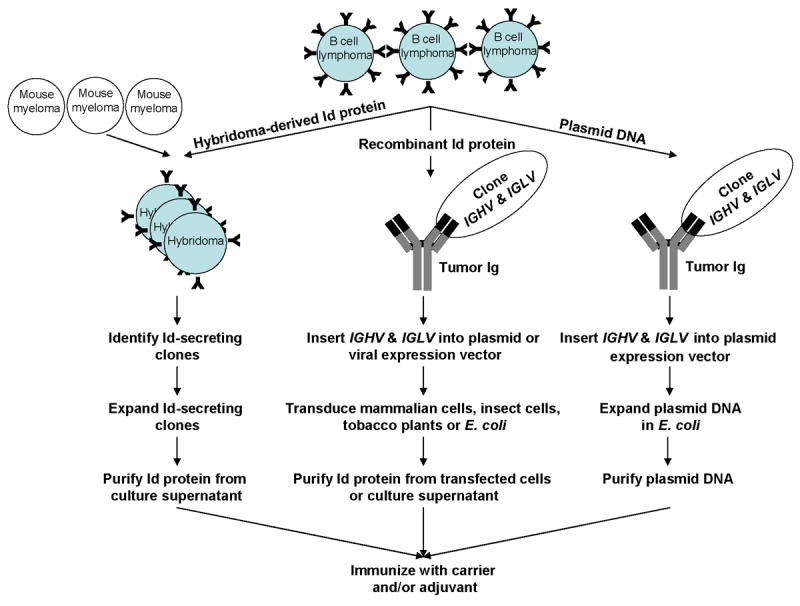
The idiotype may be used as either protein or DNA in therapeutic vaccines. In the traditional rescue hybridization technique, the idiotype protein is produced by fusing the lymphoma cells with mouse myeloma cells to generate Id-secreting hybridomas. For recombinant idiotype protein production, the variable regions of the heavy (IGHV) and light (IGLV) chains of the tumor immunoglobulin (Ig) are cloned by polymerase chain reaction and inserted into plasmid or viral vectors for expression of idiotype proteins in cell lines, tobacco plants, or Escherichia coli. For idiotype DNA vaccination, the IGHV and IGLV of the tumor immunoglobulin are cloned and inserted into a plasmid vector for naked DNA injection.
More recently, recombinant DNA technology has been used to generate idiotype proteins with the goal of shortening the vaccine production time. In this approach, the variable regions of the heavy (VH) and light (VL) chains of the tumor immunoglobulin are cloned by polymerase chain reaction (PCR) and inserted into an expression vector for production of the idiotype protein either in mammalian cells, insect cells, tobacco plants, or Escherichia coli (Hurvitz, et al 2005; McCormick, et al 1999; Kanter, et al 2007) (Fig 2). These newer approaches, although faster than the traditional hybridoma approach, still take approximately 2 months to manufacture a vaccine for each patient.
An alternative to idiotype protein vaccine is to use DNA vaccines to further shorten the vaccine production time. The most appealing aspect of DNA vaccination is its simplicity and the ease of vaccine generation. Immunoglobulin variable genes specific for the B-cell malignancies can be cloned (Hawkins, et al 1993, 1994) under the regulatory elements of a eukaryotic promoter into an expression cassette and combined into single chain variable fragment (scFv) formats, encoding a single polypeptide consisting solely of IGHV and IGLV genes linked together inframe by a short amino acid linker (Fig 2) (Benvenuti, et al 2002). The DNA vaccine is then injected via intramuscular or intradermal routes of administration or delivered into the epidermis by particle mediated bombardment of DNA-coated gold particles (gene gun). As described later in this article, preliminary studies in mice indicate that the scFv is weakly immunogenic in most cases and needs to be used together with an adjuvant to render it more immunogenic.
Development of idiotype vaccine: Preclinical studies
In the early 1970's, Lynch and colleagues demonstrated for the first time that idiotype vaccination could induce an antibody response and suppress the growth of the corresponding transplanted tumors in a mouse mineral oil-induced plasmacytoma model (Lynch, et al 1972). This observation was later confirmed in a number of different lymphoma, myeloma, and leukemia models. However, the immune responses and tumor protection were weak when mice were immunized with idiotype proteins alone (Stevenson, et al 1975; Freedman, et al 1976; Stevenson, et al 1977; Kaminski, et al 1987; Kwak, et al 1990). In 1987, Kaminski et al observed that antitumor immunity was optimally induced when the Id was conjugated to a strongly immunogenic carrier protein, such as KLH, in a syngeneic mouse 38C13 B-cell lymphoma model (Kaminski, et al 1987). The tumor protection with this formulation appeared to be predominantly mediated by an antibody response. In 1996, Kwak and colleagues demonstrated that administering low doses of GM-CSF significantly enhanced the potency of the Id-KLH vaccine in the 38C13 lymphoma model and induced both an antibody response as well as T-cell response, against the tumor (Kwak, et al 1996). In fact, the protective effect of the Id-KLH+GM-CSF vaccine in this model was dependent on effector CD4+ and CD8+ T cells. These encouraging preclinical studies provided the rationale to test the tumor idiotype as a therapeutic vaccine in human B-cell non-Hodgkin lymphoma.
Development of idiotype vaccines: Clinical studies
The optimal clinical trial setting for testing a novel therapeutic cancer vaccine formulation may not fit the traditional model developed for chemotherapy drug trials. For example, Phase I safety studies in patients with terminal disease who are immunosuppressed – either from the disease itself or from prior treatments with chemotherapeutic drugs – may not be optimal, as the toxicity of a therapeutic vaccine would likely come from the immune response itself, which may be impaired in such patients. Also, the immune responses induced by vaccination may be better at eliminating minimal residual disease rather than bulky tumors. This may require reducing the tumor burden by standard chemotherapy and allowing time for immunological recovery prior to vaccination. In addition, one may need to choose different endpoints, such as molecular remission rate and time to progression, rather than response rates. Due to these reasons, the majority of clinical trials of idiotype vaccination in lymphoma were performed after induction of clinical remission with standard chemotherapy. Another factor to consider in the design of clinical trials with idiotype vaccines in B-cell lymphomas is the use of rituximab in induction therapy. Administration of rituximab results in the depletion of both normal and malignant B cells (Maloney, et al 1994; McLaughlin, et al 1998). Consequently, patients treated with rituximab are unlikely to have peripheral blood B cells, and therefore, unlikely to generate humoral immune responses following active immunotherapy. Furthermore, animal studies have yielded conflicting results on the role of B cells in the priming of naïve T cells. Some murine models suggest that B cells may inhibit the induction of T-cell dependent immunity by competing with antigen-presenting cells for antigens, skewing the T-helper response towards a TH2 profile, and/or inducing T-cell tolerance (Qin, et al 1998; Steinman, et al 1991; Gajewski, et al 1991; Eynon, et al 1992; Bennett, et al 1998; El-Amine, et al 2000). Other experimental models, however, suggest B cells are necessary for priming, as well as generation of CD4+ and CD8+ T-cell memory (Yang, et al 1998; Rivera, et al 2001; Van Essen, et al 2000; Linton, et al 2000; Shen, et al 2003). Here, we describe the results of clinical trials with idiotype vaccines, both when used as a single agent or following chemotherapy with or without rituximab (Table 1).
Table I. Summary of immunological and clinical outcomes in idiotype vaccine trials in B-cell non-Hodgkin lymphoma.
| Formulation | Induction Therapy | No. of patients | Histology | Anti-Id/tumor immune responses (%) | Clinical response (%) | Molecular response (%) | Ref | |
|---|---|---|---|---|---|---|---|---|
| Ab | T-cell | |||||||
| Phase I/II idiotype vaccine trials after non-rituximab-based chemotherapy | ||||||||
| Id-KLH+SAF | Various | 41 | FL | 41 | 17 | 2/20 (10) | n/a |
Kwak, et al 1992
Hsu, et al 1997 |
| Id-KLH+GM-CSF | PACE | 20 | FL | 75 | 95 | n/a | 8/11 (73) | Bendandi, et al 1999 |
| Id-DC/Id-KLH-DC | None/CVP/CHOP | 35 | FL | 26 | 49 | 10/28 (36) | Reported in 1 patient |
Hsu, et al 1996
Timmerman, et al 2002a |
| Plasmid DNA | CVP | 12 | FL | 0 | 8 | 1/12 (8) | n/a | Timmerman, et al 2002b |
| Id-KLH+SAF | Various | 9 | FL | 89 | n/a | 2/9 (22) | 3/5 (60) | Barrios, et al 2002 |
| Liposomal Id/IL-2 | PACE | 10 | FL | 40 | 100 | Reported in 1 patient | n/a | Neelapu, et al 2004 |
| Id-KLH+GM-CSF | CHOP-like | 25 | FL | 52 | 72 | n/a | 3/10 (30) | Inoges, et al 2006 |
| Phase I/II idiotype vaccine trials after rituximab-based chemotherapy | ||||||||
| Id-KLH+GM-CSF | DA-EPOCH-R | 26 | MCL | 30 | 87 | n/a | n/a | Neelapu, et al 2005b |
| Id-KLH+GM-CSF | Rituximab | 89 | FL | 13 | 80 | 47% → 63%a | n/a | Koc, et al 2005 |
| Phase I/II idiotype vaccine trials as single agent | ||||||||
| Id-KLH+GM-CSF | None | 32 | FL | 20 | 67 | 4/31 (13) | n/a | Redfern, et al 2006 |
| Id(Fab)-MF59+GM-CSF | None | 18 | FL | 29 | 47 | 2/18 (11) | n/a | Bertinetti, et al 2006 |
Abbreviations: SAF: syntex adjuvant formulation; FL: follicular lymphoma; n/a: not assessable; PACE: prednisone, doxorubicin, cyclophosphamide, etoposide; DC: dendritic cell; CVP: cyclophosphamide, vincristine, prednisone; CHOP: cyclophosphamide, doxorubicin, vincristine, prednisone; DA-EPOCH-R: dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, rituximab; MCL: mantle cell lymphoma.
Clinical response rate improved from 47% after rituximab therapy to 63% after vaccination.
Phase I/II clinical trials of idiotype vaccination after non-rituximab-based chemotherapy
Kwak and colleagues conducted the first human trial of idiotype vaccination in nine patients with follicular lymphoma (Kwak, et al 1992). Patients induced into clinical remission with chemotherapy were immunized with subcutaneous injections of autologous tumor-derived idiotype protein conjugated with KLH and mixed with standard emulsion adjuvant (Syntex adjuvant formulation 1 or SAF-1). The long-term results of this Phase I clinical trial showed that 41% of the 41 patients ultimately treated on this trial developed an anti-Id antibody response and 17% developed an anti-Id T-cell response (Hsu, et al 1997). Thirty-two patients were in first remission and nine patients were in subsequent remission prior to vaccine treatments. Of the 20 patients with residual disease following chemotherapy, two patients had complete regression of the tumor in association with the development of a specific immune response. After a median follow-up of 5.3 years from last chemotherapy, the median duration of freedom from disease progression was 4.4 years. Thus, this first clinical trial demonstrated that Id-KLH+emulsion adjuvant vaccination was safe and induced an immune response against the autologous tumor idiotype in the setting of minimal tumor burden after conventional chemotherapy. However, it is important to recognize that convincing CD8+ T-cell responses were not observed, and this single-arm trial was not designed to answer the question of clinical efficacy.
Based on the preclinical observation that the addition of GM-CSF as an adjuvant to the vaccine induced tumor-specific CD8+ T cells (Kwak, et al 1996), Bendandi and colleagues conducted a Phase II clinical trial where 20 previously untreated follicular lymphoma patients received an autologous tumor-derived Id-KLH+GM-CSF vaccine (in normal saline solution) following induction of clinical remission with a uniform chemotherapy regimen [ProMACE (prednisone, methotrexate, doxorubicin, cyclophosphamide, and etoposide) without methotrexate ie, prednisone, doxorubicin, cyclophosphamide, and etoposide (PACE)] (Bendandi, et al 1999). The vaccine was injected subcutaneously in 5 monthly doses starting approximately 6 months after completing chemotherapy to allow time for immunological recovery. The vaccine was well tolerated, with the main adverse effects being injection site reactions such as erythema, induration, and pruritus. There were no autoimmune phenomena noted following the vaccination.
As previously observed on the Phase I clinical trial, anti-KLH antibody and cellular responses were induced in all patients. However, the humoral and cellular immune responses against idiotype were seen in a higher percentage of patients. Anti-idiotype antibody responses were induced in 15 out of 20 (75%) patients and Id-specific and/or tumor-specific CD4+ and CD8+ T-cell responses were observed in 19 out of 20 (95%) patients (Bendandi, et al 1999). Significant levels of HLA class I-restricted killing of autologous tumor targets were also demonstrated, suggesting the induction of a cytotoxic CD8+ T-cell response. Interestingly, the postvaccine T-cells lysed autologous tumor cells, but not the nonneoplastic, normal B cells from the same patients, suggesting that they were tumor-specific (Bendandi, et al 1999). Further characterization of anti-idiotype cellular immune responses demonstrated that the T cells specifically recognized multiple unique immunodominant epitopes within the hypervariable CDR regions, but not framework regions of the immunoglobulin heavy chain (Baskar, et al 2004).
Monitoring of the patients for minimal residual disease showed that 8 out of 11 patients with PCR-positive t(14;18) chromosomal translocation breakpoints converted to PCR negativity in their blood immediately after completing vaccination and sustained their molecular remissions for a median of 18+ months (range: 8+ to 32+ months) (Bendandi, et al 1999). Thus, these results provided the first convincing evidence of an in vivo antitumor effect of Id vaccination. Analysis of time to relapse also provided an independent indication of clinical benefit. With a median follow-up of 9.2 years, median disease-free survival (DFS) was 8 years, and the overall survival rate was 95% (Santos, et al 2005). While definitive statements cannot be made, because this was not a randomized trial, the DFS appears superior to that of a historical, ProMACE chemotherapy-treated control group (median DFS, about 2.2 years) (Longo, et al 2000). In conclusion, this phase II clinical trial demonstrated that idiotype vaccine when given to a homogeneous group of patients, all in first complete remission (CR), can induce antitumor CD8+ T-cell responses and molecular remissions.
Inoges et al recently evaluated the effects of Id-KLH+GM-CSF vaccination in 25 patients with follicular lymphoma after induction of a second complete clinical response with cyclophosphamide, doxorubicin, vincristine, prednisone (CHOP)-like chemotherapy (Inoges, et al 2006). An anti-Id antibody response was observed in 52% of the patients and anti-Id cellular response was seen in 72% of the patients. Overall, 20 (80%) out of 25 patients had either humoral or cellular anti-Id immune responses. The median duration of the second complete response among the 20 immune responders was significantly longer than the median duration of their first complete response and than the CHOP-induced second complete response. In contrast, the median duration of the second complete response among the five nonresponders was significantly shorter than their first complete response. Thus, this trial independently confirmed the immunogenicity of the Id-KLH+GM-CSF vaccine in the setting of minimal residual disease in patients with follicular lymphoma and also indicated that vaccination may be associated with clinical benefit.
To enhance the potency of idiotype vaccination, Timmerman and colleagues evaluated dendritic cells pulsed with Id or Id-KLH in 35 patients with follicular lymphoma (Hsu, et al 1996; Timmerman, et al 2002a). Ten relapsed patients with measurable lymphoma received the vaccine alone and an additional 25 patients received the vaccine after first clinical remission induced by cyclophosphamide, vincristine, prednisone (CVP)-like and/or CHOP chemotherapy. Overall, 26% of the patients developed an anti-Id antibody response and 49% developed an anti-Id T-cell response. Interestingly, among 18 patients with residual tumors at the time of vaccination, 4 (22%) had tumor regression, and 16 of 23 patients (70%) remained without tumor progression at a median of 43 months after chemotherapy. Although the Id-pulsed dendritic cell vaccine was immunogenic and clinical tumor regressions were demonstrated, a formal comparison is necessary to determine whether it is superior to Id-KLH+GM-CSF vaccination, given the technical difficulties of generating dendritic cells for each patient.
Timmerman and colleagues also evaluated naked DNA Id vaccination in a Phase I/II trial in 12 patients with follicular lymphoma (Timmerman, et al 2002b). The vaccine was administered intramuscularly and intradermally following induction of remission with chemotherapy. The vaccine was found to be safe and well-tolerated, however, the antitumor immune responses were modest, and one patient had evidence of clinical tumor regression. Thus, while the DNA vaccine has the potential to streamline the production of patient-specific Id vaccines, additional preclinical studies are needed to further optimize the immunogenicity of this vaccine formulation. In this regard, the potency of DNA vaccines could be enhanced in mouse models by fusion of the idiotype variable genes to an adjuvant, such as a gene encoding fragment C of tetanus toxin (King, et al 1998) or a proinflammatory chemokine moiety (Biragyn, et al 1999) or xenogeneic Fc fragment (Benvenuti, et al 2000 and 2001). Clinical trials using these novel formulations are being planned.
Based on preclinical studies that showed incorporation of Id into liposomes along with IL-2 may be more potent than Id-KLH vaccine (Kwak, et al 1998), Neelapu and colleagues conducted a Phase I trial to evaluate safety and immunogenicity of a liposomal Id/IL-2 vaccine formulation in patients with follicular lymphoma treated into first clinical remission with PACE chemotherapy (Neelapu, et al 2004). Antitumor T-cell responses were observed on all 10 patients treated on the study and anti-Id antibody responses were observed in 4 patients. Moreover, postvaccine – but not prevaccine – T cells lysed autologous tumor cells. There was no significant cytotoxicity against autologous normal B cells, suggesting that the lysis was tumor-specific. The tumor-specific CD4+ and CD8+ T-cell responses were sustained 18 months beyond the completion of the vaccination. After a median follow-up of 50 months, six of the 10 patients remained in continuous first complete remission, and another patient achieved a sustained second complete remission following vaccination (Neelapu, et al 2004; Neelapu, et al 2006).
Phase II clinical trials of idiotype vaccination after rituximab-based chemotherapy
The effects of rituximab-induced B-cell depletion on the immunogenicity of idiotype vaccines were recently evaluated in a pilot clinical trial in patients with mantle cell lymphoma (Neelapu, et al 2005b). Twenty-six previously untreated mantle cell lymphoma patients received 6 cycles of dose-adjusted rituximab, etoposide, vincristine, doxorubicin, cyclophosphamide, and prednisone (EPOCH-R), followed 12 weeks later with 5 monthly cycles of autologous tumor-derived Id-KLH+GM-CSF vaccination. As expected, following EPOCH-R, peripheral blood B cells were completely depleted in all patients, began to recover at approximately 6 months, and returned to baseline levels by 1 year in most patients. In contrast, CD4+ T-cell numbers decreased only slightly and recovered by the start of vaccination, with a median time of 3 months; CD8+ T-cell numbers did not change substantially.
Unexpectedly, despite rituximab administration, antibody responses against the carrier molecule KLH and Id were detected in 17 out of 23 (74%) and 7 out of 23 (30%) evaluable patients, respectively (Neelapu, et al 2005b). The humoral responses were delayed and correlated with B-cell recovery, with most detected after the fourth or fifth vaccination, compared with after the first or second vaccinations in the follicular lymphoma study in which rituximab was not administered (Neelapu, et al 2006). Additionally, in several patients, humoral responses were delayed 4 to 10 months after the last vaccination, suggesting that priming may occur at low B-cell levels, whereas detectable antibody titers required adequate B-cell expansion. In contrast, vigorous CD4+ and CD8+ antitumor and KLH T-cell responses were not delayed and were induced in 20 out of 23 (87%) and 23 out of 23 (100%) patients, respectively, in the absence of circulating B cells (Neelapu, et al 2005b) suggesting that professional antigen-presenting cells such as dendritic cells may effectively present antigens to T cells in the absence of B cells.
Among the 26 patients, EPOCH-R produced complete and partial remissions in 92% and 8% of patients, respectively; all but one remission were maintained at least to the start of vaccination. With a median potential follow-up of 46 months, overall survival probability was 89%, median event-free survival was 22 months, and five patients remained in continuous first complete remission (Neelapu, et al 2005b). Despite a high remission rate and induction of tumor-specific T-cell responses in 87% of the patients, most patients relapsed. Analysis of relapsing tumors revealed no mutations or changes in expression of the idiotype to explain its escape from therapy (Neelapu, et al 2005b). However, other potential reasons for tumor escape include inadequate induction of T cells with high avidity or frequency, inadequate T-cell trafficking to tumor sites, secretion of immunosuppressive factors by tumor cells, and/or the development of other tumor escape mechanisms such as downregulation or loss of MHC molecules. Additionally, some clinical studies suggest that antitumor humoral responses may be important, and these were only observed in 30% of patients (Hsu, et al 1997; Weng, et al 2004). Moreover, the antibody titers against KLH and the idiotype fell from their peak levels over time in 15 out of 17 (88%) and 6 out of 7 (86%) patients with a positive antibody response, respectively (Neelapu, et al 2005b), suggesting that continued late vaccination may be therapeutically beneficial. Nevertheless, the overall survival of 89% at 46 months raises the intriguing possibility that idiotype vaccination modified the natural history of mantle cell lymphoma.
Recently, Koc et al reported their experience with Id-KLH+GM-CSF vaccination after single agent rituximab induction in 89 patients with treatment naïve or relapsed/refractory follicular lymphoma (Koc, et al 2005). Anti-Id and anti-KLH T-cell responses were observed in 80% and 86% of evaluated patients, respectively, and were seen after a median of 2 doses of Id/KLH. Likewise, antibody responses to Id and KLH were seen in 13% and 69% of evaluated patients, respectively, and developed after a median of 5 to 6 doses of Id/KLH. More interestingly, the overall response rate improved to 63% after combined rituximab and Id-KLH+GM-CSF treatment, as compared with 47% at 3-month follow-up after rituximab therapy. Taken together, these two studies indicated that anti-tumor T-cell responses could be generated by idiotype vaccination when administered in the setting of B-cell depletion induced by rituximab. Furthermore, antibody responses against Id appear to be delayed but may be observed in a smaller percentage of patients.
Phase I/II clinical trials of idiotype vaccination as a single agent
The efficacy of idiotype vaccination as a single agent, without administration of cytoreductive therapy, was evaluated in two clinical trials. In a Phase II clinical trial, Redfern et al treated 32 patients with relapsed indolent non-Hodgkin lymphoma with 6 monthly doses of Id-KLH+GM-CSF vaccine followed by booster injections until disease progression (Redfern, et al 2006). Six (67%) of nine patients tested demonstrated a cellular immune response, and four (20%) of 20 patients demonstrated antibody responses against their Id. Responses were observed in 4 of 31 evaluable patients (one complete response and three partial responses – 12.9%). Median time to onset of response was 5.9 months (range, 2.3 to 14.1 months). Median duration of response was not reached, but was at least 19.4 months (range, 10.4 to 27.2+ months). Median time to progression was 13.5 months.
Bertinetti et al recently reported a Phase I trial where they evaluated recombinant idiotype Fab fragments expressed in Escherichia coli in 18 patients with previously treated advanced B-cell malignancies (Bertinetti, et al 2006). The vaccine was administered intradermally, mixed with a lipid-based adjuvant, MF59, and given in combination with GM-CSF subcutaneously at the same location. The vaccine was fairly well-tolerated. Five of 17 (29%) evaluable patients developed anti-Id antibodies and eight (47%) developed anti-Fab T-cell responses. Clinical outcome was difficult to assess on this study due to the heterogeneity of the patient population.
Phase III clinical trials of idiotype vaccination after cytoreductive therapy
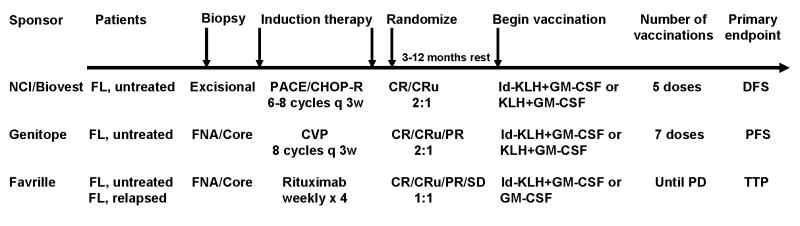
The strong immunogenicity and the induction of clinical and molecular remissions with Id-KLH+GM-CSF vaccine in the setting of low tumor burden or minimal residual disease prompted the initiation of three randomized double-blind placebo-controlled multicentre clinical trials to definitively answer the question of clinical benefit induced by idiotype vaccination (Fig 3). The first Phase III trial was initiated by the National Cancer Institute, National Institutes of Health (Bethesda, Maryland, USA) and is currently sponsored by Biovest International, Inc (Worcester, MA, USA) (Neelapu, et al 2005a). This trial was designed similarly to the Phase II trial (Bendandi, et al 1999) wherein previously untreated advanced-stage follicular lymphoma patients initially underwent an excisional lymph node biopsy and were treated into clinical remission with a PACE or CHOP-Rituximab (CHOP-R) chemotherapy regimen. Patients who achieve a CR or CRu (complete response unconfirmed) are randomized in a 2:1 manner, either to the specific vaccination arm of Id-KLH+GM-CSF or the nonspecific vaccination arm of KLH+GM-CSF. The primary endpoint for this trial is to compare the DFS between the two arms. The other two Phase III trials that evaluate idiotype vaccines in patients with follicular lymphoma differ primarily in terms of the induction therapy and the method of idiotype production. The Genitope Incorporated (Redwood City, CA, USA)-sponsored trial uses CVP chemotherapy (Vose, 2006), and the Favrille Incorporated (San Diego, CA, USA)-sponsored trial uses single agent rituximab (Hurvitz, et al 2005). Moreover, while only CR and CRu patients are vaccinated on the Biovest study, CR, CRu, and partial response (PR) patients are vaccinated on the Genitope trial, and CR, CRu, PR and stable disease (SD) patients are vaccinated on the Favrille trial. As opposed to the hybridoma method in the Biovest study, the Genitope and Favrille trials use recombinant DNA technology for production of the idiotype protein. In all trials, the idiotype protein is conjugated to KLH, and GM-CSF is used as an adjuvant. The results of interim and/or final analyses from these trials are expected within the next year, and it remains to be seen whether the idiotype vaccines can induce clinically meaningful benefits in patients with minimal residual disease (Biovest study) and/or patients with low tumor burden (Genitope and Favrille studies).
Figure 3. Schema for Phase III idiotype vaccine clinical trials.
In all three Phase III clinical trials, the patients initially undergo a biopsy to obtain tissue for vaccine production. Patients then receive induction therapy and are randomized in a 2:1 or 1:1 manner in favour of the experimental arm. The similarities and differences between the three trials are shown. NCI – National Cancer Institute; q 3w – every 3 weeks; FNA – fine needle aspiration; PD – progressive disease; DFS – disease-free survival; PFS – progression-free survival; TTP – time to progression.
Mechanism of antitumor effects following idiotype vaccination
While some murine lymphoma models have demonstrated that the antitumor efficacy of idiotype vaccine is dependent on the induction of an antibody response (Cesco-Gaspere, et al 2005, Kaminski, et al 1987; Timmerman, et al 2000; Timmerman, et al 2001), other lymphoma models have shown that protection against tumor challenge or eradication of established tumors requires a CD4+ and/or a CD8+ T-cell response (Kwak, et al 1996; Levitsky, et al 1996; Kwak, et al 1998). A recent analysis of 136 patients with follicular lymphoma treated with idiotype vaccination showed that the induction of specific anti-Id antibody responses was associated with significantly prolonged progression-free survival (PFS) (Weng, et al 2004). This study also demonstrated that patients with a favourable FCGR3A polymorphism that predicts stronger binding of the Fc of antibodies to effector cells had a longer PFS. Thus, patients with FCGR3A 158 valine/valine (V/V) genotype had a longer PFS than those with valine/phenylalanine (V/F) or phenylalanine/phenylalanine (F/F) genotypes. In contrast, no significant correlation was observed between the development of a cellular anti-Id immune responses and PFS. However, it is possible that this study may have underestimated the value of an antitumor T-cell responses for several reasons. First, cellular responses on this study were measured by a T-cell proliferation assay, which is relatively less sensitive, compared with newer assays such as enzyme-linked immunosorbent spot assay and cytokine flow cytometry (Keilholz, et al 2002). Second, the proliferation assay detects primarily a CD4+ T-cell response and may fail to detect a CD8+ T-cell response. Third, immunological assays that demonstrate recognition of the native tumor (tumor-specific) may be more clinically relevant to assessing T-cell responses following cancer vaccination, compared with assays that demonstrate recognition of tumor antigens presented on appropriate antigen-presenting cells (antigen-specific) (Malyguine, et al 2004). Fourth, 86 out of 136 (63%) patients on this study received a chemical adjuvant with the idiotype vaccine that predominantly induced a humoral immune response (Kwak, et al 1992; Hsu, et al 1997). In contrast, idiotype vaccine formulations using dendritic cells (Hsu, et al 1996; Timmerman, et al 2002a) or cytokines such as GM-CSF (Bendandi, et al 1999; Neelapu, et al 2005b; Koc, et al 2005; Redfern, et al 2006; Inoges, et al 2006) or IL-2 (Neelapu, et al 2004) as adjuvants significantly enhanced the induction of cellular immune responses. Finally, FCGR3A polymorphisms did not appear to correlate with response rate or time to progression when Id-KLH+GM-CSF vaccine was administered following rituximab induction (Maloney, et al 2007).
Several reports in the literature suggest that T-cell responses could induce tumor regression independent of a humoral response following idiotype vaccinations. Following Id-pulsed DC vaccinations, 5 follicular lymphoma patients had clinical tumor regression with the induction of a T-cell response, but without an antibody response (Hsu, et al 1996; Timmerman, et al 2002a). Similarly, following Id-KLH+GM-CSF vaccination, 3 patients achieved molecular remissions without a detectable antibody response, suggesting that cell-mediated antitumor immune responses are important for vaccine efficacy (Bendandi, et al 1999). Clinical tumor regression was also observed in a follicular lymphoma patient after administration of liposomal Id/IL-2 vaccine (OncoVAX-Id/IL-2) in the absence of an antibody response (Neelapu, et al 2004; Neelapu, et al 2006). Moreover, Nelson et al demonstrated that the precursor frequency of tumor-specific cytotoxic T lymphocytes correlated with freedom from progression following idiotype vaccination (Nelson, et al 1996). Taken together, these results suggested that T-cell responses could induce tumor regression independent of a humoral response. Furthermore, the two studies performed after rituximab-based induction therapy (Neelapu, et al 2005b; Koc, et al 2005) suggested that idiotype vaccination may be administered as early as 3 months after completion of rituximab-based therapy to induce antitumor T-cell responses. However, because the relative role of cellular versus humoral immunity for vaccine efficacy is uncertain, it may be advisable to administer booster vaccinations following B-cell recovery to optimize humoral responses.
Other lymphoma vaccine formulations
Although idiotype vaccination is immunogenic, and the results of early-phase clinical trials are encouraging, one of the major drawbacks of such vaccination is the requirement to generate a custom-made product for each patient, by a process that is expensive, laborious, and time-consuming. Therefore, novel lymphoma vaccine formulations that can be produced rapidly are needed. As mentioned earlier, using DNA vaccines instead of idiotype protein vaccines is one potential option. Another alternative for streamlining the production of individualized tumor vaccines is to directly extract membrane proteins from the tumor cells and incorporate them into liposomes along with IL-2 as an adjuvant to produce membrane-patched proteoliposomes (MPL) (Popescu, et al 2007). Such MPL vesicles may have IL-2, Id, and other unrecognized tumor-associated antigens. Testing in a mouse lymphoma model showed this formulation to be at least as potent as the prototype Id protein vaccine in inducing tumor protection (Popescu, et al 2007). The major advantage of the MPL vaccine is that it would circumvent the expensive and time-consuming steps of preparing Id protein vaccines by hybridoma or recombinant DNA technologies. Manufacturing such a vaccine would require only 24 hours after an excisional biopsy, and the vaccine would be ready for administration immediately following standard release and sterility testing.
In a pilot clinical trial, this novel membrane proteoliposomal vaccine was safe, induced autologous tumor-specific type 1 cytokine responses in 5 out of 10 advanced-stage follicular lymphoma patients, and was associated with induction of a sustained complete response in one patient when used as single agent (Neelapu, et al 2007). Other patients had large tumor burdens and progressed after a median duration of 8 months. Due to the use of total membrane proteins as antigenic material in the vaccine formulation, there was a potential risk of inducing immune responses against normal proteins, and thus, autoimmunity. However, there was no clinical or laboratory evidence of autoimmunity observed in any patient on this trial. Although this novel vaccine formulation requires the generation of a custom-made product for each patient, it offers several advantages over patient-specific idiotype vaccines. First, this formulation can be produced rapidly within a single day in contrast to the 2 to 6 months required to manufacture idiotype vaccines for each patient. Second, in addition to the membrane idiotype protein, this vaccine formulation may induce immune responses against other unrecognized tumor-associated antigens. Finally, this novel formulation may serve as a model for vaccine development against other human malignancies, including certain leukemias, lymphomas, and solid tumors where tumor-associated antigens have not been defined. The encouraging results in this pilot study of follicular lymphoma patients with bulky disease suggests that additional testing of this formulation may be warranted, particularly in the setting of low tumor burden or minimal residual disease.
Other individualized novel tumor vaccine formulations in development for non-Hodgkin lymphomas include GM-CSF transduced tumor/bystander cells (Dessureault, et al 2007), CD40 activated tumor cells (Wierda, et al 2000), T-cell receptor vaccines (Lambert, et al 2004), tumor lysate-pulsed dendritic cells (Maier, et al 2003), and tumor-derived heat shock protein peptide complexes (Oki, et al 2007). However, similar to idiotype protein vaccination, these vaccine formulations also require generating custom-made product for each patient and therefore may limit the broad applicability of this approach. Identification of universally expressed lymphoma-specific antigens will be necessary in the future to develop vaccine formulations that can be used in all lymphoma patients and are therefore easier and less costly to produce.
Future directions
In summary, results from several Phase I/II studies in patients with B-cell non-Hodgkin lymphoma suggest that idiotype vaccines are immunogenic and may be associated with improved PFS or DFS when administered in the setting of low tumor burden or minimal residual disease, respectively. Definitive results to confirm clinical benefit are expected within the next year from ongoing Phase III trials. However, to induce objective responses in patients with bulky tumors enhancement of the potency of lymphoma vaccines is needed. With the increased use of rituximab for the treatment of B-cell non-Hodgkin lymphomas, improvement in the potency of the lymphoma vaccines would require strategies to enhance the T-cell responses since humoral responses are impaired in these patients. Recent studies in animal models suggest that the T-cell immune responses against foreign or self-antigens are regulated by several immunoregulatory pathways and/or peripheral tolerance mechanisms (Zou, et al 2005). Therefore, disruption of the immunoregulatory pathways such as CD4+CD25+ regulatory T cells (Tregs), cytotoxic T lymphocyte-associated antigen (CTLA)-4, programmed cell death 1 (PD-1), B7-H1, and B7-H4 that modulate the magnitude and duration of the T-cell immune responses may enhance the potency of cancer vaccines. Data in the literature suggests that these regulatory pathways may be important in B-cell lymphomas. For example, Tregs appear to be actively recruited into the tumor microenvironment and inhibit the function of intratumoral CD4+ and CD8+ T cells (Yang, et al 2006; Yang, et al 2006). In a pilot clinical trial, administration of anti-CTLA-4 monoclonal antibody was associated with regression of tumors in patients with lymphoma (O'mahony, et al 2007). The inhibitory receptor PD-1 appears to be markedly upregulated on intratumoral and peripheral blood T cells and associated with impaired T-cell function in patients with follicular lymphoma (Nattamai, et al 2007). In several animal models, depletion of CD4+CD25+ Tregs or blockade of CTLA-4, PD-1 or B7-H1 led to improved tumor control (Sutmuller, et al 2001; Iwai, et al 2002; Iwai, et al 2005). In humans, depletion of Tregs with denileukin diftitox significantly enhanced vaccine induced or endogenous antitumor immunity in renal cell cancer and ovarian cancer patients, respectively (Dannull, et al 2005; Barnett, et al 2005). Taken together, these preclinical and early phase clinical results support the evaluation of combination immunotherapy strategies in future with the cancer vaccine to stimulate an antitumor T-cell response and the simultaneous suppression of immune regulatory pathways to augment the induced T-cell response. The existence of multiple immune regulatory pathways necessitates systematic evaluation of these approaches in clinical trials to determine the optimal combination immunotherapy regimen.
Acknowledgments
The work described in this review is supported by the American Society of Clinical Oncology Career Development Award (SSN), American Society of Hematology Junior Faculty Scholar Award in Clinical/Translational Research (SSN), National Institutes of Health grant K23CA123149 (SSN). We thank M Veech for editorial assistance.
Footnotes
Conflict of interest Sattva S. Neelapu received research funding from Biovest International, Inc and Favrille, Inc.
References
- Barnett B, Kryczek I, Cheng P, Zou W, Curiel TJ. Regulatory T cells in ovarian cancer: biology and therapeutic potential. Am J Reprod Immunol. 2005;54:369–377. doi: 10.1111/j.1600-0897.2005.00330.x. [DOI] [PubMed] [Google Scholar]
- Barrios Y, Cabrera R, Yanez R, Briz M, Plaza A, Fores R, Fernandez MN, Diaz-Espada F. Anti-idiotypic vaccination in the treatment of low-grade B-cell lymphoma. Haematologica. 2002;87:400–407. [PubMed] [Google Scholar]
- Baskar S, Kobrin CB, Kwak LW. Autologous lymphoma vaccines induce human T cell responses against multiple, unique epitopes. J Clin Invest. 2004;113:1498–1510. doi: 10.1172/JCI20312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendandi M, Gocke CD, Kobrin CB, Benko FA, Sternas LA, Pennington R, Watson TM, Reynolds CW, Gause BL, Duffey PL, Jaffe ES, Creekmore SP, Longo DL, Kwak LW. Complete molecular remissions induced by patient-specific vaccination plus granulocyte-monocyte colony-stimulating factor against lymphoma. Nat Med. 1999;5:1171–1177. doi: 10.1038/13928. [DOI] [PubMed] [Google Scholar]
- Bennett SR, Carbone FR, Toy T, Miller JF, Heath WR. B cells directly tolerize CD8(+) T cells. J Exp Med. 1998;188:1977–1983. doi: 10.1084/jem.188.11.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benvenuti F, Burrone OR, Efremov DG. Anti-idiotypic DNA vaccines for lymphoma immunotherapy require the presence of both variable region genes for tumor protection. Gene Therapy. 2000;7:605–611. doi: 10.1038/sj.gt.3301133. [DOI] [PubMed] [Google Scholar]
- Benvenuti F, Burrone OR. Anti-idiotypic antibodies induced by genetic immunisation are directed exclusively against combined V(L)/V(H) determinants. Gene Therapy. 2001;8:1555–1561. doi: 10.1038/sj.gt.3301546. [DOI] [PubMed] [Google Scholar]
- Benvenuti F, Cesco-Gaspere M, Burrone OR. Anti-idiotypic DNA vaccines for B-cell lymphoma therapy. Frontiers in Bioscience. 2002;7:d228–d234. doi: 10.2741/benvenut. Review. [DOI] [PubMed] [Google Scholar]
- Bertinetti C, Zirlik K, Heining-Mikesch K, Ihorst G, Dierbach H, Waller CF, Veelken H. Phase I trial of a novel intradermal idiotype vaccine in patients with advanced B-cell lymphoma: specific immune responses despite profound immunosuppression. Cancer Res. 2006;66:4496–4502. doi: 10.1158/0008-5472.CAN-05-4233. [DOI] [PubMed] [Google Scholar]
- Biragyn A, Tani K, Grimm MC, Weeks S, Kwak LW. Genetic Fusion of Chemokines To A Self Tumor Antigen Induces Protective, T-Cell Dependent Anti-Tumor Immunity. Nature Biotechnology. 1999;17:253–8. doi: 10.1038/6995. [DOI] [PubMed] [Google Scholar]
- Carroll WL, Thielemans K, Dilley J, Levy R. Mouse x human heterohybridomas as fusion partners with human B cell tumors. Journal of Immunological Methods. 1986;89:61–72. doi: 10.1016/0022-1759(86)90032-3. [DOI] [PubMed] [Google Scholar]
- Cesco-Gaspere M, Benvenuti F, Burrone OR. BCL1 lymphoma protection induced by idiotype DNA vaccination is entirely dependent on anti-idiotypic antibodies. Cancer Immunology and Immunotherapy. 2005;54:351–358. doi: 10.1007/s00262-004-0579-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombat P, Salles G, Brousse N, Eftekhari P, Soubeyran P, Delwail V, Deconinck E, Haioun C, Foussard C, Sebban C, Stamatoullas A, Milpied N, Boue F, Taillan B, Lederlin P, Najman A, Thieblemont C, Montestruc F, Mathieu-Boue A, Benzohra A, Solal-Celigny P. Rituximab (anti-CD20 monoclonal antibody) as single first-line therapy for patients with follicular lymphoma with a low tumor burden: clinical and molecular evaluation. Blood. 2001;97:101–106. doi: 10.1182/blood.v97.1.101. [DOI] [PubMed] [Google Scholar]
- Dannull J, Su Z, Rizzieri D, Yang BK, Coleman D, Yancey D, Zhang A, Dahm P, Chao N, Gilboa E, Vieweg J. Enhancement of vaccine-mediated antitumor immunity in cancer patients after depletion of regulatory T cells. J Clin Invest. 2005;115:3623–3633. doi: 10.1172/JCI25947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dave SS, Wright G, Tan B, Rosenwald A, Gascoyne RD, Chan WC, Fisher RI, Braziel RM, Rimsza LM, Grogan TM, Miller TP, LeBlanc M, Greiner TC, Weisenburger DD, Lynch JC, Vose J, Armitage JO, Smeland EB, Kvaloy S, Holte H, Delabie J, Connors JM, Lansdorp PM, Ouyang Q, Lister TA, Davies AJ, Norton AJ, Muller-Hermelink HK, Ott G, Campo E, Montserrat E, Wilson WH, Jaffe ES, Simon R, Yang L, Powell J, Zhao H, Goldschmidt N, Chiorazzi M, Staudt LM. Prediction of survival in follicular lymphoma based on molecular features of tumor-infiltrating immune cells. N Engl J Med. 2004;351:2159–2169. doi: 10.1056/NEJMoa041869. [DOI] [PubMed] [Google Scholar]
- Dessureault S, Noyes D, Lee D, Dunn M, Janssen W, Cantor A, Sotomayor E, Messina J, Antonia SJ. A phase-I trial using a universal GM-CSF-producing and CD40L-expressing bystander cell line (GM.CD40L) in the formulation of autologous tumor cell-based vaccines for cancer patients with stage IV disease. Ann Surg Oncol. 2007;14:869–884. doi: 10.1245/s10434-006-9196-4. [DOI] [PubMed] [Google Scholar]
- Eager R, Nemunaitis J. GM-CSF gene-transduced tumor vaccines. Molecular Therapeutics. 2005;12:18–27. doi: 10.1016/j.ymthe.2005.02.012. [DOI] [PubMed] [Google Scholar]
- El-Amine M, Melo M, Kang Y, Nguyen H, Qian J, Scott DW. Mechanisms of tolerance induction by a gene-transferred peptide-IgG fusion protein expressed in B lineage cells. J Immunol. 2000;165:5631–5636. doi: 10.4049/jimmunol.165.10.5631. [DOI] [PubMed] [Google Scholar]
- Eynon EE, Parker DC. Small B-cells as antigen-presenting cells in the induction of tolerance to soluble protein antigens. J Exp Med. 1992;175:131–138. doi: 10.1084/jem.175.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman PM, Autry JR, Tokuda S, Williams RC., Jr Tumor immunity induced by preimmunization with BALB/c mouse myeloma protein. J Natl Cancer Inst. 1976;56:735–740. doi: 10.1093/jnci/56.4.735. [DOI] [PubMed] [Google Scholar]
- Gajewski TF, Pinnas M, Wong T, Fitch FW. Murine Th1 and Th2 clones proliferate optimally in response to distinct antigen-presenting cell populations. J Immunol. 1991;146:1750–1758. [PubMed] [Google Scholar]
- Harding CV, Collins DS, Kanagawa O, Unanue ER. Liposome-encapsulated antigens engender lysosomal processing for class II MHC presentation and cytosolic processing for class I presentation. J Immunol. 1991;147:2860–2863. [PubMed] [Google Scholar]
- Harris JR, Markl J. Keyhole limpet hemocyanin: molecular structure of a potent marine immunoactivator. A review. Eur Urol. 2000;37 3:24–33. doi: 10.1159/000052389. [DOI] [PubMed] [Google Scholar]
- Hawkins RE, Winter G, Hamblin TJ, Stevenson FK, Russell SJ. A genetic approach to idiotypic vaccination. J Immunother. 1993;14:273–278. doi: 10.1097/00002371-199311000-00004. [DOI] [PubMed] [Google Scholar]
- Hawkins RE, Zhu D, Ovecka M, Winter G, Hamblin TJ, Long A, Stevenson FK. Idiotypic vaccination against human B-cell lymphoma. Rescue of variable region gene sequences from biopsy material for assembly as single-chain Fv personal vaccines. Blood. 1994;83:3279–3288. [PubMed] [Google Scholar]
- Horning SJ, Rosenberg SA. The natural history of initially untreated low-grade non-Hodgkin's lymphomas. N Engl J Med. 1984;311:1471–1475. doi: 10.1056/NEJM198412063112303. [DOI] [PubMed] [Google Scholar]
- Hsu FJ, Benike C, Fagnoni F, Liles TM, Czerwinski D, Taidi B, Engleman EG, Levy R. Vaccination of patients with B-cell lymphoma using autologous antigen-pulsed dendritic cells. Nat Med. 1996;2:52–58. doi: 10.1038/nm0196-52. [DOI] [PubMed] [Google Scholar]
- Hsu FJ, Caspar CB, Czerwinski D, Kwak LW, Liles TM, Syrengelas A, Taidi-Laskowski B, Levy R. Tumor-specific idiotype vaccines in the treatment of patients with B-cell lymphoma--long-term results of a clinical trial. Blood. 1997;89:3129–3135. [PubMed] [Google Scholar]
- Hurvitz SA, Timmerman JM. Recombinant, tumour-derived idiotype vaccination for indolent B cell non-Hodgkin's lymphomas: a focus on FavId. Expert Opin Biol Ther. 2005;5:841–852. doi: 10.1517/14712598.5.6.841. [DOI] [PubMed] [Google Scholar]
- Inoges S, Rodriguez-Calvillo M, Zabalegui N, Lopez-Diaz de Cerio A, Villanueva H, Soria E, Suarez L, Rodriguez-Caballero A, Pastor F, Garcia-Munoz R, Panizo C, Perez-Calvo J, Melero I, Rocha E, Orfao A, Bendandi M. Clinical benefit associated with idiotypic vaccination in patients with follicular lymphoma. J Natl Cancer Inst. 2006;98:1292–1301. doi: 10.1093/jnci/djj358. [DOI] [PubMed] [Google Scholar]
- Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci U S A. 2002;99:12293–12297. doi: 10.1073/pnas.192461099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwai Y, Terawaki S, Honjo T. PD-1 blockade inhibits hematogenous spread of poorly immunogenic tumor cells by enhanced recruitment of effector T cells. Int Immunol. 2005;17:133–144. doi: 10.1093/intimm/dxh194. [DOI] [PubMed] [Google Scholar]
- Kaledin VI, Matienko NA, Nikolin VP, Gruntenko YV, Budker VG, Vakhrusheva TE. Subcutaneously injected radiolabeled liposomes: transport to the lymph nodes in mice. J Natl Cancer Inst. 1982;69:67–71. [PubMed] [Google Scholar]
- Kaleem Z, Zehnbauer BA, White G, Zutter MM. Lack of expression of surface immunoglobulin light chains in B-cell non-Hodgkin lymphomas. Am J Clin Pathol. 2000;113:399–405. doi: 10.1309/28ED-MM0T-DT3B-MT4P. [DOI] [PubMed] [Google Scholar]
- Kanter G, Yang J, Voloshin A, Levy S, Swartz JR, Levy R. Cell-free production of scFv fusion proteins: an efficient approach for personalized lymphoma vaccines. Blood. 2007 Apr 15;109(8):3393–9. doi: 10.1182/blood-2006-07-030593. Epub 2006 Dec 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminski MS, Kitamura K, Maloney DG, Levy R. Idiotype vaccination against murine B cell lymphoma. Inhibition of tumor immunity by free idiotype protein. J Immunol. 1987;138:1289–1296. [PubMed] [Google Scholar]
- Keilholz U, Weber J, Finke JH, Gabrilovich DI, Kast WM, Disis ML, Kirkwood JM, Scheibenbogen C, Schlom J, Maino VC, Lyerly HK, Lee PP, Storkus W, Marincola F, Worobec A, Atkins MB. Immunologic monitoring of cancer vaccine therapy: results of a workshop sponsored by the Society for Biological Therapy. J Immunother. 2002;1997;25:97–138. doi: 10.1097/00002371-200203000-00001. [DOI] [PubMed] [Google Scholar]
- King CA, Spellerberg MB, Zhu D, Rice J, Sahota SS, Thompsett AR, Hamblin TJ, Radl J, Stevenson FK. DNA vaccines with single-chain Fv fused to fragment C of tetanus toxin induce protective immunity against lymphoma and myeloma. Nat Med. 1998;4:1281–1286. doi: 10.1038/3266. [DOI] [PubMed] [Google Scholar]
- Koc O, Redfern C, Wiernik P, et al. Extended follow-up and analysis with central radiological review of patients receiving FavId® (Id/KLH) vaccine following rituximab. Blood. 2005;106:772. [Google Scholar]
- Küppers R. Mechanisms of B-cell lymphoma pathogenesis. Nature Reviews Cancer. 2005;5:251–262. doi: 10.1038/nrc1589. [DOI] [PubMed] [Google Scholar]
- Kwak LW, Campbell MJ, Zelenetz AD, Levy R. Combined syngeneic bone marrow transplantation and immunotherapy of a murine B-cell lymphoma: active immunization with tumor-derived idiotypic immunoglobulin. Blood. 1990;76:2411–2417. [PubMed] [Google Scholar]
- Kwak LW, Campbell MJ, Czerwinski DK, Hart S, Miller RA, Levy R. Induction of immune responses in patients with B-cell lymphoma against the surface-immunoglobulin idiotype expressed by their tumors. N Engl J Med. 1992;327:1209–1215. doi: 10.1056/NEJM199210223271705. [DOI] [PubMed] [Google Scholar]
- Kwak LW, Young HA, Pennington RW, Weeks SD. Vaccination with syngeneic, lymphoma-derived immunoglobulin idiotype combined with granulocyte/macrophage colony-stimulating factor primes mice for a protective T-cell response. Proc Natl Acad Sci U S A. 1996;93:10972–10977. doi: 10.1073/pnas.93.20.10972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak LW, Pennington R, Boni L, Ochoa AC, Robb RJ, Popescu MC. Liposomal formulation of a self lymphoma antigen induces potent protective antitumor immunity. J Immunol. 1998;160:3637–3641. [PubMed] [Google Scholar]
- Lambert SL, Okada CY, Levy R. TCR vaccines against a murine T cell lymphoma: a primary role for antibodies of the IgG2c class in tumor protection. J Immunol. 2004;172:929–936. doi: 10.4049/jimmunol.172.2.929. [DOI] [PubMed] [Google Scholar]
- Lee ST, Jiang YF, Park KU, Woo AF, Neelapu SS. BiovaxID: a personalized therapeutic cancer vaccine for non-Hodgkin's lymphoma. Exp Opin Biol Ther. 2007;7:113–122. doi: 10.1517/14712598.7.1.113. [DOI] [PubMed] [Google Scholar]
- Levitsky HI, Montgomery J, Ahmadzadeh M, Staveley-O'Carroll K, Guarnieri F, Longo DL, Kwak LW. Immunization with granulocyte-macrophage colony-stimulating factor-transduced, but not B7-1-transduced, lymphoma cells primes idiotype-specific T cells and generates potent systemic antitumor immunity. J Immunol. 1996;156:3858–3865. [PubMed] [Google Scholar]
- Li S, Eshleman JR, Borowitz MJ. Lack of surface immunoglobulin light chain expression by flow cytometric immunophenotyping can help diagnose peripheral B-cell lymphoma. Amer J Clin Pathol. 2002;118:229–234. doi: 10.1309/57G0-1BNF-KB9R-L4HN. [DOI] [PubMed] [Google Scholar]
- Linton PJ, Harbertson J, Bradley LM. A critical role for B-cells in the development of memory CD4 cells. J Immunol. 2000;165:5558–5565. doi: 10.4049/jimmunol.165.10.5558. [DOI] [PubMed] [Google Scholar]
- Longo DL, Duffey PL, Gribben JG, Jaffe ES, Curti BD, Gause BL, Janik JE, Braman VM, Esseltine D, Wilson WH, Kaufman D, Wittes RE, Nadler LM, Urba WJ. Combination chemotherapy followed by an immunotoxin (anti-B4-blocked ricin) in patients with indolent lymphoma: results of a phase II study. Cancer J. 2000;6:146–150. [PubMed] [Google Scholar]
- Lynch RG, Graff RJ, Sirisinha S, Simms ES, Eisen HN. Myeloma proteins as tumor-specific transplantation antigens. Proc Natl Acad Sci U S A. 1972;69:1540–1544. doi: 10.1073/pnas.69.6.1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier T, Tun-Kyi A, Tassis A, Jungius KP, Burg G, Dummer R, Nestle FO. Vaccination of patients with cutaneous T-cell lymphoma using intranodal injection of autologous tumor-lysate-pulsed dendritic cells. Blood. 2003;102:2338–2344. doi: 10.1182/blood-2002-08-2455. [DOI] [PubMed] [Google Scholar]
- Maloney DG, Liles TM, Czerwinski DK, Waldichuk C, Rosenberg J, Grillo-Lopez A, Levy R. Phase I clinical trial using escalating single-dose infusion of chimeric anti-CD20 monoclonal antibody (IDEC-C2B8) in patients with recurrent B-cell lymphoma. Blood. 1994;84:2457–2466. [PubMed] [Google Scholar]
- Maloney DG, Pender B, McCarthy E, Gold DP. FCgamma receptor polymorphisms do not influence the outcome of treatment with rituximab followed by active immunotherapy with Mitumprotimut-T (FavId®, Id-KLH) Blood. 2007;110:3416. [Google Scholar]
- Malyguine A, Strobl SL, Shafer-Weaver KA, Ulderich T, Troke A, Baseler M, Kwak LW, Neelapu SS. A modified human ELISPOT assay to detect specific responses to primary tumor cell targets. J Transl Med. 2004;2:9. doi: 10.1186/1479-5876-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick AA, Kumagai MH, Hanley K, Turpen TH, Hakim I, Grill LK, Tuse D, Levy S, Levy R. Rapid production of specific vaccines for lymphoma by expression of the tumor-derived single-chain Fv epitopes in tobacco plants. Proc Natl Acad Sci U S A. 1999;96:703–708. doi: 10.1073/pnas.96.2.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin P, Grillo-Lopez AJ, Link BK, Levy R, Czuczman MS, Williams ME, Heyman MR, Bence-Bruckler I, White CA, Cabanillas F, Jain V, Ho AD, Lister J, Wey K, Shen D, Dallaire BK. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four-dose treatment program. J Clin Oncol. 1998;16:2825–2833. doi: 10.1200/JCO.1998.16.8.2825. [DOI] [PubMed] [Google Scholar]
- Nattamai D, Neelapu SS. PD-1 expression is markedly upregulated on intratumoral CD4+ and CD8+ T cells in follicular lymphoma and is associated with T-cell exhaustion. Blood. 2007 Nov;110:2749. [Google Scholar]
- Neelapu SS, Baskar S, Gause BL, Kobrin CB, Watson TM, Frye AR, Pennington R, Harvey L, Jaffe ES, Robb RJ, Popescu MC, Kwak LW. Human autologous tumor-specific T-cell responses induced by liposomal delivery of a lymphoma antigen. Clin Cancer Res. 2004;10:8309–8317. doi: 10.1158/1078-0432.CCR-04-1071. [DOI] [PubMed] [Google Scholar]
- Neelapu SS, Gause BL, Nikcevich DA, Schuster SJ, Winter J, Gockerman JP, Loughran T, Jr, Takeshita K, Inghirami G, McGaughey D, Watson TM, Snow S, Kubovic P, Ferraro M, Jones E, Jaffe ES, Schwartzentruber DJ, Danforth D, Sherry R, Kass E, Van Waes C, Reynolds CW, Kwak LJ. Phase III randomized trial of patient-specific vaccination for previously untreated patients with follicular lymphoma in first complete remission: protocol summary and interim report. Clin Lymphoma. 2005a;6:61–64. doi: 10.3816/clm.2005.n.031. [DOI] [PubMed] [Google Scholar]
- Neelapu SS, Kwak LW, Kobrin CB, Reynolds CW, Janik JE, Dunleavy K, White T, Harvey L, Pennington R, Stetler-Stevenson M, Jaffe ES, Steinberg SM, Gress R, Hakim F, Wilson WH. Vaccine-induced tumor-specific immunity despite severe B-cell depletion in mantle cell lymphoma. Nat Med. 2005b;11:986–991. doi: 10.1038/nm1290. [DOI] [PubMed] [Google Scholar]
- Neelapu SS, Lee ST, Qin H, Cha SC, Woo AF, Kwak LW. Therapeutic lymphoma vaccines: importance of T-cell immunity. Expert Rev Vaccines. 2006;5:381–394. doi: 10.1586/14760584.5.3.381. [DOI] [PubMed] [Google Scholar]
- Neelapu SS, Gause BL, Harvey L, Lee ST, Frye AR, Horton J, Robb RJ, Popescu MC, Kwak LW. A novel proteoliposomal vaccine induces antitumor immunity against follicular lymphoma. Blood. 2007;109:5160–5163. doi: 10.1182/blood-2006-12-063594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson EL, Li X, Hsu FJ, Kwak LW, Levy R, Clayberger C, Krensky AM. Tumor-specific, cytotoxic T-lymphocyte response after idiotype vaccination for B-cell, non-Hodgkin's lymphoma. Blood. 1996;88:580–589. [PubMed] [Google Scholar]
- Oki Y, McLaughlin P, Fayad LE, Pro B, Mansfield PF, Clayman GL, Medeiros LJ, Kwak LW, Srivastava PK, Younes A. Experience with heat shock protein-peptide complex 96 vaccine therapy in patients with indolent non-Hodgkin lymphoma. Cancer. 2007;109:77–83. doi: 10.1002/cncr.22389. [DOI] [PubMed] [Google Scholar]
- O'Mahony D, Morris JC, Quinn C, Gao W, Wilson WH, Gause B, Pittaluga S, Neelapu S, Brown M, Fleisher TA, Gulley JL, Schlom J, Nussenblatt R, Albert P, Davis TA, Lowy I, Petrus M, Waldmann TA, Janik JE. A pilot study of CTLA-4 blockade after cancer vaccine failure in patients with advanced malignancy. Clin Cancer Res. 2007;13:958–964. doi: 10.1158/1078-0432.CCR-06-1974. [DOI] [PubMed] [Google Scholar]
- Oussoren C, Storm G. Liposomes to target the lymphatics by subcutaneous administration. Adv Drug Deliv Rev. 2001;50:143–156. doi: 10.1016/s0169-409x(01)00154-5. [DOI] [PubMed] [Google Scholar]
- Popescu MC, Robb RJ, Batenjany MM, Boni LT, Neville ME, Pennington RW, Neelapu SS, Kwak LW. A novel proteoliposomal vaccine elicits potent antitumor immunity in mice. Blood. 2007;109:5407–5410. doi: 10.1182/blood-2006-08-039446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Z, Richter G, Schuler T, Ibe S, Cao X, Blankenstein T. B cells inhibit induction of T cell-dependent tumor immunity. Nat Med. 1998;4:627–630. doi: 10.1038/nm0598-627. [DOI] [PubMed] [Google Scholar]
- Rao M, Alving CR. Delivery of lipids and liposomal proteins to the cytoplasm and Golgi of antigen-presenting cells. mangala.rao@na.amedd.army.milAdv Drug Deliv Rev. 2000;41:171–188. doi: 10.1016/s0169-409x(99)00064-2. [DOI] [PubMed] [Google Scholar]
- Redfern CH, Guthrie TH, Bessudo A, Densmore JJ, Holman PR, Janakiraman N, Leonard JP, Levy RL, Just RG, Smith MR, Rosenfelt FP, Wiernik PH, Carter WD, Gold DP, Melink TJ, Gutheil JC, Bender JF. Phase II trial of idiotype vaccination in previously treated patients with indolent non-Hodgkin's lymphoma resulting in durable clinical responses. J Clin Oncol. 2006;24:3107–3112. doi: 10.1200/JCO.2005.04.4289. [DOI] [PubMed] [Google Scholar]
- Rivera A, Chen CC, Ron N, Dougherty JP, Ron Y. Role of B cells as antigen-presenting cells in vivo revisited: antigen-specific B cells are essential for T cell expansion in lymph nodes and for systemic T cell responses to low antigen concentrations. Int Immunol. 2001;13:1583–1593. doi: 10.1093/intimm/13.12.1583. [DOI] [PubMed] [Google Scholar]
- Santos C, Stern L, Katz L, Watson T, Barry G. BiovaxID™ Vaccine Therapy of Follicular Lymphoma in First Remission: Long-Term Follow-Up of a Phase II Trial and Status of a Controlled, Randomized Phase III Trial. Blood. 2005;106:2441. [Google Scholar]
- Shen H, Whitmire JK, Fan X, Shedlock DJ, Kaech SM, Ahmed R. A specific role for B cells in the generation of CD8 T cell memory by recombinant Listeria monocytogenes. J Immunol. 2003;170:1443–1451. doi: 10.4049/jimmunol.170.3.1443. [DOI] [PubMed] [Google Scholar]
- Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- Stevenson GT, Stevenson FK. Antibody to a molecularly-defined antigen confined to a tumour cell surface. Nature. 1975;254:714–716. doi: 10.1038/254714a0. [DOI] [PubMed] [Google Scholar]
- Stevenson GT, Elliott EV, Stevenson FK. Idiotypic determinants on the surface immunoglobulin of neoplastic lymphocytes: a therapeutic target. Fed Proc. 1977;36:2268–2271. [PubMed] [Google Scholar]
- Sutmuller RP, van Duivenvoorde LM, van Elsas A, Schumacher TN, Wildenberg ME, Allison JP, Toes RE, Offringa R, Melief CJ. Synergism of cytotoxic T lymphocyte-associated antigen 4 blockade and depletion of CD25(+) regulatory T cells in antitumor therapy reveals alternative pathways for suppression of autoreactive cytotoxic T lymphocyte responses. J Exp Med. 2001;194:823–832. doi: 10.1084/jem.194.6.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson KJ, Mackinnon S. Role of allogeneic transplantation in low-grade lymphoma and chronic lymphocytic leukemia. Curr Opin Hematol. 2006;13:273–279. doi: 10.1097/01.moh.0000231426.23278.32. [DOI] [PubMed] [Google Scholar]
- Timmerman JM, Levy R. Linkage of foreign carrier protein to a self-tumor antigen enhances the immunogenicity of a pulsed dendritic cell vaccine. J Immunol. 2000;164:4797–4803. doi: 10.4049/jimmunol.164.9.4797. [DOI] [PubMed] [Google Scholar]
- Timmerman JM, Caspar CB, Lambert SL, Syrengelas AD, Levy R. Idiotype-encoding recombinant adenoviruses provide protective immunity against murine B-cell lymphomas. Blood. 2001;97:1370–1377. doi: 10.1182/blood.v97.5.1370. [DOI] [PubMed] [Google Scholar]
- Timmerman JM, Czerwinski DK, Davis TA, Hsu FJ, Benike C, Hao ZM, Taidi B, Rajapaksa R, Caspar CB, Okada CY, van Beckhoven A, Liles TM, Engleman EG, Levy R. Idiotype-pulsed dendritic cell vaccination for B-cell lymphoma: clinical and immune responses in 35 patients. Blood. 2002a;99:1517–1526. doi: 10.1182/blood.v99.5.1517. [DOI] [PubMed] [Google Scholar]
- Timmerman JM, Singh G, Hermanson G, Hobart P, Czerwinski DK, Taidi B, Rajapaksa R, Caspar CB, Van Beckhoven A, Levy R. Immunogenicity of a plasmid DNA vaccine encoding chimeric idiotype in patients with B-cell lymphoma. Cancer Res. 2002b;62:5845–5852. [PubMed] [Google Scholar]
- van Essen D, Dullforce P, Brocker T, Gray D. Cellular interactions involved in Th cell memory. J Immunol. 2000;165:3640–3646. doi: 10.4049/jimmunol.165.7.3640. [DOI] [PubMed] [Google Scholar]
- Van Slooten ML, Boerman O, Romoren K, Kedar E, Crommelin DJ, Storm G. Liposomes as sustained release system for human interferon-gamma: biopharmaceutical aspects. Biochim Biophys Acta. 2001;1530:134–145. doi: 10.1016/s1388-1981(00)00174-8. [DOI] [PubMed] [Google Scholar]
- Vose JM. Personalized immunotherapy for the treatment of non-Hodgkin's lymphoma: a promising approach. Hematol Oncol. 2006;24:47–55. doi: 10.1002/hon.770. [DOI] [PubMed] [Google Scholar]
- Wassef NM, Alving CR, Richards RL. Liposomes as carriers for vaccines. Immunomethods. 1994;4:217–222. doi: 10.1006/immu.1994.1023. [DOI] [PubMed] [Google Scholar]
- Weng WK, Czerwinski D, Timmerman J, Hsu FJ, Levy R. Clinical outcome of lymphoma patients after idiotype vaccination is correlated with humoral immune response and immunoglobulin G Fc receptor genotype. J Clin Oncol. 2004;22:4717–4724. doi: 10.1200/JCO.2004.06.003. [DOI] [PubMed] [Google Scholar]; J Clin Oncol. 2005;23:248. Erratum in. [Google Scholar]
- Wierda WG, Cantwell MJ, Woods SJ, Rassenti LZ, Prussak CE, Kipps TJ. CD40-ligand (CD154) gene therapy for chronic lymphocytic leukemia. Blood. 2000;96:2917–2924. [PubMed] [Google Scholar]
- Witzig TE, Vukov AM, Habermann TM, Geyer S, Kurtin PJ, Friedenberg WR, White WL, Chalchal HI, Flynn PJ, Fitch TR, Welker DA. Rituximab therapy for patients with newly diagnosed, advanced-stage, follicular grade I non-Hodgkin's lymphoma: a phase II trial in the North Central Cancer Treatment Group. J Clin Oncol. 2005;23:1103–1108. doi: 10.1200/JCO.2005.12.052. [DOI] [PubMed] [Google Scholar]
- Yang X, Brunham RC. Gene knockout B cell-deficient mice demonstrate that B cells play an important role in the initiation of T cell responses to Chlamydia trachomatis (mouse pneumonitis) lung infection. J Immunol. 1998;161:1439–1446. [PubMed] [Google Scholar]
- Yang ZZ, Novak AJ, Stenson MJ, Witzig TE, Ansell SM. Intratumoral CD4+CD25+ regulatory T-cell-mediated suppression of infiltrating CD4+ T cells in B-cell non-Hodgkin lymphoma. Blood. 2006;107:3639–3646. doi: 10.1182/blood-2005-08-3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang ZZ, Novak AJ, Ziesmer SC, Witzig TE, Ansell SM. Attenuation of CD8(+) T-cell function by CD4(+)CD25(+) regulatory T cells in B-cell non-Hodgkin's lymphoma. Cancer Res. 2006;66:10145–10152. doi: 10.1158/0008-5472.CAN-06-1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5:263–274. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]