Abstract
Protein kinase C-related kinases (PRKs) are regulated by PI-3 kinase and Rho family GTPases. The isoform PRK1 has been characterized in detail in prostate cancer, but not in other carcinomas. We analyzed our prior microarray data for PRK1 gene expression in 175 carcinomas, and evaluated tissue microarrays for protein expression in 251 carcinomas and a comprehensive group of normal tissues. We also used immunoblotting to determine the levels and phospho-activation status of PRK1, PRK2, and PDK1 in 12 ovarian serous carcinomas, SKOV3 cells, and three samples of normal ovarian surface epithelium. The highest average level of PRK1 mRNAwas observed in ovarian serous carcinomas compared to all other carcinomas, including those of the prostate, bladder/ureter, breast, colon, stomach/esophagus, kidney, liver, pancreas, and lung (p=0.05). By immunohistochemistry, PRK1 was observed in selected normal cells including epithelium from the gynecological tract and hematolymphoid elements. All serous ovarian and endometrial endometrioid adenocarcinomas, and mesotheliomas were immunoreactive for PRK1. Nonserous ovarian and a majority of carcinomas from the prostate, breast, and pancreas were also positive but less consistently so. In comparison to ovarian surface epithelium, the serous carcinomas typically had greater pPRK1/total PRK1 (p=0.02) as well as greater pPDK/total PDK (p=0.01). The relative phosphorylation status of these two kinases correlated within each sample. In summary, PRK1 is present in various malignancies, but especially in serous carcinomas, where the increased activation status of PRK1 and its upstream regulator, PDK, as compared to normal ovarian surface epithelium suggests a role in ovarian cancer development or progression.
Introduction
Alterations in the phosphatidylinositol-3-kinase (PI-3 kinase) signaling pathway are commonly found in human cancers. Oncogenic mutation and amplification have been documented for PIK3CA, the gene that encodes the 110 kDa catalytic subunit of PI-3 kinase [1,2]. In ovarian carcinoma, PIK3CA amplification may even contribute to the development of cisplatin resistance [3]. Excessive signaling through the PI-3 kinase pathway can also result from loss of PTEN, the phosphatase that negatively regulates PI-3 kinase signaling by converting PIP3 to PIP2. PIP3 production results in activation of PDK1, which, in turn, activates AKT by direct phosphorylation. AKT phosphorylates multiple targets and regulates pathways that promote cell growth and proliferation, and suppresses apoptosis [4]. AKT2 amplification has been demonstrated in some cancers including high-grade serous carcinomas of the ovary [5,6].
PI-3 kinase signaling regulates the protein kinase C-related proteins (PRK), also known as PKN proteins, through the intermediary PDK1. PDK phosphorylates the activation loop of PRK proteins (PRK1, 2, 3), which is critical for serine/threonine kinase activity. PRK proteins are also regulated by Rho GTPases, which bind to an N-terminal hydrophobic region of PRK [7]. PRK proteins contain a C2 domain that binds phospholipids. While PRK proteins have not been studied as extensively as other PKC family members, the available data provide a reasonable model to explain how PRK undergoes activation in response to factors such as insulin which promotes PI-3 kinase-dependent signaling.
One setting where PRK1 function has been analyzed is prostate cancer in which this isoform has been shown to interact with the androgen receptor (AR) and modulate the expression of androgen-regulated genes. PRK1 phosphorylates histone H3 within the promoters of AR regulated genes, an event that promotes the recruitment of demethylases and activation of transcription [8]. PRK1 is extremely low or absent in normal prostate epithelium, while PRK1 levels are prominent in prostate cancer and increase with Gleason score [9]. Inhibition of PRK1 has been demonstrated to decrease AR-induced proliferation in cultured cells, suggesting PRK1 is an important effector of androgen signaling [8]. In addition, PRK3 expression in AR-negative PC-3 cells is important for PI-3 kinase-dependent invasive properties of these cells [10].
As in prostate cancer, androgens have been linked to the pathogenesis of ovarian carcinoma. A prospective cohort study showed significantly increased serum androgen levels in women who developed ovarian carcinoma [11], while a case-controlled study indicated that women with excess androgen syndromes were at increased risk for ovarian carcinoma. [12] Others have shown that a shorter AR gene allele is associated with a younger age at diagnosis for patients with ovarian carcinoma, similar to that for those with prostate cancer. [13] The shorter alleles inversely correlate to AR activity, and may be evidence for the use of androgen antagonists in the treatment of ovarian carcinoma. [14]
Frequent alterations in the upstream signaling pathway of PRK and its demonstrated role in AR regulation suggest that PRK proteins could be important as therapeutic targets. This warrants the development of a thorough understanding of PRK function, regulation, and tissue distribution.
Here, we describe the distribution of PRK1 protein in normal tissues and both PRK1 mRNA and protein in the ten most common types of fatal carcinoma. We found that PRK1 is overexpressed in a wide variety of human cancers, particularly including ovarian serous carcinoma. This led us to examine by immunoblotting the levels and activation states of PRK1 and PRK2 and the upstream kinase PDK1 in ovarian cancers and normal ovarian surface epithelium (OSE). The ratio of phosphorylated: total PRK1 and PRK2 was higher in most ovarian carcinomas compared to normal OSE. These data together with previous work on PRK in prostate cancer [9] suggest that PRK might be an important effector of PI-3 kinase signaling in multiple human cancers.
Materials and Methods
Oligonucleotide microarray
The University of Virginia Human Institutional Review Board for Health Sciences Research approved the use of human tissues in this study. PRK1 is one of more than 8,900 different human genes represented on U95a GeneChips (Affymetrix, Santa Clara, CA), which we used previously to develop a molecular classification of 175 human carcinomas based on patterns of gene expression [15]. In the current investigation, we focused on the PRK1 expression data from this previous more global study. With the exception of the ovarian serous carcinomas, samples from the same tumors analyzed for gene expression were also examined for PRK1 by immunohistochemistry on tissue microarrays (see below). In brief, frozen sections of profiled tumors were examined by hematoxylin-and-eosin stain, and areas rich in neoplastic cells were cut from the frozen blocks. RNA extraction and hybridization onto oligonucleotide microarrays (U95a GeneChip; Affymetrix) were performed as reported previously [16]. The processing and scaling of the hybridization data was also performed as described previously [17,18].
Tissue microarray
Tissue microarrays (TMAs) containing 1 to 3 0.6-mm cores from formalin-fixed, paraffin-embedded blocks were constructed using a tissue microarrayer (Beecher Instruments, Silver Spring, MD). These TMAs, some of which were constructed by the Cooperative Human Tissue Network, included a comprehensive selection of normal tissues (Table 1) and carcinomas from adult patients. Selected whole tissue sections were stained for comparison with the tissue microarrays. In addition, whole tissue sections were used for most of the serous ovarian carcinomas, endometrial endometrioid adenocarcinomas, and six examples of normal or reactive pleural or peritoneal mesothelium.
Table 1.
Normal tissues examined for PRK1 protein by immunohistochemistry. Tissues showing some degree of staining for PRK1 are indicated by “*”.
| Aerodigestive | Hepatic and Pancreaticobiliary |
| Alveoli | Bile ducts |
| Bronchial epithelium* | Liver |
| Salivary gland-parotid and minor | Gallbladder |
| Endocrine | Pancreas ducts* |
| Adrenal cortex | Mesothelium* |
| Adrenal medulla | Musculoskeletal |
| Parathyroid* | Cartilage |
| Pituitary | Fat |
| Thyroid | Skeletal muscle |
| Gastrointestinal | Synovium |
| Anal canal | Neural |
| Appendix | Cerebral cortex |
| Colon | Choroid plexus |
| Duodenum | Ependyma |
| Esophagus | Ganglia |
| Ileum | Hippocampus |
| Gynecological and Breast | Meninges |
| Bartholin’s gland ducts* | Nerve, peripheral |
| Breast ductal epithelium* | Motor neurons |
| Ectocervix | Purkinje cells |
| Endocervix | White matter |
| Endometrium | Skin and Lacrimal gland |
| Decidualized* | Dermis and epidermis |
| Proliferative* | Lacrimal gland |
| Secretory* | Urogenital |
| Fallopian tube epithelium* | Bladder |
| Myometrium | Epididymis, basal cells* |
| Ovary | Kidney, distal tubule epithelium* |
| Primary follicle* | Prostate |
| Surface epithelium* | Rete testes* |
| Stroma | Seminal vesicle |
| Placenta | Seminiferous tubules* |
| Amnion | Vas deferens |
| Chorionic villi | Vascular |
| Umbilical cord | Aorta, endothelium* |
| Hematolymphoid | Lymphatic endothelium |
| Bone marrow elements* | Myocardium |
| Lymph node* | Pulmonary artery* |
| Spleen* | Small vein, intestinal* |
| Thymus* | |
| Tonsil* |
Immunohistochemistry
Slides from zinc formalin-fixed, paraffin-embedded tissues were placed in citrate buffer (pH 6.0) and heated in a microwave oven for 20 minutes before application of the monoclonal antibody to PRK1 (clone 49 at 1:50 dilution with overnight incubation; BD Biosciences, San Jose, CA). After incubation with the primary antibody, and addition of the biotinylated secondary antibody, avidin-biotin immunoperoxidase was applied. Diaminobenzidine was used as the chromogen. Sections were then counterstained with hematoxylin. Tissue sections of a human prostate cancer processed in a comparable manner provided control tissue. Immunoreactivity was evaluated simultaneously by two observers without specific knowledge of the oligonucleotide microarray results. Cytoplasmic staining was graded for intensity: 0, 1 (weak), 2 (moderate), and 3 (strong); and percentage of positive cells: 0, 1 (1–33%), 2 (34–66%), and 3 (67–100%). Scores for staining intensity and percentage of positive cells were multiplied for an H-score. The H-scores for tumors with multiple cores were generally equal across cores, but discordant scores were averaged. Protein expression was then defined by the H-score: negative (0), weak (1–3), or strong (>3).
Western blot
Samples of normal ovarian surface epithelium from three cases were obtained by identifying non-pathologic ovaries removed for benign diseases of the contralateral ovary or uterus. The entire surface was scraped with a sterile surgical blade which was rinsed in RPMI solution. The RPMI solution was then centrifuged and the supernatant removed. Scrape preparations were made from the sediment and stained with hematoxylin-and-eosin to confirm the presence of normal ovarian epithelium. The pellet was then frozen in liquid nitrogen and stored in a −80°C freezer. Fresh samples of ovarian serous carcinoma were frozen in liquid nitrogen prior to storage at −80°C. Frozen section hematoxylin- and-eosin-stained slides were cut from the tumor samples to identify and selectively dissect areas rich in neoplastic tissue. This resulted in tissue sections composed of an estimated 75% serous carcinoma cells with fewer than 5% inflammatory cells in 10 of the 12 samples. SKOV3 serous carcinoma cells obtained from the American Type Culture Collection (ATCC) were also included for Western blot analysis.
The frozen tissue samples, embedded in OCT compound medium (Tissue-Tek, Sakura, Inc, Torrence, CA), were incubated in 25 mls of PBS/1 mM PMSF at room temperature with rocking. The buffer was decanted and the process was repeated. The tumor material was then resuspended in five volumes (weight:volume) of ice cold tumor extraction buffer (50mM Tris pH 8, 250mM sucrose, 50mM NaCl, 1mM EDTA, 1mM EGTA, 1 mM DTT) containing phosphatase inhibitor cocktails 1 and 2 (P2850 & P5726, Sigma, St. Louis, MO) and a protease inhibitor tablet (11873580001, Roche, Mannheim, Germany). The tumors were fragmented on ice using an electric tissue disruptor. They were then sonicated using a micro-tip sonicator (output: 4, 10 sec, 90% duty cycle) (model 250/450 Branson Ultrasonics, Danbury, CT) and then centrifuged for 15 min at 4°C. The supernatant was collected and protein concentrations were measured by the BCA method (B9643 & C2284, Sigma, St. Louis, MO). 50 ug of each tumor sample were separated by SDS PAGE (7% gels) and transferred to a nitrocellulose membrane. Antibody incubations and washes were performed at room temperature with gentle agitation. Primary antibodies were incubated with blots for 2 hrs, followed by three 5 min washes in PBS containing 0.2% Tween (PBST). Secondary antibodies were incubated with blots for 1 hr, followed by three 5 min washes in PBST. Secondary antibodies were detected by enhanced chemiluminescence (32106, Pierce, Rockford, IL) and exposure to autoradiography film (AF8100, Marsh Bio Products, Rochester, NY).
The following antibodies and dilutions were used: PRK1 and PRK2 (each at 1:1000, BD Transduction Laboratories, Bedford, MA); phospho-PRK1/2 (1:1000), PDK1 (1:1000), phospho-PDK1 (1:500)(each from Cell Signaling, Beverly, MA); HSP 70 (1:1000, Stressgen, Ann Arbor, MI); and tubulin (1:10,000, Sigma, St. Louis, MO). Peroxidase-labeled goat anti-rabbit (1:5,000) and goat anti-mouse (1:5,000, Pierce, Rockford, IL) antibodies were also used.
Statistical analysis
In comparing the gene expression for PRK1 in serous ovarian cancers with that for other carcinomas, the differences in mean log-fluorescence intensity units from the ovarian tumors minus the mean log-fluorescence units for each of the other cancers were calculated. The 95% confidence intervals for the differences, not adjusted for multiple comparisons were determined. The values re-expressed in terms of fold differences were also calculated. For the Western blot analysis, the means and standard deviations for total PRK1, PRK2, and PDK for the normal ovarian epithelium were compared with that for the ovarian cancers. The Kruskal-Wallis test was used for analysis. P-values ≤ 0.05 were considered significant.
Results
Oligonucleotide microarray analysis
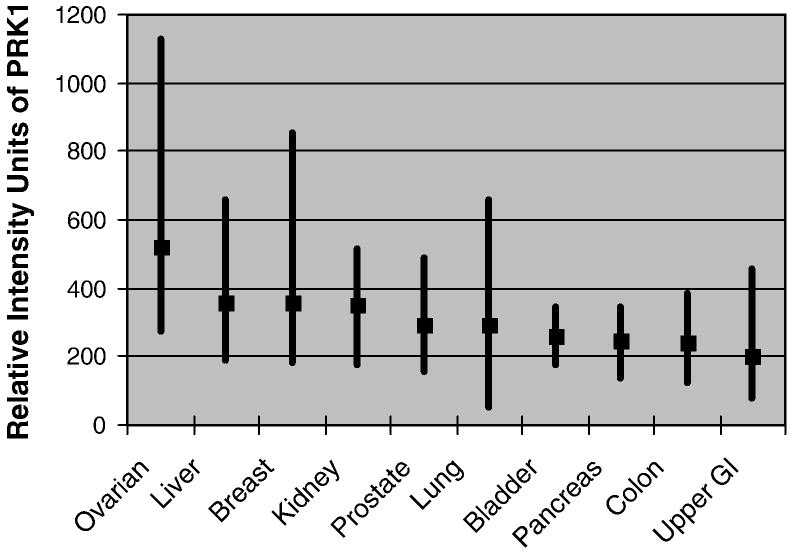
In our survey of the ten most common types of fatal human carcinomas, the highest expression levels for PRK1 were observed in the serous ovarian carcinomas (N=26) with relative intensity units averaging 520 (range 273–1130) (see Fig. 1). The remaining carcinomas had mean relative intensity units ranging from 200 to 360. Hence, the mean ratio for the ovarian carcinomas relative to the others ranged from 1.4–2.6. Although there was some overlap in PRK1 expression among individual tumor types, the difference in expression for the ovarian cancers compared with that for the other types of carcinoma was statistically significant (P<.05).
Fig. 1.
Mean and range of relative fluorescent intensity units from oligonucleotide microarray gene expression for PRK1 in the ten most common lethal carcinomas. The highest mean expression was observed in serous carcinomas of the ovary (p < 0.05).
Immunohistochemistry of normal adult tissues
Immunohistochemical staining for PRK1 in tissue microarrays was consistently demonstrated in the cytoplasm of cells in the gynecologic tract, including epithelium from the endometrium (with proliferative phase being stronger than secretory), fallopian tube, and Bartholin’s gland (with ducts stronger than acini). The ovarian surface epithelium was also positive (but variable, ranging from negative/weak to moderate in intensity) along with follicle cells (Fig. 2). Normal or reactive pleural and peritoneal mesothelium stained similarily to that of the ovarian surface epithelium.
Fig. 2.
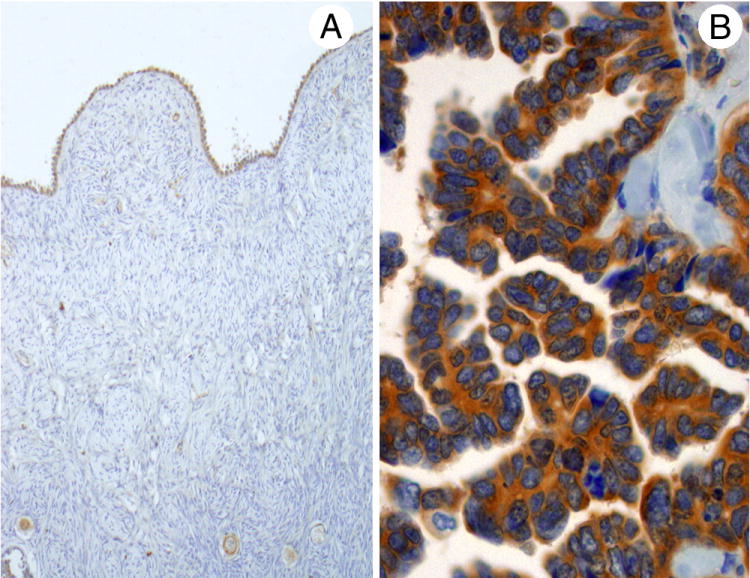
Staining for PRK1 was seen in normal ovarian surface epithelium (A) and ovarian serous carcinomas (B). Positivity for the serous carcinomas was usually strong, while that for the normal ovarian epithelium varied from negative/weak to moderate.
PRK1 was also noted in hematolymphoid tissues, including bone marrow elements, histiocytes, and lymphocytes from a reactive lymph node, spleen, thymus, and tonsillar tissue. Variable staining was detected in plasma cells and segmented neutrophils. Melanocytes and vascular endothelium were also positive, with the latter showing variable expression. Within the male genitourinary tract, reactivity was noted in Sertoli cells, rete testis epithelium, and the basal cells of the epididymis. Parathyroid glands, pancreatic ducts, breast ductules, bronchial epithelium, and renal distal convoluted tubules also demonstrated notable staining. The remaining tissues examined (Table 1) lacked immunoreactivity for PRK1.
Immunohistochemistry of malignant tissues
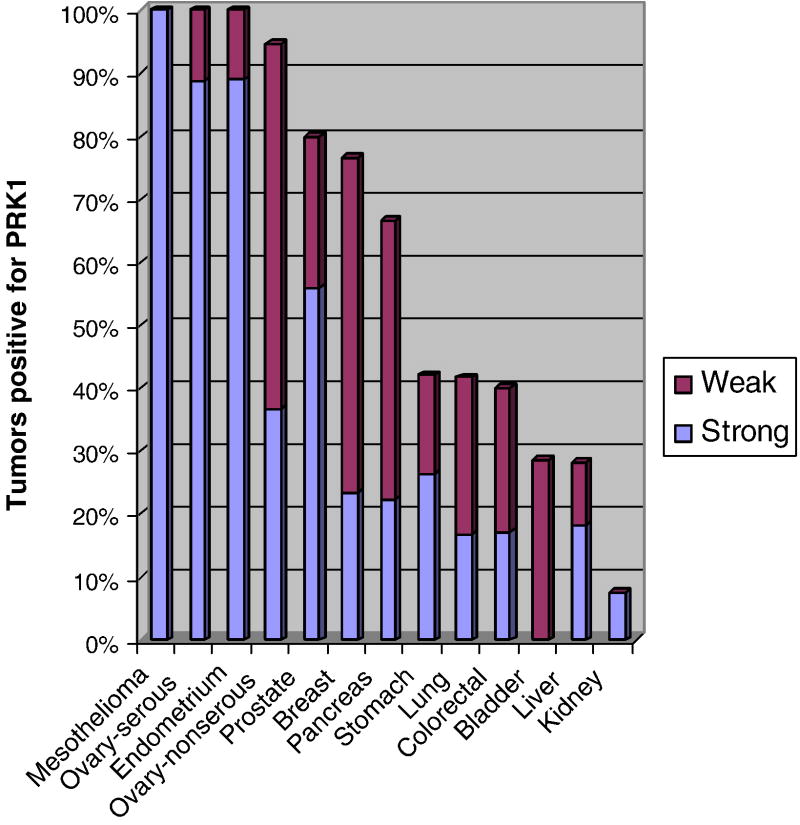
Staining for PRK1 in malignant tissues is summarized in Figure 3. All serous ovarian tumors (N=39) including three borderline tumors, four low-grade, and 32 high-grade carcinomas were positive (Fig. 2). Reactivity was also noted in six of seven endometrioid carcinomas and each of four clear cell carcinomas of the ovary. While some degree of staining was present in each of eight mucinous ovarian neoplasms, it was extremely focal and weak. Each of nine endometrioid adenocarcinomas of the endometrium was also positive.
Fig. 3.
PRK1 immunoreactivity in malignant neoplasms. Degree of staining was scored as negative (H-score = 0), weak (H-score = 1–3), and strong (H-score > 3). Tumors with the most frequent strong staining scores were ovarian serous carcinomas, endometrial carcinomas, and mesotheliomas.
All mesotheliomas (seven spindle cell and 11 epithelioid) were positive for PRK1. Immunoreactivity was also noted in 80% of 25 prostate adenocarcinomas and 76% of 30 invasive ductal breast carcinomas. Primary tumors of the digestive tract were often positive, including 66% of pancreatic (N= nine), 42% of gastric (N=19), and 40% of colorectal carcinomas (N=35). 42% of the lung carcinomas were reactive, including each of three neuroendocrine carcinomas, six of 16 adenocarcinomas, five of 15 squamous cell carcinomas, and one of two large cell undifferentiated carcinomas. Urothelial carcinomas of the bladder or ureter (N=seven) and hepatocellular carcinomas (N=11) were positive in 29% and 28% of tumors, respectively. Only a single renal cell carcinoma (N=12, conventional, clear cell-type) had PRK1 immunoreactivity.
Immunoblotting of Normal OSE and Ovarian Carcinomas
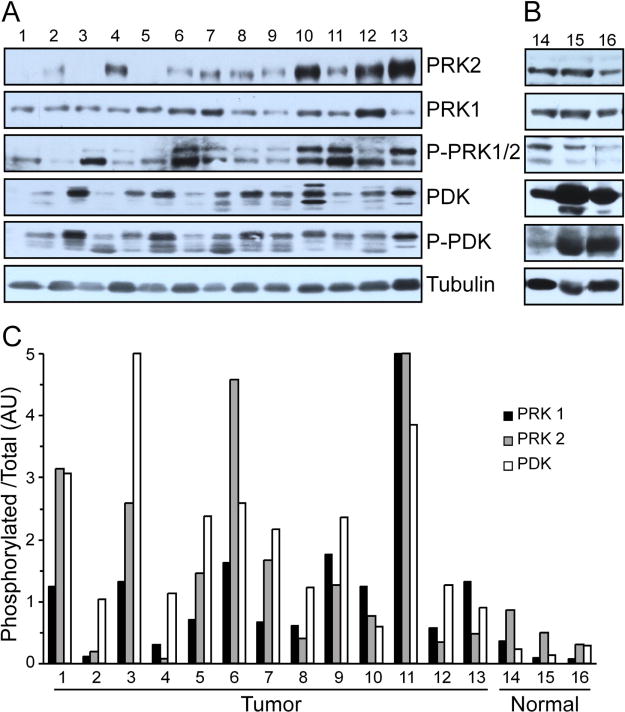
In comparison to normal OSE, the serous ovarian carcinomas had higher levels of PRK1 (p=0.02) and PDK (p=0.01), but not PRK2 (see Fig 4a–b). Ten of the 12 carcinomas had a ratio of pPRK1 to total PRK1 greater than what was observed in the normal samples (p=0.02) (see Fig 4c). All 12 carcinomas had a ratio of pPDK to total PDK greater than what was observed in the normal samples (p=0.01). The relative phosphorylation status of PRK1 and PDK correlated within each tumor sample. The SKOV3 cell line also had pPRK1: total PRK1 and pPDK: total PDK greater than that for the normal samples. While seven of the 12 carcinomas had a ratio of pPRK2 to total PRK2 greater than what was observed in the normal samples, this was not statistically significant.
Fig. 4.
(A) Twelve samples of ovarian serous carcinoma (1–12) and the SKOV3 cell line (13) had statistically higher levels of PRK1 and PDK, but not PRK2, than the (B) three samples of normal ovarian surface epithelium (14–16). (C) The phosphorylation status normalized to total for the tumor samples and cell line was significantly greater than that for the normal ovarian surface epithelium for PRK1 and PDK, but not PRK2.
Discussion
The protein kinase C-related kinases PRK1, 2, and 3 are regulated by PI-3 kinase signaling and by Rho binding [10]. PRK1 and PRK3 have been found in prostate adenocarcinomas, in which the former has been shown to enhance AR activity, even in the presence of the antagonist cyproterone acetate [9]. PRK3 function in the prostate cancer cell line PC-3 appears to be important for the invasive properties of these cells, both in 3D culture and following orthotopic injection. PRK1 has not been comprehensively analyzed by tissue microarray in normal adult tissues or cancers, including those of the ovary, which like prostate cancers, have AR signaling as an important pathway. AR has been shown to be present in normal ovarian epithelium, ovarian cancer cell lines, and human ovarian carcinomas. [19,20,21] Furthermore, in ovarian cancer cell culture, androgens promote cell growth, while flutamide, which antagonizes AR, abolishes androgen-stimulated growth. [19,21]
We observed PRK1 immunopositivity in cells of the hematolymphoid system, mesothelium, and in a wide variety of epithelial cell types. PRK1 was present in epithelial cells of the gynecologic tract including those of the endometrium, fallopian tube, and ovary. Elsewhere, PRK1 was observed in ducts of the breast and pancreas, and in bronchial epithelium. Notably, it was absent in normal epithelium of the prostate, bladder, stomach, colon, and liver. Although PRK1 was often present in carcinomas (such as tumors of the ovary, breast, and pancreas) derived from normal epithelium that expressed this protein, it was also often seen in neoplasms from sites whose normal epithelium lacked PRK1. The finding of PRK1 in carcinomas of the prostate, colon, and stomach, for instance, indicates deregulation of this kinase, and suggests the importance of the PI-3 kinase signaling pathway in these neoplasms.
Our survey of the ten most common types of fatal carcinomas in humans detected the highest average level of PRK1 mRNA in ovarian serous carcinomas, significantly higher than that for all of the others (p=0.05). Using the Oncomine database for tumor expression (www.oncomine.org), we searched for PRK1 in 1,911 neoplasms of diverse types surveyed by the International Genomics Consortium Expression Project for Oncology (Phoenix, AZ) (https://expo.intgen.org/expo/public/2006/01/01). Using Affymetrix U133 plus 2.0 arrays for neoplasms in which more than five of a specific type were examined, the highest median normalized expression was found for carcinomas of the ovary (N=241), fallopian tube (N=6), and peritoneum (N=14). At the protein level, we found a high level of positivity for PRK1 in nearly 90% of serous ovarian carcinomas. Endometrial endometrioid adenocarcinomas and mesotheliomas were not included in our gene expression array, but also demonstrated frequent strong positivity for PRK1 by immunohistochemistry. Staining of mesotheliomas and serous ovarian carcinomas is of interest given the similarities between these tumors, and the fact that both had weaker protein expression in the normal tissues compared with that of the corresponding tumors.
Using the Oncomine database for ovarian tumors from the study of Jazaeri et al., the highest median normalized expression for PRK1 was found for serous carcinomas (N=35) compared with endometrioid (N=5), clear cell (N=3), and adenocarcinomas not otherwise specified (N=16) [22]. As we also found relatively high PRK1 mRNA and protein in ovarian serous carcinomas, we explored the levels of activated PRK1, total and activated levels of its paralog PRK2, and the immediate upstream regulator of PRK1, PDK, in examples of this type of ovarian tumor and normal ovarian epithelium. Previously, Lu et al. showed that PRK1 mRNA levels were higher in serous carcinomas compared to that for normal ovarian surface epithelium (Oncomine database) [23]. Although normal ovarian surface epithelium contained PRK1, the phosphorylation states of both PRK1 and PDK were increased in ovarian carcinomas. While this could suggest a role for PRK1 in ovarian cancer development or progression, the mechanism responsible for overexpression is unclear. As PRK1 is downstream of PI-3 kinase, it is possible that in some cases amplification of PIK3CA leads to PRK1 overexpression. In one study, amplification of PIK3CA occurred in 13% of high-grade serous carcinomas [5]. More recently, PIK3R3 mRNA was found to be higher in ovarian cancers compared with that for normal ovary [24]. Chronic PI-3 kinase signaling in PC-3 cells due to loss of PTEN is partly responsible for PRK3 levels in these cells [10]. Thus, it is possible that tumors expressing higher levels of PI-3 kinase might promote PRK1 expression or protein stabilization as part of a feed forward mechanism.
In summary, our results show that PRK1 is present in normal cells of the hematolymphoid system, mesothelium and a variety of epithelia including those from the gynecologic tract. It is also variably expressed by a wide variety of carcinomas, and most consistently present in malignant mesotheliomas, endometrial adenocarcinomas, and serous ovarian carcinomas. Of the common fatal carcinomas, PRK1 mRNA is most highly expressed in serous ovarian cancers, where it and its upstream regulator, PDK1, are readily detectable in a phospho-activated state. While information regarding the direct substrates of PRK phosphorylation is currently limited to histone H3 [8], factors involved in transcriptional regulation and function of the actin cytoskeleton are clearly anticipated. Our analysis of PRK1 in ovarian carcinoma should provide a setting for defining downstream targets and developing insight into a relatively unexplored branch of the PI-3 kinase pathway. Finally, the interaction between PRK1 and AR and the subsequent modulation of the expression of androgen-regulated genes in ovarian carcinomas remains to be examined.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hennessy BT, Smith DL, Ram PT, Lu Y, Mills GB. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat Rev Drug Discov. 2005;4:988–1004. doi: 10.1038/nrd1902. [DOI] [PubMed] [Google Scholar]
- 2.Samuels Y, Ericson K. Oncogenic PI3K and its role in cancer. Curr Opin Oncol. 2006;18:77–82. doi: 10.1097/01.cco.0000198021.99347.b9. [DOI] [PubMed] [Google Scholar]
- 3.Lee S, Choi EJ, Jin C, Kim DH. Activation of PI3K/Akt pathway by PTEN reduction and PIK3CA mRNA amplification contributes to cisplatin resistance in an ovarian cancer cell line. Gynecol Oncol. 2005;97:26–34. doi: 10.1016/j.ygyno.2004.11.051. [DOI] [PubMed] [Google Scholar]
- 4.Vivanco I, Sawyers CL. The phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 5.Nakayama K, Nakayama N, Kurman RJ, et al. Sequence mutations and amplification of PIK3CA and AKT2 genes in purified ovarian serous neoplasms. Cancer Biol Ther. 2006:5. doi: 10.4161/cbt.5.7.2751. [DOI] [PubMed] [Google Scholar]
- 6.Bellacosa A, de Feo D, Godwin AK, et al. Molecular alterations of the AKT2 oncogene in ovarian and breast carcinomas. Int J Cancer. 1995;64:280–285. doi: 10.1002/ijc.2910640412. [DOI] [PubMed] [Google Scholar]
- 7.Mellor H, Parker PJ. The extended protein kinase C superfamily. Biochem J. 1998;332 (Pt 2):281–292. doi: 10.1042/bj3320281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Metzger E, Yin N, Wissmann M, et al. Phosphorylation of histone H3 at threonine 11 establishes a novel chromatin mark for transcriptional regulation. Nat Cell Biol. 2008;10:53–60. doi: 10.1038/ncb1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Metzger E, Muller JM, Ferrari S, Buettner R, Schule R. A novel inducible transactivation domain in the androgen receptor: Implications for PRK in prostate cancer. EMBO J. 2003;22:270–280. doi: 10.1093/emboj/cdg023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leenders F, Mopert K, Schmiedeknecht A, et al. PKN3 is required for malignant prostate cell growth downstream of activated PI 3-kinase. EMBO J. 2004;23:3303–3313. doi: 10.1038/sj.emboj.7600345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Helzlouer KJ, Alberq AJ, Gordon GB, et al. Serum gonadotropins and steroid hormones and the development of ovarian cancer. JAMA. 1995;274:1926–30. [PubMed] [Google Scholar]
- 12.Schildkraut JM, Schwingl PJ, Bastos E, Evanoff A, Hughes C. Epithelial ovarian cancer risk among women with polycystic ovarian syndrome. Obstet Gynecol. 1996;88:554–9. doi: 10.1016/0029-7844(96)00226-8. [DOI] [PubMed] [Google Scholar]
- 13.Levine DA, Boyd J. The androgen receptor and genetic susceptibility to ovarian cancer: results from a case series. Cancer Res. 2001;61:908–11. [PubMed] [Google Scholar]
- 14.Li AJ, Scoles DR, Armstrong KU, Karlan BY. Androgen receptor cytosine-adenine-guanine repeat polymorphisms modulate EGFR signaling in epithelial ovarian carcinomas. Gynecol Oncol. 2008;109:220–5. doi: 10.1016/j.ygyno.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 15.Su A, Welsh J, Sapinoso L, et al. Molecular classification of human carcinomas by use of gene expression signatures. Cancer Res. 2001;61:7388–7393. [PubMed] [Google Scholar]
- 16.Welsh J, Zarrinkar P, Sapinoso L, et al. Analysis of gene expression profiles in normal and neoplastic ovarian tissue samples identifies candidate molecular markers of epithelial ovarian cancer. Proc Natl Acad Sci USA. 2001;98:1176–1181. doi: 10.1073/pnas.98.3.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lockhart D, Dong H, Byrne H, et al. Expression monitoring by hybridization to high-density oligonucleotide arrays. Nat Biotechnol. 1996;14:1675–1680. doi: 10.1038/nbt1296-1675. [DOI] [PubMed] [Google Scholar]
- 18.Wodicka L, Dong H, Mittmann M, Ho M, Lockhart D. Genome-wide expression monitoring in Saccharomyces cerevisiae. Nat Biotechnol. 1997;15:1359–1367. doi: 10.1038/nbt1297-1359. [DOI] [PubMed] [Google Scholar]
- 19.Wang Y, Yang J, Gao Y, et al. Reciprocal regulation of 5alpha-dihydrotestosterone, interleukin-6 and interleukin-8 during proliferation of epithelial ovarian carcinoma. Cancer Biol Ther. 2007;6:864–71. doi: 10.4161/cbt.6.6.4093. [DOI] [PubMed] [Google Scholar]
- 20.Edmondson RJ, Monaghan JM, Davies BR. The human ovarian surface epithelium is an adrogen responsive tissue. Br J Cancer. 2002;86:879–85. doi: 10.1038/sj.bjc.6600154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahonen MH, Zhuang YH, Aine R, Ylikomi T, Tuohimaa P. Androgen receptor and vitamin D receptor in human ovarian cancer: growth stimulation and inhibition by ligands. Int J Cancer. 2000;86:40–6. doi: 10.1002/(sici)1097-0215(20000401)86:1<40::aid-ijc6>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 22.Jazaeri AA, Yee CJ, Sotiriou C, et al. Gene expression profiles of BRCA1-linked, BRCA2-linked, and sporadic ovarian cancers. J Natl Cancer Inst. 2002;94:990–1000. doi: 10.1093/jnci/94.13.990. [DOI] [PubMed] [Google Scholar]
- 23.Lu KH, Patterson AP, Wang L, et al. Selection of potential markers for epithelial ovarian cancer with gene expression arrays and recursive descent partition analysis. Clin Cancer Res. 2004;10:3291–3300. doi: 10.1158/1078-0432.CCR-03-0409. [DOI] [PubMed] [Google Scholar]
- 24.Zhang L, Huang J, Yang N, et al. Integrative genomic analysis of phosphatidylinositol 3′-kinase family identifies PIK3R3 as a potential therapeutic target in epithelial ovarian cancer. Clin Cancer Res. 2007;13:5314–5321. doi: 10.1158/1078-0432.CCR-06-2660. [DOI] [PubMed] [Google Scholar]