Abstract
Aim
To compare the clinical and microbiological effects of scaling and root planing (SRP) alone or combined with mechanical (professional plaque control - PPC) or chemical (chlorhexidine rinsing - CHX) control of supragingival plaque in the treatment of chronic periodontitis.
Methods
Sixty subjects were randomly assigned to receive SRP alone or combined with PPC (twice a week) or with CHX rinsing (twice a day). The adjunctive treatments began with SRP and continued for 42 days. Clinical and microbiological examinations were performed at baseline, 2 and 6 months post-therapy. Subgingival plaque samples were analyzed for 38 bacterial species by checkerboard DNA-DNA hybridization.
Results
The two test treatments were more effective in improving probing depth and clinical attachment level (CAL) than SRP alone, even in intermediate and deep sites. CAL gain was better maintained in the CHX group. The most beneficial microbiological changes were observed in CHX-treated subjects, who showed a significant reduction in the proportions of red and orange complexes, as well as an increase in the proportions of the host-compatible bacterial species.
Conclusion
Strict plaque control performed during and after SRP improves periodontal treatment outcomes. The greatest microbiological and clinical benefits were observed with the use of CHX rinsing.
Keywords: periodontal disease, dental plaque, biofilm, chlorhexidine, supragingival plaque control, subgingival microbiota
INTRODUCTION
The idea of introducing strict professional plaque control as part of the active phase of periodontal therapy was first described in the 70's and 80's. Different groups of investigators suggested that the removal of supragingival plaque every two weeks for prolonged periods of time would benefit the clinical and microbiological outcomes of periodontal therapy (Nyman et al. 1975, Rosling et al. 1976, Lindhe et al. 1982, Westfelt et al. 1983, Magnusson et al. 1984). After these initial studies, this concept was not investigated to a great extent during the subsequent years.
More recently, the development of new molecular genetic diagnostic tests allowed a better understanding of the composition of supra and subgingival biofilms. Some studies suggested that several periodontal pathogens may colonize the supragingival biofilm at high levels and proportions, and this could contribute for the subgingival recolonization of recently treated sites by these species (Ximénez-Fyvie et al. 2000a, Shibli et al. 2007, Haffajee et al. 2008). This knowledge brought the idea of performing strict professional plaque control (PPC) as part of the periodontal treatment back on the scene again (Ximénez-Fyvie et al. 2000a, Haffajee et al, 2003, Carvalho et al. 2004, 2005). Ximénez-Fyvie et al. (2000a) suggested that a combined protocol of scaling and root planing (SRP) and weekly PPC for 3 months would have a strikingly positive effect on the composition of the subgingival microbiota of periodontal maintenance subjects. The authors evaluated a wide range of bacterial species using whole genomic DNA probes and the checkerboard DNA-DNA hybridization diagnostic test. Interestingly, this therapy was able to create a microbial profile comparable to that observed in periodontal health, which was maintained for at least 1 year post-SRP. Subsequently, using the same diagnostic test and a similar set of DNA probes, three other studies highlighted the beneficial clinical and microbiological effects of PPC used in conjunction with SRP and systemic metronidazole in subjects with more advanced chronic periodontitis (Haffajee et al. 2003, Carvalho et al. 2004, 2005). The results of these studies were encouraging; nevertheless, one limitation of this treatment protocol is the difficulty of keeping the patients returning to the dental office to have their teeth cleaned once a week for prolonged periods of time. Since commercially available antiseptics, such as the chlorhexidine digluconate (CHX), are efficient in controlling biofilm formation (Sekino et al. 2004, Zanatta et al. 2007) and simple for the patient to use it would be worth evaluating if CHX rinsing during the active phase of periodontal treatment would be as effective as the PPC protocol. In fact, a previous publication of our group suggested adjunctive short-term clinical benefits when CHX rinsing was performed twice a day for 2 months during and after SRP (Faveri et al. 2006a). However, the long-term effects of this therapy on clinical parameters and on the composition of the subgingival microbiota have not yet been studied. Moreover, the mechanical (PPC) and chemical (CHX) strict plaque control treatment protocols have not been directly compared.
Thus, the aim of this study was to evaluate and to compare the clinical and microbiological effects of SRP alone or combined with CHX rinsing or PPC in the treatment of subjects with generalized chronic periodontitis.
MATERIAL AND METHODS
Sample size calculation
This study compared clinical and microbiological effects of three different periodontal therapies. The ideal sample size to assure adequate power to this clinical trial was calculated considering differences of at least 1 mm between groups for clinical attachment level (CAL) in initially deep periodontal sites (PD ≥ 7 mm). It was also determined that the standard deviation of CAL change at deep sites was 1.0 mm based on our earlier studies of subjects receiving SRP alone or combined with professional or chemical supragingival plaque control (Carvalho et al. 2004, Faveri et al. 2006a). Based on these calculations, it was defined that 16 subjects per group would be necessary to provide an 80% power with an α of 0.05.
Subject population
Sixty subjects with untreated previously periodontal disease were selected from the population referred to the Periodontal Clinic of Guarulhos University (Guarulhos, SP, Brazil). Detailed medical, periodontal and dental histories were obtained. Subjects who fulfilled the inclusion/exclusion criteria were invited to participate in the study. All eligible subjects were thoroughly informed of the nature, potential risks and benefits of their participation in the study and signed on an Informed Consent. This study protocol was approved previously by Guarulhos University's Ethics Committee in Clinical Research.
Inclusion and Exclusion criteria
All subjects were in good general health and were diagnosed with generalized chronic periodontitis based on the current classification of the American Academy of Periodontology (Armitage 1999). The inclusion criteria were as follows: > 30 years of age, at least 15 teeth, minimum of six teeth with at least one interproximal site with probing depth (PD) between 5 and 7 mm and CAL between 5 and 10 mm, at least 30% of the sites with PD and CAL ≥ 5 mm and presence of bleeding on probing (BOP). The exclusion criteria were as follows: previous subgingival periodontal therapy, smoking, pregnancy, nursing, systemic diseases that could affect the progression of periodontal disease (e.g. diabetes and immunological disorders), long-term administration of anti-inflammatory medication, need of antibiotic coverage for routine dental therapy, antibiotic therapy in the previous 6 months and allergy to CHX.
Experimental design
In this randomized and placebo-controlled clinical trial, subjects were randomly assigned to one of the following treatment groups: SRP + placebo rinsing; SRP + PPC + placebo rinsing and SRP + 0.12% CHX rinsing.
During the initial phase, all subjects received instruction on proper home-care techniques and full-mouth supragingival scaling. They were also given the same dentifrice to use during the period of the study (Colgate Total®, Anakol Ind. Com. Ltda-Kolynos do Brasil - Colgate Palmolive Co, São Bernardo do Campo, SP, Brazil). SRP was performed in four to six appointments and was completed in 21 days. The CHX and placebo rinsing, and the PPC procedure began along with the SRP and continued for 42 days after the end of this therapy. All subjects received microbiological and clinical monitoring at baseline, at 2 and 6 months post-therapy.
Subjects randomization and allocation to therapies
Each subject was given a code number at the enrolment visit and the study coordinators (M.Fe. and L.C.F.) used computer-generated table (in blocks of three) to randomly allocate them to one of the three therapeutic groups. Guarulhos University Pharmacy prepared the placebo and the CHX rinsing for the 60 subjects. Five hundred and forty opaque plastic tubes (360 placebos and 180 CHX 0.12%; nine per subject) containing 220 ml of the mouthwashes, in two packs, were sent to the study coordinators, who marked the code number of each subject on a set of nine tubes, according to the therapy assigned. The coded tube was given to the examining researchers (M.Fa. and L.C.G.), who at no time during the study had any access to information about the contents of the tubes or the assignment of the subjects to the three therapies. All study personnel, including the biostatistician and participants, were not aware of the treatments assignments for the duration of the study. However, this study was not considered blind due to two reasons: 1- Examiner aspect: CHX rinsing may cause stains on the tooth surface, and this might allow the clinicians to deduce whether the subjects were receiving CHX or placebo; 2- Subject aspect: subjects in the PPC group needed to return to the clinic two times a week and the others not.
Treatment protocols
SRP
All subjects received full-mouth SRP performed under local anesthesia in four to six appointments of approximately 1h each. Treatment of the entire oral cavity was completed in at most 21 days. SRP was performed by two trained periodontists (M.Fa. and L.C.G.) using mainly manual instruments.
Professional plaque control
Subjects in the SRP+PPC group received PPC twice a week for 63 days (starting at the first day of the SRP treatment) performed by two trained assistants. Supragingival plaque was removed using curettes on all accessible surfaces followed by polishing of the teeth using a rubber cup and dentifrice. In addition, all inter-proximal surfaces were cleaned using dental floss. When performing these procedures, care was taken to limit plaque removal to the supragingival area only.
CHX and placebo rinsing
All subjects were instructed to gargle with 15 ml of CHX or placebo twice a day for 63 days (starting at the first day of the SRP treatment) for 1 min, i.e., in the morning, 30 min after breakfast and tooth brushing, and at night, before going to sleep. Subjects in the SRP+CHX group rinsed with CHX, while subjects in the SRP-only and SRP+PPC groups rinsed with placebo.
Compliance
The subjects were asked to bring the tubes containing the medication once a week, even after the completion of SRP, when compliance was checked. The tubes contained 220 ml of solution, enough for 1 week of rinsing (15 ml/solution, 2 times/day for 7 days). During these visits, subjects returned the old tube and received a new one containing medication/placebo. They were also asked about any self-perceived side-effects of the mouthrinses. This inquiring was performed by two study assistants, who also had the responsibility of calling the subjects every 3 days to monitor compliance.
Clinical Monitoring
The clinical monitoring was performed by two calibrated examiners (see next item: Investigators calibration). One examiner carried out all clinical measurements in a given subject and treatment was performed by the second clinician. Thus, the monitoring clinician was masked to the treatment protocol. Subjects were clinically monitored at baseline, at 2 and 6 months post-therapy. Visible plaque (0/1), gingival bleeding (0/1), BOP (0/1), suppuration (0/1), PD (mm) and CAL (mm) were measured at six sites per tooth (mesiobuccal, buccal, distobuccal, distolingual, lingual and mesiolingual) in all teeth, excluding third molars, at each visit. The PD and CAL measurements were recorded to the nearest millimeter using a North Carolina periodontal probe (Hi-Friedy, Chicago, IL, USA).
Investigators calibration
The two examining researchers participated in the calibration exercise that was performed in 10 non-study subjects with chronic periodontitis. Each examiner measured one quadrant per subject. The quadrant chosen should have at least six teeth. If a quadrant presented fewer than six teeth, the following quadrant was chosen. For better standardization quadrant 1 was the first choice, followed by 2, 3 and 4, respectively. Initially, the first examiner measured PD and CAL in a given quadrant and 15 minutes later the second examiner measured the same quadrant. Sixty minutes later this same protocol was repeated, but the order of the examiners was changed. Therefore, all 10 subjects were probed twice in the same visit by each of the two examiners. Upon completion of all measurements, the intra and inter-examiner variability for PD and CAL measurements were assed. Calibration was made according to Araújo et al. (2003) and the standard error of measurement (SE) was calculated. Inter-examiner variability was 0.14 mm for PD and 0.31 mm for CAL. The mean intra-examiner SE variability was 0.12 mm (PD) and 0.14 mm (CAL) for the first examiner (M.Fa.), and 0.15 mm (PD) and 0.17 mm (CAL) for the second examiner (L.C.G.). These trained examiners were able to provide reproducible measures under 0.5 mm.
Microbiological monitoring
Sample collection
Subgingival plaque samples were collected at baseline, 2 and 6 months post-SRP from six non-contiguous interproximal sites per subject with PD between 5 and 7 mm and CAL between 5 and 10 mm. The selected sites were randomized in different quadrants. After the clinical parameters had been recorded, the supragingival plaque was removed and the subgingival samples were taken with individual sterile mini-Gracey curettes (11–12) and immediately placed in separate Eppendorf tubes containing 0.15 ml of TE (10mM Tris-HCl, 1 mM EDTA, pH 7.6). One hundred microliters of 0.5 M NaOH was added to each tube and the samples were dispersed using a vortex mixer.
Checkerboard DNA-DNA hybridization
Counts of 38 bacterial species were determined in each sample, using the Checkerboard DNA-DNA hybridization technique (Socransky et al. 1994). The microbiological analysis was entirely performed at the Laboratory of Microbiology of Guarulhos University. The samples were boiled for 10 min and neutralized using 0.8 ml of 5 M ammonium acetate. The released DNA was then placed into the extended slots of a Minislot 30 apparatus (Immunetics, Cambridge MA), concentrated on a 15×15 cm positively charged nylon membrane (Boehringer Mannheim, Indianapolis, IN) and fixed to the membrane by baking it at 120° C for 20 min. The membrane was placed in a Miniblotter 45 (Immunetics) with the lanes of DNA at 90° to the lanes of the device. Digoxigenin-labelled whole genomic DNA probes for 38 bacterial species were hybridized in individual lanes of the Miniblotter. After hybridization, the membranes were washed at high stringency and the DNA probes were detected using the antibody to digoxigenin conjugated with alkaline phosphatase and chemiluminescence detection. The last two lanes in each run contained standards at concentrations of 105 and 106 cells of each species. Signals were converted to absolute counts by comparison with the standards lanes on the membrane. The sensitivity of the assay was adjusted to permit detection of 104 cells of a given species by adjusting the concentration of each DNA probe.
Statistical Analysis
The primary outcome variable of this study was mean CAL change at 6 months post-SRP in sites with initial PD ≥ 7 mm. Secondary outcome variables were mean PD change in sites with initial PD ≥ 7 mm, as well as in full-mouth CAL and PD. The percentage of sites with visible plaque, gingival bleeding, BOP and suppuration, as well as mean PD and CAL were computed for each subject and then averaged across subjects in the three therapeutic groups. Similarly, the changes in PD, CAL and BOP over time were examined in subsets of sites according to the initial PD of ≤ 3mm, 4∓6 mm and ≥ 7 mm. Values for each clinical parameter were averaged separately within the three PD categories in each subject and then across subjects in the treatment groups. Mean counts (× 106) of individual bacterial species were averaged within each subject and then across subjects in the three therapeutic groups. The percentage of the total DNA probe counts was determined initially in each site, then per subject and averaged across subjects in the three groups. The significance of differences among the three groups for the clinical and microbiological parameters was sought using the Kruskal-Wallis test. If significance was achieved, the Mann-Whitney U-test was used to assess differences between two groups. The Friedman test was used to detect statistically significant differences within each group among the three time points. If significance was achieved, the Wilcoxon test was used to test differences between two experimental periods. Adjustments for multiple comparisons were made according to Socransky et al. (1991). The level of significance was set at 5%. All data analyses were performed on an “intention-to-treat” basis and with the subject as the statistical unit. Hence, all subjects who entered the study (n=60) were included in the analyses at all time points. For subjects lost during the study period (dropouts), the last available recordings were carried forward to represent all subsequent time points of evaluation.
Results
Subject retention
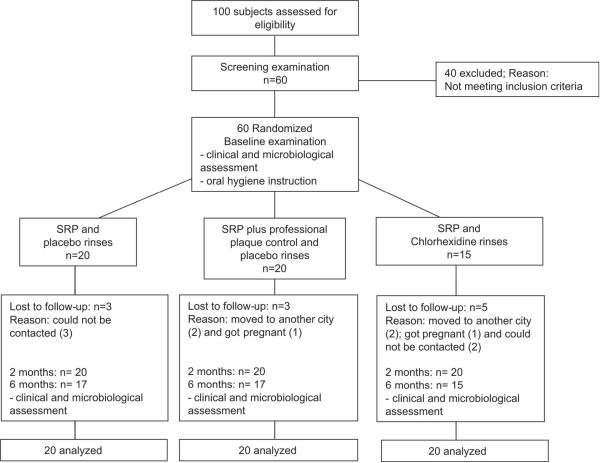
Figure 1 presents the flow chart of the study design. Eleven out of the 60 selected subjects (three from the SRP-only group, three from the PPC group, and five from the CHX group) did not return for the six months follow-up visit. Four patients moved away from Guarulhos-São Paulo due to work-related reasons, before the 6 months examination visit. Two patients became pregnant and five patients could not be contacted by phone or mail to reschedule missed appointments.
Figure 1.
Flow chart of the study design.
Adverse effects
All subjects who finished the study reported full adherence to the prescribed course of CHX/placebo. No severe adverse effects were reported by any of the subjects. Two subjects from the CHX group reported adverse events during the study, such as tooth staining and unpleasant taste. No adverse events were reported in the SRP-only and PPC groups. All subjects affirmed that the medications did not cause any major disturbance in their daily routine and that they would start the treatment again if necessary.
Clinical findings
Demographic characteristics and full-mouth mean values of periodontal clinical parameters at baseline and at 2 and 6 months after treatments are presented in Table 1. No statistically significant differences were observed among groups for any parameter at baseline. All therapies led to a statistically significant decrease in mean PD, CAL and in the percentage of sites with visible plaque, gingival bleeding, BOP and suppuration at 2 and 6 months post-SRP; however, differences were observed among treatments (Figs. 2, 3 and 4). Overall, subjects who received supragingival plaque control as part of the periodontal treatment showed the greatest improvements in clinical parameters over the course of the study. Full-mouth PD and CAL was better reduced in the CHX and PPC groups up to 6 months after treatment. However, only subjects who rinsed with CHX maintained a greater improvement in CAL at 6 months post-treatment (Fig. 2) when compared with subjects receiving SRP alone or in combination with PPC. When the sites were subset into initial PD categories (Fig. 3) an overall improvement in clinical parameters was observed for all treatments in the three PD categories, except for a slight loss in attachment in the initial shallow sites (≤ 3 mm) of the SRP and PPC groups. Subjects receiving the adjunctive treatments showed the most striking decreases in mean PD and CAL, as well as in the percentage of sites with BOP in the initially deep sites (≥ 7 mm) in comparison with the SRP-only group, at 2 and 6 months after treatment. No statistically significant differences were observed among groups for the reduction in PD for the initially intermediate sites (4∓6 mm). However, the parameters of CAL and BOP were better affected by PPC and CHX rinsing during the course of the study. In fact, at 6 months subjects who rinsed with CHX had the greatest gain in CAL for this PD category, even when compared with those subjects who received SRP in combination with PPC (p< 0.05).
Table 1.
Demographic characteristics and mean (±SD) full-mouth clinical parameters at baseline, at 2 and 6 months post-therapy in the three treatment groups. Subjects in the SRP and SRP+PPC groups rinsed with placebo.
| Treatment groups |
||||
|---|---|---|---|---|
| Variable | Time point | SRP (n = 20) | SRP + PPC (n = 20) | SRP + CHX (n = 20) |
| Gender (male/female) | baseline | 9/11 | 7/13 | 8/12 |
| Age (years) | baseline | 39.6 ± 6.1 | 42.5 ± 7.3 | 45.1 ± 9.6 |
| PD (mm) | baseline | 3.7 ± 0.7a | 3.7 ± 0.8 a | 3.7 ± 0.5 a |
| 2 months | 3.1 ± 0.5 b | 2.9 ± 0.5 b | 2.7 ± 0.4 b | |
| 6 months | 3.0 ± 0.4 b | 2.9 ± 0.5 b | 2.7 ± 0.5 b | |
| CAL (mm) | baseline | 4.1 ± 0.9 a | 4.1 ± 0.8 a | 4.5 ± 1.0 a |
| 2 months | 3.7 ± 0.8 b | 3.5 ± 0.8 b | 3.8 ± 0.9 b | |
| 6 months | 3.6 ± 0.7 b | 3.5 ± 0.7 b | 3.7 ± 0.9 b | |
| Percentage of sites with: | ||||
| Plaque accumulation | baseline | 79.3 ± 12.4 a | 86.3 ± 10.8 a | 82.0 ± 13.3 a |
| 2 months | 49.7 ± 16.9 b, A | 40.1 ± 13.1 b, A | 22.1 ± 12.8 b, B | |
| 6 months | 50.2 ± 18.4 b, A | 49.2 ± 14.7 b, A | 29.0 ± 15.6 b, B | |
| Gingival bleeding | baseline | 40.3 ± 22.5 a | 34.9 ± 19.4 a | 29.6 ± 13.8 a |
| 2 months | 10.6 ± 7.1 b | 6.3 ± 5.8 b | 6.5 ± 6.2 b | |
| 6 months | 9.1 ± 3.7 b, A | 7.7 ± 5.2 b, A | 5.7 ± 5.3 b, B | |
| Bleeding on probing | baseline | 58.6 ± 21.1 a | 60.3 ± 21.0 a | 60.7 ± 17.7 a |
| 2 months | 26.1 ± 16.9 b | 22.3 ± 12.4 b | 18.4 ± 11.3 b | |
| 6 months | 27.2 ± 17.8 b | 22.7 ± 12.2 b | 17.1 ± 12.4 b | |
| Suppuration | baseline | 2.3 ± 3.0 a | 2.7 ± 3.9 a | 2.4 ± 1.9 a |
| 2 months | 0.3 ± 0.7 b | 0.4 ± 0.9 b | 0.2 ± 0.6 b | |
| 6 months | 0.2 ± 0.5 b | 0.2 ± 0.6 b | 0.1 ± 0.3 b | |
The significance of differences among time points was assessed using the Friedman test and Wilcoxon test (different small letters indicate p<0.05). Significance of difference within pairs of groups was assessed using the Kruskal-Wallis test and Mann-Whitney U-test (different capital letters indicate p<0.05). SRP, scaling and root planning; PPC, professional supragingival plaque control; CHX, chlorhexidine digluconate; PD, probing depth; CAL, clinical attachment level.
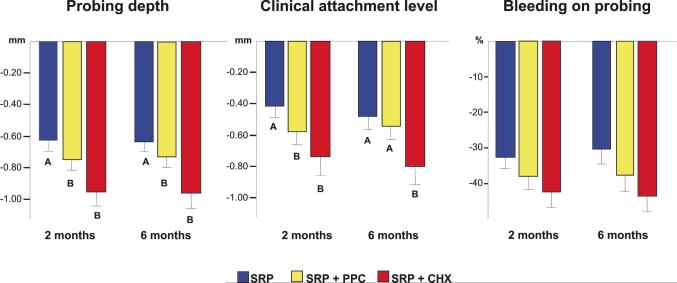
Figure 2.
Bar charts of the mean changes (±SD) in full-mouth probing depth, clinical attachment level and percentage of sites with bleeding on probing between baseline and 2 or 6 months post-SRP in the three treatment groups. Subjects in the SRP and SRP+PPC groups rinsed with placebo. The whiskers represent the SD. The significance of difference among the three treatment groups for each clinical parameter was assessed using the Kruskal-Wallis test. Subsequently, the significance of difference within pairs of groups was assessed using the Mann-Whitney U-test (different letters indicate p<0.05). SRP: Scaling and root planing; PPC: Professional plaque control; CHX: Chlorhexidine rinsing.
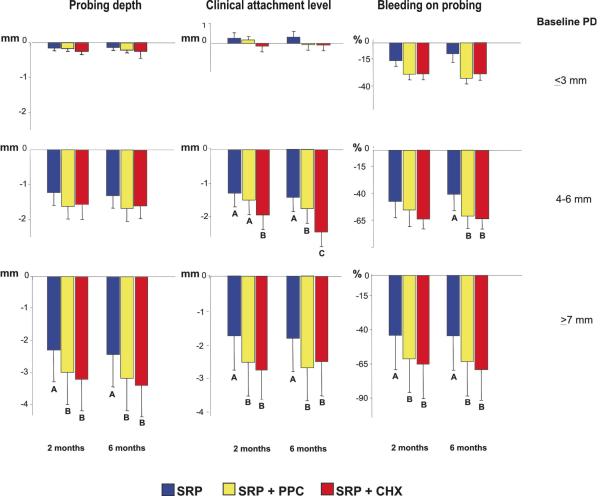
Figure 3.
Bar charts of the mean changes (±SD) in probing depth, clinical attachment level and percentage of sites with bleeding on probing at sites with initial probing depth ≤ 3, 4∓6 and ≥ 7 mm between baseline and 2 or 6 months post-SRP in the three treatment groups. Subjects in the SRP and SRP+PPC groups rinsed with placebo. The whiskers represent the SD. Significance testing is as described in Fig. 2. SRP: Scaling and root planing; PPC: Professional plaque control; CHX: Chlorhexidine rinsing.
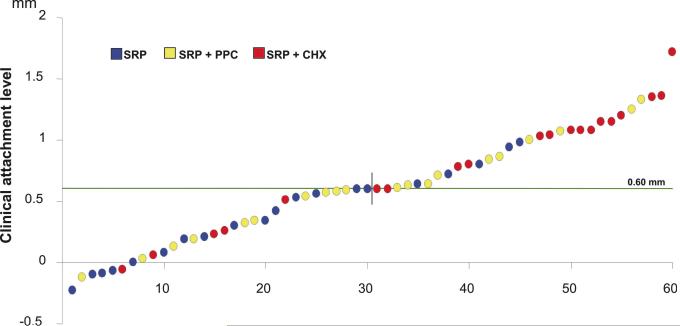
Figure 4.
Plots of the mean changes in individual full-mouth mean clinical attachment level between baseline and 6 months post-SRP of subjects in the three treatment groups. Subjects in the SRP and SRP+PPC groups rinsed with placebo. The circles represent the mean value of each subject. The dashed line represents the median of change of this clinical parameter in all 60 subjects. Positive values represent a gain in clinical attachment level (CAL), while negative values represent a loss in CAL at 6 months post-SRP. SRP: Scaling and root planing; PPC: Professional plaque control; CHX: Chlorhexidine rinsing.
Figure 4 presents changes in mean full-mouth CAL for individual subjects at 6 months post-SRP. The median of CAL change of the 60 subjects of the study was 0.60 mm. The number of subjects showing CAL change within or above this value (0.62 mm to 1.72 mm) was 15, 10 and 5 in the CHX, PPC and SRP groups, respectively. Conversely, the number of subjects presenting CAL change below 0.60 mm (0.59 mm to −0.23 mm) was 5, 10 and 15, for CHX, PPC and SRP groups, respectively.
Table 2 presents the mean percentage of sites with PD < 5 mm or ≥ 5 mm at baseline and at 2 and 6 months post-treatments. The three groups were homogeneous for these two PD categories at baseline. The % of sites with PD < 5mm significantly increased, and with PD > 5mm significantly decreased in the three treatment groups over the course of the study. However, differences were observed among groups at 2 and 6 months. At 6 months post-treatment the SRP+CHX group showed significantly less sites with PD ≥ 5 mm and more sites with PD < 5 mm than the other two groups, followed by the SRP+PPC-treated subjects.
Table 2.
Mean percentage of sites with PD < 5mm or ≥ 5mm at baseline, and at 2 and 6 months post-treatments. Subjects in the SRP and SRP+PPC groups rinsed with placebo.
| Treatment groups |
||||
|---|---|---|---|---|
| Percentage of sites with: | Time point | SRP | SRP + PPC | SRP + CHX |
| Baseline | 64.8 ± 17.1a | 68.6 ± 16.2 a | 69.8 ± 9.0 a | |
| PD < 5mm | 2 months* | 83.8 ± 9.3 b, A | 87.9 ± 13.0 b, A | 93.3 ± 3.0 b, B |
| 6 months* | 83.7 ± 9.6 b, A | 89.9 ± 12.8 b, B | 94.7 ± 1.7 b, C | |
| Baseline | 35.2 ± 17.2 a | 31.4 ± 16.2 a | 30.2 ± 9.0 a | |
| PD ≥ 5mm | 2 months* | 16.2 ± 9.3 b, A | 12.1 ± 13.0 b, A | 6.7 ± 3.0 b, B |
| 6 months* | 16.3 ± 9.2 b, A | 10.1 ± 7.2 b, B | 5.3 ± 1.6 b, C | |
The significance of differences among groups was assessed using the Kruskal-Wallis test (* p<0.05), and within pairs of groups was assessed using the Mann-Whitney U-test (different capital letters indicate p<0.05). The significance of differences among time points was assessed using the Friedman test (different small letters indicate p<0.05). Δ 6 months: mean changes between baseline and 6 months post-treatments; SRP: scaling and root planning; PPC: professional plaque control; CHX: chlorhexidine digluconate; PD: probing depth.
Microbiological findings
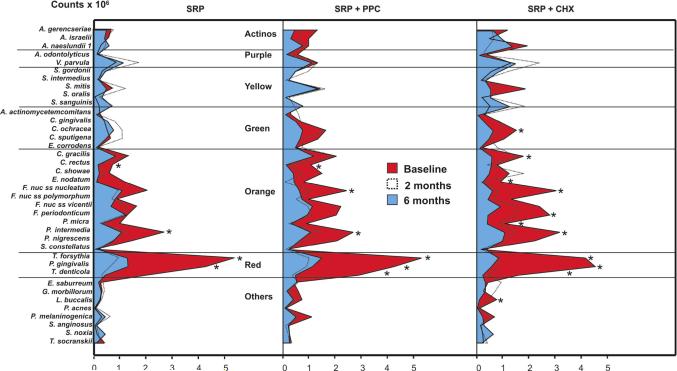
The three treatment groups were microbiologically homogeneous at the beginning of the study. No significant differences were observed among them in mean counts, proportions or prevalence of any of the test species at baseline. Figure 5 presents counts (× 106) of the 38 species evaluated over the course of the study. The species were grouped according to the microbial complexes described by Socransky et al. (1998). In general, counts of most of the host-compatible species did not change much from baseline to 6 months (Actinomyces species, purple, yellow and green complexes). The only significant change observed was a reduction in levels of Capnocytophaga achracea in the CHX group. However, a reduction in counts of several periodontal pathogens from the red and orange complexes was observed, mainly in the test groups. Four of these species were reduced in the SRP-only group, six in the PPC group and nine in the CHX group. The counts of the three pathogens from the red complex, Tannerella forsythia, Porphyromonas gingivalis and Treponema denticola, were significantly reduced by SRP+CHX and SRP+PPC therapies, while SRP alone did not significantly affect the levels of T. denticola at 6 months.
Figure 5.
Mean counts (×105) of the 38 test species at baseline, 2 and 6 months post-SRP in the three treatment groups. Subjects in the SRP and SRP+PPC groups rinsed with placebo. The species were ordered according to the microbial complexes described by Socransky et al. (1998). Counts of individual species were averaged within a subject and then across subjects in each treatment group at each time point. The significance of differences between baseline and 6 months post-SRP was assessed using the Wilcoxon test (* p<0.05), and adjusted for 38 comparisons (Socransky et al. 1991). SRP: Scaling and root planing; PPC: Professional plaque control; CHX: Chlorhexidine rinsing.
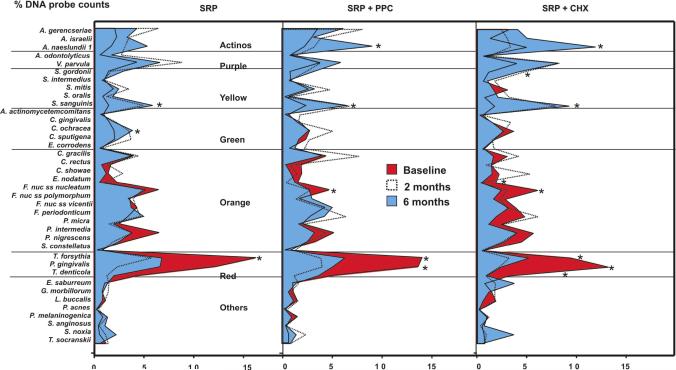
Figure 6 presents the mean percentage of DNA probe counts of the 38 individual species evaluated at all time points. The proportions of five periodontal pathogens were significantly reduced in the CHX group (Eubacterium nodatum, Fusobacterium nucleatum nucleatum, T. forsythia, P. gingivalis and T. denticola), 3 in the PPC group (F. nucleatum nucleatum, T. forsythia and P. gingivalis) and only one in the SRP group (T. forsythia). In general, the proportions of the putative periodontal pathogens from the orange complex were not deeply affected by SRP alone. There was an overall trend of increasing proportions of the majority of the host-compatible microorganisms, such as the Actinomyces species as well as the purple, yellow and green complexes after treatments, especially in the CHX group. These changes were statistically significant for Streptococcus sanguinis in all treatment groups, for Actinomyces naeslundii 1 in the CHX and PPC groups, for Streptococcus gordonii in the CHX group and for C. ochracea in the SRP group.
Figure 6.
Mean percentage of DNA probe counts of the 38 test species at baseline, 2 and 6 months post-therapy in the three treatment groups. Subjects in the SRP and SRP+PPC groups rinsed with placebo. The species were ordered according to the microbial complexes described by Socransky et al. (1998). The proportion that each species comprised of the total DNA probe count was determined at each site, and then averaged within and across subjects in each treatment group at each time point. The significance of differences between baseline and 6 months post-SRP was assessed using the Wilcoxon test (* p<0.05), and adjusted for 38 comparisons (Socransky et al. 1991). SRP: Scaling and root planing; PPC: Professional plaque control; CHX: Chlorhexidine rinsing.
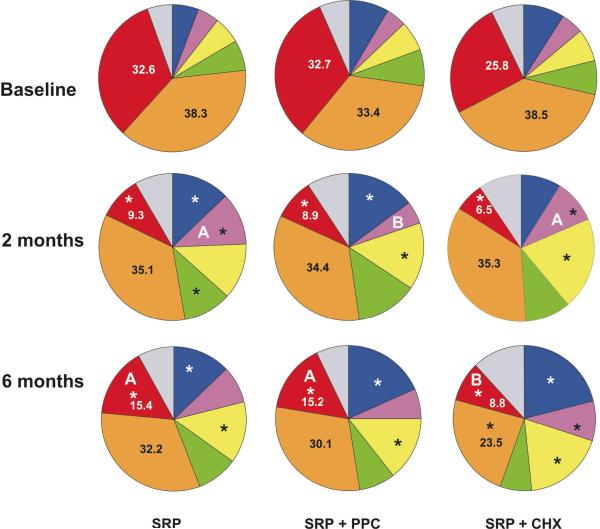
Figure 7 shows changes in the proportions of the microbial complexes in the three groups at baseline, 2 and 6 months post-treatments. The microbial profiles were profoundly changed by treatments, and the most beneficial changes were observed in subjects who received CHX as part of the treatment. These subjects showed a significant reduction in the proportions of red and orange complexes at 6 months, in comparison with baseline, as well as an increase of the beneficial Actinomyces species, purple and yellow complexes. SRP alone or combined with PPC led to a significant reduction in the proportion of red complex and a significant increase in the proportions of Actinomyces species and yellow complex. Even though no differences on the proportions of the microbial complexes were observed among the treatment groups at baseline, at 6 months post-SRP the mean proportions of red complex were significantly reduced in the CHX group, in comparison with SRP and PPC groups.
Figure 7.
Pie charts of the mean proportion of each microbial complex at baseline, 2 and 6 months post-SRP in the three treatment groups. Subjects in the SRP and SRP+PPC groups rinsed with placebo. The colors represent different microbial complexes (Socransky et al. 1998). The significance of differences between baseline and 2 months, and baseline and 6 months was assessed using the Wilcoxon test (* p<0.05). The significance of differences among treatment groups at baseline, at 2 and at 6 months post-therapy was assessed using the Kruskal-Wallis and Mann-Whitney U-test (different letters indicate p<0.05). SRP: Scaling and root planing; PPC: Professional plaque control; CHX: Chlorhexidine rinsing.
DISCUSSION
Previous studies have reported additional benefits when professional removal or chemical control of supragingival plaque are systematically implemented as part of the active phase of periodontal treatment (Ximénez-Fyvie et al. 2000a, Haffajee et al. 2003, Carvalho et al. 2004, 2005, Faveri et al. 2006a). However, these two treatment protocols have not been directly compared before. Therefore, this study evaluated and compared the effects of SRP alone or combined with PPC or CHX rinsing in the treatment of generalized chronic periodontitis. Overall, the results suggested clinical and microbiological advantages for the adjunctive treatments, especially for the CHX-treated subjects.
Clinical data
The two combined therapies were more effective than SRP alone in improving the full-mouth PD and CAL. The benefit in CAL gain was maintained up to 6 months in subjects who rinsed with CHX, but not in those who received PPC (Fig. 2). Subjects in the CHX group also showed significantly fewer sites with plaque and gingival bleeding, as well as a trend towards fewer sites with BOP when compared with the other two groups at 6 months post-SRP (Table 1).
Even though the mean full-mouth PD and CAL provides an overall picture of the differences among treatments, the magnitude of these differences is normally small, as observed in Figure 1 (from 0.2 to 0.3 mm among groups). This happens because the majority of sites are shallow, even in generalized chronic periodontitis subjects; and shallow sites do not show great changes in PD and CAL after treatment. Therefore, in order to determine more specific differences among therapies one should look at different PD categories. Nonetheless, the clinical benefits of the two adjunctive treatments were observed in all baseline PD categories, especially in the initially intermediate (4–6 mm) and deep (≥ 7 mm) periodontal pockets (Fig. 3). These sites exhibited greater improvements in the parameters of PD, CAL and BOP in subjects from the CHX or PPC groups in comparison with those who received SRP only. Apparently, the more profound effect of CHX rinsing in full-mouth attachment gain in comparison with PPC (Fig. 2) was mainly due to the additional benefit of this treatment in intermediate sites (Fig. 3). The CAL gain in intermediate sites at 6 months post-therapy was as follows (mm): SRP: 1.35 ± 0.50; SRP+PPC: 1.82 ± 0.49; SRP+CHX: 2.25 + 0.56. Even though the difference between PPC and CHX groups was less than 0.5 mm (p<0.05), the standard deviation was also low, suggesting a constant trend of the intermediate sites in the CHX group in gaining more attachment than those in the PPC group. Another interesting finding was that SRP+CHX treatment had a more striking effect on reducing the mean percentage of sites with PD ≥ 5 mm and increasing those with PD ≤ 5 mm than the other two treatments (Table 2).
The individual changes in mean CAL also suggested additional benefits of the two test treatments at a subject level (Fig. 4). 75% of subjects who rinsed with CHX showed attachment gain over the median of change of the 60 subjects involved in the study, as opposed to just 25% in the SRP-only group. The predictability of the PPC protocol in terms of the individual gain in attachment was somewhere in between SRP alone or combined with CHX, since 50% of the subjects in this group had CAL changes above the median of the studied population.
Overall, these findings agree and extend data from previous investigations that also suggested additional benefits when systematic PPC or CHX rinsing are implemented for 2 months to 2 years after surgical (Nyman et al. 1975, Rosling et al. 1976, Westfelt et al. 1983) or non-surgical (Magnusson et al. 1984, Ximénez-Fyvie et al. 2000a, Carvalho et al. 2004, Haffajee et al. 2003, Faveri et al. 2006a) periodontal treatments. An interesting finding of the current study was the striking effect of the PPC in the initially deep pockets. These data differed to a certain extent to those reported by Carvalho et al. (2004), who observed additional clinical benefits of this treatment only in shallow and intermediate sites. Haffajee et al. (2003) also reported that the most favorable effects of the association of SRP and PPC were observed in subjects with less baseline periodontal disease. These divergences can be a result of the professional plaque removal protocols used in the different investigations. In the studies of Carvalho et al. (2004) and Haffajee et al. (2003) the supragingival plaque removal began after the SRP treatment and was performed once a week; whereas, in the present study, this treatment began together with SRP and was performed twice a week. As regards the CHX rinsing protocol, the findings of the present study are in agreement with a previous investigation that reported short-term clinical benefits of this treatment, over those obtained with SRP alone, in initially intermediate and deep pockets (Faveri et al. 2006a).
Microbiological data
All three treatments led to a reduction in some periodontal pathogens and an increase in beneficial bacterial species. However, in accord with the clinical findings, the most favorable changes in the composition of the subgingival microbiota were observed with the use of SRP+CHX, followed by SRP+PPC, and by SRP alone. Levels of nine individual periodontal pathogens from the red and orange complexes were reduced after therapy in the CHX-treated subjects, six in the PPC group and four in the SRP group (Fig. 5). The only treatment that significantly reduced the individual levels and proportions of the three red complex pathogens, T. forsythia, P. gingivalis and T. denticola was SRP+CHX (Figs. 5 and 6). In addition, this therapy led to a significant reduction in the proportion of the orange complex, which was not observed in the other two groups (Fig. 7). Overall, all three treatments maintained or raised the levels and proportions of the majority of the host-compatible microorganisms from the purple, yellow and green complexes, as well as the Actinomyces species. However, the most striking effect was also observed in the CHX-treated subjects, who showed a significant increase in the proportions of three beneficial complexes at 6 months post-SRP (Fig. 7).
The favorable effects of SRP plus PPC observed in this study are in agreement with the few previous investigations that have thoroughly described the changes occurring in the subgingival microbial profile with the use of this therapy (Ximénez-Fyvie et al. 2000a, Haffajee et al. 2003, Carvalho et al. 2005). Ximénez-Fyvie et al. (2000a) reported long-term beneficial microbiological effects in subjects who received weekly professional plaque removal for 3 months. Counts of 34 bacterial species, including periodontal pathogens such as T. forsythia, P. gingivalis and Aggregatibacter actinomycetemcomitans were significantly reduced up to 1 year post-SRP. Similarly, Haffajee et al., (2003) and Carvalho et al., (2005) showed favorable microbiological effects when PPC was used for 3 months after SRP and systemically administered metronidazole. In both studies subjects receiving supragingival plaque removal showed a greater reduction in red and orange complexes species in comparison with those who received just SRP.
To our knowledge no previous studies have systematically evaluated the changes that occur in the subgingival microbial profile when CHX rinsing is associated with standard SRP treatment during the active phase of periodontal treatment. However, Faveri et al. (2006a) suggested additional short-term beneficial effect of this treatment protocol, over SRP alone, in reducing red complex species. Magnusson et al. (1984) have formerly observed that the combination of SRP, PPC and 0.2% CHX rinsing for 16 weeks were more effective in reducing motile rods and spirochetes than SRP alone. De Soete et al. (2001) employed CHX rinsing as part of the one stage full-mouth disinfection (OSFMD) protocol and evaluated the effect of this treatment in the subgingival microbial composition. The authors reported that the combination of SRP within 24hs and different forms of CHX application, including daily rinsing for 2 months, led to a greater reduction in some periodontal pathogens, such as P. gingivalis and T. forsythia, in comparison with the standard quadrant-SRP treatment. Later on, Quirynen et al. (2006) studied the OSFMD therapy, with or without adjunctive CHX, and suggested that part of the benefits of this treatment protocol was attributable to the use of this antiseptic, and part due to the completion of SRP within 24hs.
Final thoughts
The present study was designed with the aim of evaluating whether supragingival plaque control during and after SRP by means of CHX would lead to benefits similar to those obtained with the professional supragingival plaque removal protocol initially proposed by Ximénez-Fyvie et al. (2000a) and later on employed in other studies (Haffajee et al. 2003, Carvalho et al. 2004, 2005). The use of CHX would provide the great advantage of not requiring the patient to return weekly at the dental office to have professional plaque removal performed, reducing the time and the cost of treatment. It should be highlighted that the Colgate Total dentifrice containing triclosan was purposely chosen in order to allow the best possible self-performed plaque control for subjects in the three treatment groups (Mateu et al. 2008). As expected, both treatments were superior to SRP in improving clinical and microbiological parameters. These favorable results may be attributed to two main effects of the supragingival plaque control: 1- Prevention of periodontal pathogens migration to recently-scaled pockets; since it has been recognized that several of these species may colonize the supragingival environment (Ximénez-Fyvie et al. 2000b, Haffajee et al. 2008); and 2- Reduction of inflammation on the adjacent periodontal tissues, and consequently preventing the availability of nutrients necessary for bacterial multiplication (Socransky & Haffajee 2002). Surprisingly, the CHX treatment exceeded the benefits attained with the PPC protocol. It is quite impressive that the adjunctive supragingival treatment with CHX promoted such profound benefits in the subgingival microbial profile of patients with advanced periodontitis. One hypothesis that could explain the benefits of CHX rinsing, over those attained with the PPC is the effect of this antiseptic on reducing periodontal pathogen reservoirs that are not reached by the mechanical removal of supragingival plaque, such as the tongue (Faveri et al. 2006b.), saliva and oral mucosa (Mager et al. 2003).
Taken together the results of the present study suggest that strict control of supragingival plaque during SRP and through the healing phase have major benefits, over those obtained with SRP only, in the treatment of subjects with generalized chronic periodontitis. Furthermore, the greatest benefits in clinical parameters and in the subgingival microbial composition were observed with the combination of SRP and CHX rinsing.
CLINICAL RELEVANCE.
Scientific rationale for study: Repeated mechanical plaque control during and after SRP enhances the outcome of this therapy. Even though CHX rinsing seems to be a more practical alternative to this treatment strategy, no studies have compared these two clinical protocols.
Principal findings: Subjects receiving PPC or CHX rinsing showed significantly greater improvement in PD and CAL, as well as in the subgingival microbial composition. These benefits were more striking in the CHX-treated subjects.
Practical implication: The use of CHX rinsing during the active phase of therapy lead to a more beneficial re-colonization of the recently scaled pockets, and consequently to a better periodontal clinical stability over time.
Supplementary Material
Acknowledgments
This study was supported in part by Research Grants # 03/13612-1 from The State of São Paulo Research Foundation (FAPESP) and FOGARTY #5 R03 TW006269-02 from The National Institutes of Health (NIH, U.S.A.).
Footnotes
Conflict of interest and source of funding statement The authors declare that they have no conflict of interests.
References
- Araújo MW, Hovey KM, Benedek JR, Grossi SG, Dorn J, Wactawski-Wnde J, Genco RJ, Trevisan M. Reproducibility of probing depth measurement using a constant-force electronic probe: analysis of inter and intraexaminer variability. Journal of Periodontoly. 2003;74:1736–1740. doi: 10.1902/jop.2003.74.12.1736. [DOI] [PubMed] [Google Scholar]
- Armitage GC. Development of a classification system for periodontal diseases and conditions. Annals of Periodontology. 1999;4:1–6. doi: 10.1902/annals.1999.4.1.1. [DOI] [PubMed] [Google Scholar]
- Carvalho LH, D'Avila GB, Leão A, Haffajee AD, Socransky SS, Feres M. Scaling and root planing, systemic metronidazole and Professional plaque removal in the treatment of chronic periodontitis in a Brazilian population. Journal of Clinical Periodontology. 2004;31:1070–1076. doi: 10.1111/j.1600-051X.2004.00605.x. [DOI] [PubMed] [Google Scholar]
- Carvalho LH, D'Avila GB, Leão A, Gonçalves C, Haffajee AD, Socransky SS, Feres M. Scaling and root planning, systemic metronidazole and professional plaque removal in the treatment of chronic periodontitis in a Brazilian population II - microbiological results. Journal of Clinical Periodontology. 2005;32:406–411. doi: 10.1111/j.1600-051X.2005.00720.x. [DOI] [PubMed] [Google Scholar]
- De Soete M, Mongardini C, Peuwels M, Haffajee AD, Socransky SS, van Steenberghe D, Quirynen M. One-stage full-mouth disinfection. Long-term microbiological results analyzed by checkerboard DNA-DNA hybridization. Journal of Periodontology. 2001;72:374–382. doi: 10.1902/jop.2001.72.3.374. [DOI] [PubMed] [Google Scholar]
- Faveri M, Gursky LC, Feres M, Shibli JA, Salvador SL, Figueiredo LC. Scaling and root planning and chlorhexidine mouthrinses in the treatment of chronic periodontitis: a randomized, placebo-controlled clinical trial. Journal of Clinical Periodontology. 2006a;33:819–828. doi: 10.1111/j.1600-051X.2006.00994.x. [DOI] [PubMed] [Google Scholar]
- Faveri M, Feres M, Shibli JA, Hayacibara RF, Hayacibara MM, de Figueiredo LC. Microbiota of the dorsum of the tongue after plaque accumulation: an experimental study in humans. Journal of Periodontology. 2006b;77:1539–1546. doi: 10.1902/jop.2006.050366. [DOI] [PubMed] [Google Scholar]
- Haffajee AD, Socransky SS, Patel MR, Song X. Microbiological complexes in supragingival plaque. Oral Microbiology and Immunology. 2008;23:196–205. doi: 10.1111/j.1399-302X.2007.00411.x. [DOI] [PubMed] [Google Scholar]
- Haffajee AD, Arguello EI, Ximénez-Fyvie LA, Socransky SS. Controlling the plaque biofilm. International Dental Journal. 2003;53:191–199. doi: 10.1111/j.1875-595x.2003.tb00770.x. [DOI] [PubMed] [Google Scholar]
- Lindhe J, Westfelt E, Nyman S, Socransky S, Heijl L, Bratthall G. Healing following surgical/non surgical treatment of periodontal disease. Journal Clinical of Periodontology. 1982;9:115–128. doi: 10.1111/j.1600-051x.1982.tb01227.x. [DOI] [PubMed] [Google Scholar]
- Mager DL, Ximenez-Fyvie LA, Haffajee AD, Socransky SS. Distribution of selected bacterial species on intraoral surfaces. Journal Clinical of Periodontology. 2003;30:644–654. doi: 10.1034/j.1600-051x.2003.00376.x. [DOI] [PubMed] [Google Scholar]
- Magnusson I, Lindhe J, Yoneyama T, Liljenberg B. Recolonization of a subgingival microbiota following scaling in deep pockets. Journal of Clinical Periodontology. 1984;11:193–207. doi: 10.1111/j.1600-051x.1984.tb01323.x. [DOI] [PubMed] [Google Scholar]
- Mateu FA, Boneta AE, DeVizio W, Stewart B, Proskin HM. A clinical investigation of the efficacy of two dentifrices for controlling established supragingival plaque and gingivitis. Journal of Clinical Dentistry. 2008;19:85–94. [PubMed] [Google Scholar]
- Nyman S, Rosling B, Lindhe J. Effect of professional tooth cleaning on healing after periodontal surgery. Journal of Clinical Periodontology. 1975;2:80–86. doi: 10.1111/j.1600-051x.1975.tb01728.x. [DOI] [PubMed] [Google Scholar]
- Quirynen M, De Soete M, Boschmans G, Pauwels M, Couke W, Teughels W, van Sttenberghe D. Benefit of “one-stage full-mouth disinfection” is explained by disinfection and root planning within 24 hours: a randomized controlled trial. Journal of Clinical Periodontology. 2006;33:639–647. doi: 10.1111/j.1600-051X.2006.00959.x. [DOI] [PubMed] [Google Scholar]
- Rosling B, Nyman S, Lindhe J. The effect of systematic plaque control on bone regeneration in infrabony pockets. Journal of Clinical Periodontology. 1976;3:38–53. doi: 10.1111/j.1600-051x.1976.tb01849.x. [DOI] [PubMed] [Google Scholar]
- Sekino S, Ramberg P, Uzel NG, Socransky SS, Lindhe J. The effect of chlorhexidine regimen on de novo plaque formation. Journal of Clinical Periodontology. 2004;31:609–614. doi: 10.1111/j.1600-051X.2004.00526.x. [DOI] [PubMed] [Google Scholar]
- Shibli JA, Melo L, Ferrari DS, Figueiredo LC, Faveri M, Feres M. Composition of supra-and subgingival biofilms of subjects with healthy and diseased implants. Clinical Oral Implants Research. 2007;9:975–982. doi: 10.1111/j.1600-0501.2008.01566.x. [DOI] [PubMed] [Google Scholar]
- Socransky SS, Haffajee AD. Dental biofilms: Difficult therapeutic targets. Periodontology 2000. 2002;28:12–55. doi: 10.1034/j.1600-0757.2002.280102.x. [DOI] [PubMed] [Google Scholar]
- Socransky SS, Smith C, Martin L, Paster BJ, Dewhirst FE, Levin AE. “Checkerboard” DNA-DNA hybridization. Biotechniques. 1994;17:788–792. [PubMed] [Google Scholar]
- Socransky SS, Haffajee AD, Smith C, Dibart S. Relation of counts of microbial species to clinical status at the sampled site. Journal of Clinical Periodontology. 1991;18:766–775. doi: 10.1111/j.1600-051x.1991.tb00070.x. [DOI] [PubMed] [Google Scholar]
- Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL., Jr. Microbial complexes in subgingival plaque. Journal of Clinical Periodontology. 1998;25:134–144. doi: 10.1111/j.1600-051x.1998.tb02419.x. [DOI] [PubMed] [Google Scholar]
- Ximénez-Fyvie LA, Haffajee AD, Socransky SS. Microbial composition of supra-and subgingival plaque in subjects with adult periodontitis. Journal of Clinical Periodontology. 2000b;27:722–732. doi: 10.1034/j.1600-051x.2000.027010722.x. [DOI] [PubMed] [Google Scholar]
- Ximénez-Fyvie LA, Haffajee AD, Som S, Thompson M, Torresyap G, Socransky SS. The effect of repeated professional supragingival plaque removal on the composition of the supra-and subgingival microbiota. Journal of Clinical Periodontology. 2000a;27:637–647. doi: 10.1034/j.1600-051x.2000.027009637.x. [DOI] [PubMed] [Google Scholar]
- Westfelt E, Nyman S, Socransky SS, Lindhe J. Significance of frequency of professional tooth cleaning for healing following periodontal surgey. Journal of Clinical Periodontology. 1983;10:148–156. doi: 10.1111/j.1600-051x.1983.tb02203.x. [DOI] [PubMed] [Google Scholar]
- Zanatta FB, Antoniazzi RP, Rösing CK. The effect of 0.12% chlorhexidine gluconate rinsing on previously plaque-free and plaque-covered surfaces: a randomized, controlled clinical trial. Journal of Periodontology. 2007;78:2127–2134. doi: 10.1902/jop.2007.070090. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.