Abstract
Earlier we have demonstrated that IL-12 p40 homodimer (p402) induces the expression of inducible nitric oxide synthase (iNOS) in microglia. This study was undertaken to investigate underlying mechanisms required for IL-12 p402- and IL-12 p70-induced expression of iNOS in microglia. IL-12 p402 alone induced the activation of both extracellular signal-regulated kinase (ERK) and p38 mitogen-activated protein kinase (MAPK). Interestingly, the ERK pathway coupled p402 to iNOS expression via C/EBPβ, but not NF-κB, whereas the p38 pathway relayed the signal from p402 to iNOS expression via both NF-κB and C/EBPβ. Furthermore, by using microglia from IL-12Rβ1 (−/−) and IL-12Rβ2 (−/−) mice or siRNA against IL-12Rβ1 and IL-12Rβ2, we demonstrate that p402 induced the expression of iNOS in microglia via IL-12Rβ1–(ERK+p38)–(NF-κB +C/EBPβ) pathway. In contrast, both IL-12Rβ1 and IL-12Rβ2 were involved for IL-12 p70-induced microglial expression of iNOS. Although IL-12Rβ1 coupled p70 to NF-κB and C/EBPβ, IL-12Rβ2 was responsible for p70-mediated activation of GAS. This study delineates a new role of IL-12Rβ1 and IL-12Rβ2 for the expression of iNOS and production of NO in microglia that may participate in the pathogenesis of neuroinflammatory diseases.
Keywords: IL-12, p40 homodimer, IL-12 receptors, microglia, iNOS, signal transduction
INTRODUCTION
It is now well-documented that once inducible nitric oxide synthase (iNOS) is expressed in the central nervous system (CNS) in a signal-dependent fashion, NO in excess of physiological thresholds is produced and this excess NO then plays an important role in the pathogenesis of various neuroinflammatory and neurodegenerative diseases (Bo et al., 1994; Galea et al., 1992; Ghosh et al., 2007; Koprowski et al., 1993; Merrill et al., 1993; Saha and Pahan, 2006b). Evidence from several laboratories emphasizes the involvement of NO in the pathophysiology of multiple sclerosis (MS) and experimental allergic encephalomyelitis (EAE), the animal model of MS (Brahmachari and Pahan, 2007; Dasgupta et al., 2003, 2004; Kolb and Kolb-Bachofen, 1992; Koprowski et al., 1993). Analysis of cerebrospinal fluid (CSF) from MS patients has shown increased levels of nitrite and nitrate compared with normal control (Johnson et al., 1995). The reaction of NO with forms peroxynitrite, ONOO−, a strong nitrosating agent capable of nitrosating tyrosine residues of a protein to nitrotyrosine. Increased levels of nitrotyrosine have been found in demyelinating lesions of MS brains and in spinal cords of mice with EAE (Brenner et al., 1997; Hooper et al., 2000). Subsequently, analysis of iNOS mRNA in MS brains also shows markedly higher expression of iNOS mRNA in MS brains than in normal brains (Bo et al., 1994; Brosnan et al., 1994).
In contrast, interleukin-12 (IL-12) plays a critical role in the early inflammatory response to infection and in the generation of T helper type 1 Th-1 cells, which favor cell-mediated immunity (Hsieh et al., 1993). It has been found that overproduction of IL-12 can be dangerous to the host as it is involved in the pathogenesis of a number of autoimmune inflammatory diseases (e.g., MS, arthritis and insulin-dependent diabetes) (Constantinescu et al., 2000; Zipris et al., 1996). IL-12 consists of a heavy chain (p40) and a light chain (p35) linked covalently by disulfide bonds to give rise to a heterodimeric (p70) molecule (Schoenhaut et al., 1992; Wolf et al., 1991). It is known that the heterodimeric p70 molecule is the bioactive IL-12 cytokine and both subunits must be co-expressed in the same cell to generate the bioactive form (Gately et al., 1998). However, the level of p40 is much higher than that of p35 in IL-12p70-producing cells (Gately et al., 1998). Again, several reports (Constantinescu et al., 2000; Fassbender et al., 1998; Gately et al., 1998) indicate that the level of p40 mRNA in the CNS of patients with MS is much higher than the CNS of control subjects while the level of p35 mRNA is about the same or decreases compared with controls. Similarly, in mice with EAE, the expression of p40, but not p35, mRNA, increases in brain and spinal cord (Bright et al., 1998). However, IL-12 p402 (the p40 homodimer) was known as a biologically inactive molecule. The only recognized role of p402 was to antagonize the function of IL-12p70 (Gately et al., 1998). In contrast to this general notion, earlier we have reported that p402 is capable of inducing the expression of iNOS and the production of NO in microglia and macrophages via activation of NF-κB (Pahan et al., 2001).
Here, we demonstrate that p402 induced microglial expression of iNOS via IL-12Rβ1-mediated activation of ERK and p38 MAP kinases. Although ERK coupled p402 to iNOS via C/EBPβ, p38 linked p402 to iNOS via both C/EBPβ and NF-κB. Furthermore, IL-12p70 induced the expression of iNOS in microglia via both IL-12Rβ1 and IL-12Rβ2. In addition to activating NF-κB and C/EBPβ via IL-12Rβ1, IL-12p70 induced the activation of GAS via IL-12Rβ2, which was also involved in microglial expression of iNOS.
MATERIALS AND METHODS
Reagents
Fetal bovine serum, Hank’s balanced salt solution (HBSS) and DMEM/F-12 were from Mediatech. Recombinant mouse IL-12 p70 (p70) (catalog no. 419-ML-010 for carrier-containing and catalog no. 419-ML-010/CF for carrier-free) and p40 homodimer (p402) (catalog no. 499-ML-025 for carrier-containing and catalog no. 499-ML-025/CF for carrier-free) were obtained from R&D. Carrier-containing, but not carrier-free, cytokines were reconstituted in phosphate buffered saline (PBS) containing 0.1% bovine serum albumin (BSA; Fisher Scientific; catalog no. BP-1600-100). PD98059 and SB203580 were purchased from Biomol. [α-32P]dCTP was obtained from NEN. Dominant-negative mutants of C/EBPβ (ΔC/EBPβ) and p65 (Δp65) were kindly provided by Dr. Steve Smale of the University of California at Los Angeles and Dr. Sankar Ghosh of the Yale University, respectively. IL-12Rβ1 (−/−), IL-12Rβ2 (−/−) and p35 (−/−) mice and their littermate controls were purchased from Jackson Laboratories.
Isolation of Primary Mouse Microglia
Microglia were isolated from mixed glial cultures as described earlier (Jana et al., 2001, 2003, 2007) according to the procedure of Giulian and Baker (Giulian and Baker, 1986). Briefly, on Day 7–9 the mixed glial cultures were washed three times with DMEM/F-12 and subjected to a shake at 240 rpm for 2 h at 37°C on a rotary shaker. The floating cells were washed and seeded on to plastic tissue culture flasks and incubated at 37°C for 2 h. The attached cells were removed by trypsinization and seeded on to new plates for further studies. Ninety to ninety-five percent of this preparation was found to be positive for Mac-1 surface antigen. For the induction of NO production, cells were stimulated with IL-12 p70 and IL-12 p402 in serum-free DMEM/F-12.
Mouse BV-2 microglial cells (kind gift from Virginia Bocchini of University of Perugia) were also maintained and induced with different stimuli as indicated earlier.
Assay for NO Synthesis
Synthesis of NO was determined by assay of culture supernatants for nitrite, a stable reaction product of NO with molecular oxygen, using Griess reagent (Feinstein et al., 1994; Pahan et al., 1997, 1998).
Cell Viability Measurement
Mitochondrial activity was measured with the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (Sigma).
Preparation of siRNA Construct Against IL-12Rβ1 and IL-12Rβ2
We selected 19 nucleotide sequences that were immediately downstream of an AA dinucleotide in the coding sequence of IL-12Rβ1 (NM-008353) and IL-12Rβ2 (NM-008354), and the selected sequences were also submitted to a BLAST search to ensure that specifically IL-12Rβ1 or IL-12Rβ2 was targeted. Based on the chosen 19-nucleotide siRNA sequence, two DNA oligonucleotides (~55nt in size) were designed as insert sequence.
IL-12Rβ1 (Region 906–924): Forward: 5′-GAA ACA CTT GGT GCT GGT GTT CAA GAG ACA CCA GCA CCA AGT GTT TCT TTT TT-3′
Reverse: 5′-AAT TAA AAA AGA AAC ACT TGG TGC TGG TGT CTC TTG AAC ACC AGC ACC AAG TGT TTC GGC C-3′
IL-12Rβ2 (Region 255–273): Forward: 5′-GAA AGT CCA TGA CCA CAC TTT CAA GAG AAG TGT GGT CAT GGA CTT TCT TTT TT-3′
Reverse: 5′-AAT TAA AAA AGA AAG TCC ATG ACC ACA CTT CTC TTG AAA GTG TGG TCA TGG ACT TTC GGC C-3′
Complementary strands were annealed, and duplex formation was confirmed by electrophoresis. Annealed siRNA were cloned into the EcoR1 and Apa1 sites of the linearised pSilencer™ 2.1-U6 neo siRNA expression vector (Ambion). The positive clones were confirmed by sequencing the cloning site using a T3 primer.
Assay of ERK and p38 MAPK
BV-2 microglial cells were stimulated under serum-free condition and activities of ERK and p38 MAPK were measured using assay kits (Cell Signaling Technology). Briefly, at different time intervals, activated form of ERK and p38 were pulled down from cell lysates by immunoprecipitation using immobilized phospho-ERK and phospho-p38 monoclonal antibodies, respectively. Kinase assays were performed in immunoprecipitates using The pellets were washed twice with kinase buffer and finally resuspended with 50 μL kinase buffer supplemented with 200 μM ATP and either ELK-1 fusion protein (for ERK) or ATF-2 fusion protein (for p38). Following incubation at 30°C for a period of 30 min, samples were analyzed by western blot using antibodies against phospho ELK-1 or phospho ATF-2. For monitoring p402-induced activation of ERK and p38 in primary microglia, cell lysates were immunoblotted with antibodies against phospho-ERK and phospho-p38.
RT-PCR Analysis
Total RNA was isolated by RNA-Easy Qiagen kit as described earlier (Jana et al., 2007; Saha et al., 2006). To remove any contaminated genomic DNA, total RNA was digested with DNase. DNase-digested RNA (1 μg) was reverse transcribed using oligo (dT)12–18 as primer and MMLV reverse transcriptase (Clontech). The resulting cDNA was appropriately-diluted, and diluted cDNA was amplified using Titanium Taq DNA polymerase and following primers.
IL-12Rβ1: Sense: 5′-TGA AGA CGG CGC GTG GGA GTC A-3′
Antisense: 5′-TCG CGG GTA CAA CAC CTC CGG G-3′
IL-12Rβ2: Sense: 5′-GGT TGC TGG CTC CTC ACC AGG-3′
Antisense: 5′-ATG CAG CCC CTT TGC TCC GGG - 3′
iNOS (497 bp): Sense: 5′-CCC TTC CGA AGT TTC TGG CAG CAG C-3′
Antisense: 5′-GGC TGT CAG AGC CTC GTG GCT TTG G-3′
GAPDH: Sense: 5′ - GGT GAA GGT CGG TGT GAA CG-3′
Antisense: 5′ - TTG GCT CCA CCC TTC AAG TG-3′
Amplified products were electrophoresed on agarose gels and visualized by ethidium bromide staining. The relative expression of IL-12Rβ1, IL-12Rβ2 and iNOS was measured after scanning the bands with a Fluor Chem 8800 Imaging System (Alpha Innotech Corporation).
Real-Time PCR Analysis
It was performed using the ABI-Prism7700 sequence detection system (Applied Biosystems, Foster City, CA) as described earlier (Brahmachari and Pahan, 2007; Jana et al., 2007). Primers and FAM-labeled probes for mouse iNOS and GAPDH were obtained from Applied Biosystems. The mRNA expression of iNOS was normalized to the level of GAPDH mRNA. Data were processed by the ABI Sequence Detection System 1.6 software and analyzed by ANOVA.
Assay of Transcriptional Activities
To assay the transcriptional activity of NF-κB, C/EBPβ, and GAS, BV-2 microglial cells at 50–60% confluence were transfected with pNF-κB-Luc, (NF-κB-dependent reporter construct), pC/EBPβ-Luc (C/EBPβ-dependent reporter construct) and pGAS-Luc (GAS-dependent reporter construct) using the Lipofectamine Plus (Invitrogen) (Jana et al., 2001; Liu et al., 2002; Saha et al., 2006). All transfections included 50 ng/μg total DNA of pRL-TK (a plasmid encoding Renilla luciferase, used as transfection efficiency control; Promega). After 24 h of transfection, cells were treated with different stimuli for 6 h. Firefly and Renilla luciferase activities were obtained by analyzing total cell extract according to standard instructions provided in the Dual Luciferase Kit (Promega) in a TD-20/20 Luminometer (Turner Designs). Relative luciferase activity of cell extracts was typically represented as (firefly luciferase/Renilla luciferase) × 10−3.
Immunocytochemistry
Cells were fixed with chilled methanol for 5 min at −20°C, blocked with 3% BSA-PBS for 1 h at room temperature followed by incubation with primary antibodies in 1% BSA-PBS for 3 h at 37°C as described earlier (Jana and Pahan, 2007; Saha et al., 2006). Subsequently samples were washed thrice with PBS-Tween solution, incubated with Cy5 tagged secondary antibodies (Jackson ImmunoResearch), and observed under an Olympus IX81 fluorescent microscope. DAPI (Invitrogen) was added during the final wash step at a dilution of 1:1,000.
RESULTS
IL-12 p40 Homodimer (p402) Induces the Expression of iNOS in Primary Mouse Microglia Independent of p35
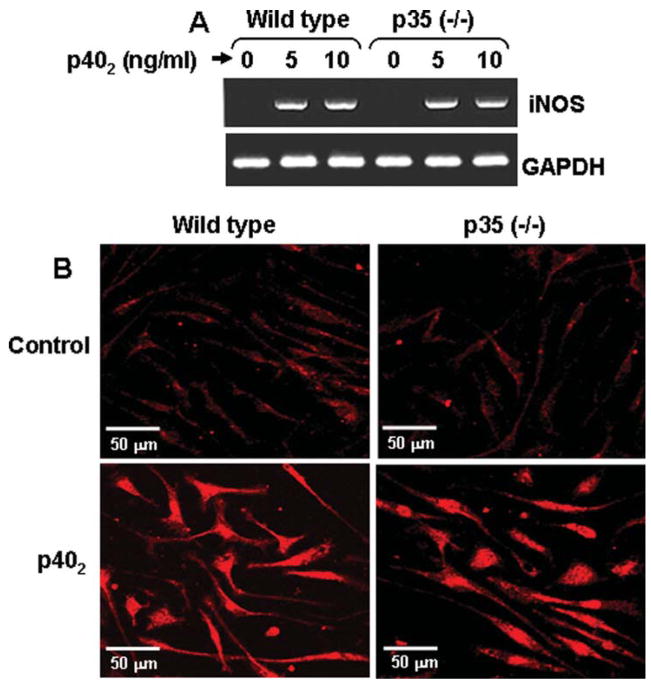
It is known that biologically active IL-12 p70 is comprised of 35 kDa (p35) and 40 kDa (p40) subunits (Gately et al., 1998). Earlier we have demonstrated that p40 homodimer (p402) induces the expression of iNOS in microglia (Pahan et al., 2001). To rule out the possibility that p402 does not need any co-operation from p35 to induce the expression of iNOS, primary microglia isolated from wild type and IL-12 p35 (−/−) mice were stimulated with p402 followed by RT-PCR and immunofluorescence analysis to monitor the level of iNOS mRNA and protein, respectively. It is clear from RT-PCR results in Fig. 1A and immunofluorescence data in Fig. 1B that p402 was equally efficient in inducing the expression of iNOS in microglia isolated from wild type and p35 (−/−) mice. These results clearly indicate that p402 is capable of inducing the expression of iNOS in microglia independent of p35.
Fig. 1.
IL-12 p40 homodimer (p402) induces the expression of iNOS in primary microglia isolated from p35 (−/−) mice. Microglia isolated from wild type and p35 (−/−) mice were stimulated with different concentrations of p402 under serum-free condition. A: After 6 h of stimulation, the mRNA expression of iNOS was monitored by RT-PCR. B: Microglia isolated from p35 (−/−) mice were stimulated with 10 ng/mL p402 for 24 h followed by immunostaining with antibodies against iNOS. Results represent three independent experiments.
Does Serum Albumin Contribute to p402-Induced Expression of iNOS in Primary Mouse Microglia?
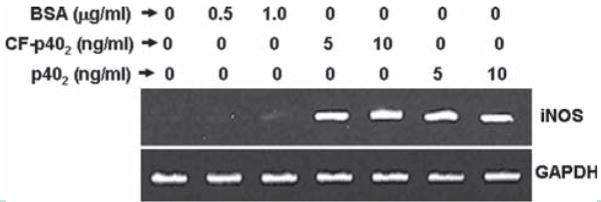
It has been reported that serum albumin increases superoxide production by cultured microglia (Nakamura et al., 2000). Furthermore, bacterial LPS-induced production of proinflammatory molecules (NO and TNF-α) in microglia is also stimulated by serum albumin (Si et al., 2000). Because our preparation of mouse recombinant p402 contained serum albumin as carrier and the cytokine was reconstituted in PBS containing 0.1% BSA, it is possible that a serum factor may contribute to the expression of iNOS in microglia following p402 stimulation. To address this concern, primary mouse microglia were stimulated with different concentrations of BSA. It is clear from Fig. 2 that BSA at concentrations of 500 and 1,000 ng/mL was not effective in inducing the expression of iNOS mRNA in microglia. Very faint increase in iNOS mRNA was observed by BSA at a concentration of 1,000 ng/mL (see Fig. 2). In other studies (Nakamura et al., 2000; Si et al., 2000), BSA was added to media at a concentration of 0.1%. This is a very high dose. It is not possible to contribute to a final concentration of 0.1% in media through the addition of cytokines containing about 0.1% BSA as carrier. To further confirm our results from another angle, carrier-free (CF) p402 was used to stimulate primary microglia. Figure 2 also shows that CF-p402 was almost equally effective as carrier-containing p402 (last two lanes) in inducing the expression of iNOS mRNA in primary mouse microglia. These results clearly demonstrate that albumin does not contribute to the expression of iNOS in p402-stimulated mouse microglia.
Fig. 2.
Effect of serum albumin on p402-induced expression of iNOS in primary mouse microglia. Microglia were stimulated with different concentrations of BSA, carrier (BSA)-free (CF) p402 and carrier (BSA)-containing p402 (p402). After 6 h of stimulation, the mRNA expression of iNOS was monitored by RT-PCR. Results represent three independent experiments.
SiRNA-Mediated Knockdown of IL-12Rβ1 and IL-12Rβ2 on p402- and p70-Induced Expression of iNOS in Mouse BV-2 Microglial Cells
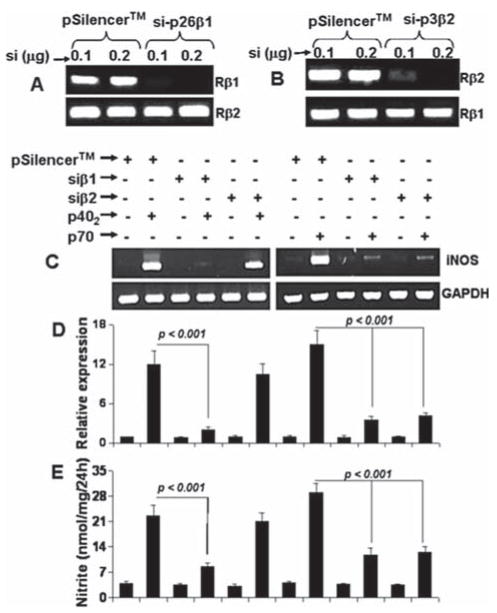
Next we investigated mechanisms by which p70 and p402 induced the expression of iNOS in microglia. IL-12 (p40:p35) is known to function on T cells via both IL-12Rβ1 and IL-12Rβ2 (Gately et al., 1998). Cua et al. (Cua et al., 2003) have demonstrated the presence of IL-12Rβ1 and IL-12Rβ2 in mouse microglia and macrophages. However, it is not known if p70 and p402 are utilizing any of these two receptors to activate microglia for the production of proinflammatory molecules. To achieve this goal, we have utilized the gene silencing approach using siRNA (short-interfering RNA) to knock down IL-12Rβ1 and IL-12Rβ2. We prepared several siRNA constructs for each of IL-12Rβ1 and IL-12Rβ2. Among several siRNA constructs tested in microglial cells, we found that si-p26β1 (si-β1), which targeted the coding sequence of IL-12Rβ1 from 906 to 924 relative to the start codon, was the most efficient one in inhibiting the mRNA expression of IL-12Rβ1 (Fig. 3A). Under the same condition, si-β1 had no effect on the expression of IL-12Rβ2 (Fig. 3A). Similarly, si-p3β2 (siβ2) for IL-12Rβ2 targeting the coding sequence of IL-12Rβ2 from 255 to 273 relative to the start codon, was very much efficient in knocking down the expression of IL-12Rβ2, but not IL-12Rβ1 (Fig. 3B).
Fig. 3.
Effect of siRNA knockdown of IL-12Rβ1 and IL-12Rβ2 on the expression of iNOS in BV-2 microglial cells. A: Cells were transfected with different amounts of the empty vector (pSilencer) and siRNA against IL-12Rβ1 (si-β1). Forty-eight hours after transfection, cells were analyzed for the expression of IL-12Rβ1 and IL-12Rβ2 by RT-PCR. B: Cells were transfected with the empty vector and siRNA against IL-12Rβ2 (si-β2). Forty-eight hours after transfection, cells were analyzed for the expression of IL-12Rβ1 and IL-12Rβ2. Twenty-four hours after transfection, cells were stimulated with 10 ng/mL p402 and p70 under serum-free condition. After 6 h of stimulation, the expression of iNOS mRNA was examined by RT-PCR (C) and quantitative real-time PCR (D). After 24 h of stimulation, concentration of nitrite (E) was measured in supernatants. Data are mean + SD of three separate experiments.
Next we examined the effect of these siRNA constructs on p70- and p402-induced expression of iNOS mRNA. As evidenced by RT-PCR analysis (Fig. 3C), quantitative real-time PCR analysis (Fig. 3D) and nitrite assay (Fig. 3E), si-β1, but not si-β2, inhibited the expression iNOS mRNA and the production of nitrite in p402-stimulated BV-2 microglial cells suggesting that p402 induces the expression of iNOS in microglia via IL-12Rβ1, but not IL-12Rβ2. In contrast, both si-β1 and si-β2 inhibited the expression of iNOS mRNA (Fig. 3C,D) and the production of nitrite (Fig. 3E) in p70-stimulated microglial cells suggesting that IL-12p70 requires the involvement of both IL-12Rβ1 and IL-12Rβ2 to induce the expression of iNOS and the production of NO in microglia.
Role of IL-12Rβ1 and IL-12Rβ2 on p402- and p70-Induced Expression of iNOS in Primary Mouse Microglia
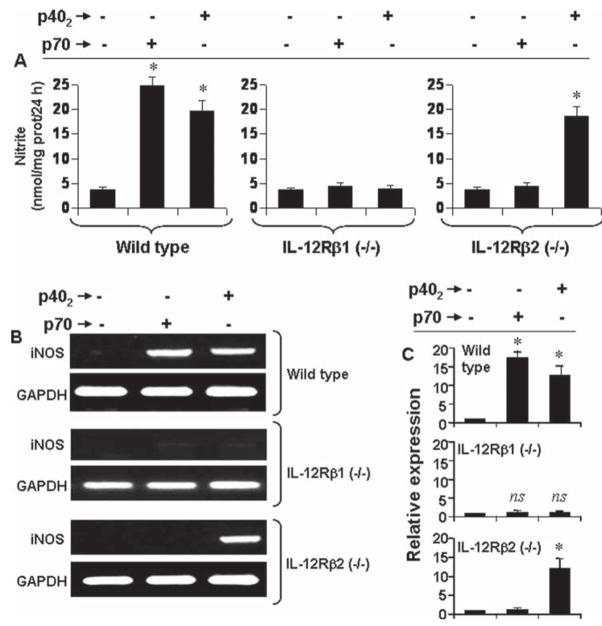
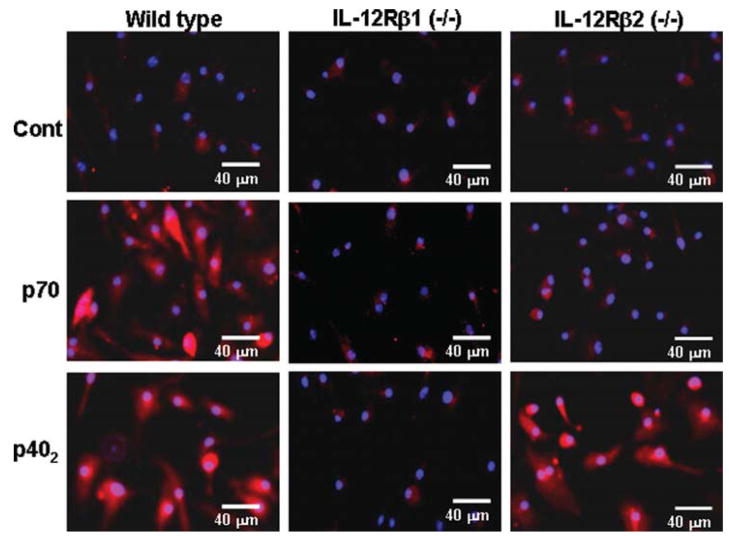
Next to confirm these results in primary cells, microglia isolated from wild type, IL-12Rβ1 (−/−) and IL-12Rβ2 (−/−) mice were challenged with p70 and p402. It is clearly evident from Fig. 4A that both p70 and p402 induced the production of nitrite in microglia isolated from wild type, but not IL-12Rβ1 (−/−) mice. In contrast, p402, but not p70, induced the production of nitrite in microglia isolated from IL-12Rβ2 (−/−) mice (Fig. 4A). Similarly, both p70 and p402 induced the expression of iNOS mRNA in microglia isolated from wild type and IL-12Rβ2 (−/−) mice (Fig. 4B,C). In contrast, p402, but not p70, induced the expression of iNOS mRNA (Fig. 4B,C) in microglia isolated from IL-12Rβ2 (−/−) mice. Immunofluorescence analysis also shows that p70 and p402 induced the expression of iNOS protein in microglia isolated from wild type and IL-12Rβ2 (−/−) mice while only p402 induced the expression of iNOS protein in IL-12Rβ1 (−/−) microglia (see Fig. 5). These results clearly indicate that p402 requires IL-12Rβ1, but not IL-12Rβ2, for the expression of iNOS and that p70 engages both IL-12Rβ1 and IL-12Rβ2 to induce iNOS in microglia.
Fig. 4.
Effect of p402 and p70 on the production of nitrite and the expression of iNOS mRNA in primary microglia isolated from wild type, IL-12Rβ1 (−/−) and IL-12Rβ2 (−/−) mice. Microglia were stimulated with 10 ng/mL p402 and p70. A: After 24 h of stimulation, the production of nitrite was monitored by ‘Griess’ reagent. Data are mean + SD of three separate experiments. *P < 0.001 vs. control. After 6 h of stimulation, the expression of iNOS mRNA was monitored by RT-PCR (B) and quantitative real-time PCR (C). *P < 0.001 vs. control.
Fig. 5.
Effect of p402 and p70 on the level of iNOS protein in primary microglia isolated from wild type, IL-12Rβ1 (−/−) and IL-12Rβ2 (−/−) mice. Microglia were stimulated with 10 ng/mL p402 and p70. After 24 h of stimulation, iNOS protein was monitored by immunofluorescence. DAPI was used to visualize nucleus. Results represent three independent experiments.
Involvement of ERK and p38 MAP Kinases in p402-Induced Expression of iNOS in Microglia
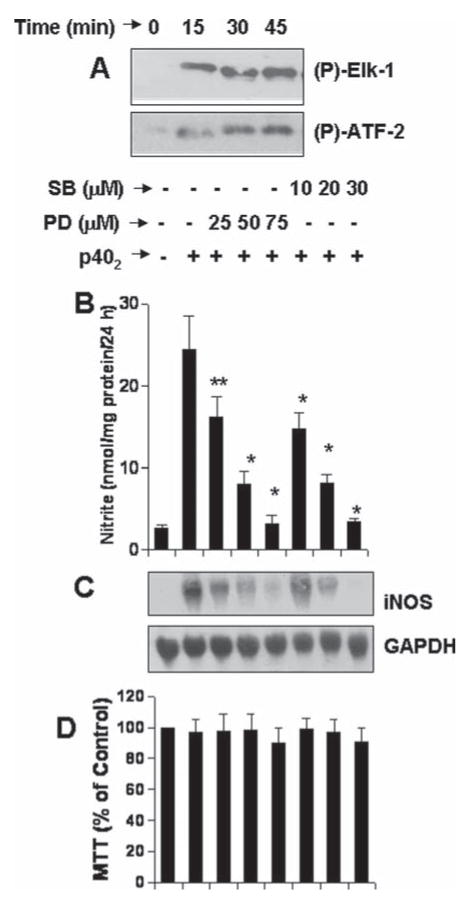
Various MAP kinases, such as ERK and p38, have been reported to be involved in glial expression of iNOS (Saha and Pahan, 2006a,b). We investigated if these two MAP kinases participate in p402-induced expression of iNOS in microglia. At first, we examined if p402 was capable of activating these two MAP kinases. It is clearly evident from Fig. 6A that p402 alone was capable of activating both ERK and p38 MAP kinases at different time points of stimulation. The activation of both the kinases began within 5 min of stimulation (Fig. 6A) and peaked at 45 min of stimulation (Fig. 6A). These results suggest that similar to other proinflammatory cytokines as described elsewhere, p402 alone is also capable of activating MAPK pathways in microglia.
Fig. 6.
Role of Erk and p38 MAP kinases in p402-induced expression of iNOS in mouse BV-2 microglial cells. A: Cells were stimulated with 10 ng/mL p402 for different minute intervals. Activities of ERK (upper panel) and p38 (lower panel) MAP kinases were assayed by immuno-complex kinase assay. Cells preincubated with different concentrations of SB203580 and PD98059 for 30 min were stimulated with 10 ng/mL p402. B: After 24 h, supernatants were used for nitrite assay. **P < 0.05 vs. p402; *P < 0.001 vs. p402. C: After 6 h of incubation, Northern blot analysis for iNOS mRNA was carried out. D: After 24 h, viability was monitored by MTT assay. Data are mean + SD of three different experiments.
Next we examined if these two MAP kinases were involved in p402-induced microglial expression of iNOS. We used SB203580 (SB), specific pharmacological inhibitor of p38, and PD98050 (PD), specific pharmacological inhibitor of MEK-ERK, for this purpose. It is clearly evident from Fig. 6B that both PD and SB dose-dependently inhibited p402-induced production of NO in BV-2 microglial cells. Northern blot analysis for iNOS mRNA clearly show that both SB and PD dose-dependently inhibited p402-induced expression of iNOS mRNA (Fig. 6C). Next to investigate if p402 induces the production of NO in primary cells via SB- and PD-sensitive pathways, we examined the effect of SB and PD on p402-induced production of NO in mouse primary microglia. Consistent to that observed in BV-2 microglial cells, both SB and PD markedly inhibited the production of NO in p402-stimulated primary microglia (Table 1). MTT results indicate that PD and SB were not toxic to microglia at any of the concentrations tested in either BV-2 microglial cells (Fig. 6D) or primary microglia (data not shown) suggesting that the inhibitory effect of PD and SB on p402-induced expression of iNOS was not due to any change in cell viability.
TABLE 1.
Effect of SB203580 and PD98059 on p402-Induced Production of Nitrite in Primary Mouse Microglia
| Nitrite (nmol/mg protein/24 h) | |||||
|---|---|---|---|---|---|
| Control | p402 | p402 + SB (20 μM) | p402 + SB (30 μM) | p402 + PD (25 μM) | p402 + PD (50 μM) |
| 3.4 ± 0.4 | 19.6 ± 2.3* | 9.1 ± 0.6** | 4.5 ± 0.8** | 12.5 ± 0.9*** | 7.8 ± 1.2** |
Primary microglia preincubated with different concentrations of SB203580 and PD98059 for 30 min were stimulated with 10 ng/mL p402. After 24 h, supernatants were used for nitrite assay. Data are mean ± S.D. of three different experiments.
P < 0.001 vs. control;
P < 0.001 vs. p402;
P < 0.05 vs. p402.
Involvement of IL-12Rβ1 and IL-12Rβ2 in p402-Induced Activation of ERK and p38 MAP Kinases in Mouse BV-2 Microglial Cells
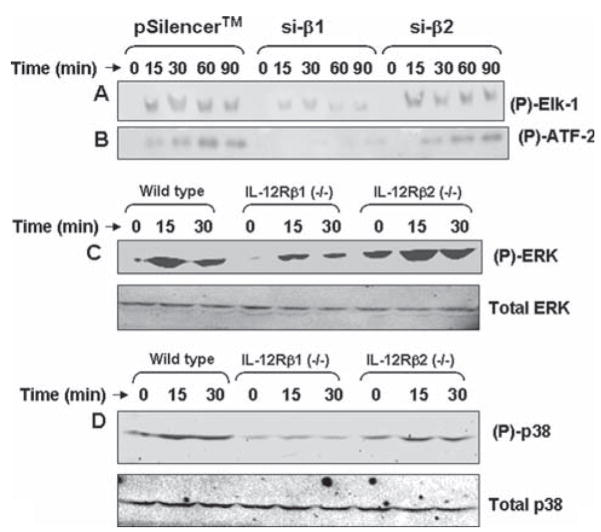
Because of our finding that p402 induces the production of NO in microglia via IL-12Rβ1 (Figs. 3–5) and that both p38 and ERK MAP kinases are also involved in p402-induced microglial expression of iNOS (see Fig. 6), we were prompted to examine the role of IL-12Rβ1 and IL-12Rβ2 in p402-induced activation of p38 and ERK MAP kinases in mouse primary microglia and BV-2 microglial cells. BV-2 cells were transfected with si-β1, si-β2 and an empty vector separately. As evident from Fig. 7, si-β1, but not si-β2, inhibited p402-induced activation of ERK (7A) and p38 (7B) MAP kinases at different time points of stimulation. Next to confirm these finding in primary cells, we isolated microglia from wild type, IL-12Rβ1 (−/−) and IL-12Rβ2 (−/−) mice. Consistent to that observed in BV-2 microglial cells, p402 markedly induced the phosphorylation of ERK in microglia isolated from wild type and IL-12Rβ2 (−/−) (Fig. 7C; top panel). However, very weak induction of ERK phosphorylation was observed by p402 in microglia isolated from IL-12Rβ1 (−/−) mice (Fig. 7C; top panel). In contrast, there was no change in the level of total ERK under different treatment conditions (Fig. 7C; bottom panel). Similarly, p402 also induced the phosphorylation of p38 MAPK at different time points of stimulation in microglia isolated from wild type and IL-12Rβ2 (−/−), but not IL-12Rβ1 (−/−), mice (Fig. 7D; top panel). Again, p402 remained unable to modulate the level of total p38 in microglia isolated from wild type and either of the two knockout mice (Fig. 7D; bottom panel). These results clearly delineate that p402 induces the activation of ERK and p38 MAP kinases in microglia via IL-12Rβ1, but not IL-12Rβ2.
Fig. 7.
Role of IL-12Rβ1 and IL-12Rβ2 on the activation of Erk and p38 MAP kinases in p402-stimulated mouse microglia. Mouse BV-2 microglial cells were transfected with the empty vector (pSilencer) and siRNA against IL-12Rβ1 (si-β1) and IL-12Rβ2 (si-β2). Twenty-four hours after transfection, cells were stimulated with 10 ng/mL p402 under serum-free condition. At different minute intervals, activities of Erk (A) and p38 (B) MAP kinases were assayed by immuno-complex kinase assay. Primary microglia isolated from wild type, IL-12Rβ1 (−/−) and IL-12Rβ2 (−/−) mice were stimulated with 10 ng/mL p402. At different minute intervals, activation status of ERK (C) and p38 (D) was monitored by western blot using antibodies against either phospho-MAP kinases or total MAP kinases. Results represent three independent experiments.
Role of NF-κB and C/EBPβ in p402-Induced Expression of iNOS in Mouse BV-2 Microglial Cells
Earlier we have shown that activation of both NF-κB and C/EBPβ are involved in the expression of iNOS in activated microglia following either neuroantigen-primed T cell contact (Dasgupta et al., 2005) or CD40 ligation (Jana et al., 2001). Others have also shown the involvement of NF-κB and C/EBPβ in LPS-induced expression of iNOS in microglia (Saha and Pahan, 2006a,b). These findings prompted us to ask whether activation of NF-κB and C/EBPβ may be responsible for p402- and p70-induced microglial expression of iNOS. Activation of NF-κB and C/EBPβ was monitored by transcriptional activities. By using the expression of luciferase from a reporter construct, pNF-κB-Luc, as an assay, here we show that both p402 and p70 were capable of inducing NF-κB-dependent transcription of luciferase (Fig. 8A; upper panel). Similarly, p402 and p70 also induced the transcriptional activity of C/EBPβ in microglial cells (Fig. 8A; lower panel).
Fig. 8.
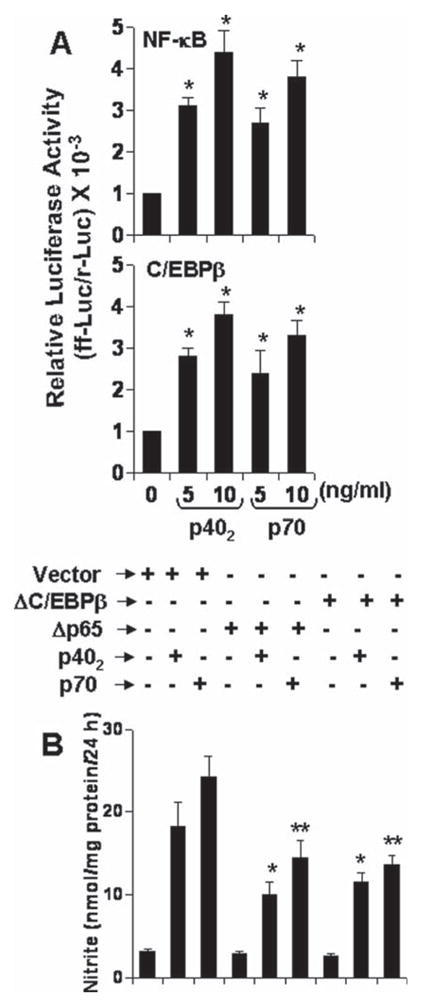
Role of NF-κB and C/EBPβ in p402- and p70-induced expression of iNOS in mouse BV-2 microglial cells. A: Cells were cotransfected with 0.2 μg of either pNF-κB-Luc or pC/EBPβ-Luc and 10 ng of pRL-TK. After 24 h of transfection, cells were stimulated with p402 and p70 for 6 h. Firefly and Renilla luciferase activities were obtained by analyzing total cell extract. *P < 0.001 vs. control. B: Cells were transfected with an empty vector, Δp65 or ΔC/EBPβ. Twenty-four hours after transfection, cells were stimulated with 10 ng/mL p402 and p70. Nitrite was assayed after 24 h. Data are mean + SD of three different experiments. *P < 0.001 vs. p402; **P < 0.001 vs. p70.
Next we examined if activation of both NF-κB and C/EBPβ is required for the expression of iNOS in p402- and p70-stimulated microglial cells. NF-κB was inhibited by a dominant-negative mutant of p65 (Δp65) (Zhong et al., 1997). Similarly we used the dominant-negative mutant of C/EBPβ (ΔC/EBPβ) (Descombes and Schibler, 1991) to inhibit the activation of C/EBPβ. Earlier we have shown that these dominant-negative mutants inhibit their respective target molecule in BV-2 microglial cells stimulated by either neuroantigen-primed T cell contact (Dasgupta et al., 2005) or CD40 ligation (Jana et al., 2001). It is apparent from Fig. 8B that both Δp65 and ΔC/EBPβ, but not the empty vector, significantly inhibited p402- and p70-induced production of nitrite in BV-2 microglial cells. These studies suggest that activation of both NF-κB and C/EBPβ is important for p402- and p70-induced microglial expression of iNOS.
Involvement of ERK and p38 MAP Kinases in p402- and p70-Induced Activation of NF-κB and C/EBPβ in Mouse BV-2 Microglial Cells
Because p402 also involved NF-κB and C/EBPβ to induce iNOS, next we examined the effect of PD and SB on p402- and p70-induced activation of NF-κB and C/EBPβ. Interestingly, PD at different doses tested had no effect on p402- and p70-mediated activation of NF-κB (Fig. 9A). In sharp contrast, SB markedly inhibited p402- and p70-induced activation of NF-κB (Fig. 9A). However, both PD and SB dose-dependently inhibited the activation of C/EBPβ in p402- and p70-stimulated microglial cells (Fig. 9B). These experiments suggest that PD inhibits p402- and p70-induced production of nitrite by inhibiting the activation of C/EBPβ, but not NF-κB, whereby SB inhibits the production of nitrite by inhibiting the activation of both NF-κB and C/EBPβ.
Fig. 9.
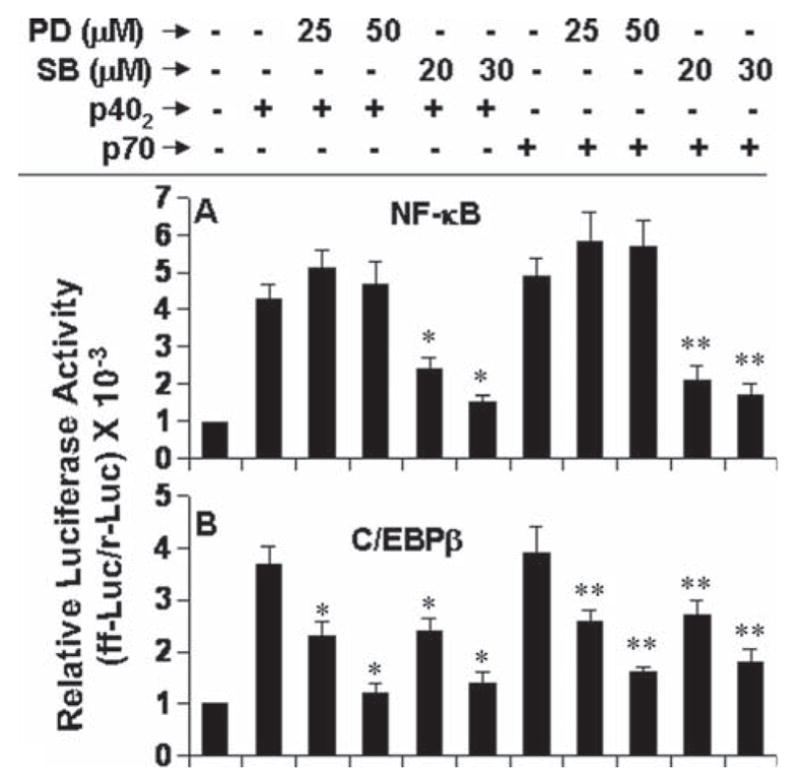
Effect of PD98059 and SB203580 on p402- and p70-induced activation of NF-κB and C/EBPβ in mouse BV-2 microglial cells. Cells were cotransfected with 0.2 μg of either pNF-κB-Luc (A) or pC/EBPβ-Luc (B) and 10 ng of pRL-TK. After 24 h of transfection, cells were treated with different concentrations of PD, SB for 30 min. Then cells were stimulated with 10 ng/mL p402 and p70 for 6 h followed by analysis of firefly and Renilla luciferase activities. Data are mean + SD of three different experiments. *P < 0.01 vs. p402; **P < 0.01 vs. p70.
Involvement of IL-12Rβ1 and IL-12Rβ2 in p402- and p70-Induced Activation of NF-κB and C/EBPβ in Mouse BV-2 Microglial Cells
Next we investigated which receptor coupled the activation of NF-κB and C/EBPβ in p402-stimulated microglia. Consistent to the involvement of IL-12Rβ1, but not IL-12Rβ2, in the activation of MAP kinases (see Fig. 7) and the regulation of NF-κB and C/EBPβ activation by MAP kinases (see Fig. 9), si-β1, but not si-β2, suppressed p402- and p70-induced activation of NF-κB (Fig. 9A) and C/EBPβ (Fig. 10B). These results suggest that p402 and p70 induce the activation of NF-κB and C/EBPβ in microglial cells via IL-12Rβ1, but not IL-12Rβ2.
Fig. 10.
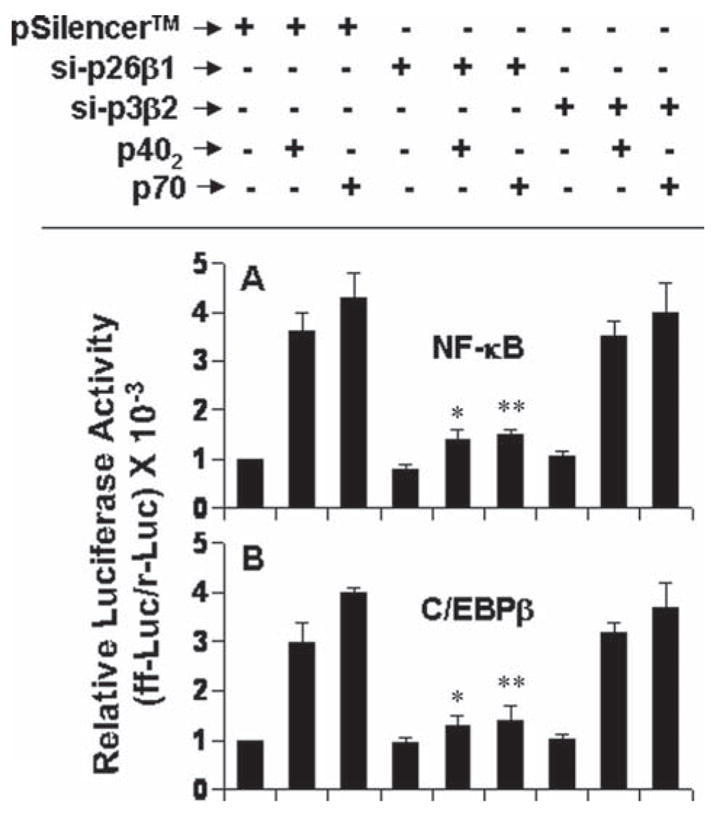
Effect of siRNA knockdown of IL-12Rβ1 and IL-12Rβ2 on the activation of NF-κB and C/EBPβ in p402- and p70-stimulated mouse BV-2 microglial cells. Cells were co-transfected with siRNA constructs against IL-12Rβ1 or IL-12Rβ2 and reporter constructs for NF-κB (A) or C/EBPβ (B). Twenty-four hours of transfection, cells were stimulated with 10 ng/mL p402 and p70 for 6 h followed by analysis of luciferase activities. Data are mean + SD of three different experiments. *P < 0.001 vs. pSilencer-p402; **P < 0.001 vs. pSilencer-p70.
How Does IL-12Rβ2 Regulate p70-Induced Expression of iNOS in Mouse BV-2 Microglial Cells?
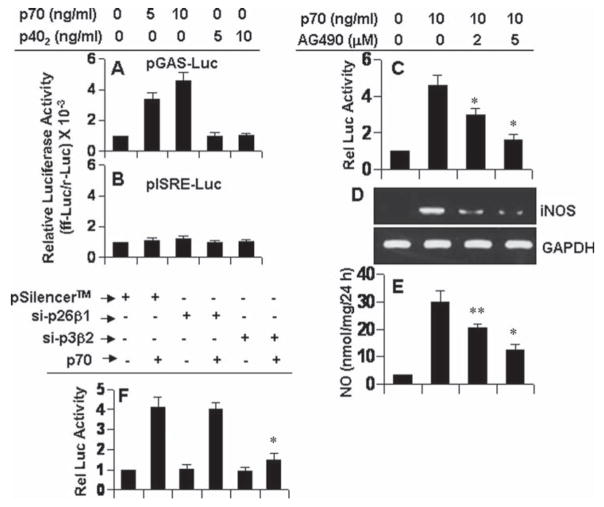
In addition to engaging IL-12Rβ1, p70 also requires the involvement of IL-12Rβ2 for the induction of iNOS in microglia (Figs. 3–5). In contrast, p402 requires the involvement of only IL-12Rβ1 for the same purpose (Figs. 3–5). Studies described earlier clearly demonstrate that IL-12Rβ1 couples the expression of iNOS via (ERK/p38)-mediated activation of NF-κB and C/EBPβ (Figs. 7–10). In addition to NF-κB and C/EBPβ, many other transcription factors play a role in the transcription of iNOS. For example, analysis of human and mouse iNOS promoter shows that it has consensus sequences for binding of several transcription factors, such as NF-κB, C/EBPβ, IRF-1 binding to ISRE, and STAT binding to GAS (Saha and Pahan, 2006a). We investigated if STAT and IRF-1 were involved in p70-induced expression of iNOS and if p70 required IL-12Rβ2 to activate these transcription factors in microglia. Interestingly, p70, but not p402, induced GAS-dependent luciferase activity in BV-2 microglial cells (Fig. 11A). In contrast, both p70 and p402 were unable to induce ISRE-dependent luciferase activity (Fig. 11B). These results suggest that STAT, but not IRF-1, may be involved in p70-induced microglial expression of iNOS. To further confirm the role of STAT/GAS in p70-induced expression of iNOS, we examined the effect of AG490, an inhibitor of JAK, on p70-induced activation of GAS and production of nitrite. As evident from luciferase activities in Fig. 11C, RT-PCR analysis in Fig. 11D and nitrite levels in Fig. 11E, AG490 dose-dependently inhibited p70-induced activation of GAS, expression of iNOS mRNA and production of nitrite suggesting the involvement of JAK/STAT in the activation of GAS and the expression of iNOS in p70-stimulated microglial cells.
Fig. 11.
Role of GAS activation in p70-induced expression of iNOS in mouse BV-2 microglial cells. Cells were cotransfected with 0.2 μg of either pGAS-Luc (an STAT-dependent reporter construct) (A) or pISRE-Luc (an IRF-dependent reporter construct) (B) and 10 ng of pRL-TK. After 24 h of transfection, cells were stimulated with different concentrations of p402 and p70 for 6 h followed by analysis of firefly and Renilla luciferase activities. C: Cells were cotransfected with pGAS-Luc and pRL-TK. After 24 h of transfection, cells were treated with different concentrations of AG490 for 30 min followed by stimulation with 10 ng/mL p70. After 6 h of stimulation, luciferase activities were analyzed. D: Cells preincubated with different concentrations of AG490 for 30 min were stimulated with 10 ng/mL p70. After 6 h of stimulation, the expression of iNOS was monitored by RT-PCR. E: After 24 h of stimulation, concentration of nitrite was measured in supernatants. *P < 0.001 and **P < 0.05 vs. p70. F: Cells were co-transfected with siRNA constructs against IL-12Rβ1 or IL-12Rβ2 and pGAS-Luc. The pRL-TK was also used as transfection efficiency control. Twenty-four hours after transfection, cells were stimulated with 10 ng/mL p70 for 6 h followed by analysis of luciferase activities. Data are mean ± SD of three different experiments. *P < 0.001 vs. pSilencer-p70.
Next we investigated which of the two or both IL-12 receptors was/were involved in p70-mediated activation of GAS in microglial cells. In contrast to the regulation of NF-κB and C/EBPβ, siRNA knockdown of IL-12Rβ1 had no effect on p70-induced activation of GAS (Fig. 11F). In contrast, siRNA knockdown of IL-12Rβ2 abrogated GAS-dependent luciferase activity in p70-stimulated microglial cells (Fig. 11F). These results show that IL-12 p70 induces the activation of GAS in microglial cells via IL-12Rβ2, but not IL-12Rβ1.
DISCUSSION
IL-12, a heterodimeric cytokine, is most noted for its ability to promote differentiation of naive T cells into Th1 cells, which is important in resistance against pathogens (Gately et al., 1998; Hsieh et al., 1993). Neither IL-12 subunits (p35 or p40) alone was found to display significant biological activity over a large range of concentrations (Gately et al., 1998; Schoenhaut et al., 1992; Wolf et al., 1991). However, several evidences indicate that p40 is expressed in excessive amount in the CNS of patients with different demyelinating diseases, such as multiple sclerosis (MS), Guillain-Barre syndrome, and animal models experimental autoimmune encephalomyelitis and neuritis (Constantinescu et al., 2000; Fassbender et al., 1998; Gately et al., 1998; Zipris et al., 1996). In contrast, the expression of p35 remains almost constant or decreases to some extent in the CNS of these demyelinating diseases compared with the CNS of control subjects (Fassbender et al., 1998; Gately et al., 1998). However, the biological significance of this overexpression of p40 in the CNS of patients with demyelinating diseases was not known. Earlier, we (Pahan et al., 2001) demonstrated that IL-12 p40 homodimer, p402, induced the expression of iNOS in mouse microglia and macrophages. This study was undertaken to delineate mechanisms by which p402 and IL-12 p70 induce the expression of iNOS in microglia.
IL-12 p402 has been shown to antagonize bioactive IL-12 p70 by binding to the IL-12 receptor complex (Gately et al., 1998). The high affinity IL-12 receptor is composed of a low affinity IL-12Rβ1 combined with a low affinity IL-12Rβ2 (Gately et al., 1998). It appears that p402 binds to IL-12Rβ1 rather than IL-12Rβ2 whereas bioactive IL-12 binds both the receptors on T cells with high affinity (Gately et al., 1998). Therefore, it is possible that p402 and p70 may engage IL-12Rs for the expression of iNOS in microglia. Here, we present evidence that p402 induces the expression of iNOS via IL-12Rβ1, but not IL-12Rβ2, and that p70 induces iNOS via both IL-12Rβ1 and IL-12Rβ2. Although IL-12Rβ1 alone can phosphorylate Tyk2 and STAT-3, this receptor has a limited role in signal transduction (Cooper and Khader, 2007). Mainly IL-12Rβ2 subunit has been reported to function as the signal transducing component of the high-affinity receptor complex (Gately et al., 1998; Naeger et al., 1999). This has been suggested due the absence of tyrosine residues in the cytoplasmic domain of IL-12Rβ1 and the presence of conserved tyrosine residues in the cytoplasmic domain of IL-12Rβ2 (Naeger et al., 1999). Therefore, our results raise an important question. How does the non signal transducing receptor IL-12Rβ1 engage itself for the transcription of iNOS by p402? Probably, one could foresee the engagement of another putative signal transducing receptor with the binding receptor IL-12Rβ1. Works are underway in this laboratory to investigate this possibility.
Mitogen-activated protein kinases (MAPK), a family of serine/threonine kinases, form an integral component of pro-inflammatory signaling cascades (Dong et al., 2002). These MAP kinases are also involved in the expression of iNOS in various cell types including glial cells (Saha and Pahan, 2006a,b). Consistently, using pharmacological inhibitors we have also found that both ERK and p38 MAP kinases play an important role in p402-induced expression of iNOS in microglia. Interestingly, p402 was able to induce the activation of ERK and p38 MAP kinases in microglia isolated from wild type and IL-12Rβ2 (−/−), but not IL-12Rβ1 (−/−), mice. Our results also indicate that p402 employ IL-12Rβ1, but not IL-12Rβ2, to induce the activation of ERK and p38 MAP kinases in microglia. However, at present, molecular mechanism(s) by which IL-12Rβ1 couple(s) the activation of ERK and p38 MAP kinases in microglia are unknown. It is possible that upstream component(s) of ERK and p38 MAP kinase pathways interact(s) with IL-12Rβ1. Experiments are underway in our laboratory to investigate if upstream modulators like Kinase suppressor of Ras (KSR), Rac, p21-activated protein kinase (PAK), and Raf interact with IL-12Rβ1 physically.
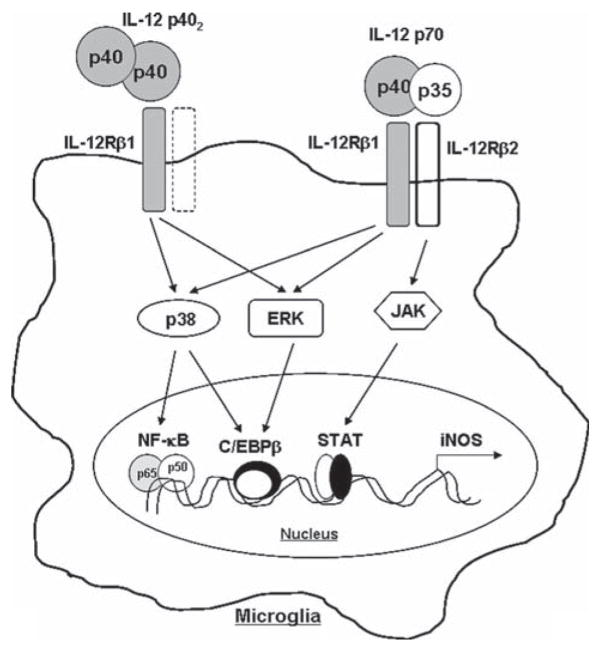
Signaling events transduced by proinflammatory cytokines and LPS for the expression of iNOS are becoming clear. Proinflammatory cytokines bind to their respective receptors and induce iNOS expression via activation of NF-κB, C/EBPβ, STAT, IRF1, etc (Saha and Pahan 2006a,b; Xie et al., 1994). Activation of both NF-κB and C/EBPβ by p70 and p402 and suppression of iNOS expression by Δp65 and ΔC/EBPβ, dominant-negative mutants of NF-κB p65 and C/EBPβ respectively, in p70-and p402-stimulated microglia suggest that p70 and p402 require the activation of NF-κB and C/EBPβ for microglial expression of iNOS. Earlier we have also demonstrated the requirement of NF-κB activation in p402-induced expression of iNOS in microglia (Pahan et al., 2001). Both ERK and p38 MAP kinases have been shown to activate a number of transcription factor in different cell types in response to various stimuli (Dong et al., 2002). We have elucidated that both p402 and p70 induce ERK to couple C/EBPβ, but not NF-κB. In contrast, p402 and p70 induce p38 to couple both NF-κB and C/EBPβ. Our studies also suggest that p402 and p70 employ IL-12Rβ1, but not IL-12Rβ2, to induce the activation of ERK and p38 (see Fig. 12). These MAP kinases ultimately couple to NF-κB and C/EBPβ for the transcription of iNOS in microglia (see Fig. 12).
Fig. 12.
Schematic presentation exhibiting the involvement of IL-12Rβ1 and IL-12Rβ2 in microglial expression of iNOS.
In contrast to the involvement of NF-κB and C/EBPβ, both p70 and p402 were unable to induce ISRE-dependent luciferase activity suggesting that IRF1 may not be involved in p70- and p402-induced expression of iNOS in microglia. In contrast, our results have shown the possible involvement of JAK-STAT pathway in p70- but not p402-induced microglial expression of iNOS. Interestingly, siRNA knockdown of IL-12Rβ2, but not IL-12Rβ1, abrogated p70-induced activation of GAS suggesting the involvement of IL-12Rβ2, but not IL-12Rβ1, in p70-mediated activation of GAS. This is consistent to earlier reports (Cooper and Khader, 2007) that identified STAT4 binding site in IL-12Rβ2, but not IL-12Rβ1. It has been also shown that a defective STAT4 activation is involved in the impaired IL-12-dependent T-cell functions with aging (Tortorella et al., 2006). Taken together, these results suggest that IL-12Rβ2 couples p70 to iNOS in microglia via JAK-STAT signaling pathway.
In summary, we have demonstrated that IL-12 p402 induces microglial expression of iNOS via IL-12Rβ1-mediated (ERK+p38)—(C/EBPβ+NF-κB) pathway (see Fig. 12). In contrast, IL-12p70 induces microglial expression of iNOS via IL-12Rβ1-mediated (ERK+p38)—(C/EBPβ+NF-κB) and IL-12Rβ2-mediated JAK-STAT pathways (see Fig. 12).
Acknowledgments
NIH; Grant number: NS39940.
References
- Bo L, Dawson TM, Wesselingh S, Mork S, Choi S, Kong PA, Hanley D, Trapp BD. Induction of nitric oxide synthase in demyelinating regions of multiple sclerosis brains. Ann Neurol. 1994;36:778–786. doi: 10.1002/ana.410360515. [DOI] [PubMed] [Google Scholar]
- Brahmachari S, Pahan K. Sodium benzoate, a food additive and a metabolite of cinnamon, modifies T cells at multiple steps and inhibits adoptive transfer of experimental allergic encephalomyelitis. J Immunol. 2007;179:275–283. doi: 10.4049/jimmunol.179.1.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner T, Brocke S, Szafer F, Sobel RA, Parkinson JF, Perez DH, Steinman L. Inhibition of nitric oxide synthase for treatment of experimental autoimmune encephalomyelitis. J Immunol. 1997;158:2940–2946. [PubMed] [Google Scholar]
- Bright JJ, Musuro BF, Du C, Sriram S. Expression of IL-12 in CNS, lymphoid organs of mice with experimental allergic encephalitis. J Neuroimmunol. 1998;82:22–30. doi: 10.1016/S0165-5728(97)00184-7. [DOI] [PubMed] [Google Scholar]
- Brosnan CF, Battistini L, Raine CS, Dickson DW, Casadevall A, Lee SC. Reactive nitrogen intermediates in human neuropathology: An overview. Dev Neurosci. 1994;16:152–161. doi: 10.1159/000112102. [DOI] [PubMed] [Google Scholar]
- Constantinescu CS, Goodman DB, Hilliard B, Wysocka M, Cohen JA. Murine macrophages stimulated with central and peripheral nervous system myelin or purified myelin proteins release inflammatory products. Neurosci Lett. 2000;287:171–174. doi: 10.1016/s0304-3940(00)01184-8. [DOI] [PubMed] [Google Scholar]
- Cooper AM, Khader SA. IL-12p40: An inherently agonistic cytokine. Trends Immunol. 2007;28:33–38. doi: 10.1016/j.it.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, Lucian L, To W, Kwan S, Churakova T, et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421(6924):744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- Dasgupta S, Zhou Y, Jana M, Banik NL, Pahan K. Sodium phenyl-acetate inhibits adoptive transfer of experimental allergic encephalomyelitis in SJL/J mice at multiple steps. J Immunol. 2003;170:3874–3882. doi: 10.4049/jimmunol.170.7.3874. [DOI] [PubMed] [Google Scholar]
- Dasgupta S, Jana M, Zhou Y, Fung YK, Ghosh S, Pahan K. Anti-neuroinflammatory effect of NF-kappaB essential modifier-binding domain peptides in the adoptive transfer model of experimental allergic encephalomyelitis. J Immunol. 2004;173:1344–1354. doi: 10.4049/jimmunol.173.2.1344. [DOI] [PubMed] [Google Scholar]
- Dasgupta S, Jana M, Liu X, Pahan K. Myelin basic protein-primed T cells of female but not male mice induce nitric-oxide synthase and proinflammatory cytokines in microglia: Implications for gender bias in multiple sclerosis. J Biol Chem. 2005;280:32609–32617. doi: 10.1074/jbc.M500299200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Descombes P, Schibler U. A liver-enriched transcriptional activator protein, LAP, and a transcriptional inhibitory protein LIP, are translated from the same mRNA. Cell. 1991;67:569–579. doi: 10.1016/0092-8674(91)90531-3. [DOI] [PubMed] [Google Scholar]
- Dong C, Davis RJ, Flavell RA. MAP kinases in the immune response. Annu Rev Immunol. 2002;20:55–72. doi: 10.1146/annurev.immunol.20.091301.131133. [DOI] [PubMed] [Google Scholar]
- Fassbender K, Ragoschke A, Rossol S, Schwartz A, Mielke O, Paulig A, Hennerici M. Increased release of interleukin-12p40 in MS: Association with intracerebral inflammation. Neurology. 1998;51:753–758. doi: 10.1212/wnl.51.3.753. [DOI] [PubMed] [Google Scholar]
- Feinstein DL, Galea E, Roberts S, Berquist H, Wang H, Reis DJ. Induction of nitric oxide synthase in rat C6 glioma cells. J Neurochem. 1994;62:315–321. doi: 10.1046/j.1471-4159.1994.62010315.x. [DOI] [PubMed] [Google Scholar]
- Galea E, Feinstein DL, Reis DJ. Induction of calcium-independent nitric oxide synthase activity in primary rat glial cultures. Proc Natl Acad Sci U S A. 1992;89:10945–10949. doi: 10.1073/pnas.89.22.10945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gately MK, Renzetti LM, Magram J, Stern AS, Adorini L, Gubler U, Presky DH. The interleukin-12/interleukin-12-receptor system: Role in normal and pathologic immune responses. Annu Rev Immunol. 1998;16:495–521. doi: 10.1146/annurev.immunol.16.1.495. [DOI] [PubMed] [Google Scholar]
- Ghosh A, Roy A, Liu X, Kordower JH, Mufson EJ, Hartley DM, Ghosh S, Mosley RL, Gendelman HE, Pahan K. Selective inhibition of NF-kappaB activation prevents dopaminergic neuronal loss in a mouse model of Parkinson’s disease. Proc Natl Acad Sci USA. 2007;104:18754–18759. doi: 10.1073/pnas.0704908104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giulian D, Baker TJ. Characterization of ameboid microglia isolated from developing mammalian brain. J Neurosci. 1986;6:2163–2178. doi: 10.1523/JNEUROSCI.06-08-02163.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper DC, Scott GS, Zborek A, Mikheeva T, Kean RB, Koprowski H, Spitsin SV. Uric acid, a peroxynitrite scavenger, inhibits CNS inflammation, blood-CNS barrier permeability changes, and tissue damage in a mouse model of multiple sclerosis. FASEB J. 2000;14:691–698. doi: 10.1096/fasebj.14.5.691. [DOI] [PubMed] [Google Scholar]
- Hsieh CS, Macatonia SE, Tripp CS, Wolf SF, O’Garra A, Murphy KM. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993;260:547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- Jana A, Pahan K. Oxidative stress kills human primary oligodendrocytes via neutral sphingomyelinase: Implications for multiple sclerosis. J Neuroimmune Pharmacol. 2007;2:184–193. doi: 10.1007/s11481-007-9066-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jana M, Liu X, Koka S, Ghosh S, Petro TM, Pahan K. Ligation of CD40 stimulates the induction of nitric-oxide synthase in microglial cells. J Biol Chem. 2001;276:44527–44533. doi: 10.1074/jbc.M106771200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jana M, Dasgupta S, Saha RN, Liu X, Pahan K. Induction of tumor necrosis factor-alpha (TNF-alpha) by interleukin-12 p40 monomer and homodimer in microglia and macrophages. J Neurochem. 2003;86:519–528. doi: 10.1046/j.1471-4159.2003.01864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jana M, Jana A, Liu X, Ghosh S, Pahan K. Involvement of phosphatidylinositol 3-kinase-mediated up-regulation of I kappa B alpha in anti-inflammatory effect of gemfibrozil in microglia. J Immunol. 2007;179:4142–4152. doi: 10.4049/jimmunol.179.6.4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AW, Land JM, Thompson EJ, Bolanos JP, Clark JB, Heales SJ. Evidence for increased nitric oxide production in multiple sclerosis. J Neurol Neurosurg Psychiatry. 1995;58:107. doi: 10.1136/jnnp.58.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb H, Kolb-Bachofen V. Nitric oxide: A pathogenetic factor in autoimmunity. Immunol Today. 1992;13:157–160. doi: 10.1016/0167-5699(92)90118-Q. [DOI] [PubMed] [Google Scholar]
- Koprowski H, Zheng YM, Heber-Katz E, Fraser N, Rorke L, Fu ZF, Hanlon C, Dietzschold B. In vivo expression of inducible nitric oxide synthase in experimentally induced neurologic diseases. Proc Natl Acad Sci USA. 1993;90:3024–3027. doi: 10.1073/pnas.90.7.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Jana M, Dasgupta S, Koka S, He J, Wood C, Pahan K. Human immunodeficiency virus type 1 (HIV-1) tat induces nitric-oxide synthase in human astroglia. J Biol Chem. 2002;277:39312–39319. doi: 10.1074/jbc.M205107200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill JE, Ignarro LJ, Sherman MP, Melinek J, Lane TE. Microglial cell cytotoxicity of oligodendrocytes is mediated through nitric oxide. J Immunol. 1993;151:2132–2141. [PubMed] [Google Scholar]
- Naeger LK, McKinney J, Salvekar A, Hoey T. Identification of a STAT4 binding site in the interleukin-12 receptor required for signaling. J Biol Chem. 1999;274:1875–1878. doi: 10.1074/jbc.274.4.1875. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Si QS, Takaku T, Kataoka K. Identification of a peptide sequence in albumin that potentiates superoxide production by microglia. J Neurochem. 2000;75:2309–2315. doi: 10.1046/j.1471-4159.2000.0752309.x. [DOI] [PubMed] [Google Scholar]
- Pahan K, Namboodiri AM, Sheikh FG, Smith BT, Singh I. Increasing cAMP attenuates induction of inducible nitric-oxide synthase in rat primary astrocytes. J Biol Chem. 1997;272:7786–7791. doi: 10.1074/jbc.272.12.7786. [DOI] [PubMed] [Google Scholar]
- Pahan K, Sheikh FG, Khan M, Namboodiri AM, Singh I. Sphingomyelinase and ceramide stimulate the expression of inducible nitric-oxide synthase in rat primary astrocytes. J Biol Chem. 1998;273:2591–2600. doi: 10.1074/jbc.273.5.2591. [DOI] [PubMed] [Google Scholar]
- Pahan K, Sheikh FG, Liu X, Hilger S, McKinney M, Petro TM. Induction of nitric-oxide synthase and activation of NF-kappaB by interleukin-12 p40 in microglial cells. J Biol Chem. 2001;276:7899–7905. doi: 10.1074/jbc.M008262200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha RN, Pahan K. Regulation of inducible nitric oxide synthase gene in glial cells. Antioxid Redox Signal. 2006a;8:929–947. doi: 10.1089/ars.2006.8.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha RN, Pahan K. Signals for the induction of nitric oxide synthase in astrocytes. Neurochem Int. 2006b;49:154–163. doi: 10.1016/j.neuint.2006.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha RN, Liu X, Pahan K. Up-regulation of BDNF in astrocytes by TNF-alpha: A case for the neuroprotective role of cytokine. J Neuroimmune Pharmacol. 2006;1:212–222. doi: 10.1007/s11481-006-9020-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenhaut DS, Chua AO, Wolitzky AG, Quinn PM, Dwyer CM, McComas W, Familletti PC, Gately MK, Gubler U. Cloning and expression of murine IL-12. J Immunol. 1992;148:3433–3440. [PubMed] [Google Scholar]
- Si Q, Nakamura Y, Kataoka K. A serum factor enhances production of nitric oxide and tumor necrosis factor-alpha from cultured microglia. Exp Neurol. 2000;162:89–97. doi: 10.1006/exnr.2000.7334. [DOI] [PubMed] [Google Scholar]
- Tortorella C, Stella I, Piazzolla G, Cappiello V, Simone O, Pisconti A, Antonaci S. Impaired interleukin-12-dependent T-cell functions during aging: Role of signal transducer and activator of transcription 4 (STAT4) and suppressor of cytokine signaling 3 (SOCS3) J Gerontol A Biol Sci Med Sci. 2006;61:125–135. doi: 10.1093/gerona/61.2.125. [DOI] [PubMed] [Google Scholar]
- Wolf SF, Temple PA, Kobayashi M, Young D, Dicig M, Lowe L, Dzialo R, Fitz L, Ferenz C, Hewick RM, et al. Cloning of cDNA for natural killer cell stimulatory factor, a heterodimeric cytokine with multiple biologic effects on T, natural killer cells. J Immunol. 1991;146:3074–3081. [PubMed] [Google Scholar]
- Xie QW, Kashiwabara Y, Nathan C. Role of transcription factor NF-kappa B/Rel in induction of nitric oxide synthase. J Biol Chem. 1994;269:4705–4708. [PubMed] [Google Scholar]
- Zhong H, SuYang H, Erdjument-Bromage H, Tempst P, Ghosh S. The transcriptional activity of NF-kappaB is regulated by the IkappaB-associated PKAc subunit through a cyclic AMP-independent mechanism. Cell. 1997;89:413–424. doi: 10.1016/s0092-8674(00)80222-6. [DOI] [PubMed] [Google Scholar]
- Zipris D, Greiner DL, Malkani S, Whalen B, Mordes JP, Rossini AA. Cytokine gene expression in islets and thyroids of BB rats. IFN-gamma and IL-12p40 mRNA increase with age in both diabetic and insulin-treated nondiabetic BB rats. J Immunol. 1996;156:1315–1321. [PubMed] [Google Scholar]