Abstract
The periodontal ligament (PDL) is a specialized connective tissue that connects the surface of the tooth root with the bony tooth socket. The healthy PDL harbors stem cell niches and extracellular matrix (ECM) microenvironments that facilitate periodontal regeneration. During periodontal disease, the PDL is often compromised or destroyed, reducing the life-span of the tooth. In order to explore new approaches toward the regeneration of diseased periodontal tissues, we have tested the effect of periodontal ECM signals, fibroblast growth factor 2 (FGF2), connective tissue growth factor (CTGF), and the cell adhesion peptide Arg-Gly- Asp (RGD) on the differentiation of two types of periodontal progenitor cells, PDL progenitor cells (PDLPs) and dental follicle progenitor cells (DFCs). Our studies documented that CTGF and FGF2 significantly enhanced the expression of collagens I & III, biglycan and periostin in tissue engineered regenerates after 4 weeks compared to untreated controls. Specifically, CTGF promoted mature PDL-like tissue regeneration as demonstrated by dense periostin localization in collagen fiber bundles. CTGF and FGF2 displayed synergistic effects on collagen III and biglycan gene expression, while effects on mineralization were antagonistic to each other: CTGF promoted while FGF2 inhibited mineralization in PDL cell cultures. Incorporation of RGD peptides in hydrogel matrices significantly enhanced attachment, spreading, survival and mineralization of the encapsulated DFCs, suggesting that RGD additives might promote the use of hydrogels for periodontal mineralized tissue engineering. Together, our studies have documented the effect of three key components of the periodontal ECM on the differentiation of periodontal progenitor populations.
Keywords: growth factors, extracellular matrix, periodontal regeneration, progenitor cells
Introduction
Adult stem cells have been identified in most mammalian tissues of the adult body and are known to support the continuous repair and regeneration of tissues (Pekovic and Hutchison., 2008). These stem cells are often located in and controlled by special tissue microenvironments known as niches - specific anatomic locations that regulate how they participate in tissue generation, maintenance and repair (Ohlstein et al., 2004; Scadden, 2006). Stem cell niches maintain adult stem cells in a balance of quiescence and activity (Moore and Lemischka, 2006) and constitute the basic unit of tissue physiology, integrating signals that mediate the balanced response of stem cells to the needs of the organism (Scadden, 2006). Through its interactions with the extracellular matrix, neighboring cells, and soluble local and systemic cues, the microenvironments of these niches influences stem cell proliferation, differentiation, and death (Geiger et al., 2001; Nelson and Bissell., 2006; Scadden, 2006). A number of stem cell niches in the adult body have been well characterized, including intestinal, hair follicle, and hematopoietic stem cell niches (Moore and Lemischka, 2006). Recent evidence suggests that teeth and their surrounding tissues contain several typical mesenchymal stem cell niches residing in the dental pulp, the apical papilla, the periodontal ligament, and the dental follicle (Luan et al., 2006; Luan et al., in press; Sonoyama et al., 2008). Among these, periodontal ligament stem cell niches are of great interest for the study of tissue maintenance and regeneration as the periodontal ligament is one of the tissues with the highest turnover rate in the human body (Stallard, 1963; Carneiro and Leblond, 1966; Melcher, 1985; Beertsen et al., 1997).
Periodontal tissues differentiate from dental follicle cells as a result of a superbly orchestrated interplay between cytokines, growth factors, and transcription factors (Sodek and McKee, 2000; Luan et al., 2006). In mammals, this finely tuned network of signals and receptors affects neural crest-derived progenitor cell populations residing in a connective tissue sac surrounding the developing tooth organ, the dental follicle (Chai et al., 2000; Luan et al., 2006). Soon after the formation of the tooth root, cementoblast progenitors in the dental follicle migrate to the root dentin surface and differentiate to form cementum (Diekwisch 2002). Dental follicles progenitor cells (DFCs) differentiate into the periodontal ligament (PDL), which retains a niche of periodontal progenitors cells (PDLPs) for its renewal (Bosshardt and Schroeder, 1996; Seo et al., 2004; Luan et al., 2006). A third periodontal tissue, the alveolar bone, also arises from the dental follicle (TenCate 1971; Sodek and McKee, 2000). In previous studies, we have provided evidence that the three mammalian mesenchymal periodontal tissues, alveolar bone, root cementum and PDL, are distinct but closely related and suggested that factors from Hertwig’s Epithelial Root Sheath might be responsible for the maintenance of the non-mineralized status of the PDL (Luan et al., 2006, 2007). The close relationship between the three periodontal tissues of mesenchymal origin provides a tempting scenario with only a limited number of key factors responsible for periodontal cytodifferentiation, which in turn might be exploited for the benefit of periodontal therapeutics.
The non-mineralized PDL sandwiched between mineralized cementum-root dentin and alveolar bone is a highly vascularized connective tissue with a heterogeneous cell population embedded in a protein-rich ECM, which is constantly remodeled in response to various stimuli. The periodontal ECM facilitates resilience of mammalian teeth by allowing limited degrees of movement and the ability to react to occlusal stresses while remaining firmly attached to the surrounding alveolar. The periodontal ECM is composed of both collagenous and non-collagenous proteins (proteoglycans and glycoproteins) along with growth factors, cytokines and matrix degradation enzymes, metalloproteinases (MMPs) (Waddington and Embery, 2001). Collagen type I (collagen I) and type III (collagen III) are the main constituents of the collagenous ECM while versican, dextran sulphated biglycan, and decorin contribute to the non-collagenous part of the ECM, which also includes glycoproteins such as fibronectin, vitronectin, thrombospondin, tenascin, periostin and osteopontin (Watanabe and Kubota, 1998; Matsuura et al., 1995; Rios et al., 2005). Many of the periodontal ECM glycoproteins contain RGD motifs, which act as cell adhesion moieties for the binding of cell surface integrins and facilitate important cellular functions such as attachment, spreading, proliferation, differentiation and ECM synthesis (Rezania and Healy,1999). In addition, several growth factors such as fibroblast growth factors (FGFs), bone morphogenetic proteins (BMPs), platelet-derived growth factors (PDGFs) and connective tissue growth factor (CTGF) feature unique temporo-spatial expression patterns during various stages of tooth root development, mesenchymal tissue regeneration and periodontal healing (Madan et al., 2005; Lalani et al., 2003; Friedrichsen et al., 2003, 2005; Lin et al., 2003). Two of the above described growth factors reveal particularly attractive features to aide targeted periodontal regeneration: FGFs because of their capability to reduce potential ankylosis of regenerated tissues, and CTGF because of its effect on collagen I synthesis (Dereka et al., 2006; Asano et al., 2005).
In the present study we have hypothesized that ECM growth factors such as FGF2 and CTGF and cell adhesion peptides such as RGD can modulate the differentiation and behavior of periodontal progenitor populations such as PDLPs and DFCs. In order to mimic the effect of growth factors during periodontal tissue differentiation and regeneration, we used in vivo and in vitro models to ask the question whether individual growth factors alone or combinations thereof elicit a response in the periodontal ECM and affect matrix molecules such as collagen I & III, biglycan and periostin. Our studies have allowed us to gain insights into the suitability of FGF and CTGF as an aide in periodontal regeneration. In addition, we were able to identify a number of mineral formation induction conditions that may be useful for the regeneration of periodontal mineralized tissues such as alveolar bone or cementum. Together, our study characterized a number of ECM -based factors that promote periodontal lineage differentiation and might be used for future periodontal tissue regeneration approaches.
Materials and methods
DF and PDL cell culture
Dental follicle tissues from impacted human 3rd molars were dissected while PDL attached to the roots of erupted 3rd molars was scraped off, and respective tissues were then digested in 5mg/ml collagenase/dispase (Roche Applied Science, Indianapolis, IN) with gentle rotation at 37°C for 40 mins. Approval for the use of human 3rd molars for our study was granted by the UIC Office for the Protection of Research Subjects. Subsequently, primary cells were plated at low density and cultured in six-well plates containing Dulbecco’s Modified Eagle medium (DMEM) supplemented with 10% fetal calf serum (FCS) and 100 units/ml of penicillin/streptomycin. The plastic adherent colonies from single cells were selected and cultured until subconfluence and expanded into 100-mm dishes (Luan et al, 2006). Clonal populations from both cell types with the highest ability to differentiate into osteogenic and adipogenic lineages (data not shown) were used as progenitor cells in all further experiments.
Differentiation induction of primary DFCs and PDLPs
Third passage DFCs and PDLPs were treated with DMEM with rhFGF (2ng/ml) (Peprotech) for a period of 2 weeks. Alternatively, PDLPs were seeded at a low density of 2×104 cells in a 100 mm tissue culture dish and treated with DMEM medium supplemented with either FGF2 (10ng/ml, Peprotech), CTGF (50ng/ml, Cell sciences) or a combination of both for a period of 10 days. Concentrations of the growth factors were chosen based on previous studies demonstrating optimum effects for these growth factors on cell proliferation and DNA synthesis in bone marrow stromal cells and mouse PDL cells at 10ng/ml or lower (FGF2) and 50ng/ml (CTGF), respectively (Hankemeier et. al., 2005; Asano et. al., 2005). PDLPs without any growth factor treatment served as the control.
Collagen gel preparation and suspension of DFCs and PDLPs
Eight ml of chilled Nutragen (Inamed Biomaterials, Fremont CA) was mixed with 1ml of 10x α-MEM and 1ml of 0.1M NaOH. The pH was adjusted to 7.4 +/− 0.2 by dropwise addition of 0.1M of endotoxin free HCl. The collagen concentration was adjusted to 2.5mg/ml for cell suspension and was used within hours of preparation. PDLPs cultured in media containing either FGF, CTGF, or no growth factors were trypsinized and suspended in collagen solution at a density of 2.5×105 cells/ml which contained either FGF2 (10ng/ml) or CTGF (50ng/ml) or a combination of both or no growth factors. About 300μl of this cell-collagen suspension along with the above mentioned concentration of growth factors was placed in each well of a 48 well plate and allowed to form gels at 37°C in 100% air for a period of 60 minutes. The constructs were subcutaneously implanted in nude mice for a period of 1 day, 2weeks and 4 weeks. Alternatively, DFCs and PDLPs in collagen gels were cultured in vitro for a period of 1day, 1 week and 2 weeks.
Synthesis of Acryloyl-PEG-RGD
Acrylated peptide containing a MW 3400 PEG (polyethylene glycol) spacer between the peptide and acrylate moiety was synthesized by mixing the peptide with acryloyl-PEG-N-hydroxysuccinimide ester as previously described (Hern and Hubble, 1998). Briefly, YRGDS (Bachem) was dissolved to a final aqueous concentration of 1mg/ml in 50mM sodium bicarbonate buffer, pH 8.4. The MW 3400 acryloyl-PEG-NHS was also dissolved in 50mM sodium bicarbonate buffer at a final molar ratio of peptide to acryloyl-PEG-NHS of 1:3. The PEG solution was added drop wise to the peptide solution. The final solution was allowed to react at room temperature for 2 hours whereupon it was dialyzed against water with a molecular weight cutoff at 1000Da overnight, followed by lyophilization to obtain the mono-vinyl PEG with a pendant RGD sequence (Acryloyl-PEG-RGD).
Cell attachment and spreading on 2D hydrogel surface
Hydrogel disks about 2mm thick were created in a 48 well plate by pipetting about 300 μl of 10% polyethylene glycol diacrylate (PEGDA) in PBS with or without the addition of 2 mM acryloyl-PEG-RGD and then photopolymerized with UV light for 10 mins. DFCs at a density of 3 × 103 cells/cm2 were seeded onto these disks. After 2 or 24 hour intervals the disks were rinsed with PBS and cell attachment was monitored using phase contrast microscopy. Attached cells were counted on a minimum of 3 random fields per disk (under 20x magnification). Cell spreading was quantified by measuring the area occupied by the cells after 2 hours of attachment using the NIH imaging software.
Photoencapsulation of DFCs in RGD-modified and unmodified hydrogels
80% confluent passage 3 DFCs were trypsinized from culture plates and centrifuged to form a cell pellet. The 10% PEGDA solution in PBS with 0.05% D2959 (Ciba-Geigy), and 1%w/v of streptomycin and penicillin with or without 2 mM acryloyl-PEG-RGD was added directly to the cells adjusting the cell concentration to about 15 million cells per ml of solution. About 50 μl of the cell/polymer solution was then pipetted into 1-ml sterile BD syringes that had their tips cut off and then photopolymerized using UV light of intensity 5mW/cm2 for 10 mins (Nuttelman et al., 2005). Hydrogels were then pushed out of the syringes and cultured in either DMEM, DMEM supplemented with 10ng/ml PDGF (Peprotech), or osteogenic differentiation medium (Cambrex) for a period of 2 weeks.
MTT viability assay
To measure cell viability DFCs-hydrogel constructs were incubated in MTT solution (2mg/ml of MTT in DMEM with 2% FBS) for 4 hours. To quantify viability, the MTT stained constructs were homogenized in HCL/Isopropanol and the absorbance was detected at 570nm with background subtraction at 630nm from centrifugal supernatants (Wang et al., 2003).
Isolation of RNA and real time RT-PCR
Total RNA was extracted from subcutaneously implanted PDLPs-collagen gel constructs at 1 day, 2 and 4 weeks and also from in vitro cultured DFCs and PDLPs cell-collagen gel constructs at 1 and 2 weeks using Trizol Reagent (Invitrogen). RNA quality and quantity were verified using agarose gel electrophoresis and spectrophotometry, respectively. About 1 μg of RNA was reverse transcribed and the cDNA was amplified using selected primers (Table 1) by Real time RT-PCR using the SuperScript III Platinum two-step qPCR kit with LUX fluorogenic primer (Invitrogen). Primer sequences for collagen I, collagen III, biglycan, periostin and GAPDH genes were designed by LUX™ Designer (FAM labeled LUX primer Invitrogen) and are shown in Table 1. Reaction conditions were as follows: 2 min at 50°C (one cycle), 10 mins at 95°C (one cycle), 15 sec at 95 °C and then 1 min at 58°C (40 cycles). PCR products were continuously monitored with an ABI PRISM 7900 detection system (RRC-CORE at UIC). Samples were normalized using human GAPDH (Joe-labeled LUX primer set, Invitrogen) as the internal control. Results are expressed as fold increases in expression over the control group by using the 2−ΔDelta;Ct method (Livak and Schmittgen, 2001).
Table 1.
| Gene | Orientation | Sequence |
|---|---|---|
| Collagen Type I α 1 | S | CGCTATCATCAGCCCGGTAGTAG[FAM]G |
| Collagen Type I α 1 | AS | TGGTTTCGACTTCAGCTTCCTG |
| Collagen Type III α 1 | S | CGGATGCTTCCAGACATCTCTATC[FAM]G |
| Collagen Type III α 1 | AS | ACAGGAAGCTGTTGAAGGAGGA |
| GAPDH | S | CGCCCATCCCTGCCTCTACTGG[JOE]G |
| GAPDH | AS | AGCTTCCCGTTCAGCTCAGG |
| Biglycan | S | CGGAGGCAGAACAACGACATCTC[FAM]G |
| Biglycan | AS | TGCTGGAGACCCTTGAAGTCAT |
| Periostin | S | CGGTGTCCTTCACTTACTTTGCAC[FAM]G |
| Periostin | AS | TCAGAATCCAAGTTGTCCCAAGC |
S = sense, AS = anti-sense
Immunohistochemistry
PDLPs-collagen gel regenerates after 4 weeks of implantation were fixed in 10% buffered formalin and prepared for paraffin sectioning. Alternatively, mandibles from 4 week post natal (pn) mice were harvested and fixed in 10% formalin and decalcified in 8% phosphate buffered EDTA (pH 7.4) for 3 weeks, dehydrated in a series of alcohol changes, cleared by xylene and embedded in paraffin. For immunohistochemistry, slides were deparaffinized and tissues were rehydrated. Immunoreactions were performed as previously described (Luan et al., 2007). Peroxidase activity was blocked by incubating the slides using a 9:1 ratio of methanol and hydrogen peroxide. The antigenic epitopes were exposed by microwave treatment of the slides in 1% citrate buffer pH 6.0 for 5 mins and then cooling it down to room temperature. Non-specific sites were blocked using serum. Immunoreactions were performed using monoclonal primary antibody for periostin (abcam) on PDLPs-collagen regenerates and collagen I, collagen III, biglycan and periostin antibodies were used on sections from 4 weeks pn mice mandibles to demonstrate localization of these proteins in the periodontium. Sections were then incubated with primary antibody at room temperature for 1 hour at a dilution of 1:500 in PBS. Sections were washed three times in PBS and incubated for 10 min with anti-mouse IgG. After three more washes in PBS, sections were incubated for 10 min with streptavidin-enzyme conjugate and then washed for three more times with PBS. Signals for immunoreactions were detected using AEC substrate-chromogen mixture (color substrate), counterstained with hematoxylin, and slides were mounted using GVA mount. The negative controls for the antibody were the ones in which the primary antibody was replaced with a similar amount of PBS.
Western blot analysis
After 4 weeks of implantation in vivo, tissue engineered PDLPs-collagen gel constructs were extracted and washed with PBS. The constructs were then homogenized in sodium dodecylsulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer and proteins extracted as described (Luan et al., 2006). Identical amounts of protein extracts from all regenerates were separated on a 10% SDS-PAGE gel and transferred to PVDF membrane in a semi-dry blotting apparatus containing transfer buffer (25mM tris, 40mM glycine, 10% methanol) for 40 minutes at 75mA. The PVDF membrane was then blocked with 2.5% BSA for 1 hour at room temperature and the blot was incubated with 1:1000 dilution of anti-human periostin (Abcam), collagen III (Abcam), biglycan (R&D Biosystems) and GAPDH (Abcam) antibodies for 2 hour, washed with TBST 3 times and incubated with 1:2500 dilution of HRP conjugated anti-rabbit or anti-mouse secondary antibody respectively (Zymed, South San Francisco, CA) for 1 hour, and further washed 3 times with TBST. HRP detection was performed using a chemiluminescent substrate (Supersignal West Pico Chemiluminescent Substrate, Pierce). The bands obtained from Western blots were then quantified for its densitometric value using the Image analysis software (Kodak Image station 440CF)
Mineralization induction in PDLPs cultures
PDLPs were seeded at a density of 5 × 104/well of a 12 well plate and cultured in DMEM media supplemented with 10% FBS and 1% antibiotics. FGF2 and CTGF were added the following day. One set of wells was treated with FGF2 (10 ng/ml) or CTGF (50 ng/ml) or a combination of both for the entire duration of 10 days. Another set of wells was treated sequentially with FGF2 for the first 4 days followed by CTGF for the next 6 days and vice versa. Wells that were cultured without addition of any growth factor served as the untreated control. All cells were cultured in an osteogenic differentiation media prepared by addition of 0.2mM ascorbic acid 2 phosphate and 4mM beta glycerophosphate in the above mentioned DMEM. At the end of 10 days of culture in mineralizing media, cell cultures were washed with PBS and fixed in 70% ethanol for 1 hr. To detect mineral deposits, cultures were stained with 40mM Alizarin Red S, pH 4.0, for 10 mins, washed with 70% ethanol and then with PBS to remove non-specific staining. Each experiment was done in triplicate and repeated 3 times. To determine the calcium levels in PDLPs cultures, the mineral in the cell-matrix was dissolved in 0.6 N HCL overnight and analyzed using the o-cresolphthalein complexone method (Baginski et al., 1973).
Mineralization analysis
After 2 weeks of in vitro culture, hydrogel constructs containing DFCs either treated with osteogenic, PDGF supplemented media, or untreated controls were fixed overnight in 10% formalin. The constructs were dehydrated through a series of alcohol changes and Citrisolve over 1 hr steps and finally in molten paraffin at 60 oC under vacuum with two changes to fresh paraffin every hour. Constructs were then embedded in paraffin and sections of 7 μm thickness were cut using a microtome (Leica). Sections were dried overnight on polylysine coated slides and stained using standard histological techniques. Von Kossa’s silver stain was used to evaluate the amount of mineralization with safranin-O as the counterstain. Micrographs were taken at magnifications of 20× and 40×.
Statistical analysis
All data are presented as means +/− standard deviation of the mean. Student’s t test was applied for data comparison and P values of less than 0.05 were judged significant.
Results
Collagens I and III, periostin, and biglycan localization in the periodontal ECM
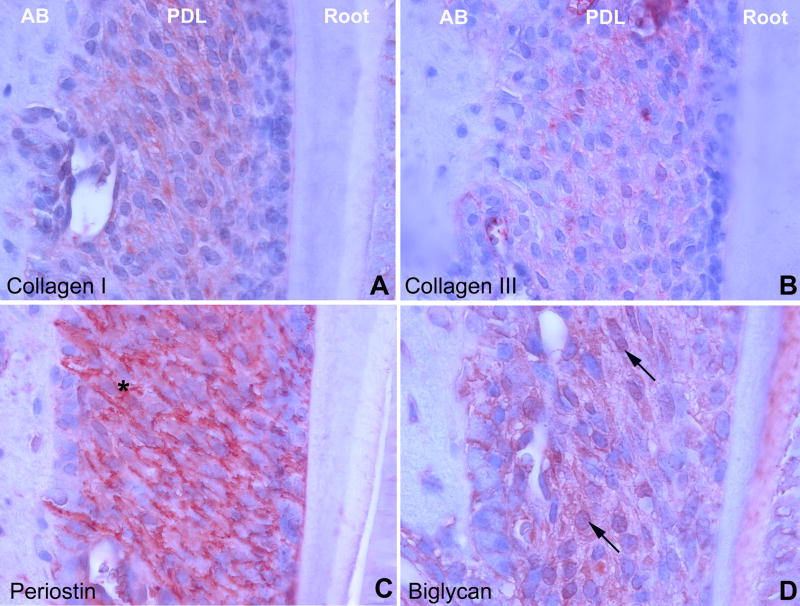
In order to map the distribution of key ECM components in the periodontium, four key periodontal matrix proteins were localized using immunohistochemistry. Collagens I and III as well as biglycan were localized in the interstitial ECM of the periodontal extracellular matrix (PDL). Sharpey’s fibers were intensely stained for periostin (asterisk, Fig. 1C), moderately for biglycan and collagen I and weakly for collagens III (Fig. 1A,1B, 1D). Note the distinct localization of biglycan in the predentin (Fig. 1D).
Fig 1. ECM components of the mouse periodontium.
Collagens I and III as well as biglycan were localized in the interstitial extracellular matrix (ECM) of the periodontal ECM. Periodontal fibers were intensely stained for periostin (asterisk). AB = alveolar bone, PDL = periodontal ligament, Root = tooth root including dentin, predentin, and cementum.
CTGF and FGF2 up-regulated collagen III and biglycan in implanted PDL/collagen gel constructs
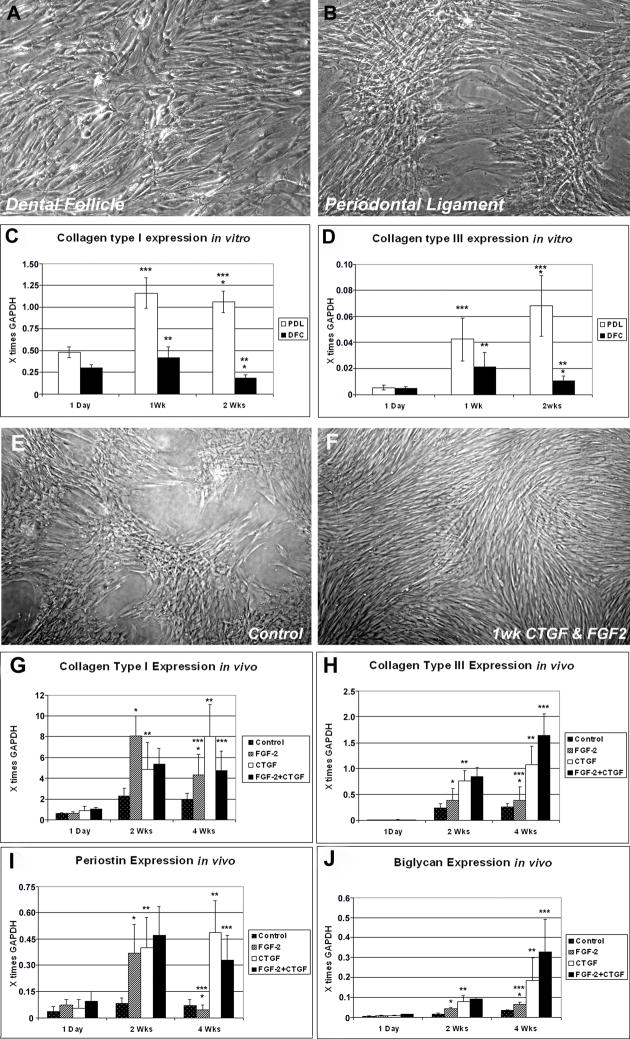
In order to compare expression levels of collagens as principle matrix components of the PDL, both DFCs and PDLPs were subjected to 2ng/ml of FGF2 and cultured at a density of 2×105 cells/ml in collagen gels for 1d, 1week and 2 weeks in DMEM. Alternatively, cells were cultured in monolayer for 1 week. After 1 week of in vitro monolayer culture, DFCs formed elongated and parallel arranged spindle shaped cells (Fig. 2A), while PDLPs assembled into dense colonies of radial symmetry (Fig. 2B). Real-time RT-PCR analysis of the cell-collagen gel constructs showed that there was significantly higher collagen I and III expression (5.88-fold (*) and 6.47-fold (*), respectively) in PDLPs compared to the DFCs after 2 weeks of in vitro culture (Fig. 2C, 2D). Both collagen I and III expression in DFCs increased at 1 week of culture but decreased 2.33-fold (**) and 2.01-fold (**) respectively after 2 weeks. In PDLPs, collagen I expression at 2 weeks remained at a similar level compared to week 1 (***), while collagen III expression continuously increased 1.6-fold after 2 weeks of culture and treatment with FGF2 (***). Findings in this study thus illustrate a difference in terms of new collagen matrix synthesis between less differentiated DFCs and lineage committed PDLPs as a result of inductive conditions. To answer questions as to how individual growth factors (FGF2, CTGF) and growth factor combinations affect cell morphology and extracellular matrix gene expression, we used monolayer culture and subcutaneously implanted cell-seeded collagen gels as a model system. On a cellular level in monolayer cultures, synergistic action of FGF2 and CTGF turned nodular cell assemblies of PDL progenitors (Fig. 2E) into densely packaged cell lawns consisting of elongated and thin individual cells (Fig. 2F). Real time RT-PCR analysis of cell-gel constructs harvested after 1 day, 2 weeks or 4 weeks of subcutaneous implantation in nude mice demonstrated that CTGF significantly increased the expression of collagen I (1.6-fold), collagen III (1.43-fold), biglycan (2.37-fold) and periostin (1.25-fold) in PDLPs constructs at 4 weeks compared to 2 weeks (Fig. 2G-J, **) while FGF2 downregulated the expression of collagen I and periostin 1.86-fold and 8.4-fold respectively (Fig. 2G, 2I, *) and upregulated the expression of biglycan 1.57-fold (Fig. 2J, *). In contrary, FGF2 and CTGF together upregulated the expression of collagen I and periostin 1.1 and 7.34-fold, respectively (Fig. 2G, 2I, ***) and synergistically increased the expression of collagen III and biglycan 4.24 and 4.92-fold, respectively (Fig. 2H, 2J, ***) after 4 weeks compared to control groups at the same time point. There was no expression of collagen type II in either group (data not shown). When compared to the untreated controls, FGF2 increased the expression of collagen I, collagen III and biglycan 2.30, 1.52 and 1.94-fold while it decreased periostin expression 1.11-fold after 4 weeks. In contrast, CTGF increased expression of collagen I, collagen III, biglycan and periostin 4.25, 4.23, 5.34, and 7.06-fold. Finally, FGF2 and CTGF together upregulated expression of collagen I, collagen III, biglycan and periostin 2.50, 6.45, 9.46 and 4.76-fold when compared to untreated controls at 4 weeks (Fig. 2G-J, 4wks). These data document the significant effect of growth factors such as FGF2 and CTGF on PDL ECM gene expression. Together, combination of FGF2 and CTGF enhanced collagen I, collagen III, biglycan and periostin gene expression after 4 weeks of in vivo implantation compared to the untreated controls.
Fig 2. Time course comparison of the expression levels of collagens (I & III) in DFCs and PDLPs and the effect of growth factors and their combinations on PDL morphology and ECM gene expression.
Figs. 2A and B illustrate elongated, parallel arrangement of DFCs (2A) and radially symmetric colonies of PDLPs after one week of monolayer culture (2B). When cultured over several weeks, there was a distinct difference in the expression of collagen I (Fig. 2C) and collagen III (Fig. 2D) between PDLPs and DFCs as revealed by real time RT-PCR analysis (Figs. 2C and D). In both studies cell-gel constructs were harvested after 1d, 1 week (Wk) or 2 weeks (Wks) of in vitro culture (Fig. 2C, D). Fig. 2E illustrates formation of dense colonies of PDLPs after one week of culture. In contrast, treatment with a combination of CTGF and FGF2 growth factors during this time period resulted in the formation of elongated and thin individual cells (Fig. 2F). In Figs. 2G–J, gene expression collagen type I (2G), collagen type III (2H), periostin (2I) and biglycan (2J) genes in cell-gel constructs was determined by Real time RT-PCR analysis and cells were harvested after 1 day, 2 weeks (Wks) or 4 weeks (Wks) of subcutaneous implantation in nude mice. While in general, gene expression levels increased after two or four weeks implantation when treated with FGF2 and/or CTGF growth factors, individual ECM gene responses to growth factors were distinctly different and growth factor combinations had either synergistic or antagonistic effects (Fig. 2G–F). In all bar graphs, gene expression levels were displayed relative to the house keeping gene GAPDH.
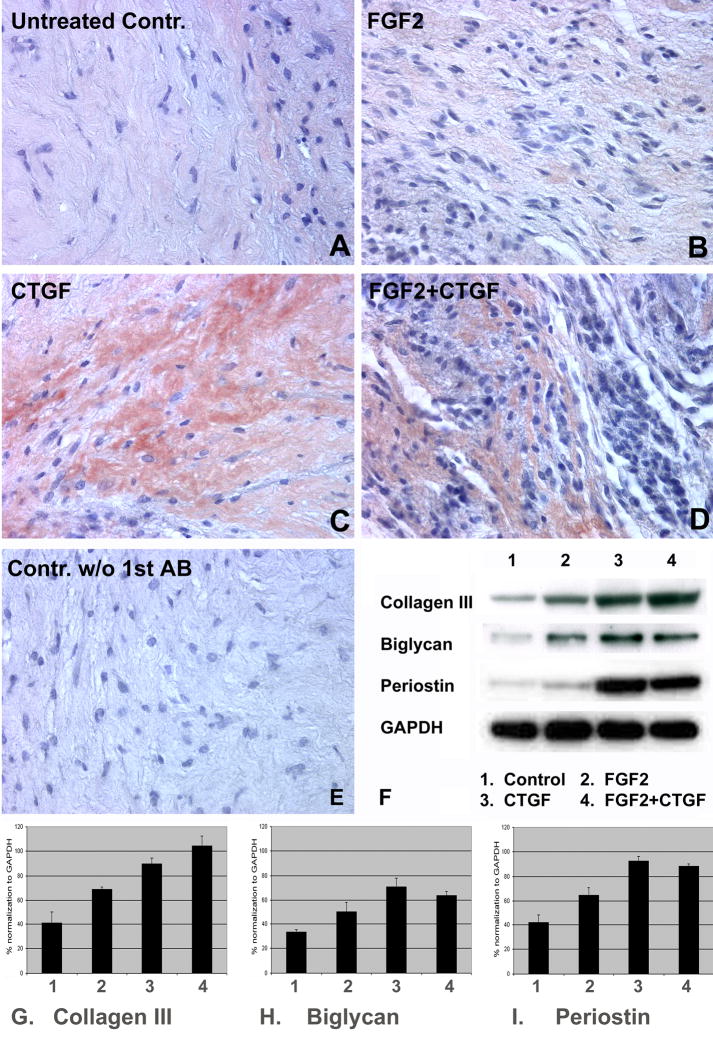
CTGF promotes the formation of a mature PDL-like tissue by enhancing the expression of the PDL ECM proteins periostin, collagen I, III, and biglycan
Periostin is a critical extracellular matrix protein important for periodontal homeostasis and PDL space maintenance (Rios et al., 2005). In order to determine whether our in vivo cell-gel implants were capable of maintaining the expression of periostin as a key protein component of the PDL matrix, collagen gels encapsulated with PDLPs were treated with FGF2 (10ng/ml, Fig. 3B, lane 2 of Figs. 3F–I), CTGF (50ng/ml, Fig. 3C, lane 3 of Figs. 3F–I), and a combination of FGF2+CTGF (10ng/ml and 50nm/ml, respectively; Fig. 3D, lane 4 of Figs. 3F–I) for a period of 10 days or left untreated (controls, Fig. 3A, lane 1 of Figs. 3F–I), and subcutaneously implanted in nude mice for 4 weeks following which regenerates were investigated for periostin localization and key ECM protein expression. Immunohistochemistry for periostin revealed distinct levels of periostin localization in PDL-constructs harvested after 4 weeks of subcutaneous implantation in nude mice as shown in Fig. 3A–E. The CTGF treated group demonstrated the highest level of periostin immunolocalization with dense immunoreactivity in collagen fiber bundles (Fig. 3C), followed by the group treated with combination of CTGF and FGF2 (Fig. 3D) and weak localization in untreated controls (Fig. 3A) and FGF2 treated groups (Fig. 3B). On Western blots, periostin levels were 1.73-times higher than untreated controls following CTGF treatment (Fig. 3I, comparing lanes 1 and 3), while FGF2 treated constructs alone had an expression level similar to that of untreated controls (1.05-times) after 4 weeks (Fig. 3I, comparing lanes 1 and 2). A combination of CTGF and FGF2 significantly increased periostin expression levels resulting in a densitometric level 1.69-times higher than the control (Fig. 3I, lanes 1 and 4). The effects of CTGF, FGF2, and combinations on collagen III and biglycan expression (Fig. 3F–H) were similar to that of periostin; however, there was a synergistic action between FGF2 and CTGF on collagen III expression (Fig. 3F(collagen III-lanes1–4), G). Together, our findings illustrate that growth factors such as CTGF and FGF2 alone or in combination significantly enhance the expression levels of PDL ECM proteins such as periostin, biglycan, and collagen III.
Fig 3. Immunohistochemical and Western blot analysis of key ECM molecules in tissue engineered PDL regenerates.
Fig. 3A–E are paraffin sections of PDLP/collagen tissue engineered constructs subcutaneously implanted in nude mice for 4 weeks. Prior to implantation, PDLPs were treated for ten days with various growth factors such as FGF2 (10ng/ml, Fig. 3B), CTGF (50ng/ml, Fig. 3C), FGF2+CTGF (10/50ng/ml Fig. 3D), or left untreated (controls, Fig. 3A). The strongest staining intensity for periostin was found in the CTGF-treated group (Fig. 3C), followed by the group treated with CTGF + FGF2 (Fig. 3D). There was little or no periostin localization in untreated controls (Fig. 3A) and in the FGF2 treated group (Fig. 3B). Fig. 3E is the negative control that was not subjected to primary antibody. In Fig. 3F we have used Western blot analysis to determine levels of collagen III, biglycan, and periostin in our growth factor-treated tissue engineered constructs; lane 1 was the control group, lane 2 treated with FGF2, lane 3 treated with CTGF, and lane 4 treated with FGF2 plus CTGF (for concentrations see above). The results of our Western blot analysis were quantified using the Kodak 1D Image analysis software, and graphs were plotted by normalizing the value of collagen III, biglycan and periostin with GAPDH as control (Fig. 3G–I). The results of this study illustrate that all three growth factor treatment modalities (FGF2, CTGF, and FGF2+CTGF) resulted in a significant increase in the expression level of ECM matrix proteins (Collagen III, Biglycan, and Periostin). Moreover, CTGF alone or combinations with CTGF and FGF2 had a stronger effect on matrix proteins than FGF2 alone.
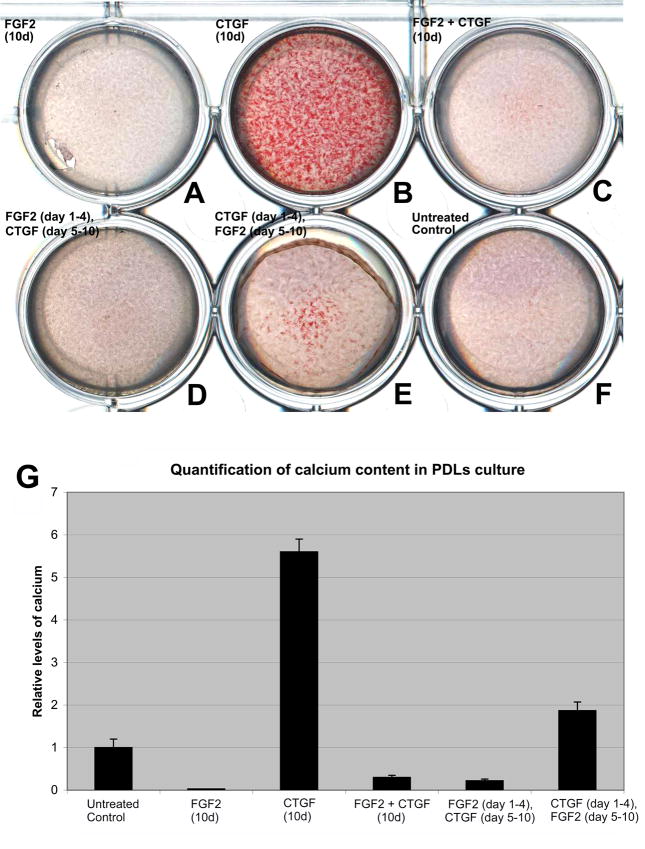
Antagonistic effects of FGF2 and CTGF on the mineralization of PDLPs in vitro
To determine whether the periodontal ECM growth factors FGF2 and CTGF had different effects on PDL matrix mineralization, we cultured PDLPs in mineralizing media for a total of 10 days under the following conditions: (i) Continuous treatment with FGF2 for 10 days, (ii) continuous treatment with CTGF for 10 days, (iii) continuous treatment with FGF2 and CTGF for 10 days, (iv) sequential treatment with FGF2 for 4 days followed by CTGF for the next 6 days, (v) sequential treatment with CTGF for 4 days followed by FGF2 for the next 6 days, and (vi) without growth factor (untreated control). The amount of mineralization was evaluated by staining cell cultures with Alizarin Red S. Our studies revealed that untreated cultures contained a moderate level of mineralization nodules (Fig. 4F, G), which were enhanced 5.6-fold by continuous treatment with CTGF (Fig. 4B, G), but completely blocked by continuous treatment with FGF2 (Fig. 4A, G). The cultures that were treated with simultaneous application of FGF2 and CTGF demonstrated a few mineralization nodules approximately, 0.3-fold of the untreated controls (Fig. 4C, G). Sequential application of FGF2 followed by CTGF also failed to form significant amounts of mineralization nodules (Fig. 4D, G), while early application of CTGF promoted mineral nodule formation, which was inhibited after application of FGF2 and resulted in a 1.87-fold increase compared to the untreated controls. (Fig. 4E, G). Taken together, these results suggest that FGF2 and CTGF have an antagonistic effect on the mineralization characteristics of PDLPs.
Fig 4. Effects of FGF2 and CTGF treatment on matrix mineralization in PDLPs.
Fig.(4 A–F) is a photograph of a culture plate containing PDLPs cultured at a density of 5 × 104 cells/well for a total of 10 days in osteogenic differentiation media with or without growth factors stained with Alizarin Red S to visualize relative levels of calcium. Cells were cultured in the following conditions: (i) continuous treatment with FGF2 for 10 days (Fig. 4A), (ii) continuous treatment with CTGF for 10 days (Fig. 4B), (iii) continuous treatment with FGF2 and CTGF (Fig. 4C), (iv) sequential treatment with FGF2 for 4 days followed by CTGF for the next 6 days (Fig. 4D), (v) sequential treatment with CTGF for 4 days followed by FGF2 for the next 6 days (Fig. 4E), and (vi) without growth factor (Fig. 4F). In Fig. 4G, relative calcium levels were calculated based on the o-cresolphthalein complexone method and spectrophotometric evaluation. Note that both CTGF treatment alone and CTGF followed by FGF2 treatment resulted in the most significant increases in calcium levels while simple addition of FGF2 and CTGF to the medium significantly decreased calcium levels (Fig. 4G).
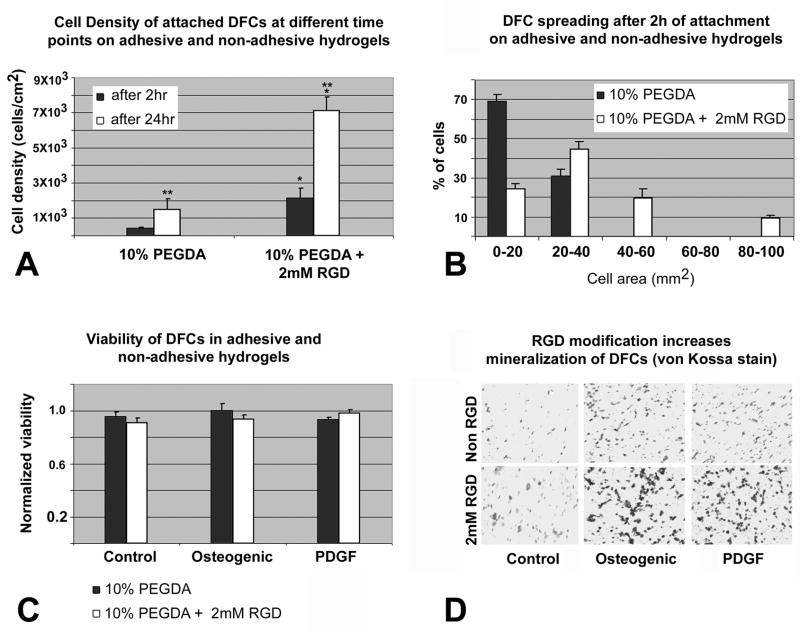
RGD-modified PEGDA hydrogels maintained cell viability, enhanced cell surface attachment and spreading and increased the mineralization of encapsulated DFCs
RGD is a tripeptide sequence present in many cell adhesion ECM proteins and glycoproteins that facilitates cell anchorage, traction for migration, proliferation and differentiation by providing binding sites for specific integrin cell surface receptors (Ruoslahti et al.,1987). In order to determine the effect of RGD peptides on DF cell behavior we monitored the attachment and spreading of DFCs on PEGDA hydrogel surfaces conjugated with PEGylated RGD and further tested their viability and mineralization potential in 3-dimensional RGD-modified or unmodified PEGDA hydrogels under the influence of osteogenic and PDGF supplemented conditions. When DFCs were allowed to attach on RGD-modified and unmodified hydrogels for 2 hrs, cell density was reduced on unmodified hydrogels (0.388 × 103 cells/cm2)compared to 2.29 × 103 cells/cm2 on RGD-modified hydrogels. After cells were allowed to attach for 24 hrs, cell density of attached cells increased from 1.49 × 103 cells/cm2 on unmodified hydrogels to 7.11 × 103 cells/cm2 on RGD-modified hydrogels (Fig. 5A), indicating that RGD-modified hydrogels not only provided a favorable substrate for cells to adhere but also increased their proliferation as the population more than doubled in 24 hrs. There was a statistical difference (p<0.05) between the number of DFCs attached on RGD-modified and unmodified hydrogels when comparing identical attachment times (Fig. 5A). Moreover, cell attachment also increased when comparing 24hrs with 2hrs incubation time (Fig. 5A). To monitor the spreading of DFCs on adhesive (10% PEGDA with 2mM Acr-PEG-RGD) and non adhesive (10% PEGDA) hydrogels, the area occupied by the cells on the two surfaces was measured after 2 hrs of attachment. The degree of cell spreading was quantified as the surface area occupied by the cells and was classified as follows: 0–20 mm2 (least spread), 20–40 mm2, 40–60 mm2, 60–80 mm2 and 80–100 mm2 (most spread). 69% of the cells attached on unmodified hydrogels maintained a round morphology and were least spread, compared to 25% of the cells attached on RGD-modified hydrogels. More than 30% of the cells on RGD-modified hydrogels had expanded in size to measure 40–100 mm2 in surface area while non of the cells on unmodified hydrogels had grown to that extend, indicating that addition of RGD peptides enhanced cell adhesion and spreading (Fig. 5B). To determine if conjugation of RGD peptide had any adverse effect on viability of encapsulated cells, DFCs in the two types of hydrogels were subjected to MTT viability assay after 3 days of culture in control, osteogenic differentiation and PDGF supplemented media. We found that there was no statistical difference (p>0.05) in the viability of DFCs in the modified and unmodified hydrogels and their treatment groups (Fig. 5C), indicating that conjugation of RGD peptides in PEGDA hydrogels did not affect the viability the DFCs. Finally, to investigate if RGD-modified hydrogels enhances the mineralization characteristics of DFCs, encapsulated DFCs in the RGD-modified and unmodified hydrogels were cultured in vitro in either control, osteogenic and PDGF supplemented media for 2 weeks at the end of which we measured the area of mineralized nodules in each group as obtained by von Kossa’s silver stain. Unmodified hydrogels accounted for 192 +− 23 mm2 of the mineralized noduleswhile the RGD-modified hydrogels accounted for 365 +− 59 mm2, resulting in a 1.90-fold increase in the mineralization area in the RGD-modified hydrogels over unmodified hydrogels, the control group. (Fig. 5D). The RGD-modified hydrogel constructs when treated with osteogenic differentiation medium increased the mineralization region to 1739 +− 116mm2 compared to the unmodified osteogenic treated constructs (499 +− 52 mm2), a 3.49-fold increase while in PDGF treated groups the area of mineralized nodules increased from 473+− 41 mm2 in unmodified hydrogels to 1408 +− 131 mm2 in RGD-modified hydrogels resulting in a 2.98-fold increase in the area of mineralization (Fig. 5D). Thus, RGD-peptide incorporation in hydrogels increased mineralization by 1.90, 3.49 and 2.98-fold in untreated, osteogenic treated and PDGF supplemented groups, respectively. Together, these data indicate that attachment, cell spreading, and mineralization were significantly increased in RGD-modified hydrogels, while cell viability was not affected, at least for the time period investigated.
Fig 5. Attachment, spreading, viability and mineralization of DFCs depending on RGD-modification of PEGDA hydrogels.
Fig. 5A. Cell density of attached DFCs after 2 and 24 hrs on 10% PEGDA without Acr-PEG-RGD or containing 2mM Acr-PEG-RGD. Values are reported as an average of three random fields times three hydrogel wells at each time point. There was a statistical difference (p<0.05) in the number of DFCs attached on the two types of hydrogels at the same time point (**) and on the same type of hydrogel at different time points (*). Fig. 5B. Histogram of DFC spreading after 2 hr attachment on either 10% PEGDA or 10% PEGDA with 2mM Acr-PEG-RGD. To visualize the morphology of the attached cells on these two types of hydrogel surfaces, micrographs were obtained following 2 hrs of attachment, and the area of cell spreading was calculated by measuring the area individual cell occupied using the NIH imaging software. Values are reported as the percentage of the attached cells spreading and occupying areas in the range specified (0–100 mm2) over three random fields per composition. Fig. 5C. Viability of DFCs in 10% PEGDA with or without 2mM of Acr-PEG-RGD after 3 days culture in osteogenic differentiation medium, PDGF supplemented medium, and control medium. Values are reported as the average of three constructs per composition per treatment. There was no statistical difference (p>0.05) in the viability of DFCs between the modified and unmodified hydrogels and their treatment groups. Fig. 5D. Von Kossa stained sections of untreated (control) and treated (osteogenic, PDGF) DFCs encapsulated in unmodified (upper row) and RGD-modified (lower row) hydrogels after 2 weeks of in vitro culture. Mineralization was significantly increased in the RGD-modified group as seen by dense mineralization nodules.
Discussion
In the present study we have tested the function of several key extracellular matrix environmental factors as they affect the differentiation potential and behavior of periodontal progenitor populations. In order to facilitate biological recognition and cell machinery survival, we have chosen collagen Type I and hydrogels as scaffolds for implantation studies. Collagen I is the major ECM protein of the PDL (Watanabe and Kubota, 1998) and provided a near ideal microenvironment for the PDLPs to attach, proliferate and form a PDL spindle like morphology. Hydrogels emerged as a second scaffold material of choice because of the elastic properties and hydrated states found in periodontal microenvironments. Using collagenous scaffolds and hydrogels, we have studied the differentiation capacities of two major periodontal progenitor populations, DFCs and PDLPs (Luan et al., 2006; Seo et al., 2004). Both DFCs and PDLPs are neural crest derived, nestin positive (neural crest marker) cell populations (Luan et al., in press), and PDLPs originate from migratory DFCs during tooth development (Palmer and Lumsden 1987; Diekwisch 2001, 2002). Here we have subjected clonally expanded, multipotent DF and PDL progenitor cells from the periodontal region to a variety of periodontium-related ECM factors and environments. To test the role of growth factors on periodontal ECM, we have chosen the periodontal connective tissue growth factors FGF2 and CTGF. In a second set of studies we have used a classic cell adhesion molecule that occurs in the integrin-rich periodontal extracellular matrix, the adhesion peptide RGD, and tested its effect on DFC function and mineralization. Together, our studies have revealed the significant effect of key periodontal extracellular matrix components on the differentiation of periodontal progenitor populations.
Our data indicated that collagens I and III, periostin, and biglycan were distributed throughout the periodontal ECM. Macromolecules such as collagens and proteoglycans are vital structural components of the periodontal ECM, and are involved in the development and regeneration of the periodontium (Matias et al., 2003). The ECM component periostin, a cell adhesion protein (Gillan et al., 2002), is essential for PDL homeostasis as its expression changes dynamically in response to tension and compression (Rios et al., 2005; Wilde et al., 2003). In our study we found periostin forming a dense network surrounding Sharpey’s fibers. Moreover, lack of periostin results in traumatic dental-alveolar stimuli and a phenotype resembling an early onset of periodontal disease (Rios et al., 2005). Together, these studies indicate that the molecules we have chosen in the present study are widely distributed throughout the periodontium and important for the physiological function of the periodontium.
Our analysis of the effects of CTGF and FGF2 on periodontal progenitor populations revealed significant effects of both factors on individual matrix gene expression as well as complex synergies between CTGF and FGF2 perhaps representative of multifactorial periodontal extracellular matrix environments. Specifically, our gene expression analysis and Western blots showed that CTGF distinctly increased periostin localization on collagen fiber bundles and upregulated collagens I and III, periostin, and biglycan expression. CTGF is a downstream factor of the TGF beta signaling pathway (Grotendorst et al.,1997) and TGF beta has been shown to induce periostin expression (Horiuchi et al.,1999), suggesting that the effect of CTGF on periostin is part of a TGF beta-related mechanism. In contrast, FGF2 inhibited expression of collagen I and periostin after four weeks of implantation compared to two weeks of implantation, supporting earlier studies that FGF family members induce proliferation of the PDL cells while inhibiting cell differentiation and mineralization via downregulation of collagen I (Okamoto et al., 1997; Murakami et al.,1999; Palmon et al., 2000; Lallier and Spencer, 2007). In comparison, FGF2 and CTGF had antagonistic effects on PDLPs mineralization under osteogenic conditions: FGF2 inhibited while CTGF promoted mineralization of PDLPs. Our finding that CTGF promotes the mineralized state of PDLPs is supported by studies indicating that CTGF expression is regulated by TGF-β1/BMP-2, which in turn enhance collagen I and alkaline phosphatase expression during PDL homeostasis in vitro (Asano et al., 2005). In addition, our study revealed that CTGF and FGF2 had synergistic effects in some instances (e.g. promotion of collagen III and biglycan gene expression) while acting in an antagonistic fashion in respect to mineralization. These seemingly opposing effects illustrate the complexities of periodontal extracellular matrix environments, suggesting that tissue architecture, microscale-homeostasis, and local sequestering of growth factors play additional roles in the presentation of matrix molecules toward their target tissue (reviewed in Nelson and Bissell, 2006).
Our data indicated that RGD-modified PEGDA hydrogels maintained cell viability, enhanced cell surface attachment and spreading, and increased the mineralization of encapsulated DFCs. Here we have shown for the first time that differentiated DFCs in a RGD-hydrogel are capable of forming mineralized matrix. Hydrogels are promising polymeric biomaterials for tissue engineering purposes due to their ability to absorb water and possess tissue-like elasticity. Cells can thus be suspended in the polymer solution and can be polymerized in situ within a defect site in the body providing strategies for tissue engineering (Peppas et al.,1987; Anseth et al., 1996; Elisseeff et al., 2005). However, the cells encapsulated in the hydrogels seem to be trapped and have restricted secretion of adhesion proteins and as a result there is limited interactions between the cell surface integrins and the surrounding gel environment (Nuttelman et al., 2005). Absence of cell-matrix interaction might result in apoptosis of the encapsulated cells (Frisch and Ruoslahti., 1997). In order to create a microenvironment conducive for cell attachment and growth we incorporated an RGD-peptide sequence into our hydrogels. As a result, our RGD-modified hydrogels resulted in increased cell attachment and cell spreading when compared to unmodified hydrogels. Increased cell adhesion and spreading on RGD-modified hydrogels is the result of the interactions between the cell surface integrins and RGD motifs on the hydrogel surface. The RGD motifs presented by the PEGDA surface provide additional integrin binding sites, supporting cell anchorage, proliferation, and differentiation, which are otherwise inhibited on unmodified hydrogels. The increase of cell attachment and proliferation on adhesive hydrogel surfaces was previously reported (Alsberg et al., 2002; Yang et al., 2005). Addition of RGD had no detrimental effects on the viability of cells when observed after 3 days of in vitro culture. Osteogenic differentiation media and PDGF supplemented media significantly enhanced the mineralization of DFCs encapsulated in RGD-modified hydrogels. Growth factors such as PDGF have been shown to act through cell surface receptors like PDGF-Rα and PDGF-Rβ to promote regeneration of bone and cementum (Giannobile et al., 2001). The higher amount of mineralization in these treatment groups can be attributed to the fact that RGD modified hydrogels promote increased cell proliferation and their subsequent differentiation. One drawback of this system however is the limited diffusivity and the non-biodegradable nature of the hydrogel. Synthesizing hydrogels that degrade during the formation of new tissues would result in clinically viable regenerates for therapeutical applications. In addition, three-dimensional scaffold architecture (microspheres and multisheets) have been shown to significantly enhance PDL cell properties and matrix synthesis under osteogenic differentiation conditions (Inanc et. al., 2006, 2007a, b) and might thus be beneficial in conjunction with RGD-based strategies to design optimum periodontal scaffolds.
In summary, our morphological, gene expression and immunohistochemical data revealed that ECM growth factors such as FGF2 and CTGF induce periodontal progenitor cells to differentiate towards a PDL-like tissue in a collagen gel microenvironment and might thus be suitable for periodontal regeneration therapy. In addition, RGD-modified hydrogels maintained cell viability, enhanced surface attachment and increased the mineralization of the encapsulated DFCs, and thus could have potential applications in healing periodontal defects by regenerating new bone and cementum.
Acknowledgments
Support for these studies by NIDCR grants DE15425 and DE17447 is gratefully acknowledged.
References
- Alsberg E, Anderson KW, Albeiruti A, Rowley JA, Mooney DJ. Engineering growing tissues. Proc Natl Acad Sci USA. 2002;99:12025–12030. doi: 10.1073/pnas.192291499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anseth KS, Bowman CN, Brannon-Peppas L. Mechanical properties of hydrogels and their experimental determination. Biomaterials. 1996;17:1647–1657. doi: 10.1016/0142-9612(96)87644-7. [DOI] [PubMed] [Google Scholar]
- Asano M, Kubota S, Nakanishi T, Nishida T, Yamaai T, Yosimichi G, Ohyama K, Sugimoto T, Murayama Y, Takigawa M. Effect of connective tissue growth factor (CCN2/CTGF) on proliferation and differentiation of mouse periodontal ligament-derived cells. Cell Commun Signal. 2005;3:11. doi: 10.1186/1478-811X-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baginski ES, Marie SS, Clark WL, Zak B. Direct microdetermination of serum calcium. Clin Chim Acta. 1973;46:49–54. doi: 10.1016/0009-8981(73)90101-0. [DOI] [PubMed] [Google Scholar]
- Beertsen W, McCulloch CA, Sodek J. The periodontal ligament: a unique, multifunctional connective tissue. Periodontology. 1997;13:20–40. doi: 10.1111/j.1600-0757.1997.tb00094.x. [DOI] [PubMed] [Google Scholar]
- Bosshardt DD, Schroeder HE. Cementogenesis reviewed: a comparison between human premolars and rodent molars. Anat Rec. 1996;245:267–292. doi: 10.1002/(SICI)1097-0185(199606)245:2<267::AID-AR12>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Carneiro J, Leblond CP. Suitability of collagenase treatment for the radioautographic identification of newly synthesized collagen labeled with 3H-glycine or 3H-proline. J Histochem Cytochem. 1966;14:334–344. doi: 10.1177/14.4.334. [DOI] [PubMed] [Google Scholar]
- Chai Y, Jiang X, Ito Y, Bringas P, Jr, Han J, Rowitch DH, Soriano P, McMahon AP, Sucov HM. Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development. 2000;127:1671–1679. doi: 10.1242/dev.127.8.1671. [DOI] [PubMed] [Google Scholar]
- Dereka XE, Markopoulou CE, Vrotsos IA. Role of growth factors on periodontal repair. Growth Factors. 2006;24:260–267. doi: 10.1080/08977190601060990. [DOI] [PubMed] [Google Scholar]
- Diekwisch TG. The developmental biology of cementum. Int J Dev Biol. 2001;45:695–706. [PubMed] [Google Scholar]
- Diekwisch TG. Pathways and fate of migratory cells during late tooth organogenesis. Connect Tissue Res. 2002;43:245–256. [PubMed] [Google Scholar]
- Elisseeff J, Puleo C, Yang F, Sharma B. Advances in skeletal tissue engineering with hydrogels. Orthod Craniofac Res. 2005;8:150–161. doi: 10.1111/j.1601-6343.2005.00335.x. [DOI] [PubMed] [Google Scholar]
- Friedrichsen S, Heuer H, Christ S, Winckler M, Brauer D, Bauer K, Raivich G. CTGF expression during mouse embryonic development. Cell Tissue Res. 2003;312:175–188. doi: 10.1007/s00441-003-0712-6. [DOI] [PubMed] [Google Scholar]
- Friedrichsen S, Heuer H, Christ S, Cuthill D, Bauer K, Raivich G. Gene expression of connective tissue growth factor in adult mouse. Growth Factors. 2005;23:43–53. doi: 10.1080/08977190512331340566. [DOI] [PubMed] [Google Scholar]
- Frisch SM, Ruoslahti E. Integrins and anoikis. Curr Opin Cell Biol. 1997;9:701–706. doi: 10.1016/s0955-0674(97)80124-x. [DOI] [PubMed] [Google Scholar]
- Geiger H, True JM, de Haan G, Van Zant G. Age- and stage-specific regulation patterns in the hematopoietic stem cell hierarchy. Blood. 2001;98:2966–2972. doi: 10.1182/blood.v98.10.2966. [DOI] [PubMed] [Google Scholar]
- Giannobile WV, Lee CS, Tomala MP, Tejeda KM, Zhu Z. Platelet-derived growth factor (PDGF) gene delivery for application in periodontal tissue engineering. J Periodontol. 2001;72:815–823. doi: 10.1902/jop.2001.72.6.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillan L, Matei D, Fishman DA, Gerbin CS, Karlan BY, Chang DD. Periostin secreted by epithelial ovarian carcinoma is a ligand for alpha(V)beta(3) and alpha(V)beta(5) integrins and promotes cell motility. Cancer Res. 2002;62:5358–5364. [PubMed] [Google Scholar]
- Grotendorst GR. Connective tissue growth factor: a mediator of TGF-beta action on fibroblasts. Cytokine Growth Factor Rev. 1997;8:171–179. doi: 10.1016/s1359-6101(97)00010-5. [DOI] [PubMed] [Google Scholar]
- Hankemeier S, Keus M, Zeichen J, Jagodzinski M, Barkhausen T, Bosch U, Krettek C, Van Griensven M. Modulation of proliferation and differentiation of human bone marrow stromal cells by fibroblast growth factor 2: potential implications for tissue engineering of tendons and ligaments. Tissue Eng. 2005;11:41–49. doi: 10.1089/ten.2005.11.41. [DOI] [PubMed] [Google Scholar]
- Hern DL, Hubbell JA. Incorporation of adhesion peptides into nonadhesive hydrogels useful for tissue resurfacing. J Biomed Mater Res. 1998;39:266–276. doi: 10.1002/(sici)1097-4636(199802)39:2<266::aid-jbm14>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Horiuchi K, Amizuka N, Takeshita S, Takamatsu H, Katsuura M, Ozawa H, Toyama Y, Bonewald LF, Kudo A. Identification and characterization of a novel protein, periostin, with restricted expression to periosteum and periodontal ligament and increased expression by transforming growth factor beta. J Bone Miner Res. 1999;14:1239–1249. doi: 10.1359/jbmr.1999.14.7.1239. [DOI] [PubMed] [Google Scholar]
- Inanc B, Elcin AE, Elcin YM. Osteogenic induction of human periodontal ligament fibroblasts under two- and three-dimensional culture conditions. Tissue Eng. 2006;12:257–266. doi: 10.1089/ten.2006.12.257. [DOI] [PubMed] [Google Scholar]
- Inanc B, Eser Elcin A, Koc A, Balos K, Parlar A, Murat Elcin Y. Encapsulation and osteoinduction of human periodontal ligament fibroblasts in chitosan-hydroxyapatite microspheres. J Biomed Mater Res A. 2007a;82:917–926. doi: 10.1002/jbm.a.31213. [DOI] [PubMed] [Google Scholar]
- Inanc B, Eser Elcin A, Murat Elcin Y. Effect of osteogenic induction on the in vitro differentiation of human embryonic stem cells cocultured with periodontal ligament fibroblasts. Artif Organs. 2007b;31:792–800. doi: 10.1111/j.1525-1594.2007.00470.x. [DOI] [PubMed] [Google Scholar]
- Lalani Z, Wong M, Brey EM, Mikos AG, Duke PJ. Spatial and temporal localization of transforming growth factor-beta1, bone morphogenetic protein-2, and platelet-derived growth factor-A in healing tooth extraction sockets in a rabbit model. J Oral Maxillofac Surg. 2003;61:1061–1072. doi: 10.1016/s0278-2391(03)00319-7. [DOI] [PubMed] [Google Scholar]
- Lallier TE, Spencer A. Use of microarrays to find novel regulators of periodontal ligament fibroblast differentiation. Cell Tissue Res. 2007;327:93–109. doi: 10.1007/s00441-006-0282-5. [DOI] [PubMed] [Google Scholar]
- Lin CG, Leu SJ, Chen N, Tebeau CM, Lin SX, Yeung CY, Lau LF. CCN3 (NOV) is a novel angiogenic regulator of the CCN protein family. J Biol Chem. 2003;278:24200–24208. doi: 10.1074/jbc.M302028200. [DOI] [PubMed] [Google Scholar]
- Livak K, Schmittgen T. Analysis of relative gene expression data using real-time quantitative PCR and the 2 (-Delta Delta C (T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Luan X, Ito Y, Dangaria S, Diekwisch TG. Dental follicle progenitor cell heterogeneity in the developing mouse periodontium. Stem Cells Dev. 2006;15:595–608. doi: 10.1089/scd.2006.15.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan X, Ito Y, Holliday S, Walker C, Daniel J, Galang TM, Fukui T, Yamane A, Begole E, Evans C, Diekwisch TG. Extracellular matrix-mediated tissue remodeling following axial movement of teeth. J Histochem Cytochem. 2007;55:127–140. doi: 10.1369/jhc.6A7018.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan X, Dangaria S, Ito Y, Walker C, Jin T, Schmidt M, Galang T, Druzinsky R, White K. Neural crest lineage segregation: A blueprint for periodontal regeneration. J Dent Res. 2009 doi: 10.1177/0022034509340641. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madan AK, Kramer B. Immunolocalization of fibroblast growth factor-2 (FGF-2) in the developing root and supporting structures of the murine tooth. J Mol Histol. 2005;36:171–178. doi: 10.1007/s10735-005-2684-1. [DOI] [PubMed] [Google Scholar]
- Matias MA, Li H, Young WG, Bartold PM. Immunohistochemical localization of fibromodulin in the periodontium during cementogenesis and root formation in the rat molar. J Periodontal Res. 2003;38:502–507. doi: 10.1034/j.1600-0765.2003.00682.x. [DOI] [PubMed] [Google Scholar]
- Matsuura M, Herr Y, Han KY, Lin WL, Genco RJ, Cho MI. Immunohistochemical expression of extracellular matrix components of normal and healing periodontal tissues in the beagle dog. J Periodontol. 1995;66:579–593. doi: 10.1902/jop.1995.66.7.579. [DOI] [PubMed] [Google Scholar]
- Melcher AH. Cells of periodontium: their role in the healing of wounds. Ann R Coll Surg Engl. 1985;67:130–131. [PMC free article] [PubMed] [Google Scholar]
- Moore KA, Lemischka IR. Stem cells and their niches. Science. 2006;311:1880–1885. doi: 10.1126/science.1110542. [DOI] [PubMed] [Google Scholar]
- Murakami S, Takayama S, Ikezawa K, Shimabukuro Y, Kitamura M, Nozaki T, Terashima A, Asano T, Okada H. Regeneration of periodontal tissues by basic fibroblast growth factor. J Periodontal Res. 1999;34:425–430. doi: 10.1111/j.1600-0765.1999.tb02277.x. [DOI] [PubMed] [Google Scholar]
- Nelson CM, Bissell MJ. Of extracellular matrix, scaffolds, and signaling: tissue architecture regulates development, homeostasis, and cancer. Annu Rev Cell Dev Biol. 2006;22:287–309. doi: 10.1146/annurev.cellbio.22.010305.104315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuttelman CR, Tripodi MC, Anseth KS. Synthetic hydrogel niches that promote hMSC viability. Matrix Biol. 2005;24:208–218. doi: 10.1016/j.matbio.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Ohlstein B, Kai T, Decotto E, Spradling A. The stem cell niche: theme and variations. Curr Opin Cell Biol. 2004;16:693–699. doi: 10.1016/j.ceb.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Okamoto T, Yatsuzuka N, Tanaka Y, Kan M, Yamanaka T, Sakamoto A, Takata T, Akagawa Y, Sato GH, Sato JD, Takada K. Growth and differentiation of periodontal ligament-derived cells in serum-free defined culture. In Vitro Cell Dev Biol Anim. 1997;33:302–309. doi: 10.1007/s11626-997-0051-0. [DOI] [PubMed] [Google Scholar]
- Palmer RM, Lumsden AG. Development of periodontal ligament and alveolar bone in homografted recombinations of enamel organs and papillary, pulpal and follicular mesenchyme in the mouse. Arch Oral Biol. 1987;32:281–289. doi: 10.1016/0003-9969(87)90022-7. [DOI] [PubMed] [Google Scholar]
- Palmon A, Roos H, Edel J, Zax B, Savion N, Grosskop A, Pitaru S. Inverse dose- and time-dependent effect of basic fibroblast growth factor on the gene expression of collagen type I and matrix metalloproteinase-1 by periodontal ligament cells in culture. J Periodontol. 2000;71:974–980. doi: 10.1902/jop.2000.71.6.974. [DOI] [PubMed] [Google Scholar]
- Pekovic V, Hutchison CJ. Adult stem cell maintenance and tissue regeneration in the ageing context: the role for A-type lamins as intrinsic modulators of ageing in adult stem cells and their niches. J Anat. 2008;213:5–25. doi: 10.1111/j.1469-7580.2008.00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peppas NA. Time- and position-dependent drug delivery in controlled-release systems. J Pharm Sci. 1987;76:267. doi: 10.1002/jps.2600760319. [DOI] [PubMed] [Google Scholar]
- Rezania A, Healy KE. Biomimetic peptide surfaces that regulate adhesion, spreading, cytoskeletal organization, and mineralization of the matrix deposited by osteoblast-like cells. Biotechnol Prog. 1999;15:19–32. doi: 10.1021/bp980083b. [DOI] [PubMed] [Google Scholar]
- Rios H, Koushik SV, Wang H, Wang J, Zhou HM, Lindsley A, Rogers R, Chen Z, Maeda M, Kruzynska-Frejtag A, Feng JQ, Conway SJ. periostin null mice exhibit dwarfism, incisor enamel defects, and an early-onset periodontal disease-like phenotype. Mol Cell Biol. 2005;25:11131–11144. doi: 10.1128/MCB.25.24.11131-11144.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruoslahti E, Pierschbacher MD. New perspectives in cell adhesion: RGD and integrins. Science. 1987;238:491–497. doi: 10.1126/science.2821619. [DOI] [PubMed] [Google Scholar]
- Scadden DT. The stem-cell niche as an entity of action. Nature. 2006;441:1075–1079. doi: 10.1038/nature04957. [DOI] [PubMed] [Google Scholar]
- Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, Young M, Robey PG, Wang CY, Shi S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364:149–155. doi: 10.1016/S0140-6736(04)16627-0. [DOI] [PubMed] [Google Scholar]
- Sodek J, McKee MD. Molecular and cellular biology of alveolar bone. Periodontology. 2000;24:99–126. doi: 10.1034/j.1600-0757.2000.2240106.x. [DOI] [PubMed] [Google Scholar]
- Sonoyama W, Liu Y, Yamaza T, Tuan RS, Wang S, Shi S, Huang GT. Characterization of the apical papilla and its residing stem cells from human immature permanent teeth: a pilot study. J Endod. 2008;34:166–171. doi: 10.1016/j.joen.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TenCate AR. Physiological resorption of connective tissue associated with tooth eruption. An electron microscopic study. J Periodontal Res. 1971;6:168–181. doi: 10.1111/j.1600-0765.1971.tb00605.x. [DOI] [PubMed] [Google Scholar]
- Waddington RJ, Embery G. Proteoglycans and orthodontic tooth movement. J Orthod. 2001;28:281–290. doi: 10.1093/ortho/28.4.281. [DOI] [PubMed] [Google Scholar]
- Wang DA, Williams CG, Li Q, Sharma B, Elisseeff JH. Synthesis and characterization of a novel degradable phosphate-containing hydrogel. Biomaterials. 2003;24:3969–3980. doi: 10.1016/s0142-9612(03)00280-1. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Kubota T. Characterization of fibromodulin isolated from bovine periodontal ligament. J Periodontal Res. 1998;33:1–7. doi: 10.1111/j.1600-0765.1998.tb02285.x. [DOI] [PubMed] [Google Scholar]
- Wilde J, Yokozeki M, Terai K, Kudo A, Moriyama K. The divergent expression of periostin mRNA in the periodontal ligament during experimental tooth movement. Cell Tissue Res. 2003;312:345–351. doi: 10.1007/s00441-002-0664-2. [DOI] [PubMed] [Google Scholar]
- Yang F, Williams CG, Wang DA, Lee H, Manson PN, Elisseeff J. The effect of incorporating RGD adhesive peptide in polyethylene glycol diacrylate hydrogel on osteogenesis of bone marrow stromal cells. Biomaterials. 2005;26:5991–5998. doi: 10.1016/j.biomaterials.2005.03.018. [DOI] [PubMed] [Google Scholar]