Abstract
The formation of adequate masses of endocrine and exocrine pancreatic tissues during embryogenesis is essential to ensure proper nutrition and glucose homeostasis at postnatal stages. We generated mice with pancreas-specific ablation of the 3-phosphoinositide–dependent protein kinase 1 (Pdk1) to investigate how signaling downstream of the phosphatidylinositol-3-OH kinase (PI3K) pathway controls pancreas development. Pdk1–conditional knock-out mice were born with conspicuous pancreas hypoplasia, and within a few weeks, they developed severe hyperglycemia. Our detailed characterization of the mutant embryonic pancreas also revealed distinct temporal, cell-type–specific requirements of Pdk1 activity in the control of cell proliferation, cell survival, and cell size during pancreas development. These results thus uncover Pdk1 as a novel, crucial regulator of pancreatic growth during embryogenesis. In addition, we provide evidence that Pdk1 activity is required differently in mature pancreatic cell types, since compensatory proliferation and possible mTORC2 activation occurred in exocrine cells but not in β cells of the Pdk1-deficient postnatal pancreas.
Keywords: Cell size, exocrine, β cell, pancreas, Pdk1, progenitors
INTRODUCTION
The mammalian pancreas is a mixed gland composed of two distinct tissues, exocrine and endocrine. The endocrine portion of the pancreas supplies insulin to the entire body and the exocrine portion produces various enzymes required for the digestion of food. The formation of adequate masses of endocrine and exocrine pancreatic tissues during embryogenesis is thus essential to ensure proper nutrition and glucose homeostasis during postnatal stages.
Morphogenesis of the mouse pancreas begins with the formation of two buds, respectively positioned on the dorsal and ventral side of the posterior foregut; these buds are clearly visible at about embryonic day (E) 10.5, and subsequently fuse into a single organ upon rotation of the gut. Most exocrine and endocrine pancreatic precursors initiate differentiation at about E14.5. The exocrine cells then proliferate extensively and start to form acini. During the last stages of embryonic development, the endocrine cells of the pancreas begin to organize into islets containing a core of cells that produce insulin (β cells) and peripheral cells that express glucagon (α cells) or other hormones (Slack, 1995). Although it is now clear that various signaling pathways regulate growth, morphogenesis, and cytodifferentiation in the developing pancreas, how the activity of specific components of those signaling pathways is required during pancreas organogenesis remains unknown (Cano et al., 2007; Murtaugh and Melton, 2003).
The growth of an organ is mediated by increases in both, cell size and cell number, through the coordinated action of cell growth and cell cycle progression (Fingar and Blenis, 2004). Many growth factors (including insulin and the insulin-like growth factor), activate the phosphatidylinositol 3-kinase (PI3K) pathway, which in turn phosphorylates phosphatidylinositol-4,5-biphosphate (PIP2) to generate phosphatidylinositol-3,4,5-triphosphate (PIP3). One of the most studied signaling events controlled by PIP3 is the activation of a group of AGC family protein kinases, including isoforms of protein kinase B (Pkb/Akt) and the ribosomal S6 kinase (S6K), which play crucial roles in regulating physiological processes relevant to metabolism, cellular growth, proliferation, and survival (Mora et al., 2004).
While the precise molecular mechanisms that control cell size in distinct developing tissues remain largely unknown, these processes most likely require the activity of the mammalian target of rapamycin (mTOR) kinase. The mTOR signaling hub comprises a distinct set of macromolecular complexes that respond to two independent cellular inputs: growth factors that activate the PI3K and PKB/Akt signaling pathways, and nutrient availability which is sensed by the AMPK (5’AMP-activated protein kinase) pathway (Hay and Sonenberg, 2008). mTOR exists as two distinct protein complexes: mTORC1 and mTORC2. mTORC1 controls cell growth and proliferation via regulating protein synthesis, whereas mTORC2 responds to growth factor signals to activate Akt (Toker, A., 2008; Oldham and Hafen, 2003; Ruvinsky and Meyuhas, 2006).
The best-characterized downstream effectors of mTor include two parallel signaling pathways involved in translational control: the protein S6 kinase (S6K) and the eukaryotic initiation factor 4E (eIF4E)-binding proteins (4E-BP1 and 4E-BP2) (Fingar and Blenis, 2002; Hay and Sonenberg, 2008; Toker, 2008). One main effector of the mTor/S6K pathway is the ribosomal protein S6 (rpS6), whose phosphorylation is directly associated with regulating cell size. The phosphorylation of rpS6 enables the efficient translation of mRNA transcripts containing a polypyrimidine tract at their 5’ transcriptional start site (the TOP motif), which encode ribosomal proteins and other translational regulators (Jefferies et al., 1997).
The 3-phosphoinositide-dependent protein kinase 1 (Pdk1) is a central mediator of cellular responses induced by PI3K signaling, as it promotes the phosphorylation-dependent activation of Pkb/Akt and S6K (Alessi et al., 1996, 1997; Biondi et al., 2001; Chung et al., 1992; Price et al., 1992; Stockoe et al., 1997; Williams et al., 2000). The lack of Pdk1 activity has serious consequences for early embryogenesis, as indicated by the fact that Pdk1-nullizygous mice do not survive beyond E9.5 and have defective formation of multiple organs (Lawlor et al. 2002). In addition, conditional ablation approaches have demonstrated that in distinct developing organs Pdk1 controls cell size, proliferation, or survival in a tissue-restricted manner (Belgardt et al., 2008; Mora et al., 2003).
Hashimoto et al. (2006) recently showed that adult mice with β-cell specific ablation of Pdk1 (βPdk1) do not have enough β-cell mass in their pancreata to preserve glucose homeostasis. These alterations were consequence of poor survival, deficient proliferation and lack of growth of mature pancreatic β cells (Hashimoto et al. 2006). In this study we conditionally inactivated Pdk1 from mouse pancreatic progenitors to investigate whether Pdk1 function is required for some aspects of pancreas organogenesis. We report that Pdk1 function contributes to expand the population of pancreatic progenitors, and we provide evidence that Pdk1 controls differently the expansion of exocrine-and β-cell masses during development.
MATERIALS AND METHODS
Animals
Pdk1flΔneo/flΔneo Pdk1ΔPanc mice were maintained and genotyped as previously described (Lawlor et al, 2002). Pdx1-Cre mice (Gu et al., 2002) were obtained from the MMRRC (UC Davis, CA). ACTB-Bgeo/ALPP (Z/AP) mice (Lobe et al., 1999) were purchased from the Jackson Laboratory (Bar Harbor, MA).
Processing of embryos and pancreatic tissues
Tissues from dissected embryos or pancreata from newborn or juvenile mice were prepared for immunohistochemical analysis in frozen sections as previously described (Wang et al., 2005). Paraffin-embedded tissues were cut (5-to 6-µm sections) and prepared for immunohistochemical analysis after deparaffinization through a xylene/ethanol series and microwave-based antigen-retrieval processes.
Histology
Paraffin sections were stained with hematoxylin and eosin (H&E) for morphology analyses. Frozen sections were stained with hPLAP following the protocol of Lobe et al. (1999).
BrdU staining and TUNEL assay
Frozen sections of embryonic pancreata were processed to visualize bromodeoxyuridine (BrdU)-labeled cells as previously described (Wang et al., 2005). Apoptotic cell death was also detected using the ApopTag Fluorescein In Situ Apoptosis Detection Kit (Chemicon International) or Klenow-Frag-EL kit (EMD Biosciences) per the manufacturers’ directions.
Immunohistochemical analysis
The following primary antibodies were used: rabbit anti-amylase (1:1,000; Sigma); goat anti-amylase (1:100; Santa Cruz); rabbit anti-Sox9 (1:250; Chemicon International); rabbit anti-Mist1 (1:500; provided by S. Konieczny); rabbit anti-elastase (1:2,000; AbCam); anti–beta catenin (1:500; Cell Signaling); anti-synaptophysin (1:1,000; Zymed); rabbit anti–pan-Akt (1:100; Cell Signaling); rabbit anti-pS473 (Akt) (1:10; Cell Signaling); rabbit anti–p-T308 (Akt) (1:10; Cell Signaling); rabbit anti–S6-P (1:500; Cell Signaling); rabbit anti-glucagon (1:50; Zymed); guinea pig anti-glucagon (1:500; Linco Research); guinea pig anti-insulin (1:250; DAKO); rabbit anti-Pdx1 (1:1,000; provided by C. Wright); rabbit anti-phospho-histone3 (PH3) (1:250; Upstate Biotechnology); rabbit anti-PLAP (1:100; Zymed); rat anti-uvomorulin/E-cadherin (1:10,000; Sigma); and rat anti–Ki67 peptide (1:1,000; Thermo Fisher Scientific). The following secondary antibodies were diluted (1:200) before use: Cy3-conjugated donkey anti–guinea pig IgG (Jackson ImmunoResearch); Cy3-conjugated donkey anti–rabbit IgG (Jackson ImmunoResearch); Cy3-conjugated donkey anti–goat IgG (Jackson ImmunoResearch); Alexa 488–conjugated donkey anti–rabbit IgG (Molecular Probes); Alexa 488–conjugated donkey anti–rat IgG (Molecular Probes); Alexa 488–conjugated goat anti–guinea pig IgG (Molecular Probes); AMCA-conjugated donkey anti–rabbit IgG (Jackson Immunoresearch), Alexa 488–conjugated donkey anti–mouse IgG (Molecular Probes). Biotinylated donkey IgG antibodies (anti-rabbit or anti-mouse; Jackson Immunoresearch) were detected by using the VECTASTAIN Elite ABC kit (Vector Laboratories). For nuclear staining, sections were covered with mounting medium containing 4’,6-diamidino-2-phenylindole (DAPI) (VECTASHIELD; Vector Laboratories) or counterstained with hematoxylin or methyl green. Images were obtained either with a Zeiss Axioskop 2 microscope or with a confocal/Multiphoton laser-scanning Zeiss LSM 510 META microscope. The blue DAPI signal was excited with a Ti:sapphire IR femtosecond laser (Coherent Inc.). Adobe Photoshop CS2 (version 9.0; Adobe Systems, Inc.) was used to process the images.
Cell counting and morphometric analyses
Whole pancreata of wild-type and Pdk1flΔneo/flΔneo;Pdx1-Cre embryos and newborns (at least 3 specimens for each stage, per genotype) were sectioned at 10 µm. Each third (E10.5), fifth (E12.5) or tenth (E15.5, E17.5, and P0) consecutive section was incubated with specific antibodies for the following analyses: 1) To estimate the average pancreatic area, we counted the number of Pdx1+ cells (E10.5 specimens), or we calculated the E-cadherin+ (Ecad+) area (E12.5, E15.5, E17.5, or P0 specimens). 2) To estimate the proliferation index, we counted the number of Pdx1+BrdU+ or Ecad+BrdU+ double-positive cells in sections from E10.5 or E15.5 specimens respectively, or the number of cells labeled with anti-phosphohistone 3 (PH3) antibodies in E17.5 sections. 3) To quantify the apoptotic index, we counted the number of TUNEL+ cells in sections from E10.5 or E17.5 specimens. 4) To estimate the proportion of endocrine cells, we counted the number of glucagon+ cells in E10.5 specimens, or we calculated the insulin+ area in E15.5 or P0 specimens. 5) To estimate the relative exocrine cell mass, we calculated the amylase+ area. 6) To determine the size of individual cells, P2 pancreatic sections were simultaneously stained with anti-amylase and anti-Ecad antibodies (for exocrine cells) or anti-insulin and anti-Ecad antibodies (for β cells). ImageJ 1.37v software (NIH) was used to estimate the Ecad+, insulin+, or amylase+ areas. Data are shown as the mean number ± standard deviation (SD) of the mean. Statistical significance was determined using the Student’s t-test (p value <0.05 indicates statistically significant differences).
Non-fasting blood glucose
Blood from the tail vein was taken, and glucose levels were measured using a CONTOUR® Blood Glucose Monitoring System (Bayer HealthCare LLC).
RESULTS
Analysis of Pdk1 activity in the developing mouse pancreas
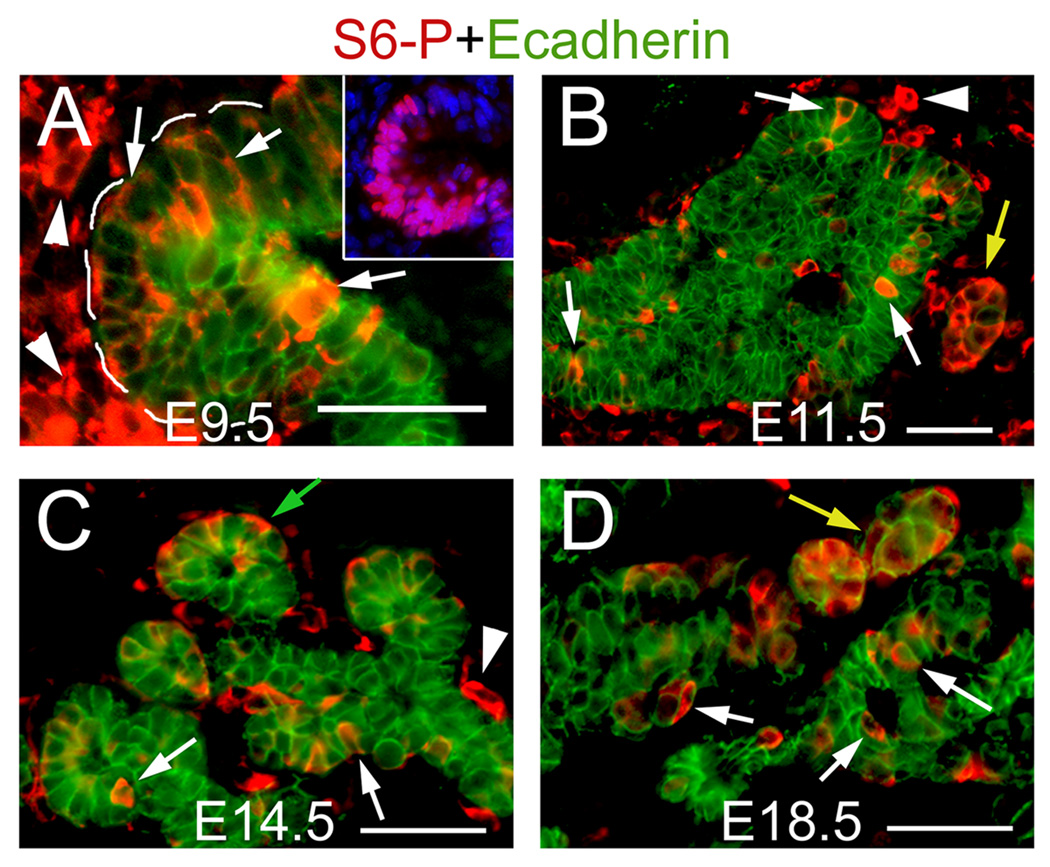
Pdk1 is required for full activation of S6K and its downstream substrate S6 (Ruvinsky and Meyuhas, 2006; Ruvinsky et al., 2005). Therefore, the phosphorylation of ribosomal protein 6 (rpS6-P or S6P) can be used as an indicator of Pdk1 activity. In E9.5 wild-type embryos stained with anti-phosphorylated ribosomal protein 6 (rpS6-P or S6P), we detected Pdk1-active (S6P+) cells in the epithelium of the emerging dorsal pancreatic bud and its associated mesenchyme (Fig. 1A). S6P proteins were also detected in epithelial and mesenchymal cells of E11.5, E14.5 and E18.5 wild-type pancreatic tissues (Fig. 1B–D). At those stages, Pdk1 appeared active in endocrine aggregates and in scattered epithelial cells (Fig. 1B–D). In E14.5 pancreata, Pdk1 was also active in cells located at the tip of the epithelial branches, where the forming acini emerge (Fig. 1C). S6P+ cells were also detected in islet and exocrine cells of the postnatal day (P) 8 pancreas (data not shown). These results indicate that PI3K signaling (and consequently, Pdk1) is active in pancreatic tissues throughout most of organogenesis and during postnatal stages.
Figure 1. Distribution of phosphorylated rpS6 proteins (S6P) throughout pancreas Development.
A, Numerous cells of the emerging E9.5 dorsal pancreatic bud express S6-P (arrows) and the surrounding mesenchymal cells (arrpowheads) express S6P proteins. Inset shows an adjacent section stained with antibodies recognizing the pancreatic marker Pdx1 (purple staining). B–D, In pancreatic tissues dissected between E11.5 and E18.5, S6P immunoreactivity can be observed in: cells scattered throughout the epithelium (white arrows), endocrine clusters (yellow arrows), the emerging acini (green arrow in C), and in associated mesenchymal cells (arrowheads). A (inset), Cell nuclei were stained with DAPI. Scale bar, 50 µm.
Loss of Pdk1 function results in pancreatic hypoplasia
To investigate whether Pdk1 activity is necessary for pancreas organogenesis, we specifically deleted Pdk1 from pancreatic progenitors of Pdk1flΔneo/flΔneo;Pdx1-Cre mice (herein called Pdk1ΔPanc mice) using a conditional knock-out approach. Pdk1flΔneo/flΔneo mice, in which exons 3 and 4 of Pdk1 are flanked by loxP sites, were crossed with Pdx1-Cre mice expressing Cre recombinase in most pancreatic progenitors (Gu et al., 2002; Lawlor et al., 2002). To assess the extent of Pdk1 inactivation, we analyzed the phosphorylation status of S6 proteins in E10.5–E18.5 Pdk1ΔPanc pancreatic tissues. Starting at around E10.5, a considerable decrease in S6P immunoreactivity was observed in the pancreatic epithelium of Pdk1ΔPanc embryos (Suppl. Fig. 1 and data not shown). In contrast, S6P was normally detected in mesenchymal cells surrounding the mutant pancreatic epithelium (Suppl. Fig. 1). These results indicate that Pdk1 was efficiently deleted in most pancreatic progenitors of Pdk1ΔPanc embryos.
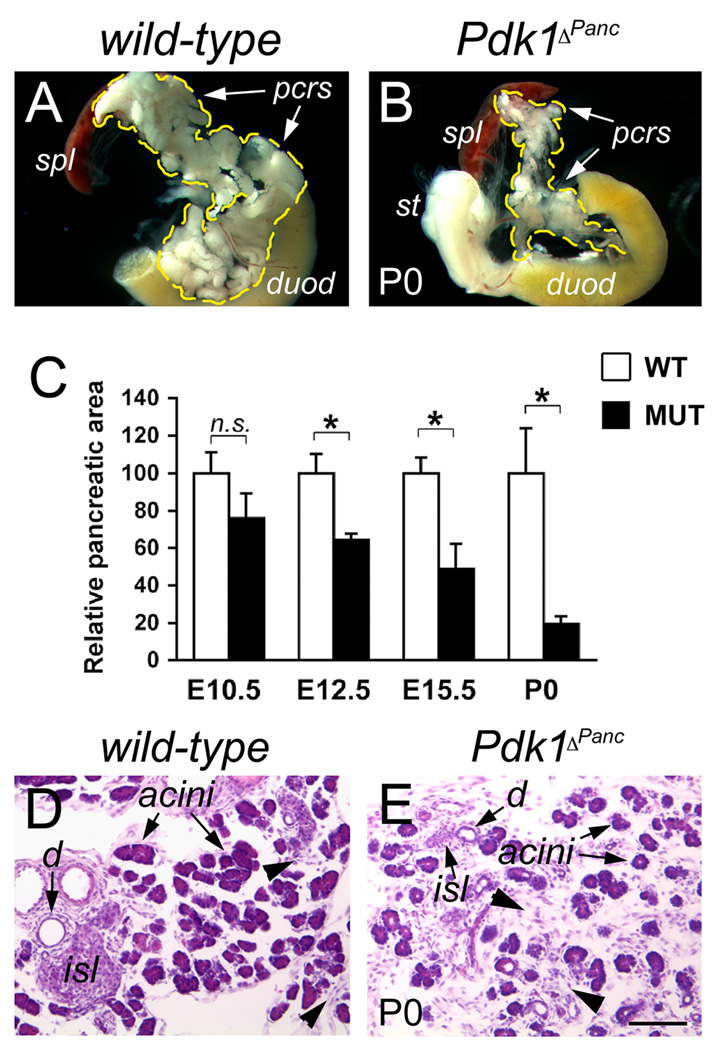
Concomitant with the observed reduction in Pdk1 activity, starting at around E10.5 the pancreas of Pdk1ΔPanc embryos appeared progressively smaller than this organ of wild-type littermates; consequently, all Pdk1ΔPanc mice were born with conspicuous pancreatic hypoplasia (Fig. 2A, B). The results of morphometric analyses further determined that the average sizes of E10.5, E12.5, E15.5, or P0 Pdk1ΔPanc pancreata were approximately 76%, 64%, 49%, and 19% the average size of the corresponding wild-type organs (Fig. 2C). Histologic analyses also revealed that the pancreata of Pdk1ΔPanc newborn mice had considerably more mesenchyme, very small islets, and noticeably less acini than those tissues of wild-type newborns (Fig. 2D,E). These results thus indicate that the activity of Pdk1 is necessary for proper pancreatic growth during embryogenesis.
Figure 2. Pdk1ΔPanc newborn mice have severe hypoplasia of the pancreas.
(A, B) The pancreas of Pdk1ΔPanc newborn mice (B) is noticeably smaller than this organ of wild-type littermates (A). C, The severity of the hypoplasia of Pdk1ΔPanc pancreatic tissues gradually increases throughout development. Asterisks=p<0.05; n.s.=not statistically significant difference (as determined by the Student t test). (D, E) wild-type newborn pancreata (D) have abundant exocrine acini, visible ducts (d) and newly formed islets (isl). Instead, Pdk1ΔPanc newborn pancreata (E) have comparatively less acini, smaller islets and overabundant mesenchyme (arrowheads). (A, B) spl: spleen; pcrs: pancreas; duod: duodenum. (D, E) are paraffin sections stained with hematoxilin-eosin. Scale bar, 50 µm.
The mass of endocrine cells is reduced in the Pdk1-conditional null embryonic pancreas
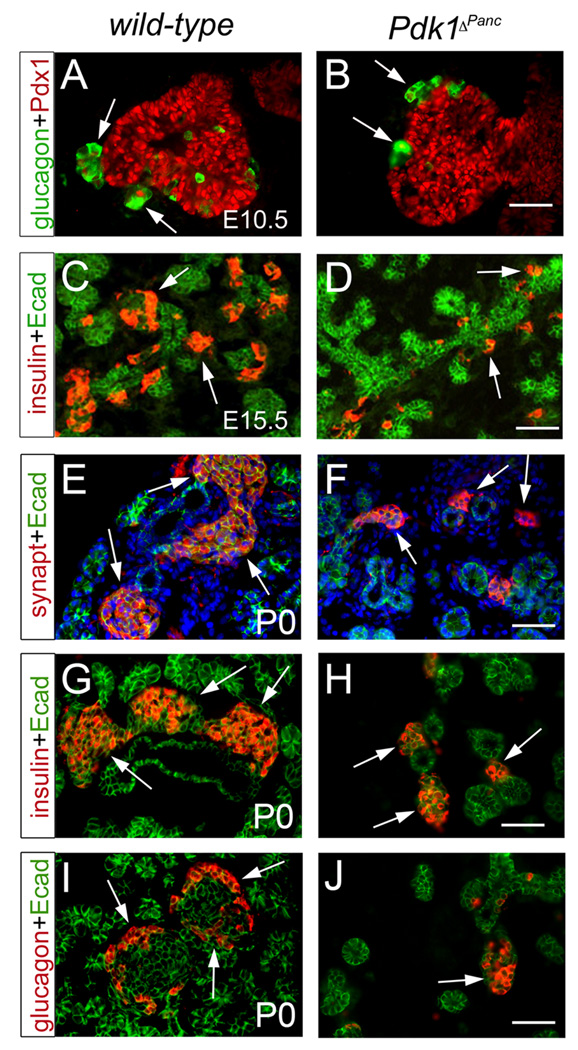
To investigate whether Pdk1 function is necessary for the formation of specific pancreatic cell types, immunohistochemical and morphometric analyses were performed on Pdk1ΔPanc or wild-type pancreata. We determined that E10.5 Pdk1ΔPanc pancreata exhibited a slight reduction in the number of glucagon-expressing cells (i.e., the main endocrine cell type of early-developing pancreata) (Fig. 3A, B and Table 1). Similarly, both E15.5 and newborn Pdk1ΔPanc pancreatic tissues had a substantial reduction in the mass of insulin-expressing β cells (i.e., the major endocrine cell type of late-developing pancreata) (Fig. 3C, D, G, H and Table 1). Furthermore, in the pancreas of Pdk1ΔPanc newborn mice the number of endocrine cells expressing glucagon, pancreatic polypeptide, ghrelin, or somatostatin also appeared substantially reduced (Fig.3I, J and data not shown). As a result, the islets of Pdk1ΔPanc newborns were abnormally small and their β cells and α cells were intermingled rather than organized into a β-cell core surrounded by an α-cell periphery (Suppl. Fig. 2A, B and Fig. 3E, F). Importantly, the loss of Pdk1 function also did not affect the expression of various typical β cell markers (e.g., insulin, Pdx1, Glut2, or MafA; data not shown). Therefore, we conclude that Pdk1 activity is necessary for the expansion of embryonic pancreatic β cells, but not for their differentiation.
Figure 3. Pdk1 deficiency decreases the production of pancreatic endocrine cells during embryogenesis.
(A, B) Early pancreatic endocrine cells (glucagon+ cells, arrows) are slightly less numerous at E10.5 in Pdk1ΔPanc pancreata (B) than in wild-type pancreata (A). (C, D) Insulin-expressing cells (arrows) are numerous at E15.5 in the pancreas of wild-type embryos (C), but scarce in these tissues of Pdk1ΔPanc littermates (D). (E, F) The pancreas of wild-type newborn mice (E) contains numerous islets (stained with anti-synaptophysin antibodies, arrows). However, Pdk1ΔPanc newborn pancreata only have a small number of islets of substantially reduced size (arrows in F). (G, I) The islets of wild-type newborn pancreata enclose a large core of β cells (insulin+, arrows in E), and peripheral β cells (glucagon+, arrows in I). (H, J) The islets of Pdk1ΔPanc newborn pancreata also have β cells (arrows in H) and β cells (arrows in J), but these cell populations noticeably less numerous than those of wild-type pancreata. The pancreatic epithelium was visualized with anti-Pdx1 antibodies (red in A, B) or with anti-Ecadherin antibodies (green in C–J). (E, F) Cell nuclei were stained with DAPI. Scale bar, 50 µm.
Table 1.
Relative endocrine cell mass of wild-type and Pdk1ΔPanc pancreata.
| E10.5 | E15.5 | P0 | ||||
|---|---|---|---|---|---|---|
| Gluc+ cell abundance (1) |
Gluc+ cells relative to WT area (2) |
Ins+ cell abundance (3) |
Ins+ area relative to WT area (4) |
Ins+ cell abundance (3) |
Ins+ area relative to WT area (4) |
|
| WT | 15.2±1.1 | 15.2 | 4.8±0.6 | 4.8 | 6.2±0.9 | 6.2 |
| MUT | 13.1±3.3 | 10.0 | 3.3±0.4 | 1.6 | 6.8±1.4 | 1.3 |
| p=0.182 | p=0.04 | p=0.52 | ||||
Percentage of pancreatic cells co-expressing glucagon+.
[(glucagon+ cells)/ Total wild-type pancreatic cells] × 100.
Percentage of Ecad+ area co-expressing insulin+.
[ins+ area/wild-type Ecad+ area] × 100.
Loss of Pdk1-pancreatic function is associated with decreased formation of exocrine cells
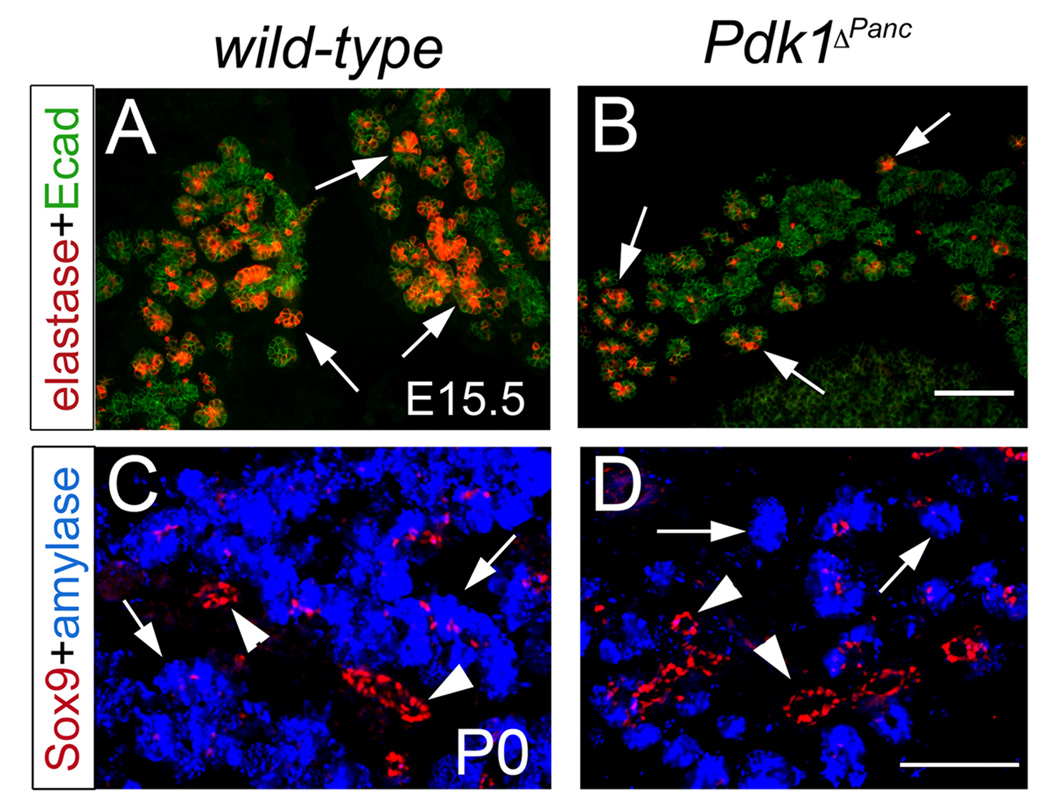
The expression of various exocrine markers (e.g., amylase, elastase, carboxypeptidase A, or Mist1) appeared unaffected in Pdk1ΔPanc embryonic and newborn pancreata (Fig. 4 and data not shown). However, as indicated by our immunohistochemical and morphometric results the pancreas of E15.5 Pdk1ΔPanc embryos exhibited a severe deficit of exocrine cells (Fig. 4A, B and Table 2). Moreover, at birth the average Pdk1ΔPanc pancreatic exocrine mass was only about 20% the size of that in wild-type littermates (Fig. 4C, D and Table 2). This deficient expansion of the exocrine tissue most likely accounted for the lack of growth of the Pdk1-deficient pancreas towards the end of gestation. Therefore, we uncovered that the function of Pdk1 is critical to produce an adequate exocrine cell mass during pancreas development.
Figure 4. Pdk1 deficiency decreases the production of pancreatic exocrine cells during embryogenesis.
(A, B) Elastase-expressing exocrine cells (arrows) are numerous at E15.5 in the pancreas of wild-type embryos (A), but scarce in these tissues of Pdk1ΔPanc littermates (B). (C) The pancreas of wild-type newborn mice contains numerous exocrine acini (amylase+ cells, blue staining and arrows) and newly formed ducts (visible with anti-Sox9 antibodies, red staining and arrowheads). (D) The pancreas of Pdk1ΔPanc newborn mice contains far less acini (arrows) than wild-type pancreata, and ducts (arrowheads) with relatively normal appearance. (A, B) The pancreatic epithelium was visualized with anti-Ecadherin antibodies (green staining). Scale bar, 100 µm.
Table 2.
Relative exocrine cell mass of wild-type and Pdk1ΔPanc pancreata.
| E15.5 | P0 | |||
|---|---|---|---|---|
| Amyl+ cell abundance (1) |
Amyl+ area relative to WT area (2) |
Amyl+ cell abundance (1) |
Amyl+ area relative to WT area (2) |
|
| WT | 54.3±1.2 | 54.3 | 86.7±8.3 | 86.7 |
| MUT | 25.2±2.3 | 12.3 | 84.3±9.6 | 16.0 |
| p=0.03 | p=0.38 | |||
Percentage of Ecad+ area co-expressing amylase+.
[amylase+ area/wild-type Ecad+ area] ×100.
Analysis of the conditional mutant pancreas showed that the loss of Pdk1 activity does not prevent formation of the pancreatic ducts (Fig. 4C, D; data not shown). However, due to the small size of most ductal cells, we could not investigate any possible quantitative changes in the ductal cell mass that could result from the lack of Pdk1 function. Overall, we conclude that during pancreas organogenesis Pdk1 does not participate in cell-type differentiation processes. However, Pdk1 activity is critical for the proper growth of this organ.
Proliferation is hindered in developing β cells, but not in developing exocrine cells, of the Pdk1-conditional null pancreas
As Pdk1 reportedly controls the proliferation of certain cell types (Hashimoto et al., 2006; Hinton et al., 2004; Nakamura et al., 2008), we compared the proliferation index (i.e., the percentage of cells incorporating BrdU or expressing the mitotic marker PH3) between pancreata of wild-type and Pdk1ΔPanc embryos.
Our quantitative results showed that, in comparison to wild-type embryos, the pancreas of Pdk1ΔPanc embryos had fewer proliferating cells (i.e, BrdU+Pdx1+ cells) at E10.5 (Table 3). Since the precursors of all pancreatic cell lineages express Pdx1 at E10.5 (Gu et al., 2002), we conclude that Pdk1 activity promotes early pancreatic growth by controlling progenitor cell proliferation.
Table 3.
Proliferation index of wild-type and Pdk1ΔPanc pancreata.
| E10.5 | E15.5 | E17.5 | ||||
|---|---|---|---|---|---|---|
| BrdU+ cell abundance (1) |
BrdU+ cell abundance relative to WT |
BrdU+ cell abundance (2) |
BrdU+ cell abundance relative to WT |
PH3+ cell abundance (3) |
PH3+ cell abundance relative to WT |
|
| WT | 44.1±7.8 | 1.0 | 3.23±0.19 | 1.0 | 14.6±6.5 | 1.0 |
| MUT | 28.2±5.7 | 0.64 | 2.93±0.39 | 0.91 | 31.2±7.1 | 2.1 |
| p=0.023 | p=0.263 | p=0.02 | ||||
Percentage of Pdx1+ cells co-expressing BrdU+.
No. of BrdU+ cells per Ecad+ area (arbitrary units × 103).
No. of PH3+ cells per Ecad+ area (arbitrary units × 103).
In the pancreas of Pdk1ΔPanc embryos we detected similar abundance of proliferating cells at E15.5, and approximately 2-fold more epithelial cells undergoing proliferation at E17.5 in comparison to those tissues of wild-type littermates (Table 3). We generated Pdk1ΔPanc;R26RLacZ embryos in which the expression of the β gal reporter gene indicates Cre recombinase activity (and consequently, Pdk1 deletion; Soriano, 1999) to uncover cell types undergoing proliferation in the Pdk1-deficient pancreas. We observed co-localization of β-gal proteins in all insulin+ cells and in some, but not all, exocrine cells in Pdk1ΔPanc;R26RLacZ pancreatic specimens dissected between E17.5 and P2 (Fig. 5 and Fig 6 and data not shown). These results suggest mosaic deletion of Pdk1 in exocrine cells, and uniform deletion of this gene in β cells, in Pdk1ΔPanc pancreata.
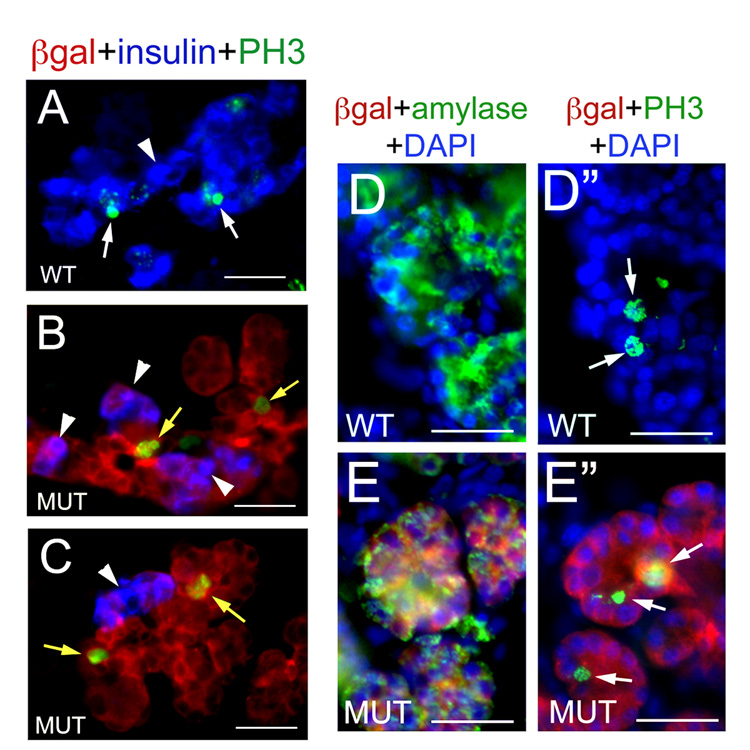
Figure 5. Exocrine cells, but not β cells, undergo proliferation in the pancreas of E17.5 Pdk1ΔPanc;R26RLacZ embryos.
(A, D, D”) At E17.5, some β cells (A, stained in blue with anti-insulin antibodies) and some exocrine cells (D”, identified with anti-amylase antibodies [green] in an adjacent section [D]) appear expressing the mitosis marker PH3 (arrows and green in A, D”) in wild-type pancreata. (B, C) PH3 (green staining) is not expressed in the vast majority of β cells (arrowheads) of the E17.5 Pdk1ΔPanc;R26RLacZ pancreatic epithelium (notice that some epithelial cells devoid of insulin are PH3+, yellow arrows). (E”) PH3 expression (green and arrows) is detected in exocrine cells (identified with anti-amylase antibodies [green] in an adjacent section [E]) of E17.5 Pdk1ΔPanc;R26RLacZ pancreata. (B, C, E, E”) Sections of E17.5 Pdk1ΔPanc;R26RLacZ pancreata were stained with anti– β-gal antibodies (red) to identify epithelial cells with presumptive Pdk1-deletion (red staining). (D–E”) Cell nuclei were stained with DAPI. Scale bar, 25 µm.
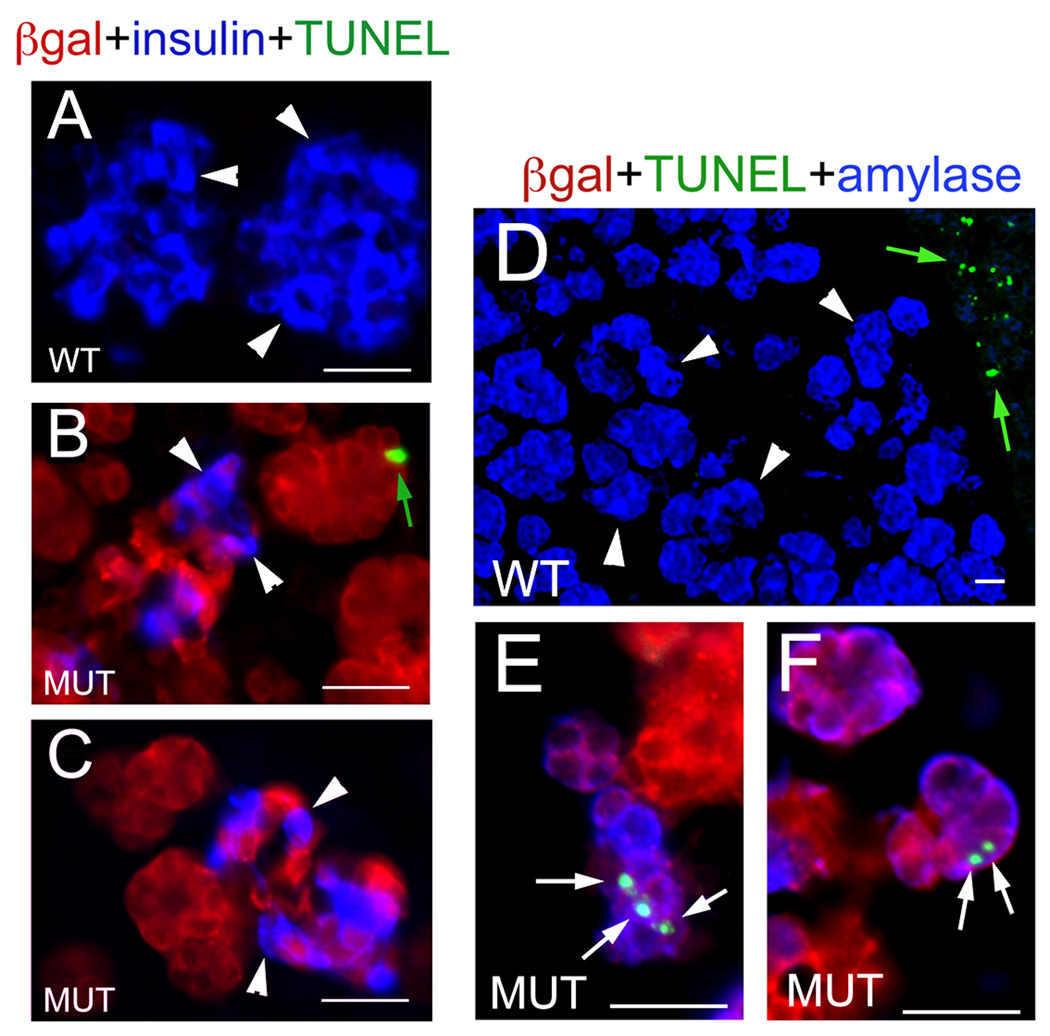
Figure 6. Exocrine cells, but not β cells, undergo apoptosis in the pancreas of E17.5 Pdk1ΔPanc;R26RLacZ embryos.
(A, D) Almost no β cells (A, insulin+ [blue]) or exocrine cells (D, amylase+ [blue]) appear to undergo apoptosis (TUNEL+ staining [green]) in the pancreas of E17.5 wild-type embryos (notice that the adjacent spleen contains numerous TUNEL+ cells, green arrows in D). (B, C) The reduced population of β cells (insulin+, blue staining and arrowheads) of E17.5 Pdk1ΔPanc;R26RLacZ pancreata is also largely negative for TUNEL staining (notice a presumptive exocrine cell staining positive for TUNEL in B [green arrow]). (E, E”) Several exocrine cells (amylase+, blue staining) in the pancreas of E17.5 Pdk1ΔPanc;R26RLacZ embryos are positive for TUNEL (arrows and green). (B, C, E, E”) Sections of E17.5 Pdk1ΔPanc;R26RLacZ pancreata were stained with anti–β-gal antibodies (red) to identify epithelial cells with presumptive Pdk1-deletion. (A) Cell nuclei were stained with DAPI. Scale bar, 25 µm.
We identified several β cells undergoing proliferation in the pancreas of E17.5 wild-type embryos (β-gal−insulin+PH3+; Fig. 5A) and, only rarely, no more than 1–2 proliferating β cells in this organ of Pdk1ΔPanc;R26RLacZ littermates (β-gal+insulin+PH3+; Fig. 5B, C and data not shown). In contrast, numerous proliferating exocrine cells (amylase+PH3+) were observed in the pancreas of E17.5 wild-type (Fig. 5D, D”) or Pdk1ΔPanc;R26RLacZ (Fig. 5E, E”) embryos. Most notable, almost all Pdk1-deleted acini (i.e., β-gal+amylase+) of Pdk1ΔPanc;R26RLacZ pancreata contained at least one exocrine cell undergoing mitosis (5E, E”). Therefore, we conclude that Pdk1 function is necessary for β cell proliferation, but is dispensable for exocrine cell proliferation, in the developing pancreas.
Loss of Pdk1 function decreases the survival of developing pancreatic exocrine cells
To investigate whether increased cell death contributed to the lack of growth of the Pdk1-deficient pancreas, we compared the apoptotic index (i.e., the percentage of TUNEL+ cells) between pancreata of wild-type and Pdk1ΔPanc embryos. Our results showed slightly increased abundance of TUNEL+ cells in Pdk1ΔPanc pancreata in comparison to wild-type pancreata at E10.5 (Table 4). On the basis of these results, we conclude that early during pancreas organogenesis (at around E10.5) Pdk1 activity is required to control the survival of pancreatic progenitors.
Table 4.
Relative apoptosis of wild-type and Pdk1ΔPanc pancreata.
| E10.5 | E17.5 | |||
|---|---|---|---|---|
| TUNEL+ cell abundance (1) |
TUNEL+ cell abundance relative to WT |
TUNEL+ cell abundance (2) |
TUNEL+ cell abundance relative to WT |
|
| WT | 1.0±0.6 | 1.0 | 1.7±0.1 | 1.0 |
| MUT | 2.2±1.6 | 2.2 | 12.6±2.3 | 7.5 |
| p=0.226 | p=0.005 | |||
Percentage of TUNEL+ pancreatic cells.
No. of TUNEL+ cells per Ecad+ area (arbitrary units ×103).
The results of quantitative analyses uncovered significantly more cells undergoing apoptosis (TUNEL+ cells) in the pancreas of Pdk1ΔPanc embryos than in this organ of wild-type littermates at E17.5 (Table 4). We stained E17.5 wild-type or Pdk1ΔPanc;R26RLacZ pancreatic specimens with specific antibodies to identify the epithelial cell types undergoing apoptosis. In wild-type pancreatic sections we observed lack of TUNEL staining in the vast majority of β cells (Fig. 6A) or exocrine cells (Fig. 6D). Pdk1-deficient β cells (β-gal+insulin+) were also largely devoid of TUNEL staining (Fig. 6B, C). In contrast, numerous Pdk1-deleted exocrine cells (β-gal+amylase+) stained positive for TUNEL in the E17.5 Pdk1ΔPanc;R26RLacZ pancreas (green arrow in Fig. 6B and white arrows in Fig. 6E, F). These results indicate that Pdk1 controls the survival of exocrine cells, but not the survival of β cells, in the embryonic pancreas.
In summary, we propose that the reduced size of Pdk1ΔPanc newborn pancreata is consequence of: 1) deficient proliferation and survival of early progenitors, 2) reduced proliferation of developing β cells, and 3) increased apoptosis of developing exocrine cells.
Loss of Pdk1 function decreases the size of embryonic exocrine-and β cells
The H&E staining of some E18.5 to P0 Pdk1ΔPanc pancreata showed entire lobes of the mutant organ with noticeably smaller acini than those of wild-type littermates (Fig. 7A, B) and other lobes containing both, relatively normal-sized and small-sized acini (Fig. 7C). The variable size of the acini (i.e., small vs. normal) of Pdk1ΔPanc pancreata was likely the consequence of mosaic deletion of Pdk1 in pancreatic exocrine progenitors, because in the larger P0 Pdk1ΔPanc;Z/AP pancreatic specimens we detected activity of the reporter human placental alkaline phosphatase (HPAP) in some, but not all, exocrine acini (Suppl. Fig. 3B, C). Conversely, nearly all exocrine cells of the smallest P0 Pdk1ΔPanc;Z/AP pancreatic specimens were HPAP+ (Suppl. Fig. 3A). HPAP activity was also uniformly detected in the ducts and islets of both, large and small P0 Pdk1ΔPanc;Z/AP pancreata (Suppl. Fig. 3A).
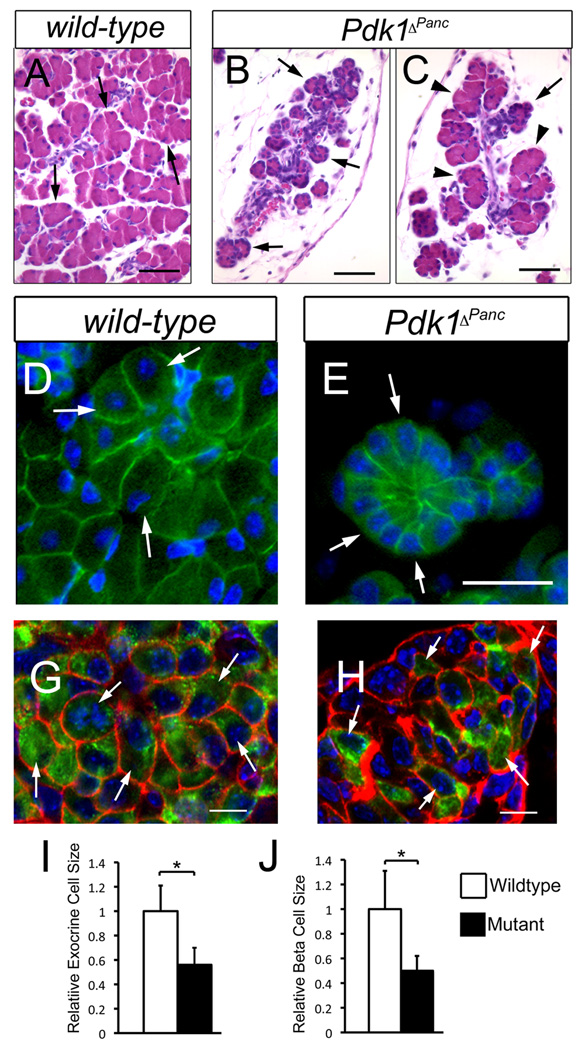
Figure 7. Loss of pancreatic Pdk1 function severely reduces the size of exocrine acini, individual exocrine cells and β cells.
(A–C) Some lobes in the pancreas of Pdk1ΔPanc newborn mice contain noticeably small acini (compare the size of the acini [arrows] between A and B), whereas other lobes have both, normal-sized acini (arrowheads in C) and small-sized acini (arrow in C). (D–H) The exocrine cells (D) and β cells (G) of wild-type newborn pancreata have a larger size and more abundant cytoplasm than the corresponding cells of Pdk1ΔPanc newborn pancreata (E, exocrine cells; H, β cells). (I, J) Graph shows the average relative sizes ± standard deviation of P2 pancreatic exocrine cells (I) or P2 pancreatic β cells (J) (*= p<0.005 versus the corresponding control value). (A–C) Paraffin sections were stained with hematoxilin-eosin. (D, E) The pancreatic epithelium was visualized with anti-Ecadherin antibodies (green) and the cell nuclei with DAPI (blue). (G, H) Confocal images of frozen sections stained with anti-Ecadherin antibodies (red), anti-insulin antibodies (green) and DAPI. Scale bar is: 50 µm (A–C), 12 µm (D, E) and 10 µm (G, H).
Immunostaining of P2 Pdk1ΔPanc or wild-type pancreata with anti–E-cadherin antibodies showed that the small mutant acini did not have fewer exocrine cells, but rather smaller exocrine cells than did the wild-type acini (Fig. 7D, E). Double-immunostaining experiments using anti–E-cadherin and anti-insulin antibodies revealed that most β cells of P2 Pdk1ΔPanc pancreata were also undersized (Fig. 7G, H). Morphometric results further established that both, the small exocrine cells and the β cells of P2 Pdk1ΔPanc pancreata were about 50% smaller than the corresponding cells of P2 wild-type pancreata (Fig. 7I, J). We conclude that Pdk1 function controls the proper size of exocrine cells and β cells during pancreas development.
Pdk1ΔPanc juvenile mice have undersized β cells and exocrine cells and are hyperglycemic
All Pdk1ΔPanc pups thrived during the first week after birth, though most were slightly smaller than their wild-type littermates. However, after P21 nearly every Pdk1ΔPanc mouse developed hyperglycemia (the average blood glucose level of random-fed wild-type mice [males and females] was 130.1 ± 19.9 mg/dL [n=16], and that of Pdk1ΔPanc mice [males and females] was 372.6 ± 119.7 mg/dL [n=19; p<0.001]). This alteration was likely caused by a severe β cell deficit, as we found that the islets of P21 Pdk1ΔPanc pancreata were minute (Suppl. Fig. 2C, D) and their endocrine cells were clearly undersized (Fig. 8I, J).
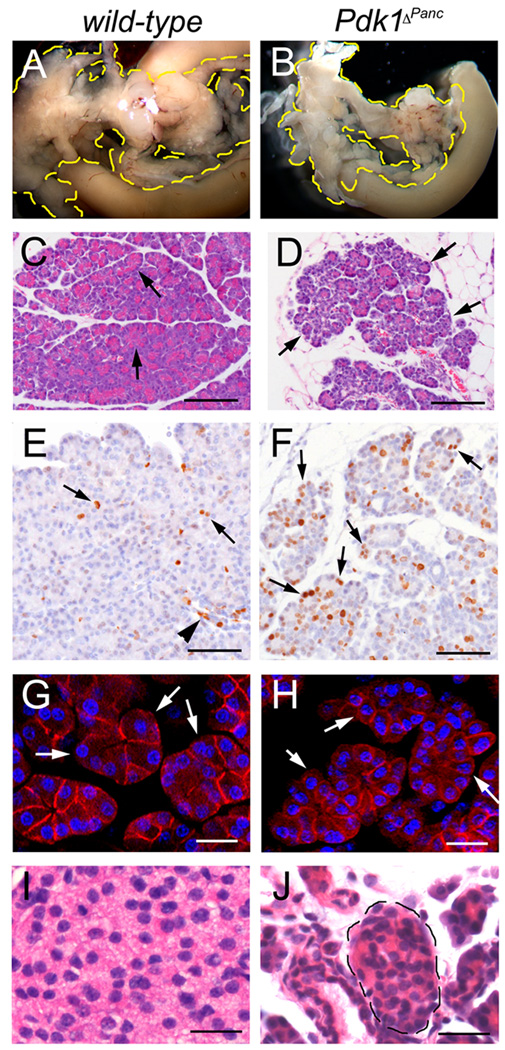
Figure 8. The pancreas of Pdk1ΔPanc juvenile mice is hypoplastic.
(A, B) At P21, the Pdk1ΔPanc pancreas (B) is markedly smaller than this organ of wild-type littermates (A). (C, D) Almost all exocrine cells in the smallest P21 Pdk1ΔPanc pancreatic specimens have less cytoplasm (arrows in D) and form smaller acini than those cells of wild-type panceata (arrowheads in C). (E) In P21 wild-type pancreata, a few exocrine cells (arrows) or ductal cells (arrowhead) appear expressing the proliferation marker Ki67 (brown staining). (F) More numerous cells express Ki67 throughout the entire P21 Pdk1ΔPanc pancreatic epithelium, including the small-sized exocrine cells (arrows). (G, H) The exocrine cells of P21 wild-type pancreata (arrows in E) have a larger size and more abundant cytoplasm than the exocrine cells of P21 Pdk1ΔPanc pancreata (arrows in F). (C, D, I, J) Paraffin sections stained with hematoxilin-eosin. (E, F) Cell nuclei were stained with hematoxilin. (G, H) Confocal images of frozen sections stained with anti-β-catenin antibodies and DAPI. Scale bar is: 25 µm (G–J) and 100 µm (C, D, E, F).
At P21, the Pdk1ΔPanc pancreas was remarkably smaller than this organ of wild-type littermates (Fig. 8A, B). Similar to what we observed at E18.5-P2, H&E staining revealed that some P21 mutant lobes had very small acini (Fig. 8C, D) formed by undersized exocrine cells (Fig. 8G, H). In contrast, other lobes of P21 Pdk1ΔPanc pancreata had relatively normal-sized acini (data not shown). Sections of P23 Pdk1ΔPanc;Z/AP pancreata stained with anti-HPAP antibodies revealed that the normal-sized acini were HPAP−, whereas the small-sized acini and nearly all islets and ducts were HPAP+ (Suppl. Fig. 4). These results indicate that the exocrine cells of Pdk1ΔPanc pancreata devoid of Pdk1 function did not recover their normal size upon maturation.
Overall, our results imply a continuous, cell-autonomous requirement of Pdk1 function in the pre- and postnatal pancreas to maintain the proper size of exocrine cells and β cells. Hashimoto et al (2006) previously reported a similar role of Pdk1 in pancreatic β cells of adult mice.
Pdk1ΔPanc juvenile mice have compensatory proliferation in their pancreatic acinar tissue
In the pancreas of P21 wild-type mice we observed expression of the proliferation marker Ki67 in some exocrine cells and in a few ductal cells or islet cells (Fig. 8E and data not shown). Conversely, in the pancreas of Pdk1ΔPanc littermates we noticed extensive Ki67 expression throughout the exocrine tissue, particularly in those acini with undersized exocrine cells (Fig. 8F). In these mutant tissues we also observed Ki67 expression in some ductal cells and, only rarely, in a few islet cells (data not shown). These observations support the notion that Pdk1 regulates the proliferation of mature pancreatic β cells (Hashimoto et al., 2006) and, conversely, uncover that Pdk1 activity is dispensable for proliferation in mature pancreatic exocrine cells.
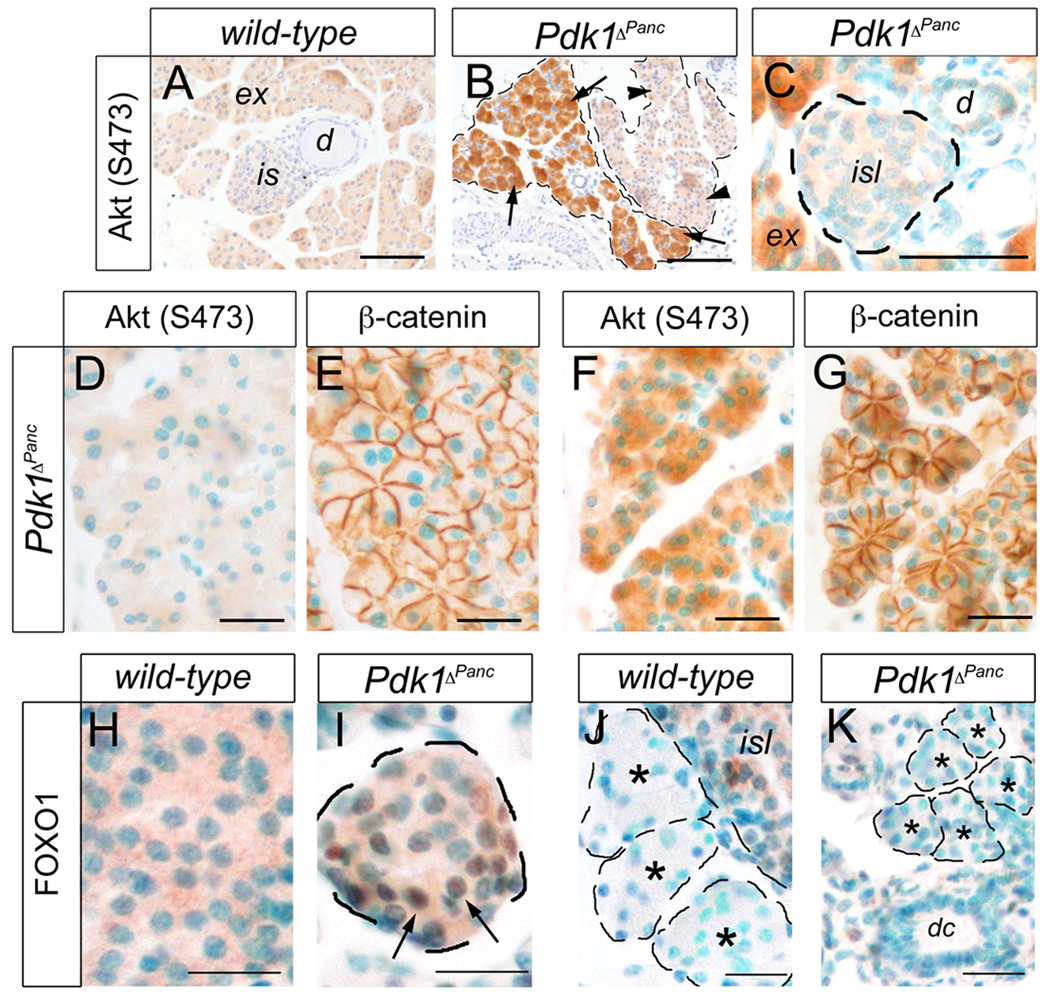
Previous results showed that in certain cell types the loss of Pdk1 function markedly stimulates the mTORC2-dependent phosphorylation of Akt at S473 (Bhaskar and Hay, 2007; Chalhoub et al., 2009; Mora et al., 2003; Williams et al., 2000). We used antibodies recognizing Akt phosphorylated at S473 (p-S473) to stain sections of P21 Pdk1ΔPanc or wild-type pancreata and showed negligible Akt phosphorylation at S473 in the wild-type tissues (Fig. 9A). Similarly, the islets (Fig. 9C), ducts (Fig. 9C), and normal-sized acini (Fig. 9D, E) of P21 Pdk1ΔPanc pancreata were devoid of Akt (S473) phosphorylation. In contrast, the p-S473 form of Akt was highly expressed in the acinar regions containing small exocrine cells of P21 Pdk1ΔPanc pancreata (Fig. 9B, F, G). This result raises the possibility that in the absence of functional Pdk1, mTORC2 kinase was activated specifically in the small-sized exocrine cells of Pdk1ΔPanc pancreata.
Figure 9. Akt phosphorylation at S-473 is seen in the small exocrine cells in the pancreas of P18–P21 Pdk1ΔPanc mice.
(A) Akt phosphorylation at S-473 is negligible in P21 wild-type pancreata. (B) Akt proteins are abundantly phosphorylated at S-473 in some lobes in the pancreas of P21 Pdk1ΔPanc mice (arrows), or expressed at negligible levels in other lobes of this mutant organ (arrowheads). (C) Phosphorylation of Akt at S-473 is observed in exocrine acini (ex), but not in islets (isl) or ducts (dc) of P21 Pdk1ΔPanc pancreata. (D, E) The exocrine cells of P21 Pdk1ΔPanc pancreata with normal size (E) do not express Akt (S473) (D). (F, G) The small exocrine cells of P21 Pdk1ΔPanc pancreata (G) express Akt (S473) proteins abundantly (F). (H) Cytoplasmic localization of Foxo1 proteins (brown staining) in an islet of P18 wild-type pancreata. (I) Distribution of Foxo1 proteins (brown staining and arrows) in the cytoplasm and nucleus of an islet of P18 Pdk1ΔPanc pancreata. (J, K) Foxo1 expression is negligible in the exocrine tissue of P18 wild-type (asterisks in J) or Pdk1ΔPanc (asterisks in K) pancreata. (E, G) Anti-β-catenin antibodies were used to delineate the exocrine cell perimeter. (D, E) and (J, K) are consecutive sections, respectively. Cell nuclei were stained with hematoxilin (A, B) or with methyl green (C–K). Scale bar is: 25 µm (C–K) and 100 µm (A, B).
Previous experimental evidence suggested that Pdk1 prevents cell cycle withdrawal of pancreatic β cells, at least in part, by promoting the Akt-mediated phosphorylation and subsequent exclusion from the nucleus of the transcription factor Foxo1 (Hashimoto et al., 2006). Accordingly, in islets of P18 wild-type pancreata we observed Foxo1 expression almost exclusively in the cytoplasm of endocrine cells (Fig. 9H). In contrast, the islets of P18 Pdk1ΔPanc pancreata had prominent nuclear Foxo1 expression, presumably in β cells (Fig. 9I). On the other hand, the wild-type acini (Fig. 9J), the normal-sized Pdk1ΔPanc acini (data not shown) and the small-sized Pdk1ΔPanc acini (Fig. 9K) were entirely devoid of Foxo1 expression. This result further suggests that Pdk1 (and consequently, Akt) does not regulate the same targets in pancreatic endocrine and exocrine cells.
DISCUSSION
In this study, we report that the conditional deletion of Pdk1 in mouse pancreatic progenitors results in severe pancreatic hypoplasia at birth and ensuing hyperglycemia at postnatal stages. We also provide evidence that these alterations are caused by inadequate formation of exocrine and endocrine cell masses during pancreas development. Therefore, we conclude that Pdk1 is a novel, crucial growth regulator in developing pancreatic tissues.
Pdk1 has multiple roles during pancreas organogenesis
The phenotypic alterations of Pdk1-deficient pancreatic tissues underscored in our analysis suggest a distinct temporal, cell-type–specific requirement of Pdk1 activity in the control of cell proliferation, cell survival, and cell size during pancreas development. Specifically, early on in pancreas organogenesis Pdk1 promotes the proliferation and survival of pancreatic progenitors. After E15.5, these early roles of Pdk1 segregate into specific cell lineages: Pdk1 continues to regulate proliferation but not survival in developing β cells whereas, reciprocally, Pdk1 activity becomes dispensable or redundant for proliferation, but not for survival, in developing exocrine cells. Finally, our study also revealed that Pdk1 function is continuously required in both, the exocrine- and β–cell lineages of the pre- and postnatal pancreas, to reach and to preserve the proper size of cells. The lack of appropriate molecular tools precluded investigating whether Pdk1 also has lineage-restricted functions in endocrine or exocrine precursors of the early embryonic pancreas.
Our finding that Pdk1 activity is differentially required in distinct pancreatic cell types is not unexpected, because in diverse organisms Pdk1 has been shown to control distinct processes required for organ growth in a tissue-restricted manner. For instance, in Drosophila dPdk1 was reported to control the growth of eye or wing imaginal disks by regulating cell size (Cho, et al., 2001). Likewise, in mouse embryos Pdk1 controls heart size by regulating the growth of individual cardiomyocytes but not the number of these cells (Mora, et al., 2003). In contrast, Pdk1 enables the expansion of pro-opiomelanocortin (POMC) cells in the mouse pituitary gland by promoting the survival of these cells (Belgardt et al., 2008).
In this study we report that the early pancreas of E10.5 Pdk1-deficient mice is hypoplastic, and we provide evidence that this defect is consequence of decreased proliferation and survival of pancreatic progenitors. These results thus indicate that the PI3K signaling mediated by Pdk1 is necessary to efficiently expand early pancreatic progenitors. It is quite possible that PI3K signaling cooperates with other cytokine-mediated pathways during this process, because the lack of Pdk1 function causes mild, but not severe size reduction in early pancreatic tissues. One likely partner in this cooperation could be the activation of MAPK by FGFs, since Bhushan et al (2001) previously showed that mice devoid of FGF10 function have very severe pancreas hypoplasia and deficient proliferation of pancreatic progenitors. It would be important to determine whether signaling downstream of PI3K/Pdk1 is also affected in the FGF10-deficient pancreas, because in certain cellular contexts FGFs have been shown to activate both, MAPK and PI3K (Schlessinger, J., 2004).
Pdk1 is a novel regulator of pancreatic exocrine cell growth
One of the major, novel findings of our study is that Pdk1 function is crucial to expand the population of developing pancreatic exocrine cells. Interestingly, similar to our results, Kido et al (2002) reported that mice with deficient insulin/insulin-like growth factor signaling (Insr−/−;Igfr1−/− mice or Igf1−/−;Igf2−/− mice) also had pancreas hypoplasia due to reduced growth of the exocrine cell mass. That fact that lack of insulin/insulin-like growth factor signaling or Pdk1 function results in similar phenotypic alterations is not unexpected because most, if not all, signaling mediated by the insulin receptor or the insulin-like growth factor receptor converges at Pdk1 (Alessi et al., 1997). However, one major difference between our study and that of Kido et al (2002) is that the pancreas of E17.5 Pdk1ΔPanc embryos exhibited increased epithelial cell proliferation (including the exocrine cells), whereas the pancreas of E18.5 Igf1−/−,Igf2−/− embryos exhibited reduced cell proliferation. Hence, while we concluded that Pdk1 is redundant or dispensable for exocrine cell proliferation, Kido et al. (2002) proposed that insulin/insulin-like growth factor signaling controls the proliferation of pancreatic exocrine cells. One possible explanation for this discrepancy is that additional, non-pancreatic defects accounted for the reduced proliferation observed in E18.5 Igf1−/−,Igf2−/− pancreatic tissues. Moreover, our results conclusively demonstrated that Pdk1 activity is required for the survival and proper size of developing pancreatic exocrine cells. It remains to be determined whether defective insulin/insulin-like growth factor signaling also leads to similar alterations, as these parameters were not evaluated in the study of Kido et al. (2002). On the other hand, our identification of increased cell proliferation in E17.5 PDK1ΔPanc pancreata also suggests that compensatory proliferation mechanisms are induced in response to the lack of pancreatic growth. The nature of this compensatory response is presently unknown. Finally, our observation that numerous exocrine cells underwent apoptosis and proliferation in the PDK1ΔPanc pancreas at E17.5, but not at postnatal stages, favors a requirement of Pdk1 activity for the survival of pancreatic exocrine cells at developmental stages but not after birth.
We also uncovered that Pdk1 function confers proper size to individual pancreatic exocrine cells. The regulation of cell size by Pdk1 involves the Akt-mediated activation of mTOR (Hay and Sonenberg, 2008). In numerous cell types, S6K proteins mediate the cell size responses induced by mTOR via the activation of diverse factors involved with protein translation and ribosome biosynthesis (Lachance et al., 2002; Montagne et al., 1999; Ruvinsky and Meyuhas, 2006). Consequently, one could predict that S6K activity regulates the size of pancreatic exocrine cells. However, a previous study showed that the lack of S6K1 function reduces the size of β cells, but does not affect the size of exocrine cells (Pende et al., 2000). Furthermore, another study suggested that the activity of rpS6 (a target of S6K) is not necessary for pancreatic exocrine cell growth, because mice expressing non-phosphorylatable rpS6 (i.e., devoid of activity) had smaller β cells but normal-sized exocrine cells (Ruvinsky et al., 2005). Although it is probable that in exocrine cells S6K2 compensates for the lack of functional S6K1, or that the size of this cell lineage requires S6K-targets different from rpS6 (e.g., SKAR, eEF2 kinase or eIF4B) (Richardson et al., 2004; Ruvinsky and Meyuhas, 2006). It is also possible that Pdk1 controls exocrine cell size via factors that do not depend on S6K activity (e.g., the mTORC1-effector eukaryotic translation initiation factor, eIF4E) (Hay and Sonenberg, 2008). Further studies are necessary to fully disclose the molecular mechanisms downstream of Pdk1 function in pancreatic exocrine cells.
Extensive phosphorylation of Akt at S473 occurs in small exocrine cells but not in normal-sized exocrine cells of Pdk1ΔPanc postnatal pancreatic tissues
Two rate-limiting steps in the pathway regulated by PI3K/Akt are the Pdk1-dependent phosphorylation of Akt at T308 and the mTORC2-dependent phosphorylation of Akt at S473 (Sarbassov et al., 2005; Toker et al., 2008). Due to the lack of anti–p-T308 antibodies suitable for immunohistochemistry we could not validate Akt phosphorylation at T308 in wild-type pancreata, or lack of thereof in Pdk1-deficient pancreata. However, we anticipate that the phosphorylation of Akt at T308 is abrogated in islets and Pdk1-deleted exocrine cells of Pdk1ΔPanc pancreata because this residue is a recognized target of Pdk1 (Alessi et al., 1997; Stokoe et al., 1997).
We used anti–p-S473 Akt antibodies appropriate for immunostaining and discovered that Akt was largely unphosphorylated on S473 residues in the pancreas of P21–P25 wild-type mice. Remarkably, although the p-S473 form of Akt was absent in normal-sized exocrine cells, islets, and ducts of the P21–P25 Pdk1ΔPanc pancreata, nearly every small-sized exocrine cell in these mutant tissues expressed (p-S473) Akt proteins abundantly. These results not only support the notion that the S473 and the T308 residues of Akt are phosphorylated independently, but also they favor the hypothesis that Pdk1 negatively regulates the kinase responsible for Akt (S473) phosphorylation (Bhaskar and Hay, 2007; Mora et al., 2003). Based on recent published evidence, we propose that mTORC2 is the kinase that phosphorylates Akt at S473 in Pdk1-deficient pancreatic exocrine cells (Bhaskar and Hay, 2007; Huang et al., 2008; Sarbassov et al., 2005).
In partial contrast with our results, it is noteworthy that Hashimoto et al. (2006) observed expression of p-S473 Akt in islets of βPdk1 mice, whereas we found that this form of Akt was absent in islets of Pdk1ΔPanc mice. Although highly speculative, we propose that abrogating Pdk1 function in β cells before they achieve maturation somehow affects the activity of mTORC2, whereas eliminating Pdk1 in fully mature β cells does not influence mTORC2 function.
Increased phosphorylation of Akt at S473 was previously reported in extracts of embryonic stem cells or cardiomyocytes lacking Pdk1 activity (Mora et al., 2003; Williams et al., 2000). More recently, Chalhoub et al. (2009) reported strong (p-S473) Akt immunoreactivity in glial cells, but not in neurons, in brains of mice lacking functional Pdk1 in the hippocampus and cortex. This unique result supported the need of strong feedback mechanisms limiting PI3K signaling in glial cells to minimize pathological conditions since, different to neurons, mature glia can be stimulated to re-enter the cell cycle (Chalhoub et al., 2009). We hypothesize that similar control mechanisms operate in mature pancreatic exocrine cells to restrict their proliferation, since we noticed abundant expression of (p-S473) Akt and Ki67 proteins in exocrine cells, but not in β cells, of Pdk1ΔPanc pancreata.
The study of Hashimoto et al. (2006) reported that in the absence of Pdk1 function, pancreatic β cells accumulated Foxo1 proteins (a target of Akt) in the nucleus. Foxo1 increases the expression of the cell cycle inhibitor p27; thus, these authors hypothesized that the Pdk1-Akt axis promotes β-cell proliferation via preventing the nuclear accumulation of Foxo1. In agreement with this result, in β cells of P18–P21 Pdk1ΔPanc pancreatic tissues, we also observed that Foxo1 proteins were largely expressed in the nucleus. In contrast, we did not detect Foxo1 expression in pancreatic exocrine cells of P18–P21 Pdk1ΔPanc mice or wild-type mice. These results support the notion that the pathways regulated by Pdk1 differ in the exocrine-and β-cell lineages of the pancreas.
Is Pdk1 function necessary to preserve global pancreas homeostasis?
Hashimoto et al. (2006) uncovered that Pdk1 function is necessary to maintain glucose homeostasis in adult mice because its activity promotes the survival, proliferation, and proper size of mature pancreatic β cells. Our detailed characterization of Pdk1ΔPanc pancreatic tissues furthered these observations by demonstrating that Pdk1 activity is also necessary to expand the population of pancreatic β cells during embryogenesis. However, different from its role in mature β cells, Pdk1 appears to control the size and proliferation, but not the survival, of developing β cells. Arguably, the survival role of Pdk1 becomes necessary in this lineage only after the cells achieve full maturation.
The results of this study also revealed that Pdk1 is a central player in the control of pancreatic exocrine growth. We also showed that, despite compensatory proliferation, the total exocrine mass of the Pdk1-deficient pancreas remains decreased at postnatal stages. Unfortunately, Pdk1ΔPanc mice are not suitable models in which to test whether defective exocrine growth leads to pancreatic insufficiency, because those animals had to be euthanized at a relatively young age (1–2 months) due to severe hyperglycemia. Addressing this important issue should require the specific inactivation of Pdk1 in the pancreatic exocrine cell lineage. Another aspect worthy of investigating is how Pdk1 activity affects pancreatic tumor formation, particularly because Stanger and colleagues previously demonstrated that the loss of activity of the PI3K negative regulator, PTEN, results in the formation of preneoplastic lesions in the pancreas (Stanger et al., 2005).
Supplementary Material
ACKNOWLEDGMENTS
We thank L. Bachaboina for excellent technical assistance; Nader Chalhoub and Melissa Fraser for providing technical advice and reagents; L. Zhang of the St. Jude Cell and Tissue Imaging Core Imaging Core for help with the confocal/multiphoton microscopy; A. McArthur for editing the manuscript; R. Klein-Geltink for helpful discussions, and G. Oliver for critical reading of the manuscript. We also thank D. Alessi for generously providing the Pdk1flΔneo/flΔneo mouse strain, D. Melton and G. Gonqiang for providing the Pdx1-Cre transgenic mice, and C. Wright and S. Konyeczny for providing antibodies. The project described was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (R01DK060542, NIH) and by the American Lebanese Syrian Associated Charities (ALSAC). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute Of Diabetes And Digestive And Kidney Diseases or the National Institutes of Health.
Grant Sponsor: National Institute of Diabetes and Digestive and Kidney Diseases, NIH (5 R01DK060542), and the American Lebanese Syrian Associated Charities (ALSAC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Alessi DR, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P, Hemmings BA. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 1996;15(23):6541–6551. [PMC free article] [PubMed] [Google Scholar]
- Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PR, Reese CB, Cohen P. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr Biol. 1997;7(4):261–269. doi: 10.1016/s0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- Belgardt BF, Husch A, Rother E, Ernst MB, Wunderlich FT, Hampel B, Klöckener T, Alessi D, Kloppenburg P, Brüning JC. PDK1 deficiency in POMC-expressing cells reveals FOXO1-dependent and -independent pathways in control of energy homeostasis and stress response. Cell Metab. 2008;7(4):291–301. doi: 10.1016/j.cmet.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Bhaskar PT, Hay N. The Two TORCs and Akt. Dev. Cell. 2007;12:487–502. doi: 10.1016/j.devcel.2007.03.020. [DOI] [PubMed] [Google Scholar]
- Bhushan A, Itoh N, Kato S, Thiery JP, Czernichow P, Bellusci S, Scharfmann R. Fgf10 is essential for maintaining the proliferative capacity of epithelial progenitor cells during early pancreatic organogenesis. Development. 2001;128(24):5109–5117. doi: 10.1242/dev.128.24.5109. [DOI] [PubMed] [Google Scholar]
- Biondi RM, Kieloch A, Currie RA, Deak M, Alessi DR. The PIF-binding pocket in PDK1 is essential for activation of S6K and SGK, but not PKB. EMBO J. 2001;20(16):4380–4390. doi: 10.1093/emboj/20.16.4380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cano DA, Hebrok M, Zenker M. Pancreatic development and disease. Gastroenterology. 2007;132:745–762. doi: 10.1053/j.gastro.2006.12.054. [DOI] [PubMed] [Google Scholar]
- Chalhoub N, Zhu G, Zhu X, Baker SJ. Cell type specificity of PI3K signaling in Pdk1- and Pten-deficient brains. Genes Dev. 2009;23(14):1–5. doi: 10.1101/gad.1799609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho KS, Lee JH, Kim S, Kim D, Koh H, Lee J, Kim C, Kim J, Chung J. Drosophila phosphoinositide-dependent kinase-1 regulates apoptosis and growth via the phosphoinositide 3-kinase-dependent signaling pathway. Proc Natl Acad Sci U S A. 2001;98(11):6144–6149. doi: 10.1073/pnas.101596998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung J, Kuo CJ, Crabtree GR, Blenis J. Rapamycin-FKBP specifically blocks growth-dependent activation of and signaling by the 70 kd S6 protein kinases. Cell. 1992;69:1227–1236. doi: 10.1016/0092-8674(92)90643-q. [DOI] [PubMed] [Google Scholar]
- Fingar DC, Blenis J. Target of rapamycin (TOR): an integrator of nutrient and growth factor signals and coordinator of cell growth and cell cycle progression. Oncogene. 2004;23(18):3151–3171. doi: 10.1038/sj.onc.1207542. [DOI] [PubMed] [Google Scholar]
- Fingar DC, Salama S, Tsou C, Harlow E, Blenis J. Mammalian cell size is controlled by mTOR and its downstream targets S6K1 and 4EBP1/eIF4E. Genes Dev. 2002;16:1472–1487. doi: 10.1101/gad.995802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu G, Dubauskaite J, Melton DA. Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development. 2002;129:2447–2457. doi: 10.1242/dev.129.10.2447. [DOI] [PubMed] [Google Scholar]
- Hashimoto N, Kido Y, Uchida T, Asahara S, Shigeyama Y, Matsuda T, Takeda A, Tsuchihashi D, Nishizawa A, Ogawa W, Fujimoto Y, Okamura H, Arden KC, Herrera PL, Noda T, Kasuga M. Ablation of PDK1 in pancreatic beta cells induces diabetes as a result of loss of beta cell mass. Nat Genet. 2006;38(5):589–593. doi: 10.1038/ng1774. [DOI] [PubMed] [Google Scholar]
- Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2008;18(16):1926–1945. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- Hinton HJ, Alessi DR, Cantrell DA. The serine kinase phosphoinositide-dependent kinase 1 (PDK1) regulates T cell development. Nat. Immunol. 2004;5(5):539–545. doi: 10.1038/ni1062. [DOI] [PubMed] [Google Scholar]
- Huang J, Dibble CC, Matsuzaki M, Manning BD. The TSC1-TSC2 complex is required for proper activation of mTOR complex 2. Mol Cell Biol. 2008;28(12):4104–4115. doi: 10.1128/MCB.00289-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferies HB, Fumagalli S, Dennis PB, Reinhard C, Pearson RB, Thomas G. Rapamycin suppresses 5'TOP mRNA translation through inhibition of p70s6k. EMBO J. 1997;16(12):3693–3704. doi: 10.1093/emboj/16.12.3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kido Y, Nakae J, Hribal ML, Xuan S, Efstratiadis A, Accili D. Effects of mutations in the insulin-like growth factor signaling system on embryonic pancreas development and beta-cell compensation to insulin resistance. J Biol Chem. 2002;277(39):36740–36747. doi: 10.1074/jbc.M206314200. [DOI] [PubMed] [Google Scholar]
- Kozma SC, Thomas G. Regulation of cell size in growth, development and human disease: PI3K, PKB and S6K. Bioessays. 2002;24(1):65–71. doi: 10.1002/bies.10031. [DOI] [PubMed] [Google Scholar]
- Lachance PE, Miron M, Raught B, Sonenberg N, Lasko P. Phosphorylation of eukaryotic translation initiation factor 4E is critical for growth. Mol Cell Biol. 2002;22(6):1656–1663. doi: 10.1128/MCB.22.6.1656-1663.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor MA, Mora A, Ashby PR, Williams MR, Murray-Tait V, Malone L, Prescott AR, Lucocq JM, Alessi DR. Essential role of PDK1 in regulating cell size and development in mice. EMBO J. 2002;21(14):3728–3738. doi: 10.1093/emboj/cdf387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobe CG, Koop KE, Kreppner W, Lomeli H, Gertsenstein M, Nagy A. Z/AP, a double reporter for cre-mediated recombination. Dev. Biol. 1999;208:281–292. doi: 10.1006/dbio.1999.9209. [DOI] [PubMed] [Google Scholar]
- Montagne J, Stewart MJ, Stocker H, Hafen E, Kozma SC, Thomas G. Drosophila S6 kinase: a regulator of cell size. Science. 1999;285(5436):2126–2129. doi: 10.1126/science.285.5436.2126. [DOI] [PubMed] [Google Scholar]
- Mora A, Komander D, van Aalten DM, Alessi DR. PDK1, the master regulator of AGC kinase signal transduction. Semin Cell Dev Biol. 2004;15(2):161–170. doi: 10.1016/j.semcdb.2003.12.022. [DOI] [PubMed] [Google Scholar]
- Mora A, Davies AM, Bertrand L, Sharif I, Budas GR, Jovanović S, Mouton V, Kahn CR, Lucocq JM, Gray GA, Jovanović A, Alessi DR. Deficiency of PDK1 in cardiac muscle results in heart failure and increased sensitivity to hypoxia. EMBO J. 2003;22(18):4666–4676. doi: 10.1093/emboj/cdg469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murtaugh LC, Melton DA. Genes, signals, and lineages in pancreas development. Annu. Rev. Cell Dev. Biol. 2003;19:71–89. doi: 10.1146/annurev.cellbio.19.111301.144752. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Sakaue H, Nishizawa A, Matsuki Y, Gomi H, Watanabe E, Hiramatsu R, Tamamori-Adachi M, Kitajima S, Noda T, Ogawa W, Kasuga M. PDK1 regulates cell proliferation and cell cycle progression through control of cyclin D1 and p27Kip1 expression. J. Biol. Chem. 2008;283(25):17702–17711. doi: 10.1074/jbc.M802589200. [DOI] [PubMed] [Google Scholar]
- Oldham S, Hafen E. Insulin/IGF and target of rapamycin signaling: a TOR de force in growth control. Trends Cell Biol. 2003;13(2):79–85. doi: 10.1016/s0962-8924(02)00042-9. [DOI] [PubMed] [Google Scholar]
- Pende M, Kozma SC, Jaquet M, Oorschot V, Burcelin R, Le Marchand-Brustel Y, Klumperman J, Thorens B, Thomas G. Hypoinsulinaemia, glucose intolerance and diminished beta-cell size in S6K1-deficient mice. Nature. 2000;408(6815):994–997. doi: 10.1038/35050135. [DOI] [PubMed] [Google Scholar]
- Price DJ, Grove JR, Calvo V, Avruch J, Bierer BE. Rapamycin-induced inhibition of the 70-kilodalton S6 protein kinase. Science. 1992;257:973–977. doi: 10.1126/science.1380182. [DOI] [PubMed] [Google Scholar]
- Richardson CJ, Bröenstrup M, Fingar DC, Jülich K, Ballif BA, Gygi S, Blenis J. SKAR is a specific target of S6 kinase 1 in cell growth control. Curr Biol. 2004;14(17):1540–1549. doi: 10.1016/j.cub.2004.08.061. [DOI] [PubMed] [Google Scholar]
- Ruvinsky I, Meyuhas O. Ribosomal protein S6 phosphorylation: from protein synthesis to cell size. TRENDS Biochem. Sci. 2006;31(6):342–348. doi: 10.1016/j.tibs.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Ruvinsky I, Sharon N, Lerer T, Cohen H, Stolovich-Rain M, Nir T, Dor Y, Zisman P, Meyuhas O. Ribosomal protein S6 phosphorylation is a determinant of cell size and glucose homeostasis. Genes Dev. 2005;19(18):2199–2211. doi: 10.1101/gad.351605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarbassov DD, Ali SM, Sabatini DM. Growing roles for the mTOR pathway. Curr Opin Cell Biol. 2005;17(6):596–603. doi: 10.1016/j.ceb.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Schelssinger J. Common and distinct elements in cellular signaling via EGF and FGF receptors. Science. 2004;306:1506–1507. doi: 10.1126/science.1105396. [DOI] [PubMed] [Google Scholar]
- Slack JM. Developmental biology of the pancreas. Development. 1995;121:1569–1580. doi: 10.1242/dev.121.6.1569. [DOI] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat. Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Stanger BZ, Stiles B, Lauwers GY, Bardeesy N, Mendoza M, Wang Y, Greenwood A, Cheng K, McLaughlin M, Brown D, DePinho RA, Wu H, Melton DA, Dor Y. Pten constrains centroacinar cell expansion and malignant transformation in the pancreas. Cancer Cell. 2005;8:185–195. doi: 10.1016/j.ccr.2005.07.015. [DOI] [PubMed] [Google Scholar]
- Stokoe D, Stephens LR, Copeland T, Gaffney PR, Reese CB, Painter GF, Holmes AB, McCormick F, Hawkins PT. Dual role of phosphatidylinositol-3,4,5-trisphosphate in the activation of protein kinase B. Science. 1997;277(5325):567–570. doi: 10.1126/science.277.5325.567. [DOI] [PubMed] [Google Scholar]
- Toker A. mTOR and Akt signaling in cancer: SGK cycles in. Mol Cell. 2008;31(1):6–8. doi: 10.1016/j.molcel.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Wang J, Kilic G, Aydin M, Burke Z, Oliver G, Sosa-Pineda B. Prox1 activity controls pancreas morphogenesis and participates in the production of "secondary transition" pancreatic endocrine cells. Dev Biol. 2005;286:182–194. doi: 10.1016/j.ydbio.2005.07.021. [DOI] [PubMed] [Google Scholar]
- Williams MR, Arthur JS, Balendran A, van der Kaay J, Poli V, Cohen P, Alessi DR. The role of 3-phosphoinositide-dependent protein kinase 1 in activating AGC kinases defined in embryonic stem cells. Curr Biol. 2000;10(8):439–448. doi: 10.1016/s0960-9822(00)00441-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.