Abstract
Reaching to grasp an object of interest requires a complex sensorimotor transformation-involving eye, head, hand and postural systems. We show here that discontinuities in development of movement in these systems are dependent not only on age but also vary according to task constraints. Providing external postural support allows us to examine the differential influences of the eye on the hand and the hand on the eye as the ability to isolate and coordinate each system changes with age. Children 4–6 years old had significant difficulty isolating eye movement from head or hand movement, whereas children 7–9 years old showed improved ability to isolate the eye, and by 10–15 years children became proficient in isolating hand movements from eye movements. Postural support had differential effects on the processes of initiation and execution of eye hand movements. The addition of postural support decreased the time needed for planning the movement, especially in the youngest children, and contributed to increased speed of isolated movements, whereas it caused differential slowing of coordinated movements depending on the child’s developmental level. We suggest that the complexity of the results reflects the complexity of changing task requirements as children transition from simpler ballistic control of all systems to flexible, independent but coordinated control of multiple systems.
Keywords: Posture, eye-hand coordination, development, children
Introduction
The “simple” act of reaching towards an object is carried out effortlessly many times daily. Yet the ease with which we accomplish this task belies the underlying complexity of the sensorimotor transformation necessary in the brain. If the target image is first visible in the peripheral visual field, planning and generating a reaching response towards it requires a transformation from eye-centered, to head-centered, body-centered, and finally hand-centered frames of reference (Snyder, 2000). In addition, accurate reaching movements are constrained by the ability to make predictive postural adjustments with the muscles of the trunk to compensate for the forces imposed on the body induced by the displacement of the arm (Bertenthal & von Hofsten, 1998). Indeed, increasing the requirement for postural stabilization in adults increases the response time of both the arm activation and postural adjustment (Cordo & Nashner, 1982). Each of these effectors moves during the course of a simple reach to a target and all of this occurs in less than a second, resulting in stereotypical smooth accurate reaching behavior regardless of target location or initial position of the head, body, and limb.
Functional coupling between the eye and the hand has been demonstrated in a number of psychophysical studies in humans and non-human primates. In adults information derived from the oculomotor system influences the planning and generation of reaching movements. Hand movements are faster and more accurate when accompanied by a saccade towards the same target (Prablanc et al., 1979). The coupling is bidirectional in that the oculomotor system is also influenced when pointing responses are made with the hand. (Epelboim et al. 1997; Lunenburger et al 2000; Snyder 2002; van Donkelaar and Lee, 1994; van Donkelaar et al 1992, 1997, 2000, 2004).
Although previous studies have examined the changes that occur in oculomotor (Fukushima et al., 2000; Salmon et al., 2005), manual motor control (Konczak et al, 1995, 1997; Hay, 1979) and anticipatory postural responses (Woollacott & von Hofsten 1998, Witherington et al, 2002; van der Hiede et al., 2003) across development, very little is known about how this development affects, or is affected by, the interactions between the eye, hand and postural motor systems. In infants it has been demonstrated that reaches to objects of interest are much more likely to occur if the object is also foveated (von Hofsten, 1982). In addition, it has been shown that children go through developmental changes in their use of visual feedback during reaching. For example 4–6 year olds make reasonably accurate movements without visual feedback; however at age 7 there is an abrupt reduction in this ability, with increased errors made when visual feedback is absent. This is followed by an increase in accuracy to adult levels by 9–11 years of age. It has been hypothesized that the age of 7–8 years is a transition period, during which there is a shift from mainly feed-forward programming of reaching to predominantly feedback control, followed by an adult-like integration of feed-forward and feed-back control by age 9 (Hay, 1978).
There is little research about the role of eye, hand, and postural interactions during childhood and adolescence. Again, in infants, it has been demonstrated that external postural stabilization of the head and trunk leads to more accurate reaching movements (Amiel-Tison & Grenier, 1980) suggesting that trunk control constrains the release of coordinated movement. Whether the same is true for the interactions between eye and hand movements is not known. Thus, the purpose of the current study was to examine the functional coupling of the eye and hand across development and determine the extent to which it was constrained by trunk postural control. For this purpose children aged 4–15 and adults made eye and hand movements either together or in isolation with and without external trunk postural support. The children were placed into one of three age groups: 4–6, 7–9, and 10–15. These age groups were chosen because 4 to 6 and 7 to 9 have been shown to be transitional periods in posture (Woollacott & Shumway Cook 1985) and feedback control, respectively (Hay 1981, Kirschenbaum 2001) while those age 10 and older have more adult like posture and eye hand parameters. We hypothesized that there would be discontinuities in the effects of postural support on oculomotor and manual motor function across these age groups. Specifically we predicted that children 4–6 years of age would be affected more by postural support, and children 7–9 years of age would be affected by the development of feedback interactions between the various systems and these would be more apparent when the systems were working in unison.
Materials and Methods
Subjects
Thirty typically developing children between 4 and 15 years of age were recruited. Ten 4–6 year olds (5 male and 5 female, 9 right handed and 1 left handed); twelve 7–9 year olds (6 male and 6 female, all right handed); and eight 10–15 year olds (5 male and 3 female, all right handed) participated in the study. Data from the children was compared with data from 10 young healthy adults, (4 male and 6 female aged 20 to 33 years, all right handed).
The study was in accord with the declaration of Helsinki guidelines and had ethical approval from the Human Subjects Committee at University of Oregon. Written consent was obtained from participants and/or their legal guardians. All subjects had normal or corrected to normal vision. Subjects were excluded if the parent/guardian reported impaired intelligence, ocular, neurologic, or psychiatric disorders or were on medication with drugs that might interfere with eye and hand movements.
Experimental set up
The experimental setup is shown in Fig 1. The subject was seated on a bench in a dark room facing a computer monitor within easy reach of the screen and with the hands resting on a table. The monitor and bench height were both adjusted so the target could be presented at eye level. The head could move freely throughout the experiment. Velcro straps attached to the bench could be used to support the pelvis and an external brace could be used to provide support at the upper trunk. SuperLab Pro was used to control the presentation of the target images on the monitor and trigger data collection.
Figure 1.
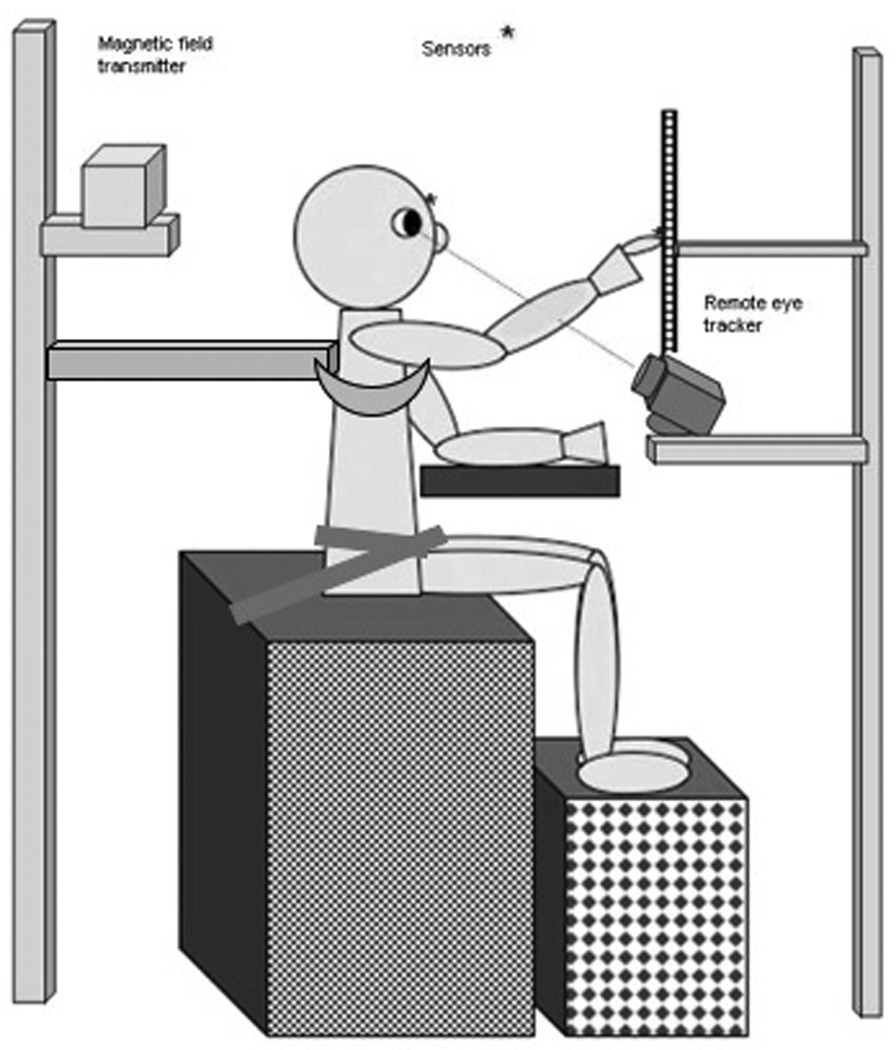
Experimental setup: children sat in front of a computer monitor in a darkened room, hands resting on a table. Supported postural condition included hip straps that keep the pelvis aligned vertically and a rigid support around the thoracic spine ~ 1 inch below arm pit. The straps were released and support bar removed for the unsupported condition. Magnetic sensors were attached to a headband and to the index finger of dominant hand.
Kinematics and Point of gaze eye tracking
An ASL remote eye tracker combined with an Ascension Flock of Birds system with two magnetic sensors was used to collect eye, head, and hand kinematic data at 60 Hz. The Flock of Birds system had a recording volume of 1m3 with a spatial accuracy of 1.8 mm. A headband was fitted such that a sensor centered on the forehead served to record head movement in 6 degrees of freedom while also providing information for the remote eye tracker. Hand kinematics were recorded by a second magnetic sensor that was taped securely to the fingernail of the index finger of the dominant hand. Corneal and pupil reflections were recorded by the eye tracker camera and transformed into horizontal and vertical point of gaze coordinates. We chose to use head free recording to avoid confounding the results of postural support and to allow more natural, less restrained movements. High frequency sampling of eye movements was limited by this decision. Helsen et al (1998) demonstrated that 60-Hz sampling of point of gaze and hand movements may provide as meaningful results as a 120-Hz sampling. They found no differences for initiation time, saccade angle, fixation duration and overall number of saccades. They did however find differences in saccade duration with the 60 Hz system overestimating saccade duration by 12 ms. and a reduction in the number of hand submovements with the 60 Hz system missing some of the smaller acceleration changes. Accordingly, eye peak velocity measurements and total number of hand submovements may be slightly underestimated in our results; however these limitations would affect all participants equally across all tasks and conditions.
Following instruction and 10–20 practice trials outside of the experimental room, the subject was seated in the test area and sensors were put in place. A foam pad was used as a home position for the hand and adjusted on the tabletop so the subject could return to the same starting point prior to each trial. It was positioned comfortably in front of the participant. The eye tracker was calibrated by having the subject visually fixate on a series of 9 calibration points on the screen. The magnetic sensor attached to the finger was calibrated by having the subject touch each of the targets.
Tasks
Each trial began with a central fixation point illuminated on the screen. After a variable delay a 2nd target appeared for 2–3 seconds in the periphery. On 66% of the trials this target was positioned 7.5 cm to the dominant hand side. These trials were submitted for further analysis. On the remaining trials the target appeared with equal probability 3.5 or 10 cm to the dominant side or 3.5 cm to the non-dominant side. These trials served to keep the participant from anticipating target direction and amplitude, thus preventing preplanned responses. These trials were not submitted for further analysis. In separate blocks of trials the subject was instructed to i) ignore the 2nd target and maintain central fixation (“Control” task), ii) look at the 2nd target (“Eyes only” task), iii) look at and point to the 2nd target (“Eye-hand” task) or iv) maintain central fixation while pointing to the 2nd target (“Hand only” task) (Figure 2). Target onset triggered a 2 second data collection for Control and Eyes only trials and 3 second data collection for Eye-hand and Hand only trials. Two separate blocks of 18 trials of each task were run in a counterbalanced order among participants.
Figure 2.
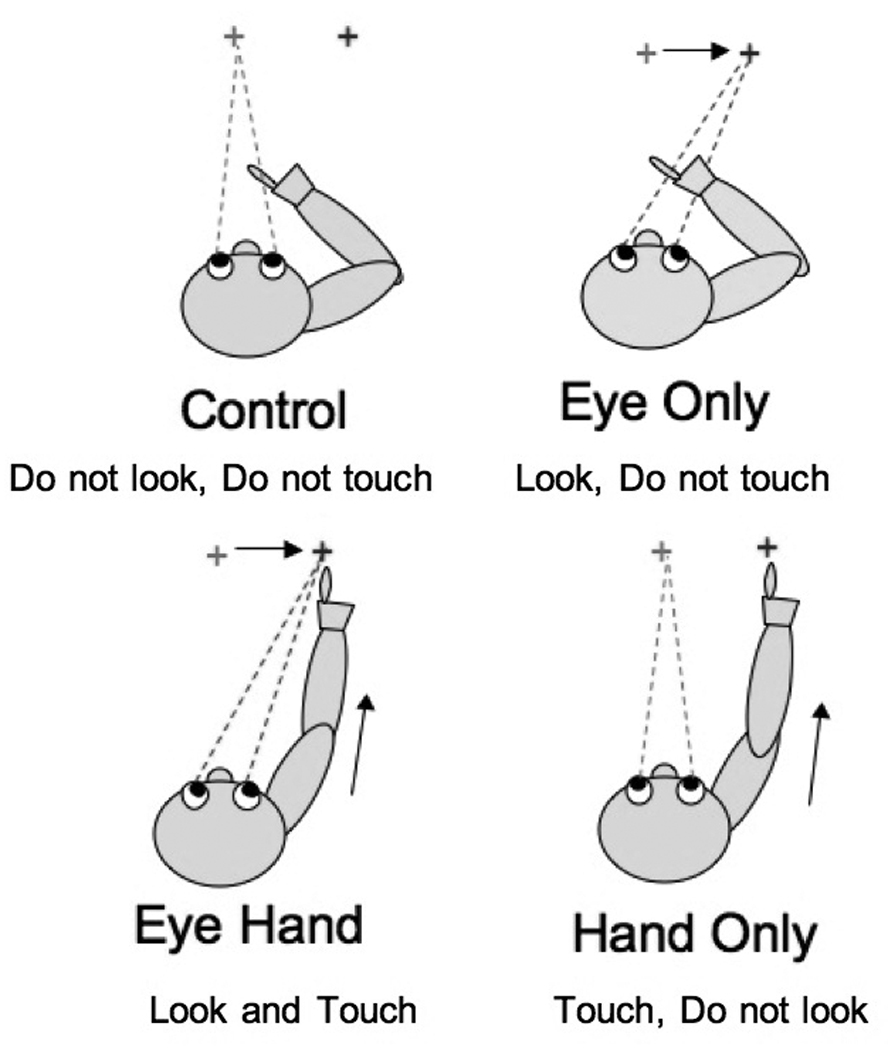
Data was collected during completion of 4 tasks a) control task, maintain visual fixation on center target (frog image) do not look at peripheral distracter (butterfly images) b)eye only task, begin with central fixation quickly look at peripheral target when it appears c) eye hand task, begin with central fixation quickly look at and touch peripheral target when it appears d) maintain central fixation, quickly touch peripheral target without looking at it.
The 4 tasks were completed under 2 different levels of external trunk postural support. In the “No Support” condition, participants sat on the bench without additional support, whereas in the “Trunk” condition, the pelvis was stabilized with straps and the external brace was positioned to provide support at the thoracic level. The 2 support conditions were completed in a counterbalanced order across subjects.
Data Reduction
Head, hand and eye movements were digitized for off-line analysis using Matlab. Primary and secondary saccade start and end times and positions were manually selected from plots of horizontal eye position for each trial. This manual procedure was carried out because of the frequent artifacts induced in the data by blinks and head motion especially in the younger children. Only trials with primary saccades that covered 90% of the distance to the target were considered for further analysis. Head azimuth minimum and maximum and hand start and stop times were marked automatically. Onset was determined by a change in resultant velocity of 5 standard deviations above baseline. End point was determined as the data point just before the finger marker reached the x coordinate matching that subject’s target calibration trial. The computer selected hand points were displayed on a computer monitor, verified by inspection and adjusted if necessary. Trials were discarded if the hand was not appropriately located at the start position, if the hand was not stationary at the beginning of the trial or if obvious artifacts were present during the reach portion of the data.
A total of 5,280 trials were collected across all the subjects. From these trials 3,520 were experimental trials and submitted to further analysis. After elimination of trials due to blinks, breaks from fixation, artifacts due to large head movements, or other discontinuities 806 trials for adults, 614 trials for 10–15 year olds, 875 trials for 7–9 year olds and 664 trials for 4–6 year olds were acceptable for use in data analysis. The number of acceptable trials for data analysis varied across participants and tasks. On average 10.2 acceptable trials per condition contributed to individual means for adults, 9 trials per mean for 10–15 year olds, 6.8 trials per mean for 7–9 year olds and 6.1 trials per individual mean for 4–6 yr olds. The tasks requiring visual fixation (control and hand only) were more difficult for the youngest children.
Data Analysis
For each participant we calculated the following parameters for each eye and hand movement: reaction time (RT) - time from target appearance to initiation of movement; movement time (MT) - time from initiation to end of movement; movement amplitude - horizontal displacement for eye point of gaze, resultant displacement for finger (x,y and z coordinates); peak velocity - maximum velocity during movement; time to peak velocity, and percent movement time at peak velocity. For each subject, the distance from the display screen depended on arm length; thus, the visual angle between the central fixation and the target varied accordingly. Eye amplitude and velocity measurements were computed in degrees of visual angle based on the distance from the head to the screen (view distance) for each subject. Hand velocity data were filtered with a zero lag 4th order low pass Butterworth filter (cut off frequency 12 Hz) prior to calculating the number of acceleration changes (submovements) for each reach. Amplitude of head azimuth (maximum-minimum) was recorded for each trial. Percentage of saccadic intrusions (i.e., breaks from fixation when the task required the eyes to remain stable) during the Control and Hand only tasks was also calculated.
Data were normally distributed for all variables except reaction times for hand and eye trials, which were significantly positively skewed for individuals as well as groups; therefore the median reaction time was used for each individual. Means of individual median reaction times were used for comparison between groups. Means and medians were calculated only for those individuals who had at least 2 good trials of each task. This resulted in exclusion of hand data for 5 children in the 4–6 year old age group due to their failure to inhibit saccades during the hand only tasks.
Eye and hand movement variables were submitted to 2 [task: eye only or hand only vs. eyehand] × 2 [levels of trunk support: no support vs. supported] × 4 [age group: 4–6 vs. 7–9 vs. 10–15 vs. adults] mixed model ANOVAs. Polynomial contrasts were included a priori to examine the effect of age. Paired t-tests and Tukey’s HSD were used for posthoc comparison of differences within and between groups.
Total head azimuth was compared across all 4 tasks, therefore, a 4 [task: control vs. eye only vs. eyehand vs. hand only] × 2 [level of trunk support] × 4 [age group] mixed design was used. Tests of homogeneity and sphericity were significant (Box’s M <.01, Mauchley’s test of sphericity for task<.01 and task* support<.01); therefore multivariate profile analysis was completed for these comparisons. The significant results were the same as for univariate tests (with Green-house Geiser adjustment) so univariate ANOVA results are reported here (Tabachnick & Fidell 2006).
Results
Table 1 shows group means, standard deviation and results of ANOVA’s for the main effect of group. Most of these developmental trends have been observed in previous studies examining eye and hand movements (Fukushima et al., 2000; Hay, 1979; Konczak et al, 1995, 1997; Salmon et al., 2005). We believe that our results accurately depict developmental trends in hand movements because we adjusted the experimental set up for each subject to allow proportionally similar reach dynamics. These adjustments created different view distances depending on the size of the subject, resulting in a significant main effect of group for view distance (see table 1). As view distance decreased visual angle increased and this is reflected in a significant group effect for visual angle (table 1). Due to these variations the significant group effect for peak velocity (table 1) must be interpreted cautiously and may be related to visual angle differences between the groups. Post hoc Tukey tests for view distance indicated that the 4–6 year olds were significantly closer than all other groups, while visual angle and peak velocity post hoc tests showed that 4–6 year olds were significantly different than 7–9 year olds and adults but not significantly different than 10–15 year olds. Interestingly, the group effect for eye movement time (MT) resulted in both significant linear and quadratic trends (table 1). On average, the 4–6 year olds completed their eye movements faster than the 7–9 year olds in spite of the fact that they were moving nearly 2 degrees further. We will now focus on those trends that were specifically related to the ability to coordinate or isolate each of the effectors and the influence of external trunk postural support on these movements.
Table 1.
Groups means showing developmental trends across task and condition
| Group means (SD)Eye parameters | 4–6 yr | 7–9 yr | 10–15 yr | adult | Eye F(3,34)= | Significance |
|---|---|---|---|---|---|---|
| omnibus(contrast test) | ||||||
| Eye RT (mean of median) |
339ms (94.6) |
356 ms (92.8) |
311 ms (61.3) |
277 ms (36.8) |
2.055 |
ns p=( .125) (linear=.039) |
| Eye MT (ms) |
97 ms (3.5) |
100 ms (7.3) |
97 ms (2.9) |
93 ms (4.2) |
3.322 |
p= .031 (linear=.043) (quadratic=.030) |
| Eye peak velocity(degrees/s) |
170.01 (22.88) |
143.41 (12.31) |
149.41 (18.77) |
140.62 (11.18) |
6.197 |
p=.002 (linear=.002) (cubic=.068) |
| Eye amplitude (degrees) |
11.05 (1.48) |
9.39 (.70) |
9.73 (1.20) |
9.19 (.78) |
6.027 |
p=.002 (linear=.002) (cubic=.083) |
| % saccadic intrusions |
60.4 (27.4) |
31.1 (14.5) |
14.2 (14.9) |
4.6 (4.8) |
19.456 |
p < .0005 (linear <.0005) |
| Head | Head F(3,28)= |
|||||
| Head azimuth (degrees) |
5.7 (1.4) |
3.2 (1.3) |
1.9 (.7) |
1.3 (.3) |
6.035 |
p < .0005 (Wilk’s) (linear <.0005) (quadratic= .037) |
| View distance (cm) |
34.93 (4.34) |
40.46 (2.32) |
39.68 (3.76) |
42.24 (4.28) |
8.815 |
p=.000 (linear=.000) (cubic=.064) |
| Hand parameters | Hand F(3,29)= |
|||||
| Hand RT (mean of median) |
518 ms (71) |
470 ms (58) |
401 ms (27) |
370 ms (35) |
13.503 |
p < .0005 (linear<.0005) |
| Hand MT |
472 ms (79) |
473 ms (86) |
416 ms (71) |
357 ms (60) |
4.958 |
p=.007 (linear=.004) |
| Hand amplitude (resultant x,y,z) |
30.6 cm (3.6) |
35.5 cm (4.0) |
35.5 cm (8.9) |
41.9 cm (6.9) |
4.196 |
p=.014 (linear=.003) |
| Hand peak velocity (meters per sec) |
2.36 (.48) |
2.79 (.27) |
2.82 (.40) |
3.33 (.65) |
3.33 (.65) |
p=.005 (linear=.001) |
| % MT at peak velocity |
56.7 (4.1) |
52.7 (8.5) |
58.0 (11.5) |
60.3 (11.3) |
1.129 |
ns p=.354 (linear= .037) |
| Hand submovements (zero acceleration crossings) |
3.86 (.92) |
3.73 (.56) |
3.42 (.73) |
2.30 (.52) |
11.079 |
p < .0005 (linear <.0005) |
Effect of postural support and task
Eye
The participants in each group were all successfully able to generate saccadic eye movements in isolation (i.e., even the youngest children never made an erroneous pointing movement during Eye only trials). However, there were systematic differences across groups and tasks in the ability to inhibit eye movements during the Control and Hand only conditions. This was characterized by calculating the frequency of saccadic intrusions during these two tasks (Table 2). This dependent variable was unaffected by the different postural support conditions so these have been collapsed. Inhibition of saccades during hand only trials was more difficult than during control trials across all groups (F(1,36)=37.106, p<.005). In addition, the ability to inhibit saccades when no concurrent hand movement was required developed earlier than the ability to inhibit saccades during hand movements, as reflected by a significant task by group interaction (F(3,36)=3.112, p=.038). Post-hoc Tukey’s tests revealed that this interaction was due to 4–6 year olds being significantly different from all the other groups on both the control and hand only tasks and the 7–9 year olds being significantly different from the adults only on the isolated hand task. These results suggest that there are developmental differences in the ability to inhibit saccades and these differences are task-dependent.
Table 2.
Mean percent saccadic intrusions (breaks from fixation) during control and hand only tasks for each group.
| saccadic intrusions Mean (SD) | 4–6 yr old | 7–9 yr old | 10–15 yr old | adult |
|---|---|---|---|---|
| Mean (SD) | ||||
| Control task |
54.2% (5.4) |
20.2% (4.9) |
9.6% (6.0) |
1.6% (5.4) |
| Hand only task |
66.6% (6.3) |
41.9% (5.7) |
18.7% (7.0) |
7.7% (6.3) |
Next, we examined how eye movements were affected by the task and the degree of postural trunk support. Across all groups, view distance was not significantly different for task (p=.054) or support (p=.073); however for each significant task and support interaction we have examined view distances changes within the specific groups. Figure 3 shows eye peak velocity for eye movements generated in isolation (a) or in combination with hand movements (b) and the influence of the additional postural support. Overall, across all groups and both tasks, peak velocity was slower when postural support was given F(1,34)=5.815, p=.021. In addition, there was a 2 way interaction between task and group (F(3,34)=5.791, p=.003) that was due to significantly increased peak velocity for eye movements paired with hand movements in the 4–6 yrs olds across both levels of support (t=3.237, p=.010). This was not due, however, to any change in view distance across these conditions in this age group (p=.145). Postural support affected eye peak velocity in the older children only when they were performing paired eye hand movements and this reached significance only in the 7–9 year olds (t= 3.226, p = .009). Again, this difference was not due to any change in view distance across these conditions in this age group (p=.329). Eye amplitude (visual angle) exhibited a similar task by group interaction (F(3,34)=3.553, p=.024). T-tests revealed that this was due to 4–6 yr olds having increased eye amplitude when eye movements were paired with hand movements (t=3.071, p=.013). The 7–9 year olds had increased mean eye amplitude on unsupported, paired eye hand movements; however, this did not reach significance (p=.066). Eye movement time exhibited a 3 way interaction which approached but did not reach significance (F(3,34)=2.776, p=.057). By contrast, saccadic reaction time was not affected by task or level of support. Taken together, this suggests that eye movements in 4–6 year olds are more sensitive to concurrent hand movements while eye movements in 7–9 year olds are especially sensitive to whether voluntary trunk control is required during the execution of coordinated eye-hand movements.
Figure 3.
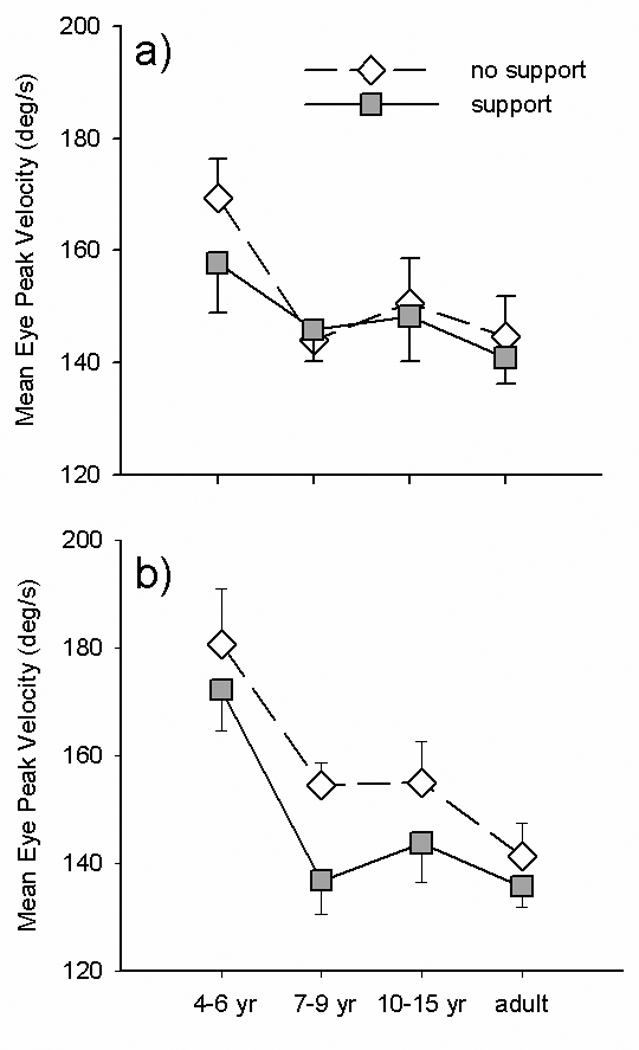
Group means for eye peak velocity during isolated (a) vs. paired (b) eye movements made with postural support (filled squares) and without support (unfilled diamonds).
Head
Because we did not explicitly ask subjects to keep their head stable during task performance, the amount of head azimuth motion varied according to task but was not affected by the different postural support conditions. Table 3 lists the mean head azimuth across the different tasks for each group. In addition to a main effect of task (F(3,84)=67.014, p<.0005) there was a significant task by group interaction (F(9,84)=4.942, p<.0005). Post-hoc Tukey tests showed that 4–6 year olds generated significantly more head movement than any other group for the two tasks involving saccades (p<.008 for all comparisons), while they were not significantly different than 7–9 year olds during Control and Hand only tasks. The 7–9 year olds were significantly different than adults only during isolated hand movements (p<.001). Taken together, these data demonstrate that the ability to maintain head stability is dependent on the task being performed and level of development.
Table 3.
Group means for head movement (degrees azimuth) during each task.
| head azimuth Mean (SD) | 4–6 yr old | 7–9 yr old | 10–15 yr old | adult |
|---|---|---|---|---|
| Control task |
1.53 (.21) |
.47 (.15) |
,32 (.18) |
.17 (.15) |
| Eye Only task |
6.04 (.99) |
2.85 (.70) |
.96 (.83) |
.21 (.70) |
| Eye Hand task |
11.67 (1.13) |
6.28 (.80) |
4.44 (.95) |
3.43 (.80) |
| Hand only task |
3.63 (.31) |
3.28 (.22) |
1.91 (.26) |
1.46 (.22) |
Hand
Finally, we characterized how the planning and execution of hand movements were influenced by the simultaneous production of eye movements and the degree of trunk postural support that was provided. Figure 4 shows hand RT for isolated hand movements (a) and hand movements paired with eye movements (b) and the influence of the additional postural support. Analysis of variance demonstrated a main effect of support (F(1,29)=22.949, p<.0005) with a support by group interaction F(3,29)=4.189, p=.014. Post hoc Tukey’s tests showed that this was due to the reduction in reaction time induced by providing postural support being significantly larger in the 4–6 year olds than the 10–15 year olds and adults. There was also a main effect of task (F(1,29)=6.287, p=.018) and a task by group interaction (F(3,29)=4.018, p=.017). Post hoc tests showed that this was due to larger reductions in reaction time with postural support for all hand movements in the 4–6 year olds, whereas 7–9 year olds had significantly reduced reaction times only for hand movements generated in isolation. Differences in reaction time between support conditions and tasks in the 10–15 yr olds and adults did not reach significance. Thus, the planning of hand movements as reflected in reaction time is influenced by the task, the degree of postural support, and the level of development.
Figure 4.
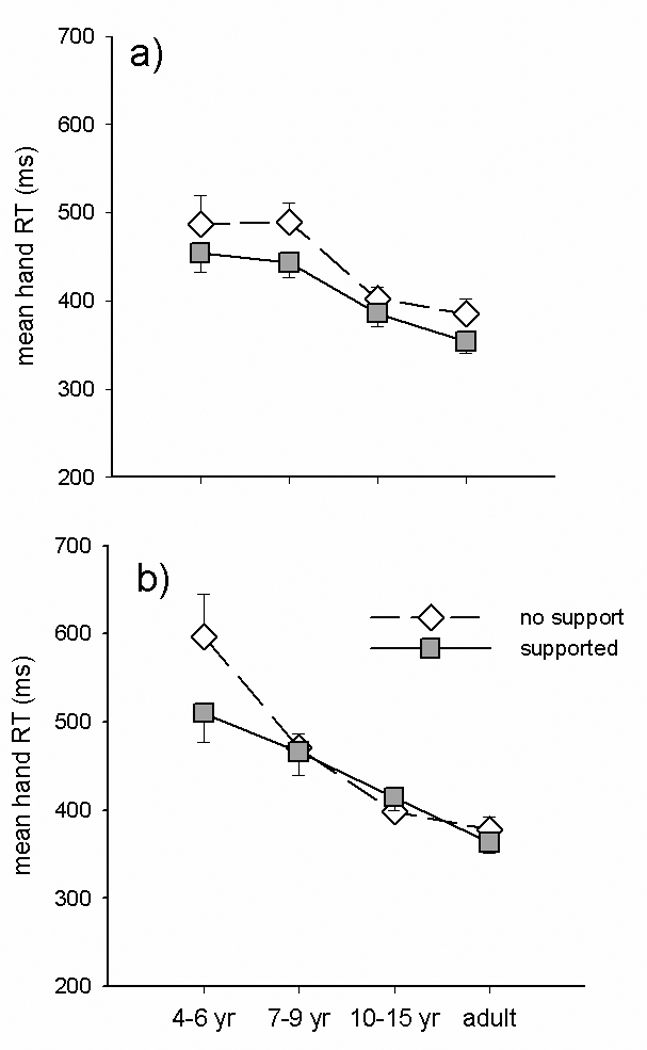
Group means for hand RT during isolated (a) vs. paired (b) hand movements made with postural support (filled squares) and without support (unfilled diamonds).
The remaining hand movement variables provided insight into the development of the execution of the hand movements. For most of these variables there was a significant effect of group (see Table 1). In addition, hand MT was faster (F(1,29)=9.007, p=.005), and percent MT at peak velocity was earlier with postural support (F(1,29)=5.537, p=.026). However, for peak hand velocity (figure 5) there was a significant task by support interaction (F(1,29)=7.155, p=.012); demonstrating that the improvement in peak hand velocity induced by providing postural support was only apparent when hand movements were generated in isolation.
Figure 5.
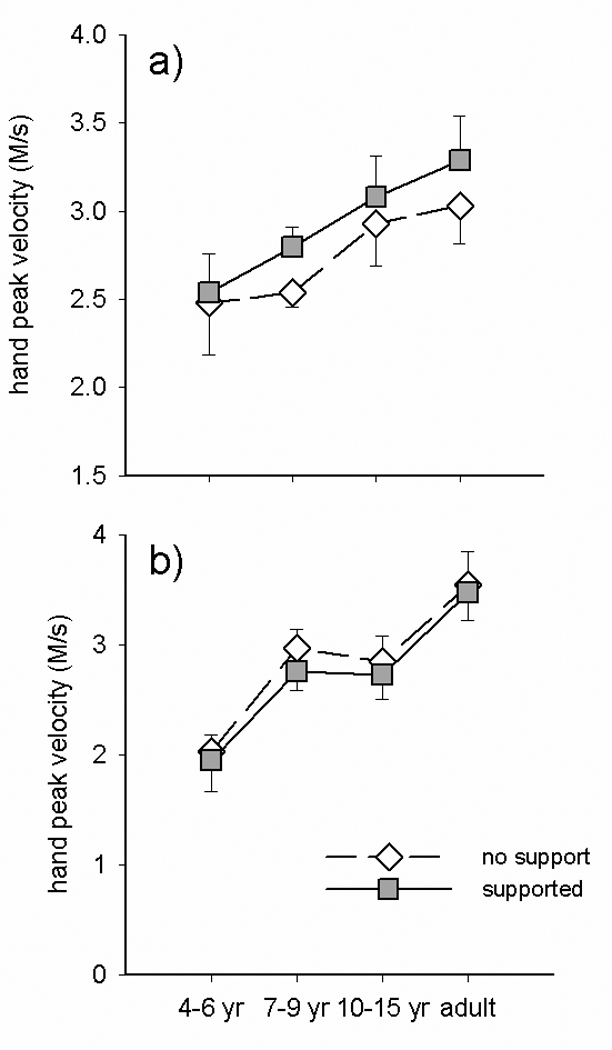
Group means for hand peak velocity during isolated (a) vs. paired (b) hand movements made with postural support (filled squares) and without support (unfilled diamonds).
Figure 6 illustrates the effect of task and postural support on the number of hand submovements during isolated hand movements (a) and during paired hand-eye movements (b). Analysis of variance revealed significant main effects of group (see Table 1), task (F(1,29)=5.383, p=.027), and support (F(1,29)=8.696, p=.006), as well as a support by group interaction (F(3,29)=5.413, p=.004) and a 3 way task by group by support interaction F(3,29)=6.452, p=.002. The 2 way interaction was due to postural support decreasing hand submovements substantially during both isolated (p=.071) and combined eye hand (p=.068) tasks in the 4–6 year olds, such that they had a significant effect of support (p=.044) across both tasks. The other groups exhibited changes in submovements with postural support when hand movements were made in combination with eye movements but not when hand movements were generated in isolation. Post-hoc tests demonstrated that the 3 way interaction was due to a reduction in the number of submovements in the 7–9 year olds (t= 3.677, p=.004) when postural support was provided and an increase in this variable under the same circumstances in the 10–15 year olds (t= −4.025, p=.007). Taken together, these results indicate that the effect of postural support depends on task. It contributed to increased speed (initiation and execution) during isolated hand movements without significantly changing the trajectory of the hand movement. Whereas it did not alter the speed of hand movements paired with eye movements but did contribute to improved trajectory (decreased submovements). In addition the effects of postural support were greater in younger children and influenced different parameters of coordination at different developmental ages.
Figure 6.
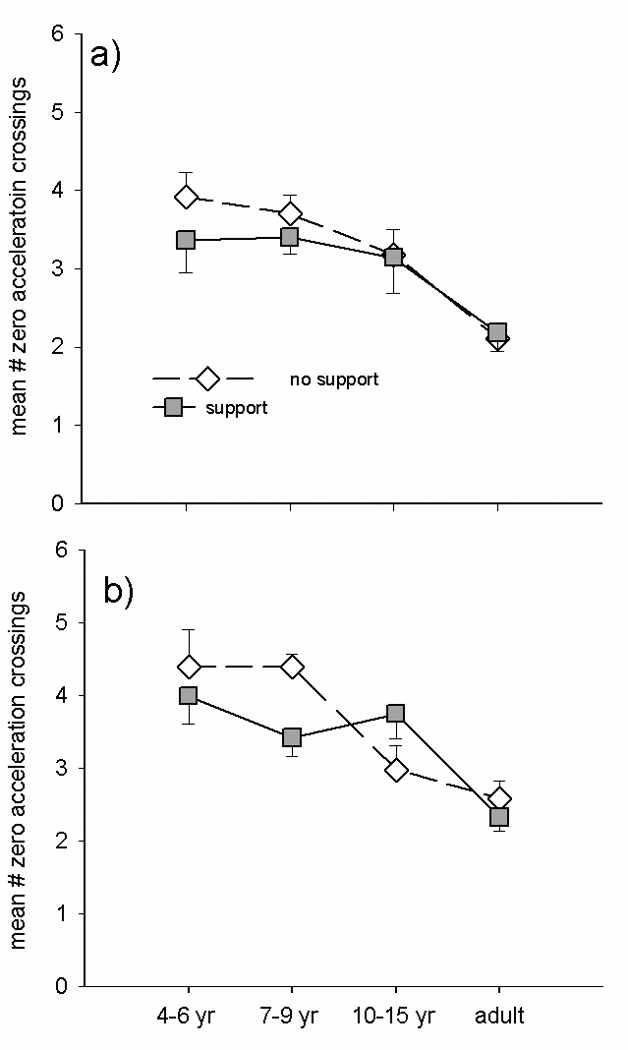
Group means for hand submovements (# of zero acceleration crossings) during isolated (a) vs. paired (b) hand movements made with postural support (filled squares) and without support (unfilled diamonds).
Discussion
In this study of eye hand coordination, we examined the functional coupling of the eye and hand across development and determined the extent to which it was constrained by trunk postural control. For this purpose children aged 4–15 and adults made eye and hand movements either together or in isolation with and without external trunk postural support. We found linear developmental trends in the ability to generate isolated eye, head and hand movements. In addition, the output of each of these effectors was differentially influenced across development by the degree of trunk postural support.. Taken together, these results demonstrate that both trunk postural control and the ability to isolate the different effectors constrain the coordination of eye, head, and hand movements during development. As has been shown previously during reaching (Hay, 1979;1990) and postural control (Kirschenbaum et al. 2001) these constraints are especially volatile in 7–9 year olds as they learn to isolate and coordinate the control of the individual effector systems.
Effects of postural support
Trunk postural control is a necessary requirement for the development of accurate reaching skills. In infants, providing external postural support allows more accurate limb movements to be generated (Amiel-Tison & Grenier, 1980 ). This influence is also observed to a much lesser degree in adults: limb movement reaction times are shorter with external trunk postural support (Cordo & Nashner, 1982 ). Our results confirm and extend these findings by characterizing the effects of trunk postural support on the ability to generate either isolated or coordinated movements of the eyes and hand across development. We find that providing external postural support improves the speed of isolated hand movements (RT shorter, peak velocity higher, MT shorter), to a greater extent in younger children than adults; whereas it improved the accuracy (decreased submovements) but not the speed of hand movements paired with eye movements specifically in the 7–9 year olds. We suggest that the fact that only isolated hand movements were improved with postural support reflects the difficulty younger children have with this task. Providing postural support reduces the planning required for the coordination between trunk control, saccade inhibition, and limb motor output, resulting in improved performance under these circumstances.
Eye movements were generally not directly affected by this manipulation. There may be a number of reasons for this. First, the low mass and inertial properties of the eye compared to arm implies that the former has a significantly smaller potential influence on trunk stability. Second, the tight coupling between the visual, oculomotor, and vestibular systems may result in head stabilization from an early stage of development (Jouen, 1989). Finally, postural control appears to develop in a top-down manner (Gesell A., 1946; Massion J. 1998) with head stability preceding trunk and standing postural control. There was, however, indirect evidence which demonstrated that providing external postural support improved hand movements generated simultaneously with eye movements: 4–6 year olds showed reduced hand reaction times during coordinated eye and hand movements when postural support was provided (Figure 4), and 7–9 year olds showed reduced eye peak velocity paired with reduced hand submovements when given support (Figure 3 and Figure 6). This suggests that information derived from the oculomotor system can influence the planning of reaching responses in the 4–6 year olds and execution of reaching responses in the 7–9 year olds, but only if trunk postural control is not required.
Ability to control isolated eye, head and hand movements
Studies of eye, head, and hand movements in infants have shown that the degree of coupling between the effectors improves with age (von Hofsten 1984,1993, von Hofsten & Rosander 1997, Rosander & von Hofsten 2000). However, these studies examined the natural tendencies for infants to generate isolated or coordinated movements. To our knowledge, this is the first study to examine changes in the ability to voluntarily isolate the movement of each of these effectors. We find that 4–6 and 7–9 year olds had difficulty generating movements of each of these effectors in isolation. In particular, the 4–6 year olds had a high degree of saccadic intrusions when required to visually fixate on a target located straight ahead in both the Control and Hand only tasks, and displayed a substantial amount of head motion in the eye only and eyehand tasks. Interestingly, these characteristics did not improve with the addition of trunk postural support. At first this appears to contradict the finding discussed above regarding improvements in isolated hand movements with postural support. However, we feel that this reflects processing differences in the planning and execution of limb movements compared to the inhibition of saccadic output. In the former situation, trunk control must be integrated into the ongoing ocular and manual motor plans; whereas in the latter situation, the prepotent response to make a saccade to the target must be inhibited. Such inhibitory planning is known to engage frontal circuits, which are involved more generally with executive function and are separate from those more directly involved with ocular and motor control. Taken together, these results suggest that coupled eye, head, and hand movements may be the default output for the CNS during early development. As the CNS matures the ability to inhibit unwanted responses improves, resulting in an increased ability to generate isolated responses when required.
7–9 year olds
Previous work by Laurette Hay (Hay, 1979; 1990) has demonstrated that reaching movements do not follow a linear developmental trend. In particular, 7–9 year olds quite often appear to perform worse than younger age groups. Children in this age range tend to be substantially less accurate than younger children when reaching movements are made without visual feedback. Moreover, when visual feedback is provided, 7–9 year olds produce a larger percentage of trajectory corrections as the hand approaches the target. The current data confirm and extend these findings by demonstrating an influence of postural support and type of task on the performance of 7–9 year olds. In particular, we find that children in this age group displayed a substantial reduction in eye peak velocity during combined eye-hand tasks when postural support was provided (Fig. 4). Moreover, there was a concurrent decrease in number of hand submovements generated under the same conditions (Fig. 6) indicating that the 7–9 year olds generated more efficient reaching movements under these conditions. In both cases, the alterations induced by providing postural support in these dependent variables in this age group were markedly different from that observed in the 4–6 and 10–15 year olds. The reduction in hand reaction time for hand movements generated in isolation implies that 7–9 year olds benefit from not having to prepare coordinated eye movements simultaneously with the hand movements. This supports the theory that this age group is especially affected by attempting to plan and prepare coordinated movements across different effectors. Clearly, the 7–9 year olds were influenced by the task constraints and experimental manipulations in a unique manner. Whether the differences in this age group observed in the present study are related to feedback mechanisms as suggested by Hay is unclear. The target and the hand were always visible in the relevant conditions. What did vary, however, was the extent of information available from the other effectors. We suggest that increased reliance on sensory input would become necessary to coordinate independent systems. Prior to the period when independent control of each system is possible there may not be a need for rapid online integration of sensory information. Perhaps the decrease in eye velocity during this transitional period allows time for the relevant sensory systems to adapt to the increasing demand for rapid assimilation of movement related information.
These results support the conclusions of Kirshenbaum et al (2001) that constriction of velocity and excursion may typify the early stages of integrating feedforward and feedback information. Their longitudinal study evaluating changes in center of pressure excursion and velocity in 5 to 8 year olds during quiet standing demonstrated similar developmental trends with a period of decreased velocity followed by a period of increased speed of online corrections. In our study the period of increased speed of online corrections is demonstrated by the 10–15 year olds who had increased number of hand submovements during combined eye hand movements (figure 6) without a concurrent decrease in MT.
Conclusions
Previous developmental studies have examined the effect of vision on eye hand coordination but to our knowledge no other studies have looked at the effect of coordinating the eye with the hand vs. performing isolated movements. Postural support had differential effects on the processes of initiation and execution of eye hand movements. The addition of postural support decreased the time needed for planning the movement, especially in the youngest children, and contributed to increased speed of isolated movements, whereas it caused differential slowing of coordinated movements depending on the child’s developmental level.
Acknowledgements
The authors wish to thank Cooper Boydston for providing computer support and Aditi Joshi for assistance with data collection. Supported by NIH grant 5R01NS038714-07 awarded to Marjorie Woollacott.
References
- Amiel-Tison C, Grenier A. Neurological evaluation of the human infant. New York: Masson; 1980. Evaluation neurologique du nouveau-ne et du nourrisson; pp. 81–102. [Google Scholar]
- Bertenthal B, Von Hofsten C. Eye, head and trunk control: The foundation for manual development. Neuroscience and Biobehavioral Reviews. 1998;22:515–520. doi: 10.1016/s0149-7634(97)00038-9. [DOI] [PubMed] [Google Scholar]
- Biguer B, Jeannerod M, et al. The Coordination of Eye, Head, and Arm Movements during Reaching at a Single Visual Target. Experimental Brain Research. 1982;46(2):301–304. doi: 10.1007/BF00237188. [DOI] [PubMed] [Google Scholar]
- Cordo PJ, Nashner LM. Properties of Postural Adjustments Associated with Rapid Arm Movements. Journal of Neurophysiology. 1982;47:287–382. doi: 10.1152/jn.1982.47.2.287. [DOI] [PubMed] [Google Scholar]
- Epelboim J, Steinman RM, et al. Gaze-shift dynamics in two kinds of sequential looking tasks. Vision Research. 1997;37(18):2597–2607. doi: 10.1016/s0042-6989(97)00075-8. [DOI] [PubMed] [Google Scholar]
- Fukushima J, Hatta T, Fukushima K. Development of voluntary control of saccadic eye movements. I. Age-related changes in normal children. Brain Development. 2000;22:173–180. doi: 10.1016/s0387-7604(00)00101-7. [DOI] [PubMed] [Google Scholar]
- Gesell A. The ontogenesis of infant behavior. In: Carmichael L, editor. Manual of child psychology. New York: Wiley; 1946. pp. 295–331. [Google Scholar]
- Hay L. Accuracy of children on an open-loop pointing task. Perceptual and Motor Skills. 1978;47:1079–1082. doi: 10.2466/pms.1978.47.3f.1079. [DOI] [PubMed] [Google Scholar]
- Hay L. Spatial-temporal analysis of movements in children: motor programs versus feedback in the development of reaching. J Mot Behav. 1979;11:189–200. doi: 10.1080/00222895.1979.10735187. [DOI] [PubMed] [Google Scholar]
- Hay L. Developmental changes in eye-hand coordination behaviors: Preprogramming versus feedback control. In: Bard C, Fleury M, Hay L, editors. Development of eye-hand coordination across the lifespan. Columbia: University of South Carolina; 1990. pp. 217–244. [Google Scholar]
- Helsen WF, Starkes JL, Elliot D, Ricker KL. Sampling frequency and the study of eye-hand coordination in aiming. Behavior Research Methods, Instruments, & Computers. 1998;30(4):617–623. [Google Scholar]
- Jouen F. Early visual-vestibular Interactions and postural development. In: Bloch H, Bertenthal BI, editors. Sensory-Motor Organizations and Development in Infancy and Early Childhood. The Netherlands: Kluwer Academic Publishers; 1989. pp. 199–216. [Google Scholar]
- Kirschenbaum N, Riach CL, Starkes JL. Non-linear development of postural control and strategy use in young children: a longitudinal study. Experimental Brain Research. 2001;140:410–431. doi: 10.1007/s002210100835. [DOI] [PubMed] [Google Scholar]
- Konczak J, Borutta M, et al. The Development of Goal-Directed Reaching in Infants - Hand Trajectory Formation and Joint Torque Control. Experimental Brain Research. 1995;106(1):156–168. doi: 10.1007/BF00241365. [DOI] [PubMed] [Google Scholar]
- Konczak J, Dichgans J. The development toward stereotypic arm kinematics during reaching in the first 3 years of life. Experimental Brain Research. 1997;117(2):346–354. doi: 10.1007/s002210050228. [DOI] [PubMed] [Google Scholar]
- Lunenburger L, Kutz DF, et al. Influence of arm movements on saccades in humans. European Journal of Neuroscience. 2000;12(11):4107–4116. doi: 10.1046/j.1460-9568.2000.00298.x. [DOI] [PubMed] [Google Scholar]
- Massion J. Postural control systems in developmental perspective. Neuroscience and Biobehavioral Reviews. 1998;22(4):465–472. doi: 10.1016/s0149-7634(97)00031-6. [DOI] [PubMed] [Google Scholar]
- Prablanc C, Echallier JF, et al. Optimal Response of Eye and Hand Motor Systems in Pointing at a Visual Target.1. Spatio-Temporal Characteristics of Eye and Hand Movements and Their Relationships When Varying the Amount of Visual Information. Biological Cybernetics. 1979;35(2):113–124. doi: 10.1007/BF00337436. [DOI] [PubMed] [Google Scholar]
- Rosander K, von Hofsten C. Visual-vestibular interaction in early infancy. Exp Brain Research. 2000;133:321–333. doi: 10.1007/s002210000413. [DOI] [PubMed] [Google Scholar]
- Snyder LH, Batista AP, Andersen RA. Saccade-related activity in the parietal reach region. Journal of Neurophysiology. 2000;83:1099–1102. doi: 10.1152/jn.2000.83.2.1099. [DOI] [PubMed] [Google Scholar]
- Salman MS, Sharpe JA, et al. Saccades in children. Vision Research. 2006;46(8–9):1432–1439. doi: 10.1016/j.visres.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Snyder LH, Calton JL, et al. Eye-hand coordination: Saccades are faster when accompanied by a coordinated arm movement. Journal of Neurophysiology. 2002;87(5):2279–2286. doi: 10.1152/jn.00854.2001. [DOI] [PubMed] [Google Scholar]
- Tabachnick FG, Fidell LS. Using Multivariate Statistics. Fifth Edition. Pearson Education, Inc; 2006. p. 330. [Google Scholar]
- van der Heide JC, Otten B, et al. Development of postural adjustments during reaching in sitting children. Experimental Brain Research. 2003;151(1):32–45. doi: 10.1007/s00221-003-1451-3. [DOI] [PubMed] [Google Scholar]
- van Donkelaar P, Lee RG, et al. Control Strategies in Directing the Hand to Moving Targets. Experimental Brain Research. 1992;91(1):151–161. doi: 10.1007/BF00230023. [DOI] [PubMed] [Google Scholar]
- van Donkelaar P. Eye-hand interactions during goal-directed pointing movements. Neuroreport. 1997;8(9–10):2139–2142. doi: 10.1097/00001756-199707070-00010. [DOI] [PubMed] [Google Scholar]
- van Donkelaar P, Siu KC, et al. Saccadic output is influenced by limb kinetics during eye-hand coordination. Journal of Motor Behavior. 2004;36(3):245–252. doi: 10.3200/JMBR.36.3.245-252. [DOI] [PubMed] [Google Scholar]
- van Donkelaar P, Staub J. Eye-hand coordination to visual versus remembered targets. Experimental Brain Research. 2000;133(3):414–418. doi: 10.1007/s002210000422. [DOI] [PubMed] [Google Scholar]
- van Donkelaar P, Lee RG, et al. The Contribution of Retinal and Extraretinal Signals to Manual Tracking Movements. Experimental Brain Research. 1994;99(1):155–163. doi: 10.1007/BF00241420. [DOI] [PubMed] [Google Scholar]
- von Hofsten C. Eye-hand coordination in the newborn. Developmental Psychology. 1982;18:450–461. [Google Scholar]
- von Hofsten C. Developmental changes in the organization of pre-reaching movements. Developmental Psychology. 1984;3:378–388. [Google Scholar]
- von Hofsten C. Studying the development of goal-directed behavior. In: Kalverboer AF, Hopkins B, Geuze R, editors. Motor development in early and later childhood:longitudinal approaches. Cambridge, UK: Cambridge University; 1993. pp. 109–124. [Google Scholar]
- von Hofsten C, Rosander K. Development of smooth pursuit tracking in young infants. Vision Research. 1997;37:1799–1810. doi: 10.1016/s0042-6989(96)00332-x. [DOI] [PubMed] [Google Scholar]
- Witherington DC, Von Hofsten C, et al. The development of anticipatory adjustments in infancy. Infancy. 2002;3:495–517. [Google Scholar]


