Abstract
Rabbits are widely used for vaccine development, and investigations of human infectious and autoimmune diseases such as Systemic Lupus Erythematosus (SLE). For these applications, we cloned, sequenced and expressed rabbit B-Cell Activating Factor (BAFF), and localized BAFF in cells and tissues of the rabbit immune system. The rabbit homolog of the human BAFF binding site (miniBR3 peptide) within the BAFF-specific receptor BR3 was synthesized. This 26-residue core domain binds to recombinant rabbit BAFF protein. Flow cytometric analyses using purified recombinant rabbit BAFF combined with real-time PCR findings revealed that BAFF detected on peripheral blood B cells from normal rabbits is probably complexed to BAFF receptors rather than produced by the B cells. BAFF was detected in developing appendix of young rabbits by immunohistochemical staining suggesting that BAFF plays a role during the period following birth when rabbit B-cell development and pre-immune antibody repertoire diversification and selection is occurring.
Keywords: MiniBR3, appendix, gut-associated lymphoid tissue, autoimmune diseases, vaccine development
Introduction
Members of the TNF/TNF-R superfamily have been shown to play significant roles in B-cell differentiation. B-cell activating factor of the TNF family (BAFF; also termed TNFSF13b, BLyS, TALL-1, and zTNF4) is a TNF-like cytokine that is essential for the survival and homeostasis of B lymphocytes [1–3] (reviewed in [4] and cited articles). BAFF is a type II transmembrane protein but also becomes a soluble ligand after cleavage at the cell surface by a furin-like protease. BAFF was initially found to be expressed and secreted by cells of the myeloid lineage [5], but there have been recent reports that BAFF is also expressed by neutrophils [6], neoplastic B cells [7, 8], activated mouse B cells [9], T cells from patients with autoimmune disorders [10], as well as by non-hematopoietic cells including astrocytes [11], and fibroblast-like synoviocytes of mesenchymal origin [12]. These mesenchymal-derived cells were shown to express functional BAFF in vitro after induction with proinflammatory cytokines such as IFN-γ and TNF-α[12]. Although BAFF is essential for development and maintenance of most B-cells, CD5-positive B-1 cells develop in mice lacking either BAFF or BAFF-receptor (BR3) [13,14]. Since BAFF was dispensable for B-1 B-cell development in mice, we investigated whether functional BAFF and it receptors are present in rabbits where the majority of B cells are CD5 positive [15]. We report here, comparative sequence and expression analyses of rabbit BAFF, and BR3 and production of recombinant BAFF (rBAFF) protein.
BAFF can bind to three distinct receptors, the BAFF receptor (BR3, BAFF-R), the transmembrane activator and calcium-modulator and cyclophilin ligand interactor (TACI) and the B cell maturation Ag (BCMA)[16,17]. BR3 binds BAFF with high affinity and is considered the only BAFF-specific receptor [18]. Failure of survival of peripheral B cells from immature transitional to mature naïve stages is observed in mice lacking BAFF or with defective BR3 [19]. TACI and BCMA bind BAFF with intermediate and low affinity, respectively. TACI plays a key role in negatively regulating mature B-cell homeostasis, but TACI also plays an important role in T-cell independent B-cell responses and class switch recombination [20,21]. Much less is known about BCMA, but recent reports suggest a role of BCMA in controlling the lifespan of long-lived plasma cells [22]. All three receptors are mainly expressed on B cells, but their expression levels change with B cell maturation.
The early development of B cells in the rabbit initiates in sites including fetal liver, omentum and bone marrow (reviewed in Mage et al.)[23]. After birth, the pre-immune antibody (Ab) repertoire is expanded and diversified in gut-associated lymphoid tissue (GALT). The molecular events that occur during Ab diversification have been intensively studied in rabbit, but less is known about the control at functional checkpoints and B-cell survival.
The present report provides the basis for further studies of the function of BAFF and its receptors in rabbit immune responses that are particularly needed now because in addition to being a valuable resource for development of diagnostic and therapeutic antibodies, rabbits are important for vaccine development and as animal models of human diseases. Our laboratory has described a model of the autoimmune disease SLE in allotype-defined pedigreed rabbits [24, 25] and (Yang, J. et al. ms in preparation). Before we could investigate the specific roles of BAFF and its receptors in rabbit B-cell development, maturation and Ab production in normal and autoimmune animals, it was necessary to first determine their cDNA and encoded protein sequences and their expression patterns in hematopoietic cells. In this study, we used a cross-species comparison strategy to design PCR primers, amplify, clone, and sequence rabbit BAFF and BR3. We found BAFF protein in developing appendix of young rabbits suggesting that BAFF plays a role during the period following birth when B-cell development, pre-immune Ab repertoire diversification, selection and expansion is occurring. We quantitated BAFF and BR3 mRNA expression and detected staining patterns on peripheral blood mononuclear (PBMC) and spleen cells of adult rabbits, expressed recombinant rabbit BAFF protein and utilized the protein for further investigations of BAFF and BR3 interactions.
Materials and methods
Animals
Rabbits were obtained from the allotype-defined pedigreed colony maintained at the National Institute of Allergy and Infectious Diseases. The animal studies described here were reviewed and approved by the animal care and use committees of NIAID/NIH (ASP LI6) and of the Spring Valley Laboratories, Inc. where the NIAID allotype-defined rabbit colony was housed. Normal adult rabbits of known Ig allotypes from our breeding colony, provided whole blood and spleens for flow cytometry, immunohistochemistry, and mRNA detection. In addition, one spleen from a 7-week old rabbit and appendix tissues of young rabbits at 4, 8 and 10 days of age were studied by immunohistochemistry.
Abs and reagents for flow cytometry, cell isolation and ELISA
BAFF was detected using biotin-conjugated goat anti-human BAFF polyclonal Ab (pAb) (Antigenix America Inc.) and rat anti-mouse BAFF monoclonal antibody (mAb) (clone 121809 R&D Systems) that we found to cross-react with rabbit BAFF. BR3 was detected using purified goat anti-human BR3 pAb (R&D Systems) that also cross-reacts with rabbit BR3. Also used were FITC-labeled mouse anti-rabbit CD14 (clone K4), FITC-labeled mouse anti-rabbit CD4 (clone KEN-4), FITC-labeled mouse anti-rabbit CD8 (clone C7) (Antigenix America Inc.), FITC-labeled goat anti-rabbit IgM, FITC-labeled goat anti-rabbit IgG (Southern Biotechnology Associates), biotin-labeled donkey anti-goat IgG, biotin-labeled goat IgG, normal goat IgG (Jackson ImmunoResearch Laboratories, Inc.), and FITC-labeled mouse IgG2a (BD Pharmingen). Biotinylated Abs were visualized by phycoerythrin-(PE)-conjugated streptavidin (Jackson ImmunoResearch Laboratories, Inc).
Isolation of cell subpopulations
For quantitative analyses of mRNA expression in cell subpopulations, spleen tissues were chopped in small pieces in PBS and pressed through a stainless steel mesh screen with rubber-tipped syringe plunger. Debris and cell clumps were removed by passing the suspension through 70 μm nylon mesh. Freshly isolated spleen cells were stained with mouse anti-rabbit CD14 (Antigenix America) or mouse anti-rabbit CD4 (Serotec) Ab and CD4+ or CD14+ cell subpopulations were isolated with goat anti-mouse IgG covalently bound to Dynabeads (Dynal). To isolate IgM positive B cell subpopulations, cells were stained with biotin-conjugated polyclonal μ-heavy chain specific goat anti-rabbit IgM (Southern Biotechnology Assoc.) followed by Streptavidin-Dynabeads. Viable peripheral blood mononuclear cells (PBMC) were isolated from heparinized blood by density gradient centrifugation on Lympholyte-Mammal (Cedarlane Laboratories Limited). This permitted recovery of lymphocytes and monocytes but eliminated most red cells and granulocytes. IgM positive subpopulations from PBMC were isolated as described above. IgM depleted PBMC (IgM− cells) were then stained with mouse anti-rabbit CD14 mAb and separated into two populations, IgM−CD14+ and IgM−CD14− using anti-mouse IgG-Dynabeads. Upon analyses by flow cytometry, fractions were shown to be at least 90% pure. After isolation, cells were immediately placed into TRIzol™ reagent (Invitrogen) for RNA isolation.
Rabbit BAFF and BR3 cloning
Total RNA was obtained from normal rabbit PBMCs that were immediately placed in TRIzol reagent. PBMC were homogenized and filtered with Qiashredder column (Qiagen). Total RNA was isolated by RNeasy spin column (Qiagen) and precipitated with ethanol. First-stand cDNA was synthesized using the SuperScript first-strand synthesis kit (Invitrogen). The BAFF primers (Table 1A) were designed after searching the rabbit whole genome shotgun (WGS) trace archives database and aligning the sequence with the highly conserved BAFF domains of multiple species. The cDNA was amplified by Platinum® pfx DNA polymerase (Invitrogen). PCR conditions were first melting at 94°C for 2 min, then 30 cycles of amplification: 15 sec at 94°C, 30 sec at 55°C, and 1 min at 68°C. A final extension was at 72°C for 10 min. The rabbit BR3 primers (Table 1A), were designed based on the ‘working draft’ sequence of genomic DNA in Oryctolagus cuniculus clone LB1-145O11 (AC145540.1) positions 124760 to 123559 on the reverse strand, that appears to contain the sequence of the rabbit homolog of human BR3. Touchdown PCR conditions were melting at 94°C for 30 sec, annealing and elongation at 72°C for 3 min. The annealing temperature was dropped from 72°C at a rate of 2°C for five cycles to 68°C for the remaining 30 cycles. The rabbit BAFF PCR products were cloned into pCR-Blunt II-TOPO (Invitrogen), and sequenced from SP6 and T7 promoter sites with SP6 and T7 primers. The rabbit BR3 PCR products were cloned into pCR 2.1 vector (Invitrogen), and sequenced from the M13 reverse priming and T7 promoter sites with M13 reverse and T7 primers. At least three independent PCR cloning and sequencing experiments were conducted to rule out errors introduced by PCR.
Table 1.
Primers and probes for cloning, expression and quantitation of rabbit BAFF and BR3
| A. Primers for cloning rabbit BAFF and BR3 | |
| Name | Sequence |
| BAFF forward | 5′ ATGGATGACTCCACGGAAAGGGA 3′ |
| BAFF reverse | 5′ TCACAACAGCTTCAGTGCACCGA 3′ |
| BR3 forward | 5′ ATGAGGCGAGGAGGACGCA 3′ |
| BR3 reverse | 5′ CTACTGTTGCTCAGGGCCGGCCGTCTT 3′ |
| B. Primers for construction of pCEP4-BAFF mammalian expression vectora | |
| P1 | 5′ AGCAGCCACAGGAGCTCACTCCGAGCACCATCACCATCACCATGCCGTTGAGGGTGTGGAAGA 3′ |
| P2 | 5′ GggtaccATGGACTGGACCTGGAGGATCCTCTTCTTGGTGGCAGCAGCCACAGGAGCTCACT 3′ |
| P3 | 5′ GAGActcgagTCACAACAGCTTCAGTGCA 3′ |
| C. Primers and probes for detection of rabbit BAFF and BR3 mRNA by Q-PCR | |
| BAFF forward | 5′ TGGTCAAAGAAACTCGGGTACTT 3′ |
| BAFF reverse | 5′ TGTTTTGAATGCAACGGAACA 3′ |
| BAFF probe | 5′ TCATATACGGTCAGGTCTT3′ |
| BR3 forward | 5′ CGGGACGGAGACCAGGA 3′ |
| BR3 reverse | 5′ TGAGTTGGGAGCTGTGGCA 3′ |
| BR3 probe | 5′ AGTCCCTGGATGATGTCA 3′ |
| PPIA forward | 5′ CAACACAAATGGCTCCCAGTT 3′ |
| PPIA reverse | 5′ CATGGCTTCCACAATGCTCAT 3′ |
| PPIA probe | 5′ ATCTGCACTGCCAAGAC 3′ |
Sequences in lower case indicate KpnI and XhoI sites. Sequences in italics indicate signal peptide, underlined (His)6 tag, in bold from rabbit BAFF extracellular domain. Sequences in red are overlapping segment for first- and second-round PCR.
Construction, expression, and purification of recombinant rabbit BAFF protein
A construct was designed with a signal sequence of human Ig heavy chain (VH1), a (His)6 tag at the N-terminus, followed by the cDNA sequence of the predicted rabbit BAFF extracellular domain (amino acids 139-290) and a stop codon. The desired product was amplified by two-rounds of PCR from the pCR-Blunt II-TOPO-BAFF construct using primers shown in Table 1B. After the second-round overlapping PCR, the specific DNA fragment was cloned by KpnI/XhoI ligation into the mammalian cell expression vector pCEP4 (Invitrogen). Following transient transfection, the (His)6-tagged rabbit BAFF protein was expressed and secreted by HEK293F cells (Invitrogen) cultured in free-Style serum-free medium (Invitrogen). Supernatants were collected after 3, 6 and 9 days of culture by centrifugation, filtered through a 0.45-μm membrane, and concentrated tenfold using an ultrafiltration device with a 5-kDa cutoff membrane (Millipore). The concentrate was loaded on a 1-ml HisTrap FF crude column (GE Healthcare) and washed with 40-volumes of washing buffer (PBS containing 500 mM NaCl and 30 mM imidazole, pH 7.4). The purified protein was eluted with elution buffer (PBS containing 500 mM NaCl and 500 mM imidazole, pH 7.4), the imidazole was immediately removed and the protein was concentrated using 5-kDa cut off centrifugal filters (Millipore). The quality and quantity of purified rabbit recombinant BAFF protein ((His)6rBAFF) was monitored by SDS-PAGE, Western blotting and A280 absorbance.
Synthesis of miniBR3
Biotinylated miniBR3 [26] was synthesized as the C-terminal amide on a 433A peptide synthesizer (Applied Biosystems) using standard Fmoc (fluorenylmethoxy carbonyl) chemistry[26] (Research Technology Branch, NIAID Peptide Synthesis and Analysis Unit). MiniBR3 was biotinylated at the amino terminus while on the resin using NHS-LC-Biotin reagent (Pierce). The peptide was cleaved from the resin using a mixture of 92% trifluoroacetic acid (TFA; Aldrich), 5% thioanisole (Aldrich), and 3% 3,6-dioxa-1,8-octanedithiol (DODT, Aldrich) for 2.5 hr at room temperature. After removal of TFA by rotary evaporation, the peptide was precipitated by addition of methyl t-butyl ether (MTBA, Burdick & Jackson), then purified by reversed-phase HPLC (acetonitrile/H2O/0.1% TFA). Peptide identity was confirmed by MALDI-TOF mass spectrometry. Spontaneous folding of the fully unprotected peptide in aqueous solution under redox control of reduced and oxidized glutathione was carried out as described [27]. The progress of the oxidation was monitored by analytical HPLC, and the final product was again purified by HPLC.
ELISA
Purified rabbit (His)6rBAFF protein was analyzed by sandwich-ELISA. Briefly, polystyrene 96-well plates (Corning) were coated with 50 μl/well of purified goat anti-human BAFF polyclonal Ab (Antigenix America Inc.) at 2 μg/ml in bicarbonate buffer (pH 9.6) and incubated overnight at 4 °C. All subsequent incubations were done for 1 h at room temperature. Plates were washed six times with 1x TBS (pH 7.4) and blocked with 100 μl of SuperBlock T20 PBS Blocking Buffer (Pierce). Wells were then incubated with serial tenfold-diluted BAFF protein or with serial tenfold-diluted human BAFF recombinant protein as a control. After washing six times with 1x TBS (pH 7.4), wells were further incubated with 50μl/well of 1/1000 diluted rat anti-mouse BAFF mAb (clone 121809, R&D Systems), then detected by horse radish peroxidase-(HRP)-conjugated goat anti-rat IgG secondary Ab (Jackson ImmunoResearch Laboratories). After washing as above, wells were developed with 3,3′, 5,5′-tetramethylbenzidine (TMB) (INOVA Diagnostics) and the resulting OD read at 450 nm. In order to detect the interaction between rabbit (His)6rBAFF and the synthesized rabbit miniBR3 homolog, rBAFF or control rabbit (His)6rCD5 [28] (600 ng/well) in carbonate buffer (pH 9.6) were coated on plates at 4 °C overnight. After washing as above, the plates were blocked with 100 μl of blocking buffer (Pierce). Wells were incubated with serial twofold dilutions of biotin-labeled miniBR3. After washing as above, wells were incubated with 40 mU/ml of streptavidin-β-peroxidase (POD) conjugate (Roche), developed with TMB and the resulting OD read at 450 nm.
Western blotting
Purified expressed rabbit (His)6rBAFF and control human (hBAFF) (Antigenix) protein were first analyzed using SDS-PAGE 4–15% Tris-HCl Ready gels (Bio-Rad, Hercules, CA) and stained with Coomassie blue. Additional separate sets of SDS gels were electrophoretically transferred onto nitrocellulose membranes and analyzed using cross-reacting biotin-conjugated polyclonal goat anti-human BAFF Ab (Antigenix) and BM Chemiluminescence Blotting kit according to the manufacturer’s instructions (Roche).
Detection of expression levels of BAFF and BR3 mRNA
Quantitative PCR to determine the relative expression levels of BAFF and BR3 mRNA was performed on a 9700HT Sequence Detection System (Applied Biosystems). Synthesized cDNA from isolated PBMCs, and subpopulations of PBMCs and splenocytes was directly used as template for real time PCR using TaqMan 2x PCR Master Mix Reagents Kit (Applied Biosystems). The total volume of the PCR was 25 μl and the PCR conditions were: 50°C for 2 min, 95°C for 10 min, followed by 40 cycles of 95°C, 15 s for denaturation and 60°C, 1 min for annealing and extension. The primers and probes for BAFF and BR3 and the primers for the control housekeeping gene Peptidylprolyl isomerase A (PPIA) are shown in Table 1C. Each sample from three independent experiments was run in duplicate. The unit number showing relative mRNA levels in each sample was determined as a value of mRNA normalized against PPIA. Previous studies in our laboratory (Rai G et al., ms in preparation), showed that there was very uniform expression of PPIA in PBMCs collected from rabbits at different time points prior to and after immunization.
Flow cytometry
Purified cells were stained using standard flow cytometric methodology. Briefly, cells were incubated on ice for 40 min with primary Ab before washing twice with cold PBS containing 1% FCS, followed by incubation with various secondary reagents or secondary Ab. A biotinylated donkey anti-goat IgG Ab (Jackson ImmunoResearch Laboratories, Inc) was used as secondary Ab to detect BR3 on cells stained with purified goat anti-human BR3 Ab (R&D Systems) that cross-reacts with rabbit BR3. Biotinylated Abs were visualized by PE-conjugated streptavidin (Jackson ImmunoResearch Laboratories, Inc). After washing, cells were analyzed using a FACS-Calibur flow cytometer (BD Pharmingen) and FlowJo analytical software (Tree Star). In order to test whether recombinant rabbit BAFF (rBAFF) would influence staining by anti-BAFF, a fixed quantity of biotin-labeled goat anti-human BAFF polyclonal Ab (Antigenix America Inc.) was incubated with different dosages of rBAFF protein on ice for 40 min, then transferred to isolated PBMC for another 30 min on ice. In additional experiments, different dosages of rBAFF were incubated with isolated rabbit PBMC on ice for 40 min. After washing, a fixed amount of biotin-labeled goat anti-human BAFF pAb was added. Biotinylated Ab was visualized by PE-conjugated streptavidin (Jackson ImmunoResearch Laboratories, Inc).
Immunohistochemistry
Cryostat serial sections (7 μm) of rabbit appendix tissues were cut and stained with primary reagents, mouse anti-human CD79a specific for the cytoplasmic domain that cross reacts with rabbit (BD Pharmingen), mouse anti-rabbit CD5 mAb (clone 2B10) [28] followed by biotin-conjugated goat anti-mouse IgG (Jackson ImmunoResearch Laboratories, Inc.) or with cross-reacting biotin-conjugated goat anti-human BAFF pAb (Antigenix). The sections were then incubated with ABC-Alkaline phosphatase kit, labeled cells visualized by Vector blue alkaline phosphatase substrate kit III, and counterstained with Nuclear Fast Red (Vector, Laboratories, Inc.). In other experiments, spleen sections were first stained with mouse anti-rabbit CD14 (Antigenix America) or mouse anti-rabbit CD4 (Serotec) Ab followed by peroxidase-conjugated goat anti-mouse IgG Ab (Jackson ImmunoResearch Laboratories, Inc.) and ABC-Peroxidase kit. Labeled cells were visualized by ABC-DAB substrate kit III. Sections were then stained with biotin-conjugated polyclonal goat anti-human BAFF Ab followed by ABC-Alkaline phosphatase kit, labeled cells were visualized by Vector blue alkaline phosphatase substrate kit III and counterstained with Nuclear Fast Red (Vector, Laboratories, Inc.).
Results
Rabbit BAFF and BR3 cloning and sequence comparison
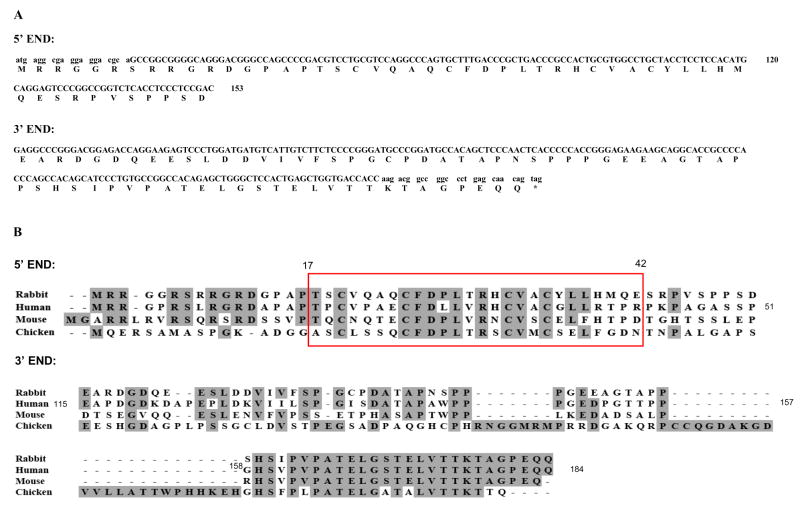
The full-length rabbit BAFF, part of BR3 cDNA sequences obtained by reverse transcriptase PCR and the conceptual amino acid sequences deduced from the cDNA sequences are shown in Figures 1A and B and Figure 2. At the protein level, full-length rabbit BAFF is five amino acids longer than human BAFF and there are two potential N-linked glycosylation sites (asterisks in Figure 1A) similar to those found in human BAFF. Because rabbit BAFF protein also contains a furin-like protease site (arrow in Figure 1A), rabbit BAFF cleaved from the membrane is predicted to become a soluble protein with a molecular weight of ~17 kDa. The deduced conceptual BAFF protein sequence we found differs by four amino acids from that reported by Guan et al. [29] (L79>P; E187>V; S209>N; P269>L). There are two amino acids not predicted by automated analysis of the low coverage trace archives of rabbit (2x) genome sequence (AAGW01000000) in Ensembl (G118 and K186) (Figure 1B). The alignments of full-length rabbit BAFF with BAFF sequences from human, mouse, and chicken are shown in Figure 1C. Rabbit BAFF cDNA sequences are 84.7 %, 65.8%, and 56.6% identical to those of human, mouse, and chicken, respectively. At the protein level, rabbit BAFF amino acid sequences shown are 82.4%, 58.8%, and 47.7% identical to human, mouse, and chicken BAFF, respectively.
Figure 1.
BAFF Sequences. (A) DNA and deduced protein sequences of rabbit BAFF. The predicted transmembrane domain (line), the potential N-glycosylation sites (stars), and the predicted natural processing site of rabbit BAFF (arrow) are indicated. Lowercase letters at the 3′ and 5′ ends indicate the location of the primer sequences used for initial PCR amplification. Our BAFF sequence was submitted to GenBank and given accession number EU982819. (B) Comparison of the predicted rabbit BAFF protein sequences from our study (Yang), reported paper (Guan) [29], and Ensembl (Ensembl). Identical residues are represented in black boxes. (C) Comparison of the BAFF protein sequences among different species. Identical residues are represented in shaded areas.
Figure 2.
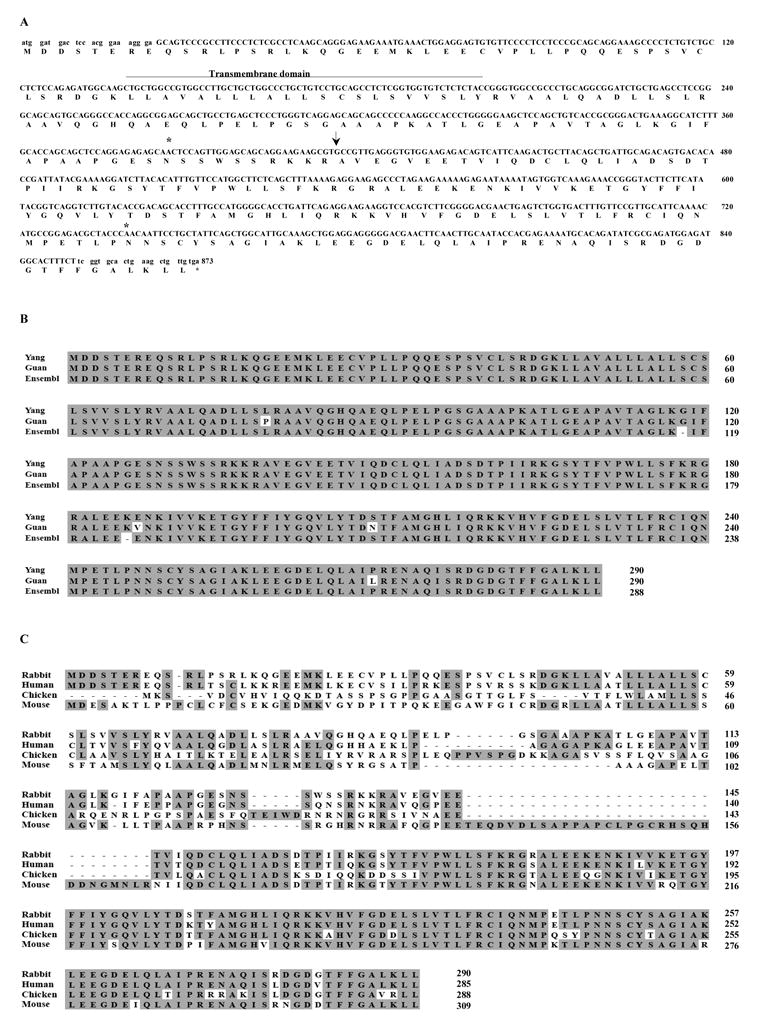
BR3 Sequences. (A) DNA and deduced protein sequences of rabbit BR3 5′-end (BAC AC145540.1 124760-124625 and 124437-124421 on reverse strand) and 3′-end (BAC AC145540.1 124150-124126 and 123740-123559 on reverse strand). Lowercase letters at the 3′ and 5′ ends indicate the location of the primer sequences used for initial PCR amplification. (B) Comparisons of rabbit BR3 protein sequences with corresponding sequences from human, mouse and chicken. Identical residues are represented in shaded areas. Red box indicates the amino acid sequences of miniBR3 homologs among different species. Amino acid numbers are shown based on the human protein sequence (GenBank accession AAK91826.1 GI:15208475) [17].
By cDNA cloning, we confirmed the sequences in BAC clone LB1-145O11 (AC145540.1) at the 5′-end and 3′-ends of rabbit BR3 that contain predicted exon 1 and the last exon of rabbit BR3 (Figure 2A). The alignment of rabbit BR3 encoded protein sequence with corresponding sequences from human, mouse, and chicken is shown in Figure 2B. Studies of human BR3 [26] reported that the BAFF binding site is contained within a 26-residue core domain (amino acids 17-42) TPCVPAECFDLLVRHCVACGLLRTPR stabilized by two disulfide bonds connecting Cys19/Cys32 and Cys24/Cys35. This miniBR3 was reported to not only adopt essentially the same structure as the full-length protein in that region, but also to bind to BAFF with the same affinity as full-length BR3 (~ 70 nM IC50). Our reported rabbit BR3 5′-end sequence encodes a similar miniBR3 core domain and conserved Cys19, Cys32, Cys24, and Cys35 residues (enclosed in a box in Figure 2). The rabbit BR3 5′-end sequence (Figure 2A) has 60.8% amino acid identity to human, 42.6% to mouse, and 32.7% to chicken BR3 and 3′-end sequence (Figure 2B) has 74.3% amino acid identity to human, 61.8% to mouse, and 26.7% to chicken BR3.
Characterization of recombinant BAFF and miniBR3
A diagram of the construct used for expression of the extracellular domain of rabbit BAFF is shown in Figure 3A. Western blotting of purified rabbit (His)6rBAFF detected a specific band at ~17 kDa (Figure 3B). The purified rBAFF was further analyzed by ELISA (Figure 3C). In a sandwich ELISA, (left panel), purified rBAFF could be captured by goat anti-human BAFF pAb and detected by rat anti-mouse mAb in a linear manner comparable to the human BAFF control. In order to prove the specificity of BAFF and BR3 interaction, 96-well plates were coated with (His)6rBAFF or control (His)6rCD5 overnight. Then serial two-fold dilutions of biotin-labeled synthesized rabbit miniBR3 peptide were added and detected by streptavidin-peroxidase peptide (right panel). The data clearly show that our synthesized miniBR3 peptide bound to (His)6rBAFF in a dose-dependent manner and not to control (His)6rCD5 at concentrations between 0.015–0.030 and 3.75 μg/ml although at higher excess concentrations, miniBR3 binding was non-specific.
Figure 3.
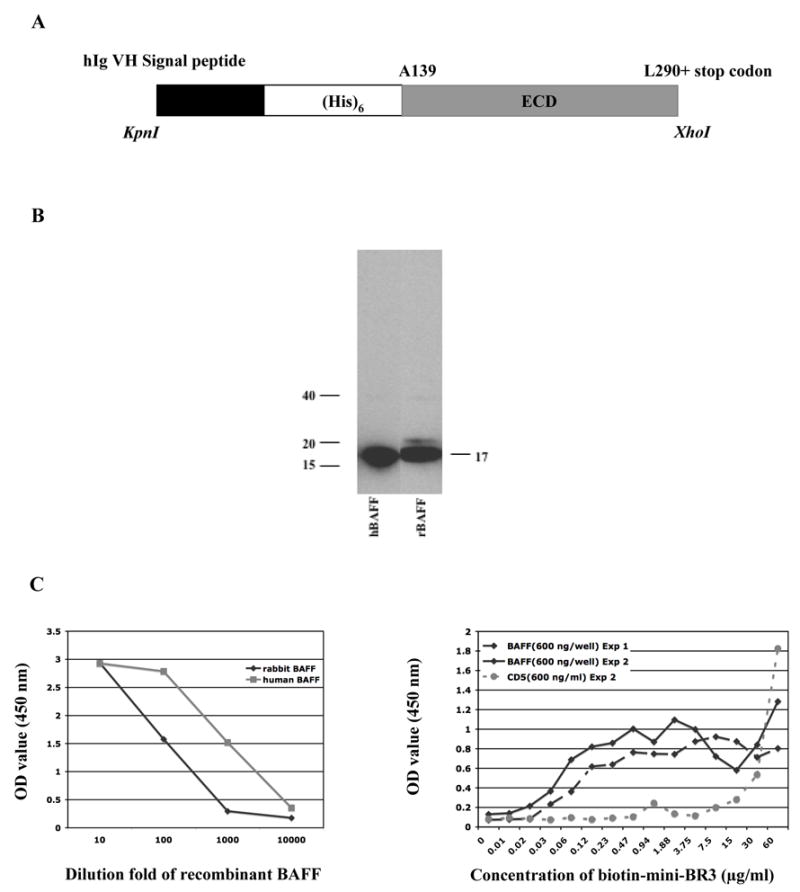
Characterization of recombinant rabbit BAFF and a synthetic rabbit miniBR3 homolog. (A) Schematic representation of BAFF expression construct. The construct encodes a human Ig heavy chain N-terminal signal peptide, a (His)6 tag, and the soluble recombinant extracellular domain of BAFF starting at Ala 139 (ECD). The construct was cloned by KpnI/XhoI ligation into the mammalian cell expression vector pCEP4. (B) Western blotting of purified recombinant rabbit BAFF (rBAFF). Recombinant human BAFF (hBAFF) was used as positive control for the detection of rabbit BAFF. (C) Left panel. For sandwich-ELISA, a 96-well plate was coated with goat-anti-human BAFF pAb overnight, then serial tenfold dilutions of recombinant rabbit BAFF were added. Rat-anti-mouse BAFF mAb was added and detected by HRP-conjugated goat anti-rat pAb. Recombinant human BAFF (hBAFF) was used as positive control. (C) Right panel. ELISA studies of the specificity of BAFF and BR3 interaction. In an initial experiment, (Exp 1), 96-well plates were coated with (His)6rBAFF overnight. In a second experiment, (Exp 2), plates were coated with (His)6rBAFF or control (His)6rCD5. In both experiments, serial two-fold dilutions of biotin-labeled rabbit miniBR3 homolog were then added and detected by streptavidin-peroxidase. Data shown have background OD 450 subtracted. The background was below 0.1.
Immunohistochemical detection of BAFF protein in B-cell areas of developing neonatal rabbit appendix
We stained sections from frozen 4-, 8- and 10-day-old rabbit appendix with mouse anti-CD79a mAb (Figure 4, left panels), goat anti-human BAFF pAb (middle) or mouse anti-rabbit CD5 mAb (right). We previously reported that B cells exhibiting VHa allotype develop later in some follicles than in the others in parallel with CD5+ cells.30 Here we show that BAFF+ and CD5+ cells also appear to develop in parallel. Particularly at day 8, some follicles that already show presence of cells of the B-lineage detected by anti-CD79a, have less evident staining by both anti-BAFF and anti-CD5 (arrows in day 8 panels).
Figure 4.

Immunohistochemical staining of serial sections from frozen 4, 8 and 10-day-old rabbit appendix. Serial sections were incubated with mouse anti-CD79a or mouse anti-rabbit CD5 mAbs followed by biotin-conjugated goat anti-mouse IgG and ABC-Alkaline phosphatase kit or with biotin-conjugated polyclonal goat anti-human BAFF Ab. Labeled cells visualized by Vector blue alkaline phosphatase substrate kit III and counterstained with Nuclear Fast Red. Positive cells are stained blue. Note that BAFF+ and CD5+ cells develop in some follicles but not in the others (arrows). Scale bar represents 200 μm.
Detection of BAFF and BR3 protein on the surface of cell populations in peripheral blood and spleen by flow cytometry
We stained for BAFF on various cell populations to further determine which cell subsets in PBMC and spleen had detectable surface BAFF using cross-reactive anti-human reagents. In peripheral blood (Figure 5A upper panels), a high percentage of IgM+ B cells (~91%), monocytes (~75% of CD14+ cells) and some IgG+ B cells had detectable surface BAFF. Although the proportions of CD4+ T cells recovered from peripheral blood were less than 5% of total PBMC, we also observed that some CD4+ T cells stained positively for BAFF protein on their cell surface. Estimates of percentages of BAFF positive cells in the gated populations of PBMC that were IgG+ or CD4+ and all cells from spleen cannot be made accurately because fluorescent intensities were low when using these cross-reactive reagents. We lacked the necessary reagents to conduct BR3 double staining of B cells but detected a large proportion of CD14+ monocytes that expressed BR3 (Figure 5E, left) and BR3 can also be detected on some CD4+ T cells in peripheral blood (Figure 5E, right).
Figure 5.
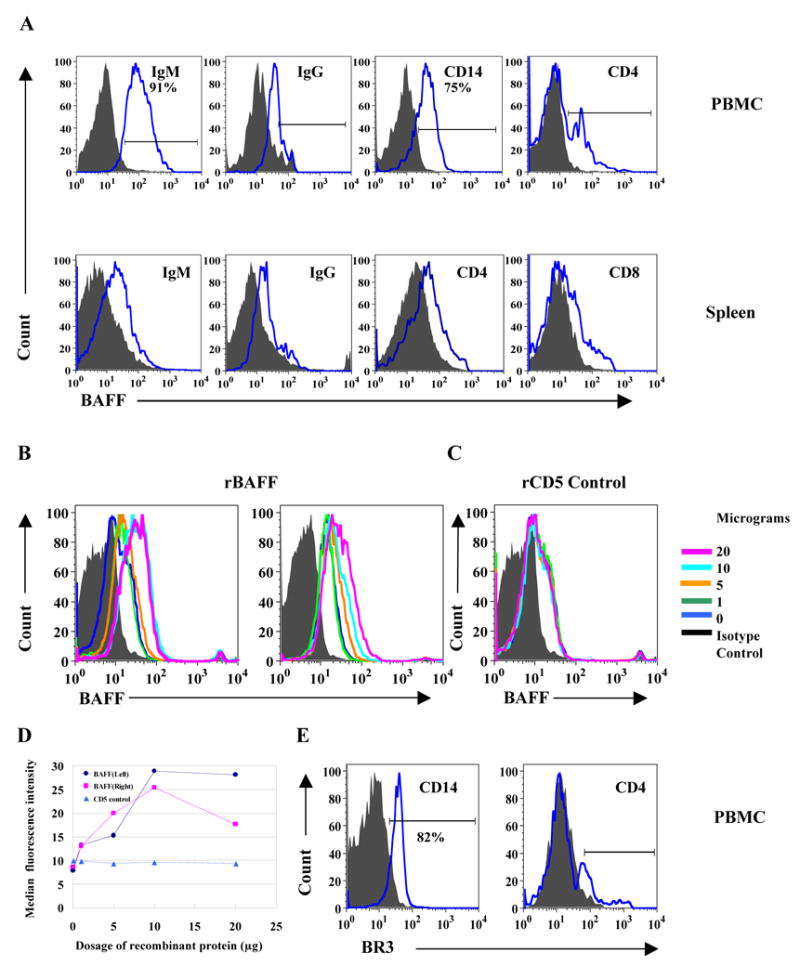
Rabbit BAFF and BR3 protein detected on the surfaces of PBMC and spleen. (A) Representative histograms showing BAFF protein on the surfaces of gated cell populations from PBMC (upper) and spleen (bottom). Data shown represent one of at least three rabbits studied. (B) Interaction of recombinant rabbit BAFF and BR3 on the cell membrane. (B left panel) A fixed quantity of biotin-labeled goat anti-human BAFF pAb was incubated with different dosages of recombinant BAFF protein on ice for 40 min, then directly transferred to isolated rabbit PBMC for further incubation on ice for 30 min. In the same experiment, addition of recombinant rabbit (His)6CD5 [28] as a negative control, had no effect (C). (B right panel) Different dosages of recombinant rabbit BAFF were incubated with isolated rabbit PBMC on ice for 40 min. After washing, a fixed amount of biotin-labeled goat anti-human BAFF pAb was added. (D) BAFF staining intensity was enhanced by addition of rabbit (His)6rBAFF in a dose-dependent manner. (E) BR3 protein on the surfaces of CD14+ cells and CD4+ T cells from PBMC. Data shown represent one of at least three rabbits studied.
Recombinant BAFF binds to PBMC
In the experiments shown in Figure 5B (left panel), we first incubated a fixed amount of biotin-labeled goat anti-human BAFF pAb with different dosages of rBAFF on ice for 40 min, then transferred to isolated PBMC for another 30 min. Instead of inhibiting the binding of goat anti-human BAFF Ab to membrane associated BAFF, the staining intensity of BAFF was significantly increased as compared to no addition of rBAFF or addition of isotype control (Figure 5B, left panel). In the same experiment, addition of recombinant rabbit (His)6CD5 as a negative control, had no effect (Figure 5C). We further investigated the observation that addition of rBAFF increased anti-BAFF binding to the cell membrane. PBMC were incubated with different dosages of rBAFF and after washing, a fixed amount of biotin-labeled goat anti-human BAFF Ab was added. Even after the washing step, BAFF staining intensity was enhanced by addition of rabbit (His)6rBAFF in a dose-dependent manner Figure 5B (right panel). Figure 5D shows the concentration dependent increases in median fluorescence intensities (MFI) under the two conditions compared to flat MFI for all concentrations of control (His)6rCD5. These results suggest that the BAFF detected on some PBMC could be bound to surface receptors rather than synthesized and expressed on the cell surface.
Measurement of rabbit BAFF and BR3 expression in mRNA extracted from normal PBMC and purified subpopulations
To further investigate whether the BAFF we detected by flow cytometry and immunohistochemistry was produced by these cells or simply complexed to its receptors, we purified IgM+ B cells and macrophage/monocytes (CD14+ cells) from PBMC and spleen and analyzed expression of BAFF and BR3 mRNA by real-time PCR. Figures 6A and B show the relative expression levels of BAFF and BR3 mRNA in PBMCs of 23 normal rabbits. The mean value is shown as the last bar in each graph. BAFF and BR3 mRNA were detected in PBMC from all normal rabbits and the relative expression levels of BR3 mRNA are ~20 fold higher than levels of BAFF mRNA (note the 20 fold difference in scales of panels A and B). Figures 6C and D show the relative expression levels of BAFF and BR3 mRNA in cells positive or negative for IgM and CD14 as well as splenic CD4+ T cells. From spleen, BR3 mRNA was highly enriched in the IgM+ B cell population, and was also detectable in CD4+ T cells, and CD14+ macrophage/monocytes. Levels of BAFF message in the identical samples (splenic mRNA from IgM+ B cells, CD4+ T cells, CD14+ macrophage/monocytes) were very low or below the limits of detection. From PBL, BR3 message was again found enriched in IgM+ B cells, and BAFF message was undetectable in the identical samples. BAFF mRNA was relatively abundant in the IgM−CD14+ cell fraction, and lower levels were present levels in IgM− CD14− cells. Low levels of BR3 mRNAs were found within the populations contained in the IgM− CD14− cell fraction, but as this is likely to be a heterogeneous group of cells, BAFF and BR3 mRNA may or may not be produced by the same cell type.
Figure 6.
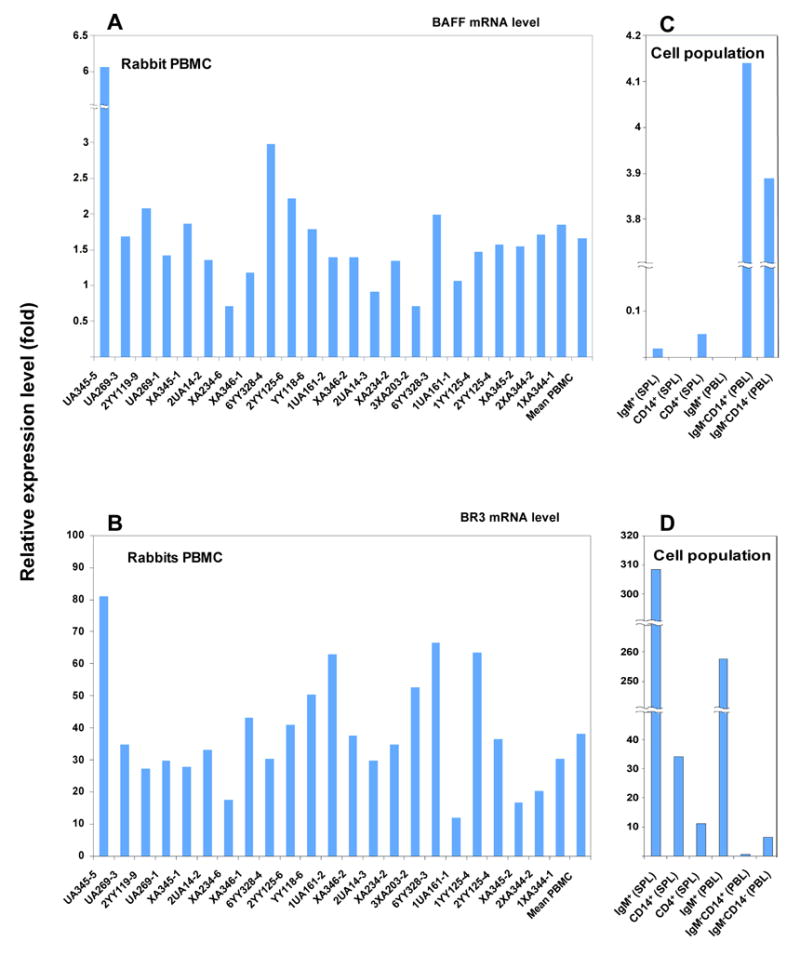
Results of quantitative real time PCR analyses. Q-PCR detecting BAFF (A) and BR3 (B) mRNA levels in Ab (PBMC, n=23) and isolated cell populations of normal rabbits (C, D). Isolated IgM−CD14+ cells and IgM−CD14− cells from peripheral blood are enriched for BAFF mRNA compared to total PBMC. Isolated IgM+ cells from peripheral blood and spleen are enriched for BR3 mRNA compared to total PBMC.
Detect of BAFF protein on cells in normal spleen by immunohistochemistry
Spleens from normal rabbits were first stained with biotin-labeled goat anti-human BAFF pAb (Figure 7A). In normal rabbit spleen, macrophages usually surround follicles that are mainly composed of CD5+ B cells (Figure 7B). Most cells both within and outside of follicles were BAFF+ (Figure 7B). Based on the structure of rabbit spleen shown in Figure 7B, BAFF is associated with most IgM+ B cells as well as with macrophage/monocytes in spleen. We further stained the spleen with mouse anti-rabbit CD14 (Figure 7C) and anti-CD4 (Figure 7D). Figures 7C and D showed that in normal spleen many CD14+ cells and a few CD4+ T cells also have detectable surface BAFF. Arrows in the high magnification panels point to double-stained cells where BAFF positive cells are stained blue. Double stained CD14 positive cells also appear brown and CD4 positive cells dark red. This supports our flow cytometry data that BAFF could be detected on the surface of both CD14+ and CD4+ T cells.
Figure 7.
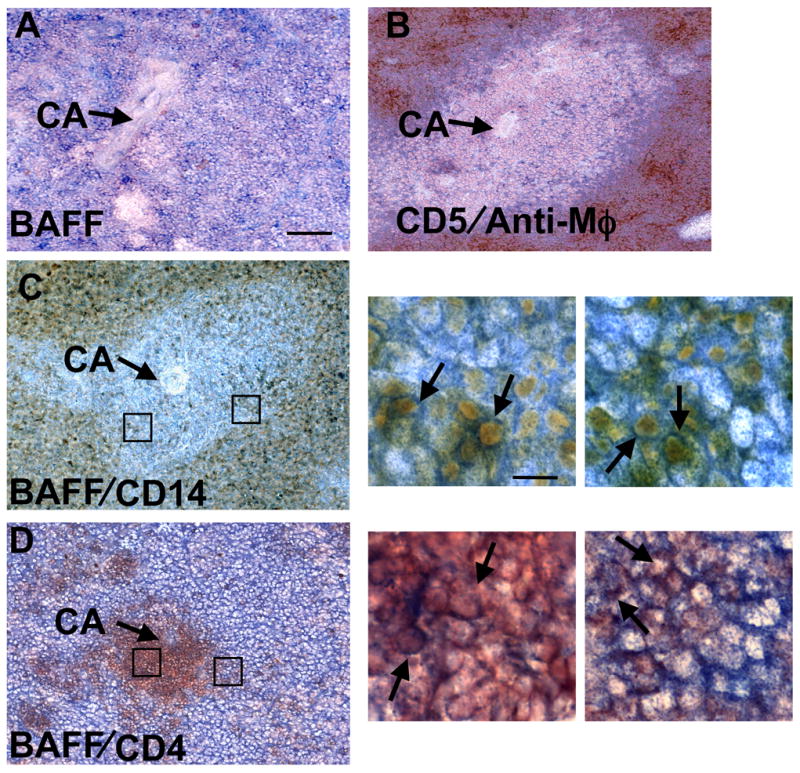
BAFF protein is found associated with B cells and monocytes in normal spleen. Detection of BAFF and other markers by immunohistochemistry. (A) Rabbit spleen section stained with biotin-conjugated polyclonal goat anti-human BAFF Ab followed by ABC-Alkaline phosphatase kit. Labeled cells were visualized by Vector blue alkaline phosphatase substrate kit III and counterstained with Nuclear Fast Red. BAFF-positive cells (blue) in follicles surrounding the central arteriole (CA) and splenic lymphocytes in red pulp are also stained blue. (B) Rabbit B cells (CD5+) detected by mouse anti-rabbit CD5 (blue), and macrophages surrounding follicles detected by mouse anti-rabbit macrophage (DAKO clone RAM11) (brown). (C) and (D). Double staining for rabbit BAFF in blue and CD14 (C) or CD4 (D) in brown. Prior to BAFF staining, tissue sections were first incubated with mouse anti-CD14 (C) or mouse anti-CD4 monoclonal Ab (D) followed by peroxidase-conjugated goat anti-mouse IgG. Labeled cells (brown) were visualized by ABC-DAB substrate kit III. Sections were then stained with biotin-conjugated polyclonal goat anti-human BAFF followed by ABC-Alkaline phosphatase kit. Labeled cells were visualized by Vector blue alkaline phosphatase substrate kit III and counterstained with Nuclear Fast Red. Scale bar for (A, B, C and D) represents 100 μm, and 10 μm in high magnification images. Boxes show regions displayed at high magnification captured from C and D in order from left to right. Arrows in high magnification panels point to examples of double-stained cells.
Discussion
BAFF and its receptors are important for B-cell homeostasis. Discovery of the BAFF system has provided immunologists with new insight into the mechanisms that control B-cell survival during maturation in the periphery. Although BAFF is a beneficial factor that promotes B-cell survival and enhances immune responses, excessive BAFF production seems to be able to subvert B-cell tolerance. Thus BAFF and its receptor expression must be carefully regulated, and decoy receptors based on TACI, BCMA, and BR3, or antibodies directly against BAFF, are potential therapeutics against B cell-mediated autoimmune diseases (reviewed in [31]). For studies conducted in rabbit models of infectious and autoimmune diseases, and for vaccine research, additional information about the roles of BAFF and its receptors in B-cell development and homeostasis in rabbits is needed.
A recent report of a rabbit BAFF cDNA sequence and distribution of mRNA, included no information about protein expression and distribution of rabbit BAFF and BR3 in hematopoietic cells [29]. The four amino acid replacements they reported (Figure 1B) may be due to differences between the Chinese (Jiangsu Academy of Agricultural Sciences) strain and the NIAID rabbit. However a third unrelated rabbit of the Thorbecke partially inbred strain was the donor of DNA for the Rabbit Genome sequence. This rabbit’s BAFF DNA sequence appears to encode amino acids identical to those we found.
Although only the 3′ end of the BR3 sequence was in the archives of the 2x sequence and assembly of the rabbit genome, we did find sequences corresponding to the entire putative rabbit BR3 homolog of human BR3 in the trace archive of the deeper coverage in progress to generate a high quality draft sequence. These sequences, although not available as an assembly yet, correspond well to those in BAC clone LB1-145O11 (AC145540.1) from a fourth unrelated rabbit. We documented (Figure 2B) that exon1 encoded a functional BAFF binding site (minBR3) as previously described for human BR3 [26] by ELISA using a biotin-labeled synthesized rabbit homolog of the miniBR3 peptide. Specific dose dependent binding to purified rabbit (His)6rBAFF was observed although non-specific binding to control (His)6rCD5 occurred at high concentrations where binding to rBAFF also increased (Figure 3C).
When we tried to clone rabbit BR3 cDNA and obtain the full cDNA sequence with the primers described here, we consistently obtained sequence for exon 1 and the last exon, but the sequences between those two exons were of variable length. The explanation may be alternative splicing or deletion during cloning due to repetitive low complexity sequences in the regions predicted to encode internal exon(s) in the genomic DNA sequence of the BAC clone. Different internal exon structures and/or alternatively spliced forms of BR3 are found in Ensembl Release 50, Ensembl Human AlignSliceView comparisons of several species including mouse (Mus musculus) and dog (Canis familiaris) (at: http://www.ensembl.org/Homo_sapiens/alignsliceview?l=22:40650991-40652728;align=opt_align_342).
Most studies of BAFF have been conducted in mouse and human, and less is known about the functional roles of BAFF and its receptors in other species. The early development of B-cell pre-immune Ab repertoire in rabbit GALT [23] has similarity to that described in chicken [32]. It was recently suggested that chicken BAFF is mainly produced by B cells both in peripheral lymphoid organs and in the bursa of Fabricius, the chicken’s unique primary lymphoid organ [33]. The rabbit appendix has structural and functional homology to the chicken bursa [32]. Our finding of BAFF in appendix of 4–10-day old rabbits suggests that BAFF functions during the neonatal period when B-cell expansion is occurring rapidly. Because BAFF is detected as early as 4-days after birth, before we detect expression of rabbit Activation Induced Deaminase (AID) protein [34] and IgA [32] in developing appendix, BAFF may initially support B-cell expansion and survival. The complexities of B-cell expansion, pre-immune repertoire development, T-independent, but AID-dependent gene conversion,-somatic hypermutation and -class-switch recombination in the gut mucosal environment make it difficult to predict what additional roles BAFF may play.
BAFF is mainly but not exclusively, expressed by cells of myeloid origin [5]. Recent studies have shown other cell types may also express BAFF under some conditions [6–12, 33]. These reports prompted us to first explore the expression pattern of BAFF and BR3 in peripheral cells of blood and spleen from normal unstimulated rabbits. We used anti-human or mouse BAFF and BR3 antibodies that cross-react with rabbit BAFF and BR3 because of lack of commercially available antibodies specific for rabbit BAFF and BR3. This is the probable explanation for the relatively low fluorescent intensities of BAFF and BR3 staining in our flow cytometry analyses. We were able to purify subpopulations of cells from PBMC and spleen and investigate BAFF and BR3 mRNA expression levels in normal rabbits. We found no evidence that BAFF message was present in non-activated IgM+ B cells. The BAFF detected on these cells appears to be present bound to BAFF receptors. Normal human peripheral blood B cells and tonsillar naïve and memory B cells were also found to have pre-bound BAFF although tonsillar activated B cells did not [35]. Occupancy of BR3 by BAFF on B-cells from Systemic Lupus Erythematosus (SLE) patients was observed to correlate with disease activity [36].
Our laboratory and others have described rabbit models of SLE. Autoantibody production and peripheral white blood cell activation is observed after immunization with branched Multiple Antigen Peptides [24,25,37]. A report of our studies of potential immunopathological roles of BAFF and its receptors in rabbits that produced lupus-like autoantibodies is in preparation. The identification of rabbit BAFF and BR3 cDNA and protein sequences will not only further our understanding of BAFF and its receptors in rabbit models of SLE, but permit investigations of their roles during normal immune responses, and generation of better specific antibodies in rabbits.
Acknowledgments
This research was supported by the intramural research program of the NIH, NIAID and the Oak Ridge Associated Universities. We thank Drs. Alejandro Schaffer and Richa Agarwala, NCBI for identification and assistance with sequence analyses of a rabbit BR3-containing BAC clone, Dr. Jan Lukszo, Peptide Synthesis and Analysis Laboratory, Research Technology Branch, NIAID for synthesis of rabbit miniBR3, Thomas Hofer and Christoph Rader for help with BAFF protein expression, Rami Zahr, Jacqueline Milton and Satyajit Ray for technical assistance, and Shirley Starnes for editorial assistance. We thank Drs. Michael Mage and Christoph Rader for helpful comments about the manuscript.
Abbreviations
- Ab
Antibody
- AID
Activation Induced Deaminase
- BAFF
B-Cell Activating Factor
- BR3
BAFF receptor
- GALT
gut-associated lymphoid tissue
- HRP
horse radish peroxidase
- mAb
monoclonal antibody
- MFI
median fluorescent intensity (or intensities)
- pAb
polyclonal antibody
- PBMC
peripheral blood mononuclear cells
- PE
phycoerythrin
- POD
streptavidin-β-peroxidase conjugate
- rBAFF
recombinant BAFF
- SLE
Systemic Lupus Erythematosus
- TBS
Tris buffered saline
- TMB
5,5′-tetramethylbenzidine
- TNF
Tumor Necrosis Factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Moore PA, Belvedere O, Orr A, Pieri K, LaFleur DW, Feng P, Soppet D, Charters M, Gentz R, Parmelee D, Li Y, Galperina O, Giri J, Roschke V, Nardelli B, Carrell J, Sosnovtseva S, Greenfield W, Ruben SM, Olsen HS, Fikes J, Hilbert DM. BLyS: member of the tumor necrosis factor family and B lymphocyte stimulator. Science. 1999;285:260–3. doi: 10.1126/science.285.5425.260. [DOI] [PubMed] [Google Scholar]
- 2.Schneider P, MacKay F, Steiner V, Hofmann K, Bodmer JL, Holler N, Ambrose C, Lawton P, Bixler S, Acha-Orbea H, Valmori D, Romero P, Werner-Favre C, Zubler RH, Browning JL, Tschopp J. BAFF, a novel ligand of the tumor necrosis factor family, stimulates B cell growth. J Exp Med. 1999;189:1747–56. doi: 10.1084/jem.189.11.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shu HB, Hu WH, Johnson H. TALL-1 is a novel member of the TNF family that is down-regulated by mitogens. J Leukoc Biol. 1999;65:680–3. [PubMed] [Google Scholar]
- 4.Mackay F, Cancro MP. Travelling with the BAFF/BLyS family: are we there yet? Semin Immunol. 2006;18:261–2. doi: 10.1016/j.smim.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 5.Nardelli B, Belvedere O, Roschke V, Moore PA, Olsen HS, Migone TS, Sosnovtseva S, Carrell JA, Feng P, Giri JG, Hilbert DM. Synthesis and release of B-lymphocyte stimulator from myeloid cells. Blood. 2001;97:198–204. doi: 10.1182/blood.v97.1.198. [DOI] [PubMed] [Google Scholar]
- 6.Scapini P, Bazzoni F, Cassatella MA. Regulation of B-cell-activating factor (BAFF)/B lymphocyte stimulator (BLyS) expression in human neutrophils. Immunol Lett. 2008;116:1–6. doi: 10.1016/j.imlet.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 7.He B, Chadburn A, Jou E, Schattner EJ, Knowles DM, Cerutti A. Lymphoma B cells evade apoptosis through the TNF family members BAFF/BLyS and APRIL. J Immunol. 2004;172:3268–79. doi: 10.4049/jimmunol.172.5.3268. [DOI] [PubMed] [Google Scholar]
- 8.Elsawa SF, Novak AJ, Grote DM, Ziesmer SC, Witzig TE, Kyle RA, Dillon SR, Harder B, Gross JA, Ansell SM. B-lymphocyte stimulator (BLyS) stimulates immunoglobulin production and malignant B-cell growth in Waldenstrom macroglobulinemia. Blood. 2006;107:2882–8. doi: 10.1182/blood-2005-09-3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chu VT, Enghard P, Riemekasten G, Berek C. In vitro and in vivo activation induces BAFF and APRIL expression in B cells. J Immunol. 2007;179:5947–57. doi: 10.4049/jimmunol.179.9.5947. [DOI] [PubMed] [Google Scholar]
- 10.Lavie F, Miceli-Richard C, Itta M, Sellam J, Gottenberg JE, Mariette X. B-cell activating factor of the tumour necrosis factor family expression in blood monocytes and T cells from patients with primary Sjogren’s syndrome. Scand J Immunol. 2008;67:185–92. doi: 10.1111/j.1365-3083.2007.02049.x. [DOI] [PubMed] [Google Scholar]
- 11.Krumbholz M, Theil D, Derfuss T, Rosenwald A, Schrader F, Monoranu CM, Kalled SL, Hess DM, Serafini B, Aloisi F, Wekerle H, Hohlfeld R, Meinl E. BAFF is produced by astrocytes and up-regulated in multiple sclerosis lesions and primary central nervous system lymphoma. J Exp Med. 2005;201:195–200. doi: 10.1084/jem.20041674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ohata J, Zvaifler NJ, Nishio M, Boyle DL, Kalled SL, Carson DA, Kipps TJ. Fibroblast-like synoviocytes of mesenchymal origin express functional B cell-activating factor of the TNF family in response to proinflammatory cytokines. J Immunol. 2005;174:864–70. doi: 10.4049/jimmunol.174.2.864. [DOI] [PubMed] [Google Scholar]
- 13.Schiemann B, Gommerman JL, Vora K, Cachero TG, Shulga-Morskaya S, Dobles M, Frew E, Scott ML. An essential role for BAFF in the normal development of B cells through a BCMA-independent pathway. Science. 2001;293:2012–3. doi: 10.1126/science.1061964. [DOI] [PubMed] [Google Scholar]
- 14.Shulga-Morskaya S, Dobles M, Walsh ME, Ng LG, MacKay F, Rao SP, Kalled SL, Scott ML. B cell-activating factor belonging to the TNF family acts through separate receptors to support B cell survival and T cell-independent antibody formation. J Immunol. 2004;173:2331–41. doi: 10.4049/jimmunol.173.4.2331. [DOI] [PubMed] [Google Scholar]
- 15.Raman C, Knight KL. CD5+ B cells predominate in peripheral tissues of rabbit. J Immunol. 1992;149:3858–64. [PubMed] [Google Scholar]
- 16.Gross JA, Johnston J, Mudri S, Enselman R, Dillon SR, Madden K, Xu W, Parrish-Novak J, Foster D, Lofton-Day C, Moore M, Littau A, Grossman A, Haugen H, Foley K, Blumberg H, Harrison K, Kindsvogel W, Clegg CH. TACI and BCMA are receptors for a TNF homologue implicated in B-cell autoimmune disease. Nature. 2000;404:949–50. doi: 10.1038/35010115. [DOI] [PubMed] [Google Scholar]
- 17.Thompson JS, Bixler SA, Qian F, Vora K, Scott ML, Cachero TG, Hession C, Schneider P, Sizing ID, Mullen C, Strauch K, Zafari M, Benjamin CD, Tschopp J, Browning JL, Ambrose C. BAFF-R, a newly identified TNF receptor that specifically interacts with BAFF. Science. 2001;293:2108–11. doi: 10.1126/science.1061965. [DOI] [PubMed] [Google Scholar]
- 18.Yan M, Brady JR, Chan B, Lee WP, Hsu B, Harless S, Cancro M, Grewal IS, Dixit VM. Identification of a novel receptor for B lymphocyte stimulator that is mutated in a mouse strain with severe B cell deficiency. Curr Biol. 2001;11:1547–52. doi: 10.1016/s0960-9822(01)00481-x. [DOI] [PubMed] [Google Scholar]
- 19.Batten M, Groom J, Cachero TG, Qian F, Schneider P, Tschopp J, Browning JL, Mackay F. BAFF mediates survival of peripheral immature B lymphocytes. J Exp Med. 2000;192:1453–66. doi: 10.1084/jem.192.10.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sakurai D, Kanno Y, Hase H, Kojima H, Okumura K, Kobata T. TACI attenuates antibody production costimulated by BAFF-R and CD40. Eur J Immunol. 2007;37:110–8. doi: 10.1002/eji.200636623. [DOI] [PubMed] [Google Scholar]
- 21.Seshasayee D, Valdez P, Yan M, Dixit VM, Tumas D, Grewal IS. Loss of TACI causes fatal lymphoproliferation and autoimmunity, establishing TACI as an inhibitory BLyS receptor. Immunity. 2003;18:279–88. doi: 10.1016/s1074-7613(03)00025-6. [DOI] [PubMed] [Google Scholar]
- 22.O’Connor BP, Raman VS, Erickson LD, Cook WJ, Weaver LK, Ahonen C, Lin LL, Mantchev GT, Bram RJ, Noelle RJ. BCMA is essential for the survival of long-lived bone marrow plasma cells. J Exp Med. 2004;199:91–8. doi: 10.1084/jem.20031330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mage RG, Lanning D, Knight KL. B cell and antibody repertoire development in rabbits: the requirement of gut-associated lymphoid tissues. Dev Comp Immunol. 2006;30:137–53. doi: 10.1016/j.dci.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 24.Rai G, Ray S, Shaw RE, DeGrange PF, Mage RG, Newman BA. Models of systemic lupus erythematosus: Development of autoimmunity following peptide immunizations of noninbred pedigreed rabbits. J Immunol. 2006;176:660–67. doi: 10.4049/jimmunol.176.1.660. [DOI] [PubMed] [Google Scholar]
- 25.Puliyath N, Ray S, Milton J, Mage RG. Genetic contributions to the autoantibody profile in a rabbit model of systemic lupus erythematosus (SLE) Vet Immunol Immunopathol. 2008;125:251–67. doi: 10.1016/j.vetimm.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kayagaki N, Yan M, Seshasayee D, Wang H, Lee W, French DM, Grewal IS, Cochran AG, Gordon NC, Yin J, Starovasnik MA, Dixit VM. BAFF/BLyS receptor 3 binds the B cell survival factor BAFF ligand through a discrete surface loop and promotes processing of NF-kappaB2. Immunity. 2002;10:515–24. doi: 10.1016/s1074-7613(02)00425-9. [DOI] [PubMed] [Google Scholar]
- 27.Kamikubo Y, Kroon G, Curriden SA, Dyson HJ, Loskutoff DJ. The reduced, denatured somatomedin B domain of vitronectin refolds into a stable, biologically active molecule. Biochemistry. 2006;45:3297–306. doi: 10.1021/bi052278f. [DOI] [PubMed] [Google Scholar]
- 28.Pospisil R, Obiakor H, Newman BA, Alexander C, Mage RG. Stable expression of the extracellular domains of rabbit recombinant CD5: development and characterization of polyclonal and monoclonal antibodies. Vet Immunol Immunopathol. 2005;103:257–67. doi: 10.1016/j.vetimm.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 29.Guan ZB, Shui Y, Zhang SQ. Two related ligands of the TNF family, BAFF and APRIL, in rabbit: molecular cloning, 3D modeling, and tissue distribution. Cytokine. 2007;39:192–200. doi: 10.1016/j.cyto.2007.07.190. [DOI] [PubMed] [Google Scholar]
- 30.Pospisil R, Alexander CB, Obiakor H, Sinha RK, Mage RG. CD5+ B cells are preferentially expanded in rabbit appendix: The role of CD5 in B cell development and selection. Dev Comp Immunol. 2006;30:711–22. doi: 10.1016/j.dci.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 31.Pego-Reigosa JM, Isenberg DA. Systemic lupus erythematosus: pharmacological developments and recommendations for a therapeutic strategy. Expert Opin Investig Drugs. 2008;17:31–41. doi: 10.1517/13543784.17.1.31. [DOI] [PubMed] [Google Scholar]
- 32.Dasso JF, Obiakor H, Bach H, Anderson AO, Mage RG. A morphological and immunohistological study of the human and rabbit appendix for comparison with the avian bursa. Dev Comp Immunol. 2000;24:797–814. doi: 10.1016/s0145-305x(00)00033-1. [DOI] [PubMed] [Google Scholar]
- 33.Kothlow S, Morgenroth I, Graef Y, Schneider K, Riehl I, Staeheli P, Schneider P, Kaspers B. Unique and conserved functions of B cell-activating factor of the TNF family (BAFF) in the chicken. Int Immunol. 2007;19:203–15. doi: 10.1093/intimm/dxl137. [DOI] [PubMed] [Google Scholar]
- 34.Yang G, Obiakor H, Sinha RK, Newman BA, Hood BL, Conrads TP, Veenstra TD, Mage RG. Activation-induced deaminase cloning, localization, and protein extraction from young VH-mutant rabbit appendix. Proc Natl Acad Sci USA. 2005;102:17083–88. doi: 10.1073/pnas.0501338102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Darce JR, Arendt BK, Wu X, Jelinek DF. Regulated expression of BAFF-binding receptors during human B cell differentiation. J Immunol. 2007;179:7276–86. doi: 10.4049/jimmunol.179.11.7276. [DOI] [PubMed] [Google Scholar]
- 36.Carter RH, Zhao H, Liu X, Pelletier M, Chatham W, Kimberly R, Zhou T. Expression and occupancy of BAFF-R on B cells in systemic lupus erythematosus. Arthritis Rheum. 2005;52:3943–54. doi: 10.1002/art.21489. [DOI] [PubMed] [Google Scholar]
- 37.James JA, Gross T, Scofield RH, Harley JB. Immunoglobulin epitope spreading and autoimmune disease after peptide immunization: Sm B/B′-derived PPPGMRPP and PPPGIRGP induce spliceosome autoimmunity. J Exp Med. 2005;181:453–61. doi: 10.1084/jem.181.2.453. [DOI] [PMC free article] [PubMed] [Google Scholar]