Abstract
Betaine-homocysteine S-methyltransferase (BHMT) catalyzes the transfer of a methyl group from betaine to l-homocysteine, yielding dimethylglycine and l-methionine. In this study, we prepared a new series of BHMT inhibitors. The inhibitors were designed to mimic the hypothetical transition state of BHMT substrates and consisted of analogues with NH, N(CH3), or N(CH3)2 groups separated from the homocysteine sulfur atom by a methylene, ethylene, or a propylene spacer. Only the inhibitor with the N(CH3) moiety and ethylene spacer gave moderate inhibition. This result led us to prepare two inhibitors lacking a nitrogen atom in the S-linked alkyl chain: (RS,RS)-5-(3-amino-3-carboxypropylthio)-3-methylpentanoic acid and (RS)-5-(3-amino-3-carboxypropylthio)-3,3-dimethylpentanoic acid. Both of these compounds were highly potent inhibitors of BHMT. The finding that BHMT does not tolerate a true betaine mimic within these inhibitors, especially the nitrogen atom, is surprising and evokes questions about putative conformational changes of BHMT upon the binding of the substrates/products and inhibitors.
Introduction
Betaine-homocysteine S-methyltransferase1 (BHMT; EC 2.1. 1.5) catalyzes a methyl transfer from betaine to l-homocysteine to form dimethylglycine and l-methionine, respectively. This enzyme is abundant in mammals but only in the liver and kidney.2 It has been predicted that BHMT is responsible for up to half of the homocysteine remethylation capacity in liver.3 BHMT follows an ordered Bi–Bi reaction mechanism with homocysteine being the first substrate to bind and methionine being the last product off.4 Cloning of human BHMT cDNA, overexpression of the enzyme in E. coli, as well as a purification protocol for preparation of homogeneous enzyme have been previously described.5 BHMT was shown to be a Zn-metalloenzyme. The metal ion is essential for its catalytic activity. Site-directed mutagenesis studies have shown that the Zn2+ ion is coordinated by three cysteine ligands.6 BHMT is a member of a family (Pfam 02574) of zinc and thiol/selenol-dependent methyltransferases.7
Our laboratory is particularly interested in the synthesis and biological evaluation of inhibitors of BHMT. During the past several years, we developed potent and selective inhibitors of human recombinant BHMT: phosphinic pseudopetides8-14 and S-alkylated derivatives of homocysteine.15 Among the series of S-alkylated homocysteines, we identified compounds 1–3 as highly potent inhibitors of BHMT. They were believed to mimic the structure of the hypothetical transition state 4 of BHMT substrates, betaine and l-homocysteine, upon binding to the active site of the enzyme. The competitive inhibitor 1 (Kiapp toward betaine of 12 nM)15,16 was cocrystallized with BHMT, and the structure of the complex was determined by X-ray crystallography.7 Inhibitor 1 was also used in a study of the intrinsic fluorescence of BHMT.17 This study helped to provide a better understanding of the catalytic mechanism of the enzyme and confirmed the ordered Bi–Bi reaction. Recently, inhibitor 1 was used in mice to study the role of BHMT in sulfur metabolism in vivo.18 This pioneering pharmacological study confirmed the role of BHMT in the synthesis of methionine from homocysteine in vivo.
Other identified and possible physiological functions of BHMT require further characterization. Betaine has been shown to be an osmolyte in rat liver cells.19 It is likely that BHMT helps to maintain the cellular osmolytic equilibrium by maintaining the correct betaine concentration.20 Potent, stable, and selective BHMT inhibitors would be useful in studies of the role of the enzyme in liver and kidney osmolytic balance. BHMT activity was shown to be increased in type 2 diabetes.21 A role for BHMT in lipid and phospholipid metabolism is highly probable, but it remains unclear and demands further investigation.22,23 It is clear that many questions about the physiological role of BHMT in mammalian organisms exist and that potent, selective, and stable inhibitors of this enzyme will have an important role in future studies, especially if BHMT-deficient mice remain unavailable.24
We are particularly interested in the structural requirements for ligand binding to the active site of BHMT. The design and synthesis of compounds that closely resemble the structure of substrates upon binding to BHMT, especially the structure of the hypothetical transition state (4), should yield highly potent inhibitors.25-27 The structures of inhibitors 1–3, which we developed previously,15 are considerably different from the structure of the transition state (4). The main difference is in the “betaine” part of their molecules. Therefore, we decided to design and synthesize new compounds that would better mimic the structure of the transition state (4), which should yield highly potent inhibitors of BHMT that could be useful in future in vivo pharmacological studies. 
Results and Discussion
We prepared two series of BHMT inhibitors (Table 1). Compounds 5–10 in series 1 were designed using the scaffold of the previously published potent inhibitor 1 to investigate the role of the number and position of sulfur atom(s) in the inhibitors. Compounds 11–21 from series 2 were designed to mimic the hypothetical transition state (4) of betaine and homocysteine substrates upon binding to the active site of BHMT.
Table 1.
Relative Inhibition of Human BHMT by Compounds 1 and 5–21a
| Compound | % Inhibitionb (average of triplicates) (0.25 mM betaine, 0.1 mM dl-homocysteine) |
IC50(μM)±SEMc (n = 3) (2 mM betaine, 1 mM dl-homocysteine) |
||
|---|---|---|---|---|
| 20 μM | 1 μM | |||
| 1 | 100.0 | 97.6 | 0.138±0.019 | |
| Series 1 | ||||
| 5 | 19.1 | Nd | Nd | |
| 6 | 97.6 | 26.3 | 3.26±0.59 | |
| 7 | 5.4 | Nd | Nd | |
| 8 | 9.8 | Nd | Nd | |
| 9 | 100.0 | 94.3 | 0.649±0.103 | |
| 10 | 2.11 | Nd | Nd | |
| Series 2 | ||||
| 11 | 0.0 | 0.0 | Nd | |
| 12 | 23.8 | 5.1 | Nd | |
| 13 | 98.5 | 58.9 | 2.50±0.19 | |
| 14 | 19.8 | Nd | Nd | |
| 15 | 15.5 | Nd | Nd | |
| 16 | 37.1 | Nd | Nd | |
| 17 | 0.0 | Nd | Nd | |
| 18 | 3.3 | 0.0 | Nd | |
| 19 | 79.01 | 22.4 | Nd | |
| 20 | 100.0 | 97.8 | 0.139±0.012 | |
| 21 | 100.0 | 98.1 | 0.084±0.004 | |
The percent inhibition of each compound was determined at 20 and 1 μM. See the Experimental Section for details.
All assays were done in triplicate, and the obtained data were reproducible within ±15%.
All data points for IC50 values were derived from assays performed in duplicate, and the values were obtained from 3 different assays. Nd means not determined.
Chemistry
Compounds 5 and 6 were synthesized directly by recombination of free unprotected acids (Scheme 1). Acidic ring-opening of γ-aminobutyrolactone in an aqueous solution containing dl-homocysteine followed by oxidation with hydrogen peroxide afforded disulfide 5 (accompanied by disulfide 22 and dl-homocystine as byproduct). Similarly, compound 6 bearing the thioformyl moiety was obtained by the phase-transfer catalyzed reaction28 of 3-mercaptopropionic acid and dl-homocysteine in dichloromethane, followed by HPLC resolution of the resulting mixture.
Scheme 1a.
a Reagents and conditions: (i) (a) HCl (20%), 60 h, rt, Ar; (b) H2O2 (30%), rt, 1 d. (ii) NaOH, CH2Cl2,H2O, benzyl triethylammonium chloride (TEBAC), rt, Ar, 1 d. (iii) (a) NaH, DMF, rt; (b) Br(CH2)3Cl, 30 °C, 1 d. (iv) Sodium 4-mercaptoethylbutanoate, EtOH, rt, 1 d. (v) 3 M HCl, reflux, 3 h.
The treatment of diethyl acetamidomalonate (DEAMa) with NaH followed by addition of 1-bromo-3-chloropropane yielded a product that is considered in the literature29,30 to be chloride 23a. However, our analysis revealed that the product was a mixture of chloride 23a and bromide 23b at a ratio of 59:41 (as determined by NMR). The coupling of a mixture of the compounds 23a and 23b with sodium 4-mercaptoethylbutanoate produced the corresponding protected sulfide 24 in good yield. The target compound 7 was obtained after treatment with 3 N HCl under reflux.
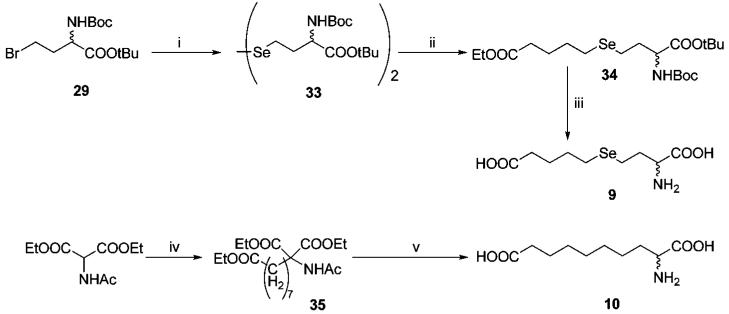
Synthesis of intermediate 29 (including its precursors 27 and 28), which was proposed as a precursor for the synthesis of compounds 8 and 9, is described in detail in Scheme S1, Supporting Information.
Attempts to prepare the oxygen-containing analogue (8) of sulfide 1 by standard Williamson ether synthesis employing alcohol 28, bromide 29, or several similar precursors were unsuccessful. Therefore, an alternative approach (Scheme 2) was used starting from DEAM, which was alkylated with 2,2′-dichloro-diethylether.31 The resulting chloride 30 was converted into iodide 31 and reacted with methylacrylate in the presence of the Zn(Cu) pair,32 which gave the desired structure 32 in high yield. The final analogue 8 was obtained after acidic deprotection.
Scheme 2a.
a Reagents and conditions: (i) (a) NaH, DMF, rt; (b) (ClCH2CH2)2O. (ii) NaI, Me2CO, 65 °C. (iii) Methyl acrylate, Zn(Cu), EtOH-H2O, ultrasound, rt. (iv) 3 M HCl, reflux.
The selenium analogue (9) of sulfide 1 was synthesized from 29 in three steps (Scheme 3). The reaction with in situ generated disodium diselenide33 afforded diselenide 33, which was then treated with NaBH4 and ethyl 5-bromovalerate to generate selenide 34. Deprotection of the latter compound yielded the desired selenide 9.
Scheme 3a.
a Reagents and conditions: (i) Se, NaOH, N2H4 · H2O, DMF, Ar, 60 °C. (ii) NaBH4, ethyl 5-bromovalerate, EtOH, Ar, 0 °C. (iii) (a) aq NaOH, rt, 1 h; (b) TFA/CH2Cl2/thioanisole, rt, 0.5 h. (iv) (a) EtONa, DMF, rt; (b) Br(CH2)7COOEt, reflux, 10 h. (v) 3 M HCl, reflux, 3 h.
Amino acid 10 was prepared according to the method of Barraclough et al.30 from DEAM and ethyl 8-bromooctanoate, followed by standard acidic decarboxylation and deprotection.
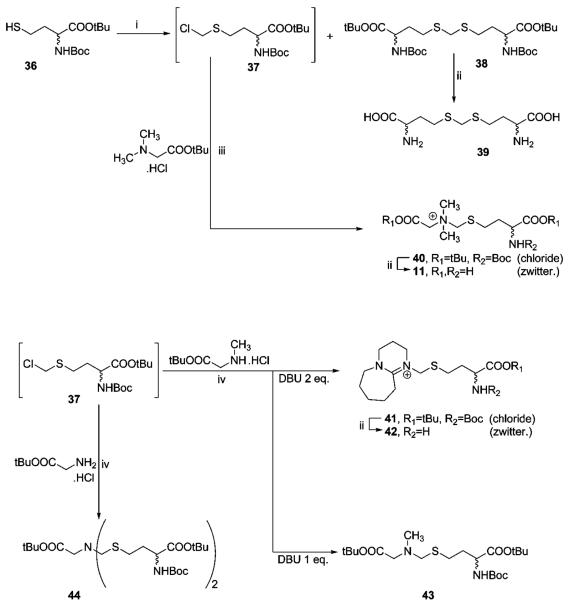
For the synthesis of the transition state analogues bearing the N-CH2-S motif (Scheme 4), a protected homocysteine derivative 36 was prepared in high yield following the synthetic method described by Zhu et al.34 Alkylating agent 37 was obtained by a phase-catalyzed reaction35 of 36 with bromochloromethane in the presence of crushed solid KOH. We did not succeed in the isolation of this compound due to its high reactivity (e.g., rapid decomposition on a silica column); however, the CH2Cl2/CH2BrCl solution of compound 37 proved to be sufficiently stable for subsequent synthetic use. During the preparation of 37, formation of the djenkolic acid36 homologue 38 was invariably observed. After standard acidic deprotection, free amino acid 39 was obtained.
Scheme 4a.
a Reagents and conditions: (i) NaOH, CH2Cl2, H2O, TEBAC, rt, Ar, 1 d. (ii) TFA/CH2Cl2/thioanisole, rt, 0.5 h. (iii) DBU, CH2Cl2, rt, 1 h. (iv) DBU, CH2Cl2, 50 °C, 6 h.
The alkylation of dimethylglycine tert-butyl ester with chloromethylsulfide 37 in the presence of one equivalent of DBU (1,8-diazabicyclo[5.4.0]undec-7-ene) at room temperature resulted in the formation of quaternary compound 40, which gave the desired amino acid 11 after deprotection.
While alkylation of the tertiary amines proceeded smoothly at room temperature, alkylation of primary and secondary amines demanded prolonged heating of the reaction mixture. Surprisingly, when 2 equiv of DBU were used in the reaction of 37 with tert-butyl-2-(methylamino)acetate hydrochloride, formation of an unknown compound was observed and 43 was not detected in the reaction mixture. Analysis of the product revealed N-alkylated DBU (41), which gave the corresponding compound 42 after deprotection. In addition, whereas the alkylation of the above amine in the presence of 1 equiv of DBU gave the expected compound 43, an attempt to prepare the respective secondary amine from β-alanine tert-butyl ester (as well as from glycine and alanine tert-butyl esters) failed, giving only bis-alkylated product 44 and, despite our best efforts, no trace of the expected secondary amine. This observation indicates a strong preference of 37 for secondary and tertiary amines over primary amines. Moreover, the acidic deprotection of 43 and 44 caused their complete decomposition, and no traces of the desired free compounds were detected.
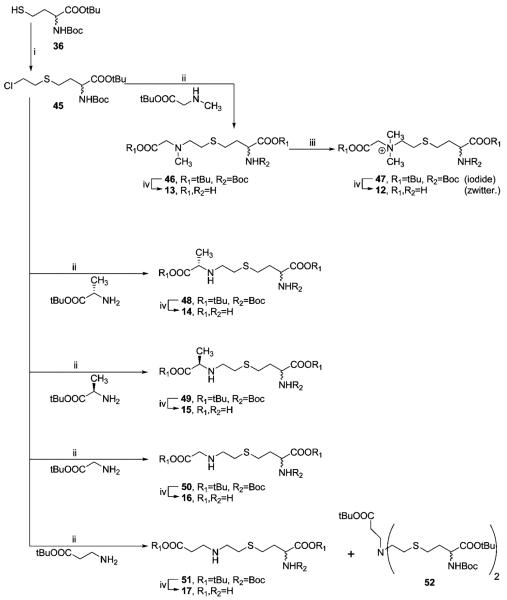
The series of compounds bearing the ethylene linker N-CH2-CH2-S and propylene linker N-(CH2)3-S was prepared from halogenides 45 and 53, respectively (Schemes 5 and 6). Unlike 37, both 45 and 53 are stable compounds, with much weaker reactivity toward amines than 37; therefore, harsh reaction conditions37,38 were required. The alkylation of dimethylglycine tert-butyl ester to quaternary amines was not feasible. This problem was successfully bypassed by the alkylation of 46 and 54 by methyl iodide. During alkylation of β-alanine tert-butyl ester, a bis-alkylated compound 52 was isolated as a byproduct.
Scheme 5a.
a Reagents and conditions: (i) ClCH2CH2Cl, DBU 1 equiv, rt, Ar, 3 h. (ii) NaI, Na2CO3, Bu4NBr, dioxane, Ar, reflux, 40 h. (iii) CH3I, dioxane, rt, 1 d. (iv) TFA/CH2Cl2/thioanisole, rt, 0.5 h.
Scheme 6a.
a Reagents and conditions: (i) Br(CH2)3Br, DBU 1 equiv, rt, 1 h. (ii) NaI, Na2CO3, Bu4NBr, dioxane, Ar, reflux, 16 h. (iii) CH3I, dioxane, rt, 1 d. (iv) TFA/CH2Cl2/thioanisole, rt, 0.5 h.
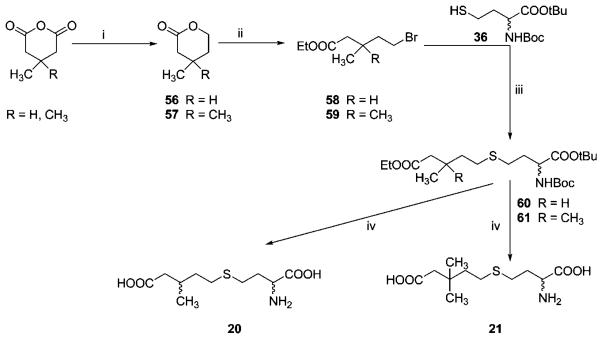
Finally, inhibitors 20 and 21, with branched alkyl side chains, were synthesized starting from 3-methyl- and 3,3-dimethylglutaranhydride, respectively, which were converted in two steps39-41 into bromide esters 58 and 59, respectively. After coupling with thiol 36, protected sulfides 60 and 61 were obtained in good yields, which, after deprotection, afforded the target compounds 20 and 21 (Scheme 7).
Scheme 7a.
a Reagents and conditions: (i) (a) NaBH4, THF, rt, 3 d (b) H+. (ii) HBr/EtOH, rt, 3 d. (iii) NaH, THF, rt, 1 d. (iv) (a) 1 M NaOH, H2O-Dx, 60 °C, 5 h; (b) TFA/CH2Cl2/thioanisole, rt, 0.5 h.
Inhibition Experiments
First, we determined the percent inhibition of BHMT using the test compounds at 20 μM. For the most potent compounds, we then determined the percent inhibition at 1 μM and their IC50 values. The percentages of inhibition were measured at relatively low concentrations of substrates, 0.25 mM betaine and 100 μM dl-homocysteine (Km values of BHMT for betaine and dl-homocysteine are 2 mM and 8 μM, respectively), in order to maximize our ability to detect inhibition. In contrast, we determined IC50 values of the most potent inhibitors at higher substrate concentrations (2 mM betaine and 1 mM dl-homocysteine) so that we could determine IC50 values in measurable concentrations more accurately. The results of the inhibition experiments are summarized in Table 1. For a clearer description of inhibitor structures, we will use the numbering of atoms as shown in a simple inhibitor scaffold (62, below). 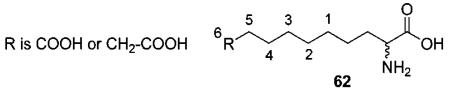
The inhibitory potencies of all tested compounds were compared to the “reference” inhibitor 1. In our previous study,15 we determined a Kiapp of 12 nM with respect to betaine for this compound. In the present series of experiments, we determined an IC50 of 0.138 μM for this compound.
Among the inhibitors 5–10, only compound 9 with a selenium atom at position 1 in scaffold 62 and with four CH2 groups in the alkyl chain strongly inhibited BHMT. Nevertheless, replacement of sulfur with a selenium atom yields an inhibitor that is 5-fold weaker than compound 1. If position 1 is occupied by an oxygen or CH2, the respective compounds 8 and 10 are almost inactive. The low inhibitory potency of compound 7, having a sulfur atom at position 2, reveals the high specificity of BHMT for the “homocysteine” moiety in inhibitors. Previously reported15 inhibitor 3, which has a second sulfur atom at position 4, displayed very strong potency toward BHMT. In this study, we prepared and tested compounds 5 and 6, which have a second sulfur atom at positions 2 and 3, respectively. Disulfide compound 5 is a very weak inhibitor of BHMT, and compound 6, which has a thioformyl moiety, displays only moderate inhibitory potency with an IC50 of 3.26 μM. The data on compounds 5–10 reveal the crucial role of a sulfur atom at position 1, which provides a good balance of nucleophilicity, electronegativity, and atom size for the interaction between inhibitors and the catalytic Zn2+ in the active site of BHMT. Hence, the potential replacement of the “homocysteine” moiety in inhibitors with a suitable mimetic remains a challenge.
Concerning the inhibitors of series 2, we first prepared compounds 11 and 12, which have a dimethylamino moiety in positions 3 and 4, respectively. These structures should ideally mimic the transition state 4; however, compound 11 was completely inactive at 20 μM, and inhibitor 12 gave only very weak inhibition at this concentration. Therefore, we prepared compounds 13–17, all of which have a nitrogen atom at position 4, not as quaternary amines, but as tertiary or secondary amines. We found that all secondary amines 14–17 were very poor inhibitors of BHMT. Only tertiary amine 13 gave promising, but still moderate, inhibition of BHMT, with an IC50 value of about 2.5 μM. Given this finding, we prepared compounds 18 and 19, both with a nitrogen atoms at position 5, with a quaternary amine in 18, and a tertiary amine in 19. The idea was to investigate if the propylene spacer placed between sulfur and nitrogen is better suited for optimal inhibition of BHMT than the ethylene spacer. The propylene spacer should provide sufficient flexibility to the inhibitors for better adaptation to the active site. However, compound 18 did not inhibit BHMT at all, and compound 19, with a tertiary amine at position 5, was only a modest inhibitor of BHMT, as it was significantly weaker than its homologue 13, which has a tertiary amine at position 4.
The above-described results indicate the following: (i) position 4 of scaffold 62 is the best suited for substitutions in the alkyl chain of inhibitors, (ii) a tertiary amine is better accommodated than a quaternary amine or secondary amine at position 4, but (iii) the BHMT enzyme does not accept a nitrogen atom at positions 3–5 of the scaffold 62 very well.
Given these data, we decided to synthesize and test compounds 20 and 21, for which position 4 of the scaffold is branched with one or two methyl groups, respectively. We found that both compounds are highly potent inhibitors of BHMT; inhibitor 20 is equipotent to the “reference” compound 1, and we found that compound 21 has an IC50 value of 84 nM toward BHMT. This value is lower than the IC50 that we determined for inhibitor 1 of about 138 nM. In a previous study,15 we determined the Kiapp of inhibitor 1 for betaine to be about 12 nM. We can competently assume that inhibitor 21 will also have a significantly stronger Kiapp than inhibitor 1. However, in this study, we did not repeat the extremely time-consuming enzyme activity assays required to determine the Kiapp, which also require high levels of radioactive betaine. Instead, we investigated the interaction of BHMT with inhibitor 21 in more detail using isothermal titration calorimetry. This technique allows for the precise determination of Kd together with the determination of changes in the total Gibbs free energy upon binding (decom-posed to enthalpic and entropic contributions).
Isothermal Titration Calorimetry (ITC)
Isothermal titration calorimetry (ITC) was used to monitor the binding of inhibitor 21 to human recombinant BHMT. The experiments were performed without the presence of BHMT substrates. Three independent titrations of compound 21 in a solution of BHMT were done separately in three buffers with different enthalpies of ionization (HEPES, ΔHion = 5.03 kcal · mol−1; ACES, ΔHion = 7.51 kcal · mol−1; Tris, ΔHion = 11.3 kcal · mol−1). These experiments indicated the same binding enthalpies, as can be seen from the superposition of the individual titration curves (Figure S1, Supporting Information), which suggests that there is no net proton transfer associated with ligand binding.42,43 The titration performed in HEPES buffer is shown in Figure 1. Compound 21 is a mixture of two enantiomers. The crystal structure of inhibitor 1 complexed with BHMT revealed that only the S-enantiomer binds to the active site of the enzyme.7 The Glu159 of BHMT forms a hydrogen bond with the amino group of the l-homocysteine moiety of inhibitor 1. Models of d-homocysteine in the active site place the amino group more than 4.0 Å away from the carboxylate of Glu159. Glu159 therefore establishes stereospecificity for the l-form of homocysteine. From these results, we presumed that only the S-enantiomer of compound 21 will interact with BHMT. Later, during the manuscript revision process, we confirmed this presumption by the synthesis and characterization of pure S- and R- enantiomers of compound 21 (compounds 63 and 64, Supporting Information). Therefore, in our calculation of enthalpy change, we considered the concentration of compound 21 to be half of the total concentration of the R,S-compound. After addition of a small amount of ligand to the protein, an unknown exothermic process was detected from the titration curve (Figure 1). The other main exothermic response was considered to be due to the interaction of the reactants. Using a model for two sets of sites using the Origin software, it was possible to separate the interaction step from the unknown exothermic process. The stoichiometry of the main interaction was estimated to be 1.1 ± 0.1, which is in a good agreement with the previous finding that one molecule of the similar inhibitor 1 binds to one BHMT monomer subunit (four inhibitor molecules per BHMT tetramer).7 Inhibitor 21 (S-enantiomer) binds to BHMT with a dissociation constant (Kd) of about 51 nM (calculated from an association constant Ka = (2.0 ± 0.6) × 107 M−1), which corresponds to a total Gibbs free energy change ΔG = −10.0 ± 0.2 kcal · mol−1. It is decomposed into enthalpic (ΔH = −29.5 ± 1.2 kcal · mol−1) and entropic (−TΔS = 19.6 ± 1.3 kcal · mol−1) contributions. This considerably large and favorable enthalpic contribution suggests a strong and direct interaction of the inhibitor with the enzyme via hydrogen bonds or ionic interactions. On the other hand, a large and positive entropic contribution is unfavorable and may reflect possible conformational changes of the enzyme upon binding of the inhibitor. Recently, we studied the binding mechanism of BHMT using its intrinsic fluorescence.17 This study confirmed the previously proposed ordered Bi–Bi mechanism of BHMT. It was shown that homocysteine is the first substrate to bind and that this binding probably induces a conformational change of the enzyme, which allows the binding of betaine, the second substrate. In 2004, it was shown that dimerization of BHMT might be required for substrate binding.44 His338 of the BHMT “dimerization arm” (residues 319–371) contributes to betaine binding at the active site of the other monomer, indicating that the complete active site is formed upon dimerization. A more recent study45 suggested that loop L2 (residues 74–79) is involved in the conformational change associated with occupancy at the betaine-binding site. Gonzalez et al.46 proposed that, in ligand-free BHMT, L2 is open, which allows ligands access to the active site, but L2 closes after formation of the ternary complex (binding of betaine). Phe76 and Tyr77 are important betaine-binding determinants in this process. It is probable that inhibitor 21 interacts with BHMT in a similar manner; first, the “homocysteine” part of the inhibitor binds, and after an induced conformational change of BHMT, the S-linked alkyl chain of the inhibitor binds. These conformational changes could explain the high and positive entropic contribution that we observed. In the above-mentioned fluorescence study,17 we determined the Kd of compound 1 (mixture of R- and S-enantiomers) for the enzyme to be about 280 nM. The Kd of inhibitor 21 for BHMT as determined in this study using ITC is significantly tighter (51 nM for S-enantiomer) and confirms the status of compound 21 as the most potent inhibitor of BMHT ever reported.
Figure 1.
Isothermal titration of inhibitor 21 with BHMT. Titration was performed at 25 °C in 50 mM HEPES/NaOH, pH 7.5, containing 5 mM 2-mercaptoethanol. Upper graph: experimental data. Lower graph: fit (line) to the integrated heats (full circles) from each injection of compound 21, corrected for the heat of dilution of this inhibitor. For details, see Experimental Section.
Considerations Regarding the Mode of Interaction between Inhibitors and BHMT
The higher inhibitory potency of inhibitor 21 suggests that this compound might represent a better mimic of transition state 4 than the previously reported inhibitor 1. Nevertheless, a number of questions arise from the inhibitory activities of our new compounds.
Molecular modeling of the BHMT–inhibitor 21 complex (see Supporting Information, Figure S2) suggests that compound 21 might bind BHMT in a similar manner as compound 1. However, the calculation of binding energies for compounds 1 and 21 (see Supporting Information) indicated that inhibitor 21 should be a weaker binder than inhibitor 1 in this type of interaction. We predicted binding energies of −8.5 and −5.9 kcal · mol−1 for compounds 1 and 21, respectively. This observation contradicts our inhibition experiments, which revealed that compound 21 was a tighter inhibitor than 1 (IC50 values of 84 and 138 nM, respectively), and it also contradicts an experimental value of ΔG of about −10.0 kcal.mol−1 for compound 21 as determined by ITC. These findings also suggest that inhibitor 21 is a stronger binder than 1. We suppose that inhibitor 21 binds to BHMT in a similar but not identical manner as inhibitor 1. This slightly different mode of interaction results in a higher binding affinity of compound 21 compared to 1.
The finding that BHMT does not tolerate betaine mimics in our inhibitors, especially the presence of the nitrogen atom, is surprising. However, the structure7 of the complex of human BHMT with inhibitor 1 reveals that the inhibitor butyl chain is surrounded by a series of aromatic residues (Phe76, Tyr77, Tyr160, Phe261, and Phe 267) and that the carboxyl group interacts with Trp44 and Tyr77. The positive charge of the quaternary amine in compounds 11, 12, and 18 would be rather disadvantageous for binding to the hydrophobic pocket of BHMT surrounding the butyl chain of inhibitor 1. It is probable that compounds with secondary (14–17) and tertiary (13 and 19) amines have protonated nitrogen atoms, which are responsible for their low inhibitory potency compared to inhibitors 1, 20, and 21. We evaluated this hypothesis by NMR spectroscopy of compounds 13 and 16 over a broad pH range. The NMR spectra of quaternary amine 12 were measured for comparison. The 1H and 13C NMR titration experiments (see Supporting Information, Figure S3) revealed a pK2 ∼ 9.5 for the secondary and tertiary amino groups in both compounds 13 and 16. These values indicate that the equilibrium between protonated and deprotonated forms of the nitrogen atoms at position 4 at pH 7.5, the pH used in our enzymatic assay, is shifted strongly to the protonated form, which may explain why they were much weaker inhibitors compared to compounds 20 and 21. This observation implies that the deprotonated forms of compounds 13 and 16 likely bind to BHMT and are responsible for the observed moderate inhibition. The higher inhibitory potency of inhibitor 13 compared to 16 could be attributed to a positive contribution of the N-linked methyl group.
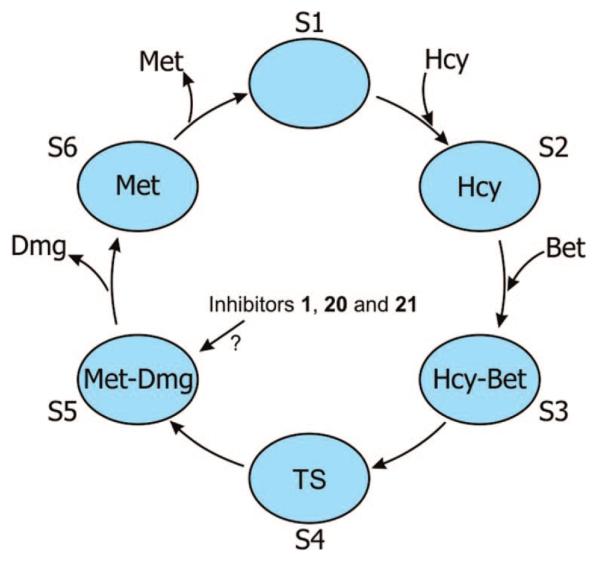
All of the above reasoning leads us to the conclusion that compound 1, and probably also compounds 20 and 21, do not correctly mimic the 3D structure of transition state 4 and that the substrates betaine and homocysteine bind BHMT differently than our inhibitors. The inhibitor-binding cavity of BHMT is relatively narrow and is buried deeply in the structure of the BMHT monomer.7 It was clearly shown that the sulfur atom of inhibitor 1 directly interacts with the Zn2+ atom in the active site of BHMT,7 and it was recently shown that the binding of ligands (substrates or inhibitor) replaces Tyr160, which is the fourth, displaceable ligand of zinc in BHMT.46,47 These facts do not support the hypothesis that our inhibitors bind outside of the active site of BHMT, but rather evoke questions about putative conformational changes of BHMT upon binding of substrates/products or our inhibitors to the active site. Interestingly, Awad et al.16,48 proposed a similar hypothesis 25 years ago. They suggested that the binding site for betaine might exist in two states, one permitting the binding of a positively charged group and the other a neutral group. It is possible that our potent inhibitors (e.g., 1, 20, and 21) bind to some transient structure of BHMT, which may be similar to a structure of the enzyme that exists when it binds methionine (Met) or dimethylglycine (Dmg), for example, but relatively different from the structure of BHMT in a complex with homocysteine (Hcy) or betaine (Bet). Along this line of reasoning, it is possible that the 3D structure of the hypothetical transition state 4 is different from the structures presented by inhibitors 1, 20, and 21. We attempted to express this hypothesis in Figure 2. We suppose that the structure of the active site of BHMT may exist in several different conformations (S1–S6 in Figure 2). Therefore, due to local rearrangements of its active site, BHMT can adapt its structure to different binders, substrates, and products (Hcy, Bet, Met, and Dmg). Structure S1 is the structure of BHMT without substrates. After binding of the first substrate, Hcy, the BHMT structure is modified such that it opens the binding site for the second substrate, Bet, and then adopts the structure S2. The transfer of a methyl group from Bet to Hcy proceeds via the structure of transition state when BHMT passes through structure S4. This process results in the formation of products Met, Dmg, and the respective BHMT structure S5. Finally, Dmg and then Met leave the active site and the BHMT structure changes from S5 to S6 and then back to the basic structure S1. It is not excluded that, for example, structures S2 and S6 are the same or that S3 does not exist because S4 is formed immediately after Bet binds. Such relatively complicated structural rearrangements of BHMT during a catalytic cycle would be in agreement with a very slow turnover rate of the enzyme.5,6,49 We propose that our strong inhibitors 1, 20, and 21 fit well into the active site of structure S5. We suppose that Dmg bound to BMHT (in S5) has a deprotonated tertiary amine nitrogen atom, probably because it is formed in the active site. On the other hand, our inhibitors that have a nitrogen atom at position 4 exist as mainly protonated molecules. For this reason, they bind weakly but their carbon-containing counterparts (20 and 21) bind strongly.
Figure 2.
Schematic representation of proposed structural changes in BHMT during binding of substrates and products. Blue ovals designated as S1–S6 represent different structural states of the BMHT monomer. Hcy is homocysteine, Bet is betaine, Met is methionine, Dmg is dimethylglycine, and TS is the putative transition state of the substrates. For details, see the text.
Conclusions
We prepared two series of new inhibitors of human recombinant BHMT and performed a systematic structure–activity study with the aim of investigating the structural requirements of the active site of the enzyme. The first series of inhibitors varies with regard to the position and number of sulfur atoms in the compounds, which are derived from the structure of the previously published inhibitor 1. We found that the presence of a well-positioned sulfur atom in this type of compound is necessary for potent inhibition of BHMT. The second series of inhibitors was designed to mimic the hypothetical transition state (4) of the BHMT substrates betaine and homocysteine upon binding to the active site. We synthesized and tested analogues with NH, N(CH3), or N(CH3)2 groups separated from the homocysteine sulfur atom by methylene, ethylene, or propylene spacers. Only the inhibitor with the N(CH3) moiety and an ethylene spacer between nitrogen and sulfur gave moderate inhibition. These results led us to prepare two inhibitors lacking a nitrogen atom in the S-linked alkyl chain, inhibitor 20 and inhibitor 21, which are both highly potent inhibitors of BHMT. We also investigated the interaction of inhibitor 21 with BHMT using isothermal titration microcalorimetry and found a highly enthalpic interaction and Kd of about 51 nM for the S-enantiomer of this inhibitor. Compound 21 is the most potent inhibitor of BHMT reported to date. The finding that BHMT does not tolerate betaine mimics in inhibitors, especially the presence of the nitrogen atom, is surprising and evokes questions about the catalytic mechanism of the reaction and about putative conformational changes of BHMT upon the binding of substrates/products in the active site. It seems reasonable to assume that the predominant effect driving the binding of inhibitors or substrates to BHMT is an electrostatic effect. However, the work reported here shows that potent inhibitors of BHMT require that the central part of the compounds be without a positive charge. We interpret these results to indicate that our inhibitors bind to the enzyme in a mode similar to the products Met and Dmg. On the other hand, good mimics of the 3D structure of transition state 4 should demand the presence of a positive charge in the central part of the molecule, however, the structure and composition of true transition state 4 mimics have thus far eluded us. Additional information about the structure of BHMT complexed with varying substrate(s)–product(s) combinations, or complexed with the new inhibitory ligands described here (e.g., inhibitors 20 and 21), will lead our future synthetic efforts to prepare BHMT ligands that deepen our understanding of the chemical events and structural changes that occur during BHMT catalysis.
Experimental Section
Chemistry: General.
Unless otherwise stated, materials were obtained from commercial suppliers (Sigma-Aldrich, Fluka, Merck) and used without purification. The solvents were evaporated at 40 °C and 2 kPa, and the products were dried over phosphorus pentoxide at rt and 13 Pa. The course of the reactions was checked on TLC plates (Fluka, Merck). More specifically, the products were detected by UV monitoring after spraying with ninhydrin (dark-blue color of amines), a 1% aqueous solution of KMnO4 (sulfur compounds), and a 1% ethanolic solution of 4-(4-nitrobenzyl)pyridine, followed by heating and treating with gaseous ammonia (blue color of esters). For flash column chromatography, silica gel 40–60 μm (Fluka) was used. TLC and preparative silica gel chromatography were carried out in the following solvent systems (v/v): chloroform–ethanol 9:1 (C1), chloroform–ethanol 19:1 (C2), ethyl acetate–acetone–ethanol–water 4:1:1:1 (H1), ethyl acetate–acetone–ethanol–water 6:1:1:0.5 (H3), 2-propanol–conc aqueous ammonia–water 7:1:2 (IPAV), 50% EtOAc–toluene (T1), 20% ethyl acetate–toluene (T2). For gradient RP-HPLC purification and analysis, a Waters LC 625 System (Milford, MA) was used. Different gradients of acetonitrile (1–80%) in water containing 0.1% (v/v) of TFA were used for the elution of compounds. Preparative RP-HPLC of the target compounds was performed using a Phenomenex (Luna C-18, 5 μm, 25 cm × 2.12 cm, Torrance, CA) column. Purified compounds were coevaporated several times with water at 75 °C to remove TFA and then lyophilized from the water. Analytical RP-HPLC was performed using a Watrex (Nucleosil 120, 5 μm, C18, 25 cm × 0.46 cm; Prague, Czech Republic) column. Anion-exchange analytical HPLC was performed using an AminoPac PA10 (0.4 cm × 25 cm, Dionex Corporation, Sunnyvale, CA) column with a BioLC system (GP50 gradient pump, ED50 electrochemical detector) from Dionex Corporation (Sunnyvale, CA). Details of the HPLC conditions are given in the Supporting Information. HRMS mass spectra were obtained on a FTMS mass spectrometer LTQ-orbitrap XL (Thermo Fisher, Bremen, Germany) in electrospray ionization mode. 1H and 13C NMR spectra were recorded on a Bruker AVANCE-500 (1H at 500.13 MHz, 13C at 125.7 MHz) and an AVANCE-600 spectrometer (1H at 600.13 MHz, 13C at 150.9 MHz) in CDCl3, DMSO-d6 or D2O solution. The 2D-H,H-COSY, 2D-H,C-HSQC, and 2D-H,CHMBC spectra were used for the structural assignment of proton and carbon signals.
Deprotection: General Procedure A
A solution of the protected compound in aqueous HCl (4 M, 10 mL/mmol) was heated under reflux for 3 h and then evaporated in vacuo. The residue was dissolved in water and purified by the standard procedure (see General part).
Deprotection: General Procedure B
A solution of the protected compound in a mixture of dioxane (5 mL/mmol) and EtOH (2 mL/mmol) was treated with aqueous NaOH (5 M, 2 mL/mmol), and the reaction mixture was stirred for 4 h at 80 °C (TLC in T1). The solution was acidified with saturated aq citric acid to pH 3 before being extracted with EtOAc (2 × 50 mL). The organic layer was washed with water (50 mL) and evaporated in vacuo. The residue was dissolved in a mixture of TFA–CH2Cl2–thioanisole–H2O (47: 47:3:3 v/v, 5 mL/mmol), and the solution was stirred at rt for 30 min. Then, H2O (10 mL/mmol) was added, and the aqueous phase was washed with Et2O(5 × 10 mL). The final product was obtained after standard purification (see General part).
Deprotection: General Procedure C
The protected compound was dissolved in a mixture of TFA–CH2Cl2–thioanisole–H2O (47: 47:3:3 v/v, 5 mL/mmol), and the solution was stirred at rt for 30 min. Then, H2O (10 mL/mmol) was added, and the aqueous phase was washed with Et2O(5 × 10 mL). The final product was obtained after standard purification (see General part).
Synthesis of compounds 27–29, 63, and 64 and spectral data for nontarget compounds 23a, 23b, 24, 30–35, 38–55, and 58–61 are given in Supporting Information.
(RS)-2-Amino-4-[(3-carboxypropyl)disulfanyl]butanoic acid (5) and 4,4′-disulfanediyldibutanoic acid (22)
A solution of dl-homocysteine (0.300 g, 2.22 mmol) and γ-thiobutyrolactone (0.576 mL, 6.66 mmol, 3 equiv) in 5 M hydrochloric acid (20 mL) was stirred at rt for 60 h under an argon atmosphere, and then hydrogen peroxide (30% aq solution, 0.454 mL, 2 equiv) was added. Formation of a white precipitate was observed after 10 min, and the reaction was allowed to proceed overnight (TLC in IPAV). The reaction mixture was then coevaporated three times with water. The residue was treated with water (20 mL), and the insoluble solid was filtered off and dried over P2O5 to give 22 as a white solid (0.523 g, 66% from the total γ-thiobutyrolactone). 1H NMR (500 MHz, D2O) 1.80 (m, 4H), 2.15 (m, 4H), 2.61 (m, 4H). 13C NMR (125.7 MHz, D2O) 25.45 (2 × C), 36.20 (2 × C), 37.86 (2 × C), 182.72 (2 × C). HRMS (ESI) calcd for C8H15O4S2 [M + H]+ 239.0412; found 239.0404.
The aqueous filtrate was concentrated in vacuo, and the crude product was purified by the standard procedure (see General part), giving 5 as a colorless hygroscopic semisolid (0.134 g, 24% from dl-homocysteine). 1H NMR (500 MHz, D2O) 1.89 (m, 2H), 2.20 (m, 1H), 2.28 (m, 1H), 2.40 (m, 2H), 2.67 (t, 2H), 2.74 (t, 2H), 4.03 (dd, 1H). 13C NMR (125.7 MHz, D2O) 23.61, 29.29, 32.22, 32.53, 36.76, 51.95, 178.05 (2 × C). HRMS (ESI) calcd for C8H16NO4S2 [M + H]+ 254.0521; found 254.0514.
(RS)-2-Amino-4-[(2-carboxyethylthio)methylthio]butanoic Acid (6)
3-Mercaptopropionic acid (386 μL, 4.44 mmol, 3 equiv) and TEBAC (0.017 g, 0.075 mmol 0.05 equiv) were added to a stirred solution of dl-homocysteine (0.200 g, 1.48 mmol) and NaOH (1.2 g, 30 mmol, 20 equiv) in H2O (10 mL). Then, dichloromethane (10 mL) was added and the reaction mixture was vigorously stirred at rt for 1 d (TLC in IPAV). The aqueous layer was passed through a column of Dowex 50 (Et3N-cycle) and evaporated to dryness. The residue was dissolved in water and purified by the standard procedure to give 6 as a white solid (0.102 g, 27%). 1H NMR (500 MHz, D2O + NaOD) 1.81 (m, 1H), 1.93 (m, 1H), 2.49 (t, 2H), 2.70 (m, 2H), 2.85 (t, 2H), 3.33 (dd, 1H), 3.80 (s, 2H). 13C NMR (125.7 MHz, D2O + NaOD) 29.83, 29.97, 36.60, 36.93, 39.77, 57.95, 183.50, 185.25. HRMS (ESI) calcd for C8H14NO4S2 [M + H]+ 252.0364; found 252.0360.
Diethyl 2-(3-Chloropropyl)-2-acetamidomalonate (23a) and Diethyl 2-(3-Bromopropyl)-2-acetamidomalonate (23b)
Diethyl acetamidomalonate (4.88 g, 22.5 mmol, 1.00 equiv) was added in five portions to an ice-cooled suspension of NaH (60% suspension in oil, 1.04 g, 26.0 mmol, 1.17 equiv) in anhydrous DMF (12.5 mL) in a flask equipped with a calcium dichloride tube, and the mixture was left to stand at rt for 1 h. Then, 1-bromo-3-chloropropane (2.46 mL, 25.0 mmol, 1.12 equiv) was added dropwise over 10 min, and the reaction was allowed to proceed at 30 °C for 24 h (TLC in T1). After removal of the solvent in vacuo, the crude product was suspended in Et2O, and the inorganic solids were filtered off. The organic phase was washed with H2O twice before being dried over Na2SO4 and evaporated in vacuo. The resulting residue was purified by flash chromatography on silica (elution with a linear gradient of EtOAc in toluene) to yield 3.70 g of a mixture of 23a and 23b as colorless needles. The mixture was used directly in the next step without further resolution.
Diethyl 2-[3-(3-Ethoxycarbonylpropylthio)propyl]-2-acetamidomalonate (24)
Sodium 4-mercaptoethylbutanoate50 (0.40 g, 2.20 mmol, 1.5 equiv) was added to a mixture of 23a and 23b (0.50 g, ca. 1.57 mmol in total) in anhydrous EtOH (10 mL), and the reaction mixture was stirred overnight (TLC in T1). The resultant white precipitate was filtered off, and the solution was evaporated in vacuo. The crude product was purified by flash chromatography on silica (elution with a linear gradient of EtOAc in toluene) to give compound 24 as a colorless solid (0.317 g, 50%).
(RS)-2-Amino-5-(3-carboxypropylthio)pentanoic Acid (7)
Compound 24 (0.310 g, 0.76 mmol) was deprotected according to general procedure A, affording 7 (0.147 g, 82%) as a white solid. 1H NMR (600 MHz, D2O) 1.70 (m, 1H), 1.77 (m, 1H), 1.90 (m, 2H), 2.02 (m, 1H), 2.06 (m, 1H), 2.50 (m, 2H), 2.62 (m, 2H), 2.64 (m, 2H), 4.04 (dd, 1H). 13 C NMR (150.9 MHz, D2O) 26.77, 26.97, 31.68, 32.83, 32.90, 35.39, 55.68, 172.66, 181.00. HRMS (ESI) calcd for C9H19N2O4S [M + H]+ 251.1066; found 251.1062.
Diethyl 2-[2-(2-Chloroethoxy)ethyl]-2-acetamidomalonate (30)
Diethyl acetamidomalonate (5 g, 23 mmol, 1 equiv) was slowly added to an intensively stirred suspension of NaH (60% dispersion in oil; 0.92 g, 23 mmol, 1 equiv) in DMF (40 mL) at 0 °C. After 20 min, sodium iodide (0.345 g, 2.3 mmol, 0.1 equiv) and bis(2-chlorethyl)ether (13.5 mL, 115 mmol, 5 equiv) were added, and the reaction mixture was stirred for 24 h at 60 °C (TLC in T1). The mixture was then concentrated in vacuo, and the dark-orange residue was partitioned between water (250 mL) and Et2O (2 × 250 mL). The organic layer was separated, washed with water (2 × 100 mL), dried over Na2SO4, and concentrated in vacuo. The crude product was purified by flash chromatography on silica (elution with a linear gradient of EtOAc in toluene) to give compound 30 as a pale-yellow viscous oil (4.24 g, 57%).
Diethyl 2-[2-(2-Iodoethoxy)ethyl]-2-acetamidomalonate (31)
Anhydrous sodium iodide (2.78 g, 18.5 mmol, 3 equiv) was added to a solution of chloride 30 (2.0 g, 6.2 mmol) in dry acetone (20 mL), and the reaction mixture was heated to 65 °C in a sealed flask for 24 h with stirring. The mixture was then diluted with Et2O (50 mL), and the precipitated inorganic salts were removed by filtration. Evaporation of the filtrate under reduced pressure gave a crude product, which was purified by flash chromatography on silica (elution with a linear gradient of EtOAc in toluene) to give compound 31 as a colorless viscous oil (2.43 g, 95%).
Diethyl 2-{2-[4-(Methoxycarbonyl)butoxy]ethyl}-2-acetamidomalonate (32)
The zinc–copper couple was prepared according to a previously described procedure32 by the sonication of zinc dust (0.50 g, 8 mmol) and CuI (0.356 g, 2.4 mmol) in water (2 mL) under argon; after 2 min, the mixture turned to a black heavy suspension. Then, EtOH (2 mL) and methyl acrylate (0.90 mL, 10 mmol, 10 equiv) were added through the septum via syringe, and finally a solution of 31 (0.415 g, 1 mmol) in iPrOH (1.5 mL) was added over a period of 1 h under sonication; the temperature was not allowed to rise above 20 °C. Sonication was continued for 1 h, and then saturated aq NaCl (5 mL) was added. The resulting precipitate was filtered over celite. The solid was extracted with Et2O (100 mL), and the filtrate was partitioned between water and Et2O, dried over Na2SO4, and concentrated in vacuo. The residue was purified by flash chromatography on silica (elution with a linear gradient of EtOAc in toluene) to give compound 32 as a colorless viscous oil (0.195 g, 52%).
(RS)-5-(3-Amino-3-carboxypropoxy)pentanoic Acid (8)
Compound 32 (0.180 g, 0.82 mmol) was deprotected according to general procedure A, yielding 8 as a white solid (0.098 g, 93%). 1H NMR (600 MHz, D2O) 1.62 (m, 4H), 2.23 (m, 2H), 2.41 (t, 2H), 2.52 (m, 2H), 3.68 (m, 2H), 4.16 (dd, 1H). 13C NMR (150.9 MHz, D2O) 23.64, 30.65, 32.10, 36.10, 54.16, 68.97, 73.22, 174.63, 181.56. HRMS (ESI) calcd for C9H18NO5 [M + H]+ 220.1185; found 220.1183.
(RS,RS)-Di-tert-butyl-4,4′-diselanediylbis[2-(tert-butoxycarbonylamino)butanoate] (33)
N2H4 · H2O (0.062 mL, 1.27 mmol, 1.1 equiv) was added to a suspension of Se (0.100 g, 1.27 mmol, 1.1 equiv) and NaOH (0.138 g, 3.45 mmol, 3 equiv) in DMF (5 mL), and the mixture was stirred at 60 °C under argon for 3 h. Then, a solution of bromide 29 (0.390 g, 1.15 mmol, 1 equiv) in DMF (3 mL) was added to the flask through the septum by syringe, and stirring was continued for a further 3 h at 60 °C (TLC in T2). The reaction mixture was concentrated in vacuo, and the residue was partitioned between Et2O and H2O. The organic phase was separated, washed with brine, and dried over Na2SO4. After evaporation, the crude product was purified by flash chromatography on silica (elution with a linear gradient of EtOAc in toluene) to give compound 33 as a yellow viscous oil (0.295 g, 76%).
(RS)-Ethyl 5-[3-tert-butoxycarbonyl-3-(tert-butoxycarbonylamino)propylselanyl]pentanoate (34)
NaBH4 (0.048 g, 1.26 mmol, 3 equiv) was added to a solution of diselenide 33 (0.285 g, 0.42 mmol) and ethyl 5-bromovalerate (0.150 mL, 0.93 mmol, 2.2 equiv) in abs EtOH (5 mL) under an argon atmosphere at 0 °C, and the mixture was stirred until the yellow color of the diselenide disappeared (ca. 5 min). The reaction was allowed to proceed at rt overnight (TLC in T2). The mixture was then partitioned between Et2O and H2O. The organic layer was washed with brine, dried over Na2SO4, and concentrated in vacuo. The crude product was purified by flash chromatography on silica (elution with a linear gradient of EtOAc in toluene) to give compound 34 as a colorless viscous oil (0.344 g, 87%).
(RS)-5-(3-Amino-3-carboxypropylselanyl)pentanoic Acid (9)
Compound 34 (0.320 g, 0.69 mmol) was deprotected according to general procedure B, yielding 9 as a white solid (0.122 g, 63%). 1H NMR (600 MHz, D2O + NaOD) 1.63 (m, 2H), 1.67 (m, 2H), 1.87 (m, 1H), 1.97 (m, 1H), 2.19 (t, 2H), 2.60 (m, 2H), 2.64 (m, 2H), 3.30 (dd, 1H). 13C NMR (150.9 MHz, D2O + NaOD) 22.02, 25.97, 28.89, 32.37, 38.53, 39.76, 58.96, 185.56, 186.44. HRMS (ESI) calcd for C9H18O4NSe [M + H]+ 284.0396; found 284.0395.
Diethyl 2-[(7-Ethoxycarbonyl)heptyl]-2-acetamidomalonate (35)
Sodium ethanolate was generated in situ by dissolving sodium (0.072 g, 3.13 mmol, 1.2 equiv) in abs EtOH (2.5 mL) at rt in a flask equipped with a reflux condenser fitted with a calcium chloride tube. DEAM (0.571 g, 2.63 mmol, 1.1 equiv) was added, and after 15 min, a solution of ethyl 8-bromooctanoate (0.600 g, 2.39 mmol, 1 equiv) in EtOH (3 mL) was introduced, and the reaction was heated to reflux for 10 h. The mixture was evaporated, partitioned between Et2O and H2O, and the organic layer was dried over Na2SO4 and concentrated in vacuo. The crude product was purified by flash chromatography on silica (elution with a linear gradient of EtOAc in toluene) to give compound 35 as a colorless viscous oil (0.547 g, 51%).
(RS)-2-Aminodecanedioic Acid (10)
Compound 35 (0.320 g, 0.69 mmol) was deprotected according to general procedure A, yielding 10 as a white solid (0.156 g, 87%). 1H NMR (600 MHz, D2O + NaOD) 1.30 (m, 2H), 1.32 (m, 4H), 1.34 (m, 2H), 1.54 (m, 2H), 1.74 (m, 1H), 1.80 (m, 1H), 2.16 (t, 2H), 3.59 (dd, 1H). 13C NMR (150.9 MHz, D2O + NaOD) 27.09, 28.54, 30.89, 31.04, 31.25, 34.16, 40.34, 57.86, 180.04, 187.08. HRMS (ESI) calcd for C10H20NO4 [M + H]+ 218,1392; found 218,1382.
(RS,RS)-Di-tert-butyl 4,4′-(methylenedithio)-2,2′-di(tert-butoxycarbonylamino)dibutanoate (38) and (RS)-1-[3-(tert-butoxycarbonylamino)-3-(tert-butoxycarbonyl)propylthio]-N-(tert-butoxycarbonylmethyl)-N,N-dimethylmethanammonium chloride (40)
A solution of chloromethylsulfide 37, which is necessary for the amine alkylation, was obtained by intensive stirring of thiol 36 (0.345 g, 1.18 mmol),34 crushed solid KOH (0.067 g, mmol, 1 equiv), and TEBAC (0.027 g, 0.12 mmol, 0.1 equiv) in a mixture of CH2BrCl (2.5 mL) and CH2Cl2 (2.5 mL) for 1 h at rt. The solid precipitate was filtered off, and the solution was directly used in subsequent reaction. A solution of 37 was added to a solution of tert-butyl 2-(dimethylamino)acetate hydrochloride (0.160 g, 0.82 mmol, 1 equiv) and DBU (0.135 mL, 0.90 mmol, 1.1 equiv) in CH2Cl2 (4 mL), and the reaction was allowed to proceed at rt for 1 h. The mixture was evaporated in vacuo, and the residue was purified by flash chromatography on silica. Elution with a linear gradient of EtOAc in toluene gave byproduct 38 as a colorless viscous oil (0.065 g, 19% from thiol 36). Further elution with a linear gradient of H1 in EtOAc yielded the desired ammonium salt 40 (0.125 g, 31% from the amine hydrochloride). Isolation of the byproduct 38 in the following procedures that employ a solution of 37 was not performed.
(RS,RS)-4,4′-(Methylenedithio)-2,2′-(diamino)dibutanoic Acid (39)
Compound 38 (0.110 g, 0.18 mmol) was deprotected according to the general procedure C, affording 39 as a white solid (0.045 g, 86%).
(RS)-2-{[(3-Amino-3-carboxypropylthio)methyl]dimethylammonium}acetate (11).
Compound 40 (0.065 g, 0.13 mmol) was deprotected according to the general procedure C, affording 11 as a white solid (0.020 g, 63%). 1H NMR (500 MHz, D2O) 2.22 (m, 1H), 2.33 (m, 1H), 2.97 (m, 1H), 3.02 (m, 1H), 3.26 (s, 6H), 4.14 (dd, 1H), 4.17 (s, 2H), 4.91 (s, 2H). 13C NMR (125.7 MHz, D2O) 32.59, 32.66, 53.20 (2 × C), 54.42, 63.54, 70.92, 170.74, 174.09. HRMS (ESI) calcd for C9H19N2O4S [M]+ 251.1066; found 251.1077.
(RS)-1-{[3-(tert-Butoxycarbonyl)-3-(tert-butoxycarbonylamino)-propylthio]methyl}-2,3,4,6,7,8,9,10-octahydropyrimido[1,2-a]azepin-1-ium Chloride (41)
The solution of 37, prepared as above, was added to a solution of tert-butyl 2-aminoacetate hydrochloride (0.200 g, 1.19 mmol, 1 equiv) and DBU (0.381 mL, 2.50 mmol, 2.1 equiv) in CH2Cl2 (4 mL), and the reaction was allowed to proceed in a sealed flask under argon at 50 °C for 6 h. The mixture was evaporated in vacuo, and the residue was purified by flash chromatography on silica. Elution with a linear gradient of H1 in EtOAc gave 41 as a white foam (0.180 g, 0.37 mmol).
(RS)-2-Amino-4-[(2,3,4,6,7,8,9,10-octahydropyrimido[1,2-a]azepin-1-ium-1-yl)methylthio]butanoate (42)
Compound 41 (0.172 g, 0.35 mmol) was deprotected according to the general procedure C, affording 42 as a white solid (0.062 g, 59%).
(RS)-tert-Butyl 2-(tert-butoxycarbonylamino)-4-{[(tert-butoxycarbonylmethyl)(methyl)amino]methylthio}butanoate (43)
The solution of 37, prepared as above, was added to a solution of tert-butyl 2-(methylamino)aminoacetate hydrochloride (0.216 g, 1.19 mmol, 1 equiv) and DBU (0.181 mL, 1.19 mmol, 1 equiv) in CH2Cl2 (4 mL), and the reaction was allowed to proceed in a sealed flask under argon at 50 °C for 6 h. Then second portion of DBU (0.181 mL, 1.19 mmol, 1 equiv) was added, the mixture evaporated in vacuo, and the residue was purified by flash chromatography on silica. Elution with a linear gradient of EtOAc in toluene gave 43 as a colorless glassy solid (0.250 g, 31%).
Di-tert-butyl (RS,RS)-4,4′-(tert-Butoxycarbonylmethylamino)bis(methylene)bis(sulfanediyl)bis[(2-tert-butoxycarbonylamino)butanoate] (44)
The solution of 37, prepared as above, was added to a solution of tert-butyl 2-aminoacetate hydrochloride (0.200 g, 1.19 mmol, 1 equiv) and DBU (0.181 mL, 1.19 mmol, 1 equiv) in CH2Cl2 (4 mL), and the reaction was allowed to proceed in a sealed flask under argon at 50 °C for 24 h. Then second portion of DBU (0.181 mL, 1.19 mmol, 1 equiv) was added, the mixture was evaporated in vacuo, and the residue was purified by flash chromatography on silica. Elution with a linear gradient of EtOAc in toluene gave 44 as a colorless glassy solid (0.143 g, 11%).
(RS)-tert-Butyl 2-(tert-butoxycarbonylamino)-4-(2-chloroethylthio)butanoate (45)
DBU (1.353 mL, 8.89 mmol, 1.1 equiv) was added to a solution of thiol 36 (2.355 g, 8.08 mmol) in 1,2-dichloroethane (20 mL), and the reaction mixture was heated under argon at 80 °C for 3 h (TLC in T2). Excessive solvent was evaporated in vacuo, and the crude product was purified by flash chromatography on silica (elution with a linear gradient of EtOAc in toluene) to give compound 45 as a white crystalline solid (2.456 g, 86%).
(RS)-tert-Butyl 2-(tert-butoxycarbonylamino)-4-{2-[(tert-butoxycarbonylmethyl)(methyl)amino]ethylthio}butanoate (46)
Chloride 45 (0.366 g, 1.03 mmol, 1 equiv), tert-butyl 2-(methylamino)acetate (0.150 g, 1.03 mmol, 1 equiv), sodium carbonate (0.165 g, 1.55 mmol, 1.5 equiv), sodium iodide (0.155 g, 1.03 mmol., 1 equiv), and tetrabutylammonium bromide (0.166 g, 0.52 mmol, 0.5 equiv) were placed in a flask, and the mixture was codistilled twice with anhydrous CH2Cl2 (10 mL) to remove traces of water. Dioxane (15 mL) was added, and the flask was equipped with a reflux condenser and heated to reflux under argon for 40 h (TLC in T2). After cooling, the mixture was filtered over celite, the solid extracted with EtOAc (50 mL), and the filtrate evaporated in vacuo. The crude product was purified by flash chromatography on silica (elution with a linear gradient of EtOAc in toluene) to give compound 46 as a colorless solid (0.298 g, 62%).
(RS)-2-[3-(tert-Butoxycarbonylamino)-3-(tert-butoxycarbonyl)-propylthio]-N-(tert-butoxycarbonylmethyl)-N,N-dimethylethanammonium Iodide (47)
A solution of 46 (0.196 g, 0.42 mmol) in dioxane (5 mL) was treated with methyl iodide (0.066 mL, 0.84 mmol, 2.5 equiv), and the reaction mixture was left to react at rt for 4 d (TLC in C1). The solution was then concentrated in vacuo, and the residue was purified by flash chromatography on silica (elution with a linear gradient of H3 in EtOAc) to give compound 47 as a dark-yellow solid (0.196 g, 89%).
(RS)-2-{[2-(3-Amino-3-carboxypropylthio)ethyl]dimethylammonium}acetate (12)
Compound 47 (0.188 g, 0.31 mmol) was deprotected according to the general procedure C, affording 12 as a white solid (0.035 g, 42%). 1H NMR (500 MHz, D2O) 2.18 (m, 1H), 2.28 (m, 1H), 2.78 (m, 2H), 2.99 (m, 2H), 3.27 (s, 6H), 3.83 (m, 2H), 4.10 (s, 2H), 4.15 (t, 1H). 13C NMR (125.7 MHz, D2O) 26.03, 29.44, 32.36, 54.54 (2×C), 54.68, 65.11, 65.82, 170.67, 174.80. HRMS (ESI) calcd for C10H21N2O4S [M]+ 265.1222; found 265.1236.
(RS)-2-Amino-4-{2-[(carboxymethyl)(methyl)amino]ethylthio}-butanoic Acid (13)
Compound 46 (0.181 g, 0.39 mmol) was deprotected according to the general procedure C, affording 13 as a white solid (0.71 g, 73%). 1H NMR (600 MHz, D2O) 2.20 (m, 1H), 2.31 (m, 1H), 2.80 (m, 2H), 2.99 (s, 3H), 3.00 (m, 2H), 3.50 (m, 2H), 4.08 (m, 2H), 4.21 (t, 1H). 13C NMR (150.9 MHz, D2O) 27.62, 28.90, 32.05, 43.82, 54.41, 58.00, 59.37, 171.27, 174.54. HRMS (ESI) calcd for C9H19N2O4S [M + H]+ 251.1066; found 251.1070.
tert-Butyl 2-(RS)-(tert-Butoxycarbonylamino)-4-[2-(S)-(1-tert-butoxycarbonylethylamino)ethylthio]butanoate (48)
Using the procedure outlined for 46, compound 48 was prepared from 45 (0.377 g, 1.06 mmol), (S)-tert-butyl 2-aminopropanoate (0.170 g, 1.17 mmol, 1.1 equiv), sodium carbonate (0.372 g, 3.51 mmol, 3.0 equiv), sodium iodide (0.175 g, 1.17 mmol, 1 equiv), and tetrabutylammonium bromide (0.190 g, 0.59 mmol, 0.5 equiv) as a colorless solid (0.234 g, 47%).
2-(RS)-Amino-4-[2-(S)-(1-carboxyethylamino)ethylthio]butanoic Acid (14)
Compound 48 (0.220 g, 0.48 mmol) was deprotected according to the general procedure C, affording 14 as a white solid (0.36 g, 30%).
tert-Butyl 2-(RS)-(tert-Butoxycarbonylamino)-4-[2-(R)-(1-tert-butoxycarbonylethylamino)ethylthio]butanoate (49)
Using the procedure outlined for 46, compound 49 was prepared from 45 (0.354 g, 1.00 mmol), (R)-tert-butyl 2-aminopropanoate (0.160 g, 1.10 mmol, 1.1 equiv), sodium carbonate (0.318 g, 3.00 mmol, 3.0 equiv), sodium iodide (0.150 g, 1.00 mmol, 1 equiv), and tetrabutylammonium bromide (0.161 g, 0.50 mmol, 0.5 equiv) as a colorless solid (0.171 g, 37%).
2-(RS)-Amino-4-[2-(R)-(1-carboxyethylamino)ethylthio]butanoic Acid (15)
Compound 49 (0.154 g, 0.33 mmol) was deprotected according to the general procedure C, affording 15 as a white solid (0.022 g, 27%). NMR: Mixture of diastereoisomers ca. 1:1; doubling of carbon signals observed. 1H NMR (600 MHz, D2O) 1.58 (d, 3H), 2.19 (m, 1H), 2.29 (m, 1H), 2.78 (m, 2H), 2.93 (m, 2H), 3.34 (m, 2H), 4.06 (q, 1H), 4.18 (t, 1H). 13C NMR (150.9 MHz, D2O) 17.01 + 17.05, 28.82 + 28.86, 29.53, 32.11, 47.41 + 47.45, 54.58 + 54.59, 58.78 + 58.82, 174.75, 175.22 + 175.24. HRMS (ESI) calcd for C9H19N2O4S [M + H]+ 251.1066; found 251.1057.
(RS)-tert-Butyl 2-(tert-Butoxycarbonylamino)-4-[2-(tert-butoxycarbonylmethylamino)ethylthio]butanoate (50)
Using the procedure outlined for 46, compound 50 was prepared from 45 (0.403 g, 1.14 mmol), tert-butyl 2-aminoacetate (0.164 g, 1.25 mmol, 1.1 equiv), sodium carbonate (0.397 g, 3.75 mmol, 3.0 equiv), sodium iodide (0.171 g, 1.14 mmol, 1 equiv), and tetrabutylammonium bromide (0.184 g, 0.57 mmol, 0.5 equiv) as a pale-yellow thick oil (0.250 g, 45%).
(RS)-2-Amino-4-[2-(carboxymethylamino)ethylthio]butanoic Acid (16)
Compound 50 (0.220 g, 0.49 mmol) was deprotected according to the general procedure C, affording 16 as a white solid (0.012 g, 10%). 1H NMR (600 MHz, D2O) 2.17 (m, 1H), 2.26 (m, 1H), 2.75 (m, 2H), 2.94 (m, 2H), 3.35 (t, 2H), 3.84 (s, 2H), 4.06 (t, 1H). 13C NMR (150.9 MHz, D2O) 28.88, 29.44, 32.36, 48.78, 50.75, 55.23, 172.63, 175.50. HRMS (ESI) calcd for C8H17N2O4S [M + H]+ 237.0909; found 237.0906.
(RS)-tert-Butyl 2-(tert-Butoxycarbonylamino)-4-[2-(2-tert-butoxycarbonylethylamino)ethylthio]butanoate (51) and Di-tert-butyl (RS,RS)-4,4′-[2,2′-(2-tert-Butoxycarbonylethylaminodiyl)bis(ethane-2,1-diyl)bis(sulfanediyl)]bis[2-(tert-butoxycarbonylamino)butanoate] (52)
The procedure outlined for 46 was followed using 45 (0.354 g, 1.00 mmol), tert-butyl 3-aminopropanoate (0.160 g, 1.10 mmol, 1.1 equiv), sodium carbonate (0.318 g, 3.00 mmol, 3.0 equiv), sodium iodide (0.150 g, 1.00 mmol, 1 equiv), and tetrabutylammonium bromide (0.161 g, 0.50 mmol, 0.5 equiv). Elution with a linear gradient of EtOAc in toluene gave 51 (0.377 g, 81%) and 52 (0.120 g, 15%) as colorless thick oils.
(RS)-2-Amino-4-[2-(2-carboxyethylamino)ethylthio]butanoic Acid (17)
Compound 51 (0.370 g, 0.80 mmol) was deprotected according to the general procedure C, affording 17 as a white solid (0.087 g, 43%). 1H NMR (600 MHz, D2O) 2.18 (m, 1H), 2.26 (m, 1H), 2.76 (m, 2H), 2.86 (t, 2H), 2.94 (m, 2H), 3.34 (t, 2H), 3.38 (t, 2H), 4.09 (t, 1H). 13C NMR (150.9 MHz, D2O) 28.96, 29.31, 32.38, 32.71, 45.66, 49.05, 55.14, 175.40, 177.10. HRMS (ESI) calcd for C9H18NO4S [M + H]+ 236.0957; found 236.0945.
(RS)-tert-Butyl 4-(3-Bromopropylthio)-2-(tert-butoxycarbonylamino)butanoate (53)
DBU (0.598 mL, 4.32 mmol, 1.1 equiv) was added to a solution of thiol 36 (1.145 g, 3.93 mmol) in 1,3-dibromopropane (10 mL), and the reaction mixture was left to stand overnight at rt (TLC in T2). Excessive solvent was evaporated in vacuo, and the crude product was purified by flash chromatography on silica (elution with a linear gradient of EtOAc in toluene) to give compound 53 as a white crystalline solid (1.32 g, 81%).
(RS)-tert-Butyl 2-(tert-butoxycarbonylamino)-4-{3-[(tert-butoxycarbonylmethyl)(methyl)amino]propylthio}butanoate (54)
Using the procedure outlined for 46, compound 54 was prepared from 53 (0.300 g, 0.73 mmol), tert-butyl 2-(methylamino)acetate hydrochloride (0.145 g, 0.80 mmol, 1.1 equiv), sodium carbonate (0.231 g, 2.18 mmol, 3.0 equiv), sodium iodide (0.109 g, 0.73 mmol, 1 equiv), and tetrabutylammonium bromide (0.117 g, 0.36 mmol, 0.5 equiv) as a colorless solid (0.290 g, 82%).
(RS)-2-Amino-4-{3-[(carboxymethyl)(methyl)amino]propylthio}butanoic Acid (19)
Compound 54 (0.225 g, 0.47 mmol) was deprotected according to the general procedure C, affording 19 as a white solid (0.69 g, 55%). 1H NMR (600 MHz, D2O) 2.06 (m, 2H), 2.18 (m, 1H), 2.28 (m, 1H), 2.68 (m, 2H), 2.74 (t, 2H), 2.97 (s, 3H), 3.28 (m, 1H), 3.41 (m, 1H), 3.96 (bd, 1H), 4.05 (bd, 1H), 4.18 (t, 1H). 13C NMR (150.9 MHz, D2O) 26.16, 29.07, 30.13, 32.21, 44.10, 54.65, 58.67, 59.49, 171.57, 174.79. HRMS (ESI) calcd for C10H21N2O4S [M + H]+ 265.1222; found 265.1219.
(RS)-3-[3-(tert-Butoxycarbonylamino)-3-(tert-butoxycarbonyl)-propylthio]-N-(carboxymethyl)-N,N-dimethylpropan-1-ammonium iodide (55)
The procedure outlined for 47 was followed using amine 54 (0.222 g, 0.47 mmol) and methyl iodide (0.072 mL, 1.16 mmol, 2.5 equiv). The reaction was complete after 2 d, and compound 55 was isolated as a dark-yellow solid (0.153 g, 53%).
(RS)-2-{[3-(3-Amino-3-carboxypropylthio)propyl]dimethylammonio}acetate (18)
Compound 55 (0.140 g, 0.23 mmol) was deprotected according to the general procedure C, affording 18 as a white solid (0.059 g, 94%). 1H NMR (600 MHz, D2O) 2.07 (m, 2H), 2.15 (m, 1H), 2.22 (m, 1H), 2.67 (m, 2H), 2.71 (t, 2H), 3.24 (s, 3H), 3.27 (s, 3H), 3.68 (m, 2H), 3.95 (s, 2H), 3.99 (t, 1H). 13C NMR (150.9 MHz, D2O) 24.77, 29.17, 30.06, 32.65, 54.33 (2 × C), 55.76, 66.00, 66.20, 171.50, 176.00. HRMS (ESI) calcd for C11H23N2O4S [M]+ 279.1379; found 279.1370.
(RS)-Ethyl 5-Bromo-3-methylpentanoate (58)
(RS)-Tetrahydro-4-methylpyran-2-one39 (0.410 g, 3.59 mmol) was treated with a saturated solution of anhydrous HBr in EtOH (15 mL), and the mixture was stirred at rt for 3 d (TLC in T2). The reaction was quenched by pouring it into 200 mL of water and extracting it with Et2O(3 × 50 mL). The combined organic extracts were sequentially washed with water (100 mL), sat. aq NaHCO3 (50 mL), and water (100 mL), dried over Na2SO4, and evaporated in vacuo. The crude product was purified by flash chromatography on silica (isocratic elution, toluene only) to give compound 58 as a colorless liquid with a camphor odor (0.660 g, 82%).
Ethyl 5-Bromo-3,3-dimethylpentanoate (59)
The procedure outlined for 58 was followed using tetrahydro-4,4-dimethylpyran-2-one39 (1.03 g, 8.00 mmol) and saturated solution of anhydrous HBr in EtOH (20 mL), giving compound 59 as a colorless liquid with a camphor odor (1.690 g, 89%).
Ethyl (RS,RS)-5-[3-(tert-Butoxycarbonylamino)-3-(tert-butoxycarbonyl)propylthio]-3-methylpentanoate (60)
NaH (60% dispersion in oil; 0.061 g, 1.52 mmol, 1.2 equiv) was added to a solution of thiol 36 (0.369 g, 1.27 mmol, 1 equiv) in anhydrous THF (5 mL) in a flask equipped with a calcium chloride tube. Bromide 58 (0.282 g, 1.27 mmol, 1 equiv) was added after 10 min, and the reaction was allowed to proceed overnight (TLC in T2). The mixture was then concentrated in vacuo, and the residue was purified by flash chromatography on silica (elution with a linear gradient of EtOAc in toluene) to give compound 60 as a colorless thick oil (0.300 g, 55%). NMR: Mixture of diastereoisomers—some carbon signals are doubled.
Ethyl (RS)-5-[3-(tert-Butoxycarbonylamino)-3-(tert-butoxycarbonyl)propylthio]-3,3-dimethylpentanoate (61)
The procedure outlined for 60 was followed using thiol 36 (0.369 g, 1.27 mmol, 1 equiv), NaH (60% dispersion in oil; 0.061 g, 1.52 mmol, 1.2 equiv), and bromide 59 (0.300 g, 1.27 mmol, 1 equiv), giving compound 61 as a colorless thick oil (0.430 g, 76%).
(RS,RS)-5-(3-Amino-3-carboxypropylthio)-3-methylpentanoic Acid (20)
Compound 60 (0.290 g, 0.67 mmol) was deprotected according to the general procedure B, affording 20 as a white solid (0.106 g, 64%). NMR: Mixture of diastereoisomers ca. 1:1; doubling of the most of carbon signals observed. 1H NMR (600 MHz, DMSO-d6) 0.89 (d, 3H), 1.40 (m, 1H), 1.53 (m, 1H), 1.93 (m, 1H), 1.97 (m, 1H), 2.02 (dd, 1H), 2.03 (m, 1H), 2.24 (dd, 1H), 2.48 (m, 1H), 2.53 (m, 1H), 2.56 (m, 1H), 2.62 (m, 1H), 3.96 (dd, 1H), 8.25 (b, 2H), 12.10 (b, 2H). 13C NMR (150.9 MHz, DMSO-d6) 19.36 + 19.39, 26.50 + 26.54, 28.39 + 28.43, 29.20 + 29.22, 30.36 + 30.38, 35.84 + 35.88, 41.09 + 41.11, 51.38, 170.98, 174.05. HRMS (ESI) calcd for C10H20NO4S[M + H]+ 250.1113; found 250.1105.
(RS)-5-(3-Amino-3-carboxypropylthio)-3,3-dimethylpentanoic Acid (21)
Compound 61 (0.430 g, 0.96 mmol) was deprotected according to the general procedure B, affording 21 as a white solid (0.154 g, 61%). 1H NMR (600 MHz, D2O) 1.02 (s, 6H), 1.63 (m, 2H), 2.18 (m, 1H), 2.27 (m, 1H), 2.28 (s, 2H), 2.61 (m, 2H), 2.72 (t, 2H), 4.14 (dd, 1H). 13C NMR (150.9 MHz, D2O) 28.51, 28.97, 29.09, 29.10, 32.32, 35.63, 43.88, 48.10, 54.69, 175.04, 180.04. HRMS (ESI) calcd for C11H22NO4S[M + H]+ 264.1270; found 264.1276.
BHMT Inhibition Assays
Human recombinant BHMT was prepared as described previously,6 and N-methyl-14C-betaine (57 mCi/mmol) was prepared and supplied by Moravek Biochemicals (Brea, CA). Compounds were tested for their ability to inhibit BHMT activity using an assay procedure that we have described previously in detail.15,49 Briefly, dl-homocysteine was freshly prepared by dissolving dl-homocysteine thiolactone hydrochloride (15.4 mg) in 400 μL of 2 M NaOH. The solution was allowed to stand for 5 min at rt. The solution was then neutralized by the addition of 600 μL of a saturated solution of KH2PO4 and immediately used in the BHMT assay.
The standard BHMT assay mixture (500 μL) used to determine percent inhibition of activity contained 0.2 μM BHMT, different concentrations of inhibitor (20 or 1 μM), 100 μM dl-homocysteine, 250 μM betaine (0.05 μCi), 10 mM β-mercaptoethanol, and 50 mM K-phosphate buffer at pH 7.5. Human recombinant BHMT was first mixed with the inhibitor(s), and then the substrates were added and the mixture was incubated at 37 °C for 30 min. The reaction was stopped by transfer of the reaction tubes to ice water and by the addition of 2.5 mL ice-cold water. The samples were applied to a Dowex 1 × 4 (200–400 mesh) column, and the nonreacted betaine was washed from the column with water. Dimethylglycine and methionine were eluted into scintillation vials with 1.5 mL of 1.5 M HCl, and then 10 mL of the scintillation mixture were added to each vial and counted. Blanks contained all of the reaction components except the enzyme; their values were subtracted from the sample values. All samples were assayed in triplicate, and the results (reproducible within ± 15%) are expressed relative (%) to a sample containing no inhibitor.
Inhibition curves for the determination of IC50 values were measured using the conditions described above, except that the concentrations of substrates used were 1 mM dl-homocysteine and 2 mM betaine (0.15 μCi). The inhibition at 10 different inhibitor concentrations in duplicate was determined for each curve. The data were analyzed by use of nonlinear regression fit using the GraphPad Prism 5 program. The experiment was repeated three times, and the IC50 data in Table 1 are presented as means of three independent experiments ± SEM.
Isothermal Titration Calorimetry (ITC)
The binding of inhibitor 21 to BHMT was monitored using a VP-ITC microcalorimeter (MicroCal Inc., Northampton, MA) at 25 °C. The solutions of reactants were prepared in 50 mM HEPES/NaOH, ACES/NaOH, and Tris/HCl buffers, at pH 7.5, containing 5 mM β-mercaptoethanol. The concentration of BHMT protein (calculated for a BHMT monomer with a relative molecular weight of 45 kDa) was determined by amino acid analysis after thorough dialysis against the respective buffer. The concentration of inhibitor 21 was determined based on elemental analysis. Typically, 9 μL aliquots of 50 μM compound 21 (calculated for the S-enantiomer) were stepwise injected into the sample cell containing 1.43 mL of 5.1 μM protein until saturation was achieved. The assay was accompanied by a control experiment in which compound 21 was injected into buffer alone. The thermodynamic parameters and association constants were estimated using MicroCal Origin software.
Supplementary Material
Acknowledgment
This work was supported by an NIH Research Grant funded by the Fogarty International Center (R01 TW0052501, T.A.G. and J.J.), by a grant from the National Institutes of Health (DK52501, T.A.G.), Research Project of the Academy of Sciences of the Czech Republic (Z40550506, J.J.), and by grants from the Ministry of Education, Youth and Sports of the Czech Republic (LC060777, Research Centre for Chemical Genetics, J.J., and 1M0508, Research Centre for New Antivirals and Antineoplastics, M.K.).
Footnotes
Supporting Information Available: Synthesis of compounds 27–29, 63, and 64. Spectral data for nontarget compounds 23a, 23b, 24, 30–35, 38–55, and 58–61. HPLC purity data for target compounds. Isothermal titration calorimetry (ITC). Molecular modeling of inhibitor 21 into the active site of BHMT. 1H and 13C NMR spectra measurements for pH titration of compounds 12, 13, and 16. Inhibition experiments with enantiomers 63 and 64. This material is available free of charge via the Internet at http://pubs.acs.org.
Abbreviations: ACES, N-(2-acetamido)-2-aminoethanesulfonic acid; DEAM, diethyl acetamidomalonate; HEPES, 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid; ITC, isothermal titration calorimetry; TEBAC, benzyl triethylammonium chloride; DBU, 1,8-diazabicyclo[5.4.0]undec-7-ene; TFA, trifluoroacetic acid; Tris, tris(hydroxymethyl)aminomethane.
References
- 1.Pajares MA, Perez-Salab D. Betaine homocysteine S-methyltransferase: just a regulator of homocysteine metabolism. Cell. Mol. Life Sci. 2006;63:2792–2803. doi: 10.1007/s00018-006-6249-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sunden SLF, Renduchintala MS, Park EI, Miklasz SD, Garrow TA. Betaine-homocysteine methyltransferase expression in porcine and human tissues and chromosomal localization of the human gene. Arch. Biochem. Biophys. 1997;345:171–174. doi: 10.1006/abbi.1997.0246. [DOI] [PubMed] [Google Scholar]
- 3.Finkelstein JD, Martin JJ. Methionine metabolism in mammals. Distribution of homocysteine between competing pathways. J. Biol. Chem. 1984;259:9508–9513. [PubMed] [Google Scholar]
- 4.Finkelstein JD, Harris BJ, Kyle WE. Methionine metabolism in mammals: kinetic study of betaine-homocysteine methyltransferase. Arch. Biochem. Biophys. 1972;153:320–324. doi: 10.1016/0003-9861(72)90451-1. [DOI] [PubMed] [Google Scholar]
- 5.Millian NS, Garrow TA. Human betaine-homocysteine methyltransferase is a zinc metalloenzyme. Arch. Biochem. Biophys. 1998;356:93–98. doi: 10.1006/abbi.1998.0757. [DOI] [PubMed] [Google Scholar]
- 6.Breksa AP, III., Garrow TA. Recombinant human liver betainehomocysteine S-methyltransferase: identification of three cysteine residues critical for zinc binding. Biochemistry. 1999;38:13991–13998. doi: 10.1021/bi991003v. [DOI] [PubMed] [Google Scholar]
- 7.Evans JC, Huddler DP, Jiracek J, Castro C, Millian NS, Garrow TA, Ludwig ML. Betaine-homocysteine methyltransferase. Zinc in a distorted barrel. Structure. 2002;10:1159–1071. doi: 10.1016/s0969-2126(02)00796-7. [DOI] [PubMed] [Google Scholar]
- 8.Collinsova M, Castro C, Garrow TA, Yiotakis A, Dive V, Jiracek J. Combining combinatorial chemistry and affinity chromatography: highly selective inhibitors of human betaine:homocysteine S-methyltransferase. Chem. Biol. 2003;10:113–122. doi: 10.1016/s1074-5521(03)00008-5. [DOI] [PubMed] [Google Scholar]
- 9.Koval D, Kasicka V, Jiracek J, Collinsova M. Separation of diastereomers of phosphinic pseudopeptides by capillary zone electrophoresis and reverse-phase high-performance liquid chromatography. J. Sep. Sci. 2003;26:660. [Google Scholar]
- 10.Koval D, Kasicka V, Jiracek J, Collinsova M. Physicochemical characterization of phosphinic pseudopeptides by capillary zone electrophoresis in highly acid background electrolytes. Electrophoresis. 2003;24:774–781. doi: 10.1002/elps.200390097. [DOI] [PubMed] [Google Scholar]
- 11.Koval D, Kasicka V, Jiracek J, Collinsova M, Garrow TA. Determination of dissociation constant of phosphinate group in phosphinic pseudopeptides by capillary zone electrophoresis. J. Chromatogr., B: Anal. Technol. Biomed. Life Sci. 2002;770:145–154. doi: 10.1016/s1570-0232(01)00595-5. [DOI] [PubMed] [Google Scholar]
- 12.Koval D, Kasicka V, Jiracek J, Collinsova M, Garrow TA. Analysis and characterization of phosphinic pseudopeptides by capillary zone electrophoresis. Electrophoresis. 2002;23:215–222. doi: 10.1002/1522-2683(200202)23:2<215::AID-ELPS215>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 13.Koval D, Kasicka V, Jiracek J, Collinsova M. Determination of pKa values of diastereomers of phosphinic pseudopeptides by CZE. Electrophoresis. 2006;27:4648–4657. doi: 10.1002/elps.200600133. [DOI] [PubMed] [Google Scholar]
- 14.Koval D, Busnel JM, Hlavacek J, Jiracek J, Kasicka V, Peltre G. Evaluation of carrier ampholyte-based capillary electrophoresis for separation of peptides and peptide mimetics. Electrophoresis. 2008;29:3759–3767. doi: 10.1002/elps.200800193. [DOI] [PubMed] [Google Scholar]
- 15.Jiracek J, Collinsova M, Rosenberg I, Budesinsky M, Protivinska E, Netusilova H, Garrow TA. S-Alkylated homocysteine derivatives: new inhibitors of human betaine-homocysteine S-methyltransferase. J. Med. Chem. 2006;49:3982–3989. doi: 10.1021/jm050885v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Awad WM, Jr., Whitney PL, Skiba WE, Mangum JH, Wells MS. Evidence for direct methyl transfer in betaine: homocysteine S-methyltransferase. J. Biol. Chem. 1983;258:12790–12792. [PubMed] [Google Scholar]
- 17.Castro C, Gratson AA, Evans JC, Jiracek J, Collinsova M, Ludwig ML, Garrow TA. Dissecting the catalytic mechanism of betaine-homocysteine S-methyltransferase by use of intrinsic tryptophan fluorescence and site-directed mutagenesis. Biochemistry. 2004;43:5341–5351. doi: 10.1021/bi049821x. [DOI] [PubMed] [Google Scholar]
- 18.Collinsova M, Strakova J, Jiracek J, Garrow TA. Inhibition of betaine-homocysteine S-methyltransferase causes hyperhomocysteinemia in mice. J. Nutr. 2006;136:1493–1497. doi: 10.1093/jn/136.6.1493. [DOI] [PubMed] [Google Scholar]
- 19.Wettstein M, Weik C, Holneicher C, Häussinger D. Betaine as an osmolyte in rat liver: metabolism and cell-to-cell interactions. Hepatology. 1998;27:787–793. doi: 10.1002/hep.510270321. [DOI] [PubMed] [Google Scholar]
- 20.Craig SAS. Betaine in human nutrition. Am. J. Clin. Nutr. 2004;80:539–549. doi: 10.1093/ajcn/80.3.539. [DOI] [PubMed] [Google Scholar]
- 21.Wijekoon EP, Brosnan ME, Brosnan JT. Homocysteine metabolism in diabetes. Biochem. Soc. Trans. 2007;35:1175–1179. doi: 10.1042/BST0351175. [DOI] [PubMed] [Google Scholar]
- 22.Devlin AM, Singh R, Wade RE, Innis SM, Bottiglieri T, Lentz SR. Hypermethylation of Fads2 and altered hepatic fatty acid and phospholipid metabolism in mice with hyperhomocysteinemia. J. Biol. Chem. 2007;282:37082–37090. doi: 10.1074/jbc.M704256200. [DOI] [PubMed] [Google Scholar]
- 23.Zeisel SH. Betaine supplementation and blood lipids: Fact or artifact? Nutr. Rev. 2006;64:77–79. doi: 10.1301/nr.2006.feb.77-79. [DOI] [PubMed] [Google Scholar]
- 24.Elmore CL, Matthews RG. The many flavors of hyperhomocyst(e)inemia: insights from transgenic and inhibitor-based mouse models of disrupted one-carbon metabolism. Antioxid. Redox Signaling. 2007;9:1911–1921. doi: 10.1089/ars.2007.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leinhard GE. Enzymatic catalysis and transition-state theory. Science. 1973;180:149–154. doi: 10.1126/science.180.4082.149. [DOI] [PubMed] [Google Scholar]
- 26.Schramm VL, Horenstein BA, Kline PC. Transition state analysis and inhibitor design for enzymatic reactions. J. Biol. Chem. 1994;269:18259–18262. [PubMed] [Google Scholar]
- 27.Wolfenden R, Radzicka A. Transition-state analogues. Curr. Opin. Struct. Biol. 1991;1:780–787. [Google Scholar]
- 28.Herriott AW, Picker D. Phase-transfer synthesis of sulfides and dithioacetals. Synthesis. 1975:447–448. [Google Scholar]
- 29.Semonsky M, Kotva R, Vachek J, Jelinek V. Substances with antineoplastic activity. 27. Some alpha-substitution derivatives of delta-(6-purinylthio)valeric and epsilon-(6-purinylthio)capronic acids. Collect. Czech. Chem. Commun. 1968;33:3823–3832. [Google Scholar]
- 30.Barraclough P, Caldwell AG, Glen RC, Harris CJ, Stepney R, Whittaker N, Whittle BJR. Synthesis and platelet aggregation inhibiting activity of acid side-chain modified hydantoin prostaglandin analogs. Arch. Pharm. 1993;326:85–95. doi: 10.1002/ardp.19933260206. [DOI] [PubMed] [Google Scholar]
- 31.Keith DD, Yang R, Tortora JA, Weigele M. Synthesis of DL-2-Amino-4-(2-aminoethoxy)-trans-but-3-enoic Acid. J. Org. Chem. 1978;43:3713–3716. [Google Scholar]
- 32.Dupuy C, Petrier C, Sarandeses LA, Luche JL. Ultrasound in organic syntheses. 19. Further studies on the conjugate additions to electron deficient olefins in aqueous media. Synth. Commun. 1991;21:643–651. [Google Scholar]
- 33.Siebum AHG, Woo WS, Raap J, Lugtenburg J. Access to any site-directed isotopomer of methionine, selenomethionine, cysteine, and selenocysteine—use of simple, efficient modular synthetic reaction schemes for isotope incorporation. E. J. Org. Chem. 2004:2905–2913. [Google Scholar]
- 34.Zhu JG, Hu XB, Dizin E, Pei DH. Catalytic mechanism of S-ribosylhomocysteinase (LuxS): Direct observation of ketone intermediates by C-13 NMR spectroscopy. J. Am. Chem. Soc. 2003;125:13379–13381. doi: 10.1021/ja0369663. [DOI] [PubMed] [Google Scholar]
- 35.Hermkens PHH, Vonmaarseveen JH, Kruse CG, Scheeren HW. Intramolecular Pictet-Spengler reaction of N-alkoxy tryptamines. 1. Synthesis of (±)-deamino-debromo-eudistomin l. Tetrahedron Lett. 1989;30:5009–5012. [Google Scholar]
- 36.Frankel M, Gertner D. Syntheses of homologues of djenkolic acid. J. Chem. Soc. 1960:898–899. [Google Scholar]
- 37.Shinohara T, Takeda A, Toda J, Sano T. Determination of ring conformation in 1-benzyl-1,2,3,4-tetrahydroisoquinolines and a new synthesis of the chiral compounds. Chem. Pharm. Bull. 1998;46:430–433. [Google Scholar]
- 38.Toda J, Ichikawa T, Saitoh T, Horiguchi Y, Sano T. A synthesis of 2,3,4,5-tetrahydro-1H-3-benzazepines via Pummerer-type cyclization of N-(2-arylethyl)-N-(2-phenylsulfinylethyl)formamides. Hetero-cycles. 2000;53:2009–2018. [Google Scholar]
- 39.Reynolds NT, Rovis T. The effect of pre-existing stereocenters in the intramolecular asymmetric Stetter reaction. Tetrahedron. 2005;61:6368–6378. [Google Scholar]
- 40.Mcintosh JM, Pillon LZ, Acquaah SO, Green JR, White GS. Enamines and iminium salts from amido acids. Can. J. Chem. 1983;61:2016–2021. [Google Scholar]
- 41.Snider BB, Lu Q. Total synthesis of (±)-leporin A. J. Org. Chem. 1996;61:2839–2844. doi: 10.1021/jo952053i. [DOI] [PubMed] [Google Scholar]
- 42.Fukada H, Takahashi K. Enthalpy and heat capacity changes for the proton dissociation of various buffer components in 0.1 M potassium chloride. Proteins. 1998;33:159–166. [PubMed] [Google Scholar]
- 43.Beres L, Sturtevant JM. Calorimetric studies of activation of chymotrypsinogen-A. Biochemistry. 1971;10:2120–2126. doi: 10.1021/bi00787a025. [DOI] [PubMed] [Google Scholar]
- 44.Szegedi SS, Garrow TA. Oligomerization is required for betainehomocysteine S-methyltransferase function. Arch. Biochem. Biophys. 2004;426:32–42. doi: 10.1016/j.abb.2004.03.040. [DOI] [PubMed] [Google Scholar]
- 45.Miller CM, Szegedi SS, Garrow TA. Conformation-dependent inactivation of human betaine-homocysteine S-methyltransferase by hydrogen peroxide in vitro. Biochem. J. 2005;392:443–448. doi: 10.1042/BJ20050356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gonzalez B, Pajares MA, Martinez-Ripoll M, Blundell TL, Sanz-Aparicio J. Crystal structure of rat liver betaine homocysteine S-methyltransferase reveals new oligomerization features and conformational changes upon substrate binding. J. Mol. Biol. 2004;338:771–782. doi: 10.1016/j.jmb.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 47.Penner-Hahn J. Zinc-promoted alkyl transfer: a new role for zinc. Curr. Opin. Chem. Biol. 2007;11:166–171. doi: 10.1016/j.cbpa.2007.02.038. [DOI] [PubMed] [Google Scholar]
- 48.Skiba WE, Taylor MP, Wells MS, Mangum JH, Awad WM., Jr. Human hepatic methionine biosynthesis. Purification and characterization of betaine:homocysteine S-methyltransferase. J. Biol. Chem. 1982;257:14944–14948. [PubMed] [Google Scholar]
- 49.Garrow TA. Purification, kinetic properties, and cDNA cloning of mammalian betaine-homocysteine methyltransferase. J. Biol. Chem. 1996;271:22831–22838. doi: 10.1074/jbc.271.37.22831. [DOI] [PubMed] [Google Scholar]
- 50.Korte F, Lohmer KH. Alpha-hydroxyalkyliden-lacton-umlagerung. 4. Die synthese von 4.5-dihydro-thiophen-carbonsaure-(3)-estern. Chem. Ber.-Recl. 1957;90:1290–1295. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.