Summary
Immunohistochemical determination of p63 protein is frequently used in the pathologic diagnosis of nonhematological solid tumors. In malignant hematological disease, p63 expression has been reported in 22% of follicular lymphoma, about 35% of diffuse large B-cell lymphoma, 23% of chronic lymphocytic leukemia, and in some cases of blast crisis of chronic myelogenous leukemia. Anaplastic large cell lymphoma is a rare disease that accounts for less than 5% of all cases of non-Hodgkin's lymphoma. There is little information concerning p63 expression in this specific type of lymphoma. In some cases, the morphological and phenotypic features between anaplastic large cell lymphoma and classical Hodgkin's lymphoma are similar, making this differential diagnosis challenging. We studied p63 expression using a tissue microarray approach in 154 cases of anaplastic large cell lymphoma, including 38% anaplastic large cell kinase positive and 62% anaplastic large cell kinase negative, and 58 Hodgkin's lymphoma cases. Sixty-eight cases of anaplastic large cell lymphoma (44%) showed p63 nuclear positivity (41% of anaplastic large cell kinase positive and 47% of anaplastic large cell kinase negative). Of 130 cases of systemic-anaplastic large cell lymphoma, 42% showed p63 positivity. The neoplastic cells expressed p63 in 38% of the cases of CD45-negative/anaplastic large cell kinase–negative null cell–type anaplastic large cell lymphoma, a subgroup that offers the most difficulties in the differential diagnosis with classical Hodgkin's lymphoma. In contrast, none of the cases of classical Hodgkin's lymphoma demonstrated any p63 expression. These results demonstrate that p63 protein expression is frequently expressed in a subset of anaplastic large cell lymphoma cases and may be used as a potential tool in the differential diagnosis between anaplastic large cell lymphoma and classical Hodgkin's lymphoma.
Keywords: p63, Anaplastic large cell lymphoma, Hodgkin's lymphoma, Immunohistochemistry, Tissue microarray
1. Introduction
p63 is a transcription factor that contains multiple isoforms with various biological activities [1]. The p63 gene locus at chromosome 3q28 bears strong homology to the tumor suppressor gene p53 and to the related gene p73. Both p63 and p73, considered p53-related genes, encode numerous proteins with transactivation, DNA binding, and tetramerization domains [2]. p63 protein exhibits a consistent expression pattern in normal tissues such as squamous epithelia, urothelium, basal cells of prostatic and breast glands, reticular epithelium of the normal thymus, and also in a subset of lymphocytes in the germinal center of morphologically normal lymph nodes [3,4]. Among hematolymphoid neoplasms, p63 expression has been reported in blast crisis in chronic myelogenous leukemia [5], follicular lymphoma (FL), diffuse large B-cell lymphoma (DLBCL) [2], isolated cases of chronic lymphocytic leukemia, marginal cell lymphoma, and in one of 2 cases of anaplastic large cell lymphoma (ALCL) [3]. Although the prognostic significance of p63 remains to be studied in lymphoid neoplasms, the correlation of p53 overexpression with aggressive disease and poor clinical outcome has been well documented in many hematological neoplastic diseases [6-8].
ALCL is a rare disease, accounting for less than 5% of all cases of non-Hodgkin's lymphoma (NHL). ALCL is an Epstein-Barr virus (EBV)–negative lymphoid neoplasm with a CD30-positive T-cell/null phenotype, with frequent expression of cytotoxic markers such as TIA-1, granzyme B, and perforin. It occurs as 2 distinct clinical entities: cutaneous (C-ALCL) and systemic (S-ALCL), as established by the World Health Organization classification [9]. Three histologic variants are recognized, including common, lymphohistiocytic, and small cell variants [9]. The differential diagnosis of ALCL includes nonhematopoietic neoplasms such as poorly differentiated carcinoma and melanoma. Among hematologic neoplasms, Hodgkin's lymphoma (HL), namely, the syncytial nodular sclerosis subtype, is often a challenging differential diagnosis. HL and ALCL may share cytologic and architectural features, such as fibrosis associated with capsular thickening, nodular formation, tumor cells resembling Reed-Sternberg cells, and atypical cells within residual lymphatic sinuses [10], besides being common in young patients. Although the use of a panel of lymphoid markers including CD15, CD45, CD3, ALK, EBV-LMP1, CD20, PAX-5, and epithelial membrane antigen is sufficient in solving the diagnostic dilemma in most cases, there are situations in which this panel may give conflicting results [11,12], namely, cases of HL that lack expression of CD15 and ALCL cases lacking expression of CD45, ALK, and T-cell markers. The distinction between these 2 types of lymphomas is of utmost importance because ALCL, an aggressive lymphoma, can be cured in about 80% of the cases with third-generation therapeutic regimens, whereas HL requires a different type of treatment [9,13]. In our daily practice, we have observed that a considerable number of ALCL cases express p63. Based on this observation, we decided to investigate the frequency of p63 expression in a large number of cases of ALCL, specifically examining the possibility that p63 may represent an additional marker to differentiate ALCL from CHL.
2. Materials and methods
2.1. Case material and clinical data
The study group included 154 cases of ALCL, of both S-ALCL (nodal and extranodal) and C-ALCL types, with available representative tissue in paraffin blocks. These cases were selected from a total of 346 cases of ALCL received in consultation between the period of June 1997 and May 2007 at Consultoria Patologia, a large reference service for anatomic pathology in Brazil. In addition, 58 consecutive cases of HL, including 51 cases of CHL and 7 cases of nodular lymphocyte predominance HL (NPLHL), all from 2007, were included in the analysis. Clinical data, including sex, age at diagnosis, and tumor anatomic location, were all obtained from the referring pathologists/oncologists or from the surgical pathology reports. The available hematoxylin and eosin–stained slides of each case were reviewed, and representative areas were selected for tissue microarray construction. A morphologic subclassification of ALCL cases was also performed, using variants included in World Health Organization classification where appropriate [9].
2.2. Tissue microarray construction
Three tissue microarray blocks were constructed, 2 for ALCL and the other for CHL, using a tissue arrayer (Beecher Instruments, Sun Prairie, WI). Three tumor cores of 0.6 mm represented each individual case for ALCL and 2-mm cores for CHL, which had been taken from the original paraffin blocks. Serial sections of 3 μm were cut from the tissue array blocks and used for immunohistochemical analysis. Proper positive and negative control cores for each marker were also included in the array block to provide adequacy of the antibodies used in the immunohistochemical study. All cases of NPLHL were studied using the original paraffin blocks.
2.3. Immunohistochemistry
Primary antibodies, source, dilution, and epitope retrieval methods are shown in Table 1. For p63 immunostaining, only tumors with more than 5% of the neoplastic cells showing nuclear expression were considered as positive. For the p63 immunostaining, cases were divided into 4 groups according to the following scores: negative, ≤5%; 1+, >5% to 10%; 2+, >10% to 50%; and 3+, >50%. Novolink polymer (Novocastra, Newcastle Upon Tyne, UK) was the detection system, and diaminobenzidine was the chromogen. We diagnosed ALK-negative ALCL according to a widely accepted consensus definition, that is, cases closely mimicking ALK-positive ALCL in its histologic and immunophe-notypic findings but without ALK expression [11]. A T-cell phenotype was defined when the lymphoma cells were positive for at least one of the specific T-cell markers tested (CD3, CD2, CD4, CD5, and CD7).
Table 1.
Antibodies used and technical details
| Antibodies to | Source | Clone | Dilution | Epitope retrieval |
|---|---|---|---|---|
| CD45RB | DAKO | 2B11+PD7 | 1:500 | MW-CB |
| CD30 | DAKO | Ber-H2 | 1:300 | MW-CB |
| p63 | Lab Vision | 4A4 | 1:300 | PC-CB |
| CD20 | DAKO | L26 | 1:1200 | MW-CB |
| CD3 | Lab Vision | SP7 | 1:200 | S-CB |
| ALK | DAKO | ALK1 | 1:200 | S-TRIS |
| CD25 | Novocastra | 4C9 | 1:200 | S-EDTA |
| CD15 | B-Dickinson | Leu-M1 | 1:50 | MW-CB |
| EMA | DAKO | E29 | 1:800 | PC-CB |
| Ki-67 | DAKO | MIB-1 | 1:4800 | PC-CB |
| CD7 | Novocastra | 272 | 1:400 | PC-CB |
| CD2 | Novocastra | 11F11 | 1:200 | MW-CB |
| CD4 | Novocastra | 4B12 | 1:100 | S-EDTA |
| CD5 | Novocastra | 4C7 | 1:150 | MW-CB |
| Clusterin | Novocastra | 7D1 | 1:50 | MW-CB |
| PAX-5 | B-Dickinson | 24 | 1:100 | S-CB |
NOTE. DAKO, Carpinteria, CA; Lab Vision Corporation, Fremont, CA, Novocastra, Newcastle Upon Tyne, United Kingdom; B-Dickinson: Becton-Dickinson Biosciences, Mountain View, CA. Heat-induced epitope retrieval was used.
Abbreviations: EMA, epithelial membrane antigen; MW, microwave oven; PC, pressure cooker; S, steamer; CB, citrate buffer.
2.4. Statistical methods
The Pearson χ2 method was used to evaluate the contributions of immunohistochemical indicators to the differential diagnosis between ALCL and CHL. The significance level adopted was 10%.
3. Results
In the ALCL group, there were 91 males and 63 females and the mean age was 43 years (4-92 years). A total of 130 cases were S-ALCL, with a nodal presentation in 104 cases (68%) and extranodal presentations in 26 cases (32%), including soft tissue, digestive tract, bone, and breast. Twenty-four cases were primary C-ALCL. Morphologically, most ALCL cases were of the common subtype (97%), with 2 cases of the small cell variant and 3 cases of the lymphohistiocytic variant; all but one of these variants were nodal.
All cases of ALCL were CD30 positive in virtually all neoplastic cells. The immunophenotype was T cell in 92 cases (60%) and null cell in the remaining 62 cases (40%). Nuclear expression of p63 protein was seen in 68 (44%) of 154 cases of ALCL; 35 cases of 1+ (51%), 14 cases of 2+ (21%), and 19 cases of 3+ (28%) (Fig. 1). In the group of S-ALCL, 58 (46%) of 130 cases showed p63 expression, most of them 1+ (53%), the rest were 2+ and 3+ in a similar proportion (Table 2). All but 2 p63-positive cases were of common type; one was a small cell variant and the other one was a lymphohistiocytic variant. ALK expression was observed in 49 (38%) of the S-ALCL cases and 20 (41%) of these cases, all nodal, demonstrated expression of p63, mostly 1+ (65%). There was no statistically significant association between p63 expression and ALK-positive or ALK-negative ALCL groups, even when the cases were stratified by phenotype (T or null) (Table 3).
Fig. 1.
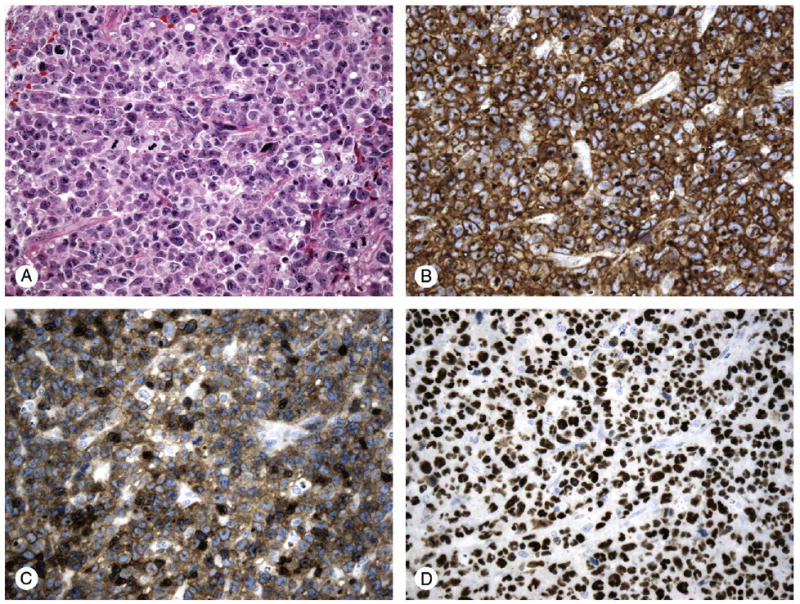
S-ALCL case, common type; A, hematoxylin-eosin (original magnification, ×200); B, CD30 positive (original magnification, ×200); C, CD3 positive, T-cell phenotype (original magnification, ×200); D, p63 nuclear expression (score 3+) (original magnification, ×200).
Table 2.
ALCL p63-positive cases: p63 scores related to anatomical type (S-ALCL and C-ALCL)
| p63 scores | ALCL (all + cases) |
S-ALCL | C-ALCL |
|---|---|---|---|
| 1+ | 35 (51.5%) | 31 (53.4%) | 4 (40%) |
| 2+ | 14 (27.9%) | 13 (22.4%) | 1 (10%) |
| 3+ | 19 (20.6%) | 14 (24.1%) | 5 (50%) |
| Total | 68 (100%) | 58 (100%) | 10 (100%) |
| (% of positive cases including all scores) | (44.2%) | (44.6%) | (41.6%) |
NOTE. 1+: >5% to 10%; 2+: >10% to 50%; and 3+: >50%.
Table 3.
Distribution of p63 expression in ALK-positive and ALK-negative-S-ALCL
| ALK expression in S-ALCL (130 cases) | |||
|---|---|---|---|
| Negative (n = 81) | Positive (n = 49) | ||
| p63 expression | p63 0 (n = 72) | 43 (53.1%) * | 29 (59.2%) |
| p63 1+ (n = 31) | 18 (22.2%) | 13 (26.5%) | |
| p63 2+ (n = 13) | 8 (9.9%) | 5 (10.2%) | |
| p63 3+ (n = 14) | 12 (14.8%) | 2 (4.1%) | |
| p63-positive cases including all scores (n = 58) | 38 (46.9%) | 20 (40.8%) | |
(%) related to ALK expression.
It is worth mentioning that CD45 was expressed in 91 cases (70%). Twelve (31%) of the 39 cases of CD45-negative ALCL showed p63 expression; 3 of these cases had a null phenotype with no expression of ALK. There was no significant association between p63 and CD45 expression in the S-ALCL group (χ2=4.94 with P = .18) (Table 4). Clusterin was expressed in 64 cases (49.2%) of S-ALCL.
Table 4.
p63 distribution in S-ALCL in relation to CD45 expression
| S-ALCL | CD45 expression (130 cases) | ||
|---|---|---|---|
| Negative (39 cases) | Positive (91 cases) | ||
| p63 expression | 0 (n = 72) | 27 (69%) * | 45 (49.5%) |
| 1+ (n = 31) | 6 (15.4%) | 25 (27.5%) | |
| 2+ (n = 13) | 2 (5.1%) | 11 (12%) | |
| 3+ (n = 14) | 4 (10.3%) | 10 (10.9%) | |
(%) related to CD45 expression.
C-ALCL presented with a T-cell immunophenotype in 10 cases (42%) and null-cell immunophenotype in 14 cases (58%). As expected, all C-ALCL were negative for ALK expression. p63 expression was observed in 10 cases (46%) of C-ACLC. Only 3 of these cases were CD45 negative, one of them expressing p63. Clusterin positivity was observed in 10 C-ALCL (41.7%) cases, 3 with p63 coexpression.
It was not possible to detect significant differences in p63 expression between C-ALCL and S-ALCL owing to the small number of patients in the C-ALCL group. The χ2 statistic was equal to 2.98 with P = .23 (Table 2).
PAX-5 expression was seen in 5 cases of ALCL, including 3 S-ALCL and 2 C-ALCL, all of T-cell phenotype; 3 of these 5 were p63 positive. On the other hand, in the group of 149 PAX-5–negative ALCL cases, 65 (44%) showed expression of p63. CD15 positivity was identified in 3 cases of ALCL; all of them PAX-5/CD20 negative and CD45 positive; one showed a T-cell phenotype and the other 2 were of null type, one of these being p63 positive. The most overlapping cases in reference to the differential diagnosis with CHL included 8 cases of CD45/ALK negative, null phenotype; 3 other cases which were CD15 positive; and 5 cases which were PAX-5 positive; 7 (44%) of these 16 cases showed p63 expression.
In the group of 58 HL cases, 25 were male and 33 female with mean age of 32 years (range, 4 to 82 years). Among the 51 CHL, 39 cases (67%) were nodular sclerosis, 8 cases (16%) mixed cellularity, 1 case lymphocyte depletion, and 3 unclassified. None of the cases of CHL showed p63 expression (Fig. 2). CD30 was expressed in all CHL cases, CD15 expression was present in 45 cases (88%), and 8 cases (16%) expressed CD20. PAX-5 was positive in 34 cases (67%), including 28, which were CD20 negative. In 17 cases of PAX-5–negative CHL, only 2 were CD20 positive and one other case was CD15 negative. All CHL cases were CD45 negative. Clusterin expression was observed in 11 CHL cases (22%). p63 expression was identified in 2 (29%, 1+) of 7 cases of nodular lymphocyte predominance HL; these cases were all CD20 and PAX-5 positive.
Fig. 2.
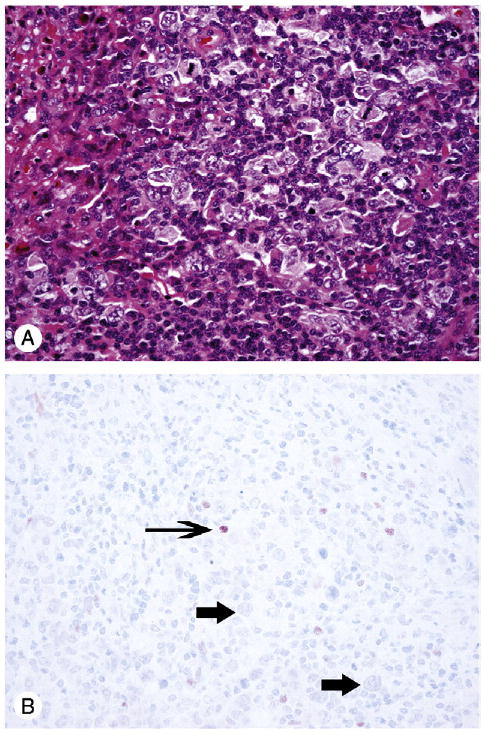
Classical HL; A, hematoxylin-eosin (original magnification, ×200); B, p63 negative in Reed-Sternberg cells and their variants (thick arrows); a few reactive lymphoid cells reveal p63 nuclear expression (thin arrow) (original magnification, ×200).
Table 5 summarizes the immunophenotype results in all cases of ALCL and HL.
Table 5.
Immunohistochemical results in ALCL and CHL
| Lymphoma group | CD30 | CD15 | p63 | ALK | PAX-5 | CD45 | EMA | Clusterin | CD20 | |
|---|---|---|---|---|---|---|---|---|---|---|
| ALCL (n = 154) (%) | Total | 154 (100) | 3 * (1.9) | 68 (44.2) | 49 (38.8) | 5 (3.2) | 91 (59) | 79 (51) | 58 (38) | 0 |
| T-cell (n = 92) | 92 (59.7) | 1 (1.1) | 46 (50) | 8 (16.3) | 5 (5.4) | 57 (62) | 35 (38) | 34 (37) | 0 | |
| N cell (n = 62) | 62 (40.3) | 2 (3.2) | 22 (35.5) | 41 (83.7) | 0 | 34 (54.8) | 44 (71) | 24 (39) | 0 | |
| HL (n = 58) (%) | CHL (n = 51) | 51 (100) | 45 (88.2) | 0 | 0 | 34 (66.7) | 0 | 4 (7.8) | 11 (22) | 8 (16) |
| NLPHL (n = 7) | 0 | 0 | 2 (28.6) | 0 | 7 (100) | 7 (100) | 0 | ND | 7 (100) |
Abbreviation: NLPHL, nodular lymphocyte predominance HL.
All 3 cases were PAX-5 and CD20 negative and LCA positive.
4. Discussion
There are few studies evaluating the expression of p63 in lymphoid malignancies, and to the best of our knowledge, none has included a large number of ALCL and HL cases. Di Como et al [3] studied p63 expression in normal and neoplastic human tissues and described a population of p63-positive cells in germinal centers of normal lymph nodes and in a subset of reactive lymphocytes in other tissues. Among hematolymphoid neoplasms, these authors found p63 positivity in DLBCL (33%), chronic lymphocytic leukemia (23%), FL (22%), one of 2 ALCL tested, none of 28 cases of CHL, and 1 of 6 nodular lymphocyte predominance HL samples. In a series of 172 cases of DLBCL, Hedvat et al [2] reported p63 expression in 32% of cases, which supports the hypothesis that p63 mutations may contribute to the development and progression of lymphoid neoplasms. Park and Oh [14] found p63 positivity in 38 cases (30%) of 126 NHLs, 53% of the DLBCL, 28% of the FL, 29% of T-cell lymphomas, and none of 5 cases of CHL. Others have found p63 overexpression in blast crisis in chronic myeloid leukemia [5]. In the present study, we observed an overall expression of p63 in 44% (68/154) of cases of ALCL. The anatomic presentation (systemic versus primary cutaneous) had no impact on the p63 expression. Cases of T-cell phenotype showed a greater number of cases that were p63 positive than cases of null type, 50% versus 37%. Interestingly, none of our cases of CHL showed expression for p63 protein in Hodgkin cells. It is well known, from a morphological and immunohistochemical perspective, that the differential diagnosis between CHL and ALCL may sometimes be challenging, and establishing a histologic diagnosis for the gray zone between CHL and ALCL is often confusing [14]. Willenbrock et al [15] in a transcriptional profiling study of ALCL cell lines and HL cell lines demonstrated that at the transcriptional level HLs are more closely related to ALK-positive ALCL than to B-cell lymphoma samples despite being a B-cell–derived lymphoma. Histopathologically, one can observe cases of ALCL with CHL-like morphology (so-called Hodgkin-like ALCL) [10]. In addition, straightforward cases of CHL may be negative for CD15 and typical cases of ALCL may show expression of CD15, making the differential diagnosis of these 2 entities somewhat problematic [9-11]. In the literature, there are several studies specifically addressing this diagnostic dilemma. Nascimento et al [16] described the use of clusterin, a ubiquitous 75- to 80-kd glycoprotein, and found positivity in 40 of 41 ALCL. Unfortunately, this high percentage of clusterin expression in ALCL has not been reproduced by others [17]. We found a relatively low frequency of clusterin positivity (49.2%) in our ALCL cases. Another tumor marker that has been used in this diagnostic situation is fascin. This is a distinct 55-kd actin-binding protein whose expression in cells may be induced by EBV infection and was initially considered to be specific for CHL [18]. However, other studies have concluded that fascin is also often expressed in the majority of cases of ALCL, including both ALK-1–positive and ALK-1–negative cases. Therefore fascin should not be used to distinguish ALCL from HL [12,19,20]. A useful marker for this differential is PAX-5, a transcription factor, with almost complete restriction to the B-cell lineage. The frequency of PAX-5 positivity in CHL varies between 65% and 88% in the literature [12,21,22]; in contrast, ALCL PAX-5 positivity is reported between 0% and 18%, mainly in ALK-negative cases [12,22]. We found PAX-5 positivity in 5 cases of ALCL, all ALK negative and 3 also positive for p63. Our finding of p63 expression in ALCL, in comparison to the consistently negative expression CHL, may be helpful in differential diagnosis, especially in ALCL-ALK–negative and/or null-phenotype cases. We found that 38% of these cases expressed p63, and when including other difficult cases such as cases of CD15-positive or Pax-5–positive ALCL, p63 positivity reached 44%.
There are many questions about the relationship between p63 overexpression and lymphoma pathobiology, classification, and prognosis. It is not clear why p63 is overexpressed in DLBCL and other lymphomas. It has been speculated that, because p63 lies close to the translocation breakpoint found in some DLBCL, genomic instability in this area may lead to p63 deregulation [23]. Among possible functions hypothesized for p63 is the regulation of cell proliferation [2]. The prognostic significance of p63 has yet to be studied in lymphoid neoplasms, although the correlation of p53 over-expression with aggressive disease and poor clinical outcome is well documented [4,24,25]; and frequent p73 overexpression is found in high-risk B-cell lymphocytic leukemia, Burkitt lymphoma, and acute lymphoblastic leukemia [26,27]. In contrast, there is an inverse correlation between p63 overexpression and advanced tumor stage and poor prognosis in other types of solid tumors [23,28]. Interestingly, a positive correlation between Ki-67 proliferation index and p63 gene copy has been observed in many solid tumors [24]. Further investigation of p63 overexpression is necessary to better understand the p53 family and the function of their protein products in lymphoid neoplasms, particularly in light of the potential for p63 as a therapeutic target.
Acknowledgments
We acknowledge the outstanding service of the Consultoria em Patologia staff for skillful technical assistance.
Footnotes
This study was partially supported by NCI 5R01CA082274, 5R01CA112217 (WH), and the (NCI) AIDS Malignancy Consortium.
References
- 1.Flores ER. The roles of p63 in cancer. Cell Cycle. 2007;6:300–4. doi: 10.4161/cc.6.3.3793. [DOI] [PubMed] [Google Scholar]
- 2.Hedvat CV, Teruya-Feldstein J, Puig P, et al. Expression of p63 in diffuse large B-cell lymphoma. Appl Immunohistochem Mol Morphol. 2005;13:237–42. doi: 10.1097/01.pai.0000142160.52670.ce. [DOI] [PubMed] [Google Scholar]
- 3.Di Como CJ, Urist MJ, Babayan I, et al. p63 expression profiles in human normal and tumor tissues. Clin Cancer Res. 2002;8:494–501. [PubMed] [Google Scholar]
- 4.Nylander K, Vojtesek B, Nenutil R, et al. Differential expression of p63 isoforms in normal tissues and neoplastic cells. J Pathol. 2002;198:417–27. doi: 10.1002/path.1231. [DOI] [PubMed] [Google Scholar]
- 5.Yamaguchi H, Inokuchi K, Tarusawa M, et al. Mutation of P51A/TAP63 g gene is associated with blastic crisis in chronic myeloid leukemia. Exp Hematol. 2000;28:76. [Google Scholar]
- 6.Preudhomme C, Fenaux P. p53 and hematologic malignancies. Pathol Biol (Paris) 1997;45:898–908. [PubMed] [Google Scholar]
- 7.Vigano MA, Mantovani R. Hitting the numbers: the emerging network of p63 targets. Cell Cycle. 2007;6:233–9. doi: 10.4161/cc.6.3.3802. [DOI] [PubMed] [Google Scholar]
- 8.Finlan LE, Hupp TR. p63: the phantom of the tumor suppressor. Cell Cycle. 2007;6:1062–71. doi: 10.4161/cc.6.9.4162. [DOI] [PubMed] [Google Scholar]
- 9.Jaffe ES, Harris NL, Stein H, Vardiman JW, editors. World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues. Lyon: IARC Press; 2001. pp. 230–5. [Google Scholar]
- 10.Vassallo J, Lamant L, Brugiers L, et al. ALK-positive anaplastic large cell lymphoma mimicking nodular sclerosis Hodgkin's disease. Am J Surg Pathol. 2006;30:223–9. doi: 10.1097/01.pas.0000179123.66748.c2. [DOI] [PubMed] [Google Scholar]
- 11.Medeiros LJ, Elenitoba-Johnson SJ. Anaplastic large cell lymphoma. Am J Clin Pathol. 2007;127:707–22. doi: 10.1309/r2q9ccuvtlrycf3h. [DOI] [PubMed] [Google Scholar]
- 12.Tamaru J, Tokuhira M, Nittsu N, et al. Hodgkin-like anaplastic large cell lymphoma (previously designated in the REAL classification) has same immunophenotypic features to classical Hodgkin lymphoma. Leuk Lymphoma. 2007;48:1127–38. doi: 10.1080/10428190701342000. [DOI] [PubMed] [Google Scholar]
- 13.Stein H, Mason DY, Gerdes J, et al. The expression of the Hodgkin's disease associated antigen Ki-1 in reactive and neoplastic lymphoid tissue: evidence that Reed-Sternberg cells and histiocytic malignancies are derived from activated lymphoid cells. Blood. 1985;66:848–58. [PubMed] [Google Scholar]
- 14.Park Ch K, Oh YH. Expression of p63 in reactive hyperplasias and malignant lymphomas. J Korean Med Sci. 2005;20:752–8. doi: 10.3346/jkms.2005.20.5.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Willenbrock K, Kuppes R, Renné C, et al. Common features and differences in the trancriptome of large cell anaplastic lymphoma and classical Hodgkin's lymphoma. Haematologica. 2006;91:596–604. [PubMed] [Google Scholar]
- 16.Nascimento A, Pinkus J, Pinkus G. Clusterin, a marker for anaplastic large cell lymphoma. Am J Clin Pathol. 2004;121:709–17. doi: 10.1309/GQ2R-LNDW-LB9W-Y6UU. [DOI] [PubMed] [Google Scholar]
- 17.Saffer H, Wahed A, Rassidakis GZ, Medeiros LJ. Clusterin expression in malignant lymphomas: a survey of 266 cases. Mod Pathol. 2002;15:1221–6. doi: 10.1097/01.MP.0000036386.87517.AA. [DOI] [PubMed] [Google Scholar]
- 18.Pinkus GS, Pinkus JL, Langhoff E, et al. Fascin, a sensitive new marker for Reed-Sternberg cells of Hodgkin's disease. Evidence for a dendritic or B cell derivation? Am J Pathol. 1997;150:543–62. [PMC free article] [PubMed] [Google Scholar]
- 19.Fan G, Kotylo P, Neiman RS, Braziel RM. Comparison of fascin expression in anaplastic large cell lymphoma and Hodgkin disease. Am J Clin Pathol. 2003;119:199–204. doi: 10.1309/EAE3-TGPP-4A5R-VA92. [DOI] [PubMed] [Google Scholar]
- 20.Bakshi NA, Finn WG, Schnitzer B, Valdez R, Ross CW. Fascin expression in diffuse large B-cell lymphoma, anaplastic large cell lymphoma, and classical Hodgkin lymphoma. Arch Pathol Lab Med. 2007;131:742–7. doi: 10.5858/2007-131-742-FEIDLB. [DOI] [PubMed] [Google Scholar]
- 21.Mhawech-Fauceglia P, Saxena R, Zhang S, et al. Pax-5 immunoexpression in various types of benign and malignant tumours: a high-throughput tissue microarray analysis. J Clin Pathol. 2007;60:709–14. doi: 10.1136/jcp.2006.039917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCune RC, Syrbu SI, Vasef MA. Expression profiling of transcription factors Pax-5, Oct-1, Oct-2, BOB.1, and PU.1 in Hodgkin's and non-Hodgkin's lymphomas: a comparative study using high throughput tissue microarrays. Mod Pathol. 2006;19:1010–8. doi: 10.1038/modpathol.3800622. [DOI] [PubMed] [Google Scholar]
- 23.Yang A, Kaghad M, Wang Y, et al. p63 and p53 homolog at 3q27-29, encodes multiple products with transactivating, death-inducing, and dominant-negative activities. Mol Cell. 1998;2:305–16. doi: 10.1016/s1097-2765(00)80275-0. [DOI] [PubMed] [Google Scholar]
- 24.Urist MJ, Di Como CJ, Lu ML, et al. Loss of p63 expression is associated with tumor progression in bladder cancer. Am J Pathol. 2002;161:1199–206. doi: 10.1016/S0002-9440(10)64396-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Massion PP, Taflan PM, Jamshedur Rahman SM, et al. Significance of p63 amplification and overexpression in lung cancer development and prognosis. Cancer Res. 2003;63:7113–21. [PubMed] [Google Scholar]
- 26.Corn PG, Kuerbitz SJ, van Noesel MM, et al. Transcriptional silencing of the p73 gene in acute lymphoblastic leukemia and Burkitt's lymphoma is associated with 5¢ CpG island methylation. Cancer Res. 1999;59:3352–6. [PubMed] [Google Scholar]
- 27.Novak U, Grob TJ, Baskaynak G, et al. Overexpression of the p73 gene is a novel finding in high-risk B-cell chronic lymphocytic leukemia. Ann Oncol. 2001;12:981–6. doi: 10.1023/a:1011153206003. [DOI] [PubMed] [Google Scholar]
- 28.Zigeuner R, Tsybrovsky O, Raschek M, Rehak P, Lipsky K, Langner C. Prognostic impact of p63 and p53 in upper urinary tract transitional cell carcinoma. Urology. 2004;63:1079–83. doi: 10.1016/j.urology.2004.01.009. [DOI] [PubMed] [Google Scholar]


