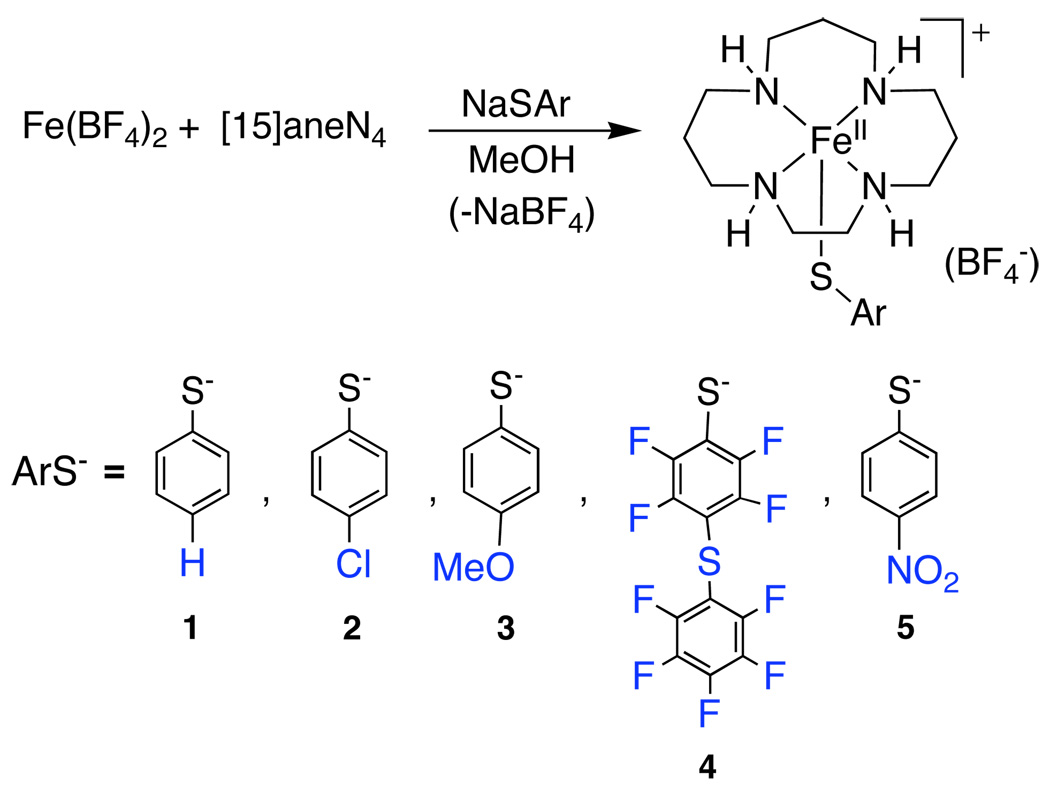
Abstract
Iron peroxide species have been identified as important intermediates in a number of non-heme iron as well as heme-containing enzymes, yet there are only a few examples of such species either synthetic or biological that have been well characterized. We describe the synthesis and structural characterization of a new series of five-coordinate (N4S(thiolate))FeII complexes that react with tert-butyl hydroperoxide (tBuOOH) or cumenyl hydroperoxide (CmOOH) to give metastable alkylperoxo-iron(III) species (N4S(thiolate)FeIII-OOR) at low temperature. These complexes were designed specifically to mimic the non-heme iron active site of superoxide reductase, which contains a five-coordinate iron(II) center bound by one Cys and four His residues in the active form of the protein. The structures of the FeII complexes are analyzed by X-ray crystallography, and their electrochemical properties are assessed by cyclic voltammetry. For the FeIII-OOR species, low-temperature UV-vis spectra reveal intense peaks between 500 – 550 nm that are typical of peroxide to iron(III) ligand-to-metal charge-transfer (LMCT) transitions, and EPR spectroscopy shows that these alkylperoxo species are all low-spin iron(III) complexes. Identification of the vibrational modes of the FeIII-OOR unit comes from resonance Raman (RR) spectroscopy, which shows ν(Fe-O) modes between 600 – 635 cm−1 and ν(O-O) bands near 800 cm−1. These Fe-O stretching frequencies are significantly lower than those found in other low-spin FeIII-OOR complexes. Trends in the data conclusively show that this weakening of the Fe-O bond arises from a trans influence of the thiolate donor, and DFT calculations support these findings. These results suggest a role for the cysteine ligand in SOR, and are discussed in light of the recent assessments of the function of the cysteine ligand in this enzyme.
Introduction
Iron peroxide species are proposed to play important roles in the function of many mononuclear iron centers in biology, including heme systems such as cytochrome P4501–6 and heme oxygenase,7 and non-heme iron centers such as bleomycin,8–10 extradiol10–13and Rieske dioxygenases,10,11 and superoxide reductases.14–19 The latter enzymes represent a new class of iron-containing proteins that is found in anaerobic and microaerophilic prokaryotes, and catalyzes the reduction of superoxide to hydrogen peroxide through a reductive pathway, O2− + e−+ 2H+ → H2O2. SOR is thought to serve as an alternative to the classical superoxide dismutases (SODs), allowing anoxic microorganisms to avoid the oxidative half-reaction of SOD, which would produce deleterious dioxygen. The metal center in SOR that carries out the reduction of O2− is comprised of an FeII ion bound to the protein by four His donors in a plane and a single axial cysteinate donor, completing a square pyramidal geometry for the high-spin ferrous (reduced) enzyme. High-resolution X-ray structures of SOR have also shown that the Cys sulfur atom is within hydrogen-bonding distance of two N-H amide groups (N-H---S bonds) from the peptide backbone.20 The mechanism of O2− reduction has been studied by several methods, and there is one intermediate, characterized by an absorption band at ~600 nm,16,17,19,21 that has been consistently observed. This species has been formulated as an iron(III)-(hydro)peroxo species. However, the structure, spin state, and protonation state of this intermediate have not been definitively determined, and the possible existence of other iron peroxide-type intermediates along the catalytic pathway remains under investigation. In addition, the thiolate ligand in SOR is unique among non-heme iron enzymes, and its role in the mechanism of O2− reduction has been a subject of much interest.18,20,22,23
We previously reported the synthesis of the SOR model complex [FeII([15]aneN4)(SC6H5)]+BF4−, and showed that it reacted with alkylhydroperoxides at low temperature in CH2Cl2 to give the metastable iron(III)-alkylperoxo species [FeIII([15]aneN4)(SC6H5)(OOR)]+ (R = cumenyl or tert-butyl).24 These complexes were characterized by UV-vis, EPR, and resonance Raman (RR) spectroscopies, and determined to be low-spin FeIII-OOR species with weak Fe-O bonds, i.e., with ν(Fe-O) ~ 90 cm−1 lower than in other low-spin FeIII-OOR species. Analysis of other low-spin and high-spin FeIII-OO(H or R) species by RR spectroscopy has led to a trend in which the low-spin complexes exhibit strong Fe-O and weak O-O bonds, while the opposite trend is observed for the high-spin species.25–32 Thus the [FeIII([15]aneN4)(SC6H5)(OOR)]+ complexes were the first examples of low-spin FeIII-OOR species to exhibit dramatically weakened Fe-O bonds. The former trend was used as precedent for the suggestion that the spin-state of the iron during O2− reduction may play a part in encouraging Fe-O bond cleavage and release of H2O2 as opposed to the O-O cleavage pathway observed for other enzymes such as cytochrome P450.16,33–35
In our earlier report we reasoned that the unexpected weakening of the Fe-O bond in [FeIII([15]aneN4)(SC6H5)(OOR)]+ may arise from a trans influence of the thiolate donor, provided that the thiolate ligand was indeed coordinated in a trans configuration. Immediately following this work a report from Kovacs and Solomon36 described the low-temperature synthesis and characterization of a high-spin FeIII-OOH model of SOR that also exhibited a significantly weakened Fe-O bond, as evident from the RR-detected Fe-O stretch for this complex (ν(Fe-O) = 419 cm−1 versus 450–639 cm−1 for other high-spin iron(III) peroxides). In this study a thiolate-induced trans influence was also invoked as the potential cause of the weak Fe-O bond. In contrast, an earlier report by Que and Halfen33 showed that increasing the electron-donating ability of a trans-oriented axial ligand (X) in a series of six-coordinate, high-spin [FeIII(N4)(X)(OOR)]+ complexes led to a decrease in the decay rate of these species. This trend is opposite to that expected for a weakening of the Fe-O bond via a trans influence of X, assuming that the decay involves Fe-O bond cleavage. Interestingly, the RR spectroscopy on these high-spin complexes displayed essentially no variance with the identity of the axial ligand. We speculated that the synthesis of a series of SOR models in which the electron-donating power of the thiolate ligand was rationally varied would greatly aid in determining the potential role of this ligand in influencing the properties of Fe-OO(H or R) species.
In this paper, we describe the modular synthesis of a series of SOR model complexes of the type [FeII([15]aneN4)(SAr)]+, wherein the thiolate ligand has been varied with regard to its electron-donating ability. These complexes have allowed us to systematically assess the influence of the thiolate donor on the structural and physical properties, as well as the reactivity of this family of SOR model complexes while holding the remaining ligand set constant. The ability of these complexes to form FeIII-OOR species is evaluated, and a mechanism of formation is proposed. The UV-vis features, spin-states and vibrational signatures of the FeIII-OOR species are determined by low-temperature electronic absorption, EPR, and RR spectroscopies. The influence of the thiolate donor on the Fe-O and O-O bond strengths is assessed. DFT calculations were performed in support of the spectroscopic data. These findings are discussed in light of recent results on SOR in which the influence of the Cys donor on the properties of an FeIII-OO(H) intermediate has been investigated through site-directed mutagenesis.18
Experimental Section
General Procedures
All reactions were carried out under an atmosphere of N2 or Ar using a drybox or standard Schlenk techniques. 1,4,8,1 2-tetraazocyclopentadecane ([15]aneN4) (98%) was purchased from Strem Chemicals. All other reagents were purchased from commercial vendors and used without further purification unless noted otherwise. Diethyl ether and dichloromethane were purified via a Pure-Solv Solvent Purification System from Innovative Technology, Inc. THF was distilled from sodium/benzophenone. Methanol was distilled over CaH2. All solvents were degassed by repeated cycles of freeze-pump-thaw and then stored in a drybox. Sodium hydride (60% in mineral oil) was washed with hexanes prior to use. Tert-butylhydroperoxide was purchased from Aldrich as an ~5.5 M solution in decane over molecular sieves. The concentration of tBuOOH was measured via titration with potassium iodide according to a published procedure.37 tBu18O18OH was synthesized by the reaction of tBuMgCl with 18O2 following a published procedure.38 Cumene18O18OH was synthesized by the reaction of cumene with 18O2 following a published procedure.39 18O2 was purchased from Icon Isotopes (99% atom purity).
Physical Methods
Electron paramagnetic resonance (EPR) spectra were obtained on a Bruker EMX EPR spectrometer controlled with a Bruker ER 041 X G microwave bridge at 15 K. The EPR spectrometer was equipped with a continuous-flow liquid He cryostat and an ITC503 temperature controller made by Oxford Instruments, Inc. Low-temperature UV-visible spectra were recorded at −78 °C on a Cary 50 Bio spectrophotometer equipped with a fiber-optic coupler (Varian) and a fiber optic dip probe (Hellma 661.302-QX-UV, 2 mm path length) for low temperature, using custom-made schlenk tubes designed for the fiber-optic dip probe. Room temperature UV-vis spectra were recorded on a Hewlett-Packard 8453 diode array spectrophotometer. Resonance Raman spectra were obtained on a custom McPherson 2061/207 spectrometer equipped with a Princeton Instrument liquid-N2 cooled CCD detector (LN-1100OB). The 514-nm excitation from an Ar laser (Innova 90, Coherent) was set at 50 mW and continuous spinning of the sample at 110 K was used to prevent adverse effect from the laser illumination. A Semrock RazorEdge filter was used to attenuate Rayleigh scattering. Sets of ~15 min accumulations were acquired at 4-cm−1 resolution. Frequencies calibrations were performed using aspirin and are accurate to ±1 cm−1. Elemental analyses were performed by Atlantic Microlab Inc., Norcross, GA.
Synthesis of [FeII([15]aneN4)(SC6H5)]BF4 (1)
To a solution of Fe(BF4)2•6H2O (0.395 g, 1.17 mmol) in MeOH (4 mL) was added a solution of [15]aneN4 (0.250 g, 1.17 mmol) in MeOH (6 mL) dropwise, and stirred for 45 minutes resulting in a pale yellow solution. A methanolic solution of NaSC6H5 (0.185 g, 1.40 mmol) (prepared from addition of benzenethiol to NaH in THF) was added to the mixture and stirred overnight to give a pale green solution. Methanol was removed under vacuum to give a green solid, which was redissolved in CH2Cl2 and filtered through celite to give a green solution. Vapor diffusion of diethyl ether into this solution resulted in colorless prisms (which appear light blue when grown together in clusters) of 1 after a week. Yield: 0.295 g (57%); Anal. Calc. for C17H31N4SFeBF4: C, 43.80; H, 6.71; N, 12.01. Found: C, 43.34; H, 6.85; N, 12.08.
Synthesis of [FeII([15]aneN4)(SC6H4-p-Cl)]BF4 (2)
To a solution of Fe(BF4)2•6H2O (0.378 g, 1.12 mmol) in MeOH (3 mL) was added a solution of [15]aneN4 (0.267 g, 1.25 mmol) in MeOH (4 mL) dropwise, and stirred for 45 minutes resulting in a pale yellow solution. A methanolic solution of NaSC6H4-p-Cl (0.236 g, 1.39 mmol) (prepared from addition of 4-chlorobenzenethiol to NaH in THF) was added to the mixture and stirred for 30 minutes to give a pale yellow solution. Methanol was removed under vacuum to give a yellow-brown solid, which was redissolved in CH2Cl2 and filtered through celite to give a forest green solution. Vapor diffusion of diethyl ether into this solution resulted in colorless prisms (which appear pale green when grown together in clusters) of 2 after 2 days. Yield: 362 mg (65%); Anal. Calc. for C17H30N4SFeClBF4: C, 40.78; H, 6.04; N, 11.09. Found: C, 40.79; H, 5.66; N, 11.33.
Synthesis of [FeII([15]aneN4)(SC6H4-p-OMe)]BF4 (3)
To a solution of Fe(BF4)2•6H2O (0.236 g, 0.700 mmol) in MeOH (4 mL) was added a solution of [15]aneN4 (0.150 g, 0.700 mmol) in MeOH (6 mL) dropwise, and stirred for 45 minutes resulting in a pale yellow solution. A methanolic solution of NaSC6H4-p-OMe (prepared from the addition of 4-methoxybenzenethiol to NaH in THF) was added to the mixture and stirred overnight to give a pale green-yellow solution. Methanol was removed under vacuum to give a green solid, which was redissolved in CH2Cl2 and filtered through celite to give a yellow-green solution. Vapor diffusion of diethyl ether into this solution resulted in colorless rods (which appear pale green when grown together in clusters) of 3 after one week. Yield: 0.226 g (65%); Anal. Calc. for C18H33N4OSFeBF4: C, 43.57; H, 6.70; N, 11.29. Found: C, 43.51; H, 6.73; N, 11.31.
Synthesis of [FeII([15]aneN4)(SC6F4-p-SC6F5]BF4 (4)
To a solution of Fe(BF4)2•6H2O (0.284 g, 0.84 mmol) in MeOH (3 mL) was added a solution of [15]aneN4 (0.180 g, 0.84 mmol) in MeOH (4 mL) dropwise, and stirred for 45 minutes resulting in a pale yellow solution. A methanolic solution of NaSC6F4-p-SC6F5 (0.266 g, 0.66 mmol) (4 mL) (prepared in situ from addition of 2,3,4,5,6-pentafluorobenzenethiol to NaH in THF) was added dropwise to give a dark yellow solution, which was stirred for 30 minutes. Methanol was removed under vacuum to give a dark yellow solid. The solid was dissolved in CH2Cl2 and filtered over celite to give a dark yellow solution. Vapor diffusion of diethyl ether into this solution resulted in colorless thick plates (which appear yellow when grown together in clusters) of 4 after 3 weeks. Anal. Calc. for C23H26N4S2FeClBF13: C, 37.52; H, 3.56; N, 7.61. Found: C, 37.33; H, 3.49; N, 8.16.
Synthesis of [FeII([15]aneN4)(SC6H4-p-NO2)]BF4 (5)
To a solution of Fe(BF4)2•6H2O (0.239 g, 0.707 mmol) in MeOH (3 mL) was added a solution of [15]aneN4 (0.152 g, 0.707 mmol) in MeOH (4 mL) dropwise, and stirred for 45 minutes resulting in a pale yellow solution. A red solution of NaSC6H5-p-NO2 (0.179 g, 1.01 mmol) in MeOH (4 mL) (prepared from the addition of 4-nitrobenzenethiol to NaH in THF) was added dropwise to give a dark red solution with orange-yellow precipitate, which was stirred for 30 minutes. The solvent was removed under vacuum to give a dark red solid interspersed with small amounts of an orange solid. The solid was dissolved in CH2Cl2 and filtered through celite to give a dark red solution. Vapor diffusion of diethyl ether into this solution resulted in dark red prisms of 5 after 1 day. Yield: 0.240 g (66%); UV-vis (CH2Cl2, 11463 M−1cm−1), λmax 370 nm, 490 nm (sh); Anal. Calc. for C17H30N5SFeO2BF4: C, 39.94; H, 5.92; N, 13.70. Found: C, 39.98; H, 5.96; N, 13.48.
X-ray crystallography
All X-ray diffraction data were collected on an Oxford Diffraction Xcalibur3 diffractometer, equipped with a Mo K-alpha sealed-tube source and a Sapphire CCD detector. Single crystals of each compound were mounted in oil under a nitrogen cold stream at 110(2) K. Data collection parameters are provided in supplementary information as CIF files. Selected crystallographic details are provided in Table 1. Data were collected, integrated, scaled and corrected for absorption using CrysAlis Pro software.40 Structures were solved and refined using the SHELXTL suite of software.41
Table 1.
Crystallographic Information for Complexes 1 – 5
| Parameter | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Formula | C17H31BF4FeN4S | C17H30BClF4FeN4S | C18H33BF4FeN4OS | C23H26BF13FeN4S2 | C17H30BF4FeN5O2S |
| Mr | 466.18 | 500.62 | 496.20 | 736.26 | 511.18 |
| cryst syst | monoclinic | Orthorhombic | Orthorhombic | monoclinic | orthorhombic |
| Space | P21/c | Pna21 | Pna21 | Cc | Pna21 |
| group | |||||
| a (Å) | 12.4027(7) | 16.1010(4) | 15.7595(4) | 8.8233(2) | 16.0267(3) |
| b (Å) | 10.1280(6) | 13.7952(4) | 14.3395(3) | 22.7707(6) | 13.6455(3) |
| c (Å) | 16.9164(11) | 9.9077(3) | 10.0194(3) | 15.2423(4) | 10.1189(2) |
| α (deg) | 90 | 90 | 90 | 90 | 90 |
| β (deg) | 92.727(5) | 90 | 90 | 103.444(3) | 90 |
| γ (deg) | 90 | 90 | 90 | 90 | 90 |
| V, Å3 | 2122.5(2) | 2200.66(11) | 2264.22(10) | 2978.46(13) | 2212.93(8) |
| Z | 4 | 4 | 4 | 4 | 4 |
| Cryst color | colorless | colorless | colorless | colorless | dark red |
| T, K | 110 | 110 | 110 | 110 | 110 |
| R1 [I > 2σ(I)] | 0.0707 | 0.0719 | 0.0947 | 0.0401 | 0.0831 |
| GOF on F2 | 1.253 | 1.071 | 1.076 | 1.047 | 1.080 |
Disorder in structures comprising the [15]aneN4 ligand is well documented.42–55 The structures presented here were refined in non-centrosymmetric space groups in order to better model the disorder in the macrocycle. Elongated thermal parameters for the macrocycle atoms suggest that these atoms are significantly disordered. Attempts to further model this disorder did not improve the refinement. In structures 1, 2, 3 and 5, the C-C and C-N bonds were restrained using values given by Blake, et al.43 The hydrogen atoms were refined in calculated positions, using appropriate riding models. The atoms in the disordered C6H4-p-OMe and C6H4-p-NO2 moieties of 3 and 5 respectively were refined isotropically. Complex 4, which is rather well-ordered was refined with no distance restraints and all non-hydrogen atoms were refined anisotropically. The N-H hydrogen atoms were located from the residual electron density maps and freely refined, while all C-H hydrogen atoms were refined in calculated positions. For further details on all structures, see CIF files in supporting information.
Electrochemistry
Cyclic voltammograms were measured with an EG&G Princeton Applied Research potentiostat/galvanostat model 263A at scan rates of 0.025–0.5 V/s. A three-electrode configuration made up of a platinum working electrode, a Ag/AgCl reference electrode (3.5 M KCl), and a platinum wire auxiliary electrode was employed. Measurements were performed with 0.1 mM analyte in CH2Cl2 at ambient temperatures under argon using 0.10 M tetra-n-butylammonium hexafluorophosphate (recrystallized 3 times from ethanol/water, dried over high vacuum for 3 days and stored in the dry box) as the supporting electrolyte. The ferrocenium/ferrocene couple (FeCp2+/0) at 0.45 V was used as an external reference.
Computational Methods
Frequency calculations were performed at optimized geometries. Initial coordinates were taken from the X-ray crystal structures of the iron(II) complexes 1 – 5. Calculations were performed with Gaussian 03 at the B3LYP/6–311G level of theory.56 The potential energy distribution was obtained by transforming the calculated force constants and normal modes from Cartesian coordinates to an internal coordinate system.57
Generation of [([15]aneN4)FeIII(SAr)(OOR)]+ for UV-vis spectroscopy
In a typical reaction, a custom-made schlenk flask was loaded with a known amount of [FeII([15]aneN4) (SAr)]BF4 and dissolved in an appropriate volume of CH2Cl2 to give a final concentration of the FeII complex between 0.3 and 3 mM. A fiber optic dip probe with a path length of 2 mm was inserted into the flask under argon and the solution was cooled to −78 °C. After recording the UV-vis spectrum of the FeII starting material, 3 equivalents of tBuOOH (stock solution in n-decane) was diluted into CH2Cl2 and added to the FeII complex and a new spectrum was immediately recorded. The colorless solution instantly turned deep red except in the case of 5 where the light orange starting material gradually turned fuchsia. The formation and decay of the new species were followed by UV-vis spectroscopy.
Generation of [FeIII([15]aneN4)(SAr)(OOR)]+ for Resonance Raman and EPR Spectroscopy
To a Wilmad WG-5M economy NMR tube or Wilmad Quartz EPR tube containing a known concentration of [FeII([15]aneN4)(SAr)]BF4 in CH2Cl2 (500 µL) cooled to −78 °C, was added 3 equivalents of ROOH (R = tBu or cumenyl) in CH2Cl2. An instant color change from colorless to deep red (light orange to fuchsia in the case of 5) was observed, indicating the formation of the FeIII-OOR species. The reaction mixture was then bubbled with Ar or N2 gas in the EPR or NMR tube to obtain a homogenous solution. Bubbling was kept to a minimum in order to minimize warming of the solution. The solution was then frozen in liquid nitrogen and stored at 77 K until either RR or EPR measurements were made.
Results and Discussion
Synthesis
The iron(II) complexes 1 – 5 that form the basis of this study were prepared according to Scheme 1. The synthesis of complex 1 was communicated previously.24 Reaction of Fe(BF4)2 with the tetraamine donor [15]aneN4 in MeOH results in the insertion of FeII into the [15]aneN4 cavity. A dark green color forms immediately upon addition of [15]aneN4 to colorless FeII(BF4)2 in MeOH, which gradually fades to a pale yellow over the course of 45 minutes. The sodium salt of the appropriate arylthiolate ligand is then added, leading to the production of complexes 1 – 5. Our initial synthesis involved changing the reaction solvent to CH2Cl2 before adding the thiolate ligand, but we have seen improved yields of the FeII complexes when adding ArS−Na+ in MeOH and then removing solvent. Recrystallization of 1 – 5 from CH2Cl2/Et2O provided crystals for X-ray diffraction, and was the general method used for preparing pure 1 – 5 for all of the spectroscopic and reactivity studies described herein. Complexes 1 and 2 recrystallize as colorless prisms, complex 3 as colorless rods, while complex 4 recrystallizes as colorless thick plates. The sodium salt of −SC6H4-p-NO2 has an intense red color from an intraligand π-π* transition, which causes crystalline complex 5 to appear as dark red prisms.
Scheme 1.
The synthesis of these complexes is sensitive to the ratio of arylthiolate ligand versus iron(II). A slight excess of the thiolate ligand (1.25 – 2.0 equiv) compared to FeII is necessary to obtain these complexes in good yield and avoid other side-products. For example, when lower ratios of ArS− are employed in the syntheses of 1 or 2, a sideproduct appears as white needle-shaped crystals, easily discernable from crystalline 1 or 2 by visual inspection. The use of too large an excess of thiolate donor (three equivalents) in the case of the p-NO2 complex 5 led to the formation of [FeII([15]aneN4)(SC6H4-p-NO2)][SC6H4-p-NO2], where the BF4− counterion has been replaced by a non-coordinating −SC6H4-p-NO2 ligand.58 For the synthesis of polyfluorinated derivative 4, the target complex was [FeII([15]aneN4)(SC6F5)]+, but nucleophilic displacement of the p-F substituent during the initial deprotonation of C6F5H with NaH led to the thioether-linked thiolate ligand shown in Scheme 1.
The modular synthetic approach taken in this study purposefully avoided covalently linking the thiolate donor to the N4 ligand in order to allow for systematic variation of the electron-donating/releasing properties of the thiolate ligand. We were pleased to find that an extensive series of mononuclear, 5-coordinate iron(II) complexes could be assembled in this manner, provided that the thiolate-to-iron ratios are carefully controlled. Other iron(II) complexes with N4S(thiolate) donor sets include [FeII(L8py2)(SAr or SC6H11)]BF4 (L8py2 = 1,5-Bis(pyridin-2-ylmethyl)-1,5-diazocane), which were prepared as models of SOR by a similar self-assembly approach,59,60 and the covalently linked, thiolate-ligated complexes [FeII(cyclam-PrS)]BPh4 and [FeII(SMe2N4(tren))]PF6 (tren = tris(2-aminoethyl)amine) from Kovacs and coworkers.36,61,62 However, this study is the first report, to our knowledge, that allows for a direct assessment of the electronic influence of the thiolate donor at parity of ligand environment for a series of N4S(thiolate)-iron complexes.
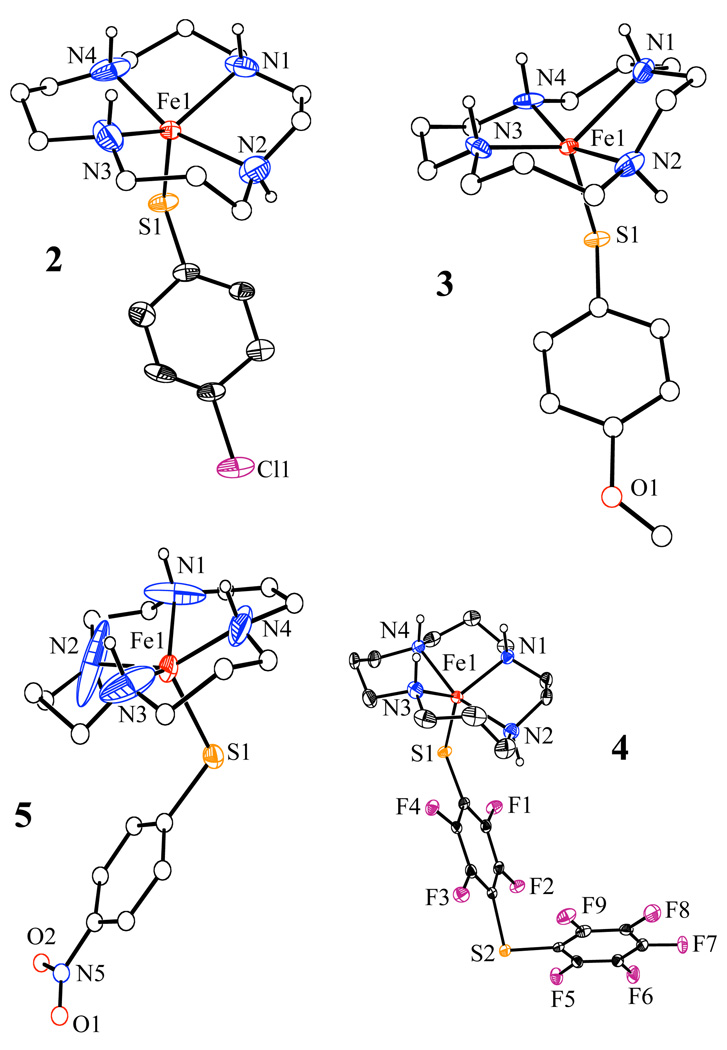
X-ray Structural Studies
The X-ray crystal structures of 2 – 5 are presented as ORTEP diagrams in Figure 1. Structural parameters are shown in Table 1, and selected bond lengths and angles for each complex are given in Table 2. The structure of 1 has been reported previously,24 but is included in Table 1 and Table 2 for comparison. Each cationic iron(II) complex is five-coordinate, with four nitrogen donors from the [15]aneN4 ligand and one sulfur donor from the ArS− ligand completing the coordination sphere. The conformation for [15]aneN4 is the same in each structure, with one N-H group (N(2)-H) on the same side of the macrocyclic plane as the thiolate ligand, while the other three N-H groups lie on the opposite side. The Fe–S bond distances for 1 – 5 range from 2.3197(12) – 2.3426(8) Å, showing little variance across the series and falling in the typical range for high-spin FeII-SR complexes.63–66 For complexes 1 – 5, the Fe-N distances range between 2.075(7) – 2.273(3) Å and are similar to other high-spin Fe(II) complexes, including the related N4S(thiolate)FeII complex [FeII(Me4cyclam)(SC6H4-p-OMe)]+ (Fe-N = 2.183(6) – 2.279(6) Å).60 For complexes 1 – 4 the Fe-N(3) distance (1: 2.138(3); 2: 2.075(5) Å; 3: 2.095(8) Å; 4: 2.088(3) Å) is slightly shorter than the other three Fe-N bonds. These short distances may be a consequence of steric constraints imposed by the [15]aneN4 ligand. The longest Fe-N distance in 1 – 4 is observed for N(2), which is also the only N-H group on the same side of the macrocycle as the sulfur donor. The p-nitro-benzenethiolate complex 5 exhibits Fe–N bond distances that are on the lower end of the range seen for 1 – 5. These shorter distances may be a consequence of the weaker donation from the NO2-substituted phenylthiolate ligand, which results in a more electropositive iron(II) center as evidenced by electrochemistry (vide infra). In addition, the NO2 complex 5 exhibits a geometry closer to square pyramidal (sp) as determined by its τ value67 of 0.27 (τ = 0.0 for idealized sp and 1.0 for idealized tbp) compared to τ ~0.5 for 1 – 4, with its thiolate ligand occupying the axial position. A combination of the electron-poor nature of 5 along with this subtle geometry change may account for the shorter Fe-N distances in 5.
Figure 1.
ORTEP diagrams of the complex cations of 2, 3, 4 and 5 showing 30% probability ellipsoids. Hydrogen atoms were omitted for clarity except for the N-H groups. The carbon atoms for the macrocycle in 2, 3, and 5 as well as the phenyl substituents of 3 and 5 are drawn as isotropic spheres for clarity.
Table 2.
Relevant Bond Distances (Å) and Angles (deg) for Complexes 1 – 5
| Compd | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Fe –N1 | 2.164(3) | 2.206(7) | 2.223(8) | 2.161(3) | 2.132(10) |
| Fe-N2 | 2.273(3) | 2.251(8) | 2.248(8) | 2.250((3) | 2.097(11) |
| Fe-N3 | 2.138(3) | 2.075(7) | 2.095(8) | 2.088(3) | 2.137(16) |
| Fe-N4 | 2.206(3) | 2.160(7) | 2.188(7) | 2.229(3) | 2.069(9) |
| Fe-S | 2.3316(11) | 2.3197(12) | 2.3240(17) | 2.3426(8) | 2.3305(15) |
| N1-Fe-N2 | 79.45(12) | 77.7(3) | 77.8(3) | 79.86(11) | 72.3(6) |
| N1-Fe-N3 | 133.56(13) | 133.4(3) | 132.5(3) | 132.06(12) | 137.4(4) |
| N1-Fe-N4 | 85.98(12) | 85.8(3) | 84.9(3) | 84.60(10) | 86.4(6) |
| N2-Fe-N3 | 94.49(12) | 96.0(3) | 95.5(3) | 95.78(12) | 95.2(5) |
| N2-Fe-N4 | 163.08(13) | 162.0(3) | 161.0(3) | 162.26(11) | 153.2(8) |
| N3-Fe-N4 | 89.30(12) | 90.1(3) | 90.4(3) | 88.28(12) | 89.4(6) |
| S-Fe-N1 | 115.33(10) | 109.4(2) | 110.8(2) | 107.85(8) | 107.0(3) |
| S-Fe-N2 | 86.10(9) | 96.6(2) | 97.3(2) | 100.04(9) | 104.2(7) |
| S-Fe-N3 | 110.03(10) | 117.2(2) | 116.7(2) | 119.83(9) | 115.5(3) |
| S-Fe-N4 | 108.10(10) | 95.59(17) | 96.06(18) | 92.79(8) | 97.5(2) |
| Fe-S-C | 116.90(12) | 111.34(15) | 111.3(4)a | 112.29(10) | 109.7 (6)a |
| 106.0(5)b | 110.3 (5)b | ||||
| Fe-S-C(12)- | 97.6(3) | −90.6(5) | −80.8(10)a | −132.8(2) | 90.3(11)a |
| C(13) | −105.9(8)b | 89.7(10)b | |||
| Fe-S-C(12)- | −87.5(3) | 94.0(5) | 102.2(12)a | 52.0(3) | −92.9(14)a |
| C(17) | 82.7(13)b | −92.7(11)b |
major component
minor component
The precise orientation of the thiolate donor in both metal-thiolate models and metalloproteins has been suggested to be important for controlling the sulfur-metal bonding interactions and related properties, including reduction potentials.60,68,69 The orientation of the arylthiolate ligands in 1 – 5 can be defined by the Fe-S-Caryl angle and Fe-S-Caryl-Caryl’ dihedral angle. The Fe-S-Caryl angles for 1 – 5 are similar, lying between 109.7(6) – 116.90(12)°. The Fe-S-Caryl-Caryl’ dihedral angles are also similar for 1 – 3 and 5, varying between 90.3(11) – 105.9(8)°, while for the fluorinated complex 4 this angle is 132.8(2)°. Interestingly, the packing diagram of complex 4 shows a π-stacking interaction in the solid state between the C6F4 ring of one molecule and C6F5 ring of a neighboring molecule (X,1-Y, −½+Z), characterized by the following geometric parameters: Ct-Ct = 3.586 Å (Ct = centroid of the ring), α = 9.30° (α = dihedral angle between the stacking planes) and D┴ = 3.296 Å and 3.403 Å (D┴ = perpendicular distances from one ring to the plane of the other ring). This π-stacking interaction may be perturbing the Fe-S-Caryl-Caryl’ dihedral angle.
Comparison to the structure of the reduced enzyme, SORred
All of the model complexes 1 – 5 are five-coordinate iron(II) complexes with one open site on the metal, as found in SORred. The N4S(thiolate) donor set of 1 – 5 is a good mimic of the coordination sphere of the iron(II) center in the active site. The FeII–S bond distances in 1 – 5, which fall in the normal range, are somewhat shorter than that found in the enzyme (X-ray structure:70 2.40 – 2.44 Å; EXAFS:71 2.37 Å). It is not clear at present why SOR exhibits an elongated iron-sulfur bond. In addition, complexes 1 – 5 show geometries between that of square pyramidal and trigonal bipyramidal, whereas the protein exhibits a more purely square pyramidal geometry at the iron(II) center.
Electrochemistry
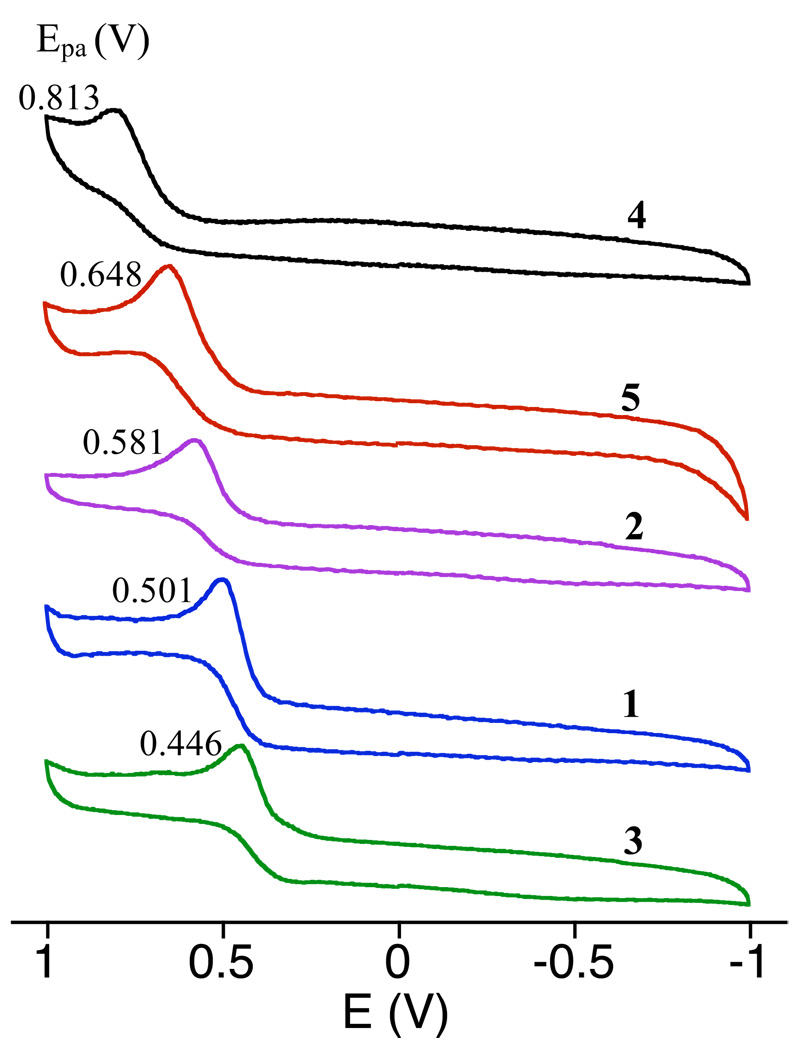
Complexes 1 – 5 were characterized by cyclic voltammetry, as shown in Figure 2. Each complex exhibits a single irreversible oxidation whose position varies with the identity of the para substituent on the arylthiolate ligand. We assign these anodic peaks (Epa) for 1 – 5 to the FeIII/II couples of these complexes, and they are listed in Table 3 together with other relevant FeIII/II redox potentials for related complexes. Although caution must be used when analyzing irreversible waves, the trends that emerge from comparing the different values in Table 3 deserve attention. There is a clear increase in the FeIII/II potential for 1 – 5 as the para substituent changes from electron-donating to electron-withdrawing, demonstrating that the thiolate donor has a dramatic influence over the FeIII/II couple. It is not easy to predict a priori where fluorinated complex 4 should appear in the series, but the CV data show that −SC6F4-p-SC6F5 has the weakest electron-donating ability, exhibiting an FeIII/II couple that is 165 mV more positive than the NO2-substituted complex 5. The p-OMe complex 3 reveals a significantly less positive oxidation potential (ΔE = 161 mV) than the related cyclam complex (FeII(Me4[14]aneN4)(SC6H4–p-OMe)]OTf.60 This complex contains the same arylthiolate ligand as 3 but includes the smaller 14-membered macrocyclic N4 donor and has N-methylated groups instead of secondary N-H groups. The change in ring size is unlikely to account for the observed difference in oxidation potentials given that the potentials for the cobalt complexes [Co[15–14]aneN4)Cl2]+ are in the opposite order ([15]aneN4 stabilizes CoII relative to [14]aneN4).72 The presence of the methyl groups on the amine donors might be expected to cause the opposite shift as well. However, experimental evidence and density functional theory (DFT) calculations for [(cyclamacetate)FeF]PF6 and (trimethylcyclamacetate)FeF]PF6 show that the N-methylated ligand is in fact a weaker donor for both steric and electronic reasons.73 Thus N-methylation may be responsible for making it significantly more difficult to oxidize (Me4[14]aneN4)FeII(SC6H4–p-OMe)]OTf compared to complex 3.
Figure 2.
Cyclic voltammograms for [FeII([15]aneN4)(SC6H4p-X)]BF4 (X = H (1), Cl (2), OMe (3), NO2 (5)) and [FeII([15]aneN4)(SC6F4p-SC6F5)]BF4 (4), measured in CH2Cl2 using a Ag/AgCl reference electrode (3.5 M KCl).
Table 3.
Electrochemical Data for (N4S(thiolate))FeII Complexes
| Complex | E (mV) vs SHE (Epa (mV) vs Ag/AgCl) |
Solvent | ref |
|---|---|---|---|
| [FeII([15]aneN4)(SC6H5)]BF4 (1) | 706a,b (501) | CH2Cl2 | this work |
| [FeII([15]aneN4)(SC6H4-p-Cl)]BF4 (2) | 786a,b (581) | CH2Cl2 | this work |
| [FeII([15]aneN4)(SC6H4-p-OMe)]BF4 (3) | 651a,b (446) | CH2Cl2 | this work |
| [FeII([15]aneN4)(SC6F4-p-SC6F5]BF4 (4) | 1018a,b(813) | CH2Cl2 | this work |
| [FeII([15]aneN4)(SC6H4-p-NO2)]BF4 (5) | 853a,b (648) | CH2Cl2 | this work |
| FeII[(Me4-cyclam) (SC6H4-p-OMe)]OTf | 812a,c | CH3CN | 60 |
| [FeII(L8py2)(SC6H4-p-CH3)]BF4 | 857c | CH3CN | 59 |
| [FeII(cyclam-PrS)]BPh4 | 462c | CH3CN | 36 |
Anodic potentials for the irreversible oxidation wave.
E versus SHE values were obtained by adding 205 mV to the recorded values versus Ag/AgCl.
E versus SHE values were obtained by adding 242 mV to the reported literature values versus SCE.
The FeIII/FeII redox potential for SORs isolated from different organisms have been reported to fall between 200 – 365 mV.71,74–76 Complexes 1 – 5 and the other model complexes in Table 3 are considerably more difficult to oxidize than the enzyme. The difference between the enzyme potentials and the models in Table 3 may arise from several environmental factors, such as solvent (organic solvents for the models versus aqueous conditions for the protein), and the presence of a Glu residue in the enzyme that coordinates to the iron(III) form of the protein. There are also N-H---S bonds in the protein that would be expected to drive the redox potential towards a more positive value,77,78 but computational models of the SOR active site show a surprising opposite effect in which the incorporation of N-H---S bonds causes a significant lowering of the FeIII/II redox potential.22 Despite the discrepancies between the models and the protein, the data for 1 – 5 provide a clear trend which shows that the nature of the thiolate donor has a strong influence over the oxidation potential, as proposed for SOR.16,22
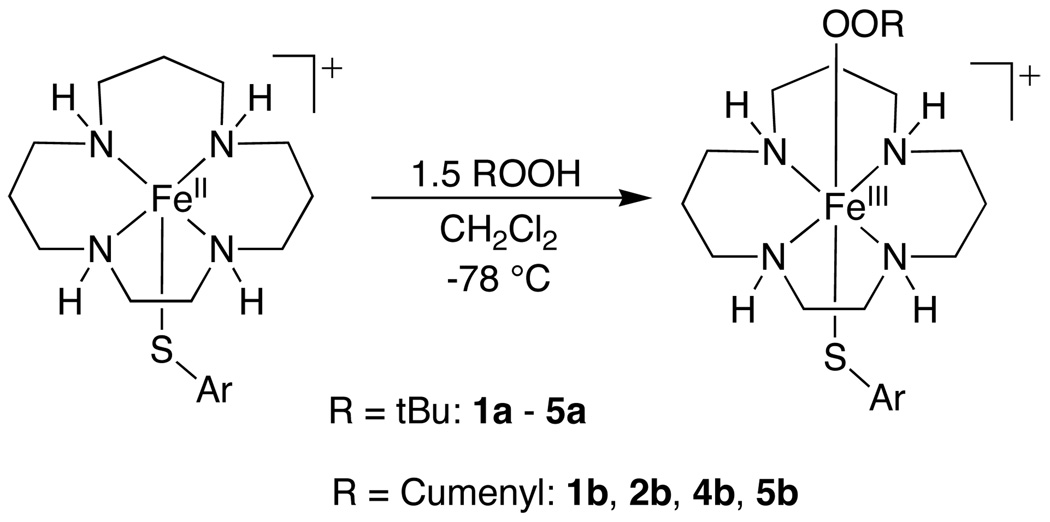
Reaction of FeII complexes with ROOH
In an earlier report we showed that 1 reacts at low temperature (−78 °C) with the alkylhydroperoxides tBuOOH and cumenylOOH to give the corresponding dark red, alkylperoxo-iron(III) species. Characterization of these low-temperature, metastable FeIII-OOR intermediates by UV-Vis, EPR, and RR spectroscopies showed that they are low-spin (S = ½) FeIII-OOR complexes with weakened Fe–O bonds. In this study we have significantly expanded this class of FeIII-OOR species via the reaction of tBuOOH and cumenylOOH with complexes 2 – 5 to give low-temperature metastable FeIII-OOR species.
UV-vis spectroscopy
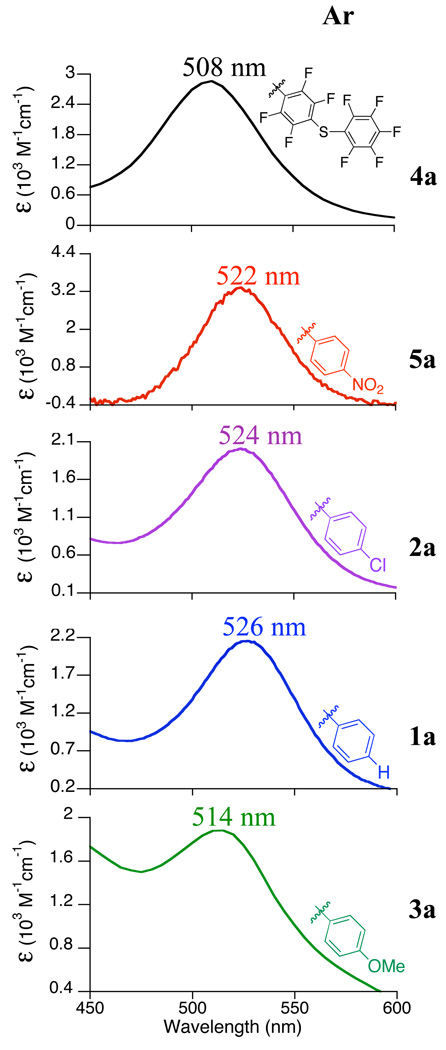
Complexes 1 – 5 react with tBuOOH at –78 °C in CH2Cl2 to give the low-temperature adducts 1a – 5a (Scheme 2). Low-temperature UV-vis spectra for these species are shown in Figure 3. The iron(II) complexes 1 – 4 are rapidly converted from colorless or light yellow to dark red upon introduction of tBuOOH at −78 °C in CH2Cl2. The p-NO2 complex 5 is red in color because of an intraligand absorption band from the thiolate donor, and the solution of 5 changes to fuchsia upon reaction with tBuOOH at −78 °C. Each complex exhibits an intense band (ε = 1800 – 3100 M−1cm−1) between 508 and 526 nm (Figure 3), for which the peak maxima and molar absorptivity are characteristic of alkylperoxo-to-iron(III) LMCT bands.26,29,30,79 This peak shifts to higher energy in the order 1a (p-H) < 2a (p-Cl) < 5a (p-NO2) < 3a (p-OMe) < 4a (SC6F4-p-SC6F5). This ordering follows an increase in the electron-withdrawing power of the para substituent and the related FeIII/II oxidation potential for each complex, with the exception of the p-OMe complex 3a. Interestingly, the shift of the peak to higher energy for complexes 1a, 2a, 4a, and 5a with an increase in the FeIII/II couple is opposite to that observed for a series of non-thiolate ligated, polypyridyl iron(III)-hydroperoxo species.80 The differences in the low-temperature UV-vis spectra for 1a – 5a provide strong evidence that the thiolate ligands remain coordinated upon oxidation to the FeIII-OOR species and exert a significant influence over the spectroscopic features of these species.
Scheme 2.
Figure 3.
UV-vis spectra of [FeIII([15]aneN4)(SAr)(OOtBu)]+ complexes 1a – 5a in CH2Cl2 at −78 °C.
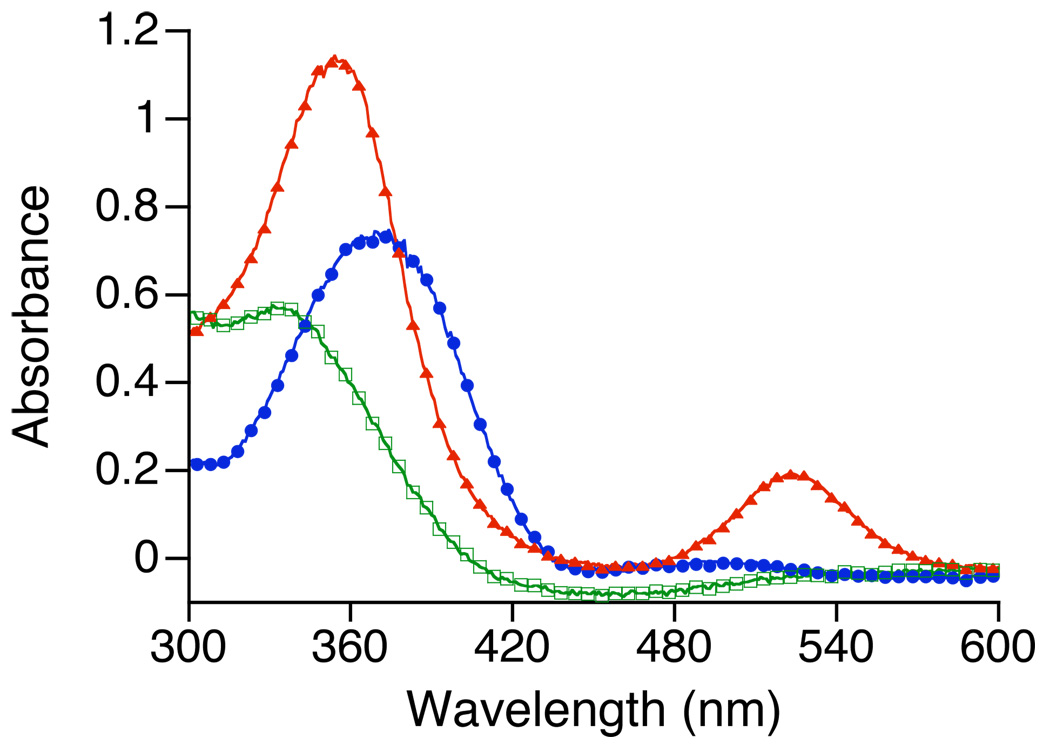
The UV-vis data for the p-NO2 complex provides more information regarding the fate of the thiolate donor because of the intraligand band associated with −SC6H4-p-NO2. The UV-vis spectra of [FeII([15]aneN4)(SC6H4-p-NO2)](BF4) 5, the corresponding FeIII-OOtBu species 5a, and the product obtained after the decay of 5a at −78 °C are shown in Figure 4. The spectrum for the dark red iron(II) complex 5 exhibits an intense, broad band at 370 nm (blue circles). The sodium salt of the free thiolate ligand (NaSC6H4-p-NO2) is also dark red in color and exhibits a peak at 422 nm in CH2Cl2:MeOH (99:1), which has been assigned as an intraligand transition81,82 (data not shown). Thus coordination of −SC6H4-p-NO2 to FeII induces a 52 nm blue-shift of this intraligand band (blue circles), and this transition is clearly sensitive to the local environment of the sulfur atom as has been shown previously.82 Upon reaction of 5 with tBuOOH to give the FeIII-OOtBu species 5a, the transition associated with the p-nitro-benzenethiolate moves to 352 nm (red triangles), concomitant with the appearance of the alkylperoxo LMCT band at 522 nm. The final spectrum exhibits a band at 327 nm that matches that of the disulfide p-NO2C6H4S– SC6H4-p-NO2 measured independently. These data indicate that the thiolate donor remains coordinated to the iron center in 5a, giving rise to the unique band at 352 nm, which converts to disulfide upon decomposition of 5a. Attempts to isolate the iron(III) product(s) after warming the reaction to room temperature have been unsuccessful, probably due to the decomposition of the complexes via disulfide formation.
Figure 4.
UV-vis spectra of [FeII([15]aneN4)(SC6H4p-NO2)]BF4(5) (blue circles), [FeIII([15]aneN4)(SC6H4p-NO2)(OOtBu)]+ (5a) (red triangles) and the decomposition product (green squares).
Proposed mechanism of formation of FeIII-OOR
A possible mechanism of formation of 1a – 5a, 1b, 2b, 4b and 5b, involves initial oxidation of the iron(II) complex by ROOH to give an FeIII-OH complex, which then reacts with ROOH by displacement of OH− to give the FeIII-OOR species and H2O. This mechanism has been proposed by Que and coworkers for the formation of similar FeIII-OOR species, and in one case the identification of an FeIII-OH complex [(6-Me3-TPA)FeIII(O2CPh)(OH)]+ (6-Me3-TPA = tris(6-methyl-2-pyridylmethyl)amine) was obtained via ESI-MS after addition of 0.5 equivalents of tBuOOH to [(6-Me3-TPA)FeII(O2CPh)]+.83 Subsequent formation of the FeIII-OOR species [(6-Me3-TPA)FeII(O2CPh)]+(OOtBu)]+ was observed upon addition of excess (5 equiv) tBuOOH.
According to the former mechanism, the stoichiometry of the first step should account for 0.5 equiv ROOH reacting with 1.0 equiv FeII to give the FeIII-OH species, and 1.0 equiv ROOH displacing the putative OH− ligand, resulting in a final stoichiometry of 1.5 ROOH : 1.0 FeII in the production of the alkylperoxo complex. We thus quantitated the stoichiometry of the reaction between 1 and tBuOOH to gain insight into the proposed mechanism. Successive addition of tBuOOH (0.2 equiv per addition, 4.0 M stock solution of tBuOOH in decane) to 1 at −78 °C revealed maximal formation of 1a (λ = 526 nm) between 1.5 and 2.0 equiv of tBuOOH (Figure S1). The overall stoichiometry of tBuOOH to FeII matches well with that predicted by the former mechanism.
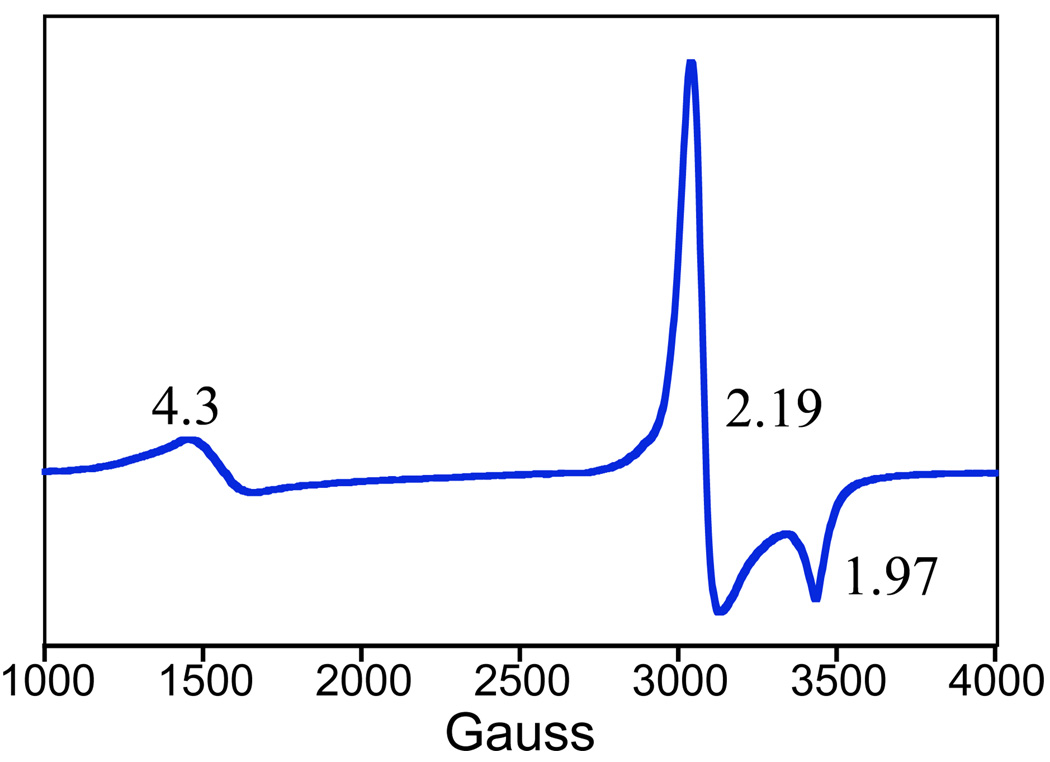
EPR spectroscopy
An X-band EPR spectrum collected at 15 K of the FeIII-OOtBu species 2a is shown in Figure 5, and is representative of the series 1a – 5a, which all show very similar EPR spectra. This spectrum is indicative of a low-spin (S = ½) FeIII complex with axial symmetry, with g values of 2.19 and 1.97. The small signal at g = 4.3 is assigned to a high-spin iron(III) impurity. It was shown previously24 that the low-spin iron(III) EPR signal for 1a decays concomitantly with the red chromophore at −78 °C, while the high-spin iron(III) signal remains unchanged. This behavior was confirmed for 2a, where a similar correlation for the decay of the EPR signal and red chromophore has been observed. These observations rule out the g = 4.3 signal as being associated with the FeIII-OOR chromophore.
Figure 5.
9 GHz X-band EPR spectrum of [FeIII([15]aneN4)(SC6H4p-Cl)(OOtBu)]+ (2a) in CH2Cl2 at 15 K. Experimental conditions: Frequency, 9.475 GHz; microwave power, 2.012 mW; modulation frequency, 100.00 kHz; modulation amplitude, 10.00 G; receiver gain, 5.02 ×103.
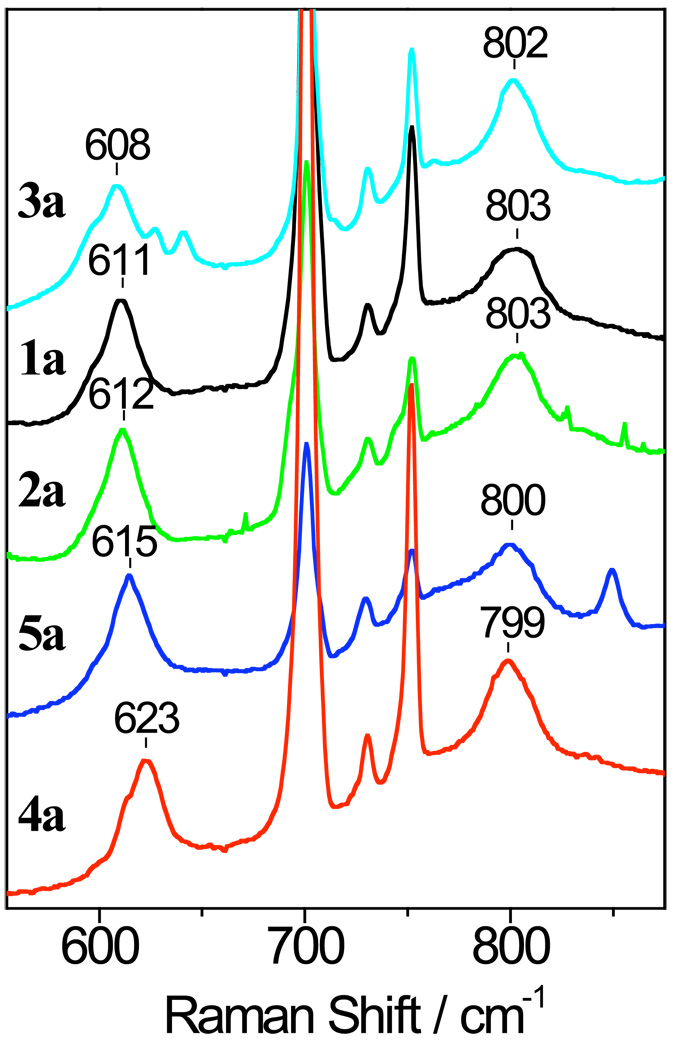
Analysis of Fe-O and O-O Bond Strengths from RR Spectroscopy
Definitive evidence for the identification of the low-temperature chromophores 1a – 5a as FeIII-OOtBu species comes from RR spectroscopy (Figure 6). The dominant band in the 600 – 650 cm−1 range is assigned to the Fe-O stretching vibration of the Fe-OOtBu unit, while the band near 800 cm−1 is assigned to the O-O stretching vibration. These vibrations are resonance-enhanced with laser excitation coinciding with the alkylperoxo-to-iron(III) LMCT band. In all compounds studied here, the RR spectra obtained with a 514-nm excitation show no evidence of resonance enhanced ν(Fe–S) modes associated with the intermediate species (data not shown). The lack of enhancement of ν(Fe–S) modes suggests that the LMCT transitions have no sulfur-to-iron(III) LMCT character. The ν(O–O) falls in the expected region for a low-spin FeIII-OOR (R = alkyl) species, but the ν(Fe–O) is lower than usual (Table 4). Confirmation of these assignments comes from the observed isotopic shifts for 1a-18O18OtBu (ν(Fe–18O) = 584 cm−1; ν(18O–18O) = 757 cm−1).24 The 28- and 46-cm−1 downshifts observed for the Δν(Fe–16/18O) and Δν(16/18O–16/18O) modes, respectively, are in perfect agreement with predicted values for isolated diatomic vibrations. Equivalent 18O-shifts are observed for the ν(Fe–O) modes of 2a, 4a, and 5a (Figure S2). Overlapping solvent bands and a Fermi splitting of the ν(18O–18O) modes makes it difficult to quantify the extent of the 18O-shifts on the ν(O–O), but the spectral changes observed are sufficient to confirm the assignment of the ν(O–O) mode.
Figure 6.
Resonance Raman spectra of [FeIII([15]aneN4)(SAr)(OOtBu)]+ complexes 1a – 5a in CH2Cl2 at 110 K obtained with 514-nm excitation. Intense Raman bands from the solvent are observed between 700 and 750 cm−1. The 849-cm−1 band in 5a is assigned to the NO2 scissoring mode.
Table 4.
Vibrational Data Obtained from Resonance Raman Spectroscopy for Low-Spin FeIII-OOtBu Complexes
| Low-spin FeIII-OOtBu complex | υ (cm−1) | υ (cm−1) | k(Fe-O)a | k(O-O)a | ref |
|---|---|---|---|---|---|
| (Fe-O) | (O-O) | (mdyn/Å) | (mdyn/ Å) | ||
| [FeIII([15]aneN4)(SC6H5)(OOtBu)]+ (1a) | 611 | 803 | 2.74 | 3.03 | b |
| [FeIII([15]aneN4)( SC6H4-p-Cl)(OOtBu)]+ (2a) | 612 | 803 | 2.75 | 3.03 | b |
| [FeIII([15]aneN4)( SC6H4-p-OMe)(OOtBu)]+ (3a) | 608 | 802 | 2.71 | 3.03 | b |
| [FeIII([15]aneN4)( SC6H4-p-NO2)(OOtBu)]+ (5a) | 615 | 800 | 2.77 | 3.02 | b |
| [FeIII([15]aneN4)( SC6F4-p-SC6F5)(OOtBu)]+ (4a) | 623 | 799 | 2.85 | 3.01 | b |
| [FeIII(TPA)(OHn)(OOtBu)]n+,c | 696 | 796 | 3.53d | 2.92d | 29 |
| [FeIII(TPA)(OOtBu)(acetone)]+ | 693 | 788 | 3.52 | 2.93 | 26 |
| [FeIII(5-Me3-TPA) (OOtBu) (acetone)]+ | 691 | 789 | 3.50 | 2.93 | 26 |
| [FeIII(bpy)2(OOtBu)(BzOH)]2+ | 678 | 808 | 3.37 | 3.08 | 30 |
| [FeIII(b-BPMCN)(OOtBu)(X)]2+,e | 685 | 793 | 3.44 | 2.96 | 79 |
| [FeIII(b-BPMCN)(OOtBu)(X)]2+,f | 680 | 789 | 3.39 | 2.93 | 79 |
Force constants calculated for a diatomic oscillator using ν(cm−1) = 1302.83[k(mdyn/Å)/μ]1/2, where μ is the diatomic reduced mass, unless otherwise noted.
This work.
Recently reported as [FeIII(TPA)(OOtBu)(NCMe]n+ in the same solvent (CH3CN).26
Obtained from DFT calculations using a combined NCA/DFT approach.
X = NCMe or H2O.
X= OTf or ROOH.
Based on the RR data for 1a, we previously noted that the Fe-O bond is surprisingly weak compared to other low-spin FeIII-OOR complexes where R is an alkyl group.24 Indeed, other low-spin FeIII-OOR complexes exhibit relatively strong Fe-O bonds and weak O-O bonds,26,29,30,79 as opposed to their high-spin analogs which give rise to weak Fe-O and strong O-O bonds.28,31,33 The vibrational frequencies observed previously for other low-spin FeIII-OOtBu complexes and those observed for 1a – 5a are given in Table 4. An earlier study of the low-spin complex [FeIII(TPA)(OHn)(OOtBu)]n+ (entry 6, Table 4) showed evidence of vibrational mixing within the Fe-OOtBu unit (exptl: Δ16/18O = 24 cm−1; theor. diatomic approx.: Δ16/18O = 31 cm−1), and a normal coordinate analysis coupled with DFT calculations was used to derive a force constant of 3.53 mdyn/Å for the Fe-O bond in [FeIII(TPA)(OHn)(OOtBu)]n+.29 This force constant together with those of the other complexes in Table 4 are significantly larger than those for 1a – 5a. The 1a to 5a series presents weaker Fe-O bonds than other iron(III)-alkylperoxo complexes lacking the thiolate ligand and implicates the unique thiolate donor in weakening this bond.
The influence of the sulfur donor can be seen in the correlation between the energy of the Fe-O vibrations for 1a – 5a and the identity of the thiolate ligand. Specifically, the ν(Fe-O) shifts to higher energy as the electron-withdrawing character of the aryl group increases. In contrast, the ν(O-O) is unaffected by the thiolate donor, and the kO-O is nearly constant in the 1a – 5a series and is similar to those of previously reported low-spin complexes lacking a thiolate ligand (Table 4). These RR data show that the electron-donating ability of the arylthiolate ligand controls the strength of the Fe-O bond without affecting the O-O bond.
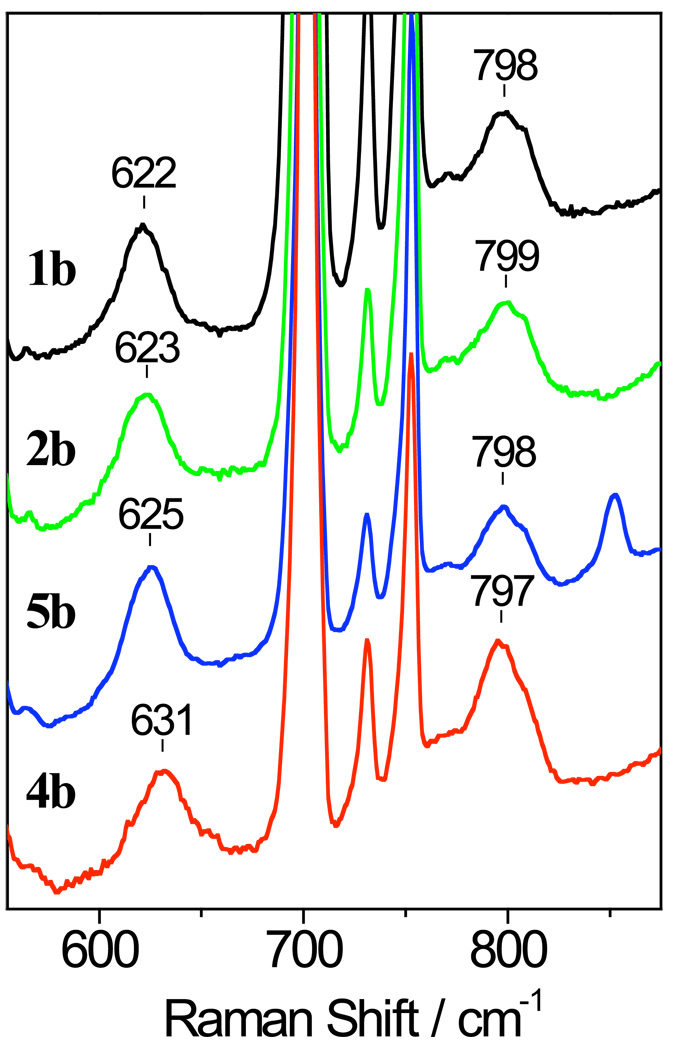
A similar dependence on the electron-donating ability of the arylthiolate ligand is observed for the FeIII-OOCm (Cm = C(CH3)2C6H5) species. The RR spectra of these complexes show ν(Fe-O) modes between 622 and 631 cm−1 (Figure 7) which are ~10 cm−1 higher than in the Fe-OOtBu complexes. As with the Fe-OOtBu complexes, the FeIII−18O18OCm complexes show RR spectra that confirm the ν(Fe-O) assignment, but the extent of the isotope shifts come short of the expected values for isolated diatomic vibrations and suggest that mode mixing occurs with the cumenyl derivatives (Figure S3). The frequency up-shifts observed with the ν(Fe-O) may be due to the different steric requirements of the cumenyl group versus the tert-butyl group and conformational changes in the Fe-OOR moiety. Despite these changes, and as seen earlier with the tBu series, the Fe-O stretching frequencies increase with increasing electron-withdrawing character from the para-substituent on the arylthiolate ligand. Once again, these results support a trans effect of the thiolate donor, where both σ- and π-bonding interactions between the iron(III) ion and the alkylperoxo ligand are weakened by the thiolate donor competing for the same metal d orbitals.
Figure 7.
Resonance Raman spectra of [FeIII([15]aneN4)(SAr)(OOCm)]+ complexes 1b, 2b, 4b, and 5b (same experimental conditions as in Figure 6).
Theoretical calculations on the alkylperoxo species were carried out to obtain further insights into the trends observed in the vibrational data. Density functional theory calculations (B3LYP/6-311G)56 were performed on a model of the alkylperoxo intermediates in which the tBu or Cm group was replaced with a methyl group, [(FeIII([15]aneN4)(OOMe)(SAr)]+. The geometries were optimized beginning from the X-ray structural coordinates of the corresponding iron(II) starting materials, and both high-spin and low-spin iron(III) configurations (S = 5/2 or 1/2) were evaluated. The low-spin species were found to be the lowest in energy for all five thiolate ligands.84 The calculated vibrational frequencies for the low-spin complexes reveal that the Fe-O stretch strengthens upon decreasing the electron-donating ability of the thiolate donor, whereas the O-O stretch shows less variation: SAr (ν(Fe-O), ν(O-O)) = SC6H4-p-OMe (586, 776 cm−1); SC6H5 (584, 774 cm−1); SC6H4-p-Cl (595, 781 cm−1); SC6H4-p-NO2 (599, 775 cm−1); SC6F4-p-SC6F5 (600, 776 cm−1). The calculated Fe-O distances also vary with the arylthiolate substituents, becoming shorter with decreasing electron-donating character, while the O-O distance shows no such correlation: SAr (d(Fe-O), d(O-O)) = SC6H4-p-OMe (1.875, 1.532 Å); SC6H5 (1.873, 1.532 Å); SC6H4-p-Cl (1.870, 1.532 Å); SC6H4-p-NO2 (1.864, 1.531 Å); SC6F4-p-SC6F5 (1.858, 1.530 Å). These findings are in agreement with the trends observed experimentally for the Fe-O and O-O vibrations with changes in the identity of the thiolate ligand, and support the idea that the thiolate donor modulates the strength of the Fe-O bond in the alkylperoxo species.
The [15]aneN4 ligand, as well as related macrocycles such as [14]aneN4 (cyclam), are flexible and potentially allow for both cis and trans geometries in complexes of the type [M(macrocycle)L2], depending on the size of the metal ion and nature of the coligands L. Although there are no structurally well-defined iron(III) complexes of the [15]aneN4 ligand,85 there are a number of other six-coordinate MIII complexes ([MIII([15]aneN4)L2] (M = Co, Cr, Rh; L = monodentate ligand)). 46,72,86–88 All of these complexes exist exclusively in the trans geometry, as opposed to smaller macrocycles such as cyclam, which exhibit both cis- and trans-isomers for complexes of the type [MIII(cyclam)L2]. Molecular mechanics calculations from Busch and Hay indicated that the trans geometry is energetically preferred for the [15]aneN4 ligand, providing a proper “fit” around the metal center.72,86 Thus we conclude that the thiolate ligands in the Fe-OOR complexes are coordinated trans to the OOR ligand and are weakening the Fe-O interaction through a trans effect.
Our spectroscopic study of these Fe-OOR complexes shows that low-spin iron(III) centers do not necessarily give rise to strong Fe-O bonds in FeIII-OOR species. Interestingly, a weakened Fe-O bond was also recently observed by Kovacs and coworkers for the high-spin complex [FeIII(cyclam-PrS)(OOH)]+, and was attributed to a trans influence of the thiolate donor.36 These observations contrast with a series of high-spin FeIII-OOR complexes [(L8py-2)(X)FeIII(OOR)]+ (L8py2 = neutral N4 donor, X = CH3-p-C6H4S−, C6H5CO2−, OTf−), where the N4 ligand enforces a trans X-Fe-OOR arrangement, and where the Fe-O bond is not affected by the nature of the trans ligand.33 The [FeIII(cyclam-PrS)(OOH)]+ complex is currently unique among high-spin FeIII-OOH species in exhibiting the same trans influence on the Fe-O bond as observed in 1a – 5a, 1b, 2b, 4b, and 5b. It should be noted that with the exception of [FeIII(cyclam-PrS)(OOH)]+, all trans-[FeIII(cyclam)L2] complexes are found in low-spin configurations. For example, even the very weak-field donor OTf− yields low-spin iron(III) in [trans-(cyclam-acetato)FeIII(OTf)]+,89 whereas cis-[FeIII(cyclam)L2] complexes are uniformly high-spin. Although a cis configuration for [FeIII(cyclam-PrS)(OOH)]+ can not be entirely ruled out based on the available data, as described this complex provides another example of a thiolate-induced trans influence for an Fe-OO(H or R) species.
Implications for the SOR mechanism
Examination of the reaction between native SOR and O2− generated by pulse radiolysis by different research groups has led to the observation of a transient intermediate characterized by a UV-vis band with λmax = 600 nm.14,17,19,21,90 Kurtz has recently reported the generation of this same 600 nm intermediate by stopped-flow mixing of SOR and KO2.91 This intermediate has been proposed to be an FeIII-OO(H) species, but has thus far eluded characterization by other spectroscopic methods. However, reaction of SOR with H2O2 has allowed for the trapping and characterization of an FeIII-peroxo species, initially formulated as an FeIII-η2-O22− (“side-on” peroxo) species.92,93 High-resolution X-ray structural data has become available on a mutant SOR (E114A) in which the crystals were first oxidized to FeIII and then soaked in H2O2 prior to freezing.15 The structural data revealed a terminal, “end-on” bound hydroperoxo-iron(III) species trapped in different configurations. Earlier predictions from density functional theory (DFT) calculations suggested that a side-on peroxide bonding mode was not sterically feasible for the SOR active site and favored a low-spin, end-on FeIII-OOH species.94 These calculations combined with the recent X-ray structural analysis argue for assignment of the species generated in solution between SOR and H2O2 as a terminally-bound FeIII-OOH species. New DFT calculations from Solomon and coworkers,22 which take into account the influence of the putative N-H---SCys hydrogen bonds in SOR, also predicts an end-on, protonated peroxide (FeIII-OOH) as the first detectable intermediate in the catalytic mechanism, although these calculations favor a high-spin FeIII ground state as opposed to the low-spin FeIII-OOH predicted in the earlier DFT study.94
Although the relationship of the H2O2-derived species to the native catalytic pathway is an open question, analysis of the structural and spectroscopic data of the H2O2-derived intermediate has provided valuable information regarding the inherent properties of an Fe-(hydro)peroxo moiety in the SOR active site. Most pertinent to the present study is recent work by Nivière and coworkers, who sought to determine the role of the cysteine donor by examining a H2O2-derived intermediate for a mutant form of SOR from D. baarsii in which the iron-sulfur bond was perturbed.18 In this study Glu114, which is found in proximity to the Fe-S bond, was replaced with Ala, causing a disruption of the dipolar interaction between Glu114 and the Fe-S unit. In the oxidized form of the mutant (obtained by treating the mutant with K2IrCl6), a downshift of the Fe3+-S vibrational modes (291/313/311 cm−1 for E114A; 299/316/323 for wild-type) was observed, indicating a significant weakening of the iron-sulfur bond. Reaction of the reduced form of the mutant with a slight excess of H2O2 followed by rapid freezing (< 5 s) allowed for freeze-trapping of an iron-peroxo intermediate and subsequent examination by RR spectroscopy. The RR spectrum showed intense ν(Fe-O) and ν(O-O) bands at 446 cm−1 and 851 cm−1. In comparison, RR data on H2O2-derived intermediates from both wild-type and E47A–SOR revealed lower Fe-O stretching frequencies (ν(Fe-O) = 438 cm−1) but the same O-O stretch (ν(O-O) = 850–851 cm−1). Thus a major conclusion from this work was that the E114A mutation induces a weakening of the Fe-S bond, which in turn causes a significant strengthening of the Fe-O bond in the (hydro)peroxo intermediate, but no change in the O-O bond.
The results presented herein are strikingly similar to the recent findings for the E114A mutant of the enzyme; namely that the weakening of the arylthiolate donor in [FeIII([15]aneN4)(SAr)(OOR)]+ induces a significant strengthening of the Fe-O bond, while leaving the O-O bond unperturbed. Although these FeIII-OOR model complexes are low-spin, while the FeIII-OOH intermediate in E114A SOR is suggested to be high-spin, our results provide strong evidence to support the hypothesis that the trans thiolate donor in SOR significantly modulates the strength of the Fe-O bond.
In addition to modulation of the Fe-O bond strength for E114A SOR, the stability of the Fe-OO(H) intermediate was also significantly increased with the weakening of the Fe-S interaction, as demonstrated by increased yields of the intermediate and slower decay kinetics.18 The kinetics of decay for 2a – 5a are complicated and do not follow a simple first-order rate law. Similarly, complex kinetic profiles were previously observed for the decay of other low-spin FeIII-OOtBu complexes.26 However, monitoring the disappearance of the LMCT bands for 1a – 5a by low-temperature UV-vis reveal a qualitative trend wherein the FeIII-OOR species decay more slowly as the electron-donating character of ArS− is reduced. For example, the fluorinated derivative 4a exhibits a remarkably slow decay rate, showing almost no decomposition at −78 °C for over 48 h, as opposed to 1.5 h for the complete decay of the p-H complex 1a. As described in the Introduction (vide supra), a study by Que and Halfen on the high-spin model complexes (N4L)FeIII-OOR (L = −OTf, −O2CC6H5, −SC6H4-p-CH3)33 showed an increased stability with electron-donating trans axial ligands (for kdecomposition, L = −OTf > −O2CC6H5 > −SC6H4-p-CH3) and no impact of the trans ligand on the ν(Fe-O) in the RR spectra. Indeed, to our knowledge compounds 1a – 5a are the first series of model complexes that provide direct evidence for such a trans influence in FeIII-OO(H or R) species.
Summary and Perspective
We have prepared a series of iron(II) complexes as models of the SOR active site. These complexes were designed to examine the influence of the thiolate donor on the structural and physical properties as well as the reactivity of the metal center at parity of ligand environment. The metrical parameters as determined by X-ray crystallography do not vary significantly with the change in thiolate donor. In contrast, the nature of the arylthiolate donor has a significant impact on the FeIII/FeII potential, in support of the suggestion that the cysteine ligand in SOR plays a major role in controlling the iron redox potential.16,22 The stabilization of FeIII-OOR species at low temperature for all of the thiolate-ligated complexes was demonstrated, and spectroscopic characterization of these alkylperoxo complexes shows that they are low-spin iron(III) species with weak Fe-O bonds. These findings provide an exception to the hypothesis that low-spin FeIII-OO(H or R) species necessarily exhibit strong Fe-O bonds in comparison to their high-spin counterparts.
The electron-donating power of the sulfur donor in the FeIII-OOR complexes described herein directly influences the strength of the Fe-O bond through a trans influence from the thiolate ligand. In contrast, the thiolate donor does not impact the O-O bond strength. DFT calculations on a simplified model for the FeIII-OOR complexes support these experimental findings. A similar observation was recently described for the E114A SOR enzyme, in which a H2O2-derived FeIII-OO(H) species was trapped and characterized by RR spectroscopy.18 Thus, our results, combined with the results for E114A SOR suggest that an important function of the Cys donor in SOR is to weaken the Fe-O bond without weakening the O-O bond, thereby favoring Fe-O over O-O bond cleavage. Recent DFT calculations on SOR also support this conclusion.22
The sulfur atom of the Cys ligand in SOR is within weak hydrogen bond contact of two nearby amide N-H groups as determined by X-ray crystallography.20 For many other cysteine-ligated iron proteins it has been proposed that N-H---S hydrogen bonds assist in tuning the S-Fe bonding interaction and the iron redox potentials,78 and similar proposals have been made for SOR.20,22 We suggest that perhaps the presence of the N-H---S bonds in SOR help to modulate the Fe-S interaction in much the same way as addition of an electron-withdrawing para substituent to the arylthiolate ligand of our model system; they provide fine-tuning of the Cys ligand’s ability to influence the Fe-O bond while leaving the O-O bond unperturbed in an FeIII-OOH intermediate, ultimately favoring protonation and formation of H2O2. Generation of FeIII-OO(H) adducts via reactions between our model complexes and H2O2 and O2− is currently being examined.
Supplementary Material
Acknowledgements
D.P.G. is grateful to the National Institute of General Medical Sciences at the NIH for funding this work (GM62309). F. N. is thankful for financial support provided by a Zeltmann Fellowship sponsored through Johns Hopkins University. Prof. V. Szalai (University of Maryland Baltimore County) is acknowledged for her help with EPR spectroscopy. We thank Dr. Andrea Caneschi (University of Florence) for collecting SQUID data.
Footnotes
Supporting Information Available: X-ray structure files for 2, 3, 4 and 5 (CIF). UV-vis spectra of the titration of [FeII([15]aneN4)(SPh)(BF4)] with tBuOOH. Resonance Raman spectra of [FeIII([15]aneN4)(SAr)(18O18OtBu)]+ and [FeIII([15]aneN4)(SAr)(18O18OCm)]+ complexes. Complete ref 56. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Sono M, Roach MP, Coulter ED, Dawson JH. Chem. Rev. 1996;96:2841–2887. doi: 10.1021/cr9500500. [DOI] [PubMed] [Google Scholar]
- 2.Sligar SG, Makris TM, Denisov IG. Biochem. Biophys. Res. Comuun. 2005;338:346–354. doi: 10.1016/j.bbrc.2005.08.094. [DOI] [PubMed] [Google Scholar]
- 3.Shaik S, Kumar D, de Visser SP, Altun A, Thiel W. Chem. Rev. 2005;105:2279–2328. doi: 10.1021/cr030722j. [DOI] [PubMed] [Google Scholar]
- 4.Meunier B, de Visser SP, Shaik S. Chem. Rev. 2004;104:3947–3980. doi: 10.1021/cr020443g. [DOI] [PubMed] [Google Scholar]
- 5.Loew GH, Harris DL. Chem. Rev. 2000;100:407–419. doi: 10.1021/cr980389x. [DOI] [PubMed] [Google Scholar]
- 6.Denisov IG, Makris TM, Sligar SG, Schlichting I. Chem. Rev. 2005;105:2253–2277. doi: 10.1021/cr0307143. [DOI] [PubMed] [Google Scholar]
- 7.Unno M, Matsui T, Ikeda-Saito M. Nat. Prod. Rep. 2007;24:553–570. doi: 10.1039/b604180a. [DOI] [PubMed] [Google Scholar]
- 8.Stubbe J, Kozarich JW, Wu W, Vanderwall DE. Acc. Chem. Res. 1996;29:322–330. [Google Scholar]
- 9.Stubbe J, Kozarich JW. Chem. Rev. 1987;87:1107–1136. [Google Scholar]
- 10.Que L, Jr, Ho RYN. Chem. Rev. 1996;96:2607–2624. doi: 10.1021/cr960039f. [DOI] [PubMed] [Google Scholar]
- 11.Kovaleva EG, Neibergall MB, Chakrabarty S, Lipscomb JD. Acc. Chem. Res. 2007;40:475–483. doi: 10.1021/ar700052v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kovaleva EG, Lipscomb JD. Science. 2007;316:453–457. doi: 10.1126/science.1134697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Costas M, Mehn MP, Jensen MP, Que L., Jr Chem. Rev. 2004;104:939–986. doi: 10.1021/cr020628n. [DOI] [PubMed] [Google Scholar]
- 14.Emerson JP, Coulter ED, Cabelli DE, Phillips RS, Kurtz DM., Jr Biochemistry. 2002;41:4348–4357. doi: 10.1021/bi0119159. [DOI] [PubMed] [Google Scholar]
- 15.Katona G, Carpentier P, Nivière V, Amara P, Adam V, Ohana J, Tsanov N, Bourgeois D. Science. 2007;316:449–453. doi: 10.1126/science.1138885. [DOI] [PubMed] [Google Scholar]
- 16.Kurtz DM., Jr Acc. Chem. Res. 2004;37:902–908. doi: 10.1021/ar0200091. [DOI] [PubMed] [Google Scholar]
- 17.Lombard M, Houée-Levin C, Touati D, Fontecave M, Nivière V. Biochemistry. 2001;40:5032–5040. doi: 10.1021/bi0023908. [DOI] [PubMed] [Google Scholar]
- 18.Mathé C, Weill CO, Mattioli TA, Berthomieu C, Houée-Levin C, Tremey E, Nivière V. J. Biol. Chem. 2007;282:22207–22216. doi: 10.1074/jbc.M700279200. [DOI] [PubMed] [Google Scholar]
- 19.Nivière V, Lombard M, Fontecave M, Houée-Levin C. Febs Letters. 2001;497:171–173. doi: 10.1016/s0014-5793(01)02468-1. [DOI] [PubMed] [Google Scholar]
- 20.Adam V, Royant A, Nivière V, Molina-Heredia FP, Bourgeois D. Structure. 2004;12:1729–1740. doi: 10.1016/j.str.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 21.Coulter ED, Emerson JP, Kurtz DM, Jr, Cabelli DE. J. Am. Chem. Soc. 2000;122:11555–11556. [Google Scholar]
- 22.Dey A, Jenney FE, Adams MWW, Johnson MK, Hodgson KO, Hedman B, Solomon EI. J. Am. Chem. Soc. 2007;129:12418–12431. doi: 10.1021/ja064167p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kovacs JA, Brines LM. Acc. Chem. Res. 2007;40:501–509. doi: 10.1021/ar600059h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krishnamurthy D, Kasper GD, Namuswe F, Kerber WD, Sarjeant AAN, Moenne-Loccoz P, Goldberg DP. J. Am. Chem. Soc. 2006;128:14222–14223. doi: 10.1021/ja064525o. [DOI] [PubMed] [Google Scholar]
- 25.Girerd JJ, Banse F, Simaan AJ. Struct. Bonding (Berlin) 2000;97:145–177. [Google Scholar]
- 26.Jensen MP, Payeras AMI, Fiedler AT, Costas M, Kaizer J, Stubna A, Munck E, Que L., Jr Inorg. Chem. 2007;46:2398–2408. doi: 10.1021/ic0607787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lehnert N, Fujisawa K, Solomon EI. Inorg. Chem. 2003;42:469–481. doi: 10.1021/ic020496g. [DOI] [PubMed] [Google Scholar]
- 28.Lehnert N, Ho RYN, Que L, Jr, Solomon EI. J. Am. Chem. Soc. 2001;123:12802–12816. doi: 10.1021/ja011450+. [DOI] [PubMed] [Google Scholar]
- 29.Lehnert N, Ho RYN, Que L, Jr, Solomon EI. J. Am. Chem. Soc. 2001;123:8271–8290. doi: 10.1021/ja010165n. [DOI] [PubMed] [Google Scholar]
- 30.Menage S, Wilkinson EC, Que L, Jr, Fontecave M. Angew. Chem. Int. Ed. 1995;34:203–205. [Google Scholar]
- 31.Wada A, Ogo S, Watanabe Y, Mukai M, Kitagawa T, Jitsukawa K, Masuda H, Einaga H. Inorg. Chem. 1999;38:3592–3593. doi: 10.1021/ic9900298. [DOI] [PubMed] [Google Scholar]
- 32.Zang Y, Kim J, Dong YH, Wilkinson EC, Appelman EH, Que L., Jr J. Am. Chem. Soc. 1997;119:4197–4205. [Google Scholar]
- 33.Bukowski MR, Halfen HL, van den Berg TA, Halfen JA, Que L., Jr Angew. Chem. Int. Ed. 2005;44:584–587. doi: 10.1002/anie.200461527. [DOI] [PubMed] [Google Scholar]
- 34.Clay MD, Cosper CA, Jenney FE, Adams MWW, Johnson MK. Proc. Natl. Acad. Sci. U.S.A. 2003;100:3796–3801. doi: 10.1073/pnas.0636858100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kovacs JA. Chem. Rev. 2004;104:825–848. doi: 10.1021/cr020619e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kitagawa T, Dey A, Lugo-Mas P, Benedict JB, Kaminsky W, Solomon E, Kovacs JA. J. Am. Chem. Soc. 2006;128:14448–14449. doi: 10.1021/ja064870d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kokatnur VR, Jelling M. J. Am. Chem. Soc. 1941;63:1432–1433. [Google Scholar]
- 38.Walling C, Buckler SA. J. Am. Chem. Soc. 1955;77:6032–6038. [Google Scholar]
- 39.Finn MG, Sharpless KB. J. Am. Chem. Soc. 1991;113:113–126. [Google Scholar]
- 40.CRysalis, CrysAlis PRO. Poland: Oxford Diffraction Ltd. Wroclaw; [Google Scholar]
- 41.Sheldrick GM. SHELXTL, Version 6.10. Madison, Wisconsin, USA: Bruker AXS Inc.; 2000. [Google Scholar]
- 42.Ballester L, Gutierrez A, Perpinan MF, Sanchez AE, Azcondo MT, Gonzalez MJ. Inorg. Chim. Acta. 2004;357:1054–1062. [Google Scholar]
- 43.Blake AJ, Li WS, Lippolis V, Schroder M. Acta. Cryst. 1998;C54:299–302. [Google Scholar]
- 44.Chen LF, Cotton FA. J. Mol. Struct. 1998;470:161–166. [Google Scholar]
- 45.Clegg W. Acta. Cryst. 1986;C42:1463–1464. [Google Scholar]
- 46.Clegg W, Leupin P, Richens DT, Sykes AG, Raper ES. Acta. Cryst. 1985;C41:530–532. [Google Scholar]
- 47.Ito T, Kato M, Ito H. Bull. Chem. Soc. Jpn. 1984;57:2634–2640. [Google Scholar]
- 48.Ito T, Kato M, Ito H. Bull. Chem. Soc. Jpn. 1984;57:2641–2649. [Google Scholar]
- 49.Jacobsen CJH, Hyldtoft J, Larsen S, Pedersen E. Inorg. Chem. 1994;33:840–842. [Google Scholar]
- 50.Kato M, Ito T. Inorg. Chem. 1985;24:509–514. [Google Scholar]
- 51.Mak TCW, Che CM, Wong KY. J. Chem. Soc., Chem. Commun. 1985:986–988. [Google Scholar]
- 52.Notni J, Gorls H, Anders E. Eur. J. Inorg. Chem. 2006:1444–1455. [Google Scholar]
- 53.Panneerselvam K, Lu TH, Chi TY, Pariya C, Liao FL, Chung CS. Acta. Cryst. 1998;C54:712–714. [Google Scholar]
- 54.Pleus RJ, Saak W, Pohl SZ. Anorg. Allg. Chem. 2001;627:250–253. [Google Scholar]
- 55.Shi S, Espenson JH, Bakac A. J. Am. Chem. Soc. 1990;112:1841–1846. [Google Scholar]
- 56.Frisch MJ, et al. Gaussian 03, Revision C.02. 2004 [Google Scholar]
- 57.Green MT. J. Am. Chem. Soc. 2006;128:1902–1906. doi: 10.1021/ja054074s. [DOI] [PubMed] [Google Scholar]
- 58.Namuswe F, Sarjeant AAN, Goldberg DP. unpublished results. [Google Scholar]
- 59.Halfen JA, Moore HL, Fox DC. Inorg. Chem. 2002;41:3935–3943. doi: 10.1021/ic025517l. [DOI] [PubMed] [Google Scholar]
- 60.Fiedler AT, Halfen HL, Halfen JA, Brunold TC. J. Am. Chem. Soc. 2005;127:1675–1689. doi: 10.1021/ja046939s. [DOI] [PubMed] [Google Scholar]
- 61.Shearer J, Scarrow RC, Kovacs JA. J. Am. Chem. Soc. 2002;124:11709–11717. doi: 10.1021/ja012722b. [DOI] [PubMed] [Google Scholar]
- 62.Shearer J, Nehring J, Lovell S, Kaminsky W, Kovacs JA. Inorg. Chem. 2001;40:5483–5484. doi: 10.1021/ic010221l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.The high-spin configuration for 1 – 3 has been confirmed by SQUID measurements, giving χMT = 3.61 – 3.70 cm3 mol−1 K at 298 K (χMT(theor.) = 3.63 cm3 mol−1 K with g = 2.2 for HS FeII)]
- 64.Krishnamurthy D, Sarjeant A, Goldberg DP, Caneschi A, Totti F, Zakharov LN, Rheingold AL. Chem. Eur. J. 2005;11:7328–7341. doi: 10.1002/chem.200500156. [DOI] [PubMed] [Google Scholar]
- 65.Mukherjee RN, Abrahamson AJ, Patterson GS, Stack TDP, Holm RH. Inorg. Chem. 1988;27:2137–2144. [Google Scholar]
- 66.Zang Y, Que L., Jr Inorg. Chem. 1995;34:1030–1035. [Google Scholar]
- 67.Addison AW, Rao TN, Reedjik J, van Rijn J, Verschoor GC. J. Chem. Soc., Dalton Trans. 1984:1349–1456. [Google Scholar]
- 68.McNaughton RL, Tipton AA, Rubie ND, Conry RR, Kirk ML. Inorg. Chem. 2000;39:5697–5706. doi: 10.1021/ic0003729. [DOI] [PubMed] [Google Scholar]
- 69.Solomon EI, Szilagyi RK, George SD, Basumallick L. Chem. Rev. 2004;104:419–458. doi: 10.1021/cr0206317. [DOI] [PubMed] [Google Scholar]
- 70.Yeh AP, Hu YL, Jenney FE, Jr, Adams MWW, Rees DC. Biochemistry. 2000;39:2499–2508. doi: 10.1021/bi992428k. [DOI] [PubMed] [Google Scholar]
- 71.Clay MD, Jenney FE, Jr, Hagedoorn PL, George GN, Adams MWW, Johnson MK. J. Am. Chem. Soc. 2002;124:788–805. doi: 10.1021/ja016889g. [DOI] [PubMed] [Google Scholar]
- 72.Hung Y, Martin LY, Jackels SC, Tait AM, Busch DH. J. Am. Chem. Soc. 1977;99:4029–4039. [Google Scholar]
- 73.Berry JF, Bill E, Garcia-Serres R, Neese F, Weyhermuller T, Wieghardt K. Inorg. Chem. 2006;45:2027–2037. doi: 10.1021/ic051823y. [DOI] [PubMed] [Google Scholar]
- 74.Jovanovic T, Ascenso C, Hazlett KRO, Sikkink R, Krebs C, Litwiller R, Benson LM, Moura I, Moura JJG, Radolf JD, Huynh BH, Naylor S, Rusnak F. J. Biol. Chem. 2000;275:28439–28448. doi: 10.1074/jbc.M003314200. [DOI] [PubMed] [Google Scholar]
- 75.Rodrigues JV, Saraiva LM, Abreu IA, Teixeira M, Cabelli DE. J. Biol. Inorg. Chem. 2007;12:248–256. doi: 10.1007/s00775-006-0182-x. [DOI] [PubMed] [Google Scholar]
- 76.Tavares P, Ravi N, Moura JJG, Legall J, Huang YH, Crouse BR, Johnson MK, Huynh BH, Moura I. J. Biol. Chem. 1994;269:10504–10510. [PubMed] [Google Scholar]
- 77.Okamura T, Takamizawa S, Ueyama N, Nakamura A. Inorg. Chem. 1998;37:18–28. doi: 10.1021/ic970640b. [DOI] [PubMed] [Google Scholar]
- 78.Dey A, Okamura T, Ueyama N, Hedman B, Hodgson KO, Solomon EI. J. Am. Chem. Soc. 2005;127:12046–12053. doi: 10.1021/ja0519031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jensen MP, Costas M, Ho RYN, Kaizer J, Payeras AMI, Munck E, Que L, Jr, Rohde JU, Stubna A. J. Am. Chem. Soc. 2005;127:10512–10525. doi: 10.1021/ja0438765. [DOI] [PubMed] [Google Scholar]
- 80.Roelfes G, Vrajmasu V, Chen K, Ho RYN, Rohde JU, Zondervan C, la Crois RM, Schudde EP, Lutz M, Spek AL, Hage R, Feringa BL, Münck E, Que L., Jr Inorg. Chem. 2003;42:2639–2653. doi: 10.1021/ic034065p. [DOI] [PubMed] [Google Scholar]
- 81.Abu-Eittah RH, Hilal RH. Appl. Spectrosc. 1972;26:270–277. [Google Scholar]
- 82.Thompson JS, Marks TJ, Ibers JA. J. Am. Chem. Soc. 1979;101:4180–4192. [Google Scholar]
- 83.Kim J, Zang Y, Costas M, Harrison RG, Wilkinson EC, Que L., Jr J. Biol. Inorg. Chem. 2001;6:275–284. doi: 10.1007/s007750000198. [DOI] [PubMed] [Google Scholar]
- 84.It should be noted that there are difficulties in determining the relative energies of spin states for open-shell metal ions by DFT calculations
- 85.Hay RW, Fraser I. Polyhedron. 1997;16:2223–2227. [Google Scholar]
- 86.Hay RW, Tarafder MTH. J. Chem. Soc., Dalton Trans. 1991:823–827. [Google Scholar]
- 87.Islam MS, Uddin MM. Polyhedron. 1993;12:423–426. [Google Scholar]
- 88.Bhattacharya PK. J. Chem. Soc., Dalton Trans. 1980:810–812. [Google Scholar]
- 89.Grapperhaus CA, Mienert B, Bill E, Weyhermuller T, Wieghardt K. Inorg. Chem. 2000;39:5306–5317. doi: 10.1021/ic0005238. [DOI] [PubMed] [Google Scholar]
- 90.Rodrigues JV, Abreu IA, Cabelli D, Teixeira M. Biochemistry. 2006;45:9266–9278. doi: 10.1021/bi052489k. [DOI] [PubMed] [Google Scholar]
- 91.Huang VW, Emerson JP, Kurtz DM. Biochemistry. 2007;46:11342–11351. doi: 10.1021/bi700450u. [DOI] [PubMed] [Google Scholar]
- 92.Mathé C, Mattioli TA, Horner O, Lombard M, Latour JM, Fontecave M, Nivière VJ. Am. Chem. Soc. 2002;124:4966–4967. doi: 10.1021/ja025707v. [DOI] [PubMed] [Google Scholar]
- 93.Horner O, Mouesca JM, Oddou JL, Jeandey C, Nivière V, Mattioli TA, Mathé C, Fontecave M, Maldivi P, Bonville P, Halfen JA, Latour JM. Biochemistry. 2004;43:8815–8825. doi: 10.1021/bi0498151. [DOI] [PubMed] [Google Scholar]
- 94.Silaghi-Dumitrescu R, Silaghi-Dumitrescu L, Coulter ED, Kurtz DM., Jr Inorg. Chem. 2003;42:446–456. doi: 10.1021/ic025684l. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.