Abstract
Objective
To test the efficacy of behavioural counselling for smoking mothers in reducing young children's exposure to environmental tobacco smoke.
Design
Randomised double blind controlled trial.
Setting
Low income homes in San Diego county, California.
Participants
108 ethnically diverse mothers who exposed their children (aged <4 years) to tobacco smoke in the home.
Intervention
Mothers were given seven counselling sessions over three months.
Main outcome measures
Children's reported exposure to environmental tobacco smoke from mothers in the home and from all sources; children's cotinine concentrations in urine.
Results
Mothers' reports of children's exposure to their smoke in the home declined in the counselled group from 27.30 cigarettes/week at baseline, to 4.47 at three months, to 3.66 at 12 months and in the controls from 24.56, to 12.08, to 8.38. The differences between the groups by time were significant (P=0.002). Reported exposure to smoke from all sources showed similar declines, with significant differences between groups by time (P=0.008). At 12 months, the reported exposure in the counselled group was 41.2% that of controls for mothers' smoke (95% confidence interval 34.2% to 48.3%) and was 45.7% (38.4% to 53.0%) that of controls for all sources of smoke. Children's mean urine cotinine concentrations decreased slightly in the counselled group from 10.93 ng/ml at baseline to 10.47 ng/ml at 12 months but increased in the controls from 9.43 ng/ml to 17.47 ng/ml (differences between groups by time P=0.008). At 12 months the cotinine concentration in the counselled group was 55.6% (48.2% to 63.0%) that of controls.
Conclusions
Counselling was effective in reducing children's exposure to environmental tobacco smoke. Similar counselling in medical and social services might protect millions of children from environmental tobacco smoke in their homes.
Introduction
The World Health Organization has estimated that the health of almost half of the world's children is threatened by exposure to environmental tobacco smoke.1 In the United States the prevalence of US children living in homes with a smoker has been estimated to be 43%, with state specific estimates of exposure in the home ranging from 12% to 34%2; nationally, about 15 million US children and adolescents are exposed.3 Similarly, about 43% of Australian children,4 33% of Canadian children,5 and 41% of British children are exposed to environmental tobacco smoke.6 Exposure increases children's risk of respiratory tract infections, otitis media, asthma, and the sudden infant death syndrome.7–9 The costs to children's medical care from exposure were $703m-$897m (£439m-£561m) in the United States, $239.5m (£150m) in Canada, and $267m (£167m) in Great Britain (in 1997 prices).10
Two trials reported significant decreases in children's exposure to environmental tobacco smoke after counselling of parents. Greenberg et al decreased children's reported exposure, but infants' urine cotinine concentrations increased.11 Hovell and colleagues found similar reductions in reported exposure, which were sustained over two years, but did not measure cotinine concentrations.12,13 We extended our earlier research by measurement of cotinine concentrations and by testing counselling (in person and by telephone) with high risk, ethnically diverse, and low income families recruited from the US supplemental nutrition programme for women, infants, and children. We hypothesised that counselling would decrease children's exposure, decrease mothers' smoking, and increase rates of stopping smoking.
Participants and methods
Protocol
Inclusion criteria
We included English and Spanish speaking mothers who smoked at least two cigarettes a day and exposed their child (aged <4 years) to the smoke from at least one cigarette a day. We excluded women who were currently breast feeding, to avoid confounded cotinine analyses,14,15 and women who did not have a telephone, to ensure exposure to the intervention.
Recruitment
Nine months' screening at sites of the supplemental nutrition programme for women, infants, and children identified 1147 possibly eligible families. Of these, we contacted 832: 162 (19.5%) qualified and were offered financial incentives ($60-$90) to participate. We enrolled the first 108 women who signed informed consent forms, an adequate sample size based on previous research.12 After we had taken baseline measures, we randomly assigned the families to counselling or control conditions.
Counselled group
Counselled mothers were told that quitting smoking was not required. They were given seven individualised counselling sessions (three in person and four by telephone) during three months. Counselling was based on shaping procedures.16 The mean duration of sessions ranged from 12.6 to 28.0 minutes. Graduate students with 20 hours of training and weekly supervision by case review provided the counselling.
At the first session, mothers set long term goals for reducing children's exposure to environmental tobacco smoke and signed contracts. Counsellors explained the shaping process (in which complex smoking practices were gradually altered to reduce exposure to the child) and assisted mothers in writing fortnightly objectives that resembled medical prescriptions. Between sessions, mothers recorded their smoking and their child's exposure on pictorial charts. Mothers were provided with “No smoking” signs and stickers to serve as cues for reducing their child's exposure. In subsequent sessions, counsellors reviewed progress and negotiated possible solutions to barriers to reducing children's exposure. New objectives and strategies were set. Contingencies included praise from counsellors and low cost “self rewards.” In the last session mothers were helped to write final goals and objectives for maintaining low exposure or for further decreasing exposure. Details about the counselling programme are published elsewhere.17
Control group
Mothers received the usual nutritional counselling of the supplemental nutrition programme and brief advice to quit smoking and not expose their children to environmental tobacco smoke.
Measures of exposure to environmental tobacco smoke
Mothers' reports
Interviews were conducted at baseline, at three months (after counselling), and at six and 12 months. The baseline interview was conducted in person in the mothers' homes, and follow up interviews were by telephone. The mean length of interviews was 57.2 (SD 15.8) minutes. Content included information on mothers' demographics and tobacco use and their child's exposure to environmental tobacco smoke.
Mothers reported their smoking and their child's exposure on typical work days and non-work days during the past seven days. They reported children's exposure to smoke from others living in and visiting the home, and from all smokers outside of the home. We measured exposure as the number of cigarettes smoked while the child was in the same room and calculated children's weekly exposure to mothers' cigarettes in the home and to all cigarettes. Acceptable test-retest reliability and validity in relation to cotinine and nicotine assays are reported elsewhere.18,19
Children's urine cotinine concentrations
Urine samples (collected at baseline and three and 12 months) were analysed for cotinine (a metabolite of nicotine and recommended biomarker)20 at the Centers for Disease Control and Prevention by means of isotope dilution liquid chromatography and tandem mass spectrometry with a limit of detection of <50 parts per trillion. We obtained samples from children who were not toilet trained by placing two sterile 15 cm cotton rolls in diapers and removing these when they were wet. The cotton rolls were packed into a sterile 20 ml syringe (without needle), and the urine was expressed into a 5 ml vial. Previous research showed that cotton rolls do not alter the cotinine concentration.21 Samples from toilet trained children were collected with a standard urine collection cup. Samples were frozen at –29°C and packed in dry ice for shipping. The laboratory was blind to subjects' identity and group assignment.
Mothers' saliva cotinine concentrations
Mothers' saliva was obtained at each interview with Episcreen collection devices (Epitope, Beaverton, OR) and stored frozen at –29°C until laboratory analysis by enzyme linked immunoassay (STC, Bethlehem, PA). The laboratory was blind to subjects' identity and group assignment. Mothers who reported stopping smoking were tested and cessation confirmed by cotinine concentrations <30 ng/ml.
Nicotine monitors
We conducted nicotine monitoring to provide objective validation of mothers' reported levels of smoking and to enhance reporting accuracy.22 Inactive monitors were placed in three rooms per household where children's greatest exposure to environmental tobacco smoke was reported. These were used to sensitise the mothers to possible confirmation of their reports of exposure. One week before the three month interview, we placed an active monitor in the room of greatest exposure for a randomly selected half of the families. The monitor was a 37 mm diameter cassette containing a Teflon coated glass fibre filter (Emfab TX 40h120WW, Pallflex, Putnam, CT) saturated with 4% sodium bisulphate and 5% ethanol and dried. Gas chromatography was used to assess nicotine levels.23,24 Assays confirmed the validity of mothers' reports.19
Assignment and masking
Random numbers were used to stratify assignment by three ethnic groups. After the baseline measures, assistants opened an envelope to reveal assignments. Measurement assistants were blind to group assignment. Control families were unaware of counselling procedures, and investigators were blind to results until all data were collected.
Statistical analyses
Analyses were based on intention to treat. We adjusted dependent variables by logarithmic or square root transformation to reduce skewness and present geometric and untransformed means. Differential rate of change in reported exposure and cotinine estimates of exposure relied on analyses of repeated measures over time. Estimated power to detect differential change between groups exceeded 0.80 for all dependent variables. We analysed the effects of counselling using the generalised estimating equations approach, with linear components of time as “within subjects” factors and the interaction as a “between subjects” factor (SAS version 6.12).25 Modelling procedures based on generalised estimating equations are superior to models based on analysis of variance in that they do not require repeated measures to be equally spaced from one another and they retain cases with missing data at one or more times. We first calculated differential change from baseline to end of follow up and then repeated this for baseline to three months (counselling effect) and from three months to end of follow up (maintenance effect).
Results
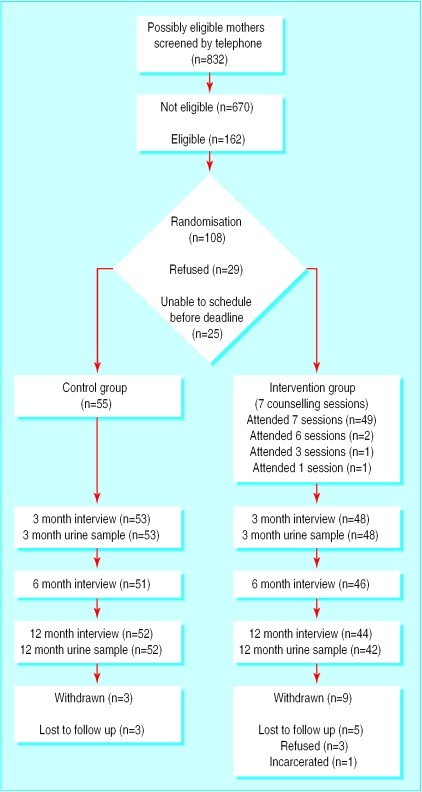
Participant flow and follow up
Figure 1 shows the number of mothers enrolled through completion of measures. Forty nine (92%) of the mothers assigned to the counselling group completed all seven counselling sessions.
Figure 1.
Flow of participants through trial
Participants
Table 1 shows the demographic characteristics of the mothers and children. Families were white, black, or Hispanic and had low income with limited education.
Table 1.
Baseline characteristics of families with a young child (<4 years old) and a mother who smoked who received either three months of counselling or standard advice to reduce smoking in the presence of the child. Values are numbers (percentages) unless stated otherwise
| Variable | Counselled families (n=53) | Control families (n=55) |
|---|---|---|
| Ethnic group: | ||
| Black | 11 (21) | 12 (22) |
| Hispanic | 14 (26) | 16 (29) |
| White | 25 (47) | 26 (47) |
| Other | 3 (6) | 1 (2) |
| Children's sex (girls) | 31 (58) | 26 (47) |
| Single parent families | 23 (43) | 27 (49) |
| Employed mothers | 8 (15) | 5 (9) |
| Mothers' education: | ||
| Less than high school or GED* | 22 (42) | 20 (36) |
| High school or GED* | 14 (26) | 13 (24) |
| Trade school | 4 (8) | 4 (7) |
| Some college | 12 (23) | 16 (29) |
| College graduate | 1 (2) | 2 (4) |
| Mean (SD) age: | ||
| Mothers' (years) | 28.5 (6.6) | 29.0 (6.9) |
| Children's (months) | 14.1 (7.0) | 14.3 (6.9) |
| Mean (SD) No of times mothers had stopped smoking for 24 hours | 11.6 (25.0) | 19.4 (48.0) |
| Mothers' mean No of cigarettes smoked/day | 12.6† | 12.2† |
GED=Generalised equivalency degree.
Means are squared estimates of means adjusted by square root transformation and so do not include standard deviations. These estimates provide an indication of the levels in clinically meaningful units.
Sampling and success of random assignment
The two groups were well matched in their demographic and dependent variables, suggesting successful random assignment.
Analyses
Reported exposure
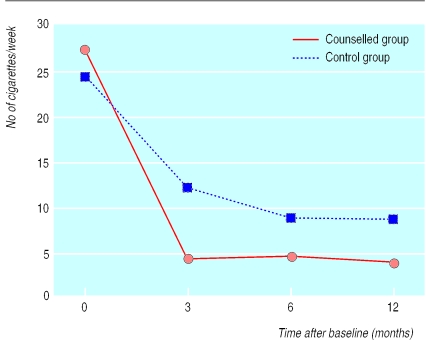
Figure 2 shows that in both groups the children's reported exposure to their mothers' tobacco smoke in the home declined steeply from baseline to three months (end of counselling) and then only slightly during follow up. Our analyses of repeated measures showed significant differences between groups by time (P=0.002), indicating that exposure declined more for the counselled group than for the control group. Analyses of changes from baseline to three months also showed significant differences between groups by time (P=0.011). From three months to 12 months, the difference between the two groups remained significant (P=0.017), but neither showed any significant change over time, suggesting that the counselling effect was maintained but that no later improvement occurred. Student's t tests showed a significant cross sectional difference between the groups at three months only (t(99)=−2.74 (95% confidence interval −1.503 to −0.240); P=0.007). Thus, the effects of counselling were obtained by three months and sustained through follow up. Table 2 shows the geometric means for children's exposure to environmental tobacco smoke at baseline, three months, and 12 months.
Figure 2.
Children's reported exposure to mothers' cigarettes in the home (No of cigarettes per week) in families with a young child (<4 years old) and a mother who smoked who received either three months of counselling or standard advice to reduce smoking in the presence of the child. Values are geometric means
Table 2.
Measures of children's exposure to environmental tobacco smoke in families with a young child (<4 years old) and a mother who smoked who received either three months of counselling or standard advice to reduce smoking in the presence of the child. Values are geometric means (interquartile ranges) unless stated otherwise
| Variable | Time
|
||
|---|---|---|---|
| Baseline | 3 months (after counselling) | 12 months | |
| Reported exposure to environmental tobacco smoke (No of cigarettes/week) | |||
| Exposure from mothers in home: | |||
| Counselled families | 27.30 (32.91) | 4.47 (26.11) | 3.66 (28.08) |
| Control families | 24.56 (31.03) | 12.08 (33.09) | 8.38 (45.99) |
| Relative value for counselled group v controls (% (95% CI))* | 32.8 (26.3 to 39.3) | 41.2 (34.2 to 48.3) | |
| Total environmental exposure: | |||
| Counselled families | 51.30 (73.43) | 12.99 (42.94) | 8.60 (44.15) |
| Control families | 50.68 (63.68) | 26.28 (59.79) | 19.23 (66.91) |
| Relative value for counselled group v controls (% (95% CI))* | 50.0 (42.8 to 57.2) | 45.7 (38.4 to 53.0) | |
| Urine cotinine concentration (ng/ml) | |||
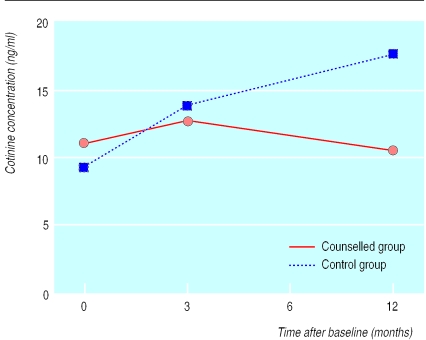
| Counselled families | 10.93 (17.29) | 12.65 (12.12) | 10.47 (24.28) |
| Control families | 9.43 (13.28) | 13.88 (18.00) | 17.47 (21.61) |
| Relative value for counselled group v controls (% (95% CI))* | 84.2 (79.0 to 89.4) | 55.6 (48.2 to 63.0) | |
Means were adjusted for baseline levels to calculate relative values.
Children's reported total exposure to environmental tobacco smoke followed a similar pattern (table 2), with the counselled group showing a significantly greater decline (P<0.008). Both groups showed significant declines from baseline to three months (P<0.001). From three months to 12 months, the difference between the two groups remained significant (P=0.043), but neither showed any significant change in exposure over time. Student's t tests showed significant differences between the two groups at three months (t(94)=−2.30 (−1.244 to −0.092); P=0.024) and 12 months (t(91)=−2.10 (−1.430 to −0.039); P=0.039), suggesting that counselling had an effect and that this was maintained.
Children's urine cotinine concentration
Figure 3 shows that children's cotinine concentrations increased from baseline to three months in both groups but that the concentration then declined slightly in the counselled group whereas it continued to increase in the control group. Our analyses of repeated measures showed significant differences between groups by time (P=0.008). Student's t tests showed significant differences between the two groups only at 12 months (t(90)=−2.05 (−0.948 to −0.015); P=0.043). These results suggested a prevention effect that lasted through follow up.
Figure 3.
Children's urine cotinine concentrations (ng/ml) in families with a young child (<4 years old) and a mother who smoked who received either three months of counselling or standard advice to reduce smoking in the presence of the child. Values are geometric means
Mothers' saliva cotinine concentration
From baseline to three months, the mothers' cotinine concentrations increased significantly in both groups—from 75.8 ng/ml to 91.2 ng/ml for counselled women and from 76.9 ng/ml to 89.7 ng/ml for controls (P<0.001). During follow up, counselled mothers' cotinine concentrations decreased to 80.6 ng/ml at 12 months, while those of the controls increased to 112.9 ng/ml. This difference between groups by time neared significance (P=0.06), suggesting a possible decrease in the relative level of smoking for counselled mothers compared with controls. There were no significant differences in the numbers of mothers who stopped smoking (six in the counselling group and four in the control group).
Discussion
This is the first study to show therapeutic benefits of counselling mothers on their children's exposure to environmental tobacco smoke based on cotinine concentrations. In the counselled group the children's cotinine concentrations decreased slightly (4%) by 12 months, whereas those in the control group increased substantially (85%), suggesting that counselling prevented an increase in exposure to environmental tobacco smoke. Reported exposure to environmental tobacco smoke decreased more after counselling and was sustained for nine months, suggesting maintenance of effects consistent with our previous findings.13
Our present results extend earlier work by showing the efficacy of counselling delivered in part by telephone to women receiving services from the supplemental nutrition programme for women, infants, and children. The successful decrease (or prevention of increase) in children's exposure to environmental tobacco smoke in this low income, racially and ethnically diverse, high risk population suggests that counselling is generalisable, as does the similarity of our results to those from earlier studies.11–13 Such counselling in medical and social services might protect millions of children from exposure to environmental tobacco smoke.
Smoking decreased slightly among counselled mothers but increased by half among controls. Counselling may have prevented an increase in mothers' smoking over time, although it did not result in more mothers quitting. Increased smoking among the controls probably contributed to their children's increased cotinine concentrations.
Parental reports of reducing their children's exposure could reflect the parents smoking in a different room but still close enough for the child to inhale smoke. Similarly, as children begin walking, they may be exposed to nicotine from dust on carpets and furniture. This would not be easily monitored or reported by parents and might account for the control mothers reporting decreased exposure to environmental tobacco smoke whereas their children had increased cotinine concentrations. Additional research is needed to determine the source of increasing cotinine concentrations in control children.
Conclusions
Both mothers' reports and cotinine analyses confirmed the benefits of counselling on children's exposure to environmental tobacco smoke. The most conservative interpretation of the results suggests that counselling prevented an increase in exposure to environmental tobacco smoke. Future studies should be directed to interventions that combine formal counselling for quitting smoking with counselling for reducing children's exposure to environmental tobacco smoke. Future studies should also extend follow up to assess how long the effects of counselling are maintained and the developmental trends in exposure to environmental tobacco smoke. These results set the stage for research to determine the effects of reducing exposure to environmental tobacco smoke on morbidity and mortality.
What is already known on this topic
The World Health Organization has estimated that the health of almost half of the world's children is threatened by exposure to environmental tobacco smoke
Two trials reported significant decreases in children's exposure to environmental tobacco smoke after counselling of mothers, but neither provided an objective outcome measure of efficacy
What this study adds
A randomised trial of counselling to reduce children's exposure to environmental tobacco smoke used measures of cotinine concentrations in addition to mothers' reports
Counselled mothers reported significantly greater decreases in exposure to environmental tobacco smoke compared with controls, and children's urine cotinine concentrations decreased slightly for counselled families while increasing substantially for controls
The findings confirm the efficacy of counselling to reduce children's exposure to environmental tobacco smoke
Acknowledgments
We thank the following investigators for their assistance during the conduct of this trial: Chris Ake, Department of Family and Preventive Medicine, University of California at San Diego; S Katharine Hammond, Environmental Health Sciences Division, School of Public Health, University of California, Berkeley; Doug Hoffman, Neurochemistry Laboratory, Dartmouth Medical School; Sarah Nordahl Larson, San Diego State University Foundation WIC programme; Brian P Leaderer, Division of Environmental Health Sciences, Yale University School of Medicine.
Footnotes
Funding: This research was supported by Grant No 027946 SFP awarded to MFH from the Robert Wood Johnson Foundation Smoke-Free Families Program, and by discretionary funds from the Center for Behavioral Epidemiology and Community Health.
Competing interests: None declared.
References
- 1.World Health Organization; Division of Noncommunicable Diseases; Tobacco Free Initiative. International consultation on environmental tobacco smoke (ETS) and child health. Consultation report. Geneva: WHO; 1999. www.who.int/toh/TFI/consult.htm (accessed 26 July 2000). [Google Scholar]
- 2.Pirkle JL, Flegal KM, Bernert JT, Brody DJ, Etzel RA, Maurer KR. Exposure of the US population to environmental tobacco smoke. The third national health and nutrition examination survey, 1988 to 1991. JAMA. 1996;275:1233–1240. [PubMed] [Google Scholar]
- 3.Centers for Disease Control. State-specific prevalence of cigarette smoking among adults, and children's and adolescents' exposure to environmental tobacco smoke—United States, 1996. MMWR Morb Mortal Wkly Rev. 1997;46:1038–1043. [PubMed] [Google Scholar]
- 4.National Health and Medical Research Council. The health effects of passive smoking. Australia: NHMRC; 1997. [Google Scholar]
- 5.Physicians for a Smoke-Free Canada. Highlight sheet No 1. Smoking in Canadian homes: Are children at risk? 1999. www.smoke-free.ca/eng_home/news_press_Jun99.htm . Available from: www.smoke-free.ca/eng_home/news_press_Jun99.htm (accessed 26 July 2000). (accessed 26 July 2000). [Google Scholar]
- 6.Jarvis MJ. Children's exposure to passive smoking: survey methodology and monitoring trends. Background paper. 1999. www.who.int/toh/TFI/consult.htm . Available from: Tobacco Free Initiative www.who.int/toh/TFI/consult.htm (accessed 7 May 2000). (accessed 7 May 2000). [Google Scholar]
- 7.US Department of Health and Human Services (PHS); NIH; US Environmental Protection Agency. Respiratory health effects of passive smoking: lung cancer and other disorders. Washington DC: Office of Research and Development, Office of Air and Radiation; 1993. . (NIH publication No 93-3605.) [Google Scholar]
- 8.Charlton A. Children and passive smoking: a review. J Fam Pract. 1994;38:267–277. [PubMed] [Google Scholar]
- 9.Klonoff-Cohen HS, Edelstein SL, Lefkowitz ES, Srinivasan IP, Kaegi D, Chang JC, et al. The effect of passive smoking and tobacco exposure through breast milk on sudden infant death syndrome. JAMA. 1995;273:795–798. doi: 10.1001/jama.1995.03520340051035. [DOI] [PubMed] [Google Scholar]
- 10.Adams EK, Melvin C, Merritt R, Worrall B. The costs of environmental tobacco smoke (ETS): an international review. 1999. www.who.int/toh/TFI/consult.htm . Available from: Tobacco Free Initiative www.who.int/toh/TFI/consult.htm (accessed 7 May 2000). (accessed 7 May 2000). [Google Scholar]
- 11.Greenberg RA, Strecher VJ, Bauman KE, Boat BW, Fowler MG, Keyes LL, et al. Evaluation of a home-based intervention program to reduce infant passive smoking and lower respiratory illness. J Behav Med. 1994;17:273–290. doi: 10.1007/BF01857953. [DOI] [PubMed] [Google Scholar]
- 12.Hovell MF, Meltzer SB, Zakarian JM, Wahlgren DR, Emerson JA, Hofstetter CR, et al. Reduction of environmental tobacco smoke exposure among asthmatic children: a controlled trial [published erratum appears in Chest 1995;107:1480] Chest. 1994;106:440–446. doi: 10.1378/chest.106.2.440. [DOI] [PubMed] [Google Scholar]
- 13.Wahlgren DR, Hovell MF, Meltzer SB, Hofstetter CR, Zakarian JM. Reduction of environmental tobacco smoke exposure in asthmatic children: a 2-year follow-up. Chest. 1997;111:81–88. doi: 10.1378/chest.111.1.81. [DOI] [PubMed] [Google Scholar]
- 14.Schulte-Hobein B, Schwartz-Bickenbach D, Abt S, Plum C, Nau H. Cigarette smoke exposure and development of infants throughout the first year of life: influence of passive smoking and nursing on cotinine levels in breast milk and infant's urine. Acta Paediatr. 1992;81:550–557. doi: 10.1111/j.1651-2227.1992.tb12293.x. [DOI] [PubMed] [Google Scholar]
- 15.Mascola MA, Vunakis HV, Tager IB, Speizer FE, Hanrahan JP. Exposure of young infants to environmental tobacco smoke: breast-feeding among smoking mothers. Am J Public Health. 1998;88:893–896. doi: 10.2105/ajph.88.6.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mattanini MA, Thyer B, editors. Finding solutions to social problems: behavioral strategies for change. Washington DC: American Psychological Association; 1996. [Google Scholar]
- 17.Hovell MF, Zakarian JM, Matt GE, Hofstetter CR, Bernert JT, Pirkle J. Decreasing environmental tobacco smoke exposure among low income children: preliminary findings. Tobacco Control. 2000;9(suppl 3):iii0–1. doi: 10.1136/tc.9.suppl_3.iii70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Emerson JA, Hovell MF, Meltzer SB, Zakarian JM, Hofstetter CR, Wahlgren DR, et al. The accuracy of environmental tobacco smoke exposure measures among asthmatic children. J Clin Epidemiol. 1995;48:1251–1259. doi: 10.1016/0895-4356(95)00021-u. [DOI] [PubMed] [Google Scholar]
- 19.Matt GE, Hovell MF, Zakarian JM, Bernert JT, Pirkle JL, Hammond SK. Measuring second-hand tobacco smoke exposure in babies: the reliability and validity of mother-reports in a sample of low-income families. Health Psychol. 2000;19:232–241. doi: 10.1037//0278-6133.19.3.232. [DOI] [PubMed] [Google Scholar]
- 20.Hovell MF, Zakarian JM, Wahlgren DR, Matt GE, Emmons KM. Measurement of environmental tobacco smoke exposure: trials and tribulations. Tobacco Control. 2000;9:0–6. doi: 10.1136/tc.9.suppl_3.iii22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matt GE, Wahlgren DR, Hovell MF, Zakarian JM, Bernert JT, Meltzer SB, et al. Measuring ETS exposure in infants and young children through urine cotinine and memory-based parental reports: empirical findings and discussion. Tobacco Control. 1999;8:282–289. doi: 10.1136/tc.8.3.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murray DM, O'Connell CM, Schmid LA, Perry CL. Validation of smoking by self-report by adolescents: a re-examination of the bogus pipeline procedures. Addict Behav. 1987;12:7–15. doi: 10.1016/0306-4603(87)90003-7. [DOI] [PubMed] [Google Scholar]
- 23.Hammond SK, Leaderer BP. A diffusion monitor to measure exposure to passive smoking. Environ Sci Technol. 1987;21:494–497. doi: 10.1021/es00159a012. [DOI] [PubMed] [Google Scholar]
- 24.Leaderer BP, Hammond SK. Evaluation of vapor-phase nicotine and respirable suspended particle mass as markers for environmental tobacco smoke. Environ Sci Technol. 1991;25:770–777. [Google Scholar]
- 25.Diggle PJ, Liang KY, Zeger SL. Analysis of longitudinal data. Oxford: Clarendon Press; 1995. [Google Scholar]