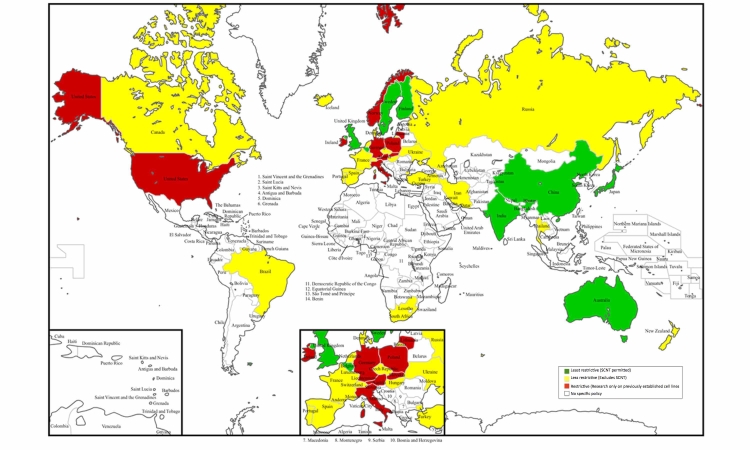
Abstract
The proliferation of stem cell research, conflated with its ethical and moral implications, has led governments to attempt regulation of both the science and funding of stem cells. Due to a diversity of opinions and cultural viewpoints, no single policy or set of rules exist to govern stem cell research. Instead, each country has developed its own policy. The following map catalogs the general legal and political milleu regarding stem cell research by country.
Japan
Japan allows scientists to conduct stem cell research for therapeutic purposes; however, there are no formal guidelines [1]. In November 2007, Japanese researchers collaborating with American scientists reprogrammed human skin cells to behave like embryonic stem cells [2].
Singapore
Singapore is widely considered “Asia’s stem cell center” [1]. It has more than 40 stem cell research groups in the country and authorizes the use, for therapeutic purposes, of embryos that are no more than two weeks old.
China
China has one of the most unrestrictive stem cell policies [1]. In 2003, guidelines were issued that required that embryos used for stem cell research be left over from in vitro fertilization (IVF); fetal cells from abortions; blastocytes from Somatic Cell Nuclear Transfer (SCNT); or germ line cells voluntarily donated. Interestingly, according to Chinese cultural attitudes, a person’s life begins with birth [3].
South Korea
In 2006, scandal arose when the country’s leading biomedical researcher, Dr. Hwang Woo-suk, was discovered to have falsely claimed that he was the first scientist to clone human embryonic stem cells. Nevertheless, South Korea continues to pursue research for the purposes of therapeutic cloning.
Australia
Australia bans all human cloning for reproduction or research [4]. It does allow for the use of embryos remaining after assisted reproduction from before April 5, 2002.
South Africa
Initially, South Africa enacted legislation that banned reproductive cloning but authorized therapeutic cloning [1]. In 2004, this country became the first African nation to create a stem cell bank.
United Kingdom
The Human Fertilization and Embryology Act (HFEA) of 1990 and the Human Reproductive Cloning Act of 2001 permit the destruction of embryos for human embryonic stem cells (hESC) and allows for SCNT [4]. This is only permissible if the proposed research increases knowledge about the development of embryos or serious disease or enables such knowledge to be applied in developing treatments for serious disease. As a result, the United Kingdom is one of the leading centers for hESC research.
Switzerland
The Swiss parliament is considering allowing research on stem cells derived from stored embryos remaining at the end of assisted reproduction for therapeutic purposes only. In 2004, a national referendum was put forth in which two-thirds of voters agreed to allow embryonic stem cell research [5].
Brazil
The Brazilian government passed legislation in March 2005 that allows the use of excess IVF embryos that have been frozen for more than three years [1]. The Brazilian Catholic Church challenged the law, arguing that embryonic stem cell research violates the right to life, but Brazil’s Supreme Court rejected the petition, thus permitting embryonic stem cell research [6].
United States
Under the auspices of the Obama administration, the National Institutes of Health plans to expand federal funding for stem cell lines that meet certain ethical requirements: the embryo was discarded after IVF; informed consent was obtained from the donors; the couple does not receive compensation (neither financial nor medical benefits) or are coerced or threatened [7]. Older stem cell lines created in the spirit of the new regulations will be considered for federal funding, whereas embryos created solely for research purposes will be excluded [8].
Mexico
While Mexico has a flourishing stem cell industry, it does not have formal regulations [9]. Mexican doctors currently are using stem cells to treat ill foreigners, including Americans, who suffer from ailments such as cerebral palsy, autism, and paralysis. The international medical community has criticized this lack of regulation.
Figure.
Stem Cell Research Policies around the World
Abbreviations
- IVF
in vitro fertilization
- SCNT
Somatic Cell Nuclear Transfer
- HFEA
Human Fertilization and Embryology Act
- hESC
human embryonic stem cells
References
- Ralston M. Pew Forum: Stem Cell Research Around the World. [cited 2009 Jul 24];The Pew Forum [Internet] 2008 Jul 17; Available from: http://pewforum.org/docs/index.php?DocID=318. [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K. et al. Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors. Cell. 2007;131(5):861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Murray F, Spar D. Bit Player or Powerhouse? China and Stem-Cell Research. N Engl J Med. 2006;355(2):1191–1194. doi: 10.1056/NEJMp068151. [DOI] [PubMed] [Google Scholar]
- The Hixton Group. Baltimore: The Johns Hopkins Berman Institute of Bioethics; c2006. [cited 2009 Jul 24]. Asia and Oceania: Policy Experts [Internet] Available from: http://www.hinxtongroup.org/wp_ao_exc.html . [Google Scholar]
- BBC News. [place unknown]: BBC News; 2008. Nov 28, [cited 2009 Jul 24]. Swiss endorse stem cell research [Internet] Available from: http://news.bbc.co.uk/2/hi/europe/4049141.stm . [Google Scholar]
- Leite M. Overcoming Opposition, Brazil Banks on Stem Cells. The Stem Cell Community [Internet] 2009. Apr 4, [cited 2009 Jul 24]. Available from: http://www.stemcellcommunity.org/metadot/index.pl?id=3072. [DOI] [PubMed]
- Vendantam S. Rules on Stem Cell Research Are Eased. Washington Post [Internet] 2009. Jul 7, [cited 2009 Jul 24]. Available from: http://www.washingtonpost.com/wp-dyn/content/article/2009/07/06/AR2009070602076.html.
- Holden C. Researchers Generally Happy With Final Stem Cell Rules. Science. 2009;325(5937):131. doi: 10.1126/science.325_131. [DOI] [PubMed] [Google Scholar]
- Zarembo A. A desperate injection of stem cells and hope. Los Angeles Times [Internet] 2005. Feb 20, [cited 2009 Jul 24]. Available from: http://www.latimes.com/features/health/medicine/la-sci-stemcells20feb20,1,4516556.story.