Abstract
(+)-Methamphetamine (MA) administered on postnatal days (P) 11–15 (four times/day) results in increased corticosterone that overlaps the stress hyporesponsive period (SHRP; P2–14) and leads to later learning and memory deficits. Elevated corticosterone during the SHRP results in neurotrophin changes and long-term effects on learning. We determined whether two known stressors could mimic the effects of MA [10 (mg/kg)/dose] administration in neonatal rats. Stressors were four 15-min sessions of forced swim or isolation (confinement in forced swim tubes without water). Saline and weighed-only controls were included and all five treatments were represented within each litter. Corticosterone in plasma and brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF) in neostriatum and hippocampus were examined after one or four treatments on P11 or P15 (0.5, 1.75, 6.5, or 24 h after first dose). MA increased corticosterone and BDNF; forced swim and isolation also increased corticosterone, but to a lesser extent than MA, and neither stressor increased BDNF. NGF was unaffected by saline treatment, but there was a minor reduction in NGF in the forced swim group compared with the weighed-only group. The data show that MA is more potent at releasing corticosterone and increasing BDNF than short-term, repeated episodes of forced swim or isolation. The possible relationship between these changes and the long-term cognitive effects of developmental MA administration are discussed.
Keywords: stress hyporesponsive period, nerve growth factor, corticosterone, BDNF, forced swim, maternal isolation, sexual dimorphism
INTRODUCTION
The prevalence of use of methamphetamine (MA) is greatest among young adults (Johnston et al., 2006). However, the use of MA by women of child-bearing age raises concerns about potential exposure of fetuses and their subsequent development (Chomchai et al., 2004). Case-control studies of newborns and infants suggest increased rates of preterm delivery, decreased head circumference, reduced birth weight and length, increased rates of neurological symptoms, and reduced performance on the Fagan test of infant intelligence (a test of novel object recognition) (Chomchai et al., 2004; Dixon, 1989; Dixon and Bejar, 1989; Little et al., 1988; Smith et al., 2003, 2006; Struthers and Hansen, 1992). Children 7- to 8-years old who were prenatally exposed to MA showed increased creatine and glutamate/glutamine spectral peaks in the striatum by magnetic resonance spectroscopy (Smith et al., 2001). Prenatal exposure to MA is also associated with reduced brain volume (in putamen, globus pallidus, caudate, and hippocampus) as shown by magnetic resonance imaging, reduced sustained attention, and delayed verbal memory (Chang et al. 2004). Although limited, these data suggest that children with a history of intrauterine exposure to MA have long-term changes in the brain and concomitant cognitive effects. However, the mechanisms underlying these effects are unknown. Recent data in animals exposed to MA developmentally suggest that stress mechanisms may be contributory (Williams et al., 2000, 2006). Human users of MA also show increases in cortisol following MA (Gouzoulis-Mayfrank et al., 1999) and neonates exposed to MA in utero exhibit similar physical changes to neonates exposed to stress in utero (Wadhwa, 2005).
The time during gestation when women use MA is variable. At least some MA users continue to use the drug to term and studies have documented positive urine and/or blood toxicology tests for MA up to and during labor and delivery (Chang et al., 2004; Chomchai et al., 2004). Brain regions critical to learning (hippocampus, basal ganglia, prefrontal cortex, and temporal cortex) develop predominantly during the second half of human gestation and may be affected by use of MA during this period. Equivalent stages of rodent brain development occur later in relation to birth than in humans. For example, cells in the dentate gyrus proliferate until postnatal day (P)19 in the rat, a stage most analogous to third trimester in humans (Bayer et al., 1993). An interspecies algorithm that uses multiple markers to compare development between species reveals that P11 rat brain is approximately equivalent to gestational week 19 for limbic regions and week 26 for cortical regions during fetal development in humans (Clancy et al., 2007). Consistent with this timetable and the aforementioned human data, we have demonstrated that rats exposed to MA on P11-20 exhibit spatial learning and memory deficits when tested as adults in the Morris water maze (Vorhees et al., 1994; Williams et al., 2002, 2003b, 2003a). Further investigation of this exposure period revealed that P11-15 MA exposure is critical for causing impaired spatial learning and memory compared with P16-20 MA exposure (Williams et al., 2003a).
Administration of MA during some and/or all of the days of this critical period results in release of corticosterone (CORT) and ACTH (Schaefer et al., 2006; Williams et al., 2000, 2006). As in other species, rodents pass through a stress hyporesponsive period (SHRP) of development (Sapolsky and Meaney, 1986; Vazquez, 1998), i.e., a period of pituitary and adrenal hypoactivity during hypothalamic-pituitary-adrenal (HPA) axis development (Dent et al., 2000). The sensitive period for the effects of MA on later learning, P11–15, overlaps with the SHRP (Sapolsky and Meaney, 1986) leading us to examine the impact of stressors delivered on the same schedule as MA at inducing changes in CORT release. Stressors selected for comparison, 15 min of forced swim (FS) or isolation (ISO), were ones that could be administered discretely and intermittently in a regimen comparable with that used for MA administration, i.e., given four times per day at 2-h intervals. The short duration of application of the stressors was used since animals given MA spend very little time outside of the litter, but still demonstrate increased plasma CORT. Others have demonstrated that longer periods of separation from the dam or litter can produce increases in CORT (McCormick et al., 1998), however we wanted stressors that did not involve long periods of separation since to do so would not be comparable to the MA treatment regimen. However, it should be noted that we were not attempting to match CORT levels between the FS-, ISO-, and MA-treatments.
Various signaling molecules are important to the normal development of the brain and disruption of these can disrupt developmental processes. For example, consistent CORT levels during the SHRP are important for hippocampal development (Sapolsky and Meaney, 1986), whereas neurotrophic factors are generally important for the survival and maintenance of neurons during development. Neurotrophic factors not only play a role in nervous system development, they have also been implicated in learning and memory processes in adult animals. For example, BDNF and trkB mRNA were shown to be increased after spatial learning (Gomez-Pinilla et al., 2001; Kesslak et al., 1998), and heterozygous BDNF or nerve growth factor (NGF) knockout mice (Chen et al., 1997; Linnarsson et al., 1997) demonstrated reduced performance in the Morris water maze. Neurotrophins can also be affected by alterations in CORT in both developing and adult animals. For example, BDNF mRNA was shown to be decreased in the dentate gyrus of adult animals following immobilization stress (Smith et al., 1995), while NGF mRNA was increased in the hippocampus following high levels of neonatal CORT exposure (Roskoden et al., 2004). We previously showed that when MA is administered from P11–14 (four times/day) and examined on P15 or administered from P11–20 (four times/day) and examined on P20, BDNF is increased in the hippocampus compared with the saline-treated animals (Skelton et al., 2007). In the present experiment, we wanted to know whether intermittent intervals of stress altered BDNF and NGF similarly to what we have seen after P11–15 MA given four times each day at 2-h intervals.
Studies in humans have reported MA daily doses of 0.25–10 g with averages in the 1–1.6 g per day range (Chang et al., 2005; Simon et al., 2000; Volkow et al., 2001). Assuming a maternal body mass of 60 kg, the range of daily doses from the aforementioned studies would be 4.2 to 166.7 mg/kg. Comparing doses on a strictly body mass basis may underestimate human exposure since species differences in basal metabolic rate, oxygen utilization, and CO2 clearance are not taken into account. An alternative approach to comparing doses across species is use of allocentric scaling formulae that have been used for therapeutic drugs to estimate human doses based on preclinical data (Mordenti and Chapell, 1989). A typical formula is Dosehuman = Doseanimal [(Weighthuman/Weightanimal)0.7]. If one assumes a maternal body mass of 60 kg, a 25-g neonatal rat, and an average human dose of 1600 mg the formula yields a per rat dose of 6.9 mg or 275.6 mg/kg with a range of 43–1721.5 mg/kg. Thus, the dose of MA used in the present experiment (10 mg/kg × four doses/day) represents a moderate to low-moderate user if one accepts dose comparisons based on body mass or interspecies scaling. It is important to note, however, that interspecies scaling has its limits (Lin, 1998) and its application to other substituted amphetamines (MDMA) has recently been questioned (Baumann et al., 2007; de la Torre and Farre, 2004).
MATERIALS AND METHODS
Subjects
Nulliparous female (151–175 g) Sprague Dawley CD IGS rats (Charles River, Raleigh, NC) were mated with males (251–275 g) of the same strain and supplier; and the offspring were the subjects in this experiment. Rats were acclimated to the homeroom (14-h dark: 10-h light, lights on at 0600 h with temperature and humidity controlled) for at least 1 week prior to breeding. Birth was considered P0. On P1, pups were removed from their mothers, weighed, sexed, and culled to five males and five females. On P11, pups were marked for identification by ear punch. Animals had ad libitum access to food and water and all protocols were approved by the Cincinnati Children’s Research Foundation’s Institutional Animal Care and Use Committee. The vivarium is accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care.
Treatments
A split-litter design was employed such that one male and female pair from each litter was administered one of the five treatments. Offspring pairs were assigned to one of five-treatment groups as follows: (1) (+)-MA, (2) FS, (3) ISO, (4) saline (SAL), or (5) weighed-only (WEIGH). Whole litters were randomly assigned to be assessed at one of eight different age–time combinations (five treatments × two sexes × two ages × four times). The age–time combinations were as follows: 0.5 or 1.75 h after a single treatment on P11; 0.5 or 18 h after the last of four treatments on P11 (hereafter referred to as 6.5 and 24 h after the first treatment, respectively); 0.5 or 1.75 h after a single treatment on P15 following four daily treatments from P11–14; and finally 0.5 or 18 h after the last of four treatments on P15 following four daily treatments from P11–14 (i.e., 6.5 or 24 h after the first treatment, respectively, on the final day of treatment). Treatments were administered at 2-h intervals, four times a day, and body weights were obtained prior to each treatment. These time points were used because we have previously shown that increases in CORT occur in MA-treated animals at 0.5 and 1.75 h after a single dose and up to 24 h after four doses on P11 and on P15 at 0.5 h after four doses on each day from P11–14 and one dose on P15, however it should be noted that on P15 levels of CORT at 1.75 h were similar between MA- and SAL-treated animals (Schaefer et al., 2006; Williams et al., 2000, 2006). For each age–time combination, eight litters were used. Pups were treated according to the schedule detailed in Figure 1.
Fig. 1.
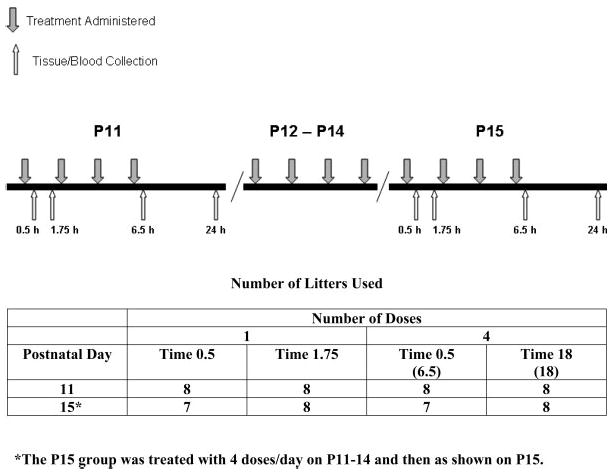
Experimental design. Diagram showing times that subjects were given one of the five treatments (WEIGH, SAL, MA, FS, ISO) and times they were sacrificed for tissue collection. Gray arrows above the line indicate when animals were administered a treatment (2-h intervals). White arrows beneath the line show times animals were sacrificed. For P11, rats were administered either one treatment and sacrificed at 0.5 or 1.75 h after treatment, or four treatments and sacrificed 6.5 h or 24 h after the first treatment. Another group was treated four times daily from P11–14 and once or four times on P15 and tissue collected. On P15, collections were made 0.5 or 1.75 h after a single treatment or 6.5 or 24 h after the first of four treatments. The numbers of litter treated per day and time points are depicted in the table on the bottom.
For the injected animals, one male and female pair received either (+)-MA HCL (MA, 10 mg/kg, expressed as the freebase; >95% pure, National Institute on Drug Abuse), or saline vehicle (SAL) subcutaneously in the dorsum in a volume of 3 ml/kg. Animals receiving treatment throughout the entire assessment period received four injections, 2-h apart per day. Sites of injection were alternated in order to avoid irritation of the skin.
In addition to the MA and SAL-treated pair in each litter, one male and female pair from each litter was placed in FS and another pair in ISO for 15 min at each treatment time. For FS, animals were placed for 15 min in a cylinder 46-cm tall and 15 cm in diameter containing 35-cm deep water (22 ± 1°C). Animals were monitored throughout the swimming period. ISO animals received 15 min in identical cylinders used in the FS procedure, with the exception that the cylinders contained no water. During ISO, cylinders were maintained at room temperature (22 ± 1°C). Upon removal from the cylinder, animals were placed back with their litters. The fifth male and female pair in each litter served as an untreated control. These animals were picked up, weighed, and replaced in the litter at each of the time points when other animals in the litter were being weighed and treated.
Tissue collection
At the time of assessment, animals were removed from the litter, transferred to another suite, and immediately decapitated (Holson, 1992) (<30 s after removal from the litter). Trunk blood was collected in polyethylene tubes containing 0.05 ml 2% EDTA and stored on ice until centrifuged. Plasma was isolated from whole blood by centrifugation at 1300g for 25 min, after which the supernatant was collected. Concurrent with blood collection, brains were removed and the hippocampus and neostriatum (caudate-putamen) dissected over ice with the aid of a brain block (Zivic-Miller, Pittsburgh, PA). The brain was first sliced coronally at the optic chiasm and the neostriatum was dissected freehand from a block of tissue that was 2-mm immediately rostral to this first coronal cut. The remaining forebrain was sliced sagitally and the hippocampus removed from each cerebral hemisphere. All tissues were frozen on dry ice immediately upon dissection and stored at −80°C until assayed. Finally, because stress is known to affect both the adrenal and thymus glands (Selye, 1936), these tissues were removed, freed of fatty tissue, and weighed. These tissue weights were expressed both as absolute weight and as a percentage of body weight.
Neurotrophin and corticosterone assessment
The concentration of BDNF and NGF in the hippocampus and neostriatum was determined on P11 and P15 using the Emax ImmunoAssay System (Promega Corp, Madison WI). The samples were homogenized in lysis buffer according to the kit instructions in 1 ml of buffer and the hippocampal samples were further diluted 1:1 and the neostriatal samples 1:10 prior to assay. All samples were assayed in duplicate according to the manufacturer’s instructions and levels were expressed against total protein (i.e., pg/mg). Protein was assayed using a BCA protein assay kit (Pierce Biotechnology, Rockford, IL). CORT levels (ng/ml) in plasma were assayed with Octeia corticosterone ELISA kits (IDS, Fountain Hills, AZ) and each sample was diluted 1:5 and assayed in duplicate according to the manufacturer’s protocol. Optical densities for the ELI-SAs were measured on a SpectraMax Plus microtiter plate reader (Molecular Devices, Sunnyvale, CA).
Statistics
Significance for all measures was determined using a mixed general linear model analysis of variance (ANOVA; Proc Mixed, SAS, Cary, NC) with treatment group (MA, Saline, FS, ISO, or WEIGH) × time (0.5, 1.75, 6.5, or 24 h) × age (P11 or 15) × sex (male or female) model and with litter as a blocking factor to control for litter effects. When significant interactions were obtained, simple-effect ANOVAs were performed to further localize differences. A posteriori group comparisons were made using the step-down Bonferroni method and significance was accepted at P ≤ 0.05. Data are presented as least square means ± standard error of the mean.
RESULTS
Body weight
Body weights were recorded on P7 and during dosing. Animals that were administered one or four treatments on P11 were similar in weight regardless of the treatment. After multiple treatments from P11–15, MA-treated animals showed decreased weight gain i.e., they weighed ~80% of the WEIGH controls; all other treatments were similar in weight to controls (not shown).
Forced swim
At the earliest ages, some rats could not tolerate 15 min of swimming because of fatigue and were therefore assisted. Assistance was defined as removal from FS if the tip of the animal’s nose did not remain above the water. When this occurred the animal was removed for the remainder of the trial and was tested again at the next scheduled trial. In the P11 condition, 17/64 animals required assistance, i.e., 13/64 animals required assistance on the first trial, 2/32 on the second trial, and 2/32 on the remaining two trials combined. In the P11–15 condition, 33/60 rats required assistance on at least one trial on P11 and 21/60 on at least one trial on P12. On P13, 9/60 required assistance, on P14 5/60, and by P15 only 1/60. Since multiple animals had difficulty with a 15-min FS, increasing the duration of FS for inducing greater stress effects on CORT release was not feasible. Despite the fact that some rats required assistance, the FS procedure resulted in significant CORT responses (see below).
Corticosterone
An ANOVA on levels of CORT demonstrated a significant main effect of treatment, F(4, 486) = 52.92, P < 0.0001, and the interactions of treatment × age, F(4, 486) = 6.68, P < 0.0001, treatment × time, F(12, 486) = 4.98, P < 0.0001, and treatment × age × time, F(12, 486) = 6.75, P < 0.0001. Examination of the three-way interaction showed that on P11 after a single treatment, levels of CORT in animals administered MA were significantly increased 0.5 h after injection compared with all other treatments (Fig. 2A). At 1.75 h, this increase in CORT in MA-administered animals was more pronounced and significantly different from all other treatments, (Fig. 2B); no other significant group differences were observed following a single dose on P11.
Fig. 2.
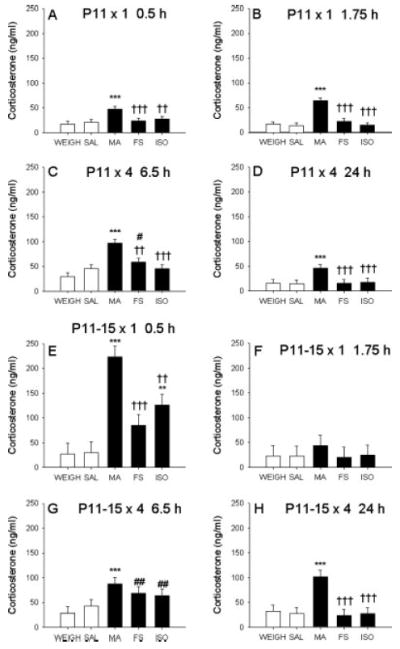
Corticosterone levels. CORT in plasma of animals treated with SAL, MA, ISO, FS, or WEIGH. (A) Increases in CORT occurred in MA-treated animals on P11 after one treatment at 0.5 h and at 1.75 h (B) on P11. All other treatments were comparable with basal levels after a single treatment. (C) At 6.5 h on P11, CORT levels were increased in MA- and FS-treated animals and at 24 h only in MA-treated animals (D). (E) When animals were treated four times daily from P11–14 and once on P15, MA-, FS-, and ISO-treated animals showed increased CORT at 0.5 h, with MA-treated animals having the highest levels. (F) At 1.75 h all groups were at baseline levels. (G) Levels of CORT were increased in MA-, FS-, and ISO-treated animals at 6.5 h on P15, but only the MA effect remained elevated at 24 h (H). *** = P < 0.001 compared with SAL and WEIGH, ** = P < 0.01 compared with SAL and WEIGH, ††† = P < 0.001 when compared with MA, †† = P < 0.01 when compared with MA, ## = P < 0.01 when compared with WEIGH, and # = P < 0.05 when compared with WEIGH.
Following four treatments on P11, animals given MA had increased CORT levels at 6.5 h after the first dose (30 min after last dose) compared with all other treatments (Fig. 2C), and the FS animals had elevated levels of CORT compared with the WEIGH group. At the 24-h time point (on P12), MA-treated animals had higher levels of CORT compared with all other treatments (Fig. 2D). No other differences were noted at 24 h.
Identical time points to those following P11 treatment were examined after multiple treatments from P11–14 with a single treatment on P15. At 0.5 h after the P15 dose, MA-treated animals had increased CORT compared with all other treatments. ISO animals had levels of CORT that were significantly increased compared with WEIGH and SAL controls, (Fig. 2E). The FS animals had intermediate levels that were different from MA-treated animals but not from WEIGH or SAL. At 1.75 h, no significant differences in CORT were observed among treatment groups (Fig. 2F).
After four treatments per day on P11–14 and four treatments on P15, at the 6.5-h time point, MA-treated animals had increased CORT compared with both WEIGH and SAL-treated animals but were not different from FS or ISO animals (Fig. 2G). CORT levels were significantly increased in the FS and ISO animals compared with WEIGH animals. At 24 h (P16), only MA-treated animals had increased levels of CORT compared with all other treatments (Fig. 2H), F(4, 66) = 6.50, P < 0.0001. No differences between males and females or between SAL and WEIGH animals were observed for CORT at any of the time points examined on either P11 or P15.
Neurotrophins
For BDNF in the hippocampus, an ANOVA revealed a significant main effect of treatment, F(4, 431) = 13.31, P < 0.0001, and age, F(1, 431) = 25.58, P < 0.0001, but no main effect of time or sex. There was also a treatment × age interaction, F(4, 431) = 8.69, P < 0.0001, but no other significant interactions with treatment. As shown in Figure 3, BDNF levels increased across ages. Examination of the interaction showed that on P11 none of the treatments altered BDNF in the hippocampus (Fig. 3 top), however there was a significant treatment main effect on P15, F(4, 235) = 18.40, P < 0.0001 (Fig. 3 bottom). Levels of BDNF in the hippocampus were increased in MA-treated animals on P15 compared with the other treatments, P < 0.01. Neither FS nor ISO significantly affected BDNF in the hippocampus. No other treatment- or sex-related differences were noted.
Fig. 3.
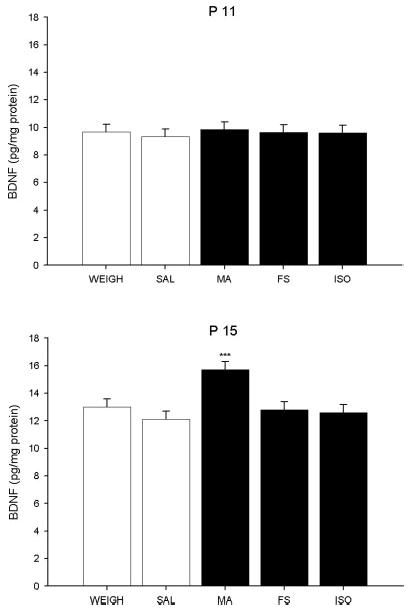
BDNF in the hippocampus. BDNF was measured in the hippocampus of animals given one of the five treatments (SAL, MA, FS, ISO, or WEIGH). No differences in BDNF were observed among treatments on P11 regardless of treatment or time point assessed (top). On P15, BDNF was increased in MA-administered animals, regardless of time assessed (bottom). *** = P < 0.001 compared with all other treatments.
For NGF in the hippocampus, there was a significant main effect of treatment, F(4, 400) = 2.66, P < 0.04, age, F(1, 400) = 4.66, P < 0.05, and sex, F(1, 400) = 11.56, P < 0.0007, observed. There were no other significant main effects or interactions. The treatment effect was the result of lower NGF in the FS group compared with the WEIGH controls (P < 0.03), (Fig. 4A); there was no effect of MA. As can be seen, although significant, the magnitude of the FS effect was small. Higher NGF levels were observed in females compared with males, (Fig. 4B) and on P15, animals had higher NGF compared with animals on P11 (Fig. 4C).
Fig. 4.
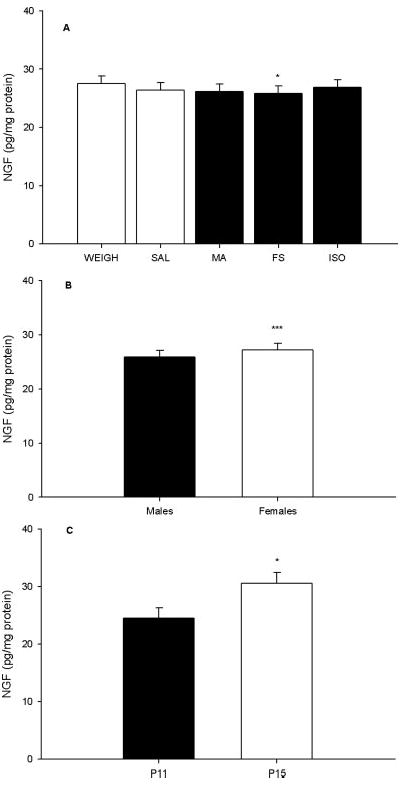
NGF in the hippocampus. (A) NGF levels. (B) Females had higher NGF levels than males averaged across treatment groups. (C) Animals examined on P15 displayed higher NGF levels than those on P11 averaged across treatment groups and sexes. WEIGH animals were used as a representative of all treatments at both time points (C). *** = P < 0.001, * = P < 0.05.
In the neostriatum, there were significant main effects on BDNF of treatment, F(4, 419) = 9.88, P < 0.0001, sex, F(1, 419) = 15.76, P < 0.0001, and a significant treatment × sex interaction (Fig. 5), F(4, 419) = 2.88, P < 0.02. Although BDNF was examined on P11 and P15, no effect of age was observed and there were no other significant main effects or interactions. ANOVAs at each level and Bonferroni step-down analyses demonstrated a significant difference between the MA group compared with all other treatments in males (Fig. 5 top); whereas the only significant difference in females was between MA and FS animals (Fig. 5 bottom). Regardless of treatment, females (13.66 ± 0.68 pg/mg protein) had greater levels of BDNF than males (12.56 ± 0.68 pg/mg protein).
Fig. 5.
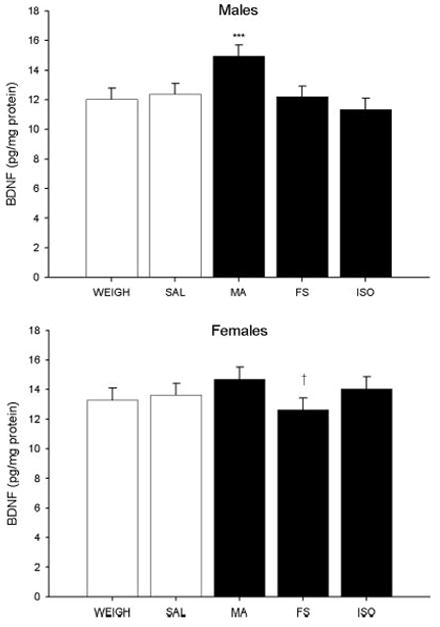
BDNF in the neostriatum. MA-treated males had increased BDNF compared with males in the other treatment groups (top). In females, levels were comparable across treatment groups; decreased BDNF was observed in FS females when compared with MA-treated females (bottom). Across treatment groups, females had higher BDNF levels than males. Data were averaged across ages (P11 and P15). *** = P < 0.001 compared to all other treatments, † = P < 0.05 compared to MA.
For NGF in the neostriatum, only a main effect of sex was observed, F(1, 305) = 14.96, P < 0.0001. Similar to BDNF in the neostriatum, females had higher levels of NGF than males (Fig. 6 bottom). There were no effects of treatment (Fig. 6 top) or any other main effects for neostriatal NGF.
Fig. 6.
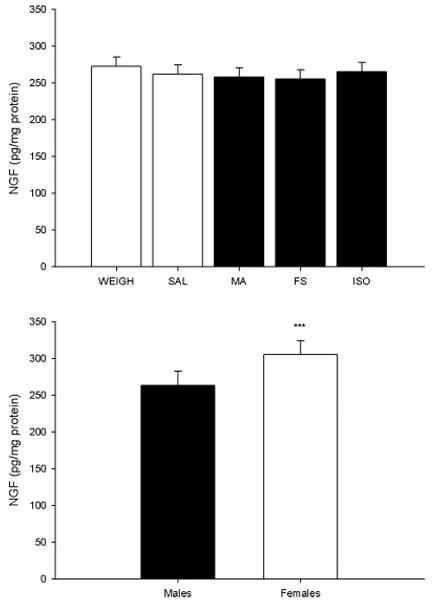
Neostriatal NGF. There were no differences in NGF observed across treatment groups in the neostriatum (top). Females displayed increased levels of NGF in the neostriatum compared with males averaged across treatment groups (bottom). *** = P < 0.001.
Adrenals and thymuses were collected and weighed. Absolute tissue weight and weight as a percentage of body weight are shown in Table I. ANOVAs on adrenal weights revealed significant main effects of treatment, F(4, 484) = 4.65, P < 0.001, and age, F(1,484) = 15.70, P < 0.0001, and the interaction of treatment × age, F(4, 484) = 3.97, P < 0.004. No other main effects were observed. Analysis of the treatment × age interaction demonstrated that the MA group had smaller adrenal weights on P15 compared with all other groups, F(4, 261) = 3.75, P < 0.006, but there was no effect on P11. ANOVAs on percentage of body weight for adrenals demonstrated a main effect of treatment, F(4, 484) = 2.61, P < 0.04, and age, F(1, 484) = 8.53, P < 0.004, as well as a significant treatment × age interaction, F(4, 484) = 2.58, P < 0.04. No other main effects or interactions with treatment were observed. The follow-up analysis of the treatment × age effect demonstrated a trend toward an effect on P15 that was similar to that seen with the unadjusted weights, i.e., reduced weight in the MA-treated group.
TABLE I.
Adrenal and thymus measurements
| Treatment | Adrenal wt. (mg) | % Body wt. | Thymus wt. (mg) | % Body wt. | |
|---|---|---|---|---|---|
| P 11 | WEIGH | 7.24 ± 0.32 | 0.030 ± 0.001 | 94.66 ± 3.80 | 0.390 ± 0.011 |
| SAL | 7.47 ± 0.32 | 0.030 ± 0.001 | 96.86 ± 3.79 | 0.388 ± 0.011 | |
| MA | 7.21 ± 0.32 | 0.030 ± 0.001 | 92.46 ± 3.79 | 0.376 ± 0.011 | |
| FS | 7.31 ± 0.32 | 0.030 ± 0.001 | 96.70 ± 3.79 | 0.391 ± 0.011 | |
| ISO | 7.44 ± 0.32 | 0.031 ± 0.001 | 91.90 ± 3.79 | 0.379 ± 0.011 | |
| P 15 | WEIGH | 10.84 ± 1.54 | 0.034 ± 0.004 | 135.40 ± 5.30 | 0.424 ± 0.013 |
| SAL | 15.24 ± 1.53 | 0.047 ± 0.004 | 128.30 ± 5.29 | 0.409 ± 0.013 | |
| MA | 8.89 ± 1.56** | 0.036 ± 0.004 | 82.61 ± 5.33**** | 0.329 ± 0.014**** | |
| FS | 11.59 ± 1.54 | 0.038 ± 0.004 | 120.3 ± 5.23††† | 0.398 ± 0.013 | |
| ISO | 11.40 ± 1.53 | 0.036 ± 0.004 | 132.3 ± 5.23 | 0.422 ± 0.013 |
Adrenal and thymus least square mean weights for weighed only (WEIGH), saline (SAL), (+)-methamphetamine (MA), forced swim (FS), and isolation (ISO) treatments for P11 and P15. For comparison, each tissue was normalized to body weight and expressed as the percentage of tissue per total body weight,
= P < 0.01,
= P < 0.0001 compared with all other treatments,
= P < 0.001 when compared with WEIGH and ISO.
For thymic weights, there were main effects of treatment, F(4, 488) = 49.23, P < 0.0001 and age, F(1, 488) = 19.01, P < 0.0001, as well as treatment × age, F(4, 488) = 42.26, P < 0.0001, and treatment × time, F(12, 488) = 1.83, P < 0.05 interactions. These were the only effects observed. The treatment × age interaction revealed that on P15, thymic weights in the MA group were less than thymic weights of all other treatments by pairwise comparison, P < 0.0001. In addition, the FS group had lower thymic weights than the WEIGH and ISO groups, P < 0.001. Follow-up of the treatment × time interaction showed a significant treatment effect at all times. The thymic weights for the MA-treated group were less than those of all other groups at all time points, P < 0.05, except MA-treated animals compared with SAL-treated animals at 1.75 h. Main effects of treatment, treatment × time, and treatment × age were also observed for thymic weight as a percentage of body weight, F(4, 488) = 17.70, P < 0.0001, F(12, 488) = 1.77, P < 0.05, and F(4, 488) = 11.62, P < 0.0001, respectively. MA-treated animals demonstrated a lower percentage of thymus to body weight on P15 compared with all other treatments, P < 0.0001. The treatment × time interaction was significant only at the 0.5- and 24-h time points, where MA-treated animals displayed lower percentages compared with all groups, P < 0.05, except MA compared with FS at the 0.5-h time point.
DISCUSSION
Previously, our lab has demonstrated that administration of MA to rats from P11–20 or from P11–15 produces spatial learning and memory deficits in the Morris water maze when the animals are tested in adulthood (Vorhees et al., 1994; Williams et al., 2002, 2003a,b). In the current study and previous studies performed in our lab we showed that MA can activate the HPA axis even during the SHRP when adrenal output is normally quiescent or blunted. The SHRP overlaps with a period of rapid hippocampal neurogenesis (Bayer et al., 1993), and the restricted CORT output (but not absence) from the adrenals is thought to be necessary to ensure neuronal survival (Sapolsky and Meaney, 1986). Although brief activation of the HPA axis can be vital and beneficial, prolonged activation, as evidenced by increased levels of glucocorticoids, can cause neuronal damage (Gould et al., 1991; McEwen et al., 1992). In this study, we were unable to match the increased adrenal output following MA when using physical or psychological stressors that are widely utilized to induce increases in CORT in adult animals. Although prolonged ISO in neonates has been shown to increase CORT (McCormick et al., 1998), few if any studies have actually examined the effect of repeated FS or ISO when applied within a day or over multiple days on the CORT response of the animals. It would have been useful had the stressors we used produced CORT increases comparable with those induced by MA without having to resort to long periods of maternal separation. Longer periods of maternal separation would make comparisons with the MA treatment difficult because of differences in maternal care and conversely lowering the MA doses to induce similar levels of CORT to the FS or ISO conditions could be problematic since lower doses of MA may have less reliable effects on spatial learning and memory (Williams et al., 2004b). Had the CORT responses in the FS and/or ISO groups been comparable with the MA-treated group we could have compared MA administration and the stressors for long-term effects in the cognitive ability of the animals. Unfortunately, we found that MA, when administered on P11, increases CORT levels more than either FS or ISO. More importantly, MA-induced increases in CORT continued for at least 18-h post-treatment (i.e., 24 h after the first treatment), thereby precluding use of these stressor methods for comparison with MA; in effect, the action of MA on CORT release proved to be too potent to match to other discrete stressors. Contrary to the protracted increases following one or four treatments of MA on P11, FS produced an increase in CORT only after four treatments (i.e., 30 min after the end of FS), with no effect at the other time points, and ISO failed to produce a significant effect at any of the time points examined on P11.
The HPA axis culminates in the release of CORT and this release is eventually halted through negative feedback at the level of the pituitary and hypothalamus and other feedback pathways through several brain regions including the hippocampus. Therefore, since CORT remains increased in MA-treated animals after 24 h, it suggests that: (1) there is still enough MA in the blood to stimulate adrenal production of CORT, (2) MA has altered the developmental state of the adrenal, and/or (3) MA exerts some effect that overrides or interferes with negative feedback mechanisms. The increases in CORT observed up to 24 h after MA administration are unlikely to be attributable to residual MA in the system since pharmacokinetic analysis of MA administered at 10 mg/kg, four times/day in the rat at these ages shows a t1/2 in plasma of 2.8 h (Cappon and Vorhees, 2001). When administered at 10 mg/kg, four times/day in the rat, <1% of the maximum concentration of MA of 6100 ng/ml is predicted to remain in the plasma after 24 h (Cappon and Vorhees, 2001). It is more likely that MA has a direct effect on the adrenal that stimulates precocious development and as a result increased release of CORT, similar to what is observed after 24 h of maternal ISO (Levine et al., 1991). Alternatively, MA could alter development of glucocorticoid (GR) and mineralocorticoid (MR) receptors, rendering negative feedback signals less effective, and while MA has been shown to alter MR and GR in adult animals (Lowy and Novotney, 1994), no such data exist in developing animals.
We have demonstrated previously that exposure to MA alters the subsequent response of the adrenal gland after 4 days of drug exposure (i.e., P11-14) as demonstrated by the fact that CORT increases were exaggerated on the fifth day of exposure (i.e., P15) compared with if the drug was delivered only on P15 (Williams et al., 2006). In the current study, we replicate that finding at the 0.5-h time point following administration from P11–14 and a single dose on P15 and extend it to show that when MA is administered four times daily from P11–15, CORT levels are elevated 24 h after the first dose on P15 (i.e. to P16). This elevation in CORT 24 h later is as large as the increase seen at the 6.5-h time point on P15. While we showed that MA produces extended increases in CORT on P11 previously and in the present experiment, this is the first experiment to demonstrate that increased CORT occurs as long as 24 h after the first dose on P15. The mechanism for this effect is unknown, but may involve increased sensitivity of the adrenal gland to ACTH. Comparison of the initial CORT responses on P11 and P15 show that maturation of the HPA axis occurred since there is a significant adrenal response to FS, ISO, and to MA on P15. As would be expected if negative feedback mechanisms are intact, levels of CORT returned to baseline levels at the 1.75-h time point following the first dose, as we have demonstrated previously (Williams et al., 2000). Regardless of the fact that FS and ISO produced some increase in CORT on P15, the data indicate that MA produces larger CORT increases in neonates than these other stressors during late phases of the SHRP.
The increased levels of CORT in MA-treated animals raise the possibility that the hippocampus may have been adversely affected since high levels of glucocorticoids are known to be neurotoxic (Sapolsky, 1996). Consistent with this hypothesis, we have shown that there is a reduction in spine numbers in the dentate gyrus in animals exposed to MA during the neonatal period (Williams et al., 2004a), suggesting that there are either fewer numbers of neurons for connections to be made, or that synaptogenesis was affected. The temporal increases in CORT during drug administration, the location of MR and GR in the hippocampus, and the previous data showing that CORT can damage neurons during development all suggest the possibility that changes in hippocampal development might be attributed to protracted increases in CORT. This hypothesis does not rule out contributions from other factors as well.
Numerous signals are important in the development of the hippocampus and these include but are not limited to the neurotrophins. Neurotrophins are necessary for neuronal survival and maintenance and as stated previously have been implicated as important factors in learning and memory (Chen et al., 1997; Kesslak et al., 1998; Linnarsson et al., 1997; Mizuno et al., 2000). Two members of the family of neurotrophins are BDNF and NGF. Levels of both proteins peak at different times during development. For instance, it was reported that in the hippocampus, NGF levels peak around P7, while BDNF levels peak at P14 (Das et al., 2001). Consistent with these previous data, we also showed that BDNF levels increased between P11 and P15 in the hippocampus whereas NGF levels in the neostriatum were consistent across ages. However, we also demonstrated that BDNF levels were unchanged in the neostriatum and NGF levels were increased over the time periods examined in the hippocampus. Therefore, our data demonstrate that NGF may not reach peak levels until a time later than P7.
Neurotrophins can also be affected by stressors or CORT administration. For example, rats administered CORT from P1–12 show increases in NGF and the neurotrophin receptors trkA, trkB, and trkC in the hippocampus (Roskoden et al., 2004). Consistent with this, we observed higher levels of NGF on P15 in the hippocampus than on P11. In contrast, we showed a small decrease in NGF levels in the hippocampus of FS-treated animals. These differences are likely the result of the timing of treatment administration. In adult animals, increases in CORT either by CORT injection or by immobilization are associated with reductions in BDNF mRNA in the hippocampus (Schaaf et al., 1997; Smith et al., 1995). Furthermore, in adult rats, repeated stress not only reduces BDNF mRNA, but also increases trkB mRNA, the primary receptor for BDNF (Nibuya et al., 1999). Because of the association that has been demonstrated between the neurotrophins and stress or CORT, as well as our previous findings (Skelton et al., 2007), we examined NGF and BDNF levels during development to determine if alterations in these neurotrophins occurred. The results demonstrate that only MA was able to induce an increase in BDNF in the hippocampus and neostriatum, but this effect only occurred on P15, and only after 4 days of a dosing regimen that began on P11. Neither ISO nor FS altered BDNF at any time point examined, showing that MA was unique in this respect. The effect of MA on BDNF appears to either require multiple days of exposure or it is protected from fluctuations by some mechanism on P11 since no changes in BDNF were observed in MA-treated animals on P11. There is a study suggesting that changes in BDNF levels are difficult to induce prior to P14 even with a known neurotoxin (Danzer et al., 2004). Although reductions in BDNF mRNA after stress or CORT injection occurs in adult animals (Schaaf et al., 1997; Smith et al., 1995), we saw increased BDNF protein in the MA-treated animals. This finding suggests that: (1) CORT may not interact with BDNF during this early period of development; (2) that very large increases in CORT are required to produce a change; or (3) that CORT actually induces an opposite effect compared with that seen in adult animals. Although the FS and ISO procedures did produce significant increases in CORT on P15, these increases were smaller than that observed for MA treatment. The increased BDNF in MA-treated animals could also arise as a response to neuronal damage. This would require cell counting and silver degeneration staining to determine and these were not performed in the present experiment.
Sexually dimorphic effects were observed in NGF levels in both the hippocampus and neostriatum as well as BDNF in the neostriatum. Females had higher levels than males in all three cases and levels of BDNF in the neostriatum were increased in MA-administered males when compared with other males. This sexually dimorphic MA effect has not been reported previously, but raises interest as to whether sex itself affects neurotrophin levels in developing animals and what role sexually dimorphic effects of BDNF may play in the developing brain. There were no sexually dimorphic effects observed in CORT in this study, which is consistent with previous studies in neonates of this age in our lab following MA administration (Williams et al., 2000, 2006) or on P11 following MDMA administration (Williams et al., 2005).
We have previously demonstrated that rats administered MA from P11–20 had a trend toward an increase in BDNF 60 min after Morris water maze testing compared with controls (Skelton et al., 2007). This study lead us to suggest that, at least in the short term, increased BDNF levels may be important for neuronal protection and survival during MA administration. It is unclear whether increases in BDNF during the SHRP contribute to the learning deficits seen later in life. It may be that protracted increases in CORT are involved in the previously observed learning deficits in MA neonates and BDNF may be involved in mediating some part of this effect. In future studies, we will determine how changes in CORT and neurotrophins are related to the learning and memory deficits observed later in life in MA-treated animals.
In conclusion, the data demonstrate that neonatal MA administration alters the profile of CORT release during the later phases of the SHRP as well as BDNF after multiple-day exposure. Further investigation is required to determine the importance of the CORT and BDNF changes in producing the long-term learning and memory deficits caused by early MA treatment.
Acknowledgments
Contract grant sponsor: NIH; Contract grant numbers: DA006733, DA014269; Contract grant sponsor: Training Grant; Contract grant number: ES07051.
Footnotes
Published online in Wiley InterScience (www.interscience.wiley.com).
References
- Baumann MH, Wang X, Rothman RB. 3,4-Methylenedioxyme-thamphetamine (MDMA) neurotoxicity in rats: A reappraisal of past and present findings. Psychopharmacology (Berl) 2007;189:407–424. doi: 10.1007/s00213-006-0322-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer SA, Altman J, Russo RJ, Zhang X. Timetables of neurogenesis in the human brain based on experimentally determined patterns in the rat. Neurotoxicology. 1993;14:83–144. [PubMed] [Google Scholar]
- Cappon GD, Vorhees CV. Plasma and brain methamphetamine concentrations in neonatal rats. Neurotoxicol Teratol. 2001;23:81–88. doi: 10.1016/s0892-0362(00)00118-5. [DOI] [PubMed] [Google Scholar]
- Chang L, Smith LM, LoPresti C, Yonekura ML, Kuo J, Walot I, Ernst T. Smaller subcortical volumes and cognitive deficits in children with prenatal methamphetamine exposure. Psychiatry Res. 2004;132:95–106. doi: 10.1016/j.pscychresns.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Chang L, Cloak C, Patterson K, Grob C, Miller EN, Ernst T. Enlarged striatum in abstinent methamphetamine abusers: A possible compensatory response. Biol Psychiatry. 2005;57:967–974. doi: 10.1016/j.biopsych.2005.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen KS, Nishimura MC, Armanini MP, Crowley C, Spencer SD, Phillips HS. Disruption of a single allele of the nerve growth factor gene results in atrophy of basal forebrain cholinergic neurons and memory deficits. J Neurosci. 1997;17:7288–7296. doi: 10.1523/JNEUROSCI.17-19-07288.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomchai C, Na MN, Watanarungsan P, Yossuck P, Chomchai S. Methamphetamine abuse during pregnancy and its health impact on neonates born at Siriraj Hospital, Bangkok, Thailand. Southeast Asian. J Trop Med Public Health. 2004;35:228–231. [PubMed] [Google Scholar]
- Clancy B, Finlay BL, Darlington RB, Anand KJ. Extrapolating brain development from experimental species to humans. Neurotoxicology. 2007 doi: 10.1016/j.neuro.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danzer SC, He X, McNamara JO. Ontogeny of seizure-induced increases in BDNF immunoreactivity and TrkB receptor activation in rat hippocampus. Hippocampus. 2004;14:345–355. doi: 10.1002/hipo.10190. [DOI] [PubMed] [Google Scholar]
- Das KP, Chao SL, White LD, Haines WT, Harry GJ, Tilson HA, Barone S., Jr Differential patterns of nerve growth factor, brain-derived neurotrophic factor and neurotrophin-3 mRNA and protein levels in developing regions of rat brain. Neuroscience. 2001;103:739–761. doi: 10.1016/s0306-4522(01)00011-2. [DOI] [PubMed] [Google Scholar]
- de la Torre R, Farre M. Neurotoxicity of MDMA (ecstasy): The limitations of scaling from animals to humans. Trends Pharmacol Sci. 2004;25:505–508. doi: 10.1016/j.tips.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Dent GW, Smith MA, Levine S. Rapid induction of corticotropin-releasing hormone gene transcription in the paraventricular nucleus of the developing rat. Endocrinology. 2000;141:1593–1598. doi: 10.1210/endo.141.5.7455. [DOI] [PubMed] [Google Scholar]
- Dixon SD. Effects of transplacental exposure to cocaine and methamphetamine on the neonate. West J Med. 1989;150:436–442. [PMC free article] [PubMed] [Google Scholar]
- Dixon SD, Bejar R. Echoencephalographic findings in neonates associated with maternal cocaine and methamphetamine use: Incidence and clinical correlates. J Pediatr. 1989;115:770–778. doi: 10.1016/s0022-3476(89)80661-4. [DOI] [PubMed] [Google Scholar]
- Gomez-Pinilla F, So V, Kesslak JP. Spatial learning induces neurotrophin receptor and synapsin I in the hippocampus. Brain Res. 2001;904:13–19. doi: 10.1016/s0006-8993(01)02394-0. [DOI] [PubMed] [Google Scholar]
- Gould E, Woolley CS, McEwen BS. Adrenal steroids regulate postnatal development of the rat dentate gyrus: I. Effects of glucocorticoids on cell death. J Comp Neurol. 1991;313:479–485. doi: 10.1002/cne.903130308. [DOI] [PubMed] [Google Scholar]
- Gouzoulis-Mayfrank E, Thelen B, Habermeyer E, Kunert HJ, Kovar KA, Lindenblatt H, Hermle L, Spitzer M, Sass H. Psychopathological, neuroendocrine and autonomic effects of 3, 4-methylenedioxyethylamphetamine (MDE), psilocybin and d-methamphetamine in healthy volunteers. Results of an experimental double-blind placebo-controlled study. Psychopharmacology (Berl) 1999;142:41–50. doi: 10.1007/s002130050860. [DOI] [PubMed] [Google Scholar]
- Holson RR. Euthanasia by decapitation: Evidence that this technique produces prompt, painless unconsciousness in laboratory rodents. Neurotoxicol Teratol. 1992;14:253–257. doi: 10.1016/0892-0362(92)90004-t. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future national survey results on drug use, 1975–2005. Volume II: College students and adults ages 19–45 (NIH Publication No 06-5884) Bethesda, MD: National Institute on Drug Abuse; 2006. [Google Scholar]
- Kesslak JP, So V, Choi J, Cotman CW, Gomez-Pinilla F. Learning upregulates brain-derived neurotrophic factor messenger ribonucleic acid: A mechanism to facilitate encoding and circuit maintenance? Behav Neurosci. 1998;112:1012–1019. doi: 10.1037//0735-7044.112.4.1012. [DOI] [PubMed] [Google Scholar]
- Levine S, Huchton DM, Wiener SG, Rosenfeld P. Time course of the effect of maternal deprivation on the hypothalamic-pituitary-adrenal axis in the infant rat. Dev Psychobiol. 1991;24:547–558. doi: 10.1002/dev.420240803. [DOI] [PubMed] [Google Scholar]
- Lin JH. Applications and limitations of interspecies scaling and in vitro extrapolation in pharmacokinetics. Drug Metab Dispos. 1998;26:1202–1212. [PubMed] [Google Scholar]
- Linnarsson S, Bjorklund A, Ernfors P. Learning deficit in BDNF mutant mice. Eur J Neurosci. 1997;9:2581–2587. doi: 10.1111/j.1460-9568.1997.tb01687.x. [DOI] [PubMed] [Google Scholar]
- Little BB, Snell LM, Gilstrap LC., III Methamphetamine abuse during pregnancy: Outcome and fetal effects. Obstet Gynecol. 1988;72:541–544. [PubMed] [Google Scholar]
- Lowy MT, Novotney S. Methamphetamine-induced decrease in neural glucocorticoid receptors: Relationship to monoamine levels. Brain Res. 1994;638:175–181. doi: 10.1016/0006-8993(94)90647-5. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Kehoe P, Kovacs S. Corticosterone release in response to repeated, short episodes of neonatal isolation: Evidence of sensitization. Int J Dev Neurosci. 1998;16:175–185. doi: 10.1016/s0736-5748(98)00026-4. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Angulo J, Cameron H, Chao HM, Daniels D, Gannon MN, Gould E, Mendelson S, Sakai R, Spencer R. Paradoxical effects of adrenal steroids on the brain: protection versus degeneration. Biol Psychiatry. 1992;31:177–199. doi: 10.1016/0006-3223(92)90204-d. [DOI] [PubMed] [Google Scholar]
- Mizuno M, Yamada K, Olariu A, Nawa H, Nabeshima T. Involvement of brain-derived neurotrophic factor in spatial memory formation and maintenance in a radial arm maze test in rats. J Neurosci. 2000;20:7116–7121. doi: 10.1523/JNEUROSCI.20-18-07116.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mordenti J, Chapell W. The use of interspecies scaling in toxicokinetics. In: Yacobi A, Kelly J, Batra V, editors. Toxicokinetics in New Drug Development. New York: Pergamon Press; 1989. pp. 42–96. [Google Scholar]
- Nibuya M, Takahashi M, Russell DS, Duman RS. Repeated stress increases catalytic TrkB mRNA in rat hippocampus. Neurosci Lett. 1999;267:81–84. doi: 10.1016/s0304-3940(99)00335-3. [DOI] [PubMed] [Google Scholar]
- Roskoden T, Otten U, Schwegler H. Early postnatal corticosterone administration regulates neurotrophins and their receptors in septum and hippocampus of the rat. Exp Brain Res. 2004;154:183–191. doi: 10.1007/s00221-003-1656-5. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Stress, glucocorticoids, and damage to the nervous system: The current state of confusion. Stress. 1996;1:1–19. doi: 10.3109/10253899609001092. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Meaney MJ. Maturation of the adrenocortical stress response: Neuroendocrine control mechanisms and the stress hyporesponsive period. Brain Res. 1986;396:64–76. doi: 10.1016/s0006-8993(86)80190-1. [DOI] [PubMed] [Google Scholar]
- Schaaf MJ, Hoetelmans RW, de Kloet ER, Vreugdenhil E. Corticosterone regulates expression of BDNF and trkB but not NT-3 and trkC mRNA in the rat hippocampus. J Neurosci Res. 1997;48:334–341. [PubMed] [Google Scholar]
- Schaefer TL, Ehrman LA, Gudelsky GA, Vorhees CV, Williams MT. A comparison of monoamine and corticosterone levels 24 hours following (+)methamphetamine, 3,4-methylenedioxyme-thamphetamine, cocaine, (+)fenfluramine, or methylphenidate administration in the neonatal rat. J Neurochem. 2006;98:1369–1378. doi: 10.1111/j.1471-4159.2006.04034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selye H. A syndrome produced by diverse nocuous agents. Nature. 1936;138:32. doi: 10.1176/jnp.10.2.230a. [DOI] [PubMed] [Google Scholar]
- Simon SL, Domier C, Carnell J, Brethen P, Rawson R, Ling W. Cognitive impairment in individuals currently using methamphetamine. Am J Addict. 2000;9:222–231. doi: 10.1080/10550490050148053. [DOI] [PubMed] [Google Scholar]
- Skelton MR, Williams MT, Schaefer TL, Vorhees CV. Neonatal (+)-methamphetamine increases brain-derived neurotrophic factor, but not nerve growth factor, during treatment and results in long-term spatial learning deficits. Psychoneuroendocrinology. 2007;32:734–745. doi: 10.1016/j.psyneuen.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith L, Yonekura ML, Wallace T, Berman N, Kuo J, Berkowitz C. Effects of prenatal methamphetamine exposure on fetal growth and drug withdrawal symptoms in infants born at term. J Dev Behav Pediatr. 2003;24:17–23. doi: 10.1097/00004703-200302000-00006. [DOI] [PubMed] [Google Scholar]
- Smith LM, Chang L, Yonekura ML, Grob C, Osborn D, Ernst T. Brain proton magnetic resonance spectroscopy in children exposed to methamphetamine in utero. Neurology. 2001;57:255–260. doi: 10.1212/wnl.57.2.255. [DOI] [PubMed] [Google Scholar]
- Smith LM, LaGasse LL, Derauf C, Grant P, Shah R, Arria A, Huestis M, Haning W, Strauss A, Della GS, Liu J, Lester BM. The infant development, environment, and lifestyle study: Effects of prenatal methamphetamine exposure, polydrug exposure, and poverty on intrauterine growth. Pediatrics. 2006;118:1149–1156. doi: 10.1542/peds.2005-2564. [DOI] [PubMed] [Google Scholar]
- Smith MA, Makino S, Kvetnansky R, Post RM. Stress and glucocorticoids affect the expression of brain-derived neurotrophic factor and neurotrophin-3 mRNAs in the hippocampus. J Neurosci. 1995;15:1768–1777. doi: 10.1523/JNEUROSCI.15-03-01768.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struthers JM, Hansen RL. Visual recognition memory in drug-exposed infants. J Dev Behav Pediatr. 1992;13:108–111. doi: 10.1097/00004703-199204000-00005. [DOI] [PubMed] [Google Scholar]
- Vazquez DM. Stress and the developing limbic-hypothalamic-pituitary-adrenal axis. Psychoneuroendocrinology. 1998;23:663–700. doi: 10.1016/s0306-4530(98)00029-8. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Leonido-Yee M, Franceschi D, Sedler MJ, Gatley SJ, Hitzemann R, Ding YS, Logan J, Wong C, Miller EN. Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. Am J Psychiatry. 2001;158:377–382. doi: 10.1176/appi.ajp.158.3.377. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, Ahrens KG, cuff-Smith KD, Schilling MA, Fisher JE. Methamphetamine exposure during early postnatal development in rats: I. Acoustic startle augmentation and spatial learning deficits. Psychopharmacology (Berl) 1994;114:392–401. doi: 10.1007/BF02249328. [DOI] [PubMed] [Google Scholar]
- Wadhwa PD. Psychoneuroendocrine processes in human pregnancy influence fetal development and health. Psychoneuroendocrinology. 2005;30:724–743. doi: 10.1016/j.psyneuen.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Williams MT, Inman-Wood SL, Morford LL, McCrea AE, Ruttle AM, Moran MS, Rock SL, Vorhees CV. Preweaning treatment with methamphetamine induces increases in both corticosterone and ACTH in rats. Neurotoxicol Teratol. 2000;22:751–759. doi: 10.1016/s0892-0362(00)00091-x. [DOI] [PubMed] [Google Scholar]
- Williams MT, Vorhees CV, Boon F, Saber AJ, Cain DP. Methamphetamine exposure from postnatal day 11 to 20 causes impairments in both behavioral strategies and spatial learning in adult rats. Brain Res. 2002;958:312–321. doi: 10.1016/s0006-8993(02)03620-x. [DOI] [PubMed] [Google Scholar]
- Williams MT, Moran MS, Vorhees CV. Refining the critical period for methamphetamine-induced spatial deficits in the Morris water maze. Psychopharmacology (Berl) 2003a;168:329–338. doi: 10.1007/s00213-003-1433-y. [DOI] [PubMed] [Google Scholar]
- Williams MT, Morford LL, Wood SL, Wallace TL, Fukumura M, Broening HW, Vorhees CV. Developmental d-methamphet-amine treatment selectively induces spatial navigation impairments in reference memory in the Morris water maze while sparing working memory. Synapse. 2003b;48:138–148. doi: 10.1002/syn.10159. [DOI] [PubMed] [Google Scholar]
- Williams MT, Brown RW, Vorhees CV. Neonatal metham-phetamine administration induces region-specific long-term neuronal morphological changes in the rat hippocampus, nucleus accumbens and parietal cortex. Eur J Neurosci. 2004a;19:3165–3170. doi: 10.1111/j.0953-816X.2004.03405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MT, Moran MS, Vorhees CV. Behavioral and growth effects induced by low dose methamphetamine administration during the neonatal period in rats. Int J Dev Neurosci. 2004b;22:273–283. doi: 10.1016/j.ijdevneu.2004.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MT, Schaefer TL, Ehrman LA, Able JA, Gudelsky GA, Sah R, Vorhees CV. 3,4-Methylenedioxymethamphetamine administration on postnatal day 11 in rats increases pituitary-adrenal output and reduces striatal and hippocampal serotonin without altering SERT activity. Brain Res. 2005;1039:97–107. doi: 10.1016/j.brainres.2005.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MT, Schaefer TL, Furay AR, Ehrman LA, Vorhees CV. Ontogeny of the adrenal response to (+)-methamphetamine in neonatal rats: The effect of prior drug exposure. Stress. 2006;9:153–163. doi: 10.1080/10253890600902842. [DOI] [PMC free article] [PubMed] [Google Scholar]


