Abstract
The goal of this project is to develop 3-D biomaterial scaffolds that present cues to direct differentiation of embryonic stem cell derived neural progenitor cells (ESNPCs) seeded inside into mature neural phenotypes, specifically neurons and oligodendrocytes. Release studies were performed to determine the appropriate conditions for retention of neurotrophin-3 (NT-3), sonic hedgehog (Shh), and platelet derived growth factor (PDGF) by an affinity-based delivery system (ABDS) incorporated into fibrin scaffolds. Embryoid bodies (EBs) containing neural progenitors were formed from mouse ES cells, using a 4−/4+ retinoic acid treatment protocol, and then seeded inside of fibrin scaffolds containing the drug delivery system. This delivery system was used to deliver various growth factor doses and combinations to the cells seeded inside of the scaffolds. Controlled delivery of NT-3 and PDGF simultaneously increased the fraction of neural progenitors, neurons, and oligodendrocytes while decreasing the fraction of astrocytes obtained compared to control cultures seeded inside of unmodified fibrin scaffolds with no growth factors present in the media. These results demonstrate that such a strategy can be used to generate an engineered tissue for the potential treatment of spinal cord injury and could be extended to study of differentiation in other tissues.
Keywords: murine embryonic stem cell, 3D culture, hydrogel, neural tissue engineering
Introduction
Combining bioactive scaffolds with embryonic stem (ES) cells provides a desirable alternative for replacement of damaged tissues (Ferreira et al., 2007; Levenberg et al., 2005; Levenberg et al., 2003; Willerth et al., 2006; Willerth et al., 2007b). For such engineered tissues to function and integrate effectively in vivo, the cues necessary for promoting optimal ES cell differentiation should be retained inside such scaffolds, allowing them to be accessible to the cells and prevent loss due to diffusion into the surrounding tissue. One of the most commonly used methods for incorporating growth factors or other cues involves covalently tethering them to the scaffold material (Beckstead et al., 2006; Fan et al., 2007; Gomez et al., 2007; Gomez and Schmidt, 2007; Ho et al., 2007; Kuhl and Griffith-Cima, 1996; Taqvi et al., 2006; Yim and Leong, 2005). These methods have been previously used to promote ES cell differentiation and survival (Beckstead et al., 2006; Ho et al., 2007; Taqvi et al., 2006; Yim and Leong, 2005). While such strategies are highly effective at retaining such cues inside scaffolds, covalent linkages can impede internalization and in turn, affect the activity and function of growth factors. Affinity-based delivery systems have been explored as alternative method of retaining growth factors inside of scaffolds via non-covalent interactions (Benoit and Anseth, 2005; Benoit et al., 2007; Edelman et al., 1991; Sakiyama-Elbert and Hubbell, 2000a, b; Taylor et al., 2004; Taylor et al., 2006; Taylor and Sakiyama-Elbert, 2006; Wissink et al., 2001). These systems utilize the interaction between heparin and a target drug to retain the drug inside of a scaffold, mimicking the way the extracellular matrix sequesters growth factors in vivo. These systems improve retention of growth factor activity and allow growth factor internalization. Such biomimetic delivery systems have shown promise as a potential treatment for spinal cord injury (SCI), but these scaffolds alone are not sufficient to produce functional recovery (Taylor et al., 2004; Taylor et al., 2006; Taylor and Sakiyama-Elbert, 2006).
The use of progenitor cells derived from ES cells as means of restoring lost cellular populations due to SCI has also been investigated with some promising results (Keirstead et al., 2005; Liu et al., 2000; McDonald et al., 1999; Nistor et al., 2005). In a study by McDonald et al., mouse ES cells were induced to form embryonic stem cell derived neural lineage precursor cells (ESNPCs) using the 4−/4+ retinoic acid treatment protocol (Bain et al., 1995) and then injected into a rat model of SCI as treatment (McDonald et al., 1999). These cells promoted a modest increase in functional recovery (~3 on the Basso, Beattie and Bresnahan scale). However, few cells (~5%) differentiated into neurons and the viability of the transplanted cells was low (~10%). Similar results have been obtained by other groups when stem cells were directly transplanted in the injured spinal cord (Cao et al., 2002; Cao et al., 2001). The glial scar promotes differentiation of stem cells into glia and discourages cell migration into the injury site. Implantable biomimetic scaffolds could provide a permissive microenvironment for transplanted stem cells and cues to promote survival and differentiation into neurons and oligodendrocytes at the injury site.
Toward this goal, previous work from our lab has identified the appropriate conditions for culturing mouse ESNPCs inside of 3-dimensional fibrin scaffolds and soluble growth factors that promote the differentiation of ESNPCs into neurons and oligodendrocytes (Willerth et al., 2006; Willerth et al., 2007b). The later study found that combining neurotrophin-3 (NT-3) with platelet derived growth factor (PDGF) and NT-3 with sonic hedgehog (Shh) increased the fraction of ESNPCs that differentiated into neurons and oligdodendrocytes compared to control cultures in unmodified fibrin with no growth factors present in the media, making them worthy of further investigation for use with our affinity-based delivery system. Additionally, these growth factors have shown beneficial effects when tested in the context of SCI. Delivery of NT-3 has been shown to enhance neural fiber sprouting into the injury site (Taylor, McDonald et al. 2004; Taylor, Rosenzweig et al. 2006) and promote regeneration of the corticospinal tract (Schnell et al., 1994; Taylor et al., 2004; Taylor et al., 2006; Tuszynski et al., 2003). Treatment with all three PDGF isoforms (PDGF-AA, PDGF-AB, and PDGF-BB) promotes angiogenesis to the injury site, which may help produce a more hospitable environment for regeneration to occur (Hiraizumi et al., 1996; Hiraizumi et al., 1992; Hiraizumi et al., 1993). Shh induces proliferation of neural progenitors when applied after SCI and when used in combination with transplanted oligodendrocyte progenitor cells, it has been shown to improve recovery and increase the amount of white matter spared from injury (Bambakidis and Miller, 2004; Bambakidis et al., 2003). Thus, these growth factors serve two purposes in vivo. They can promote the differentiation of ESNPCs into neurons and oligodendrocytes while helping to improve the microenvironment at the injury site, allowing for regeneration by the spared host cells.
The goal of this work was to generate an optimized scaffold for repair of SCI that contained an affinity-based delivery system, growth factors, and ESNPCs. To achieve this goal, delivery conditions for retention of NT-3, PDGF-AA, and Shh were determined and then the ability of these factors to direct the differentiation of ESNPCs seeded inside of scaffolds when presented by the delivery system was determined. Once optimal doses for each growth factor were found, these doses were tested in combination to determine their effectiveness at promoting differentiation of the ESNPCs into neurons and oligodendrocytes to serve as a replacement for the tissue lost to SCI. Specifically, the goal was to determine the doses of growth factor that needed to be presented by the delivery system to replicate the differentiation observed in the previous soluble growth factor studies (Willerth et al., 2007b) that would allow presentation of similar cues following cell transplantation. Additionally, control experiments were performed to confirm that the effects observed were due to the enhanced retention of growth factors by the delivery system. These scaffolds could also be used to replicate the microenvironment present during the different stages of development and may have further applications for studying the cues necessary to direct stem cell differentiation for other lineages.
Materials and Methods
All materials were purchased from Sigma-Aldrich unless otherwise noted.
Preparation of Fibrin Scaffolds Containing the Biomimetic Delivery System
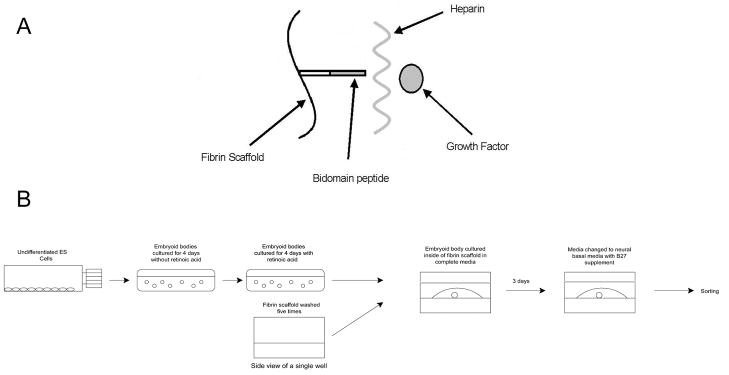
Plasminogen free fibrinogen (Calbiochem) obtained from human plasma was dissolved in tris-buffered saline (TBS, pH 7.4) for 2 hours at 37°C. Sterile fibrinogen solutions were prepared as previously described (Willerth et al., 2007c). Fibrin scaffolds (10 mg/mL) were polymerized under conditions previously determined be optimal for ES cell culture (Willerth et al., 2006). The affinity-based delivery system (Figure 1) consists of three components: a bi-domain peptide, heparin, and growth factor. The peptide contains a Factor XIIIa substrate derived from α2-plasmin inhibitor (Ichinose et al., 1983), allowing it to be covalently cross linked into the fibrin scaffold, and a heparin binding domain derived from antithrombin III (Tyler-Cross et al., 1994), which binds heparin non-covalently and retains it inside of the fibrin scaffold. The heparin can in turn bind growth factors with an affinity for heparin and retain them inside the scaffold. Thus these two reversible reactions (peptide binding to heparin and heparin binding to growth factor) allow the delivery system to retain growth factors non-covalently inside of the fibrin scaffolds. At equilibrium, five different species are present inside of the scaffold, including unbound heparin, unbound growth factor, heparin-growth factor complexes, heparin bound to the immobilized peptide, and heparin-growth factor complexes that are bound to the peptide.
Figure 1.
Diagrams showing the affinity based delivery system and cell culture process. A) Schematic of the affinity-based delivery system. The bidomain peptide becomes covalently cross linked into the fibrin scaffold during polymerization. This peptide contains heparin binding domain, allowing to non-covalently retain heparin inside of the scaffold. The heparin in turn non-covalently binds the growth factor, causing it to remain in the scaffold. B) Schematic of the process for producing embryoid bodies and followed by seeding and culture inside of fibrin scaffolds.
The goal of using this delivery system is maximize the fraction of heparin-growth factor complexes that are bound to the peptide inside of the fibrin scaffold. For promoting differentiation of ESNPCs, it is important to maximize the amount of growth factor retained inside the scaffold to ensure retention in an in vivo setting. Changing the concentration of heparin present affects the amount of growth factor retained inside of the scaffold by shifting the chemical equilibrium of the system. In the reversible reaction when heparin and growth factor bind to form a complex, adding excess unbound heparin should cause increased formation of heparin-growth factors complexes at equilibrium according to LeChatlier’s principle. These complexes can in turn bind non-covalently to the immobilized peptide, becoming immobilized inside of the scaffold. Different heparin concentrations will result in a different amounts of heparin-growth factor complexes being formed, affecting the amount of heparin-growth factor complexes that can bind to the immobilized peptide at equilibrium. In this manner, the amount of heparin present affects the rate at which growth factor is released from the scaffolds. Previous work from our lab has demonstrated that 4:1 and 40:1 peptide to heparin ratios produce sustained growth factor release from fibrin scaffolds (Taylor et al., 2004; Wood and Sakiyama-Elbert, 2008).
To produce the components needed for the delivery system, the bidomain peptide (amino acid sequence: (AcG)NQEQVSPK(βA)FAKLAARLYRKA, tranglutaminase substrate indicated by italics) was synthesized using standard Fmoc chemistry (amino acids from EMD Biosciences and peptide synthesis solvents from Applied Biosystems) and purified using reverse phase C18 chromatography with the peptide sequence being confirmed using matrix-assisted laser desorption ionization (MALDI) mass spectroscopy as previously described (Willerth et al., 2007c; Wood and Sakiyama-Elbert, 2008). Sterile porcine heparin solutions (average molecular weight: 18 kDa; concentrations: 6.25 μM and 62.5 μM) were prepared and the following growth factors were delivered using this system: NT-3 (Peprotech), PDGF-AA (R&D Systems), and Shh (R&D Systems). These components were added at the indicated concentrations during the fibrin polymerization process to ensure incorporation into the scaffolds.
Characterization of Controlled Growth Factor Release from Fibrin Scaffolds
Fibrin scaffolds (400 μL) were polymerized to contain 0.25 mM bidomain peptide, 100 ng/mL of growth factor (40 ng total) and varying concentrations of heparin. Control scaffolds contained no heparin while the experimental scaffolds contained either 6.25 μM or 62.5 μM heparin, corresponding to a 40:1 and 4:1 peptide to heparin ratios. Two different peptide to heparin ratios were tested to determine which ratio retains the maximum amount of growth factor inside of the scaffold. The peptide to heparin ratios and the heparin to growth factor ratios tested are listed in Table One.
Table 1.
Ratios of the affinity-based delivery system components
| Growth Factor | Peptide to Heparin Ratio | Heparin to Growth Factor Ratio | Estimated Fraction of Growth Factor Bound* | Fraction of Growth Factor Retained in Scaffold after 24 hours** | Fraction of Growth Factor Retained in Scaffold after 14 days** |
|---|---|---|---|---|---|
| Neurotrophin-3 | 4:1 40:1 |
1700:1 170:1 |
0.89 0.74 |
0.61 0.44 |
0.39 0.25 |
| Platelet derived growth factor | 4:1 40:1 |
1600:1 160:1 |
0.98 0.82 |
0.53 0.37 |
0.43 0.26 |
| Sonic hedgehog | 4:1 40:1 |
1300:1 130:1 |
0.74 0.64 |
0.19 0.02 |
0.10 0.01 |
Estimated retention for one wash at equilibrium. Value estimated from dissociation constants
Obtained from release studies shown in Figure 2
These acellular scaffolds were washed 5 times over the first 24 hours with 1 mL of TBS containing 2% bovine serum albumin (BSA) followed by once a day for the next 13 days, resulting a 14 day time course. Each wash was collected in a silanized tube and stored at −20°C. After the end of the study, the remaining growth factor was released from the scaffold using extraction buffer (phosphate buffered saline containing 10 mg/mL heparin, 2 M NaCl, 1% BSA, 0.01% Triton-X, pH 7.4) as previously described (Willerth et al., 2007c; Wood and Sakiyama-Elbert, 2008). The amount of NT-3 and Shh present in the washes and scaffolds was quantified using ELISA kits (R&D Systems). To quantify the amount of PDGF present, a monoclonal α-PDGF-AA antibody (clone MAB1055, R&D systems) was used as the capture antibody and a biotinylated α-PDGF-AA antibody (clone BAF1055) served as the detection antibody. Absorbance readings at 450 nm were measured using a 96 well plate reader (Multiskan RC Labsystems). The experimental wells were compared to a concentration curve of known standards to determine the quantity of growth factor present.
Embryoid Body Formation and Culture Inside of Fibrin Scaffolds
RW4 mouse ES cells were cultured on gelatin coated plates using complete media (Dubecco’s modified eagle medium containing 10% fetal bovine serum, 10% newborn calf serum, and 0.3 M nucleoside mix, all from Invitrogen except the nucleosides which were from Sigma) containing 1000 U/mL leukemia inhibitory factor (LIF) and 10−4 M β-mecaptoethanol (BME) as described previously (Willerth et al., 2006; Willerth et al., 2007b). Undifferentiated ES cells were induced to form embryoid bodies (EBs) containing ESNPCs using the 4−/4+ retinoic acid treatment protocol before being seeded onto the scaffolds (Bain et al., 1995). The cells were cultured in complete media without LIF or BME for the first four days followed by an additional four days of culture in complete media containing 100 nM retinoic acid. The media was changed every other day during this process. The 4−/4+ retinoic acid treatment protocol produces a cell population consisting of immature neural progenitors (Willerth et al., 2007a). 61 ± 6 % of these cells stain positive for the SSEA-1 marker, which is expressed by undifferentiated mouse ES cells and 71±6 % of these cells stain positive for nestin, which is a neural progenitor markers. These cells did not stain positive for any of the mature neural cells markers including β-tubulin III (early neurons), O4 (oligodendrocytes, and glial fibrilary acidic protein (GFAP, astrocytes).
For studying the effects of controlled growth factor release, 300 μL of scaffold containing delivery system and growth factors was added into each well of a 24 well plate (Corning) and allowed to polymerize for one hour at 37° C. These scaffolds were then washed 5 times with 1 mL of TBS over the next 24 hours with each wash being applied for at least 2 hours to remove unincorporated delivery system components. Following aspiration of the final wash, an individual EB was placed on each scaffold and a second layer of scaffold (100 μL) without delivery system was then added to surround each EB. After an additional hour of incubation at 37° C, 1 mL of complete media was added and the cells were cultured in scaffold for 14 days with the media being changed to neural basal media containing B27 supplement (both from Invitrogen) on day 3 as previously described (Willerth et al., 2006; Willerth et al., 2007b). A schematic of this process is shown in Figure 1B.
Fluorescence Activated Cell Sorting
After 14 days of culture, the media was aspirated from each scaffold followed by a wash with phosphate buffered saline (PBS, pH 7.4). Cells were dissociated using trypsinization and manually removed from the scaffolds then resuspended in PBS. The cells were then stained and sorted as previously described (Willerth et al., 2007b). For cell phenotype analysis, cells were stained for the following markers: stage specific embryonic antigen 1 marker (SSEA-1, undifferentiated mouse ES cells; 1:25; Chemicon), nestin (neural precursors; 1:100; Chemicon), β-tubulin III (Tuj1, early neurons; 1:1000; Covance), O4 marker (1:100; oligodendrocytes; Chemicon), and glial fibrilary acidic protein (GFAP, astrocytes; 1:40; Immunostar). Species specific secondary antibodies (AlexFluor 488, Invitrogen) were used to detect the primary antibodies. For cell surface markers (SSEA-1 and O4), live cells were blocked using PBS containing 5% normal goat serum (NGS) followed by 1 hour incubations with primary and secondary antibody. For intracellular markers (nestin, Tuj1, and GFAP), cells were fixed with 1% paraformaldehyde, permeabilized with 0.5% saponin solution, and blocked with 5% NGS solution. These cells were then incubated with primary and secondary antibodies for 30 minutes each. Cells stained with secondary antibody only were used to eliminate non specific background secondary antibody staining. A more stringent control would have been to use an isotype control primary antibody to ensure the specificity of the staining. For quantitative analysis of cell viability, cells were stained using the Live/Dead Viability/Cytotoxicity kit (Invitrogen) as previously described (Willerth et al., 2007b). The cells were incubated with PBS solutions containing 0.1 μM calecin AM and 16 μM ethidium homodimer for 30 minutes. Cellular fluorescence was detected using a FACS Calibur flow cytometer (Becton Dickinson) and analysis was performed using CellQuest software (Becton Dickinson). Representative plots showing the fluorescence for the different control and experimental groups are shown for the cultures seeded inside of unmodified fibrin scaffolds with no growth factors present and cultures seeded inside of scaffolds containing the delivery system along with PDGF and NT-3 in Supplementary Figures One and Two.
Quantitative Real Time Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
To confirm the results observed using FACS, quantitative real time RT-PCR was used to examine the expression levels of markers for different cell phenotypes for three different groups, including EBs cultured in unmodified fibrin, in scaffolds containing NT-3 and PDGF with no delivery system present, and in scaffolds containing NT-3 and PDGF with delivery system. Real time RT-PCR was used to examine the expression of the following four markers: Sox2 (neural progenitors), microtubule associated protein-2 (Map2, early neurons), platelet derived growth factor α receptor (PDGFαR, early oligodendrocytes), and vimentin (astrocytes).
To obtain RNA for real time RT-PCR analysis, the cells were cultured inside of scaffolds for 7 days. An earlier time point was chosen because mRNA upregulation often occurs before changes in protein expression are observed. Cells were then isolated from the scaffolds using trypsin as described above and the resulting cells were lysed using Buffer RLT (Qiagen). These lysates were homogenized using a Qiashredder kit and RNA was then isolated using an RNEasy kit (both from Qiagen). From the isolated RNA, cDNA was synthesized using the Quantitech Reverse Transcription kit (Qiagen) according to the manufacturer’s instructions. Using the Quantitech SYBR Green PCR Mastermix (Qiagen) combined with gene specific QuantiTect primer assays, quantitative real time PCR was performed using an Applied Biosystems 7000 Real-Time PCR System using the following PCR cycle; 50°C (2 min), 95°C (10 min), followed by 40 cycles of 95°C (15 s), 55°C (30 s), 72°C (30 s) with fluorescent signal was detected at 72°C. Target genes were normalized to β-actin expression to account for variations in cDNA concentration between samples. The differences in gene expression levels were calculated using the comparative delta Ct method, which allows for determining relative expression of RNA between two samples. In this method, the crossover threshold (Ct) refers to the cycle number when the fluorescence released by the PCR reaction begins to increase exponentially. The difference between relative Ct values for each sample was used to estimate the fold difference in expression between samples compared to unmodified fibrin scaffolds.
Statistical Analysis
For the release studies, four independent release experiments (one scaffold per experiment)were analyzed for each set of delivery system conditions. For cell phenotype analysis, three independent experiments consisting of one 24 well plate containing fibrin scaffolds with EB cultures per experiment were analyzed for each condition tested. For cell viability analysis, three independent experiments analyzing 6 wells containing fibrin scaffolds seeded with EB cultures were performed. These conditions provided enough cells (5000 per sort for each individual experiemnt) to perform FACS and statistical analysis on the outcomes. For real time RT-PCR analysis, three independent experiments analyzing 6 wells containing fibrin scaffolds seeded with EB cultures were performed. Analysis of variance applying Scheffe post-hoc tests were used to compare experimental and control groups. Significance was considered at the p < 0.05 level.
Results
Determining delivery system conditions for maximum growth factor retention inside of fibrin scaffolds
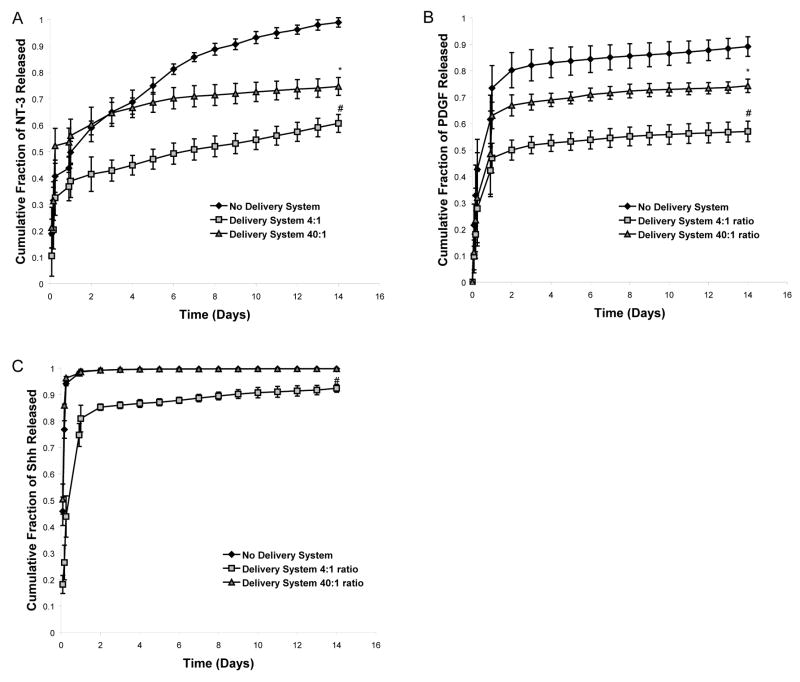
To determine the delivery system conditions that would generate the slowest release of the individual growth factors to ESNPCs, two peptide to heparin ratios were tested in the context of the delivery system by changing the concentration of heparin present. Changing the heparin concentration also alters the ratio of heparin to growth factor and all of these ratios are listed in Table One. The two chosen heparin concentrations (62.5 μM or 6.25 μM) resulted in either a 4:1 or a 40:1 heparin to peptide ratio, respectively. These ratios were selected based on previous work (Taylor et al., 2004; Wood and Sakiyama-Elbert, 2008) and had been shown to provide sustained release of growth factors that possess an affinity for heparin. The concentration of heparin changes the number of binding sites available to the growth factor present in the scaffold and thus affects the rate of growth factor release from the scaffolds. The goal of this release study was to determine which ratio resulted in the slowest growth factor release from the scaffolds and retained the maximum amount of growth factor. Release was evaluated over a two week period which corresponds roughly to the degradation time of the fibrin scaffolds in vivo. Over the first 24 hours, the scaffolds were washed extensively (5 times) to remove unincorporated components followed by one wash a day for the remaining 13 days of the study.
Figure 2A shows the results of the 14 day release study to determine the optimal peptide to heparin ratio for NT-3 delivery. When no heparin was present, virtually all of the NT-3 (~99%) was released from the scaffold by the end of 14 days. In contrast, both the 4:1 and 40:1 ratios provided sustained NT-3 delivery over the same time period. The 4:1 peptide to heparin ratio retained the most NT-3 (~40%) in the scaffold at the end of the study, which was significantly more than the amount retained when the 40:1 ratio was used. The different ratios produced two different rates of NT-3 release with the 4:1 ratio being selected for delivery of NT-3 to the ESNPCs seeded inside of the scaffolds because it generated the slowest release and maximized growth factor retention.
Figure 2.
The effect of varying the peptide to heparin ratio in the affinity-based delivery system on growth factor release over 14 day time course. A) Release profile of NT-3 from the delivery system. B) 14 day release profile of PDGF from the delivery system. C) Release profile of Shh from the delivery system. # indicates p <0.05 versus all other groups. * indicates p<0.05 versus no delivery system. Error bars indicate standard deviation.
Similar results were obtained for the release studies involving delivery of PDGF (Figure 2B). Three different rates of release were observed depending on the amount of heparin present in the delivery system. When no heparin was present, ~10% of the PDGF remained in the scaffold at the end of the study. When a 4:1 ratio was used, ~42% of the PDGF was retained in the scaffold while the 40:1 ratio resulted in ~26% retention. Thus the 4:1 peptide to heparin ratio was selected for the in vitro differentiation studies because it retained significantly more PDGF than the 40:1 ratio.
Release of Shh from the delivery system was also characterized for the different heparin to peptide ratios (Figure 2C). The 4:1 ratio generated the slowest rate of release, but it only retained ~10% of Shh at the end of the 14 day study. However, when no heparin was present or the 40:1 ratio was used, Shh was released from the system much more rapidly with 99% of the Shh released by day 2 of the 14 day study. This release rate was much faster than the rate observed for the scaffolds containing NT-3 or PDGF with no heparin present. For Shh, only the 4:1 ratio produced a release rate that was significantly different from the scaffolds containing growth factor without heparin. Overall, the 4:1 peptide to heparin ratio resulted in maximum growth factor retention inside of the scaffolds for all growth factors tested.
Determining optimal growth factor doses from the delivery system for promoting ESNPC differentiation into neurons and oligodendrocytes
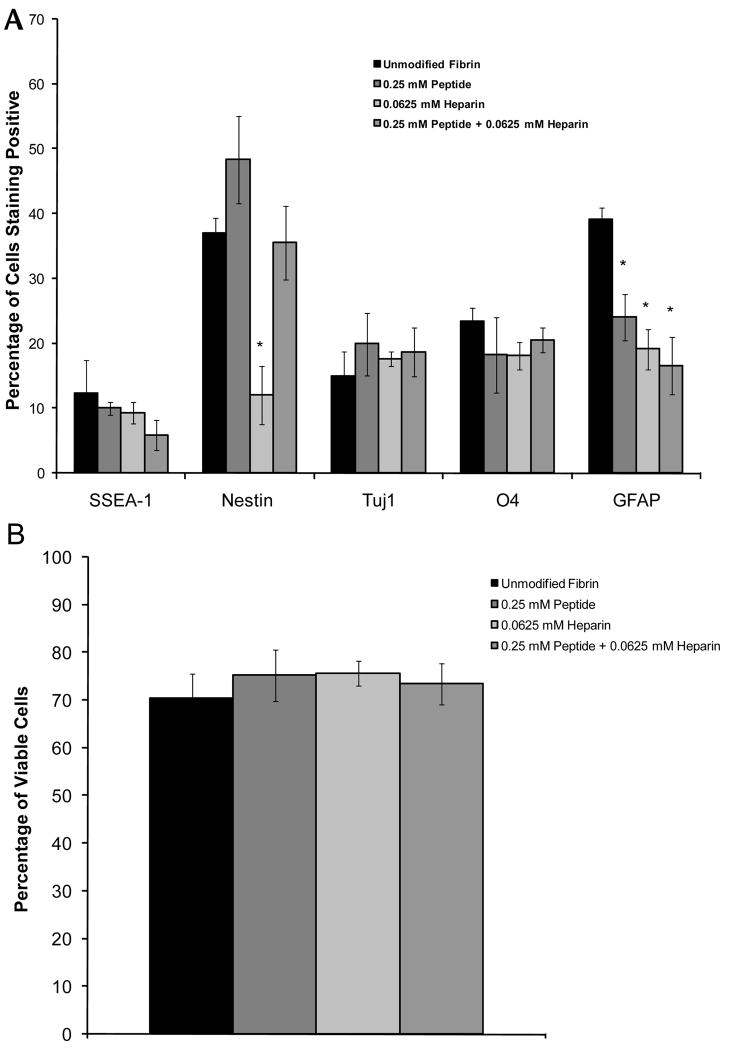
Before examining the effects of controlled growth factor delivery on the resulting cell phenotypes of the ESNPCs seeded inside, a set of control experiments was performed to determine the effects of each of the delivery system components on differentiation and cell viability. For in vitro differentiation studies, scaffolds were polymerized containing the different components at indicated concentrations and all scaffolds were washed 5 times over 24 hours before cell seeding to remove unincorporated components and to simulate the conditions of an in vivo setting where the unincorporated components would be washed away. The effect of different delivery system components on differentiation and viability after 14 days of culture can be seen in Figure 3A and 3B. When only peptide or peptide and heparin were incorporated into the scaffolds, a decrease in astrocytes was observed. When heparin alone was added to the scaffolds, a decrease in nestin (neural progenitor) staining was observed in addition to the decrease in the percentage of astrocytes. However, combining peptide with heparin restored the nestin levels similar to that of the cultures in unmodified fibrin with no growth factors present in the media. The incorporation of the delivery system components into the fibrin scaffold did not have any effect on cell viability compared to the unmodified fibrin scaffolds.
Figure 3.
The effects of the affinity-based delivery system on ESNPC differentiation and survival inside of fibrin scaffolds. A) Quantitative analysis of the effect of the different delivery system components on ESNPC differentiation inside of fibrin scaffolds. B) Quantitative analysis of the effect of the different delivery system components on ESNPC viability inside of fibrin scaffolds. * indicates p < 0.05 compared to 14 day control cultures in unmodified fibrin with no growth factors present in the media. Error bars indicate standard deviation.
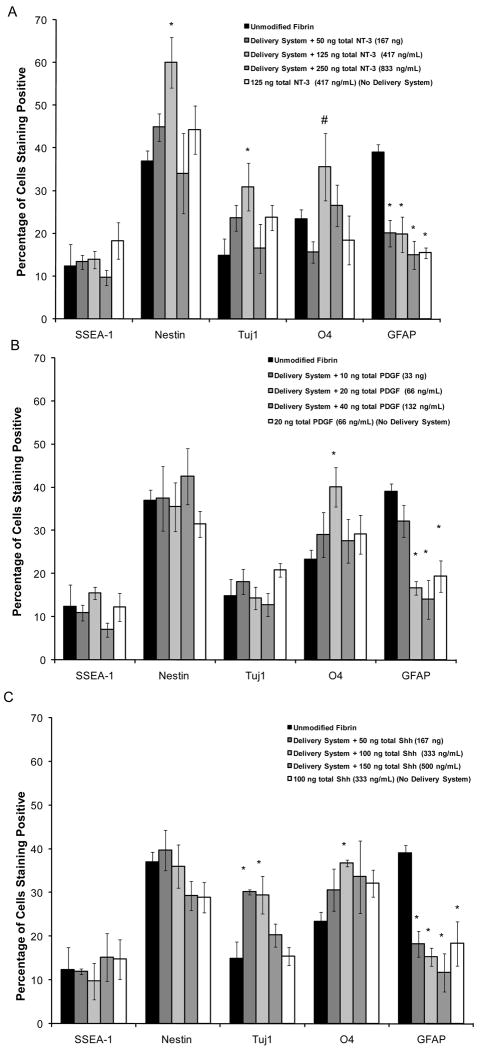
A series of different growth factor doses were delivered to ESNPCs seeded inside fibrin scaffolds. Doses were selected based on the previous studies conducted with growth factors present in the culture media (Willerth et al., 2007b) and the results of the release studies. The purpose of this study was to determine the optimal dose for promoting differentiation of neurons and oligodendrocytes from ESNPCs seeded inside of fibrin scaffolds containing the delivery system. As seen in Figure 4A, all doses of NT-3 delivered resulted in a reduction of the fraction of cells differentiating into astrocytes. Only the 125 ng (417 ng/mL) dose produced an increase the fraction of neurons and neural progenitors obtained compared to the group cultured in unmodified fibrin with no growth factors present in the media. Additionally, at this dose, an increase in the fraction of cells differentiating into oligodendrocytes was observed compared to the same dose of NT-3 when no delivery system was present. Accordingly, 125 ng dose of NT-3 was selected for use in the combination growth factor studies since it increased the fraction of cells that differentiated into both neurons and oligodendrocytes.
Figure 4.
The effects of controlled growth factor delivery on ESNPC differentiation inside of fibrin scaffolds A) The effects of controlled release of different NT-3 doses on ESNPC differentiation. B) The effects of controlled release of different PDGF doses on ESNPC differentiation. C) The effects of controlled release of different Shh doses on ESNPC differentiation. Markers examined included SSEA-1 (undifferentiated ES cells), nestin (neural progenitors), Tuj1 (early neurons), O4 (oligodendrocytes), and GFAP (astrocytes).* indicates p<0.05 versus cultures in unmodified fibrin with no growth factors present in the media. # indicates p<0.05 compared to optimal dose with no delivery system present. Error bars indicate standard deviation.
Based on previous work, different PDGF doses ranging from 10 ng to 40 ng total were delivered to ESNPCs seeded into fibrin scaffolds as seen in Figure 4B. 20 ng dose (66 ng/mL) of PDGF promoted the highest level of differentiation of ESNPCs into oligodendrocytes while reducing the fraction of cells differentiating into astrocytes compared to the group cultured in unmodified fibrin with no growth factors present in the media. For a 10 ng dose of PDGF (33 ng/mL), no differences were observed compared to controls cultured in unmodified fibrin with no growth factors present in the media and for the higher dose, only a decrease in the fraction of cells staining positive for GFAP was observed. When the 20 ng dose of PDGF was polymerized into scaffolds with no delivery system present, only a decrease in the fraction of cells differentiating into astrocytes was observed compared to the group cultured in unmodified fibrin with no growth factors present in the media.
When Shh was delivered from scaffolds containing the delivery system, the 100 ng dose resulted in increases in the fraction of cells differentiating into neurons and oligodendrocytes produced while reducing the fraction of cells differentiating into astrocytes compared to the cultures in unmodified fibrin with no growth factors present in the media (Figure 4C). The lowest Shh dose (50 ng total) only produced an increase in the fraction of cells differentiating into neurons while decreasing the fractions of cells differentiating into astrocytes present. However, no difference in the fraction of cells differentiating into oligodendrocytes was observed compared to the cultures in unmodified fibrin with no growth factors present in the media. Decreasing the fraction of cells differentiating into astrocytes produced was the only difference observed for both the highest dose of Shh delivered (150 ng) and the 100 ng Shh dose with no delivery system present.
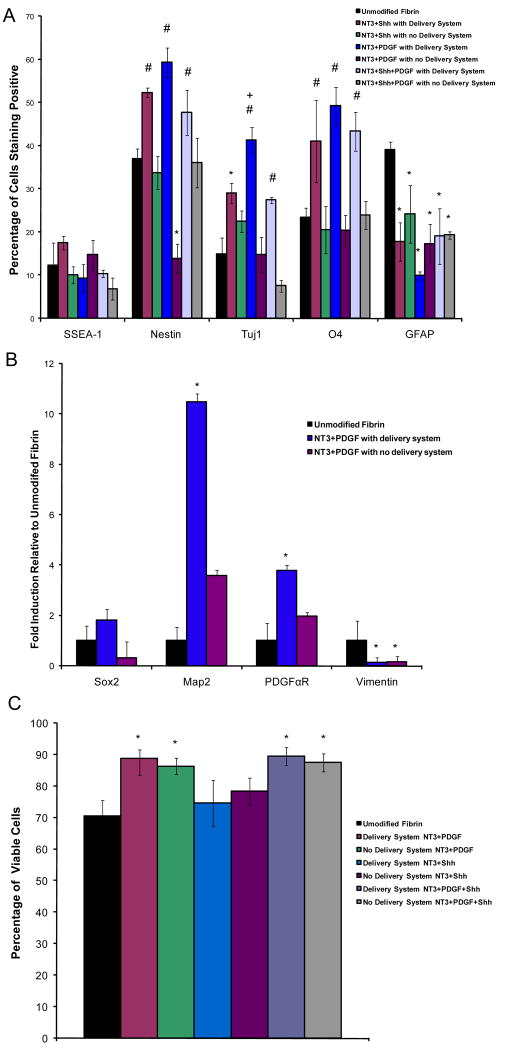
Based on these results, three different growth factor combinations, NT-3 and PDGF, NT-3 and Shh, and NT-3, PDGF, and Shh, were released from fibrin scaffolds both with and without the delivery system present and the resulting differentiation and viability quantified as seen in Figures 5A and B. The optimal dose of each growth factor for promoting ESNPC differentiation into neurons and oligodendrocytes that had been determined from the individual studies was used in these combination studies. As seen in Figure 5A, no differences in the fraction of cells staining positive for SSEA-1 was observed for any of the experimental groups, but all experimental groups did exhibit a decrease in the fraction of cells staining positive for GFAP compared to the group cultured in unmodified fibrin scaffolds with no growth factor present.
Figure 5.
The effects of controlled delivery of growth factor combinations on ESNPC cell differentiation and survival inside of fibrin scaffolds. A) Quantitative analysis of the effect of the different growth factor combinations on ESNPC differentiation after 14 days of culture. Markers examined included SSEA-1 (undifferentiated ES cells), nestin (neural progenitors), Tuj1 (early neurons), O4 (oligodendrocytes), and GFAP (astrocytes).B) Real Time RT-PCR analysis to confirm FACS results. Markers examined included Sox2 (neural progenitors), microtubule associated protein-2 (Map2, early neurons), platelet derived growth factor α receptor (PDGFαR, early oligodendrocytes), and vimentin (astrocytes) after 7 days of culture inside of scaffolds. C) Quantitative analysis of the effect of the different growth factor combinations on ESNPC survival after 14 days of culture. * indicates p<0.05 versus control cultures in unmodified fibrin with no growth factors present in the media. # indicates p>0.05 versus same growth factor combination with no delivery system present. + indicates p<0.05 versus all other groups. Error bars indicate standard deviation.
All three growth factor combinations had significant effects on the differentiation of ESNPCs compared to both the group with no growth factor present and scaffolds containing the same doses of growth factors without delivery system. These three combinations all produced an increase in the fraction of cells staining positive for nestin and O4 when compared to both the control group cultured in unmodified fibrin scaffolds with no growth factor present and the control group consisting of the same growth factor doses with no delivery system. As mentioned before, the control cultures without delivery system are important to show that the delivery system is retaining more growth factor as well as presenting it in a biologically active manner. Controlled delivery of certain growth factor combinations (the dual combination of NT-3 and PDGF, or the triple combination of NT-3, PDGF and Shh) increased the fraction of cells differentiating into neurons compared to both the group with no growth factor present and the group consisting of the same growth factor doses with no delivery system. Controlled delivery of NT-3 and Shh only increased the fraction of cells differentiating into neurons compared to the group with no growth factor present. Additionally, controlled delivery of NT-3 and PDGF also produced an increase in the fraction of cells differentiating into neurons compared to the controlled delivery of the other two growth factor combinations tested, making this combination (NT-3 and PDGF) most effective for achieving the desired goal of promoting differentiation of ESNPCs into neurons and oligodendrocytes.
To confirm the results observed using FACS, real time RT-PCR was used to examine the expression levels of four different markers for these cell phenotypes, including Sox2 (neural progenitors), Map2 (early neurons), PDGFαR (early oligodendrocytes), and vimentin (astrocytes). Figure 5B shows the results of the real time RT-PCR performed to confirm the effects of controlled delivery of NT-3 and PDGF compared to unmodified fibrin scaffolds and scaffolds containing NT-3 and PDGF with no delivery system present. For the Sox2 marker, no statistical differences were observed between groups. Increases the expression levels of Map2 and PDGFαR compared to the unmodified fibrin scaffolds were observed only for the group cultured in the scaffolds containing the delivery system with NT-3 and PDGF. Both groups treated with NT-3 and PDGF both in the presence and absence of delivery system showed a decrease in the expression of vimentin compared to the group cultured in unmodified fibrin with no growth factors present in the media. Figure 5C shows the effect of the different growth factor combinations on cell viability. All growth factor combinations that contained PDGF (both in presence and absence of the delivery system) promoted an increase in cell viability compared to the group with no growth factor present.
Discussion
To produce an engineered tissue as a treatment for SCI, delivery conditions and appropriate growth factor dosing for promoting ESNPC differentiation into neurons and oligodendrocytes were determined as both of these cells types could potentially contribute to functional recovery after SCI. Neurons could help to restore the circuitry disrupted after injury, allowing for reinnervation (Lee et al., 2007). Oligodendrocytes could restore the myelination of the spared axons present at and around the injury site and such oligodendrocyte replacement strategies have been shown to promote functional recovery in animal models of SCI (Keirstead et al., 2005; Liu et al., 2000; Nistor et al., 2005). Additionally, minimizing astrocytes production was important as they could contribute to the glial scar and inhibit regeneration (Fawcett and Asher, 1999).
Release studies determined the appropriate delivery system conditions need to retain the maximum amount of growth factor using the affinity-based delivery system. The release studies were performed in the absence of cells to quantify the amount of growth factor passively released over time. In the in vitro culture system and in vivo, the remaining growth factor is actively released from the scaffolds when cells activate plasmin, which enzymatically degrades the scaffold. In the in vitro culture system, the growth factors can act upon the ESNPCs seeded inside of the scaffolds and in vivo, the growth factors can be released by both the ESNPCs and the surviving cells that remained after the injury.
Two different heparin concentrations were tested in the context of the delivery system and the 4:1 ratio resulted in maximum growth factor retention for all growth factors tested. These results suggest that the increase in heparin concentration provided more binding sites to retain the growth factors inside of the scaffold. For the 4:1 peptide to heparin ratio, there is ~1300–1700 times excess of binding sites (heparin molecules) per growth factor while the 40:1 ratio only yields ~130–170 times excess of binding sites (Table One).
Table One shows the estimated fraction of growth factor bound at equilibrium calculated from the dissociation constants for each growth factor. The release rates of the growth factors correlate with the estimated bound fraction at equilibrium. The affinity-based delivery system was predicted to retain more PDGF and NT-3 than Shh at equilibrium. This trend was observed in the release studies with the 4:1 ratio, which was confirmed by the 14 day release studies.
For NT-3 and PDGF, the delivery system retained different amounts of growth factor depending on the peptide to heparin ratio. The pattern of growth factor release was consistent with previously published release studies of NT-3 and PDGF-BB, a different PDGF isoform (Taylor et al., 2004; Thomopoulos et al., 2007). Only the 4:1 ratio retained Shh inside of the scaffolds. Shh had never been tested previously with the affinity-based delivery system, but it has been reported to have a high affinity for heparin (Rubin et al., 2002; Zhang et al., 2007). The quick release of Shh could potentially be due to repulsive interactions between Shh and the scaffold itself. .
An initial burst of growth factor release was observed over the first twenty four hours. In an in vivo setting, this unbound growth factor would be washed away from the scaffold by cerebrospinal fluid present at the injury site. To account for this effect, all scaffolds were washed five times before cell seeding and the addition of the second scaffold layer. Different effects on ESNPC differentiation are observed when the same growth factor dose is polymerized inside of the scaffolds in the presence of delivery system compared to when no delivery system is present.
It is also important to consider the effects of the delivery system on the differentiation of ESNPCs as bioactive peptides have been previously used to promote differentiation of neural progenitors seeded on nanofibers (Silva et al., 2004). The presence of peptide and heparin, both individually and in combination, reduced the fraction of ESNPCs that differentiated into astrocytes. The reduction in the fraction of cells that differentiated into astrocytes was observed for all of the delivery system groups except when it was used to delivery 10 ng total of PDGF, suggesting that the presence of growth factor does not interfere with this beneficial effect. The presence of heparin alone decreased the fraction of neural progenitors present, but this decrease was not observed when both peptide and heparin were present, suggesting that the peptide may help maintain nestin expression. When looking at the effects of the growth factors on ESNPC differentiation, it is important to view the results in the context of these control experiments. For other tissue engineering applications, it will be important to run similar control experiments to ensure these components do not interfere with the desired stem differentiation.
For each of the growth factors tested, a range of concentrations was selected by combining the results of the release studies and previous studies that looked at the effects of growth factors in the culture media on ESNPC differentiation inside of fibrin scaffolds (Willerth et al., 2007b). Controlled delivery of 125 ng dose (417 ng/mL) from the affinity-based delivery system produced neuron and oligodendrocyte differentiation comparable to 25 ng/mL of NT-3 in the media. Soluble NT-3 has also been shown to promote the differentiation of human ES cells into neurons in three dimensional settings, suggesting these scaffolds might also be useful for human ES cell lines (Levenberg et al., 2005). NT-3 also promotes the survival of oligodendrocyte precursors (Kumar et al., 1998), which could potentially explain the increase in O4 staining observed with controlled delivery of the optimal dose (100 ng NT-3) with delivery system. . Controlled delivery of NT-3 also produced an increase in the percentage of cells staining positive for nestin, which could potentially be due to the delivery system, as this effect was not observed in the previous studies with NT-3 in the media.
From our previous work, the controlled release dose of PDGF (20 ng total) corresponds to between 2 ng/mL and 10 ng/mL of soluble PDGF and replicates the benefits of both doses, which included an increase in the fraction of cells differentiating into oligodendrocytes and a decrease in the fraction of cells differentiating into astrocytes. These results are consistent with other studies that have demonstrated PDGF’s role in promoting oligodendrocyte development (Calver et al., 1998).
Controlled delivery of 100 ng of Shh produced similar ESNPC differentiation to both 10 and 25 ng/mL of Shh in the media. The increase in neuronal differentiation was expected as many studies have demonstrated that Shh combined with retinoic acid promotes the differentiation into ES cells into motor neurons (Dutton et al., 1999; Lee et al., 2007; Li et al., 2005; Miles et al., 2004; Soundararajan et al., 2007; Soundararajan et al., 2006). Shh also plays important roles in promoting oligodendrocyte differentiation (Danesin et al., 2006; Oh et al., 2005). The poor retention of Shh by the affinity-based delivery system required polymerizing high doses of Shh into the scaffolds to promote differentiation, and more effective retention of Shh by an affinity-based delivery system might be obtained by using different scaffold material.
Controlled delivery of all three growth factor combinations produced significant changes compared to both unmodified fibrin scaffolds with no growth factors present in the media and scaffolds containing the same growth factors combinations with no delivery system. The main difference between the three combinations tested was in the fraction of cells differentiating into neurons. The combination of NT-3 and PDGF produced the largest fraction of cells differentiating into neurons compared to all the groups tested, making it the optimal combination for further testing in the context of an in vivo model of SCI. This result was also confirmed using real time RT-PCR analysis with increases in the expression levels of Map2 and PDGFαR and a decrease in vimentin expression levels compared to the cultures in unmodified fibrin scaffolds with no growth factors present.
These results are consisted with other literature that has shown different situations where combining NT-3 with PDGF produces additive effects. Previous studies have shown that this combination promotes survival of Schwann cell precursors and remyelination in rodent models of multiple sclerosis, suggesting that their signaling pathways may share an intermediate that produces these additional benefits (Fressinaud, 2005; Lobsiger et al., 2000). Also, the inclusion of PDGF in combination with other growth factors produced an increase in cell viability. This increase in viability may increase the survival rate of the transplanted cells in vivo..
This study has produced an engineered neural tissue consisting of a fibrin scaffold containing an affinity-based delivery system, growth factors and ESNPCs ready for in vivo testing as a potential treatment for SCI. The growth factor delivery system and concentration were optimized to promote the differentiation of the cells seeded within these scaffolds into neurons and oligodendrocytes and to increase cell viability. The concentrations found in this study for promoting the differentiation of ESNPCs into these cell phenotypes can be easily translated to other materials with known release properties for in vivo SCI applications. Such a strategy can be extended to generate replacements for other types of damaged tissues. Finally, this affinity-based delivery system could also be used to study signaling events during development. Its ability to mimic the extracellular matrix and present growth factors in a controlled manner allows for the reproduction of signals present during development in an in vitro setting. Thus, this delivery system allows for generation of tissue engineered scaffolds as well as providing an in vitro system for studying the development biology of stem cells.
Supplementary Material
Representative histograms of fluorescence activated cell sorting data for cultures seeded inside of unmodified fibrin scaffolds with no growth factors present. The gate marks the population of cells staining positive for a marker. Background staining for the secondary antibody alone controls was set to 1%. A) Unstained live cells B) Cells stained with secondary IgM antibody only C) Cells stained with SSEA-1 antibody D) Cells stained with O4 antibody E) Unstained fixed cells F) Cells stained with secondary IgG only G) Cells stained with Nestin antibody H) Cells stained with β-tubulin III antibody I) Cells stained with anti-rabbit antibody J) Cells stained with GFAP antibody
Representative histograms of fluorescence activated cell sorting data for cultures seeded inside of fibrin scaffolds containing the delivery system along with the growth facto combination PDGF and NT-3. The gate marks the population of cells staining positive for a marker. Background staining for the secondary antibody alone controls was set to 1%. A) Unstained live cells B) Cells stained with secondary IgM antibody only C) Cells stained with SSEA-1 antibody D) Cells stained with O4 antibody E) Unstained fixed cells F) Cells stained with secondary IgG only G) Cells stained with Nestin antibody H) Cells stained with β-tubulin III antibody I) Cells stained with anti-rabbit antibody J) Cells stained with GFAP antibody
Acknowledgments
This work was supported by NIH R01 NS051454. The authors would thank Amy Boyet, Angela Guzmán, Nicole Moore, and Matthew Wood for technical support. The authors would thank Shengzhou Wu and the Hope Center for Neurological Disorders, which is supported by NIH Neuroscience Blueprint Interdisciplinary Center Core grant (P30 NS057105), for use of the real time PCR machine.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bain G, Kitchens D, Yao M, Huettner JE, Gottlieb DI. Embryonic stem cells express neuronal properties in vitro. Developmental biology. 1995;168:342–357. doi: 10.1006/dbio.1995.1085. [DOI] [PubMed] [Google Scholar]
- Bambakidis NC, Miller RH. Transplantation of oligodendrocyte precursors and sonic hedgehog results in improved function and white matter sparing in the spinal cords of adult rats after contusion. Spine J. 2004;4:16–26. doi: 10.1016/j.spinee.2003.07.004. [DOI] [PubMed] [Google Scholar]
- Bambakidis NC, Wang RZ, Franic L, Miller RH. Sonic hedgehog-induced neural precursor proliferation after adult rodent spinal cord injury. Journal of neurosurgery. 2003;99:70–75. doi: 10.3171/spi.2003.99.1.0070. [DOI] [PubMed] [Google Scholar]
- Beckstead BL, Santosa DM, Giachelli CM. Mimicking cell-cell interactions at the biomaterial-cell interface for control of stem cell differentiation. J Biomed Mater Res A. 2006;79:94–103. doi: 10.1002/jbm.a.30760. [DOI] [PubMed] [Google Scholar]
- Benoit DS, Anseth KS. Heparin functionalized PEG gels that modulate protein adsorption for hMSC adhesion and differentiation. Acta biomaterialia. 2005;1:461–470. doi: 10.1016/j.actbio.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Benoit DS, Durney AR, Anseth KS. The effect of heparin-functionalized PEG hydrogels on three-dimensional human mesenchymal stem cell osteogenic differentiation. Biomaterials. 2007;28:66–77. doi: 10.1016/j.biomaterials.2006.08.033. [DOI] [PubMed] [Google Scholar]
- Calver AR, Hall AC, Yu WP, Walsh FS, Heath JK, Betsholtz C, Richardson WD. Oligodendrocyte population dynamics and the role of PDGF in vivo. Neuron. 1998;20:869–882. doi: 10.1016/s0896-6273(00)80469-9. [DOI] [PubMed] [Google Scholar]
- Cao QL, Howard RM, Dennison JB, Whittemore SR. Differentiation of engrafted neuronal-restricted precursor cells is inhibited in the traumatically injured spinal cord. Experimental neurology. 2002;177:349–359. doi: 10.1006/exnr.2002.7981. [DOI] [PubMed] [Google Scholar]
- Cao QL, Zhang YP, Howard RM, Walters WM, Tsoulfas P, Whittemore SR. Pluripotent stem cells engrafted into the normal or lesioned adult rat spinal cord are restricted to a glial lineage. Experimental neurology. 2001;167:48–58. doi: 10.1006/exnr.2000.7536. [DOI] [PubMed] [Google Scholar]
- Danesin C, Agius E, Escalas N, Ai X, Emerson C, Cochard P, Soula C. Ventral neural progenitors switch toward an oligodendroglial fate in response to increased Sonic hedgehog (Shh) activity: involvement of Sulfatase 1 in modulating Shh signaling in the ventral spinal cord. J Neurosci. 2006;26:5037–5048. doi: 10.1523/JNEUROSCI.0715-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutton R, Yamada T, Turnley A, Bartlett PF, Murphy M. Sonic hedgehog promotes neuronal differentiation of murine spinal cord precursors and collaborates with neurotrophin 3 to induce Islet-1. J Neurosci. 1999;19:2601–2608. doi: 10.1523/JNEUROSCI.19-07-02601.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman ER, Mathiowitz E, Langer R, Klagsbrun M. Controlled and modulated release of basic fibroblast growth factor. Biomaterials. 1991;12:619–626. doi: 10.1016/0142-9612(91)90107-l. [DOI] [PubMed] [Google Scholar]
- Fan VH, Tamama K, Au A, Littrell R, Richardson LB, Wright JW, Wells A, Griffith LG. Tethered epidermal growth factor provides a survival advantage to mesenchymal stem cells. Stem Cells. 2007;25:1241–1251. doi: 10.1634/stemcells.2006-0320. [DOI] [PubMed] [Google Scholar]
- Fawcett JW, Asher RA. The glial scar and central nervous system repair. Brain research bulletin. 1999;49:377–391. doi: 10.1016/s0361-9230(99)00072-6. [DOI] [PubMed] [Google Scholar]
- Ferreira LS, Gerecht S, Fuller J, Shieh HF, Vunjak-Novakovic G, Langer R. Bioactive hydrogel scaffolds for controllable vascular differentiation of human embryonic stem cells. Biomaterials. 2007;28:2706–2717. doi: 10.1016/j.biomaterials.2007.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fressinaud C. Repeated injuries dramatically affect cells of the oligodendrocyte lineage: effects of PDGF and NT-3 in vitro. Glia. 2005;49:555–566. doi: 10.1002/glia.20136. [DOI] [PubMed] [Google Scholar]
- Gomez N, Lu Y, Chen S, Schmidt CE. Immobilized nerve growth factor and microtopography have distinct effects on polarization versus axon elongation in hippocampal cells in culture. Biomaterials. 2007;28:271–284. doi: 10.1016/j.biomaterials.2006.07.043. [DOI] [PubMed] [Google Scholar]
- Gomez N, Schmidt CE. Nerve growth factor-immobilized polypyrrole: bioactive electrically conducting polymer for enhanced neurite extension. J Biomed Mater Res A. 2007;81:135–149. doi: 10.1002/jbm.a.31047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraizumi Y, Fujimaki E, Transfeldt EE, Kawahara N, Fiegel VD, Knighton D, Sung JH. The effect of the platelet derived wound healing formula and the nerve growth factor on the experimentally injured spinal cord. Spinal Cord. 1996;34:394–402. doi: 10.1038/sc.1996.71. [DOI] [PubMed] [Google Scholar]
- Hiraizumi Y, Transfeldt EE, Kawahara N, Fiegel VD, Knighton D, Sung JH. The effect of growth factor formula (platelet derived wound healing formula) in experimental spinal cord injuries. The Journal of the American Paraplegia Society. 1992;15:7–13. doi: 10.1080/01952307.1992.11735856. [DOI] [PubMed] [Google Scholar]
- Hiraizumi Y, Transfeldt EE, Kawahara N, Sung JH, Knighton D, Fiegel VD. In vivo angiogenesis by platelet-derived wound-healing formula in injured spinal cord. Brain research bulletin. 1993;30:353–357. doi: 10.1016/0361-9230(93)90264-c. [DOI] [PubMed] [Google Scholar]
- Ho JE, Chung EH, Wall S, Schaffer DV, Healy KE. Immobilized sonic hedgehog N-terminal signaling domain enhances differentiation of bone marrow-derived mesenchymal stem cells. J Biomed Mater Res A. 2007 doi: 10.1002/jbm.a.31355. [DOI] [PubMed] [Google Scholar]
- Ichinose A, Tamaki T, Aoki N. Factor XIII-mediated cross-linking of NH2-terminal peptide of alpha 2-plasmin inhibitor to fibrin. FEBS letters. 1983;153:369–371. doi: 10.1016/0014-5793(83)80645-0. [DOI] [PubMed] [Google Scholar]
- Keirstead HS, Nistor G, Bernal G, Totoiu M, Cloutier F, Sharp K, Steward O. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury. J Neurosci. 2005;25:4694–4705. doi: 10.1523/JNEUROSCI.0311-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl PR, Griffith-Cima LG. Tethered epidermal growth factor as a paradigm for growth factor-induced stimulation from the solid phase. Nature medicine. 1996;2:1022–1027. doi: 10.1038/nm0996-1022. [DOI] [PubMed] [Google Scholar]
- Kumar S, Kahn MA, Dinh L, de Vellis J. NT-3-mediated TrkC receptor activation promotes proliferation and cell survival of rodent progenitor oligodendrocyte cells in vitro and in vivo. Journal of neuroscience research. 1998;54:754–765. doi: 10.1002/(SICI)1097-4547(19981215)54:6<754::AID-JNR3>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Lee H, Shamy GA, Elkabetz Y, Schofield CM, Harrsion NL, Panagiotakos G, Socci ND, Tabar V, Studer L. Directed differentiation and transplantation of human embryonic stem cell-derived motoneurons. Stem Cells. 2007;25:1931–1939. doi: 10.1634/stemcells.2007-0097. [DOI] [PubMed] [Google Scholar]
- Levenberg S, Burdick JA, Kraehenbuehl T, Langer R. Neurotrophin-induced differentiation of human embryonic stem cells on three-dimensional polymeric scaffolds. Tissue engineering. 2005;11:506–512. doi: 10.1089/ten.2005.11.506. [DOI] [PubMed] [Google Scholar]
- Levenberg S, Huang NF, Lavik E, Rogers AB, Itskovitz-Eldor J, Langer R. Differentiation of human embryonic stem cells on three-dimensional polymer scaffolds. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:12741–12746. doi: 10.1073/pnas.1735463100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XJ, Du ZW, Zarnowska ED, Pankratz M, Hansen LO, Pearce RA, Zhang SC. Specification of motoneurons from human embryonic stem cells. Nature biotechnology. 2005;23:215–221. doi: 10.1038/nbt1063. [DOI] [PubMed] [Google Scholar]
- Liu S, Qu Y, Stewart TJ, Howard MJ, Chakrabortty S, Holekamp TF, McDonald JW. Embryonic stem cells differentiate into oligodendrocytes and myelinate in culture and after spinal cord transplantation. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:6126–6131. doi: 10.1073/pnas.97.11.6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobsiger CS, Schweitzer B, Taylor V, Suter U. Platelet-derived growth factor-BB supports the survival of cultured rat Schwann cell precursors in synergy with neurotrophin-3. Glia. 2000;30:290–300. doi: 10.1002/(sici)1098-1136(200005)30:3<290::aid-glia8>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- McDonald JW, Liu XZ, Qu Y, Liu S, Mickey SK, Turetsky D, Gottlieb DI, Choi DW. Transplanted embryonic stem cells survive, differentiate and promote recovery in injured rat spinal cord. Nature medicine. 1999;5:1410–1412. doi: 10.1038/70986. [DOI] [PubMed] [Google Scholar]
- Miles GB, Yohn DC, Wichterle H, Jessell TM, Rafuse VF, Brownstone RM. Functional properties of motoneurons derived from mouse embryonic stem cells. J Neurosci. 2004;24:7848–7858. doi: 10.1523/JNEUROSCI.1972-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nistor GI, Totoiu MO, Haque N, Carpenter MK, Keirstead HS. Human embryonic stem cells differentiate into oligodendrocytes in high purity and myelinate after spinal cord transplantation. Glia. 2005;49:385–396. doi: 10.1002/glia.20127. [DOI] [PubMed] [Google Scholar]
- Oh S, Huang X, Chiang C. Specific requirements of sonic hedgehog signaling during oligodendrocyte development. Dev Dyn. 2005;234:489–496. doi: 10.1002/dvdy.20422. [DOI] [PubMed] [Google Scholar]
- Rubin JB, Choi Y, Segal RA. Cerebellar proteoglycans regulate sonic hedgehog responses during development. Development (Cambridge, England) 2002;129:2223–2232. doi: 10.1242/dev.129.9.2223. [DOI] [PubMed] [Google Scholar]
- Sakiyama-Elbert SE, Hubbell JA. Controlled release of nerve growth factor from a heparin-containing fibrin-based cell ingrowth matrix. J Control Release. 2000a;69:149–158. doi: 10.1016/s0168-3659(00)00296-0. [DOI] [PubMed] [Google Scholar]
- Sakiyama-Elbert SE, Hubbell JA. Development of fibrin derivatives for controlled release of heparin-binding growth factors. J Control Release. 2000b;65:389–402. doi: 10.1016/s0168-3659(99)00221-7. [DOI] [PubMed] [Google Scholar]
- Schnell L, Schneider R, Kolbeck R, Barde YA, Schwab ME. Neurotrophin-3 enhances sprouting of corticospinal tract during development and after adult spinal cord lesion. Nature. 1994;367:170–173. doi: 10.1038/367170a0. [DOI] [PubMed] [Google Scholar]
- Silva GA, Czeisler C, Niece KL, Beniash E, Harrington DA, Kessler JA, Stupp SI. Selective differentiation of neural progenitor cells by high-epitope density nanofibers. Science (New York, NY. 2004;303:1352–1355. doi: 10.1126/science.1093783. [DOI] [PubMed] [Google Scholar]
- Soundararajan P, Lindsey BW, Leopold C, Rafuse VF. Easy and rapid differentiation of embryonic stem cells into functional motoneurons using sonic hedgehog-producing cells. Stem Cells. 2007;25:1697–1706. doi: 10.1634/stemcells.2006-0654. [DOI] [PubMed] [Google Scholar]
- Soundararajan P, Miles GB, Rubin LL, Brownstone RM, Rafuse VF. Motoneurons derived from embryonic stem cells express transcription factors and develop phenotypes characteristic of medial motor column neurons. J Neurosci. 2006;26:3256–3268. doi: 10.1523/JNEUROSCI.5537-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taqvi S, Dixit L, Roy K. Biomaterial-based notch signaling for the differentiation of hematopoietic stem cells into T cells. J Biomed Mater Res A. 2006;79:689–697. doi: 10.1002/jbm.a.30916. [DOI] [PubMed] [Google Scholar]
- Taylor SJ, McDonald JW, 3rd, Sakiyama-Elbert SE. Controlled release of neurotrophin-3 from fibrin gels for spinal cord injury. J Control Release. 2004;98:281–294. doi: 10.1016/j.jconrel.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Taylor SJ, Rosenzweig ES, McDonald JW, 3rd, Sakiyama-Elbert SE. Delivery of neurotrophin-3 from fibrin enhances neuronal fiber sprouting after spinal cord injury. J Control Release. 2006;113:226–235. doi: 10.1016/j.jconrel.2006.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SJ, Sakiyama-Elbert SE. Effect of controlled delivery of neurotrophin-3 from fibrin on spinal cord injury in a long term model. J Control Release. 2006;116:204–210. doi: 10.1016/j.jconrel.2006.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomopoulos S, Zaegel M, Das R, Harwood FL, Silva MJ, Amiel D, Sakiyama-Elbert S, Gelberman RH. PDGF-BB released in tendon repair using a novel delivery system promotes cell proliferation and collagen remodeling. J Orthop Res. 2007;25:1358–1368. doi: 10.1002/jor.20444. [DOI] [PubMed] [Google Scholar]
- Tuszynski MH, Grill R, Jones LL, Brant A, Blesch A, Low K, Lacroix S, Lu P. NT-3 gene delivery elicits growth of chronically injured corticospinal axons and modestly improves functional deficits after chronic scar resection. Experimental neurology. 2003;181:47–56. doi: 10.1016/s0014-4886(02)00055-9. [DOI] [PubMed] [Google Scholar]
- Tyler-Cross R, Sobel M, Marques D, Harris RB. Heparin binding domain peptides of antithrombin III: analysis by isothermal titration calorimetry and circular dichroism spectroscopy. Protein Sci. 1994;3:620–627. doi: 10.1002/pro.5560030410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willerth SM, Arendas KJ, Gottlieb DI, Sakiyama-Elbert SE. Optimization of fibrin scaffolds for differentiation of murine embryonic stem cells into neural lineage cells. Biomaterials. 2006;27:5990–6003. doi: 10.1016/j.biomaterials.2006.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willerth SM, Faxel TE, Gottlieb DI, Sakiyama-Elbert SE. The Effects of Soluble Growth Factors on Embryonic Stem Cell Differentiation Inside of Fibrin Scaffolds. Stem Cells. 2007a doi: 10.1634/stemcells.2007-0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willerth SM, Faxel TE, Gottlieb DI, Sakiyama-Elbert SE. The effects of soluble growth factors on embryonic stem cell differentiation inside of fibrin scaffolds. Stem Cells. 2007b;25:2235–2244. doi: 10.1634/stemcells.2007-0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willerth SM, Johnson PJ, Maxwell DJ, Parsons SR, Doukas ME, Sakiyama-Elbert SE. Rationally designed peptides for controlled release of nerve growth factor from fibrin matrices. J Biomed Mater Res A. 2007c;80:13–23. doi: 10.1002/jbm.a.30844. [DOI] [PubMed] [Google Scholar]
- Wissink MJ, Beernink R, Pieper JS, Poot AA, Engbers GH, Beugeling T, van Aken WG, Feijen J. Binding and release of basic fibroblast growth factor from heparinized collagen matrices. Biomaterials. 2001;22:2291–2299. doi: 10.1016/s0142-9612(00)00418-x. [DOI] [PubMed] [Google Scholar]
- Wood MD, Sakiyama-Elbert SE. Release rate controls biological activity of nerve growth factor released from fibrin matrices containing affinity-based delivery systems. J Biomed Mater Res A. 2008;84:300–312. doi: 10.1002/jbm.a.31269. [DOI] [PubMed] [Google Scholar]
- Yim EK, Leong KW. Proliferation and differentiation of human embryonic germ cell derivatives in bioactive polymeric fibrous scaffold. Journal of biomaterials science. 2005;16:1193–1217. doi: 10.1163/156856205774269485. [DOI] [PubMed] [Google Scholar]
- Zhang F, McLellan JS, Ayala AM, Leahy DJ, Linhardt RJ. Kinetic and structural studies on interactions between heparin or heparan sulfate and proteins of the hedgehog signaling pathway. Biochemistry. 2007;46:3933–3941. doi: 10.1021/bi6025424. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Representative histograms of fluorescence activated cell sorting data for cultures seeded inside of unmodified fibrin scaffolds with no growth factors present. The gate marks the population of cells staining positive for a marker. Background staining for the secondary antibody alone controls was set to 1%. A) Unstained live cells B) Cells stained with secondary IgM antibody only C) Cells stained with SSEA-1 antibody D) Cells stained with O4 antibody E) Unstained fixed cells F) Cells stained with secondary IgG only G) Cells stained with Nestin antibody H) Cells stained with β-tubulin III antibody I) Cells stained with anti-rabbit antibody J) Cells stained with GFAP antibody
Representative histograms of fluorescence activated cell sorting data for cultures seeded inside of fibrin scaffolds containing the delivery system along with the growth facto combination PDGF and NT-3. The gate marks the population of cells staining positive for a marker. Background staining for the secondary antibody alone controls was set to 1%. A) Unstained live cells B) Cells stained with secondary IgM antibody only C) Cells stained with SSEA-1 antibody D) Cells stained with O4 antibody E) Unstained fixed cells F) Cells stained with secondary IgG only G) Cells stained with Nestin antibody H) Cells stained with β-tubulin III antibody I) Cells stained with anti-rabbit antibody J) Cells stained with GFAP antibody