Abstract
The Plasmodium falciparum apical membrane antigen 1 (AMA1) is a leading vaccine candidate and was tested for safety and immunogenicity in human Phase I Clinical Trials. PBMC from vaccine recipients were analyzed by flow cytometric methods to determine the nature of T cell responses and AMA1-reactive memory T cells. Both CD4 and CD8 T cells produced a number of cytokines following AMA1 re-stimulation, with IL-5-producing cells at the highest frequency, consistent with a Th2 bias. The relative frequency of multifunctional cells synthesizing Th1 cytokines IFN-γ, IL-2 and TNF-α changed after each vaccination. Interestingly, median fluorescence intensity measurements revealed that cells producing more than one cytokine contributed greater quantities of each cytokine than cell populations that produced each of the cytokines alone. AMA1 vaccination also elicited the development of memory cell populations, and both central and effector memory T cells were identified concurrently after the AMA1 vaccination. The detailed profile of multifunctional T cell responses to AMA1 presented here will advance our ability to assess the immunogenicity of human malarial vaccines.
Keywords: Plasmodium falciparum apical membrane antigen 1 (PfAMA1), Multifunctional T cell responses, Central and effector memory T cells
Introduction
Malaria is a serious public health problem in tropical regions of the world and is responsible for about 1.5 million deaths yearly, mainly among sub-Saharan children under 5 years old [1]. Four species of Plasmodium, the etiologic agents of malaria, infect humans and P. falciparum is the species that has the most severe clinical consequences. In light of increasing anti-malarial drug resistance and the limited success of mosquito control programs, an effective vaccine is likely to be an important tool to decrease the burden of malaria.
P. falciparum apical membrane antigen 1 (PfAMA1) is an integral membrane protein of 83-kilodalton (kDa) that is processed to 66-kDa during late schizogony in the erythrocytic cycle of the parasite before being exported from micronemes to the merozoite surface [2]. Recent studies have revealed that AMA1 is also found in sporozoites [3]. AMA1 is thought to participate in the re-orientation and attachment of merozoites to red blood cells, a crucial phase in red blood cell invasion by the parasite [4]. AMA1 vaccination of mice and monkeys has been demonstrated to reduce parasitaemia and confer significant protection after challenge with virulent parasites, indicating the critical role of this protein during invasion [5–8]. As recently reviewed by Remarque and colleagues [9], these observations have provided a rationale for the development of recombinant AMA1 as a vaccine candidate. The mechanisms of protection are not completely known but include the generation of antibodies that block merozoite entry into red blood cells, inhibition of parasite growth and induction of parasite-directed cellular immunity [5, 8, 10, 11].
Inadequate knowledge about surrogate markers of protection against P. falciparum infection has been a major obstacle to the rational design of malarial vaccines and has complicated measurements of vaccine efficacy. Many studies have focused on antibody responses following vaccination with blood stage vaccine candidates, but a better understanding of cellular responses should assist in the development of more effective blood malaria vaccine candidates.
A number of publications have described techniques that can be used to evaluate the cellular responses and kinetics of memory responses to immunogens in vaccinated human volunteers. The IFN-γ ELISPOT is the most widely used technique [12–15]. Analysis of single-cytokine intracellular detection by flow cytometry has been used as a preferred technique in recent studies as well [12, 14]. However, these analyses provide only a partial view of the quantitative and qualitative aspects of antigen-specific T cell responses.
Recently, a study using the mouse model of leishmaniasis suggested that multifunctional cellular responses may be important in the development of protective immunity. This study showed that multifunctional CD4 T cells secreting IFN-γ, IL-2, and TNF-α may predict vaccine efficacy, memory formation and may ultimately be required for mounting a protective immune response [16]. This approach also has been applied to HIV infection [17] and vaccinia virus immunization [18].
In addition to cytokine secreting T cell frequencies, the identification of memory T cell populations elicited by vaccination would provide us with another parameter for measuring vaccine immunogenicity. Our understanding of the heterogeneity of memory T cells that are generated by natural infection and vaccination, based on phenotypic and functional markers has advanced [19, 20]. Effector memory T cells (Tem) migrate into tissues and produce cytokines that regulate effector functions of the immune responses. These cells express high levels of CD45RO but levels of L-selectin (CD62L) or the chemokine receptor CCR7 are low (or absent). Central memory T cells (Tcm) migrate through lymph nodes and are like effector memory T cells, but express high levels of the markers CD62L and CCR7. Naïve T cells also express CD62L and CCR7 but, in contrast to central memory T cells, are CD45RO negative and CD45RA positive. Using flow cytometry, it is possible to identify these memory cell subpopulations phenotypically and follow changes in these after vaccination.
The goal of this study was to perform a detailed characterization of T cell responses following vaccination of naïve human volunteers with the malaria blood stage vaccine candidate AMA1. We evaluated cellular responses using multiparameter flow cytometry and identified T cells that produced IFN-γ, IL-2 and TNF-α or combinations of these cytokines, while delineating the kinetics of AMA1-specific cytokine-producing T cell responses. We also assessed the development of memory T cell responses, demonstrating the concurrent induction of central and effector memory CD4 T cells after vaccination with AMA1. These data, derived using a novel approach to the study of AMA1-induced T cell responses in humans, provide a basis for assessment of the immunogenicity of blood stage malaria vaccines.
Materials and Methods
Study population and study sites
Samples for this study were obtained from volunteers participating in two phase 1 clinical trials of AMA1 formulated with aluminum hydroxide (Alhydrogel™) conducted in the National Institutes of Health Clinical Center (NCT00114010; www.clinicaltrials.gov; 3 enrolled volunteers who completed the vaccination schedule) and the University of Rochester ( NCT00344539: www.clinicaltrials.gov; 7 volunteers selected randomly). All subjects provided informed consent for samples collected for this study. Plasma of the volunteers was obtained at the time of enrollment and tested for anti-AMA1 antibodies with standard ELISA and showed they had no response to this antigen. The trials were conducted under an Investigational New Drug (IND) applications held by the Regulatory Compliance and Human Subjects Protection Branch and the Division of Microbiology and Infectious Diseases of the National Institutes of Allergy and Infectious Diseases at the National Institutes of Health. Related documents for both protocols were reviewed and approved by the National Institute of Allergy and Infectious Diseases Institutional Review Board and the University of Rochester Research Subjects Review Board.
Vaccination schedule and isolation of peripheral blood mononuclear cells (PBMC)
Blood samples were obtained from ten volunteers who were vaccinated with a combination of the polymorphic forms of AMA1, FVO and 3D7 (equal mixture of FVO and 3D7 allelic forms of the protein, by weight), designated AMA1-C1, formulated on Alhydrogel® on days 0, 28 and 56. In the study conducted at the NIH Clinical Center, 3 volunteers completed the vaccination schedule, and cells obtained from apharesis procedures after each vaccination were used to optimize flow cytometric methods; these samples also contributed to the analysis dataset. A subset of 7 volunteers from the second protocol conducted at the University of Rochester (NCT00344539: www.clinicaltrials.gov) [21] provided blood samples for analysis. All volunteers had the same vaccination schedule (Fig 1A). PBMC were isolated from whole blood collected one week after each vaccination and at additional time points up to day 140 post -vaccination according to the manufacturer’s protocol (BD Vacutainer® CPT tubes, Becton Dickinson, San Jose, CA). PBMC were stored in liquid nitrogen in heat inactivated fetal bovine serum (Gibco, Invitrogen Co. Carlsbad, CA) containing 8% dimethylsulfoxide (Sigma-Aldrich Co., St. Louis, MO) until analysis.
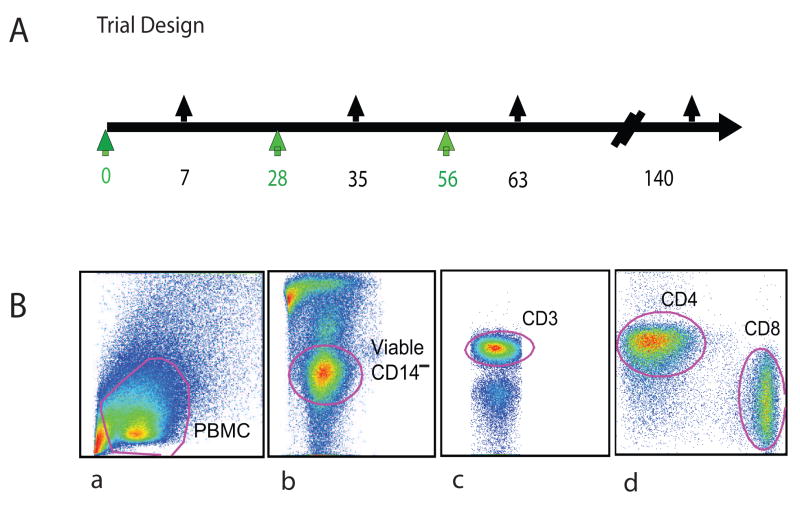
Figure 1.
(A) Study Design. AMA1-C1 (FVO+3D7) formulated with Alhydrogel® were administered at a dose of 80 μg IM to healthy American volunteers. Black arrows on the top of the time line indicate blood sampling time points in vaccinees. (B) The gating strategy for the analysis of cytometry data. For PBMC, cell debris was excluded first (a), followed by exclusion of non-viable cells and monocytes (b); the lymphocytes were identified (c), and CD4 and CD8 markers were then used to identify two populations for further characterization (d).
Processing and in vitro stimulation of PBMC
Cells were thawed in RPMI 1640 (Gibco) containing 50U/mL DNAse (Benzonase® nuclease, Novagen, WI), washed and resuspended in complete medium (RPMI 1640 containing 10% heat inactivated fetal bovine serum, Invitrogen), and incubated at 37°C with 5%CO2 in air for 24 h without stimulation. Recombinant AMA1 was produced in Pichia pastoris under cGMP conditions [11], and tested at different concentrations to re-stimulate PBMC (2×106 cells/mL). 10μg/mL was the optimal re-stimulation concentration to distinguish specific responses between vaccines and naïve volunteers. The mitogens phorbol myristoyl acetate (PMA) and ionomycin (Sigma-Aldrich Co., St. Louis, MO) at concentrations of 10 ng/ml and 500 ng/mL, respectively, were used as positive controls to assess maximal activation state or production capacity (of cytokines) for each sample.
Antibodies and flow cytometry
Co-stimulatory antibodies CD28, CD49d and Golgiplug (BD Biosciences, San Diego, CA) were added in the last 12 h of stimulation, as recommended by the manufacturers. The antibody conjugates CD14-Pacific Blue, CD8 -PE Cy7, IFN-γ-Alexafluor 647, TNF-α-Alexafluor 700, IL-5-APC, CD4-APC-Cy5.5, CCR7-PE, CD62L-FITC and CD45RO-APC were obtained from BD Biosciences. The monoclonal antibody conjugates CD4-QDot 655, CD3-PE-Alexafluor 610, IL-2-Alexafluor 488 and the live/dead fixable stain kit (ViViD) were obtained from Invitrogen. Data acquisition was performed with an LSR II Cytometer (Becton Dickinson). Fluorescent beads are run on each day of analysis as quality assessment of the LSP II instrument; and compensation beads (CompBeads BD Biosciences) were used to determine the compensation parameters of each fluorochrome. For the analysis 300,000 events were acquired; non-viable cells (ViViD+) and monocytes (CD14+) were excluded, and lymphocytes were gated with CD3. CD4 and CD8 were used to identify the two subpopulations of T cells (Fig. 1B).
Data analysis
Flow cytometric data were analyzed using FlowJo version 8.7.1 (Tree Star, Inc), and SPICE 4.1.6 (developed by Mario Roederer, Vaccine Research Center, NIAID/NIH). One-way ANOVA with Dunnett’s post test was performed for comparisons between pre-and post-vaccination time points, using GraphPad Prism version 5.00 for Windows (GraphPad Software, San Diego, CA USA).
Results
Kinetic analysis of ex vivo cytokine-producing CD4+ T cells
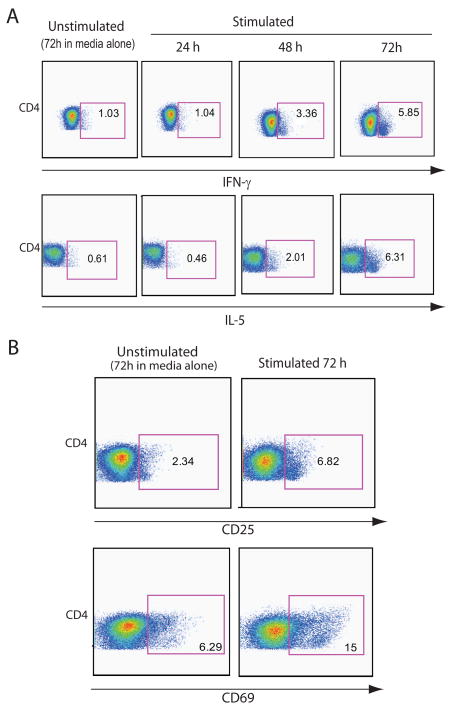
Experiments were conducted to determine the kinetics of the production of the cytokines of interest and establish the best time interval of PBMC culture for detection of cytokines in T cells. IFN-γ and IL-5 production in CD4 T cells was evaluated at 0, 24, 48 and 72 h after AMA1 re-stimulation. For both cytokines, a 72 h incubation showed the highest levels of cytokine response to AMA1 re-stimulation by flow cytometry (Fig. 2A), with increases of 5 and 10 fold, respectively, when compared to 24 h of stimulation. Cells from 3 different donors were evaluated and all showed similar kinetics in two independent experiments. The stimulation markers CD25 and CD69 were evaluated in in addition to cytokines in the same T cell subpopulation, and our results indicated that 72 h-incubation showed the highest expression of these markers among the time points studied (Fig. 2B). The pattern of cytokine production is consistent with and similar to the patterns previously described for CD8 T cell cytokine responses to SEB stimulation of human cells [22].
Figure 2.
Kinetics of CD4+T cell responses. (A) Ex vivo PBMC cytokine-secreting cell frequencies in a representative subject are shown one week after receiving the third vaccination. PBMC were thawed, stimulated with medium alone or AMA1 (10 μg/ml) for 24, 48 and 72 h; unstimulated control cells cultured for 72h are shown for comparison. Plots for CD4 T cells producing intracellular IFN-γ or IL-5 are shown at various times after in vitro stimulation. (B) Highest expression of the activation markers CD25 (IL-2R) and CD69 in CD4 T cells was detected after 72 h of stimulation with AMA1.
A subset of samples was tested for production of IL-5 with the recombinant forms of AMA1-FVO and AMA1-3D7 separately, IL-5 production was comparable for both polymorphic forms (Supplementary Table I). Subsequent experiments reported here, were carried out with an equal mixture of the 2 polymorphic forms of AMA1 (AMA-C1) to re-stimulate, of the same source and quality as the vaccine formulation.
Frequencies of IL-5 producing CD4 T cells increase following vaccination
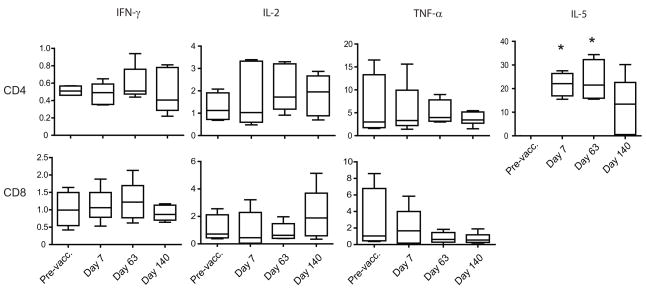
We determined the frequencies of IFN-γ, TNF-α, IL-2 or IL-5-cytokine-producing cells in the 10 PBMC samples stimulated with AMA1, to range from 0 to 4% of the total CD4 or CD8 T cell subsets (absolute frequencies) based on flow cytometric analysis. For this analysis, the frequencies of cytokine-producing populations were estimated after subtraction of values obtained in unstimulated PBMC (Fig 3). No significant changes were detected in the frequencies of IFN-γ-producing cells as a proportion of CD4 or CD8 T cells after vaccination compared to pre-vaccination frequencies. High baseline values for TNF-α-secreting cell frequencies were found in both CD4 and CD8 T cells as compared to other cytokines, but no significant change followed vaccination. IL-2-producing cell frequencies doubled in both CD4 and CD8 T cells, but differences were not found to be statistically significant. IL-5-producing cells were undetectable before vaccination but appeared after immunization, reached a peak of 21% of total CD4 T cells one week after the third vaccination (Dunnett’s test, p<0.01), and decreased to 12% of CD4 T cells by day 140. The higher frequency of IL-5 producing cells indicated a predominant Th2 type response, while other cytokines did not show statistically significant increases.
Figure 3.
Absolute frequencies of CD4 and CD8 cytokine-producing T cells. PBMC of vaccinated volunteers (n=10) were collected pre-vaccination and on days 7, 63 and 140 of the study. Cells were stimulated with AMA1 for 72h, and intracellular staining for IFN-γ, IL-2, TNF-α and IL-5 allowed determination of the frequencies of cytokine-producing populations. The horizontal lines within the boxes indicate median values; the boxes indicate 95% confidence interval, and the error bars indicate SEM. Pre-vacc: Pre-vaccination. Data are corrected for background frequencies. * Significantly different from pre-vaccination frequency, Dunnett’s test, p<0.01
Th1 cytokine responses are also elicited by vaccination with AMA1 in CD4 or CD8 T cells
Recent reports suggest the importance of multifunctional Th1 cells in the establishment of immune responses upon natural infection and vaccination to a number of infectious diseases [23]. We used multiparameter cytometry to evaluate the production of the Th1 cytokines IFN-γ, IL-2, and TNF-α in PBMC from 10 vaccinated volunteers, and identified populations that produced combinations of these cytokines. Multifunctional Th1 cell analysis was carried out in this study on Th1 cytokine producers which comprised less than 4% of total T cells. The profiles of pre-vaccination (day zero) and post vaccination time points were compared after subtraction of values obtained with unstimulated controls (the background). Comparing these time points, our results indicated that both Th1 cytokine-secreting CD4 and CD8 T cells were activated after vaccination and their cytokine profiles changed when re-stimulated in vitro with AMA1, in terms of relative proportions of cytokine producing cells (Fig. 4).
Figure 4.
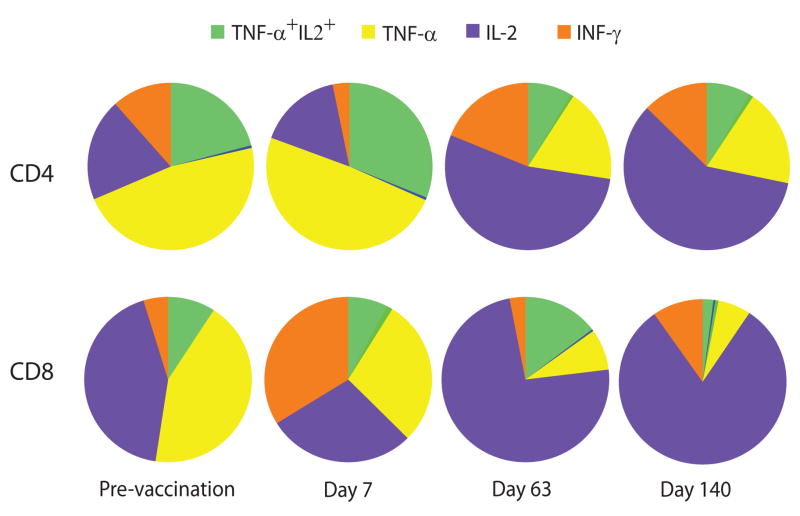
Multifunctional T cell population patterns after vaccination. Proportion of CD4 and CD8 T cells producing IFN-γ, IL-2, TNF-α, and their combinations were assessed after stimulation with AMA1 antigen in PBMC (n=10) isolated on days 0, 63 and 140. Pie charts summarize the relative proportion of the three cytokines in CD4 and CD8 T cells. Data are corrected for corresponding background frequencies of unstimulated cells.
Analysis of the relative frequencies of CD4 T cells producing combinations of these cytokines revealed that TNF-α-secreting cells constituted the largest subpopulation of cytokine-secreting cells; however, the size of this subpopulation gradually decreased and appeared to do so in inverse proportion to an increase in the frequencies of IL-2-producing cells. TNF-α+IL-2+ (double-producing) cells constituted about 20% of the total CD4 T cells and although the proportion of these cells increased after the first vaccination, at later points, only single IL-2-producing cells were detected. Frequencies of IL-2-producing T cells increased after the third vaccination and in some cases their frequencies approached more than 70% of Th1 CD4 T cell responses. A modest increase in IFN-γ-producing cells was found only after the third vaccination. The frequencies of sub-populations categorized as IFN-γ+ TNF-α+, IFN-γ+IL-2+ and triple positive (IFN-γ+ TNF-α+IL-2+ cells) were negligible and did not change after vaccination.
CD8 T cells producing only TNF-α constituted the largest subpopulation comprising ~45% pre-vaccination response but diminished after vaccination to only 15% of CD8 T cells by day 140. IFN-γ-producing CD8 T cells constituted ~25% of CD8 T cell frequencies, but this subpopulation increased after vaccination, reaching a peak on day 63 (50%) and then gradually decreasing towards day 140. Frequencies of IL-2-producing CD8 T cells showed striking increases similar to CD4 T cells. Of note, about 5% of CD8 T cells were IL-2+ TNF-α+ (double positive), and 3% were IFN-γ+IL2+; the percentage of these double positive and triple positive (IFN-γ+ TNF-α+IL-2+) cells remained low after vaccination (not shown).
Multifunctional T cells produce more cytokine per cell than single cytokine producers
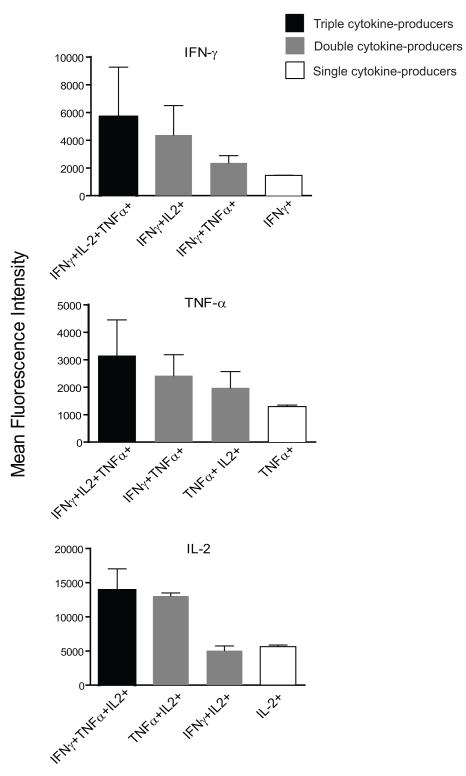
The median fluorescence intensity (MFI) of cytokines is directly related to quantitative expression of cytokine content on a per-cell basis [16]. We compared the MFI in 8 PBMC samples for each cytokine in all the combinations of cytokines analyzed and found that for CD4 T cells, albeit low in frequencies, the MFI indicates that TNF-α and IL-2 content was 2.5 times higher in triple cytokine producers than in single producers. IFN-γ production was 3-times higher in triple producers when compared to single IFN-γ producers (Fig 5). For IL-2, double producers (IL-2+ TNF-α+) showed comparable amounts of IL-2 in terms of median MFI as compared to triple producers (13846 versus 13029 MFI units). Similar analysis in CD8 T cells confirmed that triple producers produced significantly higher levels of IFN-γ, TNF-α and IL-2 (data not shown). Evaluation after 3 vaccinations with AMA1 showed a hierarchy of cytokine-producing cells, such that cells producing three cytokines produce more of each cytokine than those that produce two cytokines, and the latter produce more cytokine than cells producing a single cytokine. This finding was most consistent for IFN-γ expression.
Figure 5.
Median fluorescence intensity (MFI) of cytokines in CD4 T cells. Bars indicate the median and standard errors of fluorescence intensity values in different populations of IFN-γ-, IL-2-, and TNF-α-producing cells (n=8). Shown are the values obtained on samples collected one week after the third vaccination (Day 63), following in vitro re-stimulation with AMA1. Triple cytokine producers are shown in black bars, double cytokine producers in gray and single cytokine producers in open bars.
T cell functionality as measured by integrated median fluorescence intensity (iMFI)
The integrated median fluorescence intensity (iMFI) is a parameter that accounts for both the frequency of cells producing a given cytokine and quality of the cytokine response measured by the median fluorescence intensity. It is determined by multiplying the frequency by the MFI and has been suggested to be a better predictor of the overall effectiveness of the vaccine [16, 23].
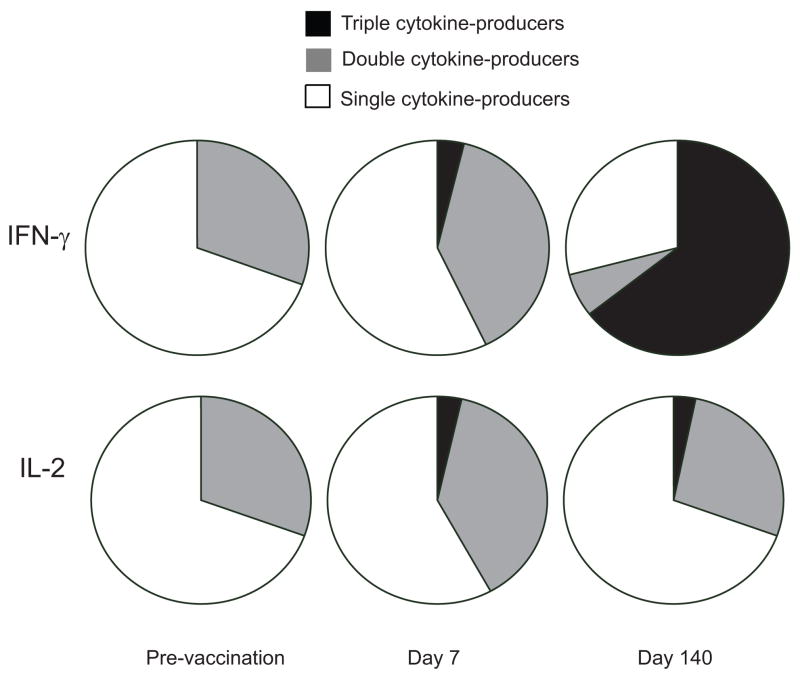
We calculated iMFI for the three cytokines assessed in CD4 T cells before and after vaccination, and determined the contribution of triple-, double-, and single-cytokine producers. We found single IFN-γ producers contributed to about 70% of IFN-γ-producers prior to vaccination and the remaining 30% was produced by IFN-γ+TNF-α+ CD4 T cells. However, following the first vaccination, IFN-γ+TNF-α+ subpopulation increased to 45%. There were no triple-cytokine producers (IFN-γ+TNF-α+IL-2+) before vaccination but IFN-γ in triple producers increased to about 7% after the first vaccination (day 7) and further increased to 60% on day 140 (Fig. 6). iMFI calculations over the course of vaccination indicated that IFN-γ production correlates to the multifunctional characteristic of cells better than TNF-α and IL-2.
Figure 6.
IFN-γ and IL-2 production induced by AMA1 vaccination. The contribution of different populations to total cytokine production is illustrated in pie charts, based on the iMFI values, a calculated parameter that accounts for both the frequency of cells producing a given cytokine and magnitude of the cytokine response reflected by the median fluorescence intensity. For these data, PBMC obtained on day zero, 7 and 140 were re-stimulated with AMA1 for 72h, then stained and analyzed (n=10).
In contrast to IFN-γ and TNF-α, IL-2 was produced mainly by single-cytokine positive cells, with 30% IL-2 production from TNF-α+IL-2+ CD4 T cells, as reflected by iMFI values. Less than 10% of total IL-2 production was observed in triple-cytokine producers 7 days after the first vaccination and remained at the same level until day 140 (Fig. 6).
TNF-α was the predominant cytokine in CD4 T cells, and in contrast to IFN-γ, single TNF-α producing T cells accounted for more than 75% of the iMFI measurement. A similar iMFI pattern was observed for TNF-α pre-vaccination and post-vaccination (data not shown).
Memory T cell development after AMA1 vaccination
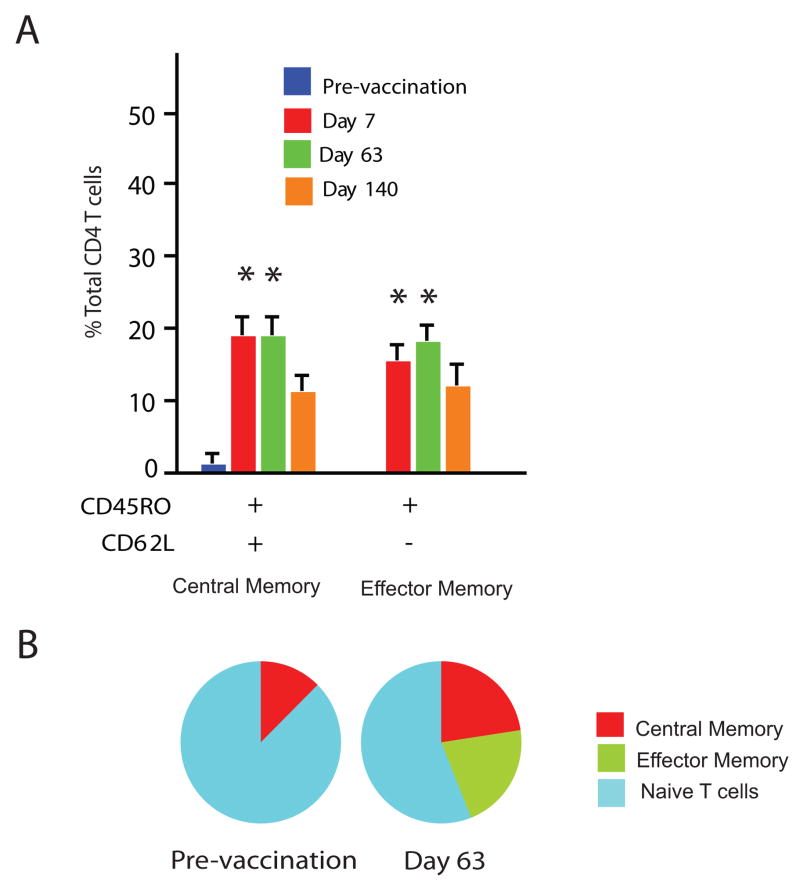
Expression of CD45RO and CD62L markers in CD4 T cells indicated a comparable increase in both effector and central memory subpopulations, as early as one week after the first vaccination (Dunnett’s test, p<0.01; Fig. 7). Effector memory CD4 T cells (CD45RO+, CD62L−) increased rapidly after vaccination, eventually representing about 20% of CD4 T cells and were maintained up to day 140, the last time point analyzed. Central memory CD4 T cells (CD45RO+, CD62L+) also showed significant increases after vaccination in a pattern similar to that of effector memory T cells.
Figure 7.
Characterization of CD4 memory T cells elicited by AMA1 vaccination. (A) PBMC were re-stimulated with AMA1 and cultured for 72 h, and then stained for markers of memory T cells (defined by expression patterns of CD45RO and CD62L). Frequencies of CD45RO+CD62L+ (representing Tcm) and CD45RO+CD62L− (representing Tem) are shown as means with standard errors from 10 PBMC samples. * Significantly different of from pre-vaccination frequency, Dunnett’s test, p<0.01. (B) Establishment of memory CD4 T cell populations following AMA1 vaccination. Pie charts summarizing the effect of AMA1 vaccination show the proportion of central memory, effector memory and naïve T cells (CD45RO-CD62L−) pre-vaccination and on day 63.
Discussion
In this study we evaluated T cell responses to malarial antigens following vaccination with a recombinant blood stage malarial antigen (AMA1) formulated with aluminum hydroxide. Samples were obtained from two clinical trials, but the vaccination schedule, antigen formulation, and study populations were essentially identical. Presence of anti-AMA1 antibodies was tested and found to be negative pre-vaccination, and 2 weeks after receiving the second and third vaccination all volunteers responded to the polymorphic forms AMA1-FVO and AMA1-3D7, as determined by both IgG antibodies and antigen-driven cytokine secretion (Supplementary Table I). Here, we focused on T cell responses which were analyzed using an approach that identifies multifunctional Th1 cells (producing IFN-γ, IL-2 and TNF-α and combinations of these cytokines) as a novel readout of vaccine-elicited T cell functionality for malarial antigens. Additionally we measured memory T cell subpopulations following each of three immunizations with the malaria antigen in a naïve population. To our knowledge, this is the first report detailing the development of malaria-associated multifunctional T cells in Ag-naïve humans.
Cellular immunity against malarial antigens has been evaluated in earlier studies, including proliferation and IFN-γ production by ELISPOT assay as measures of specific immune responses [12–15]. Doubts have been expressed about the use of ex vivo IFN-γ ELISPOT data as the most appropriate method for evaluating protective immune responses, since the frequency of IFN-γ secreting cells usually wanes and may disappear within weeks [24, 25]. Some investigators have suggested that IFN-γ ELISPOT assays performed on PBMC cultured in vitro for 10 days may provide a better correlate for memory responses and protection against malaria [25], but these assays only measure one cytokine. IL-2 is well known for its role in the induction of T-cell proliferation and recent studies have shown that the frequencies of cells that secrete a combination of IFN-γ and IL-2 were more closely associated than IFN-γ alone with immunogenicity and establishment of memory in malaria [26], and other infectious diseases [27, 28]. Intracellular staining for IFN-γ-secreting cell frequencies in whole blood samples have been utilized in the evaluation of responses to Bacillus Calmette-Guerin (BCG) in studies of vaccines against tuberculosis [29]. However, the studies performed limited analysis of cytokine secreting frequencies, focusing mainly on phenotyping of T cell subsets by surface marker expresssion. Other studies have concluded that the frequencies of T cells producing a combination of cytokines, chemokines and degranulation markers provide a better measurement of a robust immune response that may correlate with efficacy in vaccines against viral [18, 30], bacterial [31, 32] and parasitic infectious [16]. Our goal in the present study was to characterize multifunctional T cell responses, with the hope of adapting the approach to whole blood samples in the future, which would be useful in field studies of malaria vaccines.
Aluminum hydroxide has been reported to induce predominantly Th2-biased T cell responses [33–35] and, consistent with this, we found absolute frequencies of AMA1 stimulated IL-5 producing CD4 T cells to be about 20-fold higher than IFN-γ, or 3 fold more than IFN-γ and IL-2 (double-producing) cells. A recent report describing cellular immune responses to AMA1 vaccine formulated in Alhydrogel, showed increases in IFN-γ and IL-5 secretion, and a mean ratio of ELISPOT frequencies of IFN-γ/IL-5 of 1.16. In contrast, they reported more Th1-biased responses when the same antigen was formulated with Montanide ISA 720 or with AS02 as adjuvants [36]. The significance of the expansion of IL-5 producing T cells following vaccination in humans is unknown, but in combination with IL-4, IL-5 may play an important role in generation and regulation of antibody responses.
Our results evaluating Th1 cytokine-producing cells after in vitro AMA1 stimulation demonstrated that triple-cytokine producers were very low in frequency, even after 3 vaccinations. Among double cytokine producers, the population with the highest frequency was the TNF-α+IL-2+ double-positive subpopulation in both CD4 and CD8 T cell populations. Of the single cytokine producing cells, single TNF-α-producers had the highest baseline frequency within CD4 or CD8 T cell populations as previously reported [37, 38].
Although the absolute frequencies of total Th1 cytokine-producers showed no significant changes after immunization, the proportions of Th1 cytokine producing responders revealed an evolution that may represent differentiation or maturation of an immune response in both CD4 and CD8 T cells (Fig. 4). In CD4 T cells, the relative frequency of single TNFα-producing cells decreased whereas IL-2-producing cells increased strikingly from ~15% prior to vaccination (baseline) to ~80% by day 140. A transient change was detected after first vaccination where double positive TNF-α+IL-2+ producing cells increased. The proportional frequency of this double positive cell population diminished, and may have later evolved to a dominant population of single IL-2-producers. Previous studies found a clear relationship between the role of IL-2 in cell proliferation and longevity of T cells and suggested that IL-2-only secreting cells are typical of central memory T cells that persist after antigen clearance [27]. It has been further suggested that TNF-α/IL-2 producing T cells and single-IL-2-producing cells may constitute a “reservoir” population of memory T cells that can rapidly proliferate and differentiate to effector T cells upon re-exposure to infectious agent [39, 40]. Our findings are consistent with this model because of the high frequencies of these two populations and detection of memory markers.
Comparison of MFI values one week after the third vaccination suggested a hierarchy of cytokine secretion: triple cytokine-producing cells, albeit very low in absolute frequencies, produced more cytokine on a per cell basis than double-producers and the latter produced more cytokine than the single producers. This hierarchy is particularly consistent for IFN-γ production. In an animal model of leishmaniasis the iMFI values (a product of MFI by frequencies) calculated for IFN-γ correlated with protection [16]. In our experiments, AMA1 vaccination resulted in a striking change in iMFI values for CD4 T cells. These findings suggest that analysis of the product of frequencies and MFIs may be a superior approach to evaluate T cell responses to malarial vaccines or natural infection, since frequencies alone give an incomplete picture of T-cell responses. Further studies with larger numbers of samples from either case-controlled study during natural infection, or following vaccination and challenge, will help us determine if such correlations exist.
Interestingly, our results indicated that in the CD8 T cell population, the proportion of IFN-γ producing cells increased 5–6 fold after the first vaccination and, as in the CD4 T cell population mentioned above, an expanded single-IL-2-producing population appeared later. Thus, CD8 T cells also underwent cytokine profile changes after AMA1 vaccination, although it has been widely accepted that blood stage antigens are almost exclusively presented to CD4 T cells. This finding may be explained by cross-presentation, a mechanism that involves uptake and processing of exogenous antigens within the major histocompatibility complex class I pathway [41]. Of note, De Rosa and colleagues found that hepatitis B virus (HBV) immunization in humans may also induce significant CD8 responses, nearly identical in magnitude to CD4 responses [37]. Future cellular studies on malaria will help to confirm if other blood stage malaria antigens are also capable of eliciting CD8 T cell responses.
We characterized the memory subpopulations based on CD45RO and CD62L expression. Comparison of the expression patterns before and after vaccination indicates that both central and effector memory responses are induced by AMA1 vaccination and that these memory subpopulations are evident as early as one week after the first vaccination. Most of our knowledge of the development of T cell memory is based on mouse models, particularly viral infection models inducing CD8 memory T cells [42], while the maintenance and functional characteristics of memory CD4 T cells are less clear. The appearance of both CD4 Tcm and Tem memory populations within 1 week of the first vaccination is consistent with a divergent model of memory cell generation as suggested previously [43]. In a study using tetanus toxoid re-immunization with a technical approach similar to ours, Tem could be identified as early as 5 days after re-immunization [44], indicating that once a specific memory population has been established, rapid proliferation can occur upon to exposure to the target antigen. Malaria studies focusing on memory T cell populations will be important to elucidate the role of these different populations for protection, and to identify the determinants of longevity of memory responses.
In conclusion, we have demonstrated that AMA1/Alhydrogel® vaccination stimulates multifunctional cytokine producing cells in both CD4 and CD8 T cell populations. We have described the unique cytokine patterns induced by AMA1 vaccination, and also have shown that Ag-specific memory T cells, both Tem and Tcm, develop soon after immunization and can be detected in peripheral blood. These results demonstrate a successful new approach to the evaluation of cell-mediated immunogenicity of blood stage malaria vaccine candidates, which can be extended to other vaccine formulations with AMA1 and other parasite antigens. The detailed analysis of cellular immune responses based on cytokine profiles and memory cell development in this study enhances our ability to compare immune responses to specific malaria antigens qualitatively and quantitatively both in naïve individuals and in children and adults in malaria endemic areas.
Supplementary Material
Supplementary Table 1 legend. A. IgG responses to constituent polymorphic forms of AMA1 in recipients of the AMA1-C1 vaccine.
Acknowledgments
Authors thank David Stephany, Manager of the flow cytometry, NIAID, NIH, for guidance on the multi-parameter cytometry. We thank Malaria Vaccine Development Branch (MVDB), NIAID, NIH for the production and characterization of recombinant AMA1 protein. We gratefully acknowledge the assistance provided by Diane Rock, JoAnn Mican, Cathy Rehm, and members of the 8th floor clinic teams at the Clinical Center, NIH; Caroline Nolan and John Treanor at University of Rochester; Louis Miller, Head of the MVDB and Ruth Ellis at MVDB for their help in conducting the Clinical Trials. Thanks to Andrew Orcutt at MVDB for anti-AMA1 IgG ELISA test on volunteer’s plasma. We are grateful to the Division of Microbiology and Infectious Disease (DMID/NIAID/NIH) for sponsoring the clinical trial at the University of Rochester and we thank Abdi Naficy and Lee Hall of DMID for facilitating this trial. We thank Christiana Fogg for critical comments in the manuscript. We are grateful to the volunteers who participated in the clinical trials and provided samples for laboratory analysis.
Grant support
The Intramural Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health supported this study.
Abbreviations in this study
- AMA1
Apical membrane antigen 1
- PBMC
peripheral blood mononuclear cells
- APC
Allophycocyanin
- Cy-5 or Cy-7
Cy-chrome 5 or 7
- Th1
T helper 1
- Th2
T helper 2
- MFI
Median fluorescence intensity
- iMFI
integrated median fluorescence intensity
- PMA
Phorbol 12-myristate 13-acetate
- Tem
Effector memory T cells
- Tcm
Central memory T cells
- KO
knock-out
Footnotes
Data shown are ELISA units assigned with a standard serum [11]. AMA1-FVO and AMA1-3D7 are the two polymorphic forms of AMA1 that constitute the vaccine formulation AMA1-C1. Samples were collected on days 42 and 70 of the study (2 weeks after second and third vaccinations, respectively). Subjects labeled "CCxx" particpated in the NIH study, and those labeled "Rxxx" are from the Rochester study. B. IL-5 secreting cell frequencies specific for the two constituent polymorphic AMA1 forms used in the AMA1-C1 vaccine. Data represents the number of spot-forming units (SFU) per 1000 PBMC determined by Elispot assays for IL-5 [35]. Samples were collected on days 35 and 63 of the study (1 week after second and third vaccinations, respectively). Subjects labeled "CCxx" participated in the NIH study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rowe AK, Rowe SY, Snow RW, Korenromp EL, Schellenberg JR, Stein C, et al. The burden of malaria mortality among African children in the year 2000. Int J Epidemiol. 2006 Jun;35(3):691–704. doi: 10.1093/ije/dyl027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Narum DL, Thomas AW. Differential localization of full-length and processed forms of PF83/AMA-1 an apical membrane antigen of Plasmodium falciparum merozoites. Mol Biochem Parasitol. 1994 Sep;67(1):59–68. doi: 10.1016/0166-6851(94)90096-5. [DOI] [PubMed] [Google Scholar]
- 3.Silvie O, Franetich JF, Charrin S, Mueller MS, Siau A, Bodescot M, et al. A role for apical membrane antigen 1 during invasion of hepatocytes by Plasmodium falciparum sporozoites. J Biol Chem. 2004 Mar 5;279(10):9490–6. doi: 10.1074/jbc.M311331200. [DOI] [PubMed] [Google Scholar]
- 4.Mitchell GH, Thomas AW, Margos G, Dluzewski AR, Bannister LH. Apical membrane antigen 1, a major malaria vaccine candidate, mediates the close attachment of invasive merozoites to host red blood cells. Infect Immun. 2004 Jan;72(1):154–8. doi: 10.1128/IAI.72.1.154-158.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu H, Hodder AN, Yan H, Crewther PE, Anders RF, Good MF. CD4+ T cells acting independently of antibody contribute to protective immunity to Plasmodium chabaudi infection after apical membrane antigen 1 immunization. J Immunol. 2000 Jul 1;165(1):389–96. doi: 10.4049/jimmunol.165.1.389. [DOI] [PubMed] [Google Scholar]
- 6.Deans JA, Knight AM, Jean WC, Waters AP, Cohen S, Mitchell GH. Vaccination trials in rhesus monkeys with a minor, invariant, Plasmodium knowlesi 66 kD merozoite antigen. Parasite Immunol. 1988 Sep;10(5):535–52. doi: 10.1111/j.1365-3024.1988.tb00241.x. [DOI] [PubMed] [Google Scholar]
- 7.Cubillos M, Salazar LM, Torres L, Patarroyo ME. Protection against experimental P falciparum malaria is associated with short AMA-1 peptide analogue alpha-helical structures. Biochimie. 2002 Dec;84(12):1181–8. doi: 10.1016/s0300-9084(03)00002-6. [DOI] [PubMed] [Google Scholar]
- 8.Stowers AW, Kennedy MC, Keegan BP, Saul A, Long CA, Miller LH. Vaccination of monkeys with recombinant Plasmodium falciparum apical membrane antigen 1 confers protection against blood-stage malaria. Infect Immun. 2002 Dec;70(12):6961–7. doi: 10.1128/IAI.70.12.6961-6967.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Remarque EJ, Faber BW, Kocken CH, Thomas AW. Apical membrane antigen 1: a malaria vaccine candidate in review. Trends Parasitol. 2008 Feb;24(2):74–84. doi: 10.1016/j.pt.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 10.Narum DL, Ogun SA, Thomas AW, Holder AA. Immunization with parasite-derived apical membrane antigen 1 or passive immunization with a specific monoclonal antibody protects BALB/c mice against lethal Plasmodium yoelii yoelii YM blood-stage infection. Infect Immun. 2000 May;68(5):2899–906. doi: 10.1128/iai.68.5.2899-2906.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malkin EM, Diemert DJ, McArthur JH, Perreault JR, Miles AP, Giersing BK, et al. Phase 1 clinical trial of apical membrane antigen 1: an asexual blood-stage vaccine for Plasmodium falciparum malaria. Infect Immun. 2005;73(6):3677–85. doi: 10.1128/IAI.73.6.3677-3685.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Epstein JE, Charoenvit Y, Kester KE, Wang R, Newcomer R, Fitzpatrick S, et al. Safety, tolerability, and antibody responses in humans after sequential immunization with a PfCSP DNA vaccine followed by the recombinant protein vaccine RTS,S/AS02A. Vaccine. 2004 Apr 16;22(13–14):1592–603. doi: 10.1016/j.vaccine.2004.01.031. [DOI] [PubMed] [Google Scholar]
- 13.Peduzzi E, Westerfeld N, Zurbriggen R, Pluschke G, Daubenberger CA. Contribution of influenza immunity and virosomal-formulated synthetic peptide to cellular immune responses in a phase I subunit malaria vaccine trial. Clin Immunol. 2008 May;127(2):188–97. doi: 10.1016/j.clim.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 14.Todryk SM, Bejon P, Mwangi T, Plebanski M, Urban B, Marsh K, et al. Correlation of memory T cell responses against TRAP with protection from clinical malaria, and CD4 CD25 high T cells with susceptibility in Kenyans. PLoS ONE. 2008;3(4):e2027. doi: 10.1371/journal.pone.0002027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thompson FM, Porter DW, Okitsu SL, Westerfeld N, Vogel D, Todryk S, et al. Evidence of blood stage efficacy with a virosomal malaria vaccine in a phase IIa clinical trial. PLoS ONE. 2008;3(1):e1493. doi: 10.1371/journal.pone.0001493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Darrah PA, Patel DT, De Luca PM, Lindsay RW, Davey DF, Flynn BJ, et al. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med. 2007 Jul;13(7):843–50. doi: 10.1038/nm1592. [DOI] [PubMed] [Google Scholar]
- 17.Betts MR, Nason MC, West SM, De Rosa SC, Migueles SA, Abraham J, et al. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood. 2006 Jun 15;107(12):4781–9. doi: 10.1182/blood-2005-12-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Precopio ML, Betts MR, Parrino J, Price DA, Gostick E, Ambrozak DR, et al. Immunization with vaccinia virus induces polyfunctional and phenotypically distinctive CD8(+) T cell responses. J Exp Med. 2007 Jun 11;204(6):1405–16. doi: 10.1084/jem.20062363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401(6754):708–12. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 20.Tan JT, Surh CD. T cell memory. Curr Top Microbiol Immunol. 2006;311:85–115. doi: 10.1007/3-540-32636-7_4. [DOI] [PubMed] [Google Scholar]
- 21.Mullen GE, Ellis RD, Miura K, Malkin E, Nolan C, Hay M, et al. Phase 1 trial of AMA1-C1/Alhydrogel plus CPG 7909: an asexual blood-stage vaccine for Plasmodium falciparum malaria. PLoS ONE. 2008;3(8):e2940. doi: 10.1371/journal.pone.0002940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sandberg JK, Fast NM, Nixon DF. Functional heterogeneity of cytokines and cytolytic effector molecules in human CD8+ T lymphocytes. J Immunol. 2001 Jul 1;167(1):181–7. doi: 10.4049/jimmunol.167.1.181. [DOI] [PubMed] [Google Scholar]
- 23.Seder RA, Darrah PA, Roederer M. T-cell quality in memory and protection: implications for vaccine design. Nat Rev Immunol. 2008 Apr;8(4):247–58. doi: 10.1038/nri2274. [DOI] [PubMed] [Google Scholar]
- 24.Younes SA, Yassine-Diab B, Dumont AR, Boulassel MR, Grossman Z, Routy JP, et al. HIV-1 viremia prevents the establishment of interleukin 2-producing HIV-specific memory CD4+ T cells endowed with proliferative capacity. J Exp Med. 2003 Dec 15;198(12):1909–22. doi: 10.1084/jem.20031598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keating SM, Bejon P, Berthoud T, Vuola JM, Todryk S, Webster DP, et al. Durable human memory T cells quantifiable by cultured enzyme-linked immunospot assays are induced by heterologous prime boost immunization and correlate with protection against malaria. J Immunol. 2005 Nov 1;175(9):5675–80. doi: 10.4049/jimmunol.175.9.5675. [DOI] [PubMed] [Google Scholar]
- 26.Bejon P, Keating S, Mwacharo J, Kai OK, Dunachie S, Walther M, et al. Early gamma interferon and interleukin-2 responses to vaccination predict the late resting memory in malaria-naive and malaria-exposed individuals. Infect Immun. 2006 Nov;74(11):6331–8. doi: 10.1128/IAI.00774-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harari A, Vallelian F, Meylan PR, Pantaleo G. Functional heterogeneity of memory CD4 T cell responses in different conditions of antigen exposure and persistence. J Immunol. 2005 Jan 15;174(2):1037–45. doi: 10.4049/jimmunol.174.2.1037. [DOI] [PubMed] [Google Scholar]
- 28.Millington KA, Innes JA, Hackforth S, Hinks TS, Deeks JJ, Dosanjh DP, et al. Dynamic relationship between IFN-gamma and IL-2 profile of Mycobacterium tuberculosis-specific T cells and antigen load. J Immunol. 2007 Apr 15;178(8):5217–26. doi: 10.4049/jimmunol.178.8.5217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beveridge NE, Fletcher HA, Hughes J, Pathan AA, Scriba TJ, Minassian A, et al. A comparison of IFNgamma detection methods used in tuberculosis vaccine trials. Tuberculosis (Edinb) 2008 Nov;88(6):631–40. doi: 10.1016/j.tube.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 30.Lore K, Adams WC, Havenga MJ, Precopio ML, Holterman L, Goudsmit J, et al. Myeloid and plasmacytoid dendritic cells are susceptible to recombinant adenovirus vectors and stimulate polyfunctional memory T cell responses. J Immunol. 2007 Aug 1;179(3):1721–9. doi: 10.4049/jimmunol.179.3.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Forbes EK, Sander C, Ronan EO, McShane H, Hill AV, Beverley PC, et al. Multifunctional, high-level cytokine-producing Th1 cells in the lung, but not spleen, correlate with protection against Mycobacterium tuberculosis aerosol challenge in mice. J Immunol. 2008 Oct 1;181(7):4955–64. doi: 10.4049/jimmunol.181.7.4955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Soares AP, Scriba TJ, Joseph S, Harbacheuski R, Murray RA, Gelderbloem SJ, et al. Bacillus Calmette-Guerin vaccination of human newborns induces T cells with complex cytokine and phenotypic profiles. J Immunol. 2008 Mar 1;180(5):3569–77. doi: 10.4049/jimmunol.180.5.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grun JL, Maurer PH. Different T helper cell subsets elicited in mice utilizing two different adjuvant vehicles: the role of endogenous interleukin 1 in proliferative responses. Cell Immunol. 1989 Jun;121(1):134–45. doi: 10.1016/0008-8749(89)90011-7. [DOI] [PubMed] [Google Scholar]
- 34.Gupta RK. Aluminum compounds as vaccine adjuvants. Adv Drug Deliv Rev. 1998 Jul 6;32(3):155–72. doi: 10.1016/s0169-409x(98)00008-8. [DOI] [PubMed] [Google Scholar]
- 35.Huaman MC, Martin LB, Malkin E, Narum DL, Miller LH, Mahanty S, et al. Ex vivo cytokine and memory T cell responses to the 42-kDa fragment of Plasmodium falciparum merozoite surface protein-1 in vaccinated volunteers. J Immunol. 2008 Feb 1;180(3):1451–61. doi: 10.4049/jimmunol.180.3.1451. [DOI] [PubMed] [Google Scholar]
- 36.Roestenberg M, Remarque E, de Jonge E, Hermsen R, Blythman H, Leroy O, et al. Safety and immunogenicity of a recombinant Plasmodium falciparum AMA1 malaria vaccine adjuvanted with Alhydrogel, Montanide ISA 720 or AS02. PLoS ONE. 2008;3(12):e3960. doi: 10.1371/journal.pone.0003960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Rosa SC, Lu FX, Yu J, Perfetto SP, Falloon J, Moser S, et al. Vaccination in humans generates broad T cell cytokine responses. J Immunol. 2004;173(9):5372–80. doi: 10.4049/jimmunol.173.9.5372. [DOI] [PubMed] [Google Scholar]
- 38.Stubbe M, Vanderheyde N, Goldman M, Marchant A. Antigen-specific central memory CD4+ T lymphocytes produce multiple cytokines and proliferate in vivo in humans. J Immunol. 2006 Dec 1;177(11):8185–90. doi: 10.4049/jimmunol.177.11.8185. [DOI] [PubMed] [Google Scholar]
- 39.Wu CY, Kirman JR, Rotte MJ, Davey DF, Perfetto SP, Rhee EG, et al. Distinct lineages of T(H)1 cells have differential capacities for memory cell generation in vivo. Nat Immunol. 2002 Sep;3(9):852–8. doi: 10.1038/ni832. [DOI] [PubMed] [Google Scholar]
- 40.Zaph C, Uzonna J, Beverley SM, Scott P. Central memory T cells mediate long-term immunity to Leishmania major in the absence of persistent parasites. Nat Med. 2004 Oct;10(10):1104–10. doi: 10.1038/nm1108. [DOI] [PubMed] [Google Scholar]
- 41.Heath WR, Belz GT, Behrens GM, Smith CM, Forehan SP, Parish IA, et al. Cross-presentation, dendritic cell subsets, and the generation of immunity to cellular antigens. Immunol Rev. 2004 Jun;199:9–26. doi: 10.1111/j.0105-2896.2004.00142.x. [DOI] [PubMed] [Google Scholar]
- 42.Antia R, Ganusov VV, Ahmed R. The role of models in understanding CD8+ T-cell memory. Nat Rev Immunol. 2005 Feb;5(2):101–11. doi: 10.1038/nri1550. [DOI] [PubMed] [Google Scholar]
- 43.Song K, Rabin RL, Hill BJ, De Rosa SC, Perfetto SP, Zhang HH, et al. Characterization of subsets of CD4+ memory T cells reveals early branched pathways of T cell differentiation in humans. Proc Natl Acad Sci U S A. 2005 May 31;102(22):7916–21. doi: 10.1073/pnas.0409720102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cellerai C, Harari A, Vallelian F, Boyman O, Pantaleo G. Functional and phenotypic characterization of tetanus toxoid-specific human CD4+ T cells following re-immunization. Eur J Immunol. 2007 Apr;37(4):1129–38. doi: 10.1002/eji.200636885. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1 legend. A. IgG responses to constituent polymorphic forms of AMA1 in recipients of the AMA1-C1 vaccine.