Abstract
Objectives
(1) Estimate the incidence rates (IRs) of HIV testing among 13–64-year-old patients in US emergency departments (EDs); (2) Determine ED compliance with Centers for Disease Control and Prevention (CDC) recommendations for HIV testing for patients with non-sexual blood or body fluid exposures, sexually transmitted diseases (STDs), and sexual assaults; and (3) Ascertain if HIV testing in EDs varies by patient demographic characteristics.
Methods
ED visits from the 1993–2004 National Hospital Ambulatory Medical Care Survey databases were analyzed. Visits for non-sexual blood or body fluid exposures, STDs, and sexual assaults were identified using diagnosis and cause codes. IRs for HIV testing were estimated by year. Odds ratios (ORs) with 95% CIs were estimated from multivariable logistic regression models using HIV testing as the outcome and demographic characteristics as covariates.
Results
The average IR of HIV testing for 13–64-year-olds from 1993–2004 was 0.31%. 35.1% of patients with non-sexual blood or body fluid exposures, 20.4% with sexual assaults, and 2.6% with STDs were tested for HIV. HIV testing was more frequent among Hispanics (OR 1.39 [1.06–1.81]); blacks (OR 1.52 [1.19–1.94]); patients with Medicaid (OR 2.35 [1.81–3.03]), Medicare (OR 1.95 [1.20–3.16]), and self-pay/no charge/other type of insurance (OR 1.74 [1.35–2.23]), and those visiting EDs in the northeastern US (OR 1.57 [1.04–2.38]).
Conclusions
HIV testing rates are low in US EDs and have changed little over a twelve-year period. Compliance with CDC recommendations for HIV testing is poor and not in accordance with risk for infection. Hispanics, blacks, and those without private health care insurance are being tested more frequently than other ED patients.
1. Introduction
Over the past twenty years, the Centers for Disease Control and Prevention (CDC) has broadened their recommendations for which patients should be routinely tested for HIV in health care settings. Current recommendations for universal HIV screening have supplanted previous recommendations for targeted testing, and CDC recommendations for whom and the settings where HIV testing is recommended have expanded over the past twenty years. However, the CDC has consistently recommended HIV testing for patients seeking treatment in all health care settings for certain medical conditions: non-sexual blood or body fluid exposures1–5, sexually transmitted diseases/infections (STDs/STIs)5–11, and sexual assault7–10. Patients with these medical conditions frequently seek care in emergency departments (EDs). CDC recommendations released in 20015 and revised in 200611 have called for increased HIV testing in EDs, whether conducted for diagnostic testing or for screening. The extent on a national basis to which HIV testing is being conducted in EDs and how well EDs comply with CDC recommendations for potential exposures to HIV is not known.
The first objective of this study was to determine the incidence of HIV testing in US EDs among 13–64-year-old patients from 1993–2004. We were particularly interested if HIV testing rates have changed on a national basis in light of CDC encouragement of an expansion of HIV testing. The second objective was to ascertain ED compliance with HIV testing for medical conditions when testing has been specifically recommended by the CDC. The purpose of the secondary objective was to investigate if EDs are adequately responding to the needs of patients who might have recently been exposed to HIV. This response transcends any perceived need or current CDC recommendations for universal ED-based HIV screening. The third objective was to determine if ED HIV testing varies according to patient demography, as it does in the general US population.12 Males, whites, older adults, and those with private health insurance in the general population are less likely to have been tested for HIV. Such variation in HIV testing practices might indicate demographic groups for whom HIV testing is not being adequately considered in the ED, which leads to a perpetuation of the HIV epidemic.
2. Methods
2.1. Study design
We conducted a secondary analysis of ED data from the annual National Hospital Ambulatory Medical Care Survey (NHAMCS) of the CDC’s National Center for Health Statistics (NCHS) for the survey years 1993 through 2004.13 The ED data of the NHAMCS is a nationally-representative accounting of visits to non-federal, non-institutional, EDs located in general, short-stay medical care hospitals. The ED portion of the NHAMCS involves a four-stage sample of geographic areas within the US, hospitals within these geographic areas, EDs within these hospitals, and patient visits within these EDs. The study was approved by the hospital institutional review board.
2.2. Outcome measures and data analysis
All analyses were conducted using STATA 9.2 (Stata Corporation, College Station, TX) and SAS 9.2 (SAS Corporation, Cary, NC). These software programs conducted the recommended statistical adjustments of the data that take into account the complex four-stage sample of the NHAMCS. The adjustments included employing the suggested weighting scheme that inflated the data collected from the sample to produce unbiased national annual estimates. Recommended variance estimation procedures were also followed. Detailed information on the sampling techniques, survey content, and suggested statistical adjustment procedures can be found on the NCHS website.13 All sample sizes reflect the sample weights from the NHAMCS.
Summary statistics were computed for demographic characteristics of individual visits to EDs by patients 13–64-years-old. The CDC currently recommends universal screening for this age range of patients in all health care facilities, so we used this age range to establish a baseline of comparison to future studies that assess HIV testing in EDs conducted after the release of these 2006 recommendations.11 Incidence rates (IRs) with corresponding 95% confidence intervals (CIs) of HIV testing conducted during ED visits by this age group were estimated by survey year by poisson regression modeling. Tests of linear trend using poisson regression modeling were used if the graphical display of testing rates suggested a linear trend. For all analyses in the study, differences were considered significant at the α=0.05 level.
The proportion of ED patients tested over the twelve-year period was calculated for the following three medical conditions for which CDC specifically recommends HIV testing: non-sexual blood or body fluid exposures, STDs/STIs, and sexual assaults. The proportion of patients tested for HIV for any of these medical conditions (signified as a “possible HIV exposure”) was also assessed. Patient visits presenting for these medical conditions were identified using the International Classification of Disease, 9th Revision Clinical Modification (ICD-9-CM) by searching through the diagnosis and cause codes recorded in the NHAMCS databases. A case was included regardless of whether it was a primary, secondary, or tertiary ICD-9 code. Non-sexual blood or body fluid exposures were characterized using the diagnosis code V15.85 (exposure to body fluids) and the exposure code E920.5 (percutaneous exposures). STDs/STIs were identified using 50 codes for primary STDs/STIs of the anus, genitalia, and pharynx and for exposures to STDs/STIs; codes for non-specific symptoms of these infections were not included in these analyses. Sexual assaults were identified using the diagnosis codes 995.53 and 995.83 (child and adult sexual abuse), V15.41 (history of rape), V71.5 (observation following rape), and the exposure code E960.1 (rape). Two-sample binomial tests of proportions were used to compare the proportion of ED patients tested among these three medical conditions.
The proportion of ED patients tested for HIV by each demographic characteristic (age group, gender, race, ethnicity, insurance type, urban/non-urban ED, and region of the US) was calculated. Bivariate X2 analyses were conducted to determine which demographic factors were associated with HIV testing. Four multivariable logistic regression models were created using HIV diagnostic testing as the outcome and the demographic characteristics as covariates. These models examined the relative importance of the association of the demographic characteristics with HIV testing. One model examined this association for all patients and the remaining three models investigated this relationship among patients diagnosed with each of the three medical conditions of interest in this study. Models for the three medical conditions of interest were restricted to those diagnosed with these conditions. Demographic factors significant in the bivariate analyses were used as covariates. Except for age, the demographic factors with the lowest proportion of testing were used as the reference groups. Odds ratios (ORs) with 95% confidence intervals (CIs) were estimated. In addition, multivariable logistic regression was used to estimate the odds of being tested for HIV for each of the three medical conditions, after adjusting for the demographic factors that were significant in the bivariate analyses. These models estimated the adjusted ORs of being tested for HIV by comparing those diagnosed versus those not diagnosed with each of these medical conditions. A separate model was constructed that evaluated the adjusted ORs for those diagnosed with any vs. none of these medical conditions.
3. Results
3.1. ED visits and demographic characteristics
There were approximately 790 million ED visits for 13–64-year-olds during the NHAMCS survey years 1993–2004. The mean age was 35 years and slightly more than half of the participants were female. (Table 1) The majority of the study sample was white and approximately 10% self-identified as Hispanic/Latino. Approximately 64% had private or governmental-sponsored healthcare insurance. Over a third were from the south and the majority of EDs were based in an urban area.
Table 1.
Description of study sample and analysis of HIV testing by demographic characteristics and medical conditions
| All patients | Percentage tested for HIV by each factor | p-value | ||
|---|---|---|---|---|
| Factor | n=7901 | |||
| Demographic characteristics | (%)2 | n1 | (%)3 | p< 5 |
| Age (years) | 0.0001 | |||
| 13–17 | 9.3 | 73 | 0.31 | |
| 18–22 | 13.3 | 104 | 0.38 | |
| 23–27 | 12.9 | 102 | 0.36 | |
| 28–32 | 12.1 | 96 | 0.38 | |
| 33–37 | 11.7 | 92 | 0.35 | |
| 38–42 | 11.1 | 88 | 0.36 | |
| 43–47 | 9.3 | 74 | 0.27 | |
| 48–52 | 7.6 | 60 | 0.19 | |
| 53–57 | 6.0 | 48 | 0.12 | |
| 58–64 | 6.7 | 53 | 0.12 | |
| Gender | 0.02 | |||
| Female | 53.8 | 425 | 0.34 | |
| Male | 46.2 | 365 | 0.27 | |
| Ethnicity | 0.005 | |||
| Hispanic | 10.3 | 81 | 0.44 | |
| Non-Hispanic | 79.1 | 625 | 0.30 | |
| Unknown | 10.6 | 84 | 0.22 | |
| Race | 0.0001 | |||
| Asian/Pacific Islander | 2.0 | 15 | 0.34 | |
| American Indian/Alaskan Native | 0.7 | 5 | 0.28 | |
| Black | 22.1 | 175 | 0.46 | |
| White | 75.2 | 594 | 0.26 | |
| Other | 0.0 | 1 | 0.00 | |
| Healthcare insurance status | 0.0001 | |||
| Private | 42.3 | 334 | 0.18 | |
| Medicare | 4.9 | 38 | 0.30 | |
| Medicaid | 16.7 | 132 | 0.39 | |
| Self-pay/No-charge/Other | 30.3 | 240 | 0.45 | |
| Unknown | 5.8 | 46 | 0.26 | |
| Geographic region | 0.02 | |||
| Midwest | 24.9 | 197 | 0.23 | |
| Northeast | 20.1 | 159 | 0.39 | |
| South | 36.8 | 291 | 0.35 | |
| West | 18.2 | 143 | 0.24 | |
| Urban/non-urban location | 0.22 | |||
| Urban area ED | 79.8 | 631 | 0.32 | |
| Non-urban area ED | 20.2 | 159 | 0.25 | |
| Medical conditions | ||||
| Blood/body fluid exposure | 0.1 | 0.8 | 35.1 | 0.0001 |
| No blood/body fluid exposure | 99.9 | 789 | 0.27 | |
| Primary STD infection | 0.7 | 5 | 2.62 | 0.0001 |
| No primary STD infection | 99.3 | 785 | 0.29 | |
| Sexual assault | 0.09 | 0.7 | 20.4 | 0.0001 |
| No sexual assault | 99.1 | 789 | 0.29 | |
| Possible HIV exposure4 | 0.8 | 7 | 8.0 | 0.0001 |
| All other medical conditions | 99.2 | 783 | 0.24 |
in millions
indicates column percentages; may not total to 100% due to rounding
indicates row percentages
blood/body fluid exposures, primary STD infection, or sexual assault
Chi-square analyses of HIV testing vs. no HIV testing for each factor
3.2. HIV testing 1993–2004
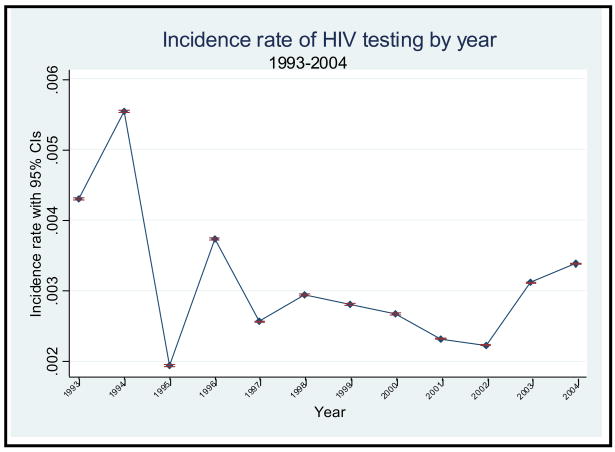
The Figure displays the IRs with corresponding 95% CIs of HIV testing for all patients from 1993–2004. HIV testing rates were highest in 1993 (0.43%) and 1994 (0.55%), and were lowest in 1995 (0.19%). Because of the large sample, each year’s IR was statistically different from all other years, although the range of values was narrow. The average IR of HIV testing within EDs for all survey years was 0.31%. There was no discernable temporal pattern in the rates across the study years. However, there was a trend towards increased testing from 2002–2004 (p<0.001).
Figure.
3.3. HIV testing by demographic characteristics
Testing was highest among 18–22- and 28–32-year-olds, females, Hispanics, blacks, self-pay/no-charge/other patients, those visiting EDs in the northeast and was lowest among 53–64-year-olds, males, whites, those with private health care insurance, and those visiting EDs in the midwest (Table 1). All demographic characteristics except presentation to an ED in an urban area were associated with HIV testing.
As shown in the multivariable logistic regression analysis involving all patients, Hispanics, blacks, and those without private health care insurance were more likely to be tested for HIV (Table 2). Patients 53–64-years-old were less likely to be tested for HIV, compared with 13–17-year-olds. Patients visiting EDs in the northeast were more likely to be tested for HIV than in the midwest. There were no differences in the odds of HIV testing between females and males, after adjusting for the other covariates.
Table 2.
Multivariable logistic regression analysis of covariates of HIV testing
| All patients | Patients with blood or body fluid exposures | Patients with sexual assault | Patients with a primary sexually transmitted infection/disease | |
|---|---|---|---|---|
| n=7901 | n=0.8011 | n=0.6781 | n=5.621 | |
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Age (years) | ||||
| 13–17 | 1.00 | 1.00 | 1.00 | 1.00 |
| 18–22 | 1.15 (0.78–1.68) | 0.84 (0.06–12.61) | 2.33 (0.76–7.18) | 3.49 (0.75–16.22) |
| 23–27 | 1.08 (0.74–1.58) | 0.79 (0.06–10.62) | 1.76 (0.54–5.72) | 4.19 (0.89–19.81) |
| 28–32 | 1.18 (0.85–1.63) | 1.53 (0.12–19.20) | 1.02 (0.18–5.77) | 0.47 (0.06–3.50) |
| 33–37 | 1.09 (0.75–1.59) | 0.48 (0.04–5.93) | 0.79 (0.11–5.62) | 1.48 (0.23–9.40) |
| 38–42 | 1.14 (0.79–1.64) | 0.91 (0.07–12.09) | <0.001 | 2.50 (0.33–19.03) |
| 43–47 | 0.88 (0.59–1.33) | 0.76 (0.06–10.45) | <0.001 | 0.77 (0.07–9.12) |
| 48–52 | 0.64 (0.40–1.02) | 0.08 (0.01–0.90) | <0.001 | <0.001 |
| 53–57 | 0.41 (0.22–0.74) | 0.68 (0.04–12.77) | <0.001 | <0.001 |
| 58–64 | 0.38 (0.22–0.66) | 0.77 (0.04–13.70) | <0.001 | <0.001 |
| Gender | ||||
| Male | 1.00 | 1.00 | 1.00 | 1.00 |
| Female | 0.83 (0.69–1.01) | 0.35 (0.16–0.78) | 1.49 (0.44–5.05) | 3.03 (1.23–7.46) |
| Ethnicity | ||||
| Non-Hispanic | 1.00 | 1.00 | 1.00 | 1.00 |
| Hispanic | 1.39 (1.06–1.81) | 0.79 (0.20–3.11) | 1.62 (0.24–11.12) | 0.22 (0.03–1.61) |
| Unknown | 0.79 (0.55–1.15) | 0.59 (0.21–1.69) | 1.22 (0.20–7.36) | 0.25 (0.04–1.64) |
| Race | ||||
| White | 1.00 | 1.00 | 1.00 | 1.00 |
| Black | 1.52 (1.19–1.94) | 0.50 (0.18–1.36) | 1.83 (0.68–4.94) | 0.75 (0.28–2.00) |
| Other | 1.26 (0.82–1.96) | 1.02 (0.24–4.46) | 2.16 (0.12–39.63) | <0.001 |
| Health Insurance | ||||
| Private | 1.00 | 1.00 | 1.00 | 1.00 |
| Medicare | 1.95 (1.20–3.16) | NA | <0.001 | 1.28 (0.16–10.17) |
| Medicaid | 2.35 (1.81–3.03) | 1.75 (0.74–4.15) | 0.31 (0.07–1.38) | 0.70 (0.22–2.27) |
| Self-pay/No-charge/Other | 1.74 (1.35–2.23) | 0.18 (0.04–0.79) | 1.46 (0.49–4.34) | 1.33 (0.50–3.52) |
| Unknown | 1.34 (0.83–2.17) | 0.46 (0.08–2.64) | 0.45 (0.16–1.28) | 2.70 (0.40–18.39) |
| Region | ||||
| Midwest | 1.00 | 1.00 | 1.00 | 1.00 |
| Northeast | 1.57 (1.04–2.38) | 2.36 (0.87–6.41) | 1.50 (0.45–5.00) | 0.53 (0.12–2.31) |
| South | 1.35 (0.90–2.01) | 1.37 (0.43–4.41) | 0.88 (0.27–2.93) | 1.92 (0.60–6.21) |
| West | 0.99 (0.67–1.46) | 1.07 (0.39–2.95) | 0.28 (0.04–1.85) | 1.96 (0.45–8.47) |
in millions
3.4. HIV testing by medical condition
Table 1 shows the percentage of patients tested for HIV for each or any of the three medical conditions. In the bivariate analyses, testing was greater for those patients diagnosed with one or any of these three medical conditions compared with other patients. The proportion of those tested for HIV was greater among those evaluated for a non-sexual blood or body fluid exposure (35.1%) than sexual assault (20.4%) (p<0.0001) or STD/STI (2.62%) (p<0.0001), and was greater among those evaluated for a sexual assault than a STD/STI (p<0.0001). After adjustment for the demographic covariates in Table 2, the adjusted ORs of being tested for HIV for ED patients diagnosed each medical condition compared to all other ED patients were: any possible HIV exposure (OR 31.3 [23.8–41.1]), blood or body fluid exposure (OR 207.8 [140.6–307.1]), STD/STI (OR 6.6 [4.0–10.7]), sexual assault (OR 82.5 [51.4–132.4]).
As shown in Table 2, there were some differences in HIV testing by demographic characteristics among ED patients diagnosed with the three medical conditions. Among patients with a blood or body fluid exposure, HIV testing was less among females and among those with self-pay/no-charge/other patients. Among patients evaluated for a sexual assault, HIV testing was less among those 38-years-old and older and was less among those with Medicare. Among patients evaluated for a STD/STI, testing was less among those age 43-years-old and older and those of other race. Testing was higher among females.
4. Discussion
HIV testing rates in US EDs are low and have not changed much over twelve years. There appears to be no significant response in EDs during 1993–2004 to CDC recommendations for increased HIV testing. The low overall HIV testing rates suggest that many opportunities for HIV testing have been lost or delayed, especially for conditions in which a potential HIV exposure has occurred. Other researchers have noted several examples of delays in the diagnosis of HIV infection of patients who could have been tested at the time of their ED visit.15–18 These delays can result in higher medical costs from the resultant opportunistic infections, continued transmission to others, and significant morbidity and mortality.
Even though CDC has consistently recommended HIV testing for patients with blood or body fluid exposures, survivors of sexual assault, and for patients with STDs/STIs, compliance with CDC recommendations is poor overall and not in accordance with risk of infection. Patients at a comparatively lower risk for infection (non-sexual blood or body fluid exposures) are tested 13.5-fold greater than those at a much higher risk for infection (STDs/STIs). It is likely that ED-based protocols facilitate routine HIV testing after occupational exposures to HIV, particularly for hospital employees. Other researchers have noted that patients presenting for possible STDs/STIs and after sexual assault are not being tested for HIV in the ED.19–21 In one study, the most common reasons ED providers gave for not offering their patients HIV testing were a lack of established mechanisms to ensure follow-up (51%), a lack of the certification perceived as necessary to provide HIV test counseling (45%), and a belief that the testing process was too time-consuming (19%).19 These data support a need for ED-based protocols for sexual assault and STDs/STIs that incorporate HIV testing as a standard of care.
HIV testing conducted in US EDs among all ED patients over the period of study was more common among patients who were members of certain demographic groups: Hispanics, blacks, and those without private health care insurance. Consistent with these findings, national samples of the US population also show that a history of prior HIV testing is more common among these demographic groups.12 We cannot determine from these data the risk of HIV infection among those tested or not tested for HIV or if members of these demographic groups were also at higher risk. On one hand, ED clinicians might be responding appropriately to the risk levels of patients under their care when ordering HIV tests or might be ordering more HIV tests for patients who are members of demographic groups who are disproportionately affected by the HIV epidemic: blacks, Hispanics, and the socioeconomically disadvantaged. On the other hand, over-reliance on patient demographic factors in ordering HIV tests might lead ED clinicians to fail to conduct adequate risk assessments on all patients, regardless of their demography. The problem with HIV testing based upon patient demography is that it can create reservoirs of undiagnosed infections in demographic groups believed to be at lower risk for an infection, which leads to further infections in the entire population. It is ironic, and potentially dangerous for these same reasons, that patients with greater health access and resources---those with private health care insurance--- were less likely to get tested for HIV in the ED. Testing also was higher in the northeastern US, which might be due to heightened awareness of HIV testing in traditionally higher prevalence areas.
There were some small demographic variations in HIV testing among patients with a blood or body fluid exposure, sexual assault, and a primary STD/STI. Differences in HIV testing by age were to be expected, given that sexual assault and primary STDs/STIs are more frequent among younger patients. It is potentially concerning, however, that males with a blood or body fluid exposure and females with a primary STD/STI were more likely to be tested for HIV than those of the opposite gender. ED clinicians should take care to provide HIV testing for these conditions without regard to gender.
This study does not address the reasons why EDs have not conducted HIV testing. Unmeasured confounders that were not variables in the database might have better explained variations in HIV testing and indicated reasons why testing did or did not occur. Perceived lack of risk for an HIV infection, negative ED clinician attitudes towards testing, barriers to testing on a local or state level, problems with arranging follow-up, lack of protocols that permit testing, time-burdensome procedures in conducting testing, lack of trained staff to conduct testing and other reasons might be preventing ED clinicians from conducting HIV testing. It is hoped that this study can provide a barometer of ED-based HIV testing and serve as a call to reduce barriers to testing. The advent of rapid HIV testing and efforts to streamline testing might provide the means for greater HIV test utilization in EDs. The trend towards greater HIV testing rates since the growing availability of rapid HIV testing in the US in the latter years of the study lends support to this claim.
There are several limitations to this study, some of which are in common with other NHAMCS secondary data analyses. These limitations include the potential for inaccuracies in the medical record, mistakes in interpreting the medical record, mistakes in coding the diagnosis and cause codes, and inaccuracies in processing of the data. These problems could have resulted in an underestimate of the number of patients tested for HIV or misclassifications of the patients identified as having one of the three conditions investigated in this study. Of more importance to this study, the efforts of ED-based HIV screening programs, which are typically conducted at places of higher HIV prevalence, would not be reflected in the data for this study. Further, referrals for HIV testing could not be captured by this database. It is possible that some EDs had reliable procedures in place for referring patients for outpatient HIV testing. However, researchers have noted that referrals for testing are not usually successful.14 Despite these limitations, these are the best estimates available of HIV testing conducted in a nationally representative sample of US EDs.
Acknowledgments
Dr. Merchant was supported by a career development grant from the National Institute for Allergy and Infectious Diseases (K23 A1060363).
Footnotes
The results of this study were presented at the Centers for Disease Control and Prevention National HIV Prevention Conference on December 3, 2007.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Update: provisional Public Health Service recommendations for chemoprophylaxis after occupational exposure to HIV. MMWR Morb Mortal Wkly Rep. 1996 Jun 7;45(22):468–480. [PubMed] [Google Scholar]
- 2.Public Health Service guidelines for the management of health-care worker exposures to HIV and recommendations for postexposure prophylaxis. Centers for Disease Control and Prevention. MMWR Recomm Rep. 1998 May 15;47(RR7):1–33. [PubMed] [Google Scholar]
- 3.Center for Disease Control and Prevention. Routinely Recommended HIV Testing at an Urban Urgent-Care Clinic --- Atlanta, Georgia, 2000. Morbidity and Mortality Weekly Reports. 2001 June 29;50(25):538–541. [PubMed] [Google Scholar]
- 4.Panlilio AL, Cardo DM, Grohskopf LA, et al. Updated U.S. Public Health Service guidelines for the management of occupational exposures to HIV and recommendations for postexposure prophylaxis. MMWR Recomm Rep. 2005 Sep 30;54(RR9):1–17. [PubMed] [Google Scholar]
- 5.Center for Disease Control and Prevention. Revised Guidelines for HIV Counseling, Testing, and Referral. Morbidity and Mortality Weekly Reports. 2001 Nov 9;50(RR19):1–58. [PubMed] [Google Scholar]
- 6.Center for Disease Control and Prevention. Perspectives in Disease Prevention and Health Promotion Public Health Service Guidelines for Counseling and Antibody Testing to Prevent HIV Infection and AIDS. Morbidity and Mortality Weekly Reports. 1987 August 14;36(31):509–515. [PubMed] [Google Scholar]
- 7.1993 sexually transmitted diseases treatment guidelines. Centers for Disease Control and Prevention. MMWR Recomm Rep. 1993 Sep 24;42(RR14):1–102. [PubMed] [Google Scholar]
- 8.1998 guidelines for treatment of sexually transmitted diseases. Centers for Disease Control and Prevention. MMWR Recomm Rep. 1998 Jan 23;47(RR1):1–111. [PubMed] [Google Scholar]
- 9.Sexually transmitted diseases treatment guidelines 2002. Centers for Disease Control and Prevention. MMWR Recomm Rep. 2002 May 10;51(RR6):1–78. [PubMed] [Google Scholar]
- 10.Workowski KA, Berman SM. Sexually transmitted diseases treatment guidelines, 2006. MMWR Recomm Rep. 2006 Aug 4;55(RR11):1–94. [PubMed] [Google Scholar]
- 11.Branson B, Handsfield HH, Lampe MA, Janssen RS, Taylor AW, Lyss SB, Clark JE. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. Morbidity and Mortality Weekly Reports Recommended Reports. 2006 Sept 22;55(RR14):1–17. [PubMed] [Google Scholar]
- 12.Lethbridge-Çejku M, Rose D, Vickerie J. Summary Health Statistics for U.S. Adults: National Health Interview Survey, 2004. National Center for Health Statistics. Vital Health Statistics. 2006;10(228):1–151. [PubMed] [Google Scholar]
- 13.National Hospital Ambulatory Medical Care Survey. Rockville, MD: National Center for Health Statistics; 1993–2004. Available at: http://www.cdc.gov/nchs. [Google Scholar]
- 14.Coil C, Haukoos JS, Witt MD, Wallace RC, Lewis RJ. Evaluation of an emergency department referral system for outpatient HIV testing. Journal of Acquired Immune Deficiency Syndrome. 2004;35:52–55. doi: 10.1097/00126334-200401010-00007. [DOI] [PubMed] [Google Scholar]
- 15.Kuo AM, Haukoos JS, Witt MD, et al. Recognition of undiagnosed HIV infection: an evaluation of missed opportunities in a predominantly urban minority population. AIDS Patient Care STDS Apr. 2005;19(4):239–246. doi: 10.1089/apc.2005.19.239. [DOI] [PubMed] [Google Scholar]
- 16.Missed opportunities for earlier diagnosis of HIV infection--South Carolina, 1997–2005. MMWR Morb Mortal Wkly Rep. 2006 Dec 1;55(47):1269–1272. [PubMed] [Google Scholar]
- 17.Liddicoat RV, Horton NJ, Urban R, et al. Assessing missed opportunities for HIV testing in medical settings. J Gen Intern Med Apr. 2004;19(4):349–356. doi: 10.1111/j.1525-1497.2004.21251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.MacDonald SR, Skor A, Socol ML, et al. Human immunodeficiency virus infection and women: a survey of missed opportunities for testing and diagnosis. Am J Obstet Gynecol Jun. 1998;178(6):1264–1271. doi: 10.1016/s0002-9378(98)70332-1. [DOI] [PubMed] [Google Scholar]
- 19.Fincher-Mergi M, Cartone KJ, Mischler J, Pasieka P, Lerner EB, Billittier AJ., IV Assessment of emergency department healthcare professionals’ behaviors regarding HIV testing and referral for patients with STDs. AIDS Patient Care STDs. 2002;16:549–553. doi: 10.1089/108729102761041100. [DOI] [PubMed] [Google Scholar]
- 20.Wilson SR, Mitchell C, Bradbury DR, et al. Testing for HIV: current practices in the academic ED. Am J Emerg Med Jul. 1999;17(4):354–356. doi: 10.1016/s0735-6757(99)90085-2. [DOI] [PubMed] [Google Scholar]
- 21.Amey AL, Bishai D. Measuring the quality of medical care for women who experience sexual assault with data from the National Hospital Ambulatory Medical Care Survey. Ann Emerg Med Jun. 2002;39(6):631–638. doi: 10.1067/mem.2002.123357. [DOI] [PubMed] [Google Scholar]