Abstract
The bacterial pathogen Legionella pneumophila replicates in a specialized vacuole within host cells. Establishment of the replication vacuole depends on the Dot/Icm translocation system that delivers a large number of protein substrates into the host cell. The functions of most substrates are unknown. Here, we analyzed a defined set of 127 confirmed or candidate Dot/Icm substrates for their effect on host cell processes using yeast as a model system. Expression of 79 candidates caused significant yeast growth defects, indicating these proteins impact essential host cell pathways. Notably, a group of 21 candidates interfered with the trafficking of secretory proteins to the yeast vacuole. Three candidates that caused yeast secretory defects (SetA, Ceg19 and Ceg9) were investigated further. These proteins impinged upon vesicle trafficking at distinct stages and had signals that allowed translocation into host cells by the Dot/Icm system. Ectopically produced SetA, Ceg19 and Ceg9 localized to secretory organelles in mammalian cells, consistent with a role for these proteins in modulating host cell vesicle trafficking. Interestingly, the ability of SetA to cause yeast phenotypes was dependent upon a functional glycosyltransferase domain. We hypothesize that SetA may glycosylate a component of the host cell vesicle trafficking machinery during L. pneumophila infection.
Introduction
Many bacterial pathogens cause disease by growing inside eukaryotic cells. One strategy for successful intracellular replication involves growth within a membrane-bound vacuole. Such pathogens interact intimately with host cell vesicle trafficking pathways to direct the biogenesis of a membranous vacuole permissive for growth (Salcedo and Holden, 2005). Establishment of a membrane-bound compartment is thought to provide pathogens with a protected niche by allowing evasion of host cell innate immune mechanisms and access to a source of nutrients (Roy and Mocarski, 2007).
Legionella pneumophila, the causative agent of Legionnaire's pneumonia, is one example of a bacterial pathogen that grows within a membrane-bound vacuole inside eukaryotic cells. L. pneumophila is a Gram-negative bacterium ubiquitously found in freshwater environments as an intracellular parasite of unicellular protozoans and in biofilms (Steinert et al., 2002). L. pneumophila causes disease in humans by growing within alveolar macrophages after the inhalation of contaminated aerosols (McDade et al., 1977; Horwitz and Silverstein, 1980). A striking feature of the bacterium's growth within both protozoan and mammalian host cells is the entry of the Legionella-containing vacuole (LCV) into a unique membrane trafficking pathway. After uptake into host cells, the LCV evades trafficking into the antimicrobial lysosomal network (Horwitz, 1983b; Roy et al., 1998). Instead, ER-derived vesicles and mitochondria are rapidly directed to the surface of the LCV, where the microorganism proceeds to grow (Horwitz, 1983a; Tilney et al., 2001; Kagan and Roy, 2002).
Central to the ability of L. pneumophila to grow within both mammalian and protozoan cells is the Dot/Icm type IV secretion system that injects a large number of protein substrates into the host cell cytosol (Segal et al., 1998; Vogel et al., 1998). These translocated substrates are believed to be essential for biogenesis of the LCV and intracellular replication of L. pneumophila (Shin and Roy, 2008). In support of this hypothesis, mutations in the dot/icm genes prevent remodeling of the LCV into an ER-like compartment and result in routing into the endocytic network (Swanson and Isberg, 1995; Tilney et al., 2001).
Recent work from several groups has identified over 80 protein substrates of the Dot/Icm translocation system (Conover et al., 2003; Luo and Isberg, 2004; Campodonico et al., 2005; Shohdy et al., 2005; Ninio and Roy, 2007; Zusman et al., 2007; Altman and Segal, 2008; de Felipe et al., 2008). Consistent with the observed ability of L. pneumophila to remodel the LCV, a group of these proteins modulate host cell vesicle trafficking pathways (Nagai et al., 2002; Derre and Isberg, 2005; Shohdy et al., 2005; Machner and Isberg, 2006; Murata et al., 2006; Ingmundson et al., 2007; Machner and Isberg, 2007; de Felipe et al., 2008; Pan et al., 2008). However, a complicating issue in understanding the precise functions of L. pneumophila Dot/Icm substrates arises from the fact that individual deletions of most substrate genes have little or no impact on intracellular growth of the bacterium. One explanation for this phenomenon may be that considerable functional redundancy exists among different substrates towards host cell targets. In addition, genetic studies indicate that multiple vesicle trafficking pathways are directed toward the LCV, suggesting that redundancy extends to the host as well as the bacterium (Dorer et al., 2006). Therefore, alternative systems for generating effector-dependent phenotypes will be important for a complete understanding of their functions during infection.
Ectopic expression in the budding yeast Saccharomyces cerevisiae has recently emerged as a powerful tool for both the identification and characterization of secreted bacterial effector proteins (Lesser and Miller, 2001; Valdivia, 2004; Kumar et al., 2006; Sisko et al., 2006; Kramer et al., 2007). Importantly, the generation of yeast phenotypes by bacterial effector proteins is specific, as non-translocated bacterial proteins rarely cause yeast phenotypes (Slagowski et al., 2008). Yeast expression has been utilized to identify and characterize L. pneumophila Dot/Icm substrates (Campodonico et al., 2005; Shohdy et al., 2005; de Felipe et al., 2008). However, these studies screened a limited number of substrates or used libraries constructed from randomly digested L. pneumophila genomic DNA, raising the possibility that these screens were not saturating. Thus, we hypothesized that more comprehensive screens will identify additional Dot/Icm substrates that modulate host cell vesicle trafficking or other essential host cell signaling pathways. In this report, we analyzed a defined set of 127 confirmed or candidate Dot/Icm substrates for their ability to generate yeast phenotypes. This directed approach allowed for the identification of a large group of proteins that interfere with cell growth and disrupt protein secretion. Interestingly, the ability of one of these proteins, SetA, to cause growth and secretory phenotypes in yeast was dependent upon a functional glycosyltransferase domain.
Results
Construction and characterization of a L. pneumophila yeast expression library
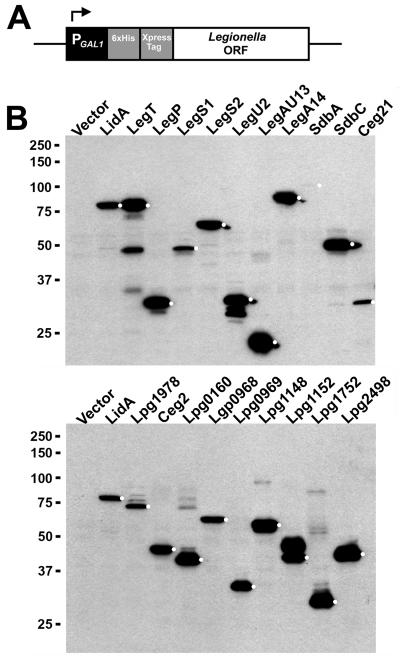
We targeted L. pneumophila ORFs for yeast expression based on several overlapping criteria: 1) ORFs that encode known Dot/Icm substrates for which little or no functional data was available; 2) ORFs predicted to encode proteins with eukaryotic-like domains (Cazalet et al., 2004; de Felipe et al., 2005); 3) ORFs that encode likely Dot/Icm substrates identified from a large-scale immunofluorescence-based screen currently ongoing in our lab (Huang et al., in preparation). As positive controls, we included the ORFs encoding the Dot/Icm substrates LidA, RalF, YlfA/LegC7 and VipD in the library as these proteins have previously been shown to generate phenotypes in yeast (Campodonico et al., 2005; Derre and Isberg, 2005; Shohdy et al., 2005; VanRheenen et al., 2006). In total, we targeted 140 ORFs for yeast expression and successfully amplified 133 of these by PCR. From this group, 127 were successfully cloned into a yeast expression plasmid by homologous recombination (Experimental Procedures). Of note, we routinely failed to clone six ORFs (lgt1, legC5/lgt3, legC8/lgt2, sdeA, lpg1489, and lpg2504), as they were extremely toxic to yeast even at low expression levels (Table 1). To control protein expression in yeast, ORFs were cloned downstream of the inducible GAL1 promoter (Fig. 1A), which is repressed by glucose and induced by galactose (Giniger et al., 1985). ORFs were cloned as fusions to an N-terminal Xpress epitope tag to facilitate detection of expressed proteins while an N-terminal 6×His tag was included to enable biochemical studies. We assessed the production of each L. pneumophila protein in yeast by immunoblot analysis with monoclonal anti-Xpress antibodies (Fig. 1B). Production of full-length protein was confirmed for 117 out of 127 candidates. Interestingly, for some proteins we observed the presence of higher molecular weight species (Fig. 1B, lower panel, see Lpg1978, Lpg0160 and Lpg1752 as examples), suggesting the presence of post-translational modifications or formation of higher-order oligomers.
Table 1.
Summary of Legionella proteins found to cause growth defects in S. cerevisiae in this study
| Lpg No. |
Alias | Growth Defecta |
Lpg No. |
Alias | Growth Defecta |
|---|---|---|---|---|---|
| 0012 | − | + | 1683 | − | + |
| 0030 | − | + | 1687 | − | + |
| 0059 | Ceg2 | + | 1701 | LegC3 | + |
| 0038 | LegA10/AnkQ | ++ | 1797 | − | + |
| 0086 | − | ++ | 1798 | − | ++ |
| 0096 | − | + | 1950 | RalF | ++ |
| 0195 | − | + | 1961 | − | ++ |
| 0210 | − | + | 1978 | SetA | ++ |
| 0227 | Ceg7 | + | 2144 | LegAU13/Ceg27/AnkB | + |
| 0246 | Ceg9 | + | 2147 | − | + |
| 0275 | SdbA | ++ | 2155 | SidJ | ++ |
| 0376 | SdhA | ++ | 2157 | SdeA | NR |
| 0390 | VipA | ++ | 2176 | LegS2 | + |
| 0401 | Ceg11 | ++ | 2199 | − | + |
| 0402 | LegA9/Ceg12/AnkY | ++ | 2257 | − | + |
| 0422 | LegY | + | 2271 | − | + |
| 0483 | LegA12/AnkC | ++ | 2298 | YlfA/LegC7 | ++ |
| 0621 | SidA | + | 2322 | LegA5/AnkK | ++ |
| 0634 | − | + | 2327 | − | + |
| 0642 | WipB | ++ | 2391 | SdbC | + |
| 0695 | LegA8/AnkN/AnkX | ++ | 2410 | VpdA | ++ |
| 0696 | − | + | 2425 | − | ++ |
| 0898 | Ceg18 | ++ | 2444 | − | + |
| 0940 | LidA | ++ | 2452 | LegA14/Ceg31/AnkF | + |
| 0944 | − | ++ | 2464 | SidM/DrrA | ++ |
| 0969 | − | ++ | 2465 | SidD | + |
| 1109 | − | + | 2482 | SdbB | ++ |
| 1111 | − | + | 2490 | LepB | ++ |
| 1121 | Ceg19 | + | 2504 | − | NR |
| 1137 | Ceg20 | ++ | 2510 | SdcA | + |
| 1152 | − | + | 2526 | − | + |
| 1154 | − | ++ | 2529 | − | + |
| 1166 | − | + | 2588 | LegS1 | + |
| 1183 | − | ++ | 2603 | − | ++ |
| 1290 | − | ++ | 2718 | WipA | + |
| 1328 | LegT | + | 2793 | LepA | + |
| 1355 | SidG | ++ | 2815 | DimB | + |
| 1368 | Lgt1 | NR | 2829 | SidH | ++ |
| 1426 | VpdC | ++ | 2831 | VipD | + |
| 1483 | LegK1 | ++ | 2862 | LegC8/Lgt2 | NR |
| 1488 | LegC5/Lgt3 | NR | 2879 | − | ++ |
| 1489 | − | NR | 2999 | LegP | + |
| 1642 | SidB | + |
A (+) symbol indicates production of the L. pneumophila protein resulted in a slow growth/intermediate phenotype in yeast, while a (++) symbol indicates the L. pneumophila protein was extremely toxic and caused a severe growth defect. NR (No Recombinants) indicates that correctly spliced recombinants could not be obtained during the cloning process, suggesting the respective L. pneumophila ORF was toxic even at low levels of expression.
Fig. 1. Generation of a yeast expression library consisting of known and candidate L. pneumophila Dot/Icm substrates.
(A) Schematic of L. pneumophila expression constructs. L. pneumophila ORFs were cloned into the yeast expression vector pYES/NT using homologous recombination (Experimental Procedures). ORFs were fused to an N-terminal Xpress epitope tag to enable detection of recombinant proteins by immunoblot and placed under control of the GAL1 promoter. Expression plasmids possess the URA3 auxotrophic marker for selection in yeast. (B) Production of Xpress-tagged L. pneumophila proteins in S. cerevisiae. Gene expression in yeast was induced by the addition of 2% galactose for 5 h (Experimental Procedures). Lysates were analyzed by immunoblot with antibodies directed against the Xpress epitope. White dots indicate bands at the predicted molecular weight for each L. pneumophila fusion protein. Positions of molecular weight markers are shown on the left side of the blot.
Production of L. pneumophila proteins inhibit yeast cell growth
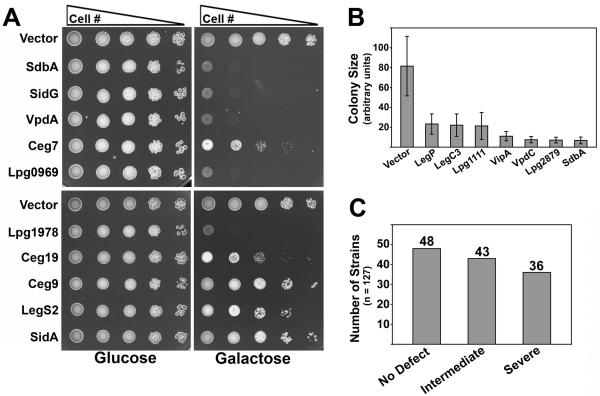
Translocated bacterial effector proteins often target conserved host cell processes essential for cell growth (Valdivia, 2004). Therefore, we tested if any of the L. pneumophila proteins in our expression bank caused yeast growth defects. The growth of strains producing L. pneumophila proteins was compared to empty vector control strains by spotting a series of 10-fold dilutions to selective media containing glucose or galactose. Strikingly, 79 of the 127 L. pneumophila proteins in our bank negatively impacted yeast cell growth. Some of these growth defects were intermediate in nature, with strains growing at a reduced rate compared to the control strain (Fig. 2A, see Ceg7, Ceg19, Ceg9, LegS2, and SidA as examples), while another group exhibited severe growth defects with the strains growing extremely slowly or not at all (Fig. 2A, see SdbA, SidG, VpdA, Lpg0969, and Lpg1978 as examples). These results suggest that these proteins impact processes essential for yeast cell growth. In total, we found that 43 proteins caused an intermediate growth defect, 36 severely impacted growth, while 48 did not impact growth in a discernible manner (Fig. 2C and Table 1). Of the 48 that did not cause a growth phenotype, 41 of these were produced as assessed by immunoblot, indicating that the lack of phenotype was not due to lack of protein synthesis (Table S1). Determination of colony size provided quantitative support for the phenotypic growth defect categorization of strains (Fig. 2B).
Fig. 2. Production of L. pneumophila proteins in yeast causes a spectrum of growth defects.
(A) Known and candidate Dot/Icm substrates cause defects in yeast cell growth. 10-fold serial dilutions of yeast strains were spotted onto selective plates containing 2% glucose or 2% galactose. (B) Quantitation of yeast growth defects generated by expression of L. pneumophila ORFs. A subset of yeast expression strains were spotted to selective plates and incubated at 30°C for 45 h. For each strain, ∼25 colonies were digitally imaged and colony diameter measured using IP lab software. Average colony diameter (pixels) was normalized to an empty vector control strain ± standard deviation and plotted on a bar graph. (C) Summary of yeast growth phenotypes.
Identification of L. pneumophila proteins that delay trafficking of host cell secretory proteins to the yeast vacuole
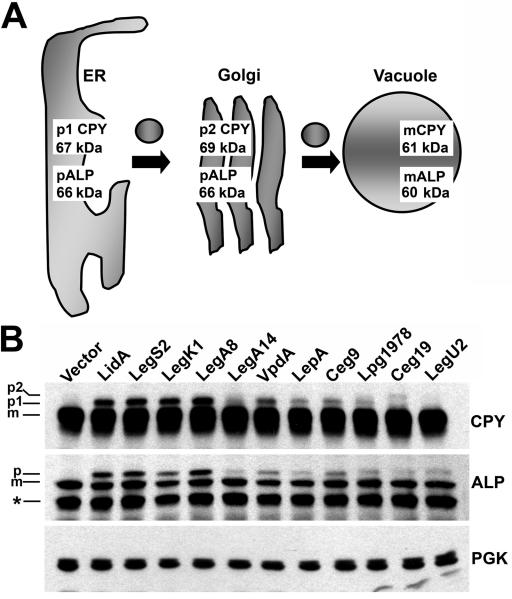
We next tested if any of the L. pneumophila proteins included in the expression bank impinged upon the sorting of secretory proteins to the yeast vacuole, the equivalent of the mammalian lysosome (Bowers and Stevens, 2005). Proteins that cause this phenotype are likely to interact with host cell vesicle trafficking factors and therefore may be involved in LCV biogenesis during infection (Derre and Isberg, 2005; Shohdy et al., 2005; de Felipe et al., 2008). To this end, we analyzed protein sorting by assaying the trafficking of the well-characterized secretory proteins carboxypeptidase Y (CPY) and alkaline phosphatase (ALP). These proteins are subjected to organelle-specific post-translational modifications as they are trafficked from the ER to the yeast vacuole via transport vesicles. These modifications alter their electrophoretic mobility as assessed by SDS-PAGE and immunoblotting, and therefore they provide sensitive and stage-specific read-outs of secretory processes (Fig. 3A). CPY is resident in the ER as the p1 precursor form of 67 kDa and then modified in the Golgi to produce the p2 form of 69 kDa. Finally, CPY is proteolytically processed in the vacuole to yield the mature form of 61 kDa (Stevens et al., 1982). Similarly, the ER form of ALP is a p1 precursor of 66 kDa, and is transported through the Golgi and cleaved in the vacuole to generate the 60 kDa mature form (Klionsky and Emr, 1989).
Fig. 3. Confirmed and candidate Dot/Icm substrates cause defects in the trafficking of secretory proteins to the yeast vacuole.
(A) Schematic of CPY and ALP trafficking from the ER to the vacuole in yeast. CPY and ALP undergo organelle specific post-translational modifications during trafficking from the ER to the Golgi and finally the vacuole via vesicular intermediates. These modifications alter the molecular weights of CPY and ALP and enable monitoring of their trafficking by immunoblot. (B) Analysis of CPY and ALP trafficking in yeast strains producing L. pneumophila proteins. Yeast strains were grown in media containing 2% galactose to induce production of L. pneumophila proteins (Experimental Procedures). Samples were analyzed by immunoblot with antibodies directed against CPY and ALP. PGK immunoblot is a control to show equivalent loading. The positions of the mature and precursor forms of CPY and ALP are indicated. Asterisk indicates an ALP degradation product. A LidA producing strain is included as a positive control for a protein known to cause secretory defects (Derre and Isberg, 2005).
In control strains containing empty vector, CPY and ALP were detected primarily as the mature forms, indicating efficient transport to the vacuole (Fig. 3B). Interestingly, immunoblot analysis revealed that production of 21 different L. pneumophila proteins out of the 127 tested caused the accumulation of precursor forms of CPY and/or ALP to varying degrees (see Fig. 3B for a representative immunoblot). This group included the substrates LidA, VipD, SidM/DrrA, RalF and LegA8/AnkN/AnkX, each of which has previously been shown to manipulate host cell secretory processes (Nagai et al., 2002; Derre and Isberg, 2005; Machner and Isberg, 2006; Pan et al., 2008). Of the remaining 16 candidates, 7 caused strong or intermediate accumulation of both CPY and ALP precursors when produced in yeast (Fig. 3B and Table 2). This group included the proteins LegS2, LegK1, VpdA, LepA, Ceg9, Ceg19, and Lpg1978. Four candidates caused weaker defects in both CPY and ALP processing (Lpg0518, Lpg0634, Lpg2603 and LegA14/Ceg31/AnkF), while the remaining 5 appeared to cause selective defects either in CPY transport (LegA7/AnkG/AnkZ and LegA12/AnkC) or ALP transport (Lpg1108, Lpg1961 and LegU2/LubX) (Table 2). A full compilation of yeast expression data, growth defects, and secretory defects is provided in Table S1.
Table 2.
Summary of L. pneumophila proteins found to cause secretory defects in S. cerevisiae in this study
| Lpg No. | Alias | CPY Defecta | ALP Defecta |
|---|---|---|---|
| 0246 | Ceg9 | ++ | ++ |
| 0403 | LegA7/AnkG/AnkZ | + | − |
| 0483 | LegA12/AnkC | + | − |
| 0518 | − | + | + |
| 0634 | − | + | + |
| 0695 | LegA8/AnkN/AnkX | +++ | +++ |
| 0940 | LidA | +++ | +++ |
| 1108 | − | − | ++ |
| 1121 | Ceg19 | ++ | ++ |
| 1483 | LegK1 | +++ | +++ |
| 1950 | RalF | +++ | +++ |
| 1961b | − | − | + |
| 1978 | SetA | ++ | ++ |
| 2176 | LegS2 | +++ | +++ |
| 2410 | VpdA | +++ | +++ |
| 2452 | LegA14/Ceg31/AnkF | + | + |
| 2464 | SidM/DrrA | +++ | +++ |
| 2603 | − | + | + |
| 2793 | LepA | ++ | ++ |
| 2830 | LegU2/LubX | − | + |
| 2831 | VipD | ++ | ++ |
L. pneumophila proteins were assayed by immunoblot for their ability to cause defects in the trafficking of CPY and/or ALP to the yeast vacuole. A (−) symbol indicates production of the protein did not impinge upon trafficking. A (+) symbol indicates a mild defect in trafficking, a (++) symbol indicates an intermediate defect while a (+++) symbol indicates the L. pneumophila protein caused a strong delay in the trafficking process.
Paralog of SetA/Lpg1978
Given the secretory defects associated with the production of these proteins in yeast, we hypothesized that they represent strong candidates for Dot/Icm substrates that modulate host cell vesicle trafficking during infection. Since Lpg1978, Ceg19 and Ceg9 caused significant secretory phenotypes and were relatively uncharacterized, we investigated these candidates further. Based on the ability of Lpg1978 to impinge upon yeast protein trafficking, we have termed this protein SetA for subversion of eukaryotic vesicle trafficking A.
SetA, Ceg19 and Ceg9 target vesicle trafficking at different stages
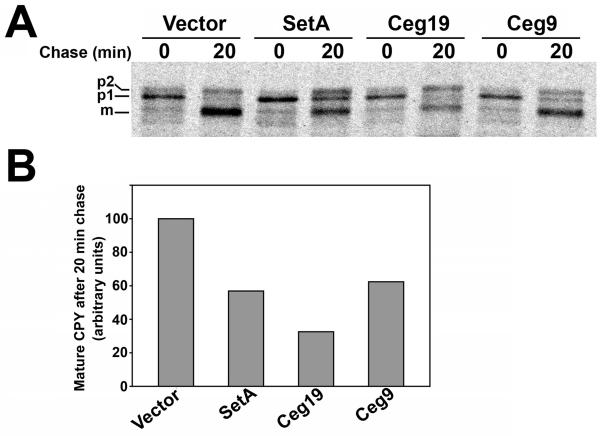
We next analyzed the secretory defects caused by production of SetA, Ceg19 and Ceg9 in greater detail. Specifically, we examined the transport kinetics of CPY in yeast strains producing SetA, Ceg19 and Ceg9 compared to a strain containing empty vector. Strains were pulse labeled for 7 min with [35S] methionine and cysteine and then chased with excess unlabeled amino acids for 20 min. Maturation of CPY was monitored by immunoprecipitation with anti-CPY antibodies. Most of the CPY was converted to the mature form after 20 min chase in the control strain (Fig. 4A). In contrast, strains producing SetA accumulated both the p1 and p2 forms of CPY, indicating a delay in trafficking between the ER and the Golgi as well as between the Golgi and the vacuole. As shown in Fig. 4B, densitometry analysis revealed that SetA producing cells converted ∼40% less CPY to the mature form relative to the empty vector strain over the chase period. Production of Ceg19 caused the specific accumulation of the p2 form of CPY, indicating a block in trafficking between the Golgi and the vacuole. Ceg19 producing cells converted ∼60% less CPY to the mature form relative to the control strain during the chase period (Fig. 4B). Lastly, production of Ceg9 caused the specific accumulation of p1 CPY, indicating a delay in trafficking between the ER and the Golgi complex. These cells converted ∼45% less CPY to the mature vacuolar form relative to the control strain over the chase period. Taken together, these data indicate that SetA, Ceg19 and Ceg9 cause distinct trafficking defects in yeast. Therefore, these proteins likely modulate host cell vesicle trafficking by different molecular mechanisms.
Fig. 4. Production of SetA, Ceg19 and Ceg9 cause distinct trafficking defects in yeast.
(A) Pulse-chase analysis of yeast expression strains. Yeast strains grown in the presence of 2% galactose were pulsed for 7 min with 35S-labeled cysteine/methionine to label nascent proteins and then chased for 20 min with excess unlabeled amino acids. Pulse-labeled CPY was immunoprecipitated, resolved on an 8% acrylamide gel, and visualized by autoradiography. The positions of mature and precursor forms of CPY are indicated on the left side of the autoradiogram. (B) Quantification of CPY trafficking defects in yeast strains producing SetA, Ceg19 and Ceg9. Mature CPY (mCPY) levels for each strain were visualized as described in (A) and quantified using densitometry. Levels were normalized to the mature CPY signal from the empty vector control strain and plotted as a bar graph. Data is representative of 2 separate experiments.
SetA, Ceg19 and Ceg9 have tropism for eukaryotic secretory organelles
The subcellular localization of ectopically produced bacterial effector proteins in eukaryotic cells can provide important clues as to their function (Sisko et al., 2006). Since production of SetA, Ceg19 and Ceg9 caused secretory defects in yeast, we hypothesized that these proteins may localize to organelles of the eukaryotic secretory pathway. EGFP fusions to the N-terminus of SetA, Ceg19 and Ceg9 were produced in transiently transfected HeLa cells and analyzed by fluorescence microscopy.
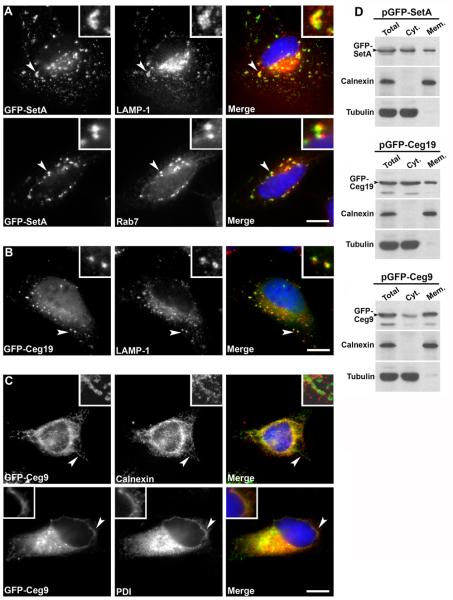
We observed that EGFP-SetA localized to pleiomorphic vesicular structures that were found in a perinuclear locale as well as throughout the cell (Fig. 5A). A significant portion of these structures co-stained with antibodies directed against the late endosomal/lysosomal proteins LAMP-1 and Rab7. The EGFP-SetA positive structures did not co-stain with antibodies directed against ER proteins, Golgi markers, or the autophagy marker Atg12 (Fig. S1 and data not shown) indicating the SetA localization pattern was specific and that SetA was not targeted to an autophagic compartment. These results indicate that SetA localizes to late endosomal/lysosomal compartments when ectopically produced in mammalian cells. EGFP-Ceg19 localized to relatively smaller vesicular structures that could be found in a perinuclear position as well as throughout the cell periphery. In some cells, a portion of EGFP-Ceg19 also exhibited a cytoplasmic distribution. A significant number of these EGFP-Ceg19 positive compartments co-stained with anti-LAMP1 antibodies, indicating they were endosomal in nature (Fig. 5B). Interestingly, these structures rarely stained positively for Rab7, suggesting they may be a different class of late endosomal compartments as compared to those targeted by SetA.
Fig. 5. Ectopically produced EGFP fusions to SetA, Ceg19 and Ceg9 localize to secretory organelles in mammalian cells.
(A) EGFP-SetA localizes to late endosomal/lysosomal compartments. EGFP-SetA was produced in HeLa cells by transient transfection for 8 h. Cells were fixed and stained with antibodies directed against the late endosomal proteins LAMP-1 and Rab7. Left panels, EGFP-SetA; center top panel, LAMP-1; center lower panel, Rab7; right panels, merge (DAPI-stained DNA in blue, EGFP-SetA in green, and LAMP-1 or Rab7 in red). Bar, 10 μm. Arrows indicate examples of structures that stain positively for EGFP-SetA and either LAMP-1 or Rab7. (B) EGFP-Ceg19 localizes to late endosomal compartments. EGFP-Ceg19 was produced in HeLa cells by transient transfection for 16 h. Cells were fixed and stained with antibodies directed against the late endosomal protein LAMP-1. Left panel, EGFP-Ceg19; center panel, LAMP-1; right panel, merge (DAPI-stained DNA in blue, EGFP-Ceg19 in green, and LAMP-1 in red). Bar, 10 μm. Arrow indicates examples of structures that stain positively for both EGFP-Ceg19 and LAMP-1. (C) EGFP-Ceg9 has tropism for ER membranes. EGFP-Ceg9 was produced in HeLa cells by transient transfection for 16 h. Cells were fixed and stained with antibodies directed against the ER resident proteins calnexin or PDI. Left panels, EGFP-Ceg9; center top panel, calnexin; center lower panel, PDI; right panels, merge (DAPI-stained DNA in blue, EGFP-SetB in green, and calnexin or PDI in red). Bar, 10 μm. Arrows indicate examples of structures that that stain positively for EGFP-Ceg9 and either calnexin or PDI. (D) Co-fractionation of EGFP fusions to SetA, Ceg19 and Ceg9 with eukaryotic cell membranes. Fusion proteins were produced in HEK-293T cells by transient transfection for 20 h. Cells were lysed and fractionated as described (Experimental Procedures). Total: crude lysate cleared of unbroken cells; Cyt. (cytosol): high-speed supernatant, cleared of membranous material; Mem. (membrane): high-speed pellet containing cellular membranes. For each fraction, samples were removed, volume adjusted with buffer, mixed with sample buffer and boiled for 5 min. Samples were resolved on SDS-PAGE gels, transferred to PVDF membranes, and immunoprobed with antibodies directed against GFP, calnexin (marker for host cell membranes) and tubulin (marker for host cell cytosol). Arrows indicate positions of GFP fusion proteins.
In contrast to the subcellular localization patterns displayed by SetA and Ceg19, EGFP-Ceg9 localized to the nuclear rim, juxtanuclear compartments, and membrane tubules that extended towards the cell periphery (Fig. 5C). This localization pattern was heterogeneous, as some transfected cells contained EGFP-Ceg9 primarily in tubular structures while other cells contained EGFP-Ceg9 mainly at the nuclear rim and in juxtanuclear structures. Regardless, these structures co-stained with antibodies directed against the ER proteins calnexin and PDI, indicating ectopically produced EGFP-Ceg9 has tropism for ER membranes. We also observed that EGFP fusions to SetA, Ceg19 and Ceg9 co-fractionated with membranes to varying extents when ectopically produced in mammalian cells (Fig. 5D). Taken together, these data indicate that SetA, Ceg19 and Ceg9 localize to secretory organelles when produced in mammalian cells, consistent with the ability of these proteins to cause yeast secretory defects.
SetA, Ceg19 and Ceg9 have Dot/Icm translocation signals
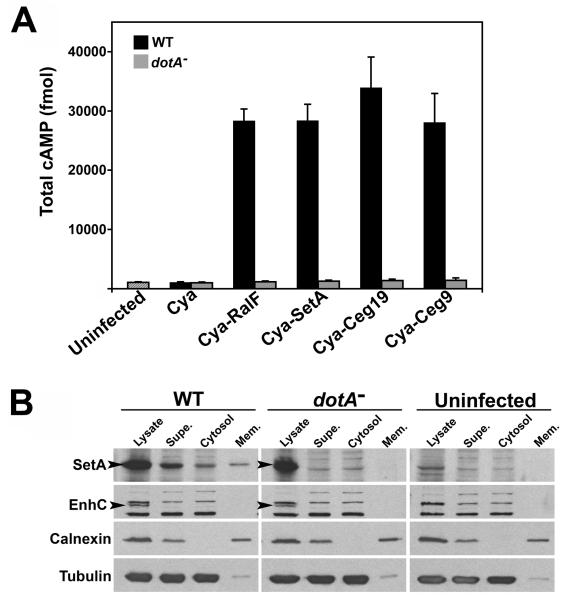
Since production of SetA, Ceg19 and Ceg9 caused specific phenotypes in eukaryotic cells, we hypothesized that these proteins are substrates of the Dot/Icm system. To test this possibility, we employed the CyaA fusion assay. This assay has been successfully used to study the translocation of many bacterial effector proteins into eukaryotic cells (Chen et al., 2004; de Felipe et al., 2005; Shohdy et al., 2005; Cambronne and Roy, 2007). CyaA activity is dependent on host cell calmodulin for activity, so increased cellular cAMP levels after infection with strains producing Cya fusion proteins indicates Cya translocation from the bacteria to the host cytosol (Sory and Cornelis, 1994). L. pneumophila strains harboring the catalytic domain of CyaA fused to the N-terminus of SetA, Ceg19 or Ceg9 were used to challenge U937 cells at multiplicity of infection (MOI) = 1 for 30 min. We observed a ∼30 fold increase in cAMP levels relative to the uninfected control following incubation of cells with each of the wild-type L. pneumophila strains producing Cya fusion proteins (Fig. 6A). This increase in cAMP was comparable to the amount generated by a strain producing Cya-RalF, a known Dot/Icm substrate (Nagai et al., 2002). Furthermore, the increase in cAMP was dependent on a functional Dot/Icm system, as no increase in cAMP levels was observed for dotA− strains producing Cya fusions. This was not due to differential production of the Cya fusion proteins in the wild-type and dotA− strain backgrounds, as assessed by immunoblot with anti-Cya antibodies (Fig. S2). These data indicate that SetA, Ceg19 and Ceg9 are delivered into the cytosol of host cells in a Dot/Icm-dependent manner.
Fig. 6. SetA, Ceg19 and Ceg9 are translocated into the cytosol of host cells by the Dot/Icm system.
(A) Wild-type (WT) and translocation-defective dotA− L. pneumophila strains producing the indicated Cya fusion proteins were used to infect U937 cells at MOI = 1 for 30 min. For each infection condition, cAMP levels are presented as the average of triplicate samples ± standard deviation (Experimental Procedures). (B) A pool of SetA fractionates with host cell membranes during infection. Wild-type (WT) and dotA− strains over-producing SetA were incubated with U937 cells at MOI = 5 for 30 min. Lysed cells were fractionated as described (Experimental Procedures). Supe: low-speed supernatant containing cytosol and membrane vesicles; Cytosol: high-speed supernatant, cleared of membranous material; Mem. (membrane): high-speed pellet from Supe. fraction. For each fraction, samples were removed, volume adjusted with buffer, mixed with sample buffer and boiled for 5 min. Samples were resolved on SDS-PAGE gels, transferred to PVDF membranes, and immunoprobed with antibodies directed against SetA, EnhC (marker for a non-translocated L. pneumophila protein), calnexin (marker for host cell membranes) and tubulin (marker for host cell cytosol). Arrow indicates positions of the SetA and EnhC bands, respectively.
We next assessed the subcellular distribution of SetA during infection. The mammalian cell localization data suggest that a pool of SetA may associate with host cell membranes during infection. To test this possibility, we performed fractionation experiments on infected cells. U937 cells were incubated at MOI = 5 for 30 min with wild-type or dotA− L. pneumophila strains over-producing SetA. Lysates were collected, subjected to low-speed spins to remove bacteria and unbroken host cells, and the low-speed supernatant was subjected to further fractionation by centrifugation at 100,000 × g. The low-speed, bacteria-free supernatant obtained from host cells infected with wild-type L. pneumophila contained a significant amount of SetA, indicating the protein was translocated (Fig. 6B, supe.). In contrast, the non-translocated protein EnhC was not detected in this fraction (Liu et al., 2008). Importantly, the presence of SetA in the supernatant was dependent on a functional Dot/Icm system, as SetA was not detected in low-speed supernatants obtained from a dotA− incubation. After high-speed centrifugation, SetA fractionated with both host cell cytosol and membrane fractions in a Dot/Icm-dependent manner (Fig. 6B; cytosol, mem.). These results indicate that a portion of SetA associates with host cell membranes after translocation by the Dot/Icm system, consistent with our observations that SetA has tropism for late endocytic compartments (Fig. 5A).
SetA is induced in the post-exponential phase of growth
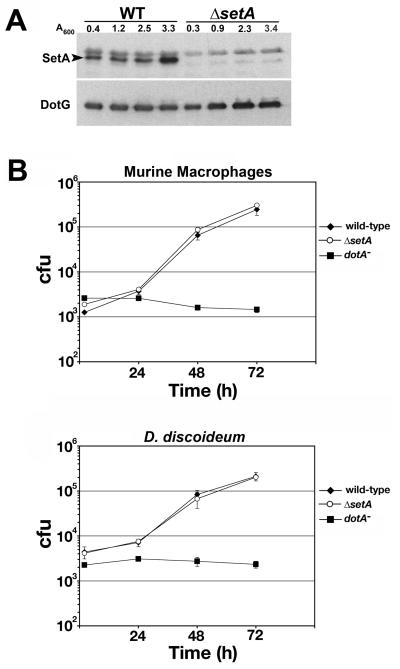
A large body of evidence indicates that the expression of factors important for L. pneumophila virulence, including several Dot/Icm substrates, is increased during the post-exponential phase of growth (Byrne and Swanson, 1998; Hammer and Swanson, 1999; Nagai et al., 2002; Luo and Isberg, 2004; Bardill et al., 2005; Bruggemann et al., 2006; VanRheenen et al., 2006; Banga et al., 2007; Zusman et al., 2007). Affinity purified anti-SetA antibodies were used to immunoprobe bacterial lysates harvested from L. pneumophila strains at different stages of growth. SetA protein levels were increased ∼3-fold in the post-exponential phase of growth. This protein was absent in lysates derived from a ΔsetA strain, indicating the antibodies were specific (Fig. 7A).
Fig. 7. SetA is induced during the post-exponential phase of growth.
(A) Wild-type (WT) or ΔsetA L. pneumophila strains were cultured in broth and equivalent A600 units were harvested at different stages of growth. Lysates were prepared and immunoprobed with affinity-purified antibodies directed against recombinant SetA. DotG immunoblot is a control for equivalent loading conditions. Cell density is indicated in A600 units at the top of the blot. Arrow indicates the band corresponding to SetA. (B) Primary bone-marrow derived macrophages from A/J mice and D. discoideum were infected with the indicated strains. Viable counts were determined as described in Experimental Procedures. The means and standard deviations are shown for each time point; experiments were done in triplicate.
Intracellular growth characteristics of a ΔsetA mutant strain
To determine whether SetA is important for efficient intracellular growth of L. pneumophila, the ΔsetA mutant strain was analyzed for growth in both murine bone marrow-derived macrophages and the soil amoeba Dictyostelium discoideum (Fig. 7B). The ΔsetA mutant strain grew similarly to wild-type L. pneumophila in both host cell types, indicating that SetA is not required for efficient intracellular replication of L. pneumophila under laboratory growth conditions, typical of knockouts that effect most of the Dot/Icm substrate genes.
A functional glycosyltransferase domain is required for SetA to interfere with yeast cell function
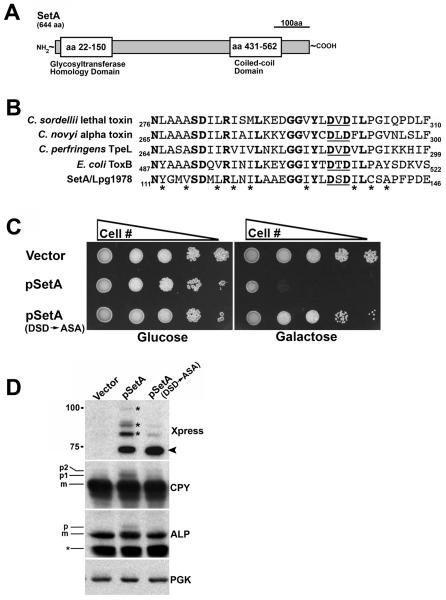
Inspection of the amino acid sequence of SetA revealed structural features that may provide clues as to function. First, analysis of the SetA sequence with the MARCOIL algorithm predicted the presence of coiled-coil structure in the C-terminal region of the protein (Fig. 8A). Several confirmed Dot/Icm substrates are predicted to possess coiled-coil domains (Conover et al., 2003; Campodonico et al., 2005; de Felipe et al., 2005). Second, BLAST searches revealed significant homology of the N-terminal region of SetA with both prokaryotic and eukaryotic glycosyltransferases (Fig. 8A).
Fig. 8. A functional glycosyltransferase domain is required for the ability of SetA to cause yeast phenotypes.
(A) Schematic of predicted domains of SetA. The N-terminal region of SetA has significant homology to eukaryotic and prokaryotic glycosyltransferases while the C-terminal region has a high probability of assuming a coiled-coil structure as determined by the MARCOIL algorithm. (B) The N-terminus of SetA has significant homology to bacterial glucosylating cytotoxins. Residues 111-146 of SetA are shown aligned to homologous regions of known and putative bacterial glucosylating cytotoxins. Identical amino acid residues are shown in bold, while residues of similar character are labeled with an asterisk (*). The position of the conserved DxD motif in each protein is underlined. GenBank accession numbers of the aligned proteins are as follows: Lpg1978/SetA, AAU28047; C. sordelli lethal toxin, CAA57959; C. novyi alpha toxin, CAA88565; C. perfringens TpeL, BAF46125; E. coli ToxB, BAA31815. Alignments were generated using the ClustalW program. (C) The toxicity of SetA to yeast cell growth requires an intact DxD motif. Yeast strains were spotted onto selective plates containing either 2% glucose or 2% galactose. Plates were incubated at 30°C for 72 h. (D) Mutation of the DxD motif blocks the ability of SetA to cause secretory defects in yeast. Yeast strains were grown in 2% galactose, equivalent A600 units of each strain were harvested and cell lysates were prepared (Experimental Procedures). Lysates were immunoblotted with antibodies directed against the Xpress epitope to assess SetA levels. Lysates were also probed with antibodies directed against CPY, ALP and PGK as controls. Positions of mature and precursor forms of CPY and ALP are indicated on the left side of the blot. Arrow indicates position of unmodified Xpress-SetA. Small asterisk indicates an ALP degradation product. Large asterisks; higher molecular weight forms of Xpress-SetA.
Glycosyltransferases catalyze the transfer of a sugar moiety from an activated sugar donor onto carbohydrate and protein acceptors (Breton et al., 2006). Some secreted bacterial toxins are known to act as glycosyltransferases toward host cell targets, such as the clostridial glucosylating toxins which catalyze the transfer of a glucose moiety onto host cell Rho GTPases (Aktories and Just, 2005). Recently, three paralogous L. pneumophila proteins (Lgt1, Lgt2/LegC8 and Lgt3/LegC5) were shown to possess cytotoxin-like glucosylation activity towards the host cell translation elongation factor eEF1A (Belyi et al., 2003; Belyi et al., 2006; Belyi et al., 2008). At least 2 of these proteins (Lgt2/LegC8 and Lgt3/LegC5) have also been shown to be Dot/Icm substrates (de Felipe et al., 2005).
Alignment of the catalytic regions of known and putative glucosylating toxins with the glycosyltransferase domain of SetA revealed a conserved D×D motif, shown to be essential for enzymatic activity of these proteins (Fig. 8B) (Busch et al., 1998). Given these findings, we tested if the DSD motif in the glycosyltransferase domain of SetA was important for the generation of yeast growth and secretory phenotypes. Mutation of the DSD motif to ASA alleviated the toxicity of SetA to yeast growth (Fig. 8C). This was not due to instability of the ASA mutant protein as assessed by immunoblot (Fig. 8D). Furthermore, the ASA mutant protein was no longer able to cause the accumulation of precursor forms of CPY and ALP (Fig. 8D). These results demonstrate that a functional glycosyltransferase domain is required for the ability of SetA to interfere with yeast cell function. These data are consistent with a model where SetA may act as a glycosyltransferase toward host cell targets involved in vesicle trafficking.
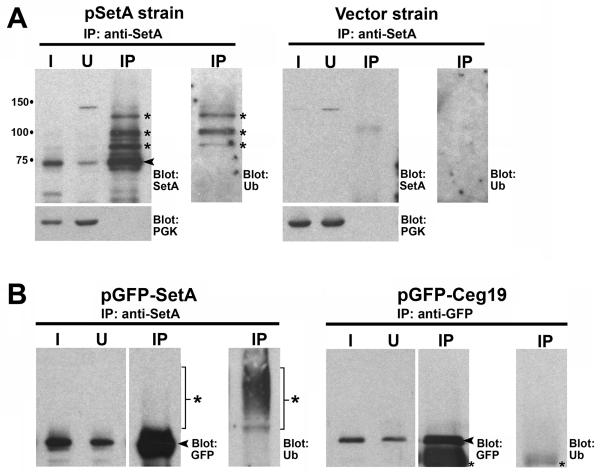
SetA is ubiquitinated in eukaryotic cells
Immunoblot analysis of SetA produced in yeast consistently revealed the presence of higher molecular weight forms of SetA (Fig. 8D). These higher forms were present for the DSD to ASA mutant protein, although they were less intense. We also observed that EGFP-SetA localized to endocytic organelles in mammalian cells (Fig. 5A), organelles known to be associated with the ubiquitin-dependent sorting of membrane proteins (Babst, 2005; Piper and Katzmann, 2007; Piper and Luzio, 2007). Therefore, we hypothesized that the larger forms of SetA may represent ubiquitinated species of the protein. To test this idea, we prepared detergent-solublized lysates from yeast strains harboring an empty vector or a vector encoding epitope-tagged SetA and performed immunoprecipitations with affinity-purified antibodies directed against SetA. SetA and its higher order forms could be efficiently immunoprecipitated in this manner, as SetA was depleted from the input material (compare Input and Unbound lanes) and enriched in the immunoprecipitate (IP lane) (Fig. 9A). This procedure was specific as the cytosolic protein PGK was absent from the immunoprecipitate. Immunoblot analysis of the immunoprecipitate with anti-ubiquitin antibodies revealed the presence of ubiquitinated proteins migrating at similar positions as the larger forms of SetA (Fig. 9A, anti-ubiquitin blot). This result was specific, as no labeling with anti-ubiquitin antibodies occurred on immunoblots of immunoprecipitates from an empty vector control strain. To exclude the possibility that ubiquitination of SetA was an artifact of yeast expression, we also performed immunoprecipitations from mammalian cells producing EGFP-SetA. EGFP-SetA was efficiently immunoprecipitated under these conditions (Fig. 9B). Again, we observed larger molecular weight forms of SetA that cross-reacted with anti-ubiquitin antibodies (Fig. 9B, IP: anti-SetA, Blot: Ub). At least some of these higher forms represent polyubiquitinated SetA, as determined by immunoblot analysis of the immunoprecipitates with polyubiquitin specific antibodies (Fig. S3). EGFP-Ceg19 was not ubiquitinated when produced in mammalian cells, indicating ubiquitination of SetA was specific. Taken together, these results indicate that SetA is a substrate for ubiquitination when ectopically produced in both yeast and mammalian cells. These findings suggest that SetA may interact with the ubiquitination machinery present at late endosomal compartments (Babst, 2005; Piper and Luzio, 2007).
Fig. 9. SetA is ubiquitinated when ectopically produced in eukaryotic cells.
(A) Immunoprecipitation of Xpress-SetA from yeast cells. Extracts of yeast cells producing Xpress-SetA or cells harboring empty vector were immunoprecipitated with affinity purified anti-SetA antibodies (Experimental Procedures). Input lane (I) represents 1% of the total starting material, while unbound lane (U) represents material remaining after IP. 30% of the total immunoprecipitated material was loaded in the lane marked IP. Antibodies used for immunoblotting are indicated on the right side of each panel. Arrow indicates position of unmodified Xpress-SetA, while asterisks indicate higher molecular weight forms of Xpress-SetA. (B) Immunoprecipitation of EGFP-SetA and EGFP-Ceg19 from mammalian cells. HEK-293T cells were transfected with the indicated plasmids for 16 h. Lysates were subjected to immunoprecipitation with anti-SetA antibodies (for EGFP-SetA) or anti-GFP antibodies (for EGFP-Ceg19). Input lane (I) represents 1% of the total starting material, while unbound lane (U) represents material remaining after IP. 10% of the total immunoprecipitated material was loaded in the lane marked IP. Antibodies used for immunoblotting are indicated on the right side of each panel. Arrows indicate positions of unmodified EGFP-SetA and EGFP-Ceg19. Large asterisks indicate higher molecular weight forms of EGFP-SetA, while small asterisks indicate position of IgG heavy chain.
Discussion
L. pneumophila utilizes the Dot/Icm translocation system to deliver protein substrates into the cytosol of eukaryotic cells. A large number of translocated substrates of the Dot/Icm apparatus have been identified, but there are few strategies available to identify their roles in the lifestyle of L. pneumophila. Only on rare occasions has the loss of a single substrate gene resulted in an obvious intracellular growth phenotype as compared to wild-type strains (Bardill et al., 2005; Laguna et al., 2006; Liu and Luo, 2007). One explanation for this phenomenon is that functional redundancy may exist among different Dot/Icm substrates towards host cell targets, and it also appears that multiple host membrane trafficking pathways feed into biogenesis of the replication vacuole (Dorer et al., 2006).
Given these complexities, alternative strategies for studying the functions of Dot/Icm substrates will be important. Specifically, a large-scale characterization of known and predicted Dot/Icm substrates is necessary to overcome these complexities. Previous studies have shown that ectopic expression of a limited set of effector genes or random L. pneumophila genomic libraries in yeast has uncovered distinct phenotypes for individual substrates. This has allowed the identification of several substrates, and has also demonstrated that L. pneumophila proteins can perturb sorting of a model secretory protein to the yeast vacuole at distinct steps in the trafficking pathway (Campodonico et al., 2005; Shohdy et al., 2005; de Felipe et al., 2008).
In this report, we screened a defined set of 127 confirmed or candidate Dot/Icm substrates for their ability to generate yeast growth and secretory phenotypes. We found that 79 L. pneumophila proteins significantly impacted yeast cell growth. Furthermore, we suspect that 6 other genes caused such severe growth defects that they were unclonable by the yeast homologous recombination cloning strategy we employed (Table 1). Specifically, we utilized a multi-copy expression vector, and this likely allowed for low-level production of L. pneumophila proteins due to “leaky” expression from the GAL1 promoter even under repressive conditions. Recent evidence indicates that the generation of yeast growth phenotypes by the ectopic expression of bacterial proteins is a unique property of translocated substrates, as expression of non-translocated proteins rarely causes growth defects (Slagowski et al., 2008). Therefore, the growth phenotypes we observed are likely due to disruption of specific host cell targets. Importantly, these growth defects are genetically tractable phenotypes and provide means to investigate the action of these substrates towards host cell factors.
We also identified a group of L. pneumophila proteins that inhibited the trafficking of secretory proteins to the yeast vacuole. Nearly all of these proteins also caused growth phenotypes (Tables 1 and 2). Of the 21 proteins in our expression library that disrupted trafficking of the secretory proteins CPY and/or ALP to the yeast vacuole, 5 (LidA, SidM/DrrA, RalF VipD and LegA8/AnkN/AnkX) had already been implicated in modulating secretory processes, and thus served as an important validation of our approach (Nagai et al., 2002; Derre and Isberg, 2005; Shohdy et al., 2005; Machner and Isberg, 2006; Pan et al., 2008). We focused on 3 uncharacterized proteins (SetA/Lpg1978, Ceg19/Lpg1121 and Ceg9/Lpg0246) identified from our secretion screen for further study. Lpg1121 and Lpg0246 have been previously annotated as Ceg proteins (coregulated with effector encoding genes) (Zusman et al., 2007). The ceg genes are predicted to be under transcriptional control of the response-regulator PmrA, an important regulator of virulence factor expression in both L. pneumophila and C. burnetii (Zusman et al., 2007). We demonstrated that SetA, Ceg19 and Ceg9 were translocated into host cells by the Dot/Icm system, as assessed by the Cya translocation assay. Furthermore, SetA co-fractionated with host cell cytosol and membranes in a Dot/Icm-dependent manner during infection, providing a second line of evidence for translocation. Interestingly, Ceg9 is predicted to possess a hydrophobic putative membrane-spanning region. Other Dot/Icm substrates are also predicted to contain hydrophobic domains (Campodonico et al., 2005; de Felipe et al., 2005; Cambronne and Roy, 2007; Kubori et al., 2008). These observations indicate that the Dot/Icm system is capable of translocating proteins with significant hydrophobic character, and raises mechanistic questions as to the folding state of such proteins as they are engaged by and pass through the Dot/Icm complex. Since Ceg9 has a robust translocation signal, this substrate may serve as a useful model to investigate the mechanisms by which hydrophobic substrates interface with the Dot/Icm machinery.
In vivo labeling experiments revealed that SetA, Ceg19 and Ceg9 modulated yeast protein trafficking in distinct ways. The precise molecular mechanisms by which these proteins impinge upon vesicular trafficking are unknown at present. However, some information can be gained from inspection of their protein sequences and subcellular localization patterns. Ceg19 is predicted to possess an N-terminal coiled-coil region and displays tropism for late endocytic organelles. We also observed that Ceg19 localized to the vacuolar membrane when ectopically produced in yeast cells (data not shown), the equivalent of the mammalian lysosomal membrane. Further, Ceg19 overproduction in yeast specifically delayed CPY transport between the Golgi complex and the vacuole, consistent with its localization to late endocytic compartments. Given these data, we speculate that Ceg19 may interact through its coiled-coil domain with host cell proteins involved in vesicular transport between the Golgi complex and late endocytic organelles. Similarly, Ceg9 has no primary sequence homology to any known protein. However, since this protein delays CPY transport between the ER and the Golgi complex and exhibits tropism for ER membranes, we hypothesize that Ceg9 interacts with host cell factors involved in ER-to-Golgi membrane trafficking, perhaps to recruit or redirect ER-derived vesicles to the LCV surface.
We have focused much of our attention on the SetA protein. SetA is a coiled-coil domain containing protein with homology to large clostridial glucosylating toxins. These toxins target host cell Rho GTPases by catalyzing the transfer of a glucose moiety to a conserved threonine residue within their catalytic region, resulting in inactivation of the GTPase and disruption of downstream signaling events (Aktories and Just, 2005). This homology raises the possibility that SetA may act as a toxin-like factor that glucosylates a host cell protein involved in vesicle trafficking. Consistent with this model, we found that mutation of a conserved D×D motif within the glycosyltransferase domain of SetA blocked the ability of the protein to cause yeast growth and secretory defects. One possibility is that SetA glucosylates a component of the endosomal ubiquitin-dependent protein sorting machinery. Through the glucosylation and inactivation of such a factor, SetA might inhibit ubiquitin-dependent endocytic trafficking and therefore prevent entry of the LCV into the endocytic network. Three lines of evidence are consistent with this hypothesis: 1) Overproduction of SetA in yeast inhibits CPY and ALP transport to the vacuole/lysosome; 2) Ectopically produced EGFP-SetA localizes to late endosomal/lysosomal organelles, compartments known to harbor ubiquitin-dependent sorting complexes; and 3) A pool of SetA is ubiquitinated during ectopic expression in host cells, suggesting that a subset of SetA is in close proximity to the endosomally localized ubiquitination machinery.
Based on these properties, SetA may be a member of an emerging class of Dot/Icm substrates that prevent entry of the LCV into the endocytic network. Recent work indicates that the substrate LegA8/AnkN/AnkX may also be in this functional class of effectors, as this protein appears to inhibit microtubule-dependent fusion of the LCV with LAMP1 positive compartments (Pan et al., 2008). Alternatively, glucosylation of a host cell trafficking factor by SetA may act to promote the recruitment of ER-derived vesicles to the LCV. Glucosylation of a host trafficking factor might alter its function such that the downstream effect is a rerouting of ER-derived material to the LCV surface.
It should be noted that there are other explanations for the function of SetA ubiquitination. The most obvious model is that the function of ubiquitination is to simply direct SetA to proteasomes for degradation once SetA function is no longer required. Previous work, however, raises another possibility that this modification could play a role in the dislocation of the protein from the LCV. Ubiquitinated proteins have been observed surrounding the LCV membrane during infection of host cells (Dorer et al., 2006). The identities of these ubiquitinated proteins are unknown, although their dislocation from the LCV surface is promoted by cytosolic components of the host cell ER-associated degradation pathway (ERAD). It has been proposed that L. pneumophila may hijack the ERAD pathway to promote removal of ubiquitinated Dot/Icm substrates from the LCV membrane. Therefore, ubiquitination could be a strategy to facilitate removal of SetA from the vacuolar membrane and movement to other sites in the cell. At present, we have been unable to detect ubiquitination of SetA during L. pneumophila infection of host cells. This may be due to technical issues or may be due to inhibition of SetA ubiquitination by the activity of other Dot/Icm substrates. For example, L. pneumophila produces several Dot/Icm substrates that modulate or are predicted to impact host cell ubiquitination pathways, including a deubiquitinase (Cazalet et al., 2004; Catic et al., 2007; Kubori et al., 2008).
In summary, we have utilized a defined yeast expression library to identify a large group of confirmed and candidate L. pneumophila Dot/Icm substrates that cause yeast growth and secretory defects. These growth phenotypes will provide new opportunities for functional analysis of these proteins towards host cell targets using yeast genetic approaches. Furthermore, we demonstrated that 21 L. pneumophila proteins subvert yeast protein trafficking, 16 of which had not been previously implicated in this process. For 3 of these proteins (SetA, Ceg19 and Ceg9), our initial characterization studies are consistent with a model where they function during infection to facilitate biogenesis of the LCV by manipulating host cell vesicle trafficking factors. Future studies on the precise modes of action of SetA, Ceg19 and Ceg9 toward host cell targets will provide insight towards the molecular mechanisms by which L. pneumophila manipulates host cell membrane trafficking to establish a replication vacuole.
Experimental Procedures
Strains and media
Bacterial strains, yeast strains, and plasmids used in this study are listed in Table S2. The L. pneumophila strains used are derivatives of the L. pneumophila strain Philadelphia-1 strain Lp02 (thyA Δ[hsdR-lvh] rpsL) (Berger and Isberg, 1993), and were grown on casamino acids-yeast extract (CYE) solid medium or in ACES-yeast extract (AYE) broth. For L. pneumophila, antibiotics were used at the following concentrations: chloramphenicol, 5 μg ml−1; and kanamycin, 40 μg ml−1. The growth media was supplemented with thymidine at 100 μg ml−1 or 5% sucrose when appropriate. The L. pneumophila strains used in all assays were grown to post-exponential phase (A600 ∼ 3.2-3.5) unless stated otherwise. Lp02 ΔsetA harboring an in-frame deletion of setA was constructed as described previously (Dumenil and Isberg, 2001; Luo and Isberg, 2004).
For E. coli strains, ampicillin was added to 100 μg ml−1 when appropriate. Yeast strains were grown in complete synthetic medium (CSM) dropout mixes (US Biological) supplemented with glucose or galactose as indicated in the text. All yeast methods were based on protocols as outlined (Sherman, 1991).
Construction of an L. pneumophila yeast expression library
L. pneumophila ORFs were cloned into the commercially available yeast expression plasmid pYES/NT (Invitrogen) using yeast homologous recombination (Sisko et al., 2006). Oligonucleotides (IDT) were designed such that the 3′ ends were specific to the gene of interest and the 5′ ends were complementary to the sequences flanking the EcoRI-XhoI sites within the multi-cloning site of pYES/NT (a complete list of oligonucleotides used is available in Table S3). Pfu Ultra II DNA polymerase (Stratagene) was used for the amplification of L. pneumophila ORFs from Lp02 genomic DNA. PCR amplified L. pneumophila ORFs were cotransformed with EcoRI-XhoI digested pYES/NT into BY4742 yeast cells using the lithium acetate/PEG method (Sherman, 1991). Transformation reactions were plated to CSM media lacking uracil to select for recombinants. Plasmids were recovered from recombinant yeast strains using the ’smash and grab’ method (Sherman, 1991), sequenced to confirm correct insertion of the amplified L. pneumophila ORF and retransformed into the S. cerevisiae strain BY4742. For immunoblot analysis of L. pneumophila proteins produced in yeast, strains were grown to saturation at 30°C (A600 ∼5) in CSM-ura media containing 2% glucose. Strains were then back-diluted to an A600 of 1.5 in CSM-ura media containing 2% galactose to induce protein expression and grown for 5 h at 30°C. Cells were harvested (6 A600 units) and lysed by vigorous agitation with glass beads in M2 buffer (20 mM Hepes pH 7.0, 150 mM NaCl) containing a protease inhibitor cocktail (Roche). Cleared lysates were mixed with sample buffer, boiled for 5 min, resolved on 8% or 10% polyacrylamide gels, transferred to PVDF membranes and probed with anti-Xpress monoclonal antibodies (Invitrogen). For analysis of yeast secretory defects, expression strains were grown as described above and lysates were immunoblotted with antibodies against CPY, ALP and PGK (Molecular Probes).
Pulse-chase experiments
Labeling of growing yeast strains was performed as previously described (Belden and Barlowe, 1996) with some modifications. Yeast expression strains and an empty vector control strain were grown to stationary phase (A600 ∼5) in CSM-ura containing 2% glucose. Cells were then back-diluted to an A600 of 0.2 in 30 ml low-sulfate CSM-ura medium (US Biological) containing 2% glucose and grown for 4 h at 30°C (approximately 2 cell doublings). Cells were then pelleted, resuspended in low-sulfate CSM-ura media containing 2% galactose, and grown for 5 h to induce expression of L. pneumophila proteins. Next, 15 A600 units of each culture were pelleted, resuspended in 5 ml medium, pre-cultured for 10 min, and pulse-labeled with 12 μl ProMix [35S] methionine and cysteine (Amersham) for 7 min. Cultures were then chased by the addition of excess unlabeled methionine and cysteine (10 μl of a 20 mg/ml solution). Cell samples were taken at the end of the pulse period and after 20 min of chase. Cell lysates were prepared by glass bead lysis and labeled species were precipitated from a common extract with specific antibodies for CPY (a kind gift of Dr. C. Barlowe, Darmouth Medical School, Hanover NH). Immunoprecipitations were performed as described below.
Analysis of yeast growth defects
To determine the effect of L. pneumophila protein production on yeast cell growth, strains were grown to saturation in CSM-ura media containing 2% glucose. Strains were then back-diluted to an A600 of 1.0, diluted 10-fold serially, and 5 μl of each dilution was spotted onto CSM-ura plates containing either 2% glucose or 2% galactose. Plates were incubated at 30°C for 72 h. For all experiments, growth of yeast strains was compared to a control strain harboring empty vector. Strains were scored visually for growth defects and placed in 3 categories: (1) no defect, (2) intermediate growth defect and (3) severe growth defect. For more quantitative analysis of growth defects, strains were grown to saturation in CSM-ura containing 2% glucose. Cultures were adjusted to an A600 of 1.0, diluted 1:1000 with sterile water, and 100 μl was plated to CSM-ura plates containing either 2% glucose or 2% galactose. Plates were incubated at 30°C for 45 h. For each strain, ∼25 colonies were imaged using SPOT microscope digital imaging software. Images were exported to IP Lab Spectrum for measurement of colony diameter in pixels. The average colony diameter and standard deviations for each strain was normalized to the empty vector control strain for each experiment.
Mammalian cell culture, transfections and immunofluorescence
HeLa cells and HEK-293T cells were maintained in DMEM containing 10% heat-inactivated FBS (Gibco). Macrophage-like U937 cells were cultured as described (Berger and Isberg, 1993). For transfection of EGFP-SetA, EGFP-Ceg19 and EGFP-Ceg9 expression plasmids, HeLa cells were seeded onto glass coverslips in 24-well dishes and were transfected the next day with 0.5 μg plasmid DNA using Lipofectamine 2000 (Invitrogen). Transfections were allowed to proceed for 8-16 h, cells were fixed with 4% paraformaldehyde for 10 min at RT, permeabilized with PBS/0.1% Triton X-100 for 10 min at RT, and then blocked for ∼16 h in PBS/4% goat serum at 4°C. Cells were then stained with the appropriate antibodies and coverslips were mounted using FluoroGuard Antifade Reagent (BioRad). For immunofluorescence, the following antibodies and dilutions were employed: anti-calnexin (1:100, BD Biosciences), anti-PDI (1:50, BD Biosciences), anti-LAMP1 (1:100, Abcam), anti-Rab7 (1:100, Santa Cruz Biotechnology), and anti-Atg12 (1:100, Cell Signalling). Texas red-conjugated goat anti-rabbit or anti-mouse IgG (Molecular Probes) were utilized as secondary antibodies.
For subcellular frationation experiments, 1 × 106 HEK-293T cells were transiently transfected with the indicated plasmids for 16-20 h. Cells were pelleted and resuspended in 250 μl lysis buffer (25 mM Tris-HCl pH 7.5, 150 mM NaCl, 0.25 M sucrose, 2 mM MnCl2, 1 mM MgCl2) containing protease inhibitors and lysed on ice by 30 passes through a 26-gauge needle. Lysates were centrifuged at 500 × g for 2 min at 4°C to remove unbroken cells. Cleared lysates were then centrifuged at 100,000 × g for 12 min at 4°C to generate cytosol and membrane fractions. Samples were volume adjusted with buffer, mixed with sample buffer, boiled for 5 min and then analyzed by immunoblot.
Translocation assay
Translocation of SetA, Ceg19 and Ceg9 into host cells was assessed using the Cya fusion system (Sory and Cornelis, 1994). Specifically, the L. pneumophila ORFs setA (lpg1987), ceg19 (lpg1121) and ceg9 (lpg0246) were amplified by PCR from Lp02 genomic DNA, digested with BamHI and ligated into the CyaA fusion vector pJB2581 (a kind gift of Dr. J. Vogel, Washington University, St. Louis MO). Sequenced plasmids were transformed into WT and dotA− L. pneumophila strains by electroporation. Production of Cya fusion proteins was assessed by immunoblotting using monoclonal antibodies directed against CyaA (Santa Cruz Biotechnology). For the translocation assay, differentiated U937 cells were plated at a density of 2.5 × 106 cells per well of a 24-well tissue culture dish. The next day, the monolayers were infected with post-exponential phase (A600 ∼ 3.5) L. pneumophila strains expressing the Cya fusion proteins of interest at MOI = 1. Incubations were allowed to proceed for 30 min, cells were washed 3 times with warm media, and the monolayer was lysed by the addition of a solution containing 50 mM HCl and 0.1% Triton X-100. Lysates were then boiled for 5 min, the pH was neutralized with NaOH, 0.2 ml cold ethanol was added to the lysate, and samples were incubated on ice for 5 min. Samples were spun at 13,000 × g for 5 min, the supernatant was transferred to a new tube, and dried under a vacuum. cAMP levels were measured using the cAMP Biotrak Enzymeimmunoassay System (GE healthcare/Amersham). Values were plotted graphically and are the result of three independent infections ± standard deviation.
Intracellular growth assays
Bone marrow-derived macrophages from A/J mice were prepared as previously described (Conover et al., 2003). D. discoideum AX4 was plated in MB medium prior to incubation with L. pneumophila (Li et al., 2005). For assays of L. pneumophila growth within bone-marrow-derived macrophages or D. discoideum, host cells were cultured in 24-well plates and infected with L. pneumophila at MOI = 0.05 for 2 h. At each time point, monolayers were lysed with saponin, dilutions of the lysate were plated onto bacteriological media, and cfu were determined from triplicate wells at each time point. Each time point represents the mean number of bacteria recovered from three independent wells ± standard deviation.
Fractionation of infected U937 cells
To detect the fractionation of SetA with host cell membranes after contact with L. pneumophila, differentiated U937 cells were plated at a density of 4 × 107 cells in 10 cm tissue culture dishes. The next day, monolayers were incubated at MOI = 5 for 30 min with post-exponential phase cultures of L. pneumophila wild-type or dotA− strains over-producing SetA from the plasmid pJB908. Monolayers were washed 3 times with PBS to remove extracellular bacteria, cells were lifted with 1 ml trypsin/EDTA, washed once with cold PBS, and pelleted. Cells were then resuspended on ice in 1.5 ml cold lysis buffer (PBS w/250 mM sucrose) containing 1 mM EDTA, protease inhibitor cocktail (Roche), and 5 μl Halt phosphatase inhibitor (Thermo Scientific). Cells were mechanically lysed on ice by 12 passes within a chilled Dounce homogenizer. Lysates were spun at 4000 × g for 5 min at 4°C to pellet unbroken cells and reduce the viscosity of the sample. The sample was then spun again at 10,000 × g for 5 min at 4°C to pellet any uninternalized bacteria and intact bacterial phagosomes. The resulting bacteria-free, vesicle-rich supernatant was then spun at 100,000 × g to separate host cell cytosol from host cell membranes. Samples were collected from each fraction, volume adjusted with lysis buffer, mixed with sample buffer and boiled for 5 min. Samples were analyzed by immunoblot with antibodies directed against SetA, EnhC (Liu et al., 2008), calnexin, and tubulin (Sigma).
For analysis of growth stage-specific induction of SetA, L. pneumophila strains were grown at 37°C in AYE broth supplemented with thymidine. The equivalent of 0.5 A600 units of each strain was harvested at various phases of growth, pelleted, resuspended in 100 μl sample buffer and boiled for 5 min. Samples were resolved on SDS-PAGE gels, transferred to PVDF membranes and immunoprobed with antibodies directed against SetA and DotG (VanRheenen et al., 2004).
Immunoprecipitations
For immunoprecipitations from yeast cells, 25 A600 units of cells expressing Xpress-SetA or an empty vector control were pelleted, resuspended in 0.3 ml lysis buffer (PBS, 250 mM sorbitol, and protease inhibitor cocktail) and disrupted by glass bead lysis. Lysates were cleared of unbroken cells by centrifugation at 500 × g for 2 min at 4°C. Lysates were solubilized with 1% Triton-X 100 and clarified by centrifugation at 16,000 × g for 10 min at 4°C. Solubilized material (150 μl) was transferred to a new tube on ice, diluted 5-fold with ice-cold PBS, and SetA was immunoprecipitated by the addition of 0.2 μg of affinity-purified anti-SetA antibodies and 50 μl 20% protein A-sepharose beads (Amersham). After binding for 3 h at 4°C, beads with bound protein were washed four times with lysis buffer/0.1% Trition X-100. Finally, the bound protein was released from the beads by the addition of 40 μl sample buffer and boiled for 5 min. Proteins from the immunoprecipitates were resolved on polyacrylamide gels and analyzed by immunoblot.
For immunoprecipitations from tissue culture cells, 1 × 106 HEK-293T cells were transfected for 16 h with pEGFP-SetA, pEGFP-Ceg19 or empty vector. Cells were harvested, pelleted, and solubilized in 0.3 ml buffer (PBS, 250 mM sucrose, and protease inhibitors) containing 1% Trition X-100. Samples were centrifuged at 16,000 × g for 10 min at 4°C to remove debris. Solubilized material (0.2 ml) was transferred to a new tube on ice, diluted 5-fold with buffer, and proteins were immunoprecipitated by the addition of affinity-purified anti-SetA antibodies (0.2 μg) or anti-GFP antibodies (2 μg) and 50 μl 20% protein A-sepharose beads. Monoclonal antibodies directed against ubiquitin were obtained from BD Biosciences, monoclonal antibodies specific for polyubiquitin were acquired from Biomol International, and polyclonal anti-GFP antibodies were obtained from Invitrogen.
Generation of anti-SetA antibodies
Full-length setA was amplified by PCR and cloned into the bacterial expression vector pQE80 (Qiagen) to generate a 6×His-SetA fusion construct (Table S2). 6×His-SetA was expressed and purified from BL21-DE3 E. coli by incubating bacterial cell lysates with Ni-NTA agarose, extensive washing of the resin, and elution of 6×His-SetA with PBS/250 mM imidazole. The fusion protein was used to immunize rabbits by standard procedures (Pocono Rabbit Farm and Laboratory Inc., Canadensis, PA).
Supplementary Material
EGFP-SetA does not colocalize with the autophagy protein Atg12. HeLa cells were transfected for 8 h with pEGFP-SetA, fixed, permeabilized and stained with antibodies directed against Atg12. Left panel, EGFP-SetA; center panel, Atg12; right panel, merge (DAPI-stained DNA in blue, EGFP-SetA in green, and Atg12 in red). Bar, 10 μm.
Cya fusions to SetA, Ceg19 and Ceg9 are produced in both wild-type and dotA− L. pneumophila strains. Immunoblot of whole-cell bacterial extracts from wild-type (WT) and dotA- bacterial strains producing the indicated Cya fusion proteins. Samples were immunoprobed with antibodies directed against CyaA to assess fusion protein levels. Immunoblot of ICDH is a control to show equivalent loading. White dots indicate bands of the predicted molecular weight for each fusion protein, while positions of molecular weight markers are indicated on the left side of the blot.
A subset of EGFP-SetA is polyubiquitinated when ectopically produced in eukaryotic cells. HEK-293T cells were transfected with pEGFP-SetA for 16 h. Lysates were subjected to immunoprecipitation with anti-SetA antibodies (Experimental Procedures). Input lane (I) represents 0.2% of the total starting material. 4% of the total immunoprecipitated material was loaded into the lane marked IP. Antibodies used for immunoblotting are indicated on the right side of each panel. Arrow indicates position of EGFP-SetA. Large asterisks indicate higher molecular weight forms of EGFP-SetA. Small asterisk indicates the position of a band that likely represents a truncated polyubiquitinated form of EGFP-SetA.
Compilation of yeast expression phenotypes (production of L. pneumophila proteins, growth defects and secretory defects).
Strains, primers and plasmids utilized in this study.
Primers used for amplification and cloning of L. pneumophila ORFs by homologous recombination in yeast.
Acknowledgements
We thank Matthias Machner, Alex Ensminger, Tamara O'Connor and Sina Mohammadi for careful review of the manuscript. We thank Charles Barlowe, Joe Vogel, and Vicki Losick for providing plasmids and antibodies used in this work. This study was supported by the Howard Hughes Medical institute and an NIH NRSA fellowship to M.H. R.R.I. is a Howard Hughes Medical Institute investigator. The authors have declared that no competing financial interests exist.
References
- Aktories K, Just I. Clostridial Rho-inhibiting protein toxins. Curr Top Microbiol Immunol. 2005;291:113–145. doi: 10.1007/3-540-27511-8_7. [DOI] [PubMed] [Google Scholar]
- Altman E, Segal G. The response regulator CpxR directly regulates expression of several Legionella pneumophila icm/dot components as well as new translocated substrates. J Bacteriol. 2008;190:1985–1996. doi: 10.1128/JB.01493-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babst M. A protein's final ESCRT. Traffic. 2005;6:2–9. doi: 10.1111/j.1600-0854.2004.00246.x. [DOI] [PubMed] [Google Scholar]
- Banga S, Gao P, Shen X, Fiscus V, Zong WX, Chen L, Luo ZQ. Legionella pneumophila inhibits macrophage apoptosis by targeting pro-death members of the Bcl2 protein family. Proc Natl Acad Sci U S A. 2007;104:5121–5126. doi: 10.1073/pnas.0611030104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardill JP, Miller JL, Vogel JP. IcmS-dependent translocation of SdeA into macrophages by the Legionella pneumophila type IV secretion system. Mol Microbiol. 2005;56:90–103. doi: 10.1111/j.1365-2958.2005.04539.x. [DOI] [PubMed] [Google Scholar]
- Belden WJ, Barlowe C. Erv25p, a component of COPII-coated vesicles, forms a complex with Emp24p that is required for efficient endoplasmic reticulum to Golgi transport. J Biol Chem. 1996;271:26939–26946. doi: 10.1074/jbc.271.43.26939. [DOI] [PubMed] [Google Scholar]
- Belyi I, Popoff MR, Cianciotto NP. Purification and characterization of a UDP-glucosyltransferase produced by Legionella pneumophila. Infect Immun. 2003;71:181–186. doi: 10.1128/IAI.71.1.181-186.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belyi Y, Niggeweg R, Opitz B, Vogelsgesang M, Hippenstiel S, Wilm M, Aktories K. Legionella pneumophila glucosyltransferase inhibits host elongation factor 1A. Proc Natl Acad Sci. 2006;103:16953–16958. doi: 10.1073/pnas.0601562103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belyi Y, Tabakova I, Stahl M, Aktories K. Lgt: a family of cytotoxic glucosyltransferases produced by Legionella pneumophila. J Bacteriol. 2008 doi: 10.1128/JB.01798-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger KH, Isberg RR. Two distinct defects in intracellular growth complemented by a single genetic locus in Legionella pneumophila. Mol Microbiol. 1993;7:7–19. doi: 10.1111/j.1365-2958.1993.tb01092.x. [DOI] [PubMed] [Google Scholar]
- Bowers K, Stevens TH. Protein transport from the late Golgi to the vacuole in the yeast Saccharomyces cerevisiae. Biochim Biophys Acta. 2005;1744:438–454. doi: 10.1016/j.bbamcr.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Breton C, Snajdrova L, Jeanneau C, Koca J, Imberty A. Structures and mechanisms of glycosyltransferases. Glycobiology. 2006;16:29R–37R. doi: 10.1093/glycob/cwj016. [DOI] [PubMed] [Google Scholar]
- Bruggemann H, Hagman A, Jules M, Sismeiro O, Dillies MA, Gouyette C, Kunst F, Steinert M, Heuner K, Coppee JY, Buchrieser C. Virulence strategies for infecting phagocytes deduced from the in vivo transcriptional program of Legionella pneumophila. Cell Microbiol. 2006;8:1228–1240. doi: 10.1111/j.1462-5822.2006.00703.x. [DOI] [PubMed] [Google Scholar]
- Busch C, Hofmann F, Selzer J, Munro S, Jeckel D, Aktories K. A common motif of eukaryotic glycosyltransferases is essential for the enzyme activity of large clostridial cytotoxins. J Biol Chem. 1998;273:19566–19572. doi: 10.1074/jbc.273.31.19566. [DOI] [PubMed] [Google Scholar]
- Byrne B, Swanson MS. Expression of Legionella pneumophila virulence traits in response to growth conditions. Infect Immun. 1998;66:3029–3034. doi: 10.1128/iai.66.7.3029-3034.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambronne ED, Roy CR. The Legionella pneumophila IcmSW Complex interacts with Multiple Dot/Icm Effectors to Facilitate Type IV Translocation. PLoS Pathog. 2007;3:e188. doi: 10.1371/journal.ppat.0030188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campodonico EM, Chesnel L, Roy CR. A yeast genetic system for the identification and characterization of substrate proteins transferred into host cells by the Legionella pneumophila Dot/Icm system. Mol Microbiol. 2005;56:918–933. doi: 10.1111/j.1365-2958.2005.04595.x. [DOI] [PubMed] [Google Scholar]
- Catic A, Misaghi S, Korbel GA, Ploegh HL. ElaD, a Deubiquitinating protease expressed by E. coli. PLoS ONE. 2007;2:e381. doi: 10.1371/journal.pone.0000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazalet C, Rusniok C, Bruggemann H, Zidane N, Magnier A, Ma L, et al. Evidence in the Legionella pneumophila genome for exploitation of host cell functions and high genome plasticity. Nat Genet. 2004;36:1165–1173. doi: 10.1038/ng1447. [DOI] [PubMed] [Google Scholar]
- Chen J, de Felipe KS, Clarke M, Lu H, Anderson OR, Segal G, Shuman HA. Legionella effectors that promote nonlytic release from protozoa. Science. 2004;303:1358–1361. doi: 10.1126/science.1094226. [DOI] [PubMed] [Google Scholar]
- Conover GM, Derre I, Vogel JP, Isberg RR. The Legionella pneumophila LidA protein: a translocated substrate of the Dot/Icm system associated with maintenance of bacterial integrity. Mol Microbiol. 2003;48:305–321. doi: 10.1046/j.1365-2958.2003.03400.x. [DOI] [PubMed] [Google Scholar]
- de Felipe KS, Pampou S, Jovanovic OS, Pericone CD, Ye SF, Kalachikov S, Shuman HA. Evidence for acquisition of Legionella type IV secretion substrates via interdomain horizontal gene transfer. J Bacteriol. 2005;187:7716–7726. doi: 10.1128/JB.187.22.7716-7726.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Felipe KS, Glover RT, Charpentier X, Anderson OR, Reyes M, Pericone CD, Shuman HA. Legionella eukaryotic-like type IV substrates interfere with organelle trafficking. PLoS Pathog. 2008;4:e1000117. doi: 10.1371/journal.ppat.1000117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derre I, Isberg RR. LidA, a translocated substrate of the Legionella pneumophila type IV secretion system, interferes with the early secretory pathway. Infect Immun. 2005;73:4370–4380. doi: 10.1128/IAI.73.7.4370-4380.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorer MS, Kirton D, Bader JS, Isberg RR. RNA interference analysis of Legionella in Drosophila cells: exploitation of early secretory apparatus dynamics. PLoS Pathog. 2006;2:e34. doi: 10.1371/journal.ppat.0020034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumenil G, Isberg RR. The Legionella pneumophila IcmR protein exhibits chaperone activity for IcmQ by preventing its participation in high-molecular-weight complexes. Mol Microbiol. 2001;40:1113–1127. doi: 10.1046/j.1365-2958.2001.02454.x. [DOI] [PubMed] [Google Scholar]
- Giniger E, Varnum SM, Ptashne M. Specific DNA binding of GAL4, a positive regulatory protein of yeast. Cell. 1985;40:767–774. doi: 10.1016/0092-8674(85)90336-8. [DOI] [PubMed] [Google Scholar]
- Habyarimana F, Al-khodor S, Kalia A, Graham JE, Price CT, Teresa Garcia M, Kwaik YA. Role for the ankyrin eukaryotic-like genes of Legionella pneumophila in parasitism of protozoan hosts and human macrophages. 2008;10:1460–1474. doi: 10.1111/j.1462-2920.2007.01560.x. [DOI] [PubMed] [Google Scholar]
- Hammer BK, Swanson MS. Co-ordination of Legionella pneumophila virulence with entry into stationary phase by ppGpp. Mol Microbiol. 1999;33:721–731. doi: 10.1046/j.1365-2958.1999.01519.x. [DOI] [PubMed] [Google Scholar]
- Horwitz MA, Silverstein SC. Legionnaires' disease bacterium (Legionella pneumophila) multiples intracellularly in human monocytes. J Clin Invest. 1980;66:441–450. doi: 10.1172/JCI109874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz MA. Formation of a novel phagosome by the Legionnaires' disease bacterium (Legionella pneumophila) in human monocytes. J Exp Med. 1983a;158:1319–1331. doi: 10.1084/jem.158.4.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz MA. The Legionnaires' disease bacterium (Legionella pneumophila) inhibits phagosome-lysosome fusion in human monocytes. J Exp Med. 1983b;158:2108–2126. doi: 10.1084/jem.158.6.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingmundson A, Delprato A, Lambright DG, Roy CR. Legionella pneumophila proteins that regulate Rab1 membrane cycling. Nature. 2007;450:365–369. doi: 10.1038/nature06336. [DOI] [PubMed] [Google Scholar]
- Kagan JC, Roy CR. Legionella phagosomes intercept vesicular traffic from endoplasmic reticulum exit sites. Nat Cell Biol. 2002;4:945–954. doi: 10.1038/ncb883. [DOI] [PubMed] [Google Scholar]
- Klionsky DJ, Emr SD. Membrane protein sorting: biosynthesis, transport and processing of yeast vacuolar alkaline phosphatase. Embo J. 1989;8:2241–2250. doi: 10.1002/j.1460-2075.1989.tb08348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer RW, Slagowski NL, Eze NA, Giddings KS, Morrison MF, Siggers KA, Starnbach MN, Lesser CF. Yeast functional genomic screens lead to identification of a role for a bacterial effector in innate immunity regulation. PLoS Pathog. 2007;3:e21. doi: 10.1371/journal.ppat.0030021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubori T, Hyakutake A, Nagai H. Legionella translocates an E3 ubiquitin ligase that has multiple U-boxes with distinct functions. Mol Microbiol. 2008;67:1307–1319. doi: 10.1111/j.1365-2958.2008.06124.x. [DOI] [PubMed] [Google Scholar]
- Kumar Y, Cocchiaro J, Valdivia RH. The obligate intracellular pathogen Chlamydia trachomatis targets host lipid droplets. Curr Biol. 2006;16:1646–1651. doi: 10.1016/j.cub.2006.06.060. [DOI] [PubMed] [Google Scholar]
- Laguna RK, Creasey EA, Li Z, Valtz N, Isberg RR. A Legionella pneumophila-translocated substrate that is required for growth within macrophages and protection from host cell death. Proc Natl Acad Sci. 2006;103:18745–18750. doi: 10.1073/pnas.0609012103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesser CF, Miller SI. Expression of microbial virulence proteins in Saccharomyces cerevisiae models mammalian infection. Embo J. 2001;20:1840–1849. doi: 10.1093/emboj/20.8.1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Solomon JM, Isberg RR. Dictyostelium discoideum strains lacking the RtoA protein are defective for maturation of the Legionella pneumophila replication vacuole. Cell Microbiol. 2005;7:431–442. doi: 10.1111/j.1462-5822.2004.00472.x. [DOI] [PubMed] [Google Scholar]
- Liu M, Conover GM, Isberg RR. Legionella pneumophila EnhC is required for efficient replication in tumor necrosis factor alpha-stimulated macrophages. Cell Microbiol. 2008;10:1906–1923. doi: 10.1111/j.1462-5822.2008.01180.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Luo ZQ. The Legionella pneumophila Effector SidJ Is Required for Efficient Recruitment of Endoplasmic Reticulum Proteins to the Bacterial Phagosome. Infect Immun. 2007;75:592–603. doi: 10.1128/IAI.01278-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo ZQ, Isberg RR. Multiple substrates of the Legionella pneumophila Dot/Icm system identified by interbacterial protein transfer. Proc Natl Acad Sci. 2004;101:841–846. doi: 10.1073/pnas.0304916101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machner MP, Isberg RR. Targeting of host Rab GTPase function by the intravacuolar pathogen Legionella pneumophila. Dev Cell. 2006;11:47–56. doi: 10.1016/j.devcel.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Machner MP, Isberg RR. A bifunctional bacterial protein links GDI displacement to Rab1 activation. Science. 2007;318:974–977. doi: 10.1126/science.1149121. [DOI] [PubMed] [Google Scholar]
- McDade JE, Shepard CC, Fraser DW, Tsai TR, Redus MA, Dowdle WR. Legionnaires' disease: isolation of a bacterium and demonstration of its role in other respiratory disease. N Engl J Med. 1977;297:1197–1203. doi: 10.1056/NEJM197712012972202. [DOI] [PubMed] [Google Scholar]
- Murata T, Delprato A, Ingmundson A, Toomre DK, Lambright DG, Roy CR. The Legionella pneumophila effector protein DrrA is a Rab1 guanine nucleotide-exchange factor. Nat Cell Biol. 2006;8:971–977. doi: 10.1038/ncb1463. [DOI] [PubMed] [Google Scholar]
- Nagai H, Kagan JC, Zhu X, Kahn RA, Roy CR. A bacterial guanine nucleotide exchange factor activates ARF on Legionella phagosomes. Science. 2002;295:679–682. doi: 10.1126/science.1067025. [DOI] [PubMed] [Google Scholar]
- Ninio S, Roy CR. Effector proteins translocated by Legionella pneumophila: strength in numbers. Trends Microbiol. 2007;15:372–380. doi: 10.1016/j.tim.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Pan X, Luhrmann A, Satoh A, Laskowski-Arce MA, Roy CR. Ankyrin repeat proteins comprise a diverse family of bacterial type IV effectors. Science. 2008;320:1651–1654. doi: 10.1126/science.1158160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper RC, Katzmann DJ. Biogenesis and function of multivesicular bodies. Annu Rev Cell Dev Biol. 2007;23:519–547. doi: 10.1146/annurev.cellbio.23.090506.123319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper RC, Luzio JP. Ubiquitin-dependent sorting of integral membrane proteins for degradation in lysosomes. Curr Opin Cell Biol. 2007;19:459–465. doi: 10.1016/j.ceb.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy CR, Berger KH, Isberg RR. Legionella pneumophila DotA protein is required for early phagosome trafficking decisions that occur within minutes of bacterial uptake. Mol Microbiol. 1998;28:663–674. doi: 10.1046/j.1365-2958.1998.00841.x. [DOI] [PubMed] [Google Scholar]
- Roy CR, Mocarski ES. Pathogen subversion of cell-intrinsic innate immunity. Nat Immunol. 2007;8:1179–1187. doi: 10.1038/ni1528. [DOI] [PubMed] [Google Scholar]
- Salcedo SP, Holden DW. Bacterial interactions with the eukaryotic secretory pathway. Curr Opin Microbiol. 2005;8:92–98. doi: 10.1016/j.mib.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Segal G, Purcell M, Shuman HA. Host cell killing and bacterial conjugation require overlapping sets of genes within a 22-kb region of the Legionella pneumophila genome. Proc Natl Acad Sci U S A. 1998;95:1669–1674. doi: 10.1073/pnas.95.4.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman F. Getting started with yeast. Methods Enzymol. 1991;194:3–21. doi: 10.1016/0076-6879(91)94004-v. [DOI] [PubMed] [Google Scholar]
- Shin S, Roy CR. Host cell processes that influence the intracellular survival of Legionella pneumophila. Cell Microbiol. 2008;10:1209–1220. doi: 10.1111/j.1462-5822.2008.01145.x. [DOI] [PubMed] [Google Scholar]
- Shohdy N, Efe JA, Emr SD, Shuman HA. Pathogen effector protein screening in yeast identifies Legionella factors that interfere with membrane trafficking. Proc Natl Acad Sci. 2005;102:4866–4871. doi: 10.1073/pnas.0501315102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisko JL, Spaeth K, Kumar Y, Valdivia RH. Multifunctional analysis of Chlamydia-specific genes in a yeast expression system. Mol Microbiol. 2006;60:51–66. doi: 10.1111/j.1365-2958.2006.05074.x. [DOI] [PubMed] [Google Scholar]
- Slagowski NL, Kramer RW, Morrison MF, LaBaer J, Lesser CF. A functional genomic yeast screen to identify pathogenic bacterial proteins. PLoS Pathog. 2008;4:e9. doi: 10.1371/journal.ppat.0040009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sory MP, Cornelis GR. Translocation of a hybrid YopE-adenylate cyclase from Yersinia enterocolitica into HeLa cells. Mol Microbiol. 1994;14:583–594. doi: 10.1111/j.1365-2958.1994.tb02191.x. [DOI] [PubMed] [Google Scholar]
- Steinert M, Hentschel U, Hacker J. Legionella pneumophila: an aquatic microbe goes astray. FEMS Microbiol Rev. 2002;26:149–162. doi: 10.1111/j.1574-6976.2002.tb00607.x. [DOI] [PubMed] [Google Scholar]
- Stevens T, Esmon B, Schekman R. Early stages in the yeast secretory pathway are required for transport of carboxypeptidase Y to the vacuole. Cell. 1982;30:439–448. doi: 10.1016/0092-8674(82)90241-0. [DOI] [PubMed] [Google Scholar]
- Swanson MS, Isberg RR. Association of Legionella pneumophila with the macrophage endoplasmic reticulum. Infect Immun. 1995;63:3609–3620. doi: 10.1128/iai.63.9.3609-3620.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney LG, Harb OS, Connelly PS, Robinson CG, Roy CR. How the parasitic bacterium Legionella pneumophila modifies its phagosome and transforms it into rough ER: implications for conversion of plasma membrane to the ER membrane. J Cell Sci. 2001;114:4637–4650. doi: 10.1242/jcs.114.24.4637. [DOI] [PubMed] [Google Scholar]
- Valdivia RH. Modeling the function of bacterial virulence factors in Saccharomyces cerevisiae. Eukaryot Cell. 2004;3:827–834. doi: 10.1128/EC.3.4.827-834.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanRheenen SM, Dumenil G, Isberg RR. IcmF and DotU are required for optimal effector translocation and trafficking of the Legionella pneumophila vacuole. Infect Immun. 2004;72:5972–5982. doi: 10.1128/IAI.72.10.5972-5982.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanRheenen SM, Luo ZQ, O'Connor TJ, Isberg RR. Members of a Legionella pneumophila family of proteins with ExoU (phospholipase A) active sites are translocated to target cells. Infect Immun. 2006;74:3597–3606. doi: 10.1128/IAI.02060-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel JP, Andrews HL, Wong SK, Isberg RR. Conjugative transfer by the virulence system of Legionella pneumophila. Science. 1998;279:873–876. doi: 10.1126/science.279.5352.873. [DOI] [PubMed] [Google Scholar]
- Zusman T, Aloni G, Halperin E, Kotzer H, Degtyar E, Feldman M, Segal G. The response regulator PmrA is a major regulator of the icm/dot type IV secretion system in Legionella pneumophila and Coxiella burnetii. Mol Microbiol. 2007;63:1508–1523. doi: 10.1111/j.1365-2958.2007.05604.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
EGFP-SetA does not colocalize with the autophagy protein Atg12. HeLa cells were transfected for 8 h with pEGFP-SetA, fixed, permeabilized and stained with antibodies directed against Atg12. Left panel, EGFP-SetA; center panel, Atg12; right panel, merge (DAPI-stained DNA in blue, EGFP-SetA in green, and Atg12 in red). Bar, 10 μm.
Cya fusions to SetA, Ceg19 and Ceg9 are produced in both wild-type and dotA− L. pneumophila strains. Immunoblot of whole-cell bacterial extracts from wild-type (WT) and dotA- bacterial strains producing the indicated Cya fusion proteins. Samples were immunoprobed with antibodies directed against CyaA to assess fusion protein levels. Immunoblot of ICDH is a control to show equivalent loading. White dots indicate bands of the predicted molecular weight for each fusion protein, while positions of molecular weight markers are indicated on the left side of the blot.
A subset of EGFP-SetA is polyubiquitinated when ectopically produced in eukaryotic cells. HEK-293T cells were transfected with pEGFP-SetA for 16 h. Lysates were subjected to immunoprecipitation with anti-SetA antibodies (Experimental Procedures). Input lane (I) represents 0.2% of the total starting material. 4% of the total immunoprecipitated material was loaded into the lane marked IP. Antibodies used for immunoblotting are indicated on the right side of each panel. Arrow indicates position of EGFP-SetA. Large asterisks indicate higher molecular weight forms of EGFP-SetA. Small asterisk indicates the position of a band that likely represents a truncated polyubiquitinated form of EGFP-SetA.
Compilation of yeast expression phenotypes (production of L. pneumophila proteins, growth defects and secretory defects).
Strains, primers and plasmids utilized in this study.
Primers used for amplification and cloning of L. pneumophila ORFs by homologous recombination in yeast.