Abstract
Rationale:
Nicotine nasal spray (NNS) may be better for relieving acute cigarette cravings than other nicotine replacement and it may help smokers with schizophrenia because of its rapid onset of action.
Objectives:
We tested whether NNS was more effective than a nicotine patch (NP; 21 mg) in reducing cue-induced craving during a 3-day abstinence.
Methods:
Twenty-five smokers with schizophrenia or schizoaffective disorder (SA) were randomized to open-label NNS or NP treatment after baseline measures of craving were assessed. NNS users were instructed to dose at a minimum of 1/hour and up to a maximum of 40/day. Averages from a 4-item visual analogue scale (need, urge, want to smoke, crave a cigarette) measured craving.
Results:
Five subjects who smoked (4 NP, 1 NNS) were excluded, leaving 21 (11 NP, 10 NNS) for analyses. No differences were detected between groups on baseline craving. On day 3, NNS users reported significantly less craving in response to smoking cues compared to NP users (mean craving scores: NNS, 7.0; NP, 20.3; p = .014). A repeated measure ANCOVA demonstrated significantly reduced craving in the NNS group compared to the NP group from baseline to day 3 (F = 5.09; p = .037). NNS users took an average of 20 doses/day, and NNS was rated as being as easy to use as NP.
Conclusions:
The potential utility of NNS in smokers with schizophrenia supports the need for placebo-controlled studies.
Keywords: Nicotine replacement, smoking cessation, craving, cue reactivity, nicotine nasal spray, schizophrenia
INTRODUCTION
Individuals with schizophrenia (SCZ) or shizoaffective disorder (SA) smoke at rates nearly three times that of the general population, with prevalence rates of at least 70% in a variety of studies (Hughes, Hatsukami, Mitchell, & Dahlgren, 1996; de Leon, Abraham, Nair, Verghese, McGrory, & McCann, 1995). Smokers with SCZ are often highly nicotine-dependent and have higher blood nicotine levels and lower than expected success rates in attempts to stop smoking, compared with smokers without this disorder (Williams, Ziedonis, Abanyie, Steinberg, Foulds, & Benowitz, 2005; Williams & Foulds, 2007). High nicotine levels in SCZ may be optimal for activation of low-affinity α7 nicotinic receptors that are reduced in both number and function in SCZ (Freedman, Adams, & Leonard, 2000; Breese et al., 2000). Acute tobacco cravings provoked by exposure to smoking cues can be potent triggers for smoking relapse, and it has been speculated that altered dopamine neurotransmission in mesolimbic systems in SCZ may enhance drug craving and reward mechanisms in these individuals (Chambers, Krystal, & Self, 2001; Smelson et al., 2002). Thus, smokers with SCZ represent a population at particularly high risk for relapse during quit attempts, and there is a need to better understand their craving in order to develop more effective treatments.
Acute nicotine cravings provoked by exposure to smoking cues in the environment can be important triggers for smoking relapse (Abrams, Monti, Carey, Pinto, & Jacobus, 1988; Tiffany, 1990; Niaura, Abrams, Demuth, Pinto, & Monti, 1989; Niaura, Shadel, Abrams, Monti, Rohsenow, & Sirota, 1998). Craving is an inducible state, highly responsive to drug-related stimuli, and retrospective analyses of craving can be influenced by recall bias (Sayette, Shiffman, Tiffany, Niaura, Martin, & Shadel, 2000). Cue exposure offers a human laboratory methodology for inducing craving in a controlled laboratory setting by exposing the individual to specific cues and measuring subsequent changes in craving (Childress, Hole, Ehrman, Robbins, McLellan, & O'Brien, 1993; Rickard-Figueroa & Zeichner, 1985; Niaura, Abrams, Pedraza, Monti, & Rohsenow, 1992; Carter & Tiffany, 1999; Droungas, Ehrman, Childress, & O'Brien, 1995). Cue exposure can also be used to test out the efficacy of anticraving medications (Ehrman, Robbins, Childress, & O'Brien, 1992). Common cue modalities used in research protocols include videotapes, scripted imagery, as well as in vivo tests involving manual handling of cigarettes and paraphernalia and simulated smoking (Shadel, Niaura, & Abrams, 2001; Conklin & Tiffany, 2001; Maude-Griffin & Tiffany, 1996; Drobes & Tiffany, 1997). These stimuli have all been reported to increase urges to smoke. Nicotine and smoking cues have been studied extensively in the general population as a proven paradigm for studying craving for nicotine in a laboratory setting (Carter & Tiffany, 2001; Lazev, Herzog, & Brandon, 1999).
Increasingly, studies of nicotine craving and nicotine replacement have incorporated cue reactivity paradigms. This technology could substantially improve smoking cessation outcomes by determining which individuals are high cravers and in need of varying interventions. The nicotine patch, for example, reduces overall levels of craving associated with smoking abstinence yet in studies does not appear to be helpful in reducing cue-induced cravings (Tiffany, Cox, & Elash, 2000; Waters, Shiffman, Sayette, Paty, Gwaltney, & Balabanis, 2004). In contrast, nicotine gum is effective in reducing cue-induced cravings (Shiffman et al., 2003). To date, there have been no studies examining nicotine nasal spray in craving paradigms with smokers with SCZ.
Preliminary studies of cue exposure methodology have demonstrated that this is a useful technique to study smokers with SCZ, as they seem to respond to cues in the laboratory setting with increased urges to smoke (Tidey, Rohsenow, Kaplan, & Swift, 2005; Fonder et al., 2005). Fonder et al. (2005) conducted a study of craving in smokers with and without SCZ who were given doses of the nicotinic receptor antagonist mecamylamine. Cues consisted of photographs of faces shown in a slideshow presentation, which may be less likely to induce urges to smoke compared to other methods (Glautier & Tiffany, 1995), since only 50% of smokers with or without SCZ displayed smoking cue reactivity in this study. This may have been due to the cues themselves since live cues are known to produce a more robust craving response than still pictures (Drobes & Tiffany, 1997). Tidey et al. (2005) described a procedure for cue exposure involving handling and lighting cigarettes in 25 smokers with SCZ that demonstrated increased urges to smoke. Both authors conclude that cue reactivity models are useful for investigating potential smoking treatments for smokers with SCZ and that more studies are needed.
To date, clinical trials of nicotine replacement in smokers with SCZ have been done almost exclusively with nicotine patches and have yielded quit rates that are only about half that of other smokers (Ziedonis & George, 1997; Addington, 1998; Williams & Hughes, 2003). There may be limitations to this method of nicotine delivery, which is passive and continuous and does not allow control of nicotine levels in response to cravings. Nicotine patches may also cause the greatest amount of desensitization of the α7 nicotinic receptor. Pilot work has suggested that abnormal electrophysiological measures and eye movements in SCZ are corrected or reversed only when nicotine is administered via smoking cigarettes or with nicotine gum or nasal spray (Adler, Hoffer, Wiser, & Freedman, 1993; Olincy, Ross, Young, Roath, & Freedman, 1998; Sherr, Myers, Avila, Elliott, Blaxton, & Thaker, 2002). Of interest, sleep is another mechanism that corrects the P50; however, the sleep effect is blocked when the nicotine patch is administered, presumably due to receptor desensitization (Griffith, O'Neill, Petty, Garver, Young, & Freedman, 1998). It is possible that abnormalities of electrophysiology and eye movements that are corrected by specific forms of nicotine replacement could be linked to smoking cessation outcomes with these products; however, this has not been explicitly studied. Nicotine nasal spray has unique features such as intermittent dosing, a rapid onset of action, and more immediate craving relief, which may make it more desirable for persons with SCZ as compared to the patch. In addition, nicotine nasal spray has demonstrated particular efficacy in highly dependent smokers (Sutherland, Stapleton, et al., 1992; Hjalmarson, Franzon, Westin, & Wiklund, 1994).
Smokers with SCZ seem to respond well to the nicotine nasal spray, with excellent compliance and few side effects, making this a promising treatment approach (Williams & Foulds, 2007; Williams, Ziedonis, & Foulds, 2004). We reported on a case series of 12 smokers with SCZ or SA who had not succeeded with previous treatments for tobacco dependence and did well with nicotine nasal spray treatment. Many patients remained abstinent or significantly reduced their smoking, and tolerability and overall use of the spray was better than published reports of its use in the general population (Williams et al., 2004).
The purpose of this research study was to measure the effects of nicotine nasal spray compared to nicotine patches on cue-induced urges to smoke in a randomized, 3-day open-label trial for smokers with SCZ or schizoaffective disorder.
METHODS
Participants
Subjects were smokers with SCZ or SA recruited from outpatient mental health centers (all subtypes of SCZ and SA were included). They were informed that this was not a treatment study, but a research study to measure the effects of nicotine nasal spray and nicotine patches on urges to smoke. At screening, subjects had to be willing to abstain from all tobacco products for 3 days. Because we were interested in the clinical applicability of this craving study, we wished to study subjects in the abstinent state and offered subjects monetary incentives to abstain temporarily from smoking although they were not otherwise trying to quit. Contingency management has been shown to be an effective technique for promoting cigarette abstinence (Heil, Tidey, Holmes, Badger, & Higgins, 2003) and is effective in laboratory studies of SCZ in which subjects are motivated to abstain for the anticipation of monetary reinforcement (Tidey, O'Neill, & Higgins, 2002).
After participants were provided with a description of the study, written informed consent was obtained. The institutional review board of the Robert Wood Johnson Medical School (RWJMS) approved the protocol. Study procedures were carried out at the RWJMS Department of Psychiatry Craving/Physiology laboratory. All subjects had a chart diagnosis of SCZ or SA and smoked more than 10 cigarettes per day. Seriously cognitively impaired patients were excluded, and subjects were required to score 22 or higher on the Folstein Mini-Mental Status Examination (Folstein, Folstein, & McHugh, 1975) to be eligible. Eligibility requirements were as follows: age 18–70 years; no use of tobacco other than cigarettes; enrolled in mental health treatment and stable on antipsychotic medications; no use of clonidine, bupropion, or any nicotine replacement products; no medical history of unstable angina pectoris, myocardial infarction, or significant cardiac arrhythmia (including atrial fibrillation) in the past 90 days; not pregnant, lactating, or planning to become pregnant; and no problematic substance abuse in the last month. All subjects were required to bring their own cigarettes for testing procedures.
We screened 35 smokers with SCZ or SA. Of these, 8 were deemed ineligible (1 for incorrect diagnosis, 3 for transportation issues, 1 for use of bupropion, 1 for smoking fewer than 10 cigarettes per day, 2 did not call back). We consented 27 smokers with SCZ or SA, although all the data from 1 subject were subsequently excluded when it was discovered that there were marked inconsistencies in his reports of his smoking behavior.
Baseline Assessments
Following the screening, review, and signing of consent forms, subjects completed baseline assessments for demographic information, smoking history, and nicotine dependence. Women of childbearing capacity had a urine pregnancy test. Chart review was conducted to verify psychiatric diagnosis and current medication dose. Expired carbon monoxide (CO) readings were assessed by having participants take a deep breath and hold it for 15 seconds before exhaling into a handheld carbon monoxide monitor (EC-50 Smokerlyzer, Bedfont Scientific).
Subjects completing all baseline assessments including cue exposure received $25. Participants were paid an additional $5 for each subsequent visit to the laboratory on day 2 and day 3, but only subjects who remained abstinent were eligible to participate in the 2-hour testing session on day 3 and receive the additional $75 compensation. Subjects who were not abstinent were dropped from the study. At study end, all subjects received a referral for treatment to one of the services provided in the New Jersey Comprehensive Tobacco Control Program (NJQuitline, Quitnet, or Quitcenters), including the Tobacco Dependence Program at the University of Medicine and Dentistry, New Jersey-School of Public Health.
Baseline Cue Exposure
Subjects were instructed to smoke as usual on the morning of the session and to bring their pack of cigarettes with them. They were told not to consume any alcohol for 12 hours prior to each test session and to eat breakfast. All laboratory sessions occurred during approximately the same 4-hour period (10 am–2 pm), with follow-up laboratory procedures scheduled at the same time of day to reduce circadian effects. Immediately upon arrival, subjects were instructed to smoke one of their own cigarettes and the exact time of this cigarette was recorded. Research staff collected the used cigarette butt and cigarette packs for the subsequent testing.
In a temperature-controlled and sound-attenuated room in the cue exposure laboratory, patients were seated in a comfortable chair in an effort to minimize any distractions. Immediately upon arrival to the laboratory, an expired CO reading and a non-deprived craving assessment was performed (Session A; See Table 1 for description of Sessions A–G).
TABLE 1.
Description of Complete Cue Exposure Session
| Session | |
|---|---|
| A | Immediate arrival to laboratory; on baseline (smoking) day, this measurement immediately follows a cigarette. |
| B | 60 minutes after arrival to laboratory; paced breathing task in which subjects are instructed to breathe at a rate of 10 breaths/second for a period of 5 minutes. |
| C | Neutral cue consisting of guided imagery of library/reading newspaper scenario, with visualization of newspaper for a period of 5 minutes. |
| D | Active cue consisting of guided imagery of waiting for ride and wanting to have a cigarette, with visualization of subject's pack of cigarettes and extinguished cigarette butt for a period of 5 minutes. |
| E | 10 minutes after start of active cue. |
| F | 15 minutes after start of active cue. |
| G | 20 minutes after start of active cue. |
| Day 1 | Subjects were instructed to abstain completely from use of all tobacco products on this day and to begin using their assigned study medication. They did not visit the study center on this day. |
| Day 2 | Participants returned to the study center for a treatment adherence check to verify abstinence from cigarettes and measure any medication side effects. Any subject who reported smoking or had a carbon monoxide value >15 ppm was dropped from the study. |
| Day 3 | Subjects who remained abstinent returned for repeat laboratory sessions including test of cue exposure. Any subject who reported smoking or had a carbon monoxide value >10 ppm was dropped from the study and did not complete the testing sessions. |
Subjective measurements of craving were measured with a 4-item visual analogue scale. Responses to each item were rated on a scale of 0 (not at all) to 10 (strong), with responses summed over the 4 items to produce a total craving score at each session (maximum score = 40). Smokers rated the following 4 items: need to smoke, urge to smoke, want to smoke, and crave a cigarette, which has a reliability (Cronbach's α) >.95 (Shiffman et al., 2003; Shiffman, Paty, Gnys, Kassel, & Hickcox, 1996). We defined a positive craving response as an increase in the total score by 1 or more unit.
Cue exposure procedures occurred approximately 60 minutes after smoking the timed cigarette. The delay in testing procedures allowed the subjects to become accustomed to the laboratory and physiological monitoring and also to assess craving in a moderately nicotine-deprived state (60 minutes since last cigarette). Testing sessions for both neutral and active cues used a combination cue that incorporated audiotape-guided imagery and the in vivo presentation of smoking paraphernalia (see Table 2 for neutral and active cue scripts). A nicotine craving scale and physiological measurements were used to assess nicotine craving at baseline and after each presentation of nicotine cues or neutral cues. To standardize procedures, all instructions were scripted and read aloud by a research assistant. Recordings of physiology were monitored unobtrusively via closed-circuit video and equipment located outside the laboratory room.
TABLE 2.
Neutral and Active Cue Scripts
| Neutral Cue Script | |
| You are about to visit the public library. You see the building ahead of you. It is tall and white. There are long white columns on the front side. The many windows are colored dark so you can't see in. In front of the library is a large green lawn. The grass has been freshly cut and you can smell it. The brick walkway turns to the left and right under your feet as you make your way to the front door. The library is open and the lights are on. You see the lights shining in from the front door window. As you pass through the door, you see the librarian sitting at her desk. She is looking down at her work and does not notice you pass by. In front of her are stacks of books piled high. Next to her desk is a black wastebasket. The clock on the wall ticks quietly. Some people are behind the bookshelves reading. It is quiet. You can hear the sounds of pages turning. You walk by a rack of magazines that reaches all the way to the ceiling. Folded newspapers are displayed on a round table next to the magazine rack. You select today's newspaper from the counter and sit down at an empty table. You don't start to read immediately but instead look around the room. Other people are looking down at their books and magazines reading quietly. You feel calm as you look around the room and see the newspaper in front of you on the table. Please close your eyes and continue to imagine yourself in this scenario sitting quietly in the library. Now open your eyes, see the newspaper in front of you, and imagine yourself getting ready to read it after we finish. Okay… stop. |
|
| Active Cue Script | |
| Imagine yourself outside on a cool but sunny day waiting for a ride. You are wearing a light jacket, so you feel comfortable but wish your ride would get there to pick you up already. You are drinking a cup of coffee from a Styrofoam cup while you wait outside alone. You're getting a little bored from waiting by yourself with no one to talk to and nothing to do. You wish you could look busy. You notice the smell the hot coffee, and you feel the cool breeze and warm sun on your face. Time keeps passing, and still no one to pick you up. You're feeling bored and watch other people as they walk by. You notice someone taking a cigarette pack out of their pocket. It's your brand. You're bored and start to think how much you'd like a cigarette too. This person stops near you. He taps the end of the cigarette pack and takes out a cigarette. He puts the cigarette in his mouth and protects the end of the cigarette from the light breeze as he lights it with a match. You can see his lips purse as he takes his first drag to light the cigarette and watch him slowly exhale the smoke. You take another sip of your coffee and think about how great a cigarette would go with it. The smoker you were watching keeps walking by, but you can smell the smoke in the air. You're feeling pretty bored from waiting. As you wonder when your ride will get there, you reach for your cigarettes. You're relieved that you still have a couple left. Please close your eyes and continue to imagine yourself in this scenario waiting for a ride and getting ready to smoke a cigarette. Now open your eyes, see your cigarettes in front of you, and imagine yourself having a cigarette when you leave here after we finish. Okay… stop. |
|
Because we are interested in studies of craving methodology and psychophysiology, we conducted an additional placebo assessment condition that consisted of a paced breathing exercise (session B). Subjects were instructed to match their breathing rate to a paced marker shown on a computer screen directly in front of them that was set to 10 breaths/minute, which is considered a placebo rate in studies of biofeedback with nonsignificant changes in baroreflex sensitivity (Bernardi et al., 2002; Bernardi, Porta, Gabutti, Spicuzza, & Sleight, 2001; Halamek et al., 2003). Session B began 60 minutes after session A and lasted for 5 minutes, during which time physiological data were recorded. At the completion of 5 minutes of paced breathing, subjects were immediately shown the visual analogue scale and responses were recorded by the research assistant. The remaining sessions (C–G) described below were subsequently continued at 5-minute intervals until the procedure was completed.
For the neutral cue (session C), an audiotape was played of a prerecorded guided imagery script (see Table 2 for full text of neutral and active scripts). At the same time, a tray with an object was presented directly in front of the subject by the research assistant. The tape instructed the subject to look at the object (newspaper) and imagine the scenario being described. The subject was instructed not to move or speak during testing procedures. Five minutes of physiological measuring were also recorded. The audiotape and physiological measurements were stopped after 5 minutes and craving responses were recorded.
For the active cue (session D), an audiotape was played of a prerecorded guided imagery script. At the same time, a tray of objects was presented directly in front of the subject by the research assistant. The tape instructed the subject to look at the objects (lighter, extinguished cigarette butt in ashtray, subject's pack of cigarettes) and imagine the scenario being described. The subject was instructed not to move or speak during the testing procedures. Five minutes of physiological measures were also recorded. The audiotape and physiological measurements were stopped after 5 minutes and craving responses were recorded.
Subjects were then instructed to sit silently as they underwent three more assessments of craving at 5-minute intervals for a total elapsed time of 20 minutes (sessions E, F, and G).
Medication Procedures
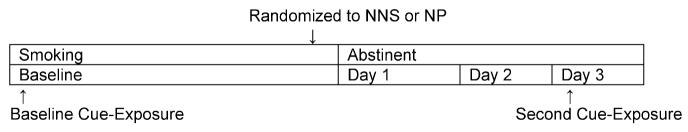
Following the baseline cue exposure procedures, subjects were randomly assigned to nicotine nasal spray (Nicotrol NS) or a 21-mg nicotine patch (Nicoderm CQ) in an open-label design (see Figure 1 for single-subject study timeline). We used a random sequences block randomization procedure with block sizes of 2, 3, and 4 (at random). Investigational agents were packaged by the study team, and a 3-day supply (of either three 21-mg patches or three 10-mL bottles of nasal spray) was provided to subjects.
FIGURE 1.
Single-Subject Timeline for Smoking Cue Exposure Sessions
Subjects were instructed to use the nicotine product they were assigned for a 3-day period (day 1–day 3), during which time they should abstain completely from tobacco. The 3-day lead-in allowed subjects to become habituated to the use of the nasal spray and minimized side effects during testing procedures. The 3-day lead-in has been used by other investigators for nicotine cue exposure studies in order to compare anticraving medications during a period of abstinence (Shiffman et al., 2003). The medication start date was defined by the research team to coincide with the testing schedule. Patients were given verbal and written instructions about the use of nicotine medications.
Nicotrol NS is an aqueous solution of nicotine intended for administration as a metered spray to the nasal mucosa. Each 10-mL spray bottle contains 100 mg nicotine (10 mg/mL) and is manufactured by Pfizer/Pharmacia Corporation, which provided product support for this study. Those randomized to the nicotine nasal spray received a demonstration on how to use it and then took a test dose with the research staff observing, although they were not to start the nicotine nasal spray until awakening on day 1. They were instructed to use the nasal spray within a range of 1 dose/hour (minimum 8 doses/day) to a maximum of 40 doses/day. One dose was measured as two sprays, one into each nostril. They were instructed to carry the nasal spray with them and bring it to follow-up sessions. Subjects were instructed about the most commonly reported side effects of nasal irritation, sneezing, lacrimation, and rhinorrhea, which usually remit with continued use.
The Nicoderm CQ patch (GlaxoSmithKline) is a 21-mg/24-hour patch used in most studies of smokers with SCZ that provides continuous delivery of nicotine throughout the day. Those randomized to the nicotine patch received instructions to put on the patch at home upon awakening on day 1 and wear it for 24 hours, changing patches at approximately the same time each morning. Subjects were instructed about the most commonly reported side effects from the patch including mild skin irritation and were educated about how to administer the patch, what to do if the patch came off, how to handle common issues such as taking a shower, and how to dispose of the patch.
Subjects were informed that those who were unable to abstain completely from tobacco during the 3 study days would cease participation in the study. Subjects always started medications on Tuesday or Wednesday as day 1 so as to have day 3 testing on Thursday or Friday. Subjects were instructed to abstain completely from all tobacco products upon awakening on day 1 and to begin using their assigned study medication. On day 1 (quit date), subjects did not visit the study center but research staff called them to ask about smoking and remind about the visit on day 2. No other pharmacological or behavioral treatment was provided.
All subjects made brief visits to the office on day 2 to verify abstinence and check for medication adherence and medication side effects. On day 2 or day 3, subjects who reported smoking or had a CO value >15 ppm or 10 ppm, respectively, were withdrawn from the study. On day 3, subjects returned for a 2-hour laboratory testing session for repeat cue exposure (sessions A–G), in which they were under constant observation by a research assistant. Immediately upon arrival at laboratory, they had an expired CO reading and visual analogue scale craving assessment. Subjects receiving nicotine nasal spray were permitted to use the spray at any time in the sessions (except for 5-minute testing periods when they were not allowed to move), and a research assistant recorded the timing of use.
Statistical Procedures
Baseline variables were compared between nicotine nasal spray and nicotine patch groups using the two-sample t-tests and chi-square tests, as appropriate. For baseline and day 3, separate two-sample t-tests were carried out to compare mean craving scores between nicotine nasal spray and nicotine patch groups at each session (A-G). In addition, to evaluate the changes in craving scores from baseline to day 3 in each session, we used separate repeated measures ANCOVA, which included 2 medication types and 2 days (baseline and day 3), controlling for cigarettes per day. All statistical analyses was performed using SPSS version 14.0
RESULTS
Subject Attrition
Most (21/26; 80.8%) of the subjects met the study's abstinence criteria at all sessions. The data from the 5 subjects who smoked (4 randomized to the nicotine patch, 1 to nicotine nasal spray) were excluded from all subsequent analyses. Subjects who did not remain abstinent were more nicotine-dependent than completers, smoked more cigarettes per day at baseline, and had higher CO values and Fagerstrom Test of Nicotine Dependence scores, although the differences were not significant in this small sample. The mean expired CO values for abstinent subjects at day 3 were not significantly different between groups (nicotine nasal spray, 2.0 ppm vs. nicotine patch, 1.4 ppm). The mean expired CO levels in nonsmoking subjects at day 2 and day 3 were 2.53 and 1.67 ppm, respectively.
Demographics
Table 3 presents the demographic and smoking data by medication group for participants completing the study. No significant differences were observed between groups on all variables, although there was a trend for nicotine patch users to smoke more cigarettes per day and report greater levels of nicotine dependence.
TABLE 3.
Baseline Characteristics of Smokers With Schizophrenia or Schizoaffective Disorder Randomized to Nicotine Nasal Spray (NNS) or Nicotine Patch (NP) (N = 21)*
| NNS Group (n = 10) Mean (SD) |
NP Group (n = 10) Mean (SD) |
p value | |
|---|---|---|---|
| Cigarettes per day | 23.9 (12.02) | 29.6 (12.70) | .302 |
| Baseline CO (ppm) | 15.80 (4.63) | 19.36 (9.43) | .294 |
| FTND | 5.50 (2.32) | 7.00 (1.34) | .082 |
| Years smoked | 18.6 (7.38) | 17.2 (2.75) | .559 |
| Age of first smoking | 11.2 (5.03) | 14.64 (3.72) | .089 |
| Age | 50.40 (7.92) | 44.5 (9.3) | .139 |
| Past quit attempts | 3.90 (2.42) | 4.09 (3.44) | .886 |
| Age at onset of psychiatric problems |
21.78 (14.90) | 22.00 (8.075) | .967 |
| CPZ equivalents | 263.9 (158.8) | 412.2(291.4) | .189 |
| Race/ethnicity | W 8/AA 1/O 1 | W 6/AA 2/O 3 | .333 |
| Sex | M 6/F 4 | M 8/F 3 | .537 |
| Antipsychotic drug class | 9 ATP/1 TYP | 9 ATP/1 TYP/1 BOTH | .620 |
| Diagnosis | SCZ 7/SA 3 | SCZ 9/SA 2 | .525 |
Note.
5 subjects excluded for failure to remain abstinent during the study period.
M = male; F = female; W = white; AA = African American; O = other; CO = carbon monoxide; FTND = Fagerstrom Test for Nicotine Dependence; ATP = atypical antipsychotic; TYP = typical antipsychotic; SCZ = schizophrenia; SA = schizoaffective disorder; CPZ = chlorpromazine.
Medication Use
Compliance with both nicotine nasal spray and nicotine patch regimens was excellent, and no one dropped out of the study due to adverse effects from study medications. The average self-reported number of doses of nicotine nasal spray per day over the first 2 full days of use was 19.7 doses per day.
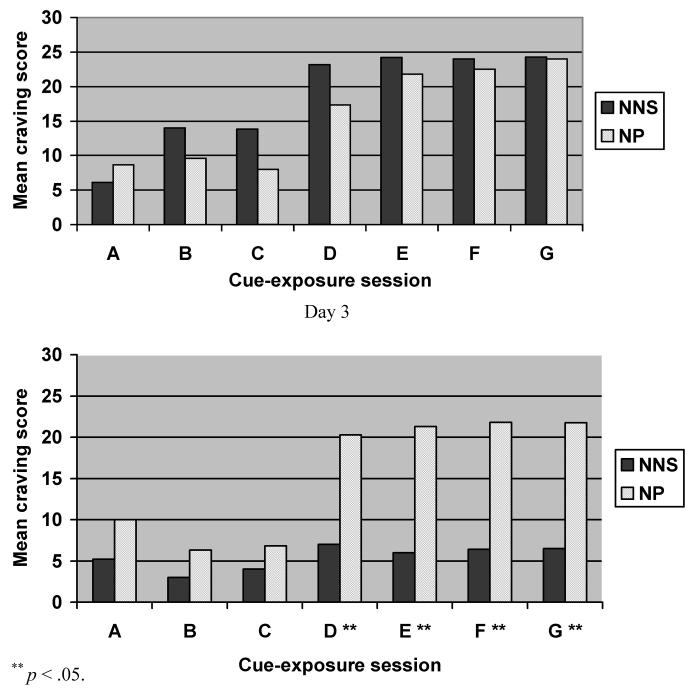
Baseline Craving Scores
Figure 2 presents the mean ratings of craving at all Sessions (A–G) on baseline and day 3 in each medication group. On the baseline (smoking) day, mean craving scores were not different between nicotine nasal spray and nicotine patch groups at all test sessions (see upper panel of Figure 2). The response to active cues was robust for the entire sample, with an overall increase in craving score of 87% at baseline (mean total craving score of 20.1 for active cue compared to 10.8 for neutral cue). Both the neutral cue and paced breathing exercise produced a similarly low craving response. Craving provoked by the active cue continued to increase in the first 10 minutes (D–E) and then remained stable over the subsequent 10 minutes (F–G).
FIGURE 2.
Mean Score of Nicotine Craving at Baseline and Day 3 Baseline (Pre-nicotine Medication)
Day 3 Craving Scores
On Day 3, nicotine nasal spray users reported significantly less craving in response to smoking cues compared to nicotine patch users (mean craving scores: nicotine nasal spray, 7.0; nicotine patch, 20.3; p = .014). A repeated measures ANCOVA controlling for cigarettes per day evaluated the changes in craving (session D) from baseline to day 3. As compared to the nicotine patch group, the nicotine nasal spray group demonstrated significantly reduced craving in response to the active cue (F = 5.09; df = 1; p = .037).
Timing of Nicotine Nasal Spray on Day 3
Eight of the 11 nicotine nasal spray subjects used it during the laboratory session on say 3. Although the timing of this dose was not standardized, most subjects used it within a 15-minute period just before or after the active cue. We did an analysis of mean craving scores based on subjects who used the nicotine nasal spray prior to the active cue (n = 4), after the active cue (n = 4), or not at all (n = 3) during the testing session and found no significant differences in these subgroups.
DISCUSSION
This study demonstrates that nicotine nasal spray significantly reduced cravings in response to smoking cues, compared to cravings in people using the nicotine patch, in smokers with SCZ. In considerations of craving, it is important to distinguish “background” craving from “episodic” craving. Background craving is experienced as a kind of steady state throughout the day that requires no triggering environmental stimuli, whereas episodic craving that is occasional may be more intense and triggered by environmental (people, places, things) or affective cues (Shiffman, 2000). Episodic craving is associated with relapse and can be manipulated in the laboratory through exposure to smoking cues (Shiffman et al., 1996; Marlatt, 1990). Use of a nicotine patch may reduce background craving (Rose, Herskovic, Trilling, & Jarvik, 1985) but has not been found to attenuate cue-provoked craving in studies of smokers in the general population (Tiffany et al., 2000; Waters et al., 2004). We chose to test the nicotine nasal spray in comparison with the 21-mg (24-hour) patch preparation, as this dose was tested in clinical trials of smokers with SCZ and because it is associated with less craving than a 15-mg 16-hour patch (Shiffman et al., 2000).
Nicotine nasal spray might then be expected to predictably outperform nicotine patches in measures of response to cue-induced craving, although both products have similar efficacy rates (Silagy, Lancaster, Stead, Mant, & Fowler, 2004; Hajek, West, Foulds, Nilsson, Burrows, & Meadow, 1999). Nicotine nasal spray differs from other forms of nicotine replacement medications in that nicotine is absorbed rapidly through the nasal mucosa and produces physiological effects that more closely resemble smoking (Benowitz, Zevin, & Jacob, 1997). Nicotine nasal spray produces peak plasma nicotine levels within 5 minutes of use (Russell, Jarvis, Feyerabend, & Ferno, 1983; Sutherland, Russell, Stapleton, Feyerabend, & Ferno, 1992), which is expected to speed relief of withdrawal and is associated with less craving than the slower-acting nicotine patch (Perkins, Lerman, Keenan, Fonte, & Coddington, 2004). Doses can be self-administered in quick succession, up to 5 in a 1-hour period, and nicotine nasal spray has the advantage of giving patients a greater sense of control and ability to self-medicate relief during a craving crisis (Schneider et al., 1995). This is in contrast to the nicotine skin patch, which has a slow absorption, rising gradually to a relatively flat nicotine level in 6 hours and providing no immediate control over nicotine craving.
Despite this seemingly advantageous profile, use of the nicotine nasal spray in the general population lags far behind other forms of nicotine replacement. Irritation to the nasal mucosa is common with many smokers who discontinue the product early on due to these unpleasant adverse effects (Sutherland, Stapleton, et al., 1992; Hjalmarson et al., 1994; West, Hajek, Foulds, Nilsson, May, & Meadows, 2000; Jimenez Ruiz et al., 1999), although the most common adverse effects (runny nose, nasal irritation, throat irritation, watering eyes, and sneezing) decrease significantly in the first 7 days of use (Hurt, Dale, Croghan, Croghan, Gomez-Dahl, & Offord, 1998). The minimum of 8 doses per day is recommended because less is unlikely to be effective (Hurt et al., 1998), and the mean daily frequency in clinical efficacy trials was 7 to 15 doses/day (Sutherland, Stapleton, et al., 1992; Hjalmarson et al., 1994; Hurt et al., 1998; Schneider et al., 2003).
In this sample subjects, used nicotine nasal spray at an average of 20 doses per day in only the first 3 days of use. This is consistent with our published report that use of nicotine nasal spray in SCZ appears to be higher than in other groups (Williams et al., 2004). More frequent use of nicotine nasal spray in the general population is associated with improved quit rates (Hajek et al., 1999; Jones, Nguyen, & Man, 1998); however, we cannot conclude that greater craving relief in our sample of smokers with SCZ was achieved by a greater total dose of nicotine replacement in the nicotine nasal spray versus patch groups, since we did not measure nicotine or cotinine levels.
Our entire sample of smokers with SCZ or SA responded to cues with increased craving. This is in contrast to at least one other study that showed only a 50% cue reactivity in a sample of smokers with and without SCZ (Fonder et al., 2005). One possible explanation was that our methods produced a more robust response due to our choice of cues. Imagined and in vivo smoking cues are equally effective at eliciting high levels of self-reported urges and may produce a more robust craving effect than still photos (Drobes & Tiffany, 1997).
Study Limitations
This was a pilot study that did not use placebo medications and could be influenced by the open-label design. In addition, the nicotine nasal spray group had the added behavioral advantage of being actively used in the laboratory session, which may have alleviated craving. This raises the issue of expectancies and how this might have influenced the study outcomes. Stronger expectancy effects for nicotine nasal spray compared to nicotine patches are expected given the strong side effects of nicotine nasal spray. Only future studies of a placebo-controlled design can address this issue completely.
However, this difference mirrors the real-life characteristics of the products. Limitations of the study include that cues were not counterbalanced. This was to avoid a carryover response that could occur in response to active cues and effect neutral cues (Sayette & Hufford, 1994; Rohsenow & Niaura, 1999). Craving in smokers with SCZ in particular can be influenced by this carryover effect since a ceiling effect has been described for smokers with SCZ in that they tend to report high levels of craving near the end of the measurement scale, even before exposure to cues (Tidey & Williams, 2007). Another limitation was that we did not assess nicotine blood levels or conduct assessments of mood or nicotine withdrawal. Future studies should include placebo groups and also consider the possible effects of sex on smoking cue reactivity.
In summary, this study found that abstaining smokers with SCZ had similar craving levels in response to no or neutral cues while wearing a nicotine patch or using a nicotine nasal spray but that the nicotine nasal spray resulted in much lower craving ratings than the patch in response to active smoking cues. Compliance with both medications was very good, and these results suggest that nicotine nasal spray may have particular advantages for preventing cue-induced relapse in smokers with SCZ who are trying to quit.
Acknowledgments
This work was supported by grants from the National Institute on Drug Abuse (K23-DA140090-01 to JMW and K23-DA 018203-02 to MLS) and Pfizer, Inc, who also supplied product support. The authors (JMW, JF, MLS) are also supported in part by grants from the New Jersey Department of Health and Senior Services through funds from New Jersey's Comprehensive Tobacco Control Program.
The authors wish to thank Dr. Mikael Franzon for his assistance with this study.
REFERENCES
- Abrams DB, Monti PM, Carey KB, Pinto RP, Jacobus SI. Reactivity to smoking cues and relapse: Two studies of discriminant validity. Behavior Research and Therapy. 1988;26(3):225–233. doi: 10.1016/0005-7967(88)90003-4. [DOI] [PubMed] [Google Scholar]
- Addington J. Group treatment for smoking cessation among persons with schizophrenia. Psychiatric Services. 1998;49(7):925–928. doi: 10.1176/ps.49.7.925. [DOI] [PubMed] [Google Scholar]
- Adler LE, Hoffer LD, Wiser A, Freedman R. Normalization of auditory physiology by cigarette smoking in schizophrenic patients. American Journal of Psychiatry. 1993;150:1856–1861. doi: 10.1176/ajp.150.12.1856. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Zevin S, Jacob P., 3rd. Sources of variability in nicotine and cotinine levels with use of nicotine nasal spray, transdermal nicotine, and cigarette smoking. British Journal of Clinical Pharmacology. 1997;43(3):259–267. doi: 10.1111/j.1365-2125.1997.00566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardi L, Porta C, Gabutti A, Spicuzza L, Sleight P. Modulatory effects of respiration. Autonomic Neuroscience. 2001;90(1–2):47–56. doi: 10.1016/S1566-0702(01)00267-3. [DOI] [PubMed] [Google Scholar]
- Bernardi L, Porta C, Spicuzza L, Bellwon J, Spadacini G, Frey AW, et al. Slow breathing increases arterial baroreflex sensitivity in patients with chronic heart failure. Circulation. 2002;105(2):143–145. doi: 10.1161/hc0202.103311. [DOI] [PubMed] [Google Scholar]
- Breese CR, Lee MJ, Adams CE, Sullivan B, Logel J, Gillen KM, et al. Abnormal regulation of high affinity nicotinic receptors in subjects with schizophrenia. Neuropsychopharmacology. 2000;23(4):351–364. doi: 10.1016/S0893-133X(00)00121-4. [DOI] [PubMed] [Google Scholar]
- Carter BL, Tiffany ST. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94(3):327–340. [PubMed] [Google Scholar]
- Carter BL, Tiffany ST. The cue-availability paradigm: The effects of cigarette availability on cue reactivity in smokers. Experimental and Clinical Psychopharmacology. 2001;9(2):183–190. doi: 10.1037//1064-1297.9.2.183. [DOI] [PubMed] [Google Scholar]
- Chambers RA, Krystal JH, Self DW. A neurobiological basis for substance abuse comorbidity in schizophrenia. Biological Psychiatry. 2001;50(2):71–83. doi: 10.1016/s0006-3223(01)01134-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childress AR, Hole AV, Ehrman RN, Robbins SJ, McLellan AT, O'Brien CP. Cue reactivity and cue reactivity interventions in drug dependence. NIDA Research Monograph. 1993;137:73–95. [PubMed] [Google Scholar]
- Conklin CA, Tiffany ST. The impact of imagining personalized versus standardized urge scenarios on cigarette craving and autonomic reactivity. Experimental and Clinical Psychopharmacology. 2001;9(4):399–408. doi: 10.1037//1064-1297.9.4.399. [DOI] [PubMed] [Google Scholar]
- de Leon J, Abraham G, Nair C, Verghese C, McGrory A, McCann E. Nicotine addiction in chronic schizophrenic inpatients. Biological Psychiatry. 1995;37:593–683. [Google Scholar]
- Drobes DJ, Tiffany ST. Induction of smoking urge through imaginal and in vivo procedures: Physiological and self-report manifestations. Journal of Abnormal Psychology. 1997;106(1):15–25. doi: 10.1037//0021-843x.106.1.15. [DOI] [PubMed] [Google Scholar]
- Droungas A, Ehrman RN, Childress AR, O'Brien CP. Effect of smoking cues and cigarette availability on craving and smoking behavior. Addictive Behaviors. 1995;20(5):657–673. doi: 10.1016/0306-4603(95)00029-c. [DOI] [PubMed] [Google Scholar]
- Ehrman RN, Robbins SJ, Childress AR, O'Brien CP. Conditioned responses to cocaine-related stimuli in cocaine abuse patients. Psychopharmacology. 1992;107:523–529. doi: 10.1007/BF02245266. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-Mental State: A practical method for grading the state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fonder MA, Sacco KA, Termine A, Boland BS, Seyal AA, Dudas MM, et al. Smoking cue reactivity in schizophrenia: Effects of a nicotinic receptor antagonist. Biological Psychiatry. 2005;57(7):802–808. doi: 10.1016/j.biopsych.2004.12.027. [DOI] [PubMed] [Google Scholar]
- Freedman R, Adams CE, Leonard S. The alpha7-nicotinic acetylcholine receptor and the pathology of hippocampal interneurons in schizophrenia. Journal of Chemical Neuroanatomy. 2000;20:299–306. doi: 10.1016/s0891-0618(00)00109-5. [DOI] [PubMed] [Google Scholar]
- Glautier S, Tiffany ST. Methodological issues in cue reactivity research. In: Drummond DC, Tiffany ST, Glautier G, Remington B, editors. Addictive behaviour: Cue exposure theory and practice. John Wiley & Sons; New York: 1995. [Google Scholar]
- Griffith JM, O'Neill JE, Petty F, Garver D, Young D, Freedman R. Nicotinic receptor desensitization and sensory gating deficits in schizophrenia. Biological Psychiatry. 1998;44(2):98–106. doi: 10.1016/s0006-3223(97)00362-4. [DOI] [PubMed] [Google Scholar]
- Hajek P, West R, Foulds J, Nilsson F, Burrows S, Meadow A. Randomized comparative trial of nicotine polacrilex, a transdermal patch, nasal spray and an inhaler. Archives of Internal Medicine. 1999;159:2033–2038. doi: 10.1001/archinte.159.17.2033. [DOI] [PubMed] [Google Scholar]
- Halamek J, Kara T, Jurak P, Soucek M, Francis DP, Davies LC, et al. Variability of phase shift between blood pressure and heart rate fluctuations: A marker of short-term circulation control. Circulation. 2003;108(3):292–297. doi: 10.1161/01.CIR.0000079222.91910.EE. [DOI] [PubMed] [Google Scholar]
- Heil SH, Tidey JW, Holmes HW, Badger GJ, Higgins ST. A contingent payment model of smoking cessation: Effects on abstinence and withdrawal. Nicotine & Tobacco Research. 2003;5(2):205–213. doi: 10.1080/1462220031000074864. [DOI] [PubMed] [Google Scholar]
- Hjalmarson A, Franzon M, Westin A, Wiklund O. Effect of nicotine nasal spray on smoking cessation. Archives of Internal Medicine. 1994;154(28):2567–2572. [PubMed] [Google Scholar]
- Hughes JR, Hatsukami DK, Mitchell JE, Dahlgren LA. Prevalence of smoking among psychiatric outpatients. American Journal of Psychiatry. 1996;143:993–997. doi: 10.1176/ajp.143.8.993. [DOI] [PubMed] [Google Scholar]
- Hurt RD, Dale LC, Croghan GA, Croghan IT, Gomez-Dahl LC, Offord KP. Nicotine nasal spray for smoking cessation: Pattern of use, side effects, relief of withdrawal symptoms, and cotinine levels. Mayo Clinic Proceedings. 1998;73(2):118–125. doi: 10.1016/S0025-6196(11)63642-2. [DOI] [PubMed] [Google Scholar]
- Jimenez Ruiz CA, Florez Martin S, Ramos Pineda A, Lorza JJ, Hernandez-Mezquita MA, Solano Reina S, et al. Nasal nicotine spray in smoking cessation. Results of a multicenter study. Archives of Broncopneumologia. 1999;35(11):535–538. doi: 10.1016/s0300-2896(15)30005-3. [DOI] [PubMed] [Google Scholar]
- Jones RL, Nguyen A, Man SFP. Nicotine and cotinine when nicotine nasal spray is used to quit smoking. Psychopharmacology. 1998;137:345–350. doi: 10.1007/s002130050629. [DOI] [PubMed] [Google Scholar]
- Lazev AB, Herzog TA, Brandon TH. Classical conditions of environmental cues to cigarette smoking. Experimental and Clinical Psychopharmacology. 1999;7(1):56–63. doi: 10.1037//1064-1297.7.1.56. [DOI] [PubMed] [Google Scholar]
- Marlatt GA. Cue exposure and relapse prevention in the treatment of addictive behaviors. Addictive Behaviors. 1990;15(4):395–399. doi: 10.1016/0306-4603(90)90048-3. [DOI] [PubMed] [Google Scholar]
- Maude-Griffin PM, Tiffany ST. The production of smoking urges through imagery: The impact of affect and smoking abstinence. Experimental and Clinical Psychopharmacology. 1996;4:198–208. [Google Scholar]
- Niaura R, Abrams D, Demuth B, Pinto R, Monti P. Responses to smoking-related stimuli and early relapse to smoking. Addictive Behaviors. 1989;14(4):419–428. doi: 10.1016/0306-4603(89)90029-4. [DOI] [PubMed] [Google Scholar]
- Niaura R, Abrams DB, Pedraza M, Monti PM, Rohsenow DJ. Smokers' reactions to interpersonal interaction and presentation of smoking cues. Addictive Behaviors. 1992;17(6):557–566. doi: 10.1016/0306-4603(92)90065-4. [DOI] [PubMed] [Google Scholar]
- Niaura R, Shadel WG, Abrams DB, Monti PM, Rohsenow DJ, Sirota A. Individual differences in cue reactivity among smokers trying to quit: Effects of gender and cue type. Addictive Behaviors. 1998;23(2):209–224. doi: 10.1016/s0306-4603(97)00043-9. [DOI] [PubMed] [Google Scholar]
- Olincy A, Ross RG, Young DA, Roath M, Freedman R. Improvement in smooth pursuit eye movements after cigarette smoking in schizophrenic patients. Neuropsychopharmacology. 1998;18(3):175–185. doi: 10.1016/S0893-133X(97)00095-X. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Lerman C, Keenan J, Fonte C, Coddington S. Rate of nicotine onset from nicotine replacement therapy and acute responses in smokers. Nicotine & Tobacco Research. 2004;6(3):501–507. doi: 10.1080/14622200410001696547. [DOI] [PubMed] [Google Scholar]
- Rickard-Figueroa K, Zeichner A. Assessment of smoking urge and its concomitants under an environmental smoking cue manipulation. Addictive Behaviors. 1985;10(3):249–256. doi: 10.1016/0306-4603(85)90005-x. [DOI] [PubMed] [Google Scholar]
- Rohsenow DJ, Niaura RS. Reflections on the state of cue-reactivity theories and research. Addiction. 1999;94(3):343–344. [PubMed] [Google Scholar]
- Rose JE, Herskovic JE, Trilling Y, Jarvik ME. Transdermal nicotine reduces cigarette craving and nicotine preference. Clinical Pharmacology & Therapeutics. 1985;38:450–456. doi: 10.1038/clpt.1985.203. [DOI] [PubMed] [Google Scholar]
- Russell MAH, Jarvis MJ, Feyerabend C, Ferno O. Nasal nicotine solution: A potential aid to giving up smoking. British Medical Journal (Clinical Research Ed.) 1983;286:683–684. doi: 10.1136/bmj.286.6366.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayette MA, Hufford MR. Effects of cue exposure and deprivation on cognitive resources in smokers. Journal of Abnormal Psychology. 1994;103(4):812–818. doi: 10.1037//0021-843x.103.4.812. [DOI] [PubMed] [Google Scholar]
- Sayette MA, Shiffman S, Tiffany ST, Niaura RS, Martin CS, Shadel WG. The measurement of drug craving. Addiction. 2000;95(Supplement 2):S189–S210. doi: 10.1080/09652140050111762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider MP, van Melle G, Uldry C, Huynh-Ba M, Fallab Stubi CL, Iorillo D. Electronic monitoring of long-term use of the nicotine nasal spray and predictors of success in a smoking cessation program. Nicotine & Tobacco Research. 2003;5(5):719–727. doi: 10.1080/14622200310001608545. [DOI] [PubMed] [Google Scholar]
- Schneider NG, Olmstead R, Mody FV, Doaan K, Franzon M, Jarvik MA, et al. Efficacy of a nicotine nasal spray in smoking cessation: A placebo-controlled, double blind trial. Addiction. 1995;90:1671–1682. doi: 10.1046/j.1360-0443.1995.901216719.x. [DOI] [PubMed] [Google Scholar]
- Shadel WG, Niaura R, Abrams DB. Effect of different cue stimulus delivery channels on craving reactivity: Comparing in vivo and video cues in regular cigarette smokers. Journal of Behavior Therapy & Experimental Psychiatry. 2001;32(4):203–209. doi: 10.1016/s0005-7916(01)00035-0. [DOI] [PubMed] [Google Scholar]
- Sherr JD, Myers C, Avila MT, Elliott A, Blaxton TA, Thaker GK. The effects of nicotine on specific eye tracking measures in schizophrenia. Biological Psychiatry. 2002;52(7):721–728. doi: 10.1016/s0006-3223(02)01342-2. [DOI] [PubMed] [Google Scholar]
- Shiffman S. Comments on craving. Addiction. 2000;95(Supplement 2):S171–S175. doi: 10.1080/09652140050111744. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Elash CA, Paton SM, Gwaltney CJ, Paty JA, Clark DB, et al. Comparative efficacy of 24-hour and 16-hour transdermal nicotine patches for relief of morning craving. Addiction. 2000;95(8):1185–1195. doi: 10.1046/j.1360-0443.2000.95811855.x. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Paty JA, Gnys M, Kassel JA, Hickcox M. First lapses to smoking: Within-subjects analysis of real-time reports. Journal of Consulting & Clinical Psychology. 1996;64(2):366–379. doi: 10.1037//0022-006x.64.2.366. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Shadel WG, Niaura R, Khayrallah MA, Jorenby DE, Ryan CF, et al. Efficacy of acute administration of nicotine gum in relief of cue-provoked cigarette craving. Psychopharmacology (Berlin) 2003;166(4):343–350. doi: 10.1007/s00213-002-1338-1. [DOI] [PubMed] [Google Scholar]
- Silagy C, Lancaster T, Stead L, Mant D, Fowler G. Nicotine replacement therapy for smoking cessation. Cochrane Database of Systematic Reviews. 2004;3:CD000146. doi: 10.1002/14651858.CD000146.pub2. [DOI] [PubMed] [Google Scholar]
- Smelson DA, Losonczy MF, Kilker C, Starosta A, Kind J, Williams J, et al. An analysis of cue reactivity among persons with and without schizophrenia who are addicted to cocaine. Psychiatric Services. 2002;53:1612–1616. doi: 10.1176/appi.ps.53.12.1612. [DOI] [PubMed] [Google Scholar]
- Song HS, Lehrer PM. The effects of specific respiratory rates on heart rate and heart rate variability. Applied Psychophysiology & Biofeedback. 2003;28(1):13–23. doi: 10.1023/a:1022312815649. [DOI] [PubMed] [Google Scholar]
- Sutherland G, Russell MAH, Stapleton J, Feyerabend C, Ferno O. Nasal nicotine spray: A rapid nicotine delivery system. Psychopharmacology. 1992;108:512–518. doi: 10.1007/BF02247430. [DOI] [PubMed] [Google Scholar]
- Sutherland G, Stapleton JA, Russell MA, Jarvis MJ, Hajek P, Belcher M, et al. Randomized controlled trial of nicotine nasal spray in smoking cessation. Lancet. 1992;340:324–329. doi: 10.1016/0140-6736(92)91403-u. [DOI] [PubMed] [Google Scholar]
- Tidey JW, O'Neill SC, Higgins ST. Contingent monetary reinforcement of smoking reductions, with and without transdermal nicotine, in outpatients with schizophrenia. Experimental & Clinical Psychopharmacology. 2002;10(3):241–247. doi: 10.1037//1064-1297.10.3.241. [DOI] [PubMed] [Google Scholar]
- Tidey JW, Rohsenow DJ, Kaplan GB, Swift RM. Subjective and physiological responses to smoking cues in smokers with schizophrenia. Nicotine & Tobacco Research. 2005;7(3):1–9. doi: 10.1080/14622200500125724. [DOI] [PubMed] [Google Scholar]
- Tidey JW, Williams JM. Clinical indices of tobacco use in people with schizophrenia. Journal of Dual Diagnosis. 2007;3(34):79–98. [Google Scholar]
- Tiffany ST. A cognitive model of drug urges and drug-use behavior: Role of automatic and nonautomatic processes. Psychological Review. 1990;97(2):147–168. doi: 10.1037/0033-295x.97.2.147. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Cox LS, Elash CA. Effects of transdermal nicotine patches on abstinence-induced and cue-elicited craving in cigarette smokers. Journal of Consulting & Clinical Psychology. 2000;68(2):233–240. doi: 10.1037//0022-006x.68.2.233. [DOI] [PubMed] [Google Scholar]
- Waters AJ, Shiffman S, Sayette MA, Paty JA, Gwaltney CJ, Balabanis MH. Cue-provoked craving and nicotine replacement therapy in smoking cessation. Journal of Consulting & Clinical Psychology. 2004;72(6):1136–1143. doi: 10.1037/0022-006X.72.6.1136. [DOI] [PubMed] [Google Scholar]
- West R, Hajek P, Foulds J, Nilsson F, May S, Meadows A. A comparison of the abuse liability and dependence potential of nicotine patch, gum, spray and inhaler. Psychopharmacology. 2000;149:198–202. doi: 10.1007/s002130000382. [DOI] [PubMed] [Google Scholar]
- Williams JM, Foulds J. Successful tobacco dependence treatment in schizophrenia. American Journal of Psychiatry. 2007;164(2):222–227. doi: 10.1176/ajp.2007.164.2.222. [DOI] [PubMed] [Google Scholar]
- Williams JM, Hughes JR. Pharmacotherapy treatments for tobacco dependence among smokers with mental illness or addiction. Psychiatric Annals. 2003;22(7):457–466. [Google Scholar]
- Williams JM, Ziedonis DM, Abanyie F, Steinberg ML, Foulds J, Benowitz NL. Increased nicotine and cotinine levels in smokers with schizophrenia and schizoaffective disorder is not a metabolic effect. Schizophrenia Research. 2005;79(2–3):323–335. doi: 10.1016/j.schres.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Williams JM, Ziedonis DM, Foulds J. A case series of nicotine nasal spray in the treatment of tobacco dependence among patients with schizophrenia. Psychiatric Services. 2004;55(9):1064–1066. doi: 10.1176/appi.ps.55.9.1064. [DOI] [PubMed] [Google Scholar]
- Ziedonis DM, George TP. Schizophrenia and nicotine use: Report of a pilot smoking cessation program and of neurobiological and clinical issues. Schizophrenia Bulletin. 1997;23(2):247–254. doi: 10.1093/schbul/23.2.247. [DOI] [PubMed] [Google Scholar]