Abstract
Objectives
The APOE ε4 allele and a history of depression are each separate risk factors for cognitive decline (CD). However, little research has investigated whether a history of depression influences the relationship between APOE ε4 and CD. The present study examined whether depressive symptoms had greater influence on subsequent CD among participants with APOE ε4 than those without the allele.
Design
Prospective six year longitudinal study.
Setting
Community in-home interviews.
Participants
A biracial sample of community dwelling older adults (N=1992) from the Duke Established Populations for Epidemiologic Studies of the Elderly (EPESE).
Measurements
Data were drawn from Waves 1 and 3 of the EPESE, which were conducted 6 years apart. Cognitive functioning and depressive symptoms were assessed at both waves, and APOE genotyping was completed during the Wave 3 assessment.
Results
Regression analyses revealed that depressive symptoms and the APOE ε4 allele independently predicted CD. Importantly, the influence of depressive symptoms on CD was greater for individuals with the APOE ε4 allele compared to those without the allele.
Conclusion
Depressive symptoms and the APOE ε4 allele are independent contributors to CD. Moreover, the influence of depressive symptoms on CD is greater among individuals with the APOE ε4 allele. Depression and the APOE ε4 allele may act together in disrupting neurological functioning, which may in turn lower an individual's cognitive reserve capacity. Given the efficacious treatments currently available for depression, future research should investigate the extent to which interventions for depression may reduce the risk for subsequent CD.
Keywords: APOE, depressive symptoms, cognitive decline
Cognitive decline (CD) is a significantly increasing problem as individuals age. Mild cognitive impairment affects between 22% and 56% of older adults [1, 2], and the prevalence of dementia in individuals 65 years and older is approximately 6 to 10%. Further, the prevalence of dementia climbs above 30% among individuals over age 85 [3]. Given the pervasiveness of cognitive impairment in our society, further investigation of factors that influence the onset and course of CD is merited. Identifying such factors may lead to greater insight into ways to prevent or slow the progression of CD. Two variables that have each been individually identified as placing a person at an increased risk for CD are the apolipoproteinE ε4 allele (APOE ε4) and a history of depression or depressive symptoms.
APOE ε4 is the most widely recognized genetic risk factor for Alzheimer's disease (AD) [4-6]. Nevertheless, other environmental or biological factors are also likely operating to influence CD. In particular, there is growing evidence that a history of depression may increase the risk for CD [7]. Although many studies have examined APOE ε4 and depressive symptoms separately as specific risk factors for CD, there is a paucity of research examining how these factors may interact to influence subsequent CD. In the present study, we examined whether the influence of depressive symptoms on CD was greater among participants with the ε4 allele than those without the allele. That is: are the effects of APOE ε4 and depressive symptoms simply independent risk factors for CD, or is there a synergistic relationship between the two factors?
The APOE ε4 allele on Chromosome 19 is a major susceptibility gene for late-onset AD, the most common form of dementia [4]. It is related to earlier and faster CD in persons with AD [8]. Longitudinal studies have demonstrated that this genotype is associated with CD in initially high-functioning community-dwelling elderly [9, 10]. The mechanisms by which the APOE ε4 allele confers risk are not altogether clear. However, the APOE ε4 allele has been implicated in contributing to higher levels of Aβ plaque and tangle formations, which are associated with AD [11].
Depression has also been identified as a risk factor for CD. Cross-sectional studies have frequently reported an association between depressive symptoms and poorer cognitive functioning [12, 13]. In some cases, up to half of those with dementia also have comorbid depression [14]. Although depression may occur in response to changes in cognitive abilities, depressive disorders may also lead to neurological changes that increase the risk for CD. In fact, longitudinal evidence has provided growing support for this idea. For instance, a history of depressive symptoms was associated with AD, even when the onset of depressive symptoms preceded the onset of AD by more than 25 years [15]. Moreover, Sachs-Ericsson and colleagues (2005) found that, in a large sample of community-dwelling older adults, depressive symptoms were associated with increased cognitive errors three years later. Additionally, results from a meta-analysis of case-control and prospective studies determined that a history of depression approximately doubles the risk for developing dementia [16]. Nonetheless, some studies have failed to find an association between prior depressive symptoms and the onset of CD [17-19].
Although both APOE genotype and depressive symptoms are implicated as risk factors for CD, few studies have examined their combined effect in increasing risk for CD. Among studies that have examined the combined effect, results have been mixed. Geda and colleagues (2006) found in a sample of 840 cognitively normal elderly subjects that the joint effect of depression and APOE genotype was significantly greater than the sum of their independent effects in increasing the risk for mild cognitive impairment (MCI) three and a half years after baseline [20]. Other studies have not found an interaction between prior depression and APOE genotype in their effects on AD risk or subsequent CD [21-23]. Some of these studies, however, have had methodological limitations. Foremost, limited power due to small sample sizes may explain why some studies have failed to find an interaction between APOE ε4 and depression. Further, some studies relied on participants' memory of previous episodes of depression rather than directly measuring current depressive symptoms. No prospective epidemiological studies, however, have examined whether the influence of depressive symptoms on CD would be greater among carriers of the APOE ε4 allele than noncarriers.
It is important to distinguish this investigation from other studies that have examined whether having the APOE ε4 allele is related to increased risk for depression, irrespective of rate of CD. Although some have found that the presence of the APOE ε4 allele is associated with increased risk of being severely depressed [24], Blazer and colleagues found using the Duke EPESE data that the APOE ε4 did not predict depression [25].
In the current study, in contrast, we examined whether APOE genotype and depressive symptoms interact in predicting CD over a six year period. In light of the current literature, we predicted that depressive symptoms and the APOE ε4 allele would both independently predict subsequent CD. Moreover, we predicted that the influence of depressive symptoms on CD would be greater among those with the ε4 allele than those without the ε4 allele.
This study has several methodological strengths. First, it included a large biracial sample of community dwelling elders and examined changes in cognitive functioning prospectively. Current depressive symptoms were assessed at baseline using the Center for Epidemiological Studies-Depression Scale (CES-D) [26], and therefore we did not rely on participants' memory of past depression. Cognitive functioning was assessed both at baseline and follow-up; thus we were able measure change in cognitive functioning over time. Finally, we were able to control for variables that have been associated with both depressive symptoms and CD (e.g., age, race, gender, health problems, physical functioning, education, income, and literacy).
Methods
Participants
The data used in the present analyses were drawn from the Duke Established Populations for Epidemiologic Studies of the Elderly (EPESE) project, a multi-center epidemiologic investigation of the physical, psychological, and social functioning of community-dwelling adults 65 years and older. The Duke-EPESE sample was comprised of residents selected from five contiguous counties in North Carolina. The current study focuses on data from Wave 1 (n = 4,162, 1986-87) and Wave 3 (n = 2,840, 1992-93). The sampling design strategy has been reported in detail elsewhere [27]. To summarize: the data were collected through in-person interviews. Participants were administered a questionnaire that included detailed information about demographics, psychological, physical, social, and cognitive functioning. At Wave 3 genotype information was obtained (see more detail below). In the current study, we chose to use data from Waves 1 and Wave 3 because this was the largest sample of participants for whom we had an assessment of both cognitive functioning and genotype.
Control Variables
We controlled for several variables that have been found to be associated with depressive symptoms and CD, including demographic variables (age, race, gender), education, literacy [28], income, as well as health functioning (e.g., diabetes, high blood pressure, heart attack, stroke) and physical functioning variables [29].
Measures
A comprehensive demographic section documented the age, gender, race, income, years of education, and literacy of the participants. Participants were asked to select an income category best representing their income during the last year. Income categories were: 1 (0 to 1,999), 2 (2,000 to 2,999), 3 (3,000 to 3,999), etc.
Measurement of Depression
The CES-D scale [26] was administered at Wave 1 (1986) (Cronbach's α = .82). For ease of administration, a modified version of the CES-D was used [30]. For the purpose of the study, a dichotomous response scale was used for each item, coded: 0 `No,' 1 `Yes.' Responses across the 20 items were summed to create a CES-D scale score (0 to 20). Higher scores indicate more depressive symptoms.
Assessment of genotype
At Wave 3 (six years after baseline), blood was drawn from 1,999 participants who gave their personal consent. Cheek swabs were obtained approximately four years later from 77 additional participants who had not undergone the initial blood draw. APOE genotype was determined by DNA extraction and polymerase chain reaction by methods described elsewhere [10]. The validity of the genotyping is indicated by the concordance of the allele frequency with Hardy-Weinberg Equilibrium for the sample as a whole χ2 (df = 3, N = 2076) = 7.21, p. > .25).
In this present study, participants were divided into two groups: those who had at least one APOE ε4 allele and those who had no ε4 allele. As some participants refused to undergo blood draw, genotype information is not available for all participants. Among the 2840 participants interviewed at Wave 3, there were 746 for whom we did not have genotype information. Additionally, there were 186 participants with other missing data. Thus, we included 1992 participants in the current study who had complete data on all variables.
Cognitive Functioning
The 10-item SPMSQ [31] was used to measure global cognition at Wave 1 (α = .74), and Wave 3 (α = .93). Items assessed orientation and knowledge, such as the date and current president. Participants' errors were summed across items to form a continuous scale (0 to 10 errors), with higher scores indicating more difficulty with cognitive functioning.
For some additional analyses, a binary measure of the SPMSQ was computed. Specifically, the errors on the Time-2 SPMSQ were dichotomized, with a score of `2' representing approximately the lowest 10% of the population (i.e., those with the greatest number of errors). The remaining 90% of the sample was coded `1'.
Physical Functioning
At baseline, three items from the Rosow-Breslau functional health scale [32] were used to assess physical functioning. Items involved unaided ability to do heavy housework, walk up and down stairs, and walk one-half mile (α = .79).
Health problems
At baseline, participants were asked to indicate whether a physician had informed them that they had certain health problems, including heart attack, diabetes, hypertension, stroke, and hip fracture. Responses were coded: 1 `No,' 2, `possibly,' 3 `Yes.'
Chronic health problems
Physicians provided ratings to indicate the impact of each medical condition described above on physical health. When a condition was present, the respondent was given a score equal to the mean physician rating for that condition. These scores were then summed across conditions. This measure is an index of the severity of overall health problems.
Assessment of Literacy
Interviewers assessed participants' literacy based on their ability to read several pieces of written information given to them during the first interview (See Sachs-Ericsson [28]).
Data Analytic Strategy
Initial analyses were conducted to describe the demographics of the participants, as well as the relevant variables included in the subsequent regression analyses. These variables are presented in Table 1.
Table 1.
Description of the sample by APOE ε4 status at Time 1a
| Sample as Whole (N = 1993) | Non-APOE ε4 carriers (N = 1362) | APOE ε4 carriers (N = 631) | F-statistic or χ2 | p-value | |
|---|---|---|---|---|---|
| Race | Caucasian: 45.3% African-American: 54.3.% |
Caucasian: 76.5% African-American: 61.3% |
Caucasian: 23.5% African-American: 38.7% |
χ2 = 52.3 | p < .01 |
| Gender | Male: 33.1% | Male: 32.9% | Male: 34.4% | χ2 = .81 | p = .42 |
| Age | 71.6 (5.37) | 71.9 (5.40) | 71.1 (5.27) | F = 8.3 | p < .01 |
| Education | 9.0 years (4.03) | 9.0 (4.04) | 8.94 (3.99) | F = .47 | p = .49 |
| Income | $11,532.07 (10,725.70) | $11,965.13 (11,009.37) | $10,597.32 (10,045.33) | F = 7.0 | p < .01 |
| Literacy | Literate: 90.9% | Literate: 91.3% | Literate: 90.0% | χ2 = .37 | p = .21 |
| Heart Attack | No: 86.6% Maybe: 2.7% Yes: 9.7% |
No: 87.6% Maybe: 2.7% Yes: 9.7% |
No: 87.6% Maybe: 2.5% Yes: 9.8% |
χ2 = .06 | p = .97 |
| Stroke | No: 95.1% Maybe: 1.6% Yes: 4.3% |
No: 95.7% Maybe: 0.7% Yes: 3.5% |
No: 93.8% Maybe: 0.3% Yes: 5.9% |
χ2 = 6.9 | p = .03 |
| Diabetes | No: 82.3% Maybe: 2.8% Yes: 14.9% |
No: 82.5% Maybe: 3.2% Yes: 14.2% |
No: 81.9% Maybe: 1.9% Yes: 16.2% |
χ2 = 3.8 | p = .15 |
| HBP | No: 42.9% Maybe: 3.3% Yes: 53.8% |
No: 43.8% Maybe: 3.2% Yes: 53.0% |
No: 40.9% Maybe: 3.5% Yes: 55.6% |
χ2 = 1.6 | p = .46 |
| Chronicity of health problemsb | .88 | .89 | .86 | F = .55 | p = .46 |
| Physical Functioning Problemsb |
.62 | .61 | .63 | F = .10 | p = .75 |
| CES-D Wave 1b | 2.9 | 2.8 | 3.1 | F = 2.8 | p = .09 |
| SPMSQ Wave 1b | 1.35 | 1.33 | 1.40 | F = 2.4 | p = .23 |
| SPMSQ Wave 3b | 1.73 | 1.58 | 2.06 | F = 28.6 | p < .01 |
Degrees of freedom for each variable was df = 1, 1993, except for the specific health problems, in which df = 2, 1993.
Higher scores represent more problems
Several analyses were completed to examine the influence of depressive symptoms on the relationship between the APOE ε4 allele and CD. First, hierarchical linear regression analyses were conducted in which we examined the main effect of depressive symptoms and the main effect of APOE ε4 on CD while controlling for variables associated with cognitive functioning. Next, we examined whether the influence of depressive symptoms on CD was greater for those with the ε4 allele than for those without the allele by including in the analyses the standardized interaction term between depressive symptoms and the genotype.
A secondary logistic regression analysis was conducted using a dichotomous measure of errors on the SPMSQ with a threshold at approximately the tenth percentile (i.e., 10% of the population with the highest number of errors vs. the remaining population). This additional analysis was conducted to provide further information regarding the risk conferred by the interaction of the APOE ε4 allele and depressive symptoms (i.e., the odds ratio). A hierarchical logistic analysis was performed using the same order of inclusion of the variables as in the hierarchical linear regression analysis described above.
To reduce the variance across allele types another hierarchical linear regression analysis was also performed in which the sample was restricted to ε3/3 and ε3/4 carriers. Because our original analyses grouped several different variations of genotypes that are related to varying degrees of risk for CD, we decided to isolate participants with two distinguishable genotypes (ε3/3 and ε3/4). We specifically chose to compare ε3/3 and ε3/4 carriers for several reasons. First, the literature suggests that possessing at least one ε4 allele is related to an increased risk for AD and an earlier age of onset [4]. Additionally, although sample size is reduced by eliminating participants with other allele combinations, participants with the ε 3/3 (n=1075) and ε3/4 (n=482) variations comprised the two largest groups among the six genotype variations (i.e., 76% of the population).
Results
Descriptive statistics
Participants (N=1992) were 45.3% White and 33.1% male. The average age of participants at Wave 1 was 71.6 (SD=5.37). Among the participants, 32% had at least one ε4 allele. Specifically, less than one percent were ε2/2 carriers, 14% were ε2/3 carriers, 4% were ε2/4 carriers, 54% were ε3/3 carriers, 24% were ε3/4 carriers, and 3% ε4/4 carriers. As reported previously [33], African Americans had a higher rate of the APOE ε4 allele than Caucasians (38.7% vs. 23.5), χ2 (N=1992) = 52.3, p < .01. Importantly, the negative impact of the APOE ε4 allele was found in a previous study to equally affect African Americans and Caucasians [33]. Table 1 provides descriptive statistics for the participants by genotype.
Prediction of CD: Linear regression analysis
We performed linear regression analysis with Time-1 depressive symptoms and APOE ε4 allele as the independent variables and CD as the dependent variable, controlling for demographic variables (age, gender, and race), indices of SES (income, literacy, and years of education), physical functioning, specific health variables and chronicity of health problems. The regression analysis is summarized in Table 2. Because the sample as a whole tended to have more correct responses than incorrect responses on the SPMSQ, the frequency distribution of cognitive errors was not normally distributed at Time-2. Nevertheless, in Step 1 of the regression analysis we controlled for the number of incorrect responses at Time-1 in predicting incorrect responses at Time-2. Thus, we actually examined the difference in SPSMQ errors between Time-1 and Time-2. The difference in cognitive errors between Time-1 and Time-2 was normally distributed, which is important with regard to satisfying the assumption of normality in the regression analysis.
Table 2.
Regression Model
| Unstandard Coefficients | F | Sig. | 95% Confidence Interval | Corr | |||||
|---|---|---|---|---|---|---|---|---|---|
| df | B | Std. Error | p-value | Lower | Upper | Partial | |||
| Stepl | F(4,1988) = 271.8 p <.001 (Constant) | -3.358 | .371 | 81.92 | p <.001 | -4.085 | -2.630 | ||
| SPMSQ errors Wave 1 | 1,1988 | .571 | .022 | 653.91 | p <.001 | .527 | .615 | .50 | |
| Age | 1,1988 | .044 | .005 | 72.12 | p <.001 | .034 | .054 | .19 | |
| Gender | 1,1988 | -.026 | .058 | .21 | p = .65 | -.140 | .088 | -.01 | |
| Race | 1,1988 | .479 | .057 | 70.88 | p <.001 | .367 | .590 | .19 | |
| Step 2 | F(7,1985) = 271.8 p<.001 | ||||||||
| Education | 1,1985 | -.066 | .009 | 56.87 | p <.001 | -.083 | -.049 | -.17 | |
| Literacy | 1,1985 | -.627 | .099 | 39.95 | p <.001 | -.821 | -.432 | -.14 | |
| Income | 1,1985 | .000 | .000 | .02 | p = .89 | .000 | .000 | .00 | |
| Step 3 | F(14,1978) = 94.5 p <.001 | ||||||||
| Physical Functioning | 1,1978 | .111 | .029 | 14.57 | p <.001 | .054 | .168 | .09 | |
| Chronic Health Problems | 1,1978 | -.022 | .043 | .26 | p = .61 | -.107 | .063 | -.01 | |
| Heart Attack | 1,1978 | .023 | .048 | .22 | p = .64 | -.072 | .118 | .01 | |
| HBP | 1,1978 | -.020 | .029 | .48 | p = .49 | -.078 | .037 | -.02 | |
| Diabetes | 1,1978 | .031 | .044 | .52 | p = .47 | -.054 | .117 | .02 | |
| Broken Hip | 1,1978 | -.011 | .092 | .01 | p = .91 | -.192 | .170 | -.003 | |
| Stroke | 1,1978 | .073 | .067 | 1.19 | p = .28 | -.059 | .205 | .02 | |
| Step 4 | F(16,1976) = 84.8, p <.001 | ||||||||
| Time 1 CES-D | 1,1976 | .031 | .009 | 12.42 | p <.001 | .014 | .048 | .08 | |
| APOE ε4 status | 1,1976 | .163 | .057 | 8.27 | p <.01 | .052 | .275 | .07 | |
| Step 5 | F(17,1975) = 80.7, p <.001 | ||||||||
| Interaction Time 1 | |||||||||
| CES-D and APOE ε4 status | 1,1975 | .081 | .027 | 8.98 | p <.01 | .028 | .135 | .07 | |
In Step 1, we first controlled for Time-1 cognitive errors on the SPMSQ, F(1,1988) = 653.9, p < .01. By controlling for Time 1 errors, we could then examine the influence of each of the other variables in relation to the change in errors over time (e.g., CD). We found increasing age, F(1,1988) = 72.2, p < .01, and African American race, F(1,1988) = 70.9, p < .01 to predict CD. However, gender was unrelated to CD. In Step 2, we included indices of social economic status. Income was unrelated to CD. However, fewer years of education, F(1,1985) = 56.9 p < .01, and literacy, F(1,1985) = 39.9, p < .01, predicted CD.
In Step 3, we entered each of the specific health problems, the index of chronicity of health problems, and physical functioning. Only physical functioning problems were related to CD, F(1,1978) = 14.6, p < .01. In Step 4, we entered the main effects of depressive symptoms and the main effects of the APOE ε4 allele. Depressive symptoms, F(1,1976) = 12.4, p < .01, and the APOE ε4 allele, F(1,1976) = 8.3 p < .01, both predicted CD.
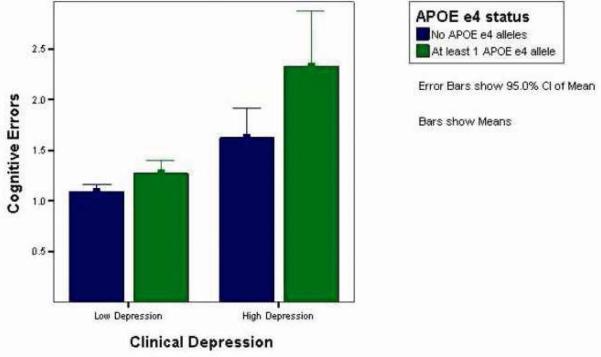
In Step 5, we entered the interaction term of the APOE ε4 allele and depressive symptoms, which was significant, F(1,1975) = 8.97, p < .01. As illustrated in Figure 1, the interaction was such that depressive symptoms had a greater impact on those who were carriers of the APOE ε4 allele to those who were non-carriers.
Figure 1.
Prediction of CD: Dichotomized SPMSQ
In order to determine the additional risk conferred by the interaction of depressive symptoms and the APOE ε4 allele, we conducted a logistic regression analysis using the dichotomous measure of errors on the SPMSQ (e.g., approximately 10% with the highest number of errors coded `2' and the remaining participants coded `1'). We found that each of the variables that was significant in the above-described linear regression analysis was also significant in the logistic regression analysis, with one exception. The main effect of depression was unrelated to the dichotomous SPMSQ score, Wald (1, 1976) = .203, p = .652, OR = 1.02; 95% CI: .946-1.092. However, the APOE ε4 allele predicted the dichotomized SPMSQ measure, Wald (1, 1976) = 5.3, p = .02, OR = 1.34; 95% CI: 1.044-1.716. Importantly, as we found in the above linear regression analysis, the interaction of depressive symptoms and the APOE ε4 allele predicted the dichotomized SPMSQ measure, Wald (1, 1975) = 5.3, p = .03, OR = 1.1; 95% CI: 1.008-1.151, such that depressive symptoms had a greater influence on those who possessed the APOE ε4 allele compared to those who did not.
APOE ε3/3 and the ε3/4 carriers
We performed an additional linear regression analysis using a subsample of participants with the APOE ε3/3 and the ε3/4 genotype combinations. As in the original analysis, the dependent variable, cognitive errors at Time 2, was continuous. First, we found that Time 1 depressive symptoms predicted CD, F(1, 1540) = 10.579, p < .01. However, there was no main effect of genotype F(1, 1540) = 2.372, p = .124. Importantly, as we found in the original analysis, the interaction of depressive symptoms and the APOE combination predicted CD, such that the influence of depressive symptoms was greater for individuals with the APOE ε3/4 combination compared to those with the ε3/3 combination, F(1, 1539) = 4.04, p < .05. The failure of the APOE ε3/4 gene to predict CD may represent a loss of power with the exclusion of 149 participants with either the ε2/4 or ε4/4 genotype.
Race, the APOE ε4 allele and depressive symptoms
There is controversy surrounding the predictive power of the APOE ε4 allele among African Americans, with some studies finding that the genotype predicts CD among Whites but not among African Americans [33]. To further explore this issue, we repeated the above linear regression analyses for African Americans and Whites separately. We found that the pattern of the results were consistent with those described above. That is, for both Whites and African Americans separately there was a main effect of depressive symptoms, a main effect of the APOE genotype, and a significant interaction of the two variables in predicting change in cognitive errors.
Effect of age
Age is a critical factor in Alzheimer and dementia (with risk increasing with age); thus it was of interest to determine whether, after controlling for the main effect of age, there was an increased risk associated with the APOE genotype, depression and their interaction with increasing age. Thus, we conducted subsequent linear regression analyses including the interactions of the APOE genotype and age as well as age and depression. Although there was (as reported earlier) a main effect of age, none of the interactions with age were significant; that is, after controlling for the main effect of age, increasing age did not confer further risk in association with the APOE ε4 allele or depression.
Discussion
The APOE ε4 allele and depression are each independent risk factors for cognitive decline (CD); however, there has been little research to date examining whether depressive symptoms moderate the relationship between APOE ε4 and CD. The purpose of the current study, therefore, was to determine whether depressive symptoms had a greater influence on CD among participants with the ε4 allele than those without the allele.
Using prospective linear regression analyses based on data from a large sample of community-dwelling older adults, we found depressive symptoms and the APOE ε4 allele to each separately predict CD over a 6 year period. Importantly, and consistent with our prediction, depressive symptoms moderated the association between the APOE ε4 allele and CD such that the influence of depressive symptoms was greater for individuals with the APOE ε4 allele compared to those without the allele.
Important for explaining the association between depressive symptoms and CD, several studies have shown a history of depressive symptoms to be associated with neuropathological changes in the brain that may impact cognition. In particular, depression has been shown to affect the hippocampus, a brain region which plays a pivotal role in memory formation [34]. The hippocampus is thought to be affected through a depression-initiated glucocorticoid cascade associated with the dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis. In support of this theory, Sheline and colleagues found a significant correlation between total lifetime duration of depression and hippocampal volume loss [35]. Sheline and colleagues also demonstrated that middle-aged and older individuals with a history of recurrent major depression had smaller hippocampal gray matter volumes than a group of pair-wise matched normal controls [36]. Additionally, some studies have found depression to have greater influence on the neurological functioning of individuals with AD compared to those without AD. For example, one study found that patients with AD and a lifetime history of depression showed both more rapid CD and higher levels of amyloid-beta (Aβ) plaque and tangle formation within the hippocampus than patients with AD but without a history of depression [37]. Rapp and colleagues also found that persons with AD and comorbid depression had more advanced stages of neurofibrillary tangles than patients with AD without comorbid depression [38].
Although the mechanisms through which APOE affects cognitive functioning are not entirely understood [39], the APOE ε4 allele has also been shown to predict structural abnormalities in the brain. It has also been suggested that the APOE gene may be involved in stimulating Aβ deposition as well as in enhancing its production [39]. For example, the APOE ε4 allele is associated with higher stages of both intraneuronal neurofibrillary changes and extraneuronal Aβ deposition [11], as well as greater hippocampal volume loss [40].
Although both APOE ε4 and depressive symptoms are implicated in pathological abnormalities within the brain, few previous studies have examined the combined effect of depressive symptoms and APOE ε4 in increasing risk for CD. This interaction may be best explained within the context of a cognitive reserve model. Specifically, the brain attempts to respond to brain insults by using neural networks that are less susceptible to disruption [41]. When negative factors act on the brain, the brain may actively compensate for pathology through the use of other routes that have not been affected by this disruption. However, if the brain is damaged by multiple factors, it may be increasingly difficult to recruit alternative neurological networks because there are fewer unaffected routes from which to choose.
The influence of depression on individuals who already have neurological damage is likely to be one of the contributing causes to individual differences in cognitive reserve, or one's capacity to cope with advancing brain pathological abnormalities. Moreover, genetic factors (e.g., APOE ε4) may interact with other factors (such as depression) to produce even greater variations in cognitive functioning. Thus, the synergistic interaction observed between depressive symptoms and the APOE ε4 allele may be a result of the inability of the brain to compensate for this combined assault.
In evaluating this study, several limitations should be considered. First, although we found depressive symptoms to moderate the association between APOE genotype and CD, even after controlling for several known risk factors for CD, other potential third variables may have been operating and may account for this relationship. These variables may be genetic, environmental, or both.
Second, this study used single-item self report measures of health problems. Self report measures have demonstrated good reliability, validity, and agreement with physician's diagnoses [42]. Nonetheless health assessments were dependent on the participants' ability to accurately recall medical diagnoses and also on their seeking health care. Therefore, some health problems may have been underreported, and the relationships between APOE ε4, depressive symptoms, and cognitive decline may be explained, in part, by unmeasured health problems.
Third, the present study assessed cognitive functioning using the SPMSQ, a brief, ten-point measure of global cognition. Although the SPMSQ has been shown to have good reliability and validity [31], it is a broad, overall measure of cognitive functioning and is less sensitive to subtle changes in cognitive functioning. This may have obscured the associations among depressive symptoms, genotype and CD.
Fourth, it should be noted that we only included participants' current depressive symptoms in the analysis. Thus, although we used Time-1 depressive symptoms to predict Time-2 cognitive functioning, our measure of depression did not assess participants' past history of depression. Indeed, chronic or prolonged depressive symptoms appear to have a greater impact on cognition than more transient symptoms of depression [43]. Our measure of depressive symptoms at Time-1 may be conceptualized as a proxy variable for a history of depression However, this is less precise than a measure of lifetime clinical depression, and it introduces two important confounds. Our analyses failed to take into account participants' history of depression if they had no depressive symptoms at Time-1. Second, depressive symptoms assessed at Time-1 may have been symptomatic of prodromal signs of dementia that were not yet observable on our measure of cognitive functioning. Thus, this possible prodromal sign of dementia may have increased, or even caused, the apparent relationship between depressive symptoms and CD.
Finally, it should be noted that our study had significant missing data due to participants' deaths, refusal of genotyping, and severe cognitive dysfunction interfering with their reports. Moreover, this missing data was not random. Participants with missing data were found to have higher levels of depressive symptoms and cognitive errors at Wave 1. Thus, missing data may have attenuated the gene-depression interaction found in the current study. However, we performed additional analyses to address this issue. In these analyses we assumed all participants with missing data to be carriers of the APOE ε4 allele. Results from these analyses were consistent with our original results (i.e., main effects of depressive symptoms, main effects of APOE genotype, and a significant interaction of depressive symptoms and APOE genotype). Nonetheless, given the large number of participants with missing data, the study's findings may not generalize to the population as a whole.
In sum, our findings suggest that the influence of depressive symptoms on CD is greater among participants with the APOE ε4 allele than those without the allele. Future research should investigate the mechanisms by which both depression and APOE together affect the internal structure of the brain and contribute to CD. Moreover, although the genetic vulnerability for CD cannot be modified, treatment for depression may help reduce the risk for subsequent CD or slow its progression. Given the availability of efficacious pharmacological and psychological treatments for depression as well as the recent interest and promising nature of depression prevention research in old age [44-47], future research should explore the impact of interventions for depression on CD.
Acknowledgments
Supported, in part, by grant 5R01-AG20614 from the National Institute on Aging
References
- 1.DeCarli C. Mild cognitive impairment: Prevalence, prognosis, aetiology, and treatment. The Lancet Neurology. 2003;2(1):15. doi: 10.1016/s1474-4422(03)00262-x. [DOI] [PubMed] [Google Scholar]
- 2.Lopez O, et al. Prevalence and classification of mild cognitive impairment in the Cardiovascular Health Study Cognition Study: Part 1. Archives of Neurology. 2003;60(10):1385. doi: 10.1001/archneur.60.10.1385. [DOI] [PubMed] [Google Scholar]
- 3.Hendrie H. Epidemiology of dementia and Alzheimer's disease. American Journal of Geriatric Psychiatry. 1998;6(2):S3. doi: 10.1097/00019442-199821001-00002. [DOI] [PubMed] [Google Scholar]
- 4.Corder EH, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993;261(5123):921. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 5.Saunders AM, et al. Association of apolipoprotein E allele [epsilon]4 with late-onset familial and sporadic Alzheimer's disease. Neurology. 1993;43(8):1467. doi: 10.1212/wnl.43.8.1467. [DOI] [PubMed] [Google Scholar]
- 6.Strittmatter WJ, et al. Apolipoprotein E: High-avidity binding to {beta}-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proceedings of the National Academy of Sciences; 1993. p. 1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sachs-Ericsson N, et al. The influence of depression on cognitive decline in community-dwelling elderly persons. Am. J. Geriatr. Psychiatry. 2005;13(5):402. doi: 10.1176/appi.ajgp.13.5.402. [DOI] [PubMed] [Google Scholar]
- 8.Martins CAR, et al. APOE alleles predict the rate of cognitive decline in Alzheimer disease: A nonlinear model. Neurology. 2005;65(12):1888. doi: 10.1212/01.wnl.0000188871.74093.12. [DOI] [PubMed] [Google Scholar]
- 9.Bretsky P, et al. The role of APOE-{epsilon}4 in longitudinal cognitive decline: MacArthur Studies of Successful Aging. Neurology. 2003;60(7):1077. doi: 10.1212/01.wnl.0000055875.26908.24. [DOI] [PubMed] [Google Scholar]
- 10.Fillenbaum G, et al. The relationship of APOE genotype to cognitive functioning in African American and White community resident elderly. Journal of the American Geriatrics Society. 2001;49(9):1148. doi: 10.1046/j.1532-5415.2001.49230.x. [DOI] [PubMed] [Google Scholar]
- 11.Ohm TG, et al. Apolipoprotein E polymorphism influences not only cerebral senile plaque load but also Alzheimer-type neurofibrillary tangle formation. Neuroscience. 1995;66(3):583. doi: 10.1016/0306-4522(94)00596-w. [DOI] [PubMed] [Google Scholar]
- 12.Lopez OL, et al. Risk factors for mild cognitive impairment in the Cardiovascular Health Study Cognition Study: Part 2. Archives of Neurology. 2003;60:1394. doi: 10.1001/archneur.60.10.1394. [DOI] [PubMed] [Google Scholar]
- 13.Rabbitt P, et al. Unique and interactive effects of depression, age, socioeconomic advantage, and gender on cognitive performance of normal healthy older people. Psychology and Aging. 1995;10(3):301. doi: 10.1037//0882-7974.10.3.307. [DOI] [PubMed] [Google Scholar]
- 14.Zubenko G, et al. A collaborative study of the emergence and clinical features of the major depressive syndrome of Alzheimer's disease. American Journal of Psychiatry. 2003;160(5):857. doi: 10.1176/appi.ajp.160.5.857. [DOI] [PubMed] [Google Scholar]
- 15.Green RC, et al. Depression as a risk factor for Alzheimer disease. Archives of Neurology. 2003;60:753. doi: 10.1001/archneur.60.5.753. [DOI] [PubMed] [Google Scholar]
- 16.Jorm AF. Is depression a risk factor for dementia or cognitive decline? A review. Gerontology. 2000;46(219):227. doi: 10.1159/000022163. [DOI] [PubMed] [Google Scholar]
- 17.Dufoil C, et al. Longitudinal analysis of the association between depressive symptomatology and cognitive deterioration. American Journal of Epidemiology. 1996;144(7):634. doi: 10.1093/oxfordjournals.aje.a008974. [DOI] [PubMed] [Google Scholar]
- 18.Chen P, et al. The temporal relationship between depressive symptoms and dementia. Archives of General Psychiatry. 1999;56(261):266. doi: 10.1001/archpsyc.56.3.261. [DOI] [PubMed] [Google Scholar]
- 19.Bassuk SS, Berkman LF, Wypij D. Depressive symptomatology and incident cognitive decline in an elderly community sample. Archives of General Psychiatry. 1998;55:1073. doi: 10.1001/archpsyc.55.12.1073. [DOI] [PubMed] [Google Scholar]
- 20.Geda Y, et al. Depression, apolipoprotein E genotype, and the incidence of mild cognitive impairment: A prospective cohort study. Archives of Neurology. 2006;63:435. doi: 10.1001/archneur.63.3.435. [DOI] [PubMed] [Google Scholar]
- 21.Steffens DC, et al. A twin study of late-onset depression and apolipoprotein E ε4 as risk factors for Alzheimer's disease. Biological Psychiatry. 1997;41(8):851. doi: 10.1016/S0006-3223(96)00247-8. [DOI] [PubMed] [Google Scholar]
- 22.Forsell Y, et al. Depression and dementia in relation to apolipoprotein E polymorphism in a population sample age 75+ Biological Psychiatry. 1997;42(10):898. doi: 10.1016/S0006-3223(96)00468-4. [DOI] [PubMed] [Google Scholar]
- 23.Lavretsky H, et al. Apolipopotein e4 allele status, depressive symptoms, and cognitive decline in middle-aged and elderly persons without dementia. American Journal of Geriatric Psychiatry. 2003;11(6):667. doi: 10.1176/appi.ajgp.11.6.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yen Y, et al. ApoE4 allele is associated with late-life depression: A population-based study. American Journal of Geriatric Psychiatry. 2007;15:858–868. doi: 10.1097/JGP.0b013e3180f63373. [DOI] [PubMed] [Google Scholar]
- 25.Blazer D, Burchette B, Fillenbaum G. APOE E4 and low cholesterol as risks for depression in a biracial elderly community sample. American Journal of Geriatric Psychiatry. 2002;10:515–520. [PubMed] [Google Scholar]
- 26.Radloff L. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measures. 1977;1(385):401. [Google Scholar]
- 27.Cornoni-Huntley J, et al. In: Established Populations for Epidemiologic Studies of the Elderly: Resource data book. Anonymous, editor. National Institute on Aging; Bethesda, MD: 1990. [Google Scholar]
- 28.Sachs-Ericsson N, Blazer DG. Racial differences in cognitive decline in a sample of community-dwelling older adults: The mediating role of education and literacy. American Journal of Geriatric Psychiatry. 2005;13(11):968. doi: 10.1176/appi.ajgp.13.11.968. [DOI] [PubMed] [Google Scholar]
- 29.Brayne C, et al. Vascular risks and incident dementia: results from a cohort study of the very old. Dementia & Geriatric Cognitive Disorders. 1998;9(3):175–80. doi: 10.1159/000017043. [DOI] [PubMed] [Google Scholar]
- 30.Blazer D, et al. The association of age and depression among the elderly: an epidemiologic exploration. Journal of Gerontology: Social Sciences. 1991;46:M210. doi: 10.1093/geronj/46.6.m210. [DOI] [PubMed] [Google Scholar]
- 31.Pfeiffer E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. Journal of the American Geriatrics Society. 1975;23(433):441. doi: 10.1111/j.1532-5415.1975.tb00927.x. [DOI] [PubMed] [Google Scholar]
- 32.Rosow I, Breslau N. A Guttman health scale for the aged. Journal of Gerontology. 1966;21:556. doi: 10.1093/geronj/21.4.556. [DOI] [PubMed] [Google Scholar]
- 33.Sawyer K, et al. Racial differences in the influence of the APOE epsilon 4 allele on cognitive decline in a sample of community dwelling older adults. 2008. In press. [DOI] [PubMed] [Google Scholar]
- 34.Kumaran D, Maguire EA. The human hippocampus: Cognitive maps or relational memory? J. Neurosci. 2005;25(31):7254–7259. doi: 10.1523/JNEUROSCI.1103-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sheline YI, et al. Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression. J. Neurosci. 1999;19(12):5034–5043. doi: 10.1523/JNEUROSCI.19-12-05034.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sheline YI, et al. Hippocampal atrophy in recurrent major depression. Proceedings of the National Academy of Sciences; 1996. p. 3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rapp MA, et al. Increased hippocampal plaques and tangles in patients with Alzheimer disease with a lifetime history of major depression. Archives of General Psychiatry. 2006;63(2):161. doi: 10.1001/archpsyc.63.2.161. [DOI] [PubMed] [Google Scholar]
- 38.Rapp MA, et al. Increased neurofibrillary tangles in patients with Alzheimer disease with comorbid depression. American Journal of Geriatric Psychiatry. 2008;16(2):168–174. doi: 10.1097/JGP.0b013e31816029ec. [DOI] [PubMed] [Google Scholar]
- 39.Mahley RW, Weisgraber KH, Huang Y. Apolipoprotein E4: A causative factor and therapeutic target in neuropathology including Alzheimer's disease. Proceedings of the National Academy of Sciences; 2006. p. 5644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moffat SD, et al. Longitudinal change in hippocampal volume as a function of apolipoprotein E genotype. Neurology. 2000;55(1):134. doi: 10.1212/wnl.55.1.134. [DOI] [PubMed] [Google Scholar]
- 41.Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. Journal of the International Neuropsychological Society. 2003;8:443. [PubMed] [Google Scholar]
- 42.Markides KS, Martin HW. Predicting self-related health among the aged. Research on Aging. 1979;1(1):97–112. [Google Scholar]
- 43.Dotson VM, Resnick SM, Zonderman AB. Differential association of concurrent, baseline, and average depressive symptoms with cognitive decline in older adults. American Journal of Geriatric Psychiatry. 2008;16:318–330. doi: 10.1097/JGP.0b013e3181662a9c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smits F, et al. An Epidemiological Approach to Depression Prevention in Old Age. American Journal of Geriatric Psychiatry. 2008;16(6):444–453. doi: 10.1097/JGP.0b013e3181662ab6. [DOI] [PubMed] [Google Scholar]
- 45.Rovner BW, Casten JR. Preventing late-life depression in age-related macular degeneration. American Journal of Geriatric Psychiatry. 2008;16(6):454–459. doi: 10.1097/JGP.0b013e31816b7342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cole MG. Brief interventions to prevent depression in older subjects: A systematic review of feasibility and effectiveness. American Journal of Geriatric Psychiatry. 2008;16(6):435–443. doi: 10.1097/JGP.0b013e318162f174. [DOI] [PubMed] [Google Scholar]
- 47.Reynolds CF. Preventing depression in old age: It's time. American Journal of Geriatric Psychiatry. 2008;16(6):433–434. doi: 10.1097/JGP.0b013e31816c7b67. [DOI] [PubMed] [Google Scholar]