Abstract
Background
Age-related alterations of left ventricular (LV) structure and function that may predispose to cardiovascular events are not well understood.
Methods and Results
We used cardiac magnetic resonance imaging (MRI) to examine age-related differences in LV structure and function in 5004 participants without overt cardiovascular disease when enrolled in the Multi-Ethnic Study of Atherosclerosis; 1099 participants received additional strain analyses by MRI tagging. We also assessed the relation of age-associated remodeling with cardiovascular outcomes using Cox proportional hazard models adjusting for cardiovascular risk factors. Although LV mass decreased with age (−0.3 g per year), the mass-to-volume ratio markedly increased (+5 mg/mL per year, p<0.0001), driven by a substantial reduction in end-diastolic volume (−0.8 mL per year, p<0.0001). Age was also associated with a significant fall in stroke volume (−0.4 mL per year, p<0.0001) along with strain patterns reflecting systolic (p<0.0001) as well as diastolic (p<0.01) myocardial dysfunction – despite a modestly enhanced ejection fraction (+0.1% per year, p<0.0001). Increased mass-to-volume ratio conferred a significant risk for total cardiovascular events; this trend was strongest among younger (<65 years, HR 3.69 [CI 1.34–10.10]) versus older (≥65 years, HR 1.68 [CI 0.77–3.68]) individuals with the highest compared to lowest mass-to-volume ratio quintile (Pinteraction=0.013).
Conclusion
Age is associated with a phenotype of LV remodeling marked by increased mass-to-volume ratio and accompanied by systolic, as well as diastolic, myocardial dysfunction that is not reflected by preserved ejection fraction. This pattern of ventricular remodeling confers significant cardiovascular risk, particularly when present earlier in life.
Keywords: aging, magnetic resonance imaging, myocardium, remodeling
Introduction
While age is one of the most powerful risk factors for cardiovascular disease, the mechanisms by which aging predisposes to cardiovascular morbidity and mortality remain incompletely understood.1–3 It is well known that age is associated with left ventricular (LV) hypertrophy, but less clear is the degree to which this remodeling is accompanied by age-specific relative changes in LV mass,4–11 volumes,11–17 chamber performance,13,15,16 and intrinsic myocardial function in humans.10,11,14,18 Recent work has described aging-related cellular8,19,20 and molecular21–23 processes that likely contribute to myocardial dysfunction. Although the relation of advancing age with diastolic dysfunction is widely recognized, associated changes in systolic function are less clear.11,16,24–27 Conventional diagnostic methods may be limited in their ability to detect intrinsic age-associated changes in human myocardial function in relation to structural chamber remodeling. Furthermore, their link to cardiovascular morbidity and mortality remains elusive.
In a large cohort of ethnically diverse individuals free of cardiovascular disease at baseline, we sought to comprehensively assess age-associated changes in LV structure and function using cardiac magnetic resonance imaging (MRI), and also determine if these changes predict cardiovascular outcomes. Cardiac MRI with myocardial tagging is considered the reference standard for assessing LV structural abnormalities and also offers the ability to assess myocardial function in fine detail. However, cardiac MRI tagging has not previously been used in large studies to concurrently investigate structural and functional aspects of LV aging.9,15–17,28,29 Therefore, we used a range of MRI techniques to characterize the phenotype of age-specific LV remodeling in detail, and to determine whether prominent aspects of such a phenotype are related to incident cardiovascular events.
Methods
Study Population
The original cohort included 6814 community-dwelling individuals enrolled in the Multi-Ethnic Study of Atherosclerosis (MESA). The design and objectives of MESA have been described elsewhere.30 In brief, study participants were 45–84 years of age, represented 4 racial/ethnic groups (non-Hispanic white, black, Hispanic, and Chinese), and had no history of clinical cardiovascular disease (diagnosed coronary artery disease, peripheral arterial disease, cerebrovascular disease, or heart failure) at enrollment. As part of the baseline examination, consenting participants underwent cardiac MRI studies at 6 centers across the United States.31 Of the 5004 participants who underwent cardiac MRI, 1099 received additional tissue tagging as described previously.31 Institutional review boards at each center approved the study protocol.
MRI Standard and Tagging Acquisition Protocols
Cardiac MRI images were obtained using 1.5-T MR scanners: Signa LX or CVi (GE Medical Systems, Waukesha, WI); and, Somatom Vision or Sonata (Siemens Medical Systems, Erlangen, Germany). Images were acquired using segmented k-space, ECG-triggered gradient echo pulse sequence17 during breathholds lasting 12–18 seconds. Two and 4-chamber cine MR images and short-axis cine images were obtained from above the mitral valve plane to the LV apex.
For the 1099 participants with tissue tagging performed, 3 tagged short-axis slices were obtained, uniformly spaced, from base to apex. Parallel stripe tags were prescribed separately in 2 orthogonal orientations (0°, 90°) using spatial modulation of magnetization. Parameters for tagged images were: field of view, 40 cm; slice thickness, 8–10 mm; repetition time, 6 ms (range, 3.5–7.2 ms); echo time, 3.0 ms (range, 2.0–4.2 ms); flip angle, 12°; matrix size, 256×96 to 140; phase-encoding views per segment, 4–9; tag spacing, 7 mm; and, temporal resolution, 20–40 ms. Myocardial strain was analyzed by harmonic phase imaging.
Parameters of Ventricular Structure and Function
Indices of LV structure and function included LV mass, end-diastolic volume (LVEDV), end-systolic volume (LVESV), stroke volume (SV), and ejection fraction (EF) obtained using commercially available software (MASS 4.2, MEDIS, Leiden, The Netherlands). Mass-to-volume (M/V) ratio was calculated as LV mass divided by LVEDV. Myocardial midwall circumferential strain was measured as (Ld − Lr) / Lr, where Ld is length in the deformed state and Lr is length in the relaxed state. By convention, positive strain denotes lengthening and negative strain denotes shortening. Peak systolic strain (Ecc) and early diastolic strain rate (SRE) were determined in each of 4 LV wall regions (anterior, lateral, septal, and inferior); mean midwall Ecc and SRE were calculated for each individual.
Clinical Events
Participants were followed via telephone interviews for incident cardiovacular events at intervals of 9–12 months from baseline. Details regarding the MESA processes and criteria for verifying, classifying, and adjudicating cardiovascular events have been reported.32 Medical records from approximately 98% of hospitalizations for cardiovascular events and from 95% of outpatient cardiovascular diagnostic encounters were obtained. A cardiovascular event was defined as: a new diagnosis of coronary artery disease (CAD) including myocardial infarction, definite or probable angina if revascularized at the same time or afterwards, resuscitated cardiac arrest, or CAD death; heart failure (HF); stroke; stroke death; other atherosclerotic death; or, other cardiovascular death as previously described.32 Diagnosis of HF required: symptoms; a physician diagnosis; medical treatment for HF; and, a) pulmonary edema/congestion by chest x-ray and/or b) ventricular dilatation, poor LV function, or evidence of LV diastolic dysfunction by echocardiography or ventriculography.
Statistical Analysis
Mean (95% confidence interval [CI]) values of all LV structural and functional indices were calculated in the sample divided into 8 equal groups by age. We considered mass and volumes as absolute measures and as indexed to body size (body surface area [BSA], body mass index [BMI], and height). We also quantified mean Ecc and SRE across age quartiles.
Independent associations between increasing age and all LV indices were performed using linear regression with multivariable adjustments for demographic characteristics and risk factors: Model 1 was unadjusted; Model 2 adjusted for sex, race/ethnicity, systolic blood pressure (SBP), diastolic blood pressure (DBP), pulse pressure, and BMI; and, Model 3 included the variables from Model 2 and also further adjusted for total cholesterol, low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, diabetes (fasting plasma glucose ≥7.0 mmol/L [126 mg/dL]), smoking status (current, former, or never), and taking medications to control hypertension, dyslipidemia, and/or diabetes. All analyses were also performed by sex, racial/ethnic group, and hypertension status (BP ≥140/90 or taking anti-hypertensive medication); two-way ANOVA was used to assess for the possible interaction of sex, race/ethnicity, or hypertension status on the relation of age to each of the LV indices.
For outcomes analyses, Cox proportional hazard models were calculated for each LV measure separately as continuous variables (per standard deviation increments) and then for LV mass and LVEDV jointly in the same model. All models were adjusted for age, sex, race/ethnicity, baseline height and weight, hypertension status, LDL cholesterol, diabetes, and smoking status. Analyses were repeated for incident cardiovascular events using M/V ratio quintiles, and then repeated again by age group (<65 and ≥65 years). All analyses were performed using SPSS 13.0 and Stata 10.0. The authors had full access to and take full responsibility for the integrity of the data. All authors have read and agree to the manuscript as written.
Results
Table 1 shows the demographic and clinical characteristics of the 5004 study participants in this analysis by age group. Notably, older adults had higher SBP, lower DBP, and higher pulse pressure when compared to younger adults; these trends were more pronounced across increasing age (Supplement, Figure 1).
Table 1.
Baseline characteristics.*
| Characteristic | Age <65 years n=3125 | Age ≥65 years n=1879 |
|---|---|---|
| Female sex (%) | 52.7 | 52.9 |
| Race/ethnicity (%) | ||
| Caucasian | 37.9 | 41.3 |
| Chinese American | 12.9 | 13.4 |
| African American | 25.5 | 26.1 |
| Hispanic | 23.8 | 19.2 |
| Body mass index (kg/m2) | 28.1±5.2 | 27.2±4.6 |
| Body surface area (m2) | 1.9±0.2 | 1.8±0.2 |
| Systolic blood pressure (mm Hg) | 120.6±19.1 | 133.4±22.4 |
| Diastolic blood pressure (mm Hg) | 72.4±10.2 | 70.8±10.3 |
| Pulse pressure (mm Hg) | 48.2±13.7 | 62.6±17.7 |
| Hypertension (%) | 26.7 | 49.7 |
| Diabetes (%) | 10.4 | 17.3 |
| Cigarette smoking (%) | ||
| Former | 32.7 | 40.9 |
| Current | 15.8 | 7.5 |
| Total cholesterol (mg/dL) | 195.3±35.6 | 192.6±34.9 |
| Triglycerides (mg/dL) | 133.3±90.7 | 127.9±75.0 |
| LDL cholesterol (mg/dL) | 118.6±31.6 | 114.9±30.8 |
| HDL cholesterol (mg/dL) | 50.5±14.7 | 52.4±15.3 |
| Taking medication for hypertension (%) | 24.8 | 43.1 |
| Taking medication for cholesterol (%) | 11.6 | 22.4 |
| Taking medication for diabetes (%) | 7.4 | 11.4 |
| LV mass (g) | 147.2±39.3 | 142.1±39.6 |
| LV mass index (g/m2) † | 77.9±15.9 | 78.0±17.0 |
| LVEDV (mL) | 131.8±31.0 | 117.4. ±30.3 |
| LVEDV index (mL/m2) † | 70.1±13.0 | 64.8±14.0 |
| LVESV (mL) | 42.5±17.1 | 35.8±16.3 |
| LVESV index (mL/m2) † | 22.5±7.9 | 19.6±8.1 |
| LV mass-to-volume ratio (g/mL) | 1.1±0.2 | 1.2±0.3 |
| LV stroke volume (mL) | 89.3±19.5 | 81.6±19.5 |
| LV stroke volume index (mL/m2) † | 47.6±8.7 | 45.2±9.4 |
| LV ejection fraction (%) | 68.3±7.2 | 70.2±7.6 |
| Peak systolic strain‡ | −17.4±2.6 | −17.0±2.7 |
| Diastolic strain rate‡ | 1.6±0.5 | 1.6±0.6 |
LDL = low-density lipoprotein; HDL = high-density lipoprotein; LV = left ventricular; LVEDV = left ventricular end-diastolic volume; LVESV = left ventricular end-systolic volume.
Data presented as means (± SD) or frequencies (%).
Values are indexed to body surface area.
Strain measures assessed a subset of 1099 participants: 449 aged <65 years and 650 aged ≥65 years.
Left Ventricular Structure and Aging
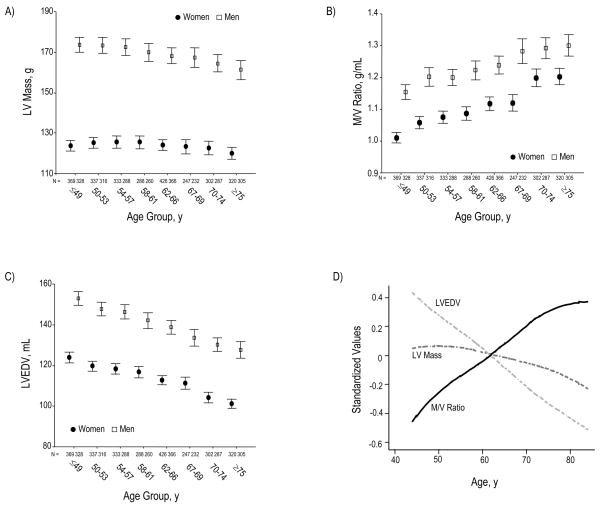
In age-based analyses, absolute LV mass incrementally decreased across increasing age group by −0.3g per year (p<0.0001), and this trend was observed in both sexes (Figure 1A). When LV mass was indexed to BSA, this inverse relationship was diminished but remained statistically significant after adjusting for multiple variables including sex, race/ethnicity, and blood pressure (Table 2). While LV mass slightly declined across increasing age groups, M/V ratio substantially increased by 5 mg/mL (0.005 g/mL) for every year increase in age (p<0.0001, Figure 1B). The mean M/V ratio was almost 25% higher in the oldest compared to youngest age group (1.25 versus 1.08 g/mL).
Figure 1.
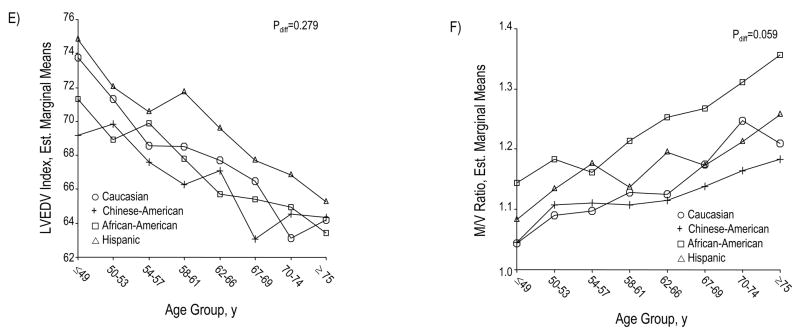
Across increasing age groups, women and men demonstrate a marked reduction in LVEDV, out of proportion to a slight decrease in LV mass (Panels A and C). Accordingly, M/V ratio markedly increases for women and men (Panel B). Panel D summarizes these structural findings by showing age-associated changes in standardized values for LV mass, LVEDV, and M/V ratio. LVEDV (indexed to BSA) declines with age similarly across the 4 ethnic/racial groups (Panel E); the age-associated trend of greater M/V augmentation among African Americans was of only borderline statistical significance (Panel F). Values for panels A, B, and C are presented as means (95% CI).
Table 2.
Association of age with indices of LV structure and function.
| Coefficient* (95% CI) | |||
|---|---|---|---|
| Model 1 | Model 2 | Model 3 | |
| LV mass (g) | −0.261 (−0.369, −0.153) † | −0.449 (−0.540, −0.357)† | −0.477 (−0.570, −0.384) † |
| LV mass index (g/m2) | 0.015 (−0.029, 0.060) | −0.146 (−0.190, −0.101) † | −0.161 (−0.206, −0.115) † |
| LVEDV (mL) | −0.771 (−0.854, −0.687) † | −0.888 (−0.974, −0.803) † | −0.872 (−0.960, −0.784) † |
| LVEDV index (mL/m2) | −0.282 (−0.318, −0.245) † | −0.396 (−0.438, −0.354) † | −0.396 (−0.440, −0.353) † |
| LVESV (mL) | −0.341 (−0.387, −0.296) † | −0.332 (−0.381, −0.284) † | −0.310 (−0.360, −0.260) † |
| LVESV index (mL/m2) | −0.142 (−0.164, −0.120) † | −0.152 (−0.177, −0.128) † | −0.144 (−0.169, −0.118) † |
| SV (mL) | −0.429 (−0.482, −0.376) † | −0.556 (−0.614, −0.499) † | −0.562 (−0.621, −0.504) † |
| SV index (mL/m2) | −0.139 (−0.164, −0.115) † | −0.244 (−0.272, −0.215) † | −0.249 (−0.279, −0.220) † |
| Ejection fraction (%) | 0.089 (0.069, 0.109) † | 0.047 (0.024, 0.070) † | 0.048 (0.011, 0.058) § |
| Peak systolic strain | 0.020 (0.004, 0.037) § | 0.049 (0.030, 0.068) † | 0.041 (0.022, 0.060) † |
| Diastolic strain rate** | −0.005 (−0.009, −0.001) ‡ | −0.007 (−0.011, −0.003) ‡ | −0.007 (−0.012, −0.003) ‡ |
LV = left ventricular; LVEDV = LV end-diastolic volume; LVESV = LV end-systolic volume; SV = stroke volume.
Coefficients represent change in dependent variable per 1 year increase in age, with adjustments for multiple variables: Model 1 is unadjusted. Model 2 is adjusted for sex, race/ethnicity, SBP, DBP, pulse pressure, and BMI; and, Model 3 is further adjusted for total cholesterol, LDL cholesterol, HDL cholesterol, diabetes, smoking, and taking medications to control hypertension, dyslipidemia, and diabetes.
p<0.00001;
p<0.01;
p<0.05
These are mean values calculated as the average value from the 4 wall regions (anterior, lateral, septal, inferior) for each individual in the subset 1099 participants who had tissue tagging as part of their MRI studies.
The marked age-associated increase in M/V ratio was driven by a proportionately greater magnitude of age-associated decline in LVEDV (0.8–0.9 mL per year) compared to that of LV mass (0.2–0.5 g per year, Table 2, Figures 1C and 1D). Moreover, LVEDV index declined at similar rates in individuals of different race/ethnicity (Figure 1E) and, correspondingly, the M/V ratio increased across all race/ethnic groups with a non-significant trend towards a sharper incline among African Americans (Figure 1F). In addition, when LV mass and volumes were normalized to indices of body size other than BSA (Table 2), such as BMI and height, these age-based trends remained the same. All age-associated differences in LV mass and volumes remained highly significant (p<0.00001) after adjustment for multiple variables including cardiovascular risk factors (Table 2).
Left Ventricular Function and Aging
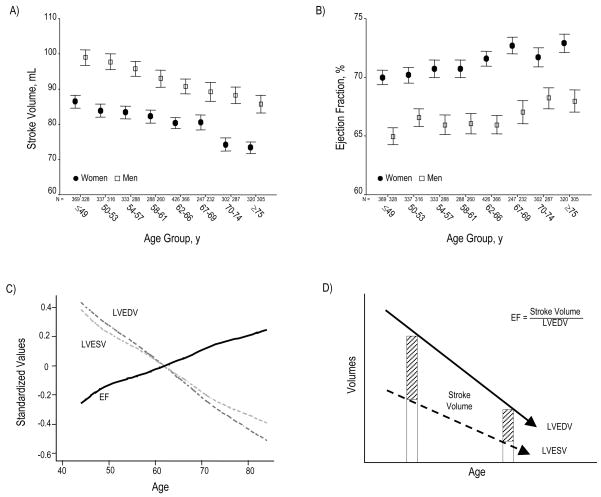
The age-associated decline in LVEDV exceeded that of LVESV (0.3–0.4 mL per year), generating a corresponding age-associated decline in the SV (Table 2, Figure 2A). Conversely, EF increased modestly with age, in the face of this age-associated decline in LV volumes (Table 2; Figures 2B, 2C, and 2D). Age-associated differences in SV and EF remained significant in multivariable analyses.
Figure 2.
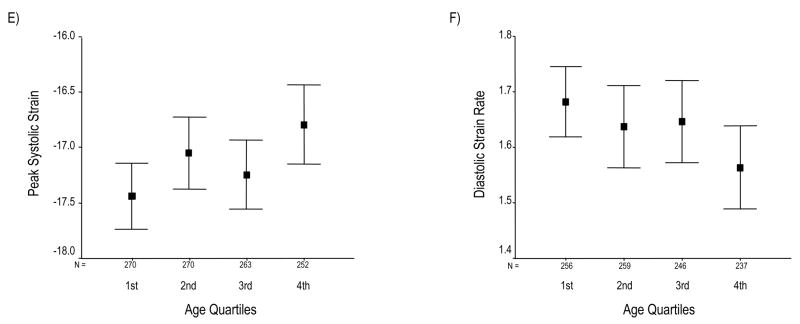
SV declines across increasing age group (Panel A). Conversely, EF progressively rises (Panel B). Panel C summarizes the relation of age to these global functional indices by showing age-associated changes in standardized values for LVEDV, LVESV, and EF. Panel D depicts a schematic overview of changes in LV volumes and EF with increasing age. Although SV progressively falls, EF paradoxically increases; this appears due to the progressive decline in all LV volumes, particularly LVESV. Panel E depicts peak systolic strain (Ecc), reflecting the magnitude of myocardial systolic deformation, across increasing age quartiles. As age increases, midwall circumferential shortening becomes less negative, i.e. decreases since sysolic strain is conventionally negative during systole. Because diastolic strain rate is conventionally a positive value, reduction of its magnitude reflects slower circumferential lengthening during LV filling or progressively reduced myocardial relaxation with age (Panel F). Values in panels A, B, E, and F are presented as means (95% CI).
The effect of hypertension status on LV indices of structure and function was independent of age. Similarly, the effects of sex and race/ethnicity (Figure 1E and 1F) were also independent. Age did appear to augment differences in EF between women and men, but this heterogeneity was not statistically significant (Supplement, Figure 2). Similarly, the relations of age with LV indices of structure and function were not significantly different in hypertensive compared to normotensive individuals (Supplement, Figure 3).
Finally, among the 1099 participants who had LV strain analysis performed, older age was associated with impaired systolic strain, represented by reduced circumferential shortening and less negative mean Ecc, particularly after multivariable adjustment (Table 2, Figure 2E). As well, mean early diastolic strain rate (SRE) significantly decreased with age in both unadjusted and adjusted models, consistent with lower rates of early circumferential lengthening (SRE) during diastole in older compared to younger adults (Table 2, Figure 2F).
Age-Associated Ventricular Remodeling and Outcomes
Of the 5004 participants who had baseline MRI studies, 36 participants were lost to follow up after the baseline exam and so were excluded from outcomes analyses. Over the 5.2 years of MESA cohort follow-up (median 4.0 years) for the remaining 4968 participants, there were 180 total cardiovascular events: 45 myocardial infarctions, 71 anginal episodes, 48 HF cases, 39 strokes, and 13 CAD deaths.
Structural LV features were significantly associated with total cardiovascular events; in particular, the M/V ratio was consistently associated with a higher rate of cardiovascular events in the total sample (HR 1.23 [95% CI 1.09–1.39], p=0.001). Moreover, we observed significant age-based heterogeneity with respect to the relation of structural indices to cardiovascular events: when comparing outcomes between age groups, elevated M/V ratio was associated with a higher hazard ratio for total cardiovascular – events with wider confidence intervals – in younger compared to older adults (Pinteraction=0.013). This finding remained unchanged in models adjusting for multiple risk factors (Table 3, Figure 3).
Table 3.
Relation of selected LV indices with total CV events by age group.*
| Age<65 |
Age≥65 |
|||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| LV mass (1 SD = 39.5 g) | 1.45 (1.11, 1.90) | 0.007 | 1.48 (1.22, 1.81) | <0.001 |
| LVEDV (1 SD = 31.5 mL) | 0.89 (0.66, 1.21) | 0.470 | 1.23 (1.00, 1.50) | 0.050 |
| LV mass (1 SD = 39.5 g) | 1.86 (1.33, 2.62) | <0.001 | 1.50 (1.17, 1.92) | 0.001 |
| LVEDV (1 SD = 31.5 mL) | 0.65 (0.46, 0.92) | 0.015 | 0.98 (0.77, 1.26) | 0.900 |
| M/V ratio (1 SD = 0.25 g/mL) | 1.51 (1.23, 1.85) | <0.001 | 1.12 (0.96, 1.32) | 0.160 |
| M/V ratio quintiles | ||||
| 1st quintile | referent | –– | referent | –– |
| 2nd quintile | 1.67 (0.56, 4.93) | 0.350 | 1.08 (0.44, 2.61) | 0.870 |
| 3rd quintile | 0.75 (0.21, 2.63) | 0.650 | 1.48 (0.65, 3.38) | 0.350 |
| 4th quintile | 2.68 (0.96, 7.49) | 0.060 | 1.79 (0.81, 3.92) | 0.150 |
| 5th quintile | 3.69 (1.34, 10.10) | 0.011 | 1.68 (0.77, 3.68) | 0.190 |
Models are adjusted for age, sex, race/ethnicity, height, weight, hypertension status, LDL cholesterol, diabetes, and smoking; HR = hazards ratio; for continuous variables, hazards ratios are for 1 standard deviation (SD) change.
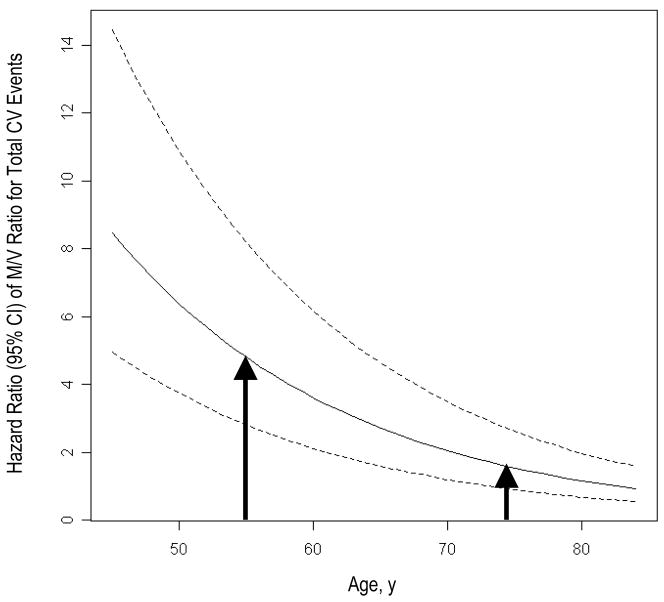
Figure 3.
Fitted curves represent hazard ratios (95% CI) of M/V ratio with respect to total CV events across increasing age while adjusting for age, sex, race/ethnicity, height, weight, hypertension status, LDL cholesterol, diabetes, and smoking. As shown, the risk is greater for those individuals who develop the “typical” age-associated LV remodeling phenotype at a younger compared to older age (arrows).
Discussion
This study demonstrates marked age-associated differences in LV structure and function in the largest cohort of multi-ethnic individuals studied to date with cardiac MRI. In this cohort, age was associated with a phenotype of LV remodeling marked by several specific structural features and coincident with impaired systolic as well as diastolic myocardial function. Furthermore, this LV remodeling conferred significant risk for cardiovascular events. Taken together, these findings suggest that processes related to biologic aging likely contribute to alterations in LV structure and function that, in turn, predispose to greater cardiovascular risk.
The major age-associated differences in LV structure and function found in this study were three-fold. First, age was associated with a markedly increased M/V ratio, consistent with the age-associated LV hypertrophic remodeling7,33 and increase in relative wall thickness34 found in prior studies. However, the increased M/V ratio in this study was not due to increased LV mass, as suggested by some prior population studies,7 but instead driven by a substantial decline in LV volumes out of proportion to any alterations in mass. Earlier investigations used echocardiographic methods for estimating LV mass, including M-mode, which are less able than cardiac MRI to distinguish between concentric remodeling and concentric hypertrophy.34–36 On the other hand, prior MRI studies in smaller samples have found similarly preserved or reduced LV mass in older compared to younger adults.17,28 An explanation for age-associated hypertrophy, in the absence of increased mass, is offered by histomorphometric studies in humans and animals showing that as the absolute number of myocytes decreases in older age, remaining myocytes tend to hypertrophy in size.8,19,37,38 These studies also found that the number of collagen fibers and nonenzymatic cross-linking in myocardium increases with age. Therefore, despite progressive myocyte dropout in aging, myocyte hypertrophy and increased collagen concentration appear to relatively preserve but not increase LV mass. This type of age-based ventricular remodeling is likely also related to the well-described coupling of ventricular and vascular stiffening processes that can occur over a life course.34,39 Indeed, our ventricular findings were observed independent from but also in the setting of a concurrent age-associated rise in pulse pressure, a recognized index of arterial stiffness.
Secondly, we found an age-associated decline in SV that was accompanied, paradoxically, by a modestly enhanced EF. Physiologic studies have previously documented an age-associated deterioration in global LV function as represented by indices of SV but not EF.27 Conversely, other reports have described increased fractional shortening34 or at least preserved EF40 in older compared to younger subjects. In our study, the apparent paradox of rising EF in the face of a falling SV appeared driven by the overall decline in LV chamber volumes, particularly in LVESV despite a correspondingly greater reduction in LVEDV across all age groups (Table 2, Figure 2C). Given that EF is the ratio of SV over LVEDV, we were able to observe that as SV decreased, EF actually increased (see hatched bars in Figure 2D). Prior studies have suggested that age is associated with preserved EF but greater LV volumes.41–43 However, these earlier studies assessed LV volumes using radionuclide ventriculography, which is limited by varying attenuation between anterior and posterior walls, background subtraction errors, and geometric assumptions when compared to cardiac MRI.44
Finally, in the setting of falling SV and rising EF, age was also associated with abnormal systolic and diastolic strain, representing early myocardial dysfunction. Age-related myocyte depletion, combined with increased myocardial collagen deposition, may well contribute to impaired contractility and abnormal diastolic function as well as reduced LV volumes. Additionally, the discrepancy between the age-associated increase in ejection fraction and decline in circumferential shortening may be in part due to the phenomenon of cross fiber shortening,45,46 whereby the ventricle compensates for impaired contractile function through concentric hypertrophy and associated reliance on shortening of cross fibers to maintain systolic wall thickening and ejection fraction. Prior studies have found abnormal relaxation and diastolic strain in older adults34 but overall myocardial systolic function was generally thought to remain intact. On the other hand, reduced systolic strain in the presence of LV hypertrophy and preserved EF has been found in association with hypertension and, in the MESA cohort, systolic circumferential strain was progressively lower in participants with increasing M/V ratios.47 This study suggests that these same features are associated with age even after adjusting for hypertension and BP indices. Age-associated abnormalities in myocardial strain may be yet another sign of abnormal coupling of ventricular and vascular remodeling, particularly as we have previously observed abnormal myocardial strain in the setting of increased arterial stiffness.48
The most representative feature of age-associated remodeling was increased M/V ratio, reflective of a hypertrophic process occurring in the setting of progressively reduced LV volumes. Prior studies have reported on the relation of LV hypertrophy with a variety of important cardiovascular outcomes.33,49 Interestingly, we found that an increased M/V ratio conferred a higher hazard for total cardiovascular events in younger compared to older age. This finding may reflect survivor bias in the MESA population and/or suggest that individuals who develop hypertrophic remodeling earlier in life have greater risk. This higher risk in younger adults may be related to subclinical coronary artery disease and/or hypertensive heart disease. However, the relation of M/V ratio to cardiovascular outcomes was significant even after adjustment for risk factors including hypertension.
The generalizability of our results may be limited by selection and survivor biases. Since MESA participants had no known cardiovascular disease at baseline, older individuals in this cohort represent a particularly healthy sample; therefore, present findings may underestimate true age-related differences. Our sample did not include persons <45 or >84 years of age. Additionally, analyses of age-associated LV remodeling were cross-sectional across a large cohort of individuals of varying age groups and did not account for the possible influence of birth cohort. Therefore, temporal relationships cannot be inferred for analyses of age-associated LV remodeling, although these may be the subject of future longitudinal investigations using serial cardiac MRI. We also were not able to adjust measures of peak systolic and diastolic strain for wall stress, which should ideally be considered in detailed investigations of contractile function and relaxation.50
Our findings suggest that human aging is associated with specific alterations of LV structure and function marked by an increase in M/V ratio, driven by a reduction in LV volumes out of proportion to declining LV mass. Moreover, age is independently associated with a parallel reduction in SV and progressive systolic and diastolic myocardial dysfunction, despite a modestly enhanced EF. Additionally, this study demonstrates a significant relationship between this phenotype of LV remodeling and adverse cardiovascular outcomes. Further investigations are needed to elucidate the etiology of such age-associated processes.
Supplementary Material
Acknowledgments
We thank the other investigators, the staff, and the participantsof the MESA study for their valuable contributions. A full listof participating MESA investigators and institutions can befound at http://www.mesa-nhlbi.org.
Funding/Support
This study was supported by a NIH/NHLBI grant (R01-HL66075-01) and MESA study contracts (N01-HC-9808, N01-HC-95168, and N01-HC-95169). Dr. Lima is also supported by the Reynolds Foundation and Dr. Fernandes was a recipient of a research grant from CAPES, Ministry of Education, Brazil Government.
Footnotes
Clinical Perspective
Age-related alterations of left ventricular (LV) structure and function that may predispose to cardiovascular events are not well understood. Therefore, we used cardiac magnetic resonance imaging to examine age-related differences in LV structure and function among 5004 participants of the Multi-Ethnic Study of Atherosclerosis who had no cardiovascular disease at baseline; additional strain analyses were performed for 1099 of these participants. We also assessed the relation of age-associated remodeling with cardiovascular outcomes while adjusting for traditional risk factors. In multivariable regression models, age was associated with a decrease in LV mass but also a marked increased in the LV mass-to-volume ratio driven by substantial reduction in LV end-diastolic volume. Age was also associated with a significant fall in stroke volume along with strain patterns reflecting systolic as well as diastolic myocardial dysfunction – despite a modestly enhanced ejection fraction. Higher mass-to-volume ratio conferred a significant risk for total cardiovascular events, and this trend was strongest among younger (<65 years, HR 3.69, P=0.011) versus older (≥65 years, HR 1.68, P=0.190) individuals with the highest compared to lowest mass-to-volume ratio quintile (Pinteraction=0.013). These findings suggest that age is associated with a phenotype of LV remodeling marked by increased mass-to-volume ratio and accompanied by systolic, as well as diastolic, myocardial dysfunction that is not reflected by preserved ejection fraction. This pattern of ventricular remodeling appears to confer significant cardiovascular risk, especially when present earlier in life.
Disclosures
None.
References
- 1.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a “set up” for vascular disease. Circulation. 2003;107:139–46. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- 2.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part II: the aging heart in health: links to heart disease. Circulation. 2003;107:346–54. doi: 10.1161/01.cir.0000048893.62841.f7. [DOI] [PubMed] [Google Scholar]
- 3.Lakatta EG. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part III: cellular and molecular clues to heart and arterial aging. Circulation. 2003;107:490–7. doi: 10.1161/01.cir.0000048894.99865.02. [DOI] [PubMed] [Google Scholar]
- 4.Dannenberg AL, Levy D, Garrison RJ. Impact of age on echocardiographic left ventricular mass in a healthy population (the Framingham Study) Am J Cardiol. 1989;64:1066–8. doi: 10.1016/0002-9149(89)90816-3. [DOI] [PubMed] [Google Scholar]
- 5.Lindroos M, Kupari M, Heikkila J, Tilvis R. Echocardiographic evidence of left ventricular hypertrophy in a general aged population. Am J Cardiol. 1994;74:385–90. doi: 10.1016/0002-9149(94)90408-1. [DOI] [PubMed] [Google Scholar]
- 6.Ganau A, Saba PS, Roman MJ, de Simone G, Realdi G, Devereux RB. Ageing induces left ventricular concentric remodelling in normotensive subjects. J Hypertens. 1995;13:1818–22. [PubMed] [Google Scholar]
- 7.Gardin JM, Siscovick D, Anton-Culver H, Lynch JC, Smith VE, Klopfenstein HS, Bommer WJ, Fried L, O’Leary D, Manolio TA. Sex, age, and disease affect echocardiographic left ventricular mass and systolic function in the free-living elderly. The Cardiovascular Health Study. Circulation. 1995;91:1739–48. doi: 10.1161/01.cir.91.6.1739. [DOI] [PubMed] [Google Scholar]
- 8.Olivetti G, Giordano G, Corradi D, Melissari M, Lagrasta C, Gambert SR, Anversa P. Gender differences and aging: effects on the human heart. J Am Coll Cardiol. 1995;26:1068–79. doi: 10.1016/0735-1097(95)00282-8. [DOI] [PubMed] [Google Scholar]
- 9.Hees PS, Fleg JL, Lakatta EG, Shapiro EP. Left ventricular remodeling with age in normal men versus women: novel insights using three-dimensional magnetic resonance imaging. Am J Cardiol. 2002;90:1231–6. doi: 10.1016/s0002-9149(02)02840-0. [DOI] [PubMed] [Google Scholar]
- 10.Salmasi AM, Alimo A, Jepson E, Dancy M. Age-associated changes in left ventricular diastolic function are related to increasing left ventricular mass. Am J Hypertens. 2003;16:473–7. doi: 10.1016/s0895-7061(03)00846-x. [DOI] [PubMed] [Google Scholar]
- 11.Claessens TE, Rietzschel ER, De Buyzere ML, De Bacquer D, De Backer G, Gillebert TC, Verdonck PR, Segers P. Noninvasive assessment of left ventricular and myocardial contractility in middle-aged men and women: disparate evolution above the age of 50? Am J Physiol Heart Circ Physiol. 2007;292:H856–65. doi: 10.1152/ajpheart.00759.2006. [DOI] [PubMed] [Google Scholar]
- 12.Gerstenblith G, Frederiksen J, Yin FC, Fortuin NJ, Lakatta EG, Weisfeldt ML. Echocardiographic assessment of a normal adult aging population. Circulation. 1977;56:273–8. doi: 10.1161/01.cir.56.2.273. [DOI] [PubMed] [Google Scholar]
- 13.Merino A, Alegria E, Castello R, Martinez-Caro D. Influence of age on left ventricular contractility. Am J Cardiol. 1988;62:1103–8. doi: 10.1016/0002-9149(88)90557-7. [DOI] [PubMed] [Google Scholar]
- 14.Pearson AC, Gudipati CV, Labovitz AJ. Effects of aging on left ventricular structure and function. Am Heart J. 1991;121:871–5. doi: 10.1016/0002-8703(91)90201-r. [DOI] [PubMed] [Google Scholar]
- 15.Sandstede J, Lipke C, Beer M, Hofmann S, Pabst T, Kenn W, Neubauer S, Hahn D. Age-and gender-specific differences in left and right ventricular cardiac function and mass determined by cine magnetic resonance imaging. Eur Radiol. 2000;10:438–42. doi: 10.1007/s003300050072. [DOI] [PubMed] [Google Scholar]
- 16.Nikitin NP, Loh PH, de Silva R, Witte KK, Lukaschuk EI, Parker A, Farnsworth TA, Alamgir FM, Clark AL, Cleland JG. Left ventricular morphology, global and longitudinal function in normal older individuals: a cardiac magnetic resonance study. Int J Cardiol. 2006;108:76–83. doi: 10.1016/j.ijcard.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 17.Natori S, Lai S, Finn JP, Gomes AS, Hundley WG, Jerosch-Herold M, Pearson G, Sinha S, Arai A, Lima JA, Bluemke DA. Cardiovascular function in multi-ethnic study of atherosclerosis: normal values by age, sex, and ethnicity. AJR Am J Roentgenol. 2006;186:S357–65. doi: 10.2214/AJR.04.1868. [DOI] [PubMed] [Google Scholar]
- 18.Slotwiner DJ, Devereux RB, Schwartz JE, Pickering TG, de Simone G, Ganau A, Saba PS, Roman MJ. Relation of age to left ventricular function in clinically normal adults. Am J Cardiol. 1998;82:621–6. doi: 10.1016/s0002-9149(98)00390-7. [DOI] [PubMed] [Google Scholar]
- 19.Olivetti G, Melissari M, Capasso JM, Anversa P. Cardiomyopathy of the aging human heart. Myocyte loss and reactive cellular hypertrophy. Circ Res. 1991;68:1560–8. doi: 10.1161/01.res.68.6.1560. [DOI] [PubMed] [Google Scholar]
- 20.Capasso JM, Fitzpatrick D, Anversa P. Cellular mechanisms of ventricular failure: myocyte kinetics and geometry with age. Am J Physiol. 1992;262:H1770–81. doi: 10.1152/ajpheart.1992.262.6.H1770. [DOI] [PubMed] [Google Scholar]
- 21.Raizada V, Pathak D, Nakouzi A, Malhotra A. Prevention of age-related V1 myosin isozyme decrement in the adult rat heart. J Mol Cell Cardiol. 1994;26:293–6. doi: 10.1006/jmcc.1994.1037. [DOI] [PubMed] [Google Scholar]
- 22.Roth DA, White CD, Podolin DA, Mazzeo RS. Alterations in myocardial signal transduction due to aging and chronic dynamic exercise. J Appl Physiol. 1998;84:177–84. doi: 10.1152/jappl.1998.84.1.177. [DOI] [PubMed] [Google Scholar]
- 23.Lindsey ML, Goshorn DK, Squires CE, Escobar GP, Hendrick JW, Mingoia JT, Sweterlitsch SE, Spinale FG. Age-dependent changes in myocardial matrix metalloproteinase/tissue inhibitor of metalloproteinase profiles and fibroblast function. Cardiovasc Res. 2005;66:410–9. doi: 10.1016/j.cardiores.2004.11.029. [DOI] [PubMed] [Google Scholar]
- 24.Fleg JL, O’Connor F, Gerstenblith G, Becker LC, Clulow J, Schulman SP, Lakatta EG. Impact of age on the cardiovascular response to dynamic upright exercise in healthy men and women. J Appl Physiol. 1995;78:890–900. doi: 10.1152/jappl.1995.78.3.890. [DOI] [PubMed] [Google Scholar]
- 25.Mantero A, Gentile F, Gualtierotti C, Azzollini M, Barbier P, Beretta L, Casazza F, Corno R, Giagnoni E, Lippolis A, et al. Left ventricular diastolic parameters in 288 normal subjects from 20 to 80 years old. Eur Heart J. 1995;16:94–105. doi: 10.1093/eurheartj/16.1.94. [DOI] [PubMed] [Google Scholar]
- 26.Fioranelli M, Piccoli M, Mileto GM, Risa MP, Sgreccia F, Azzolini AP, Puglisi A. Modifications in cardiovascular functional parameters with aging. Minerva Cardioangiol. 2001;49:169–78. [PubMed] [Google Scholar]
- 27.Salmasi AM, Dore C. Variation of aortic blood velocity with age at rest and during exercise in normal subjects. Clin Auton Res. 1995;5:19–23. doi: 10.1007/BF01845494. [DOI] [PubMed] [Google Scholar]
- 28.Alfakih K, Plein S, Thiele H, Jones T, Ridgway JP, Sivananthan MU. Normal human left and right ventricular dimensions for MRI as assessed by turbo gradient echo and steady-state free precession imaging sequences. J Magn Reson Imaging. 2003;17:323–9. doi: 10.1002/jmri.10262. [DOI] [PubMed] [Google Scholar]
- 29.Hudsmith LE, Petersen SE, Francis JM, Robson MD, Neubauer S. Normal human left and right ventricular and left atrial dimensions using steady state free precession magnetic resonance imaging. J Cardiovasc Magn Reson. 2005;7:775–82. doi: 10.1080/10976640500295516. [DOI] [PubMed] [Google Scholar]
- 30.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR, Jr, Kronmal R, Liu K, Nelson JC, O’Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–81. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 31.Edvardsen T, Detrano R, Rosen BD, Carr JJ, Liu K, Lai S, Shea S, Pan L, Bluemke DA, Lima JA. Coronary artery atherosclerosis is related to reduced regional left ventricular function in individuals without history of clinical cardiovascular disease: the Multiethnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol. 2006;26:206–11. doi: 10.1161/01.ATV.0000194077.23234.ae. [DOI] [PubMed] [Google Scholar]
- 32.Lakoski SG, Greenland P, Wong ND, Schreiner PJ, Herrington DM, Kronmal RA, Liu K, Blumenthal RS. Coronary artery calcium scores and risk for cardiovascular events in women classified as “low risk” based on Framingham risk score: the multi-ethnic study of atherosclerosis (MESA) Arch Intern Med. 2007;167:2437–42. doi: 10.1001/archinte.167.22.2437. [DOI] [PubMed] [Google Scholar]
- 33.Levy D. Left ventricular hypertrophy. Epidemiological insights from the Framingham Heart Study. Drugs. 1988;35 (Suppl 5):1–5. doi: 10.2165/00003495-198800355-00002. [DOI] [PubMed] [Google Scholar]
- 34.Redfield MM, Jacobsen SJ, Borlaug BA, Rodeheffer RJ, Kass DA. Age- and gender-related ventricular-vascular stiffening: a community-based study. Circulation. 2005;112:2254–62. doi: 10.1161/CIRCULATIONAHA.105.541078. [DOI] [PubMed] [Google Scholar]
- 35.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–63. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 36.Alfakih K, Bloomer T, Bainbridge S, Bainbridge G, Ridgway J, Williams G, Sivananthan M. A comparison of left ventricular mass between two-dimensional echocardiography, using fundamental and tissue harmonic imaging, and cardiac MRI in patients with hypertension. Eur J Radiol. 2004;52:103–9. doi: 10.1016/j.ejrad.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 37.Anversa P, Hiler B, Ricci R, Guideri G, Olivetti G. Myocyte cell loss and myocyte hypertrophy in the aging rat heart. J Am Coll Cardiol. 1986;8:1441–8. doi: 10.1016/s0735-1097(86)80321-7. [DOI] [PubMed] [Google Scholar]
- 38.Anversa P, Palackal T, Sonnenblick EH, Olivetti G, Meggs LG, Capasso JM. Myocyte cell loss and myocyte cellular hyperplasia in the hypertrophied aging rat heart. Circ Res. 1990;67:871–85. doi: 10.1161/01.res.67.4.871. [DOI] [PubMed] [Google Scholar]
- 39.Kass DA. Age-related changes in venticular-arterial coupling: pathophysiologic implications. Heart Fail Rev. 2002;7:51–62. doi: 10.1023/a:1013749806227. [DOI] [PubMed] [Google Scholar]
- 40.Chen CH, Nakayama M, Nevo E, Fetics BJ, Maughan WL, Kass DA. Coupled systolic-ventricular and vascular stiffening with age: implications for pressure regulation and cardiac reserve in the elderly. J Am Coll Cardiol. 1998;32:1221–7. doi: 10.1016/s0735-1097(98)00374-x. [DOI] [PubMed] [Google Scholar]
- 41.Port S, Cobb FR, Coleman RE, Jones RH. Effect of age on the response of the left ventricular ejection fraction to exercise. N Engl J Med. 1980;303:1133–7. doi: 10.1056/NEJM198011133032001. [DOI] [PubMed] [Google Scholar]
- 42.Rodeheffer RJ, Gerstenblith G, Becker LC, Fleg JL, Weisfeldt ML, Lakatta EG. Exercise cardiac output is maintained with advancing age in healthy human subjects: cardiac dilatation and increased stroke volume compensate for a diminished heart rate. Circulation. 1984;69:203–13. doi: 10.1161/01.cir.69.2.203. [DOI] [PubMed] [Google Scholar]
- 43.Nussbacher A, Gerstenblith G, O’Connor FC, Becker LC, Kass DA, Schulman SP, Fleg JL, Lakatta EG. Hemodynamic effects of unloading the old heart. Am J Physiol. 1999;277:H1863–71. doi: 10.1152/ajpheart.1999.277.5.H1863. [DOI] [PubMed] [Google Scholar]
- 44.Bellenger NG, Burgess MI, Ray SG, Lahiri A, Coats AJ, Cleland JG, Pennell DJ. Comparison of left ventricular ejection fraction and volumes in heart failure by echocardiography, radionuclide ventriculography and cardiovascular magnetic resonance; are they interchangeable? Eur Heart J. 2000;21:1387–96. doi: 10.1053/euhj.2000.2011. [DOI] [PubMed] [Google Scholar]
- 45.MacGowan GA, Shapiro EP, Azhari H, Siu CO, Hees PS, Hutchins GM, Weiss JL, Rademakers FE. Noninvasive measurement of shortening in the fiber and cross-fiber directions in the normal human left ventricle and in idiopathic dilated cardiomyopathy. Circulation. 1997;96:535–41. doi: 10.1161/01.cir.96.2.535. [DOI] [PubMed] [Google Scholar]
- 46.de Simone G, Ganau A, Roman MJ, Devereux RB. Relation of left ventricular longitudinal and circumferential shortening to ejection fraction in the presence or in the absence of mild hypertension. J Hypertens. 1997;15:1011–7. doi: 10.1097/00004872-199715090-00012. [DOI] [PubMed] [Google Scholar]
- 47.Rosen BD, Edvardsen T, Lai S, Castillo E, Pan L, Jerosch-Herold M, Sinha S, Kronmal R, Arnett D, Crouse JR, 3rd, Heckbert SR, Bluemke DA, Lima JA. Left ventricular concentric remodeling is associated with decreased global and regional systolic function: the Multi-Ethnic Study of Atherosclerosis. Circulation. 2005;112:984–91. doi: 10.1161/CIRCULATIONAHA104.500488. [DOI] [PubMed] [Google Scholar]
- 48.Fernandes VR, Polak JF, Cheng S, Rosen BD, Carvalho B, Nasir K, McClelland R, Hundley G, Pearson G, O’Leary DH, Bluemke DA, Lima JA. Arterial stiffness is associated with regional ventricular systolic and diastolic dysfunction: the Multi-Ethnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol. 2008;28:194–201. doi: 10.1161/ATVBAHA.107.156950. [DOI] [PubMed] [Google Scholar]
- 49.Bluemke DA, Kronmal RA, Lima JA, Olson J, Burke GL, Folsom AR. Incident congestive heart failure in the MESA study: relationship to left venticular mass. Circulation. 2005;112:II_800. [Google Scholar]
- 50.de Simone G, Devereux RB, Koren MJ, Mensah GA, Casale PN, Laragh JH. Midwall left ventricular mechanics. An independent predictor of cardiovascular risk in arterial hypertension. Circulation. 1996;93:259–65. doi: 10.1161/01.cir.93.2.259. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.