Abstract
Sudden cardiac death remains a leading cause of mortality in the Western world, accounting for up to 20% of all deaths in the U.S.1, 2 The major causes of sudden cardiac death in adults age 35 and older are coronary artery disease (70–80%) and dilated cardiomyopathy (10–15%).3 At the molecular level, a wide variety of mechanisms contribute to arrhythmias that cause sudden cardiac death, ranging from genetic predisposition (rare mutations and common polymorphisms in ion channels and structural proteins) to acquired electrophysiological and structural remodeling in left ventricular hypertrophy and failure.4, 5 A growing body of evidence suggests that altered ion channel function is closely linked to changes in metabolic activity in a wide variety of pathological conditions. In this review we focus on the mechanisms by which altered metabolic function impacts cardiac electrophysiology. We will review the specific molecular targets that allow cardiomyocytes to recognize alterations in their metabolic state and translate this information into changes in membrane excitability in various pathophysiological conditions including ischemia-reperfusion, heart failure (HF), left ventricular hypertrophy, diabetic cardiomyopathy and atrial fibrillation. A comprehensive understanding of the interrelated processes of metabolic and electrical remodeling promises to identify new molecular targets for the treatment of cardiac arrhythmias.
Keywords: Metabolism, Ischemia, Diabetes mellitus, Arrhythmia, Ion channels
1. Maintenance of sodium and calcium homeostasis is critical for electrical stability
Free energy released from the hydrolysis of high-energy phosphates is essential for maintenance of cellular homeostasis. Maintenance of Na+ and K+ gradients across the plasma membrane is among the most energy-intensive cellular processes in the heart. Ultimately, all transmembrane ionic movements are driven by the free energy released from ATP hydrolysis by P-type (P for phosphorylated intermediate) ATPases, including the Na+-K+-ATPase and the sarcoplasmic reticulum Ca2+-ATPase. The electrochemical gradient generated by the Na+-K+-ATPase establishes the gradients of Na+ and K+ across the sarcolemma, thus establishing the driving force for the passage of these ions across the myocyte cell membrane through voltage-dependent ion selective channels. The energy stored in the electrochemical Na+ gradient is used to move other ions across the sarcolemma. For instance, it provides the driving force for Ca2+ removal from the cytoplasm to the extracellular space via the Na+-Ca2+-exchanger (NCX1 encoded by SLC8A1). Removal of Ca2+ during diastole is also achieved through a second P-type ATPase located in the sarcoplasmic reticulum membrane (SERCA2 encoded by ATP2A2) which transports Ca2+ from the cytosol of the cell to the lumen of the sarcoplasmic reticulum (SR) at the expense of ATP hydrolysis during muscle relaxation.
Under physiological conditions, ATP is mainly supplied by mitochondrial oxidative phosphorylation and to a lesser degree by glycolysis. Commensurate with the large metabolic demand of contraction of the heart, mitochondria occupy approximately 30% of the volume of ventricular cardiomyocytes6 and form a network around the myofilaments, thus placing the sites of ATP production immediately adjacent to the sites of ATP consumption.7 The critical dependence of the ion homeostasis on sufficient energy supply becomes evident in pathophysiological conditions as diverse as ischemia/reperfusion, HF and ventricular hypertrophy, where a mismatch in ATP supply and ATP utilization may lead to electrical and mechanical instability.
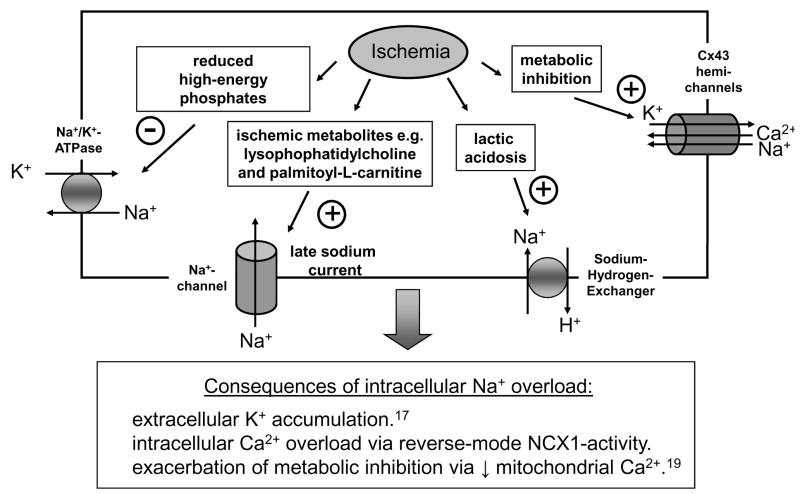
During severe ischemia, for instance, oxidative metabolism comes to a halt. Even though anaerobic glycolysis increases in a compensatory fashion, this process is unable to supply sufficient amounts of ATP to maintain long-term viability and function. As a consequence, concentrations of ATP may fall by more than 70% within 20min after onset of ischemia,8 thereby limiting the function of the Na+-K+-ATPase, reducing the extrusion of sodium ions and dissipating the transmembrane ion and voltage gradients. Anaerobic glycolysis may further aggravate intracellular sodium loading, as accumulating protons (generated from lactate) activate the Na+-H+-exchanger (NHE1 encoded by SLC9A1) which exchanges intracellular H+ for extracellular Na+. Metabolic inhibition, as observed in ischemia, also promotes opening of connexin-43 hemichannels. Connexin proteins are assembled together in groups of six to form hemichannels (or connexons) which can either combine end-to-end to form gap junction channels or occur as single connexons in non-junctional regions. If activated in the nonjunctional plasma membrane by metabolic inhibition, hemichannels are non-selective cation channels and permit K+, Na+ and Ca2+ to move down their respective electrochemical gradients across the sarcolemma.9, 10 Based on their large unitary conductance (120pS) and high open probability, evidence suggests that only a small number of open connexin-43 hemichannels is sufficient to double the Na+ influx of a normal beating myocyte.10, 11 The notion that intracellular sodium ([Na+]i) overload contributes to electrical instability is supported by the fact that high [Na+]i has been found to be a strong predictor of ventricular fibrillation (VF) in experimental models of myocardial ischemia.12 In addition to ischemia, profound metabolic abnormalities with elevation of [Na+]i are also encountered in pathophysiological states as diverse as left ventricular hypertrophy, HF or diabetes mellitus – all of which are associated with an increased incidence of arrhythmias.13–15 In diabetes, it has been shown that hyperglycemia directly inhibits the Na+-K+-ATPase,16 thus promoting intracellular Na+ overload. In left ventricular hypertrophy and HF, a rise in [Na+]i may partially compensate for depressed contractility by raising cytosolic Ca2+ via the reverse-mode NCX1-current.
Intracellular Na+ loading is linked to arrhythmias by a number of mechanisms, as summarized in Figure 1: First, intracellular Na+ accumulation leads to net K+ loss because of the need to maintain electroneutrality and osmotic balance.17 Accumulation of extracellular K+ not only promotes diastolic injury currents which trigger extrasystoles, but also progressively slows conduction and alters refractoriness, generating both the trigger and substrate for reentry.18 Second, elevation of [Na+]i may further exacerbate a mitochondrial energetic deficit, observed in various pathophysiological states. Recent evidence suggests that cardiac mitochondria take up Ca2+ rapidly via the Ca2+ uniporter (MCU).19 Under physiological conditions, the extrusion rate of mitochondrial calcium from the mitochondrial matrix via the mitochondrial sodium-calcium exchanger (mNCX) is slower than the mitochondrial Ca2+ uptake,19, 20 so that, at higher heart rates, Ca2+ accumulates in the mitochondrial matrix, activating several enzymes of the tricarboxylic acid (TCA) cycle and increasing NADH production. Together with the high-energy phosphotransfer enzymes creatine kinase (CK) and adenylate kinase (AK), mitochondrial Ca2+ accumulation thereby facilitates the matching of NADH/NAD+ redox potential to ATP production in periods of increased energetic demand.21 The balance between mitochondrial uptake and release of Ca2+ can be modified by a variety of factors, including mitochondrial inorganic phosphate, ADP and also [Na+]i.22, 23 In conditions where cytosolic Na+ is elevated, the decay rate of the mitochondrial calcium transient is increased and mitochondrial Ca2+ accumulation is impaired as more calcium is extruded via the mitochondrial NCX.19, 22 Consequently, high intracellular Na+ will lead to a vicious cycle exacerbating the mismatch between ATP demand and ATP supply, as the reduction of Ca2+ in the mitochondrial matrix leads to NADH oxidation.19
Figure 1. Pathophysiological processes associated with intracellular sodium overload during ischemia.
Finally, elevation of [Na+]i in myocardial ischemia and reperfusion activates reverse-mode Na+-Ca+-exchanger, thereby promoting intracellular calcium overload, as the cell exchanges three sodium ions for one calcium ion.
2. Metabolic alterations of calcium homeostasis and excitation-contraction coupling
Following the rapid depolarization of the action potential, Ca2+ enters the cell via L-type calcium channels (ICa,L) and triggers calcium-induced calcium release from the sarcoplasmic reticulum. During metabolic inhibition, multiple studies have documented a reduction of the amplitude of ICa,L in ventricular myocytes of guinea pigs,24, 25 rats26 and rabbits,27, 28 thought to have important functional consequences: First, the reduction in ICa,L contributes to shortening of the action potential (AP) duration during ischemia together with activation of sarcolemmal KATP channels (see section 4). However, the overall contribution of ICa,L to shortening of the AP during ischemia is still a matter of debate, as Verkerk and colleagues have demonstrated that IKATP - induced AP shortening occurred independent of changes in ICa,L.29 Second, reduction in ICa,L amplitude will decrease the calcium-induced calcium release from the sarcoplasmic reticulum. Metabolic inhibition, intracellular acidification, a reduction in ATP and a rise in ADP concentrations will increase the cytoplasmic free calcium ([Ca2+]i)30 and inhibit both the SR Ca2+ uptake (via SERCA2)31 and the SR Ca2+ release (via the ryanodine receptor RyR2);31–33 thus, the net effect on the calcium transient is complex and multifactorial. Overall, in ischemia the reduction in SR Ca2+ release appears to predominate over the reduction in SR Ca2+ uptake, manifest as a decrease in the frequency of spontaneous release of Ca2+ via RyR231 and a marked decrease in the area of the cell exhibiting organized Ca2+ release.34 Consequently, SR Ca2+ content tends to increase during metabolic stress and ischemia.31 Upon reperfusion, removal of the inhibitory effect on RyR2 could give rise to spontaneous, arrhythmogenic waves of Ca2+ release. Perhaps more importantly, these complex alterations in subcellular Ca2+ handling have been implicated in the genesis of calcium transient (CaT) alternans which can give rise to AP- or T-wave alternans, preceding VF.35–37 Of note, at physiological pacing frequencies, neither CaTnor AP alternans are observed in normally perfused myocardium, but occur exclusively in the ischemic area or the border zone after 2 to 4 minutes of ischemia.38 Consequently, the spatial heterogeneity of the CaT during the early phase of ischemia could produce electrical instability and arrhythmias.35
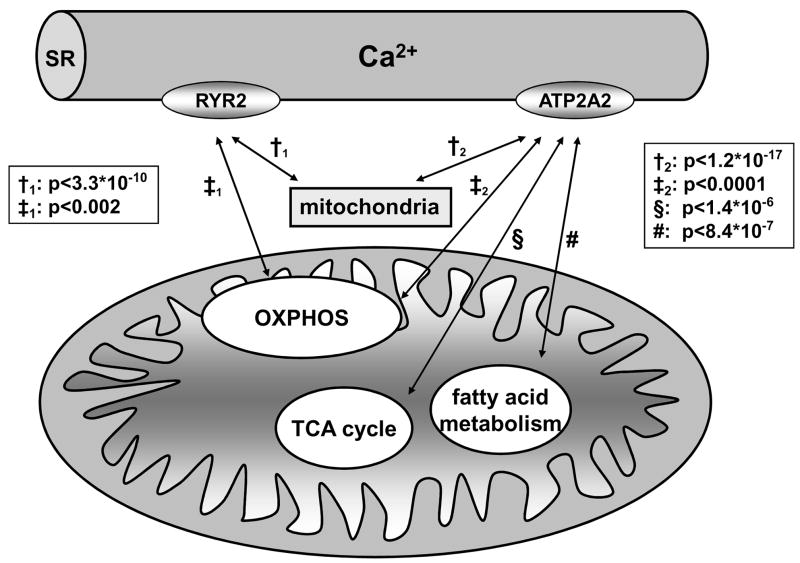
In HF, it is well established that altered functional expression of SERCA2 plays an important role in the abnormal Ca2+ homeostasis, however the mechanisms underlying SERCA2 dysregulation are not fully understood. Using a genome-wide transcriptional approach in a canine tachypacing HF model, we demonstrated that changes in SERCA2 mRNA occurred as early as 3 days after initiation of tachypacing and were accompanied by concomitant prolongation of the AP.39 What is more, about half of the transcripts associated with SERCA2 expression (18 out of 37) were directly linked to oxidative phosphorylation, ATP synthesis, fatty acid β-oxidation, and the TCA cycle.39 This suggests a coordinate dysregulation shared by SERCA2 and energetic pathways during tachypacing-induced HF. To our knowledge, this was the first report linking changes in AP duration and SERCA2 mRNA transcript levels to metabolic activity in left ventricular dysfunction. Importantly, the close transcriptional correlation between mitochondrial genes and SERCA2 is not confined to the pacing-induced HF model, but is also observed in mouse myocardium (Figure 2) and human HF (e.g. Gene Expression Omnibus accession number GSE5406). Recent data showing that the mitochondrial transcription factors Tfam and Tfb2m40 bind to the SERCA2 promoter and regulate SERCA2 transcript levels, provide first mechanistic insights into the coordinate regulation of ATP production and expenditure in mammalian myocardium.41
Figure 2. Transcriptional co-regulatory patterns for SERCA2 (ATP2A2) and RYR2 in mouse myocardium.
Genes that were co-regulated with SERCA2 and RYR2 were identified by StarNet102 and grouped into Gene Ontology categories. Mitochondrial transcripts showed the most significant co-expression pattern with SERCA2 and RYR2. (Bonferroni corrected p-values for the correlation coefficient across 239 mouse microarrays are indicated). OXPHOS = oxidative phosphorylation.
3. Metabolic activity alters expression and function of repolarizing potassium currents
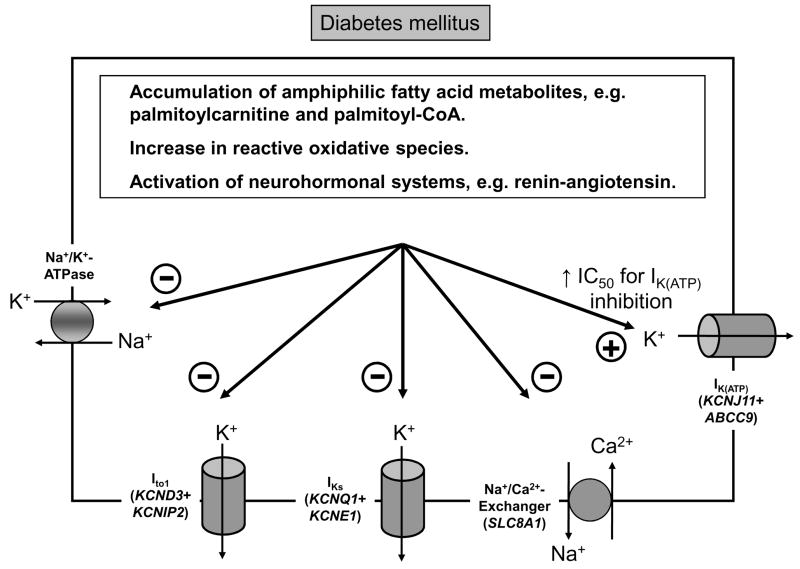
Besides regulating intracellular Na+ and Ca2+ homeostasis, metabolic activity has also been shown to critically affect repolarizing K+ currents. Other than KATP, one of the first K+ channels recognized to be modulated by metabolic inhibition was the Ca2+-independent transient outward K+ current, Ito. Inhibition of oxidative phosphorylation, using inhibitors such as 2,4-dinitrophenol (DNP) and cyanide, markedly decreased the magnitude of the 4-AP sensitive Ito.42 Remodeling of cardiac Ito has also been implicated in diabetes mellitus, one of the leading metabolic abnormalities associated with arrhythmias. Compared to healthy individuals, diabetic patients show a higher incidence of AF, VF and sudden cardiac death.14, 43 Electrocardiographically, diabetic patients often display QT-interval prolongation and T wave abnormalities.14 Studies designed to elucidate the cellular mechanisms of diabetes-induced repolarization abnormalities have consistently demonstrated AP prolongation in myocytes isolated from diabetic animals. This was mainly due to a net decrease in outward repolarizing currents, including Ito.44–46 A primary role of metabolic changes in regulation of Ito is suggested since down regulation of Ito in cardiomyocytes from diabetic hearts could be reversed by short-term treatment with insulin.44 Recent studies have shed some light on the mechanisms by which diabetes mellitus alters the functional expression of cardiac ion channels. Peroxisome proliferator-activated receptor alpha (PPARα), a key regulator of fatty acid and glucose metabolism is up regulated in diabetic hearts and has been found to lead to remodeling of a wide variety of ion channels, most prominently Ito.47 Transgenic mice with chronic, cardiac-specific activation of PPARα displayed decreased Ito density, concomitant with reduced protein expression of the alpha (Kv4.2) and beta (KChIP2) subunits of Ito channels.47 In contrast, transgenic animals with deletion of the PPARα–gene, showed increased density of Ito.47 Of note, in PPARα-overexpressing animals, these changes occurred well before any signs of LV hypertrophy or LV dysfunction became apparent, suggesting that altered metabolic activity directly affects expression of Ito. In addition to up regulation of PPARα, a rise in plasma free fatty acids in diabetes mellitus increases the rate of β-oxidation. Amphiphilic fatty acid metabolites, such as palmitoylcarnitine and palmitoyl-CoA have been shown to directly suppress Ito in rodents.48 Activation of neurohormonal systems, including the renin-angiotensin-system, also contribute to potassium channel remodeling in the diabetic heart.49 Likewise, diabetes-induced down regulation of Ito involves the formation of reactive oxygen species (ROS) and incubation of cells isolated from diabetic hearts with reduced glutathione (GSH) was able to reverse down regulation of Ito.44
While most of these studies have been carried out in rodents, recent studies demonstrate that diabetes-induced remodeling of potassium channels also holds true for larger mammals, which share a far greater similarity of ventricular repolarization to humans. In dogs, for instance, diabetes-induced repolarization abnormalities were associated with prominent down regulation of Ito.50 In addition, a decrease in the density of the slow component of the delayed rectifier current IKs, and a reduction in protein levels of the beta-subunit, minK (encoded by KCNE1) were observed.50 Functional down regulation of potassium channel subunits and currents can be regarded as acquired long QT syndrome whereby the reduced repolarization reserve increases the predisposition to early afterdepolarizations, triggered arrhythmias, and ultimately sudden cardiac death. The pathophysiological processes contributing to electrical instability in the diabetic heart are summarized in Figure 3.
Figure 3. Pathophysiological processes associated with electrical instability in diabetic hearts.
4. Sarcolemmal ATP-dependent potassium channels (KATP) link metabolic activity to excitability
KATP channels act as immediate metabolic sensors coupling electrical excitability to the cellular high-energy phosphate pool of cardiomyocytes. These channels occur in high density in ventricular cardiomyocytes and are hetero-octamers comprised of the pore-forming Kir6.2 (KCNJ11) subunits and the regulatory sulfonylurea receptor SUR2A (ABCC9).51–53 ATP is the main inhibitory channel ligand which binds to Kir6.2 subunits and keeps the channel closed under physiological conditions when intracellular ATP levels are high (~6–10 mM). O’Rourke and colleagues described repetitive and self-sustaining oscillations of IKATP which were closely associated with cyclical changes in the mitochondrial NADH/NAD+ redox state in cardiac myocytes subjected to metabolic stress.54 However, glycolytically generated ATP seems to be more critical in regulating IKATP in mammalian ventricular myocytes than ATP generated by oxidative phosphorylation.55 These observations were complemented by recent data showing that glyceraldehyde 3-phosphate dehydrogenase (GAPDH), a key glycolytic enzyme, serves as an accessory protein of the cardiac sarcolemmal KATP channel complex.56, 57 The physical association between Kir6.2 and GAPDH may have significant functional consequences on the KATP-channel, as this enzyme catalyzes the reaction of 1,3-bisphosphoglycerate production. 1,3-bisphosphoglycerate has been suggested to be an endogenous KATP-channel opener that has the ability to activate sarcolemmal KATP channels even in the presence of high levels of intracellular ATP.58 Modulation of ATP binding by additional metabolic factors is also suggested by a two-fold higher IC50 for ATP-dependent inhibition of KATP channels in diabetic hearts compared to controls.59 As a result, hypoxia-induced shortening of the AP due to activation of the KATP channels, occurs to a much greater extent in ventricular cells from diabetic rats.60 Of note, this sudden increase in KATP conductance during ischemia is expected to produce a greater dispersion of refractoriness in the diabetic vs. non-diabetic heart, as AP prolongation due to down regulation of major repolarizing K+ channels is characteristically seen in diabetic myocardium (see Section 3).
During hypoxia or ischemia, global and especially local ATP concentrations may fall below a critical level, leading to opening of the KATP channel. Interestingly, the ST-segment elevation often observed on the electrocardiogram in the ischemic heart is postulated to result from excess opening of ATP-sensitive potassium channels.61 The IKATP-induced shortening of the cardiac AP is cardioprotective by decreasing Ca2+ influx through ICa,L, thus preventing cellular Ca2+ overload and further decline of the ATP pool. In line with this finding, Kir6.2 (KCNJ11) knock-out mice developed lethal arrhythmias upon sympathetic stimulation.62 Contraction bands, pathognomonic of cytosolic calcium loading, were visible on autopsy throughout the ventricular myocardium of the Kir6.2-knockout, but not wild-type mice.62 These results suggest that Kir6.2 plays an essential role in the myocardial response to stress. However, excessive AP shortening, resulting from activation of IKATP, can also increase the dispersion of repolarization between ischemic and non-ischemic regions of the myocardium and predispose to reentrant arrhythmias including VF. In contrast to the general cardioprotective action ascribed to IKATP opening, several animal studies also suggest a beneficial effect of IKATP-blockade by reducing the incidence of VT/VF in the setting of ischemia reperfusion.63, 64 In the following sections we will review recent evidence regarding the upstream signaling events leading to opening of KATP channels.
5. Collapse of mitochondrial membrane potential activates IKATP thereby creating regions of non-excitability (“metabolic sinks”)
Mitochondria are the “cellular power plants”, as they supply most of the cell’s chemical energy in form of ATP. New evidence has emerged over the last decade, demonstrating that mitochondria are involved in a wide range of additional cellular processes, such as cell signaling, apoptosis, ischemic preconditioning, control of cellular differentiation and cell cycle regulation.65 Important mediators that link metabolic activity to various cell signaling pathways are ROS. As a byproduct of activity in the electron transfer chain, it is estimated that up to 5% of the molecular oxygen consumed in mitochondria is converted to superoxide anions by complexes I and III.7, 66, 67 As ROS can be highly toxic to nucleic acids, proteins, lipids, and other cellular components, cardiomyocytes possess a highly efficient antioxidant defense system, consisting of superoxide dismutase, catalase and glutathione peroxidase. Ischemia/reperfusion or metabolic stress can produce local elevations of ROS in the myocardium68 which, by a positive feedback mechanism described by Zorov et al., can trigger additional ROS-production (“ROS-induced ROS release”).69 When the balance between ROS production and ROS scavenging is shifted towards ROS production, the accumulation of ROS (and particularly superoxide anion, O2−) increases the open probability of ion channels located in the inner mitochondrial membrane thereby depolarizing the mitochondrial membrane potential (ΔΨm).70–72 Importantly, local mitochondrial perturbations are transmitted to neighboring mitochondria with ROS implicated as key signal transducers.73 As a result, a local collapse of the mitochondrial membrane potential can have an impact on the entire mitochondrial network.71, 74, 75 As variations in ΔΨm and AP duration are highly correlated through opening of sarcolemmal KATP channels,70 large regions of conduction block may be created in the setting of ischemia/reperfusion, producing a substrate for stable arrhythmias upon reperfusion. Therefore, this “metabolic sink” would be distinct from other forms of block of electrical propagation, including loss of cell-cell coupling by closure of gap junctions, regional uncoupling by anatomical barriers to conduction (e.g. scar tissue) or dynamic functional block due to heterogeneity of intrinsic electrophysiological restitution properties.76
6. Role of non-ion channel genes linking metabolic activity to arrhythmias
Adenosine monophosphate–activated protein kinase (AMPK) is a serine/threonine kinase abundantly expressed in myocardial tissue.77 It is comprised of a catalytic α-subunit and regulatory β- and γ-subunits assembled as a heterotrimer.78, 79 AMP-activated protein kinase functions as a metabolic sensor in cardiomyocytes. AMPK activation by an elevated ratio of AMP to ATP stimulates ATP-generating pathways and down regulates ATP-consuming pathways. Therefore, AMPK has been termed the “guardian of energy status” in the heart.79–81 Specific mutations in the γ2 regulatory subunit of AMP-activated protein kinase (PRKAG2) have been shown to alter myocardial AMPK activity and cause a glycogen storage cardiomyopathy characterized by ventricular pre-excitation, atrial fibrillation, progressive conduction system disease and a variable degree of left ventricular hypertrophy.82–84 The structural abnormalities, including glycogen accumulation and left ventricular hypertrophy, suggest that electrical instability associated with PRKAG2 mutations might arise secondary to extensive structural remodeling rather than as a primary result of the PRKAG2 mutation. In this sense, it has been suggested that glycogen-filled cardiomyocytes contribute to the formation of muscular bundles between atria and ventricles that mediate ventricular preexcitation.85 Alternatively, the high incidence of ventricular pre-excitation and atrioventricular conduction abnormalities observed across several metabolic gene defects may result from disruption of the developmental patterning of the atrioventricular ring.86 The notion that arrhythmias arise secondary to structural abnormalities has been challenged by the discovery of a family with a mutation in PRKAG2, in which affected family members displayed an arrhythmogenic phenotype starting in early childhood at a time when structural abnormalities and cardiac hypertrophy were absent.86 Mechanistically, AMPK could directly modulate the activity of sarcolemmal ion channels, as demonstrated for the cardiac sodium channel.87 Additionally, AMPK activity could affect electrophysiological processes via a wide variety intracellular signaling pathways. Most notably, AMPK can phosphorylate endothelial nitric oxide synthase88 and p38 MAP kinase81, both of which have pleiotropic actions in the heart, including direct effects on ion channels. Even though the precise cellular mechanisms linking AMPK mutations to arrhythmias are still elusive, the finding that a mutation in a major regulator of cardiac energy metabolism causes arrhythmogenic activity supports the notion that alterations in cardiac bioenergetics are sufficient to cause arrhythmias independent of a precipitating event like ischemia/reperfusion.
In addition to AMPK mutations, several monogenic disorders affecting genes of lipid and glycogen metabolism present with arrhythmias. For instance, Bonnet et al. reported a series of 24 children in whom arrhythmias or conduction defects were the presenting symptom of various fatty acid oxidation disorders.89 They suggested that the accumulation of arrhythmogenic intermediary metabolites of fatty acids, such as long-chain acylcarnitines, may be responsible for the arrhythmias.89 However, these rare inherited disorders of fatty acid metabolism are commonly associated with cardiomyopathy, making it difficult to separate the arrhythmogenic predisposition from the underlying heart failure.
7. Altered metabolism in atria: cause and consequence of arrhythmias
Cha and colleagues have demonstrated that the propensity for sustained atrial fibrillation in dogs with pacing-induced heart failure robustly correlates with the cellular energetic state in the atria.90 Failing atrial myocardium displays a profound bioenergetic deficit with reduced activities of the phosphotransfer enzymes creatine kinase (CK) and adenylate kinase (AK), and depletion of high-energy phosphates. Despite significant increases in the atrial effective refractory period (ERP) in failing hearts, an enhanced propensity to sustained AF was observed in HF. Intriguingly, AF duration correlated inversely with atrial ATP concentration, as well as AK and CK activity.90 Likewise, atrial tissue samples from patients who developed atrial fibrillation post-operatively had significantly lower concentrations of glucose, beta-hydroxybutyrate, and acetate compared to patients who remained in sinus rhythm after surgery.91 These observations suggest that perturbations in bioenergetic homeostasis and myocardial substrate use contribute to electrical instability in atrial myocardium.
There is a growing body of evidence that metabolic activity and arrhythmias are interdependent: An impaired cellular energetic state not only predisposes to atrial arrhythmias, but atrial rhythm disturbances also influence metabolic activity. Transcriptomic studies in permanent human AF, for instance, identified a prominent dysregulation of transcripts with metabolic function in tissue samples from patients with AF compared to patients in sinus rhythm. Notably, there was a coordinated transcriptional down regulation of enzymes controlling fatty acid oxidation and concomitant up regulation of enzymes involved in glucose utilization.92 In a goat model of AF, phosphocreatine decreased by 60% in atrial myocytes after one week of AF, suggesting an enhanced demand for high-energy phosphates.93 As phosphocreatine levels increased thereafter and returned to baseline levels after 16 weeks of AF,93 compensatory mechanisms are assumed to be activated to restore the metabolic balance between energy demand and supply. The finding of a prominent up regulation of transcripts involved in metabolic processes in human92 and experimental models of AF94 supports this notion. Consistent with these microarray expression data, a recent proteomic and metabolomic study of human atrial AF samples showed that the majority of changes observed in fibrillating atria were related to energy metabolism.91
8. Correction of metabolic abnormalities: A novel antiarrhythmic strategy?
Data reviewed in this article has highlighted that altered metabolism can lead to arrhythmias via a variety of cellular pathways in myocardial cells. Life-threatening ventricular tachyarrhythmias are prevalent in virtually every cardiovascular condition associated with structural and/or functional abnormalities. Clinical trials such as CAST95 and SWORD96 revealed that, at least in post-infarction patients, ion channel-targeted antiarrhythmic therapy may lead to increased rather than reduced mortality. In AF, large clinical trials have failed to prove the superiority of a “rhythm control” over a “rate control strategy”,97, 98 possibly due to an increased mortality associated with antiarrhythmic therapy.99 Thus, modulating only the electrical activity (i.e. ion channel function) has proven ineffective to prevent arrhythmias in the long-term. Instead, additional strategies are needed in order to correct the underlying pathophysiological processes and metabolic abnormalities that lead to maladaptive ion channel remodeling.
In this respect, several drugs that either increase metabolic efficiency by augmenting glucose metabolism and blocking muscle mitochondrial free fatty acid uptake (e.g. perhexiline, trimetazidine, ranolazine) and/or block late sodium currents (ranolazine) have shown benefits in small preliminary clinical trials.100 Moreover, emerging evidence regarding the central role of mitochondria in maintaining electrical stability in the heart has led to the identification of several novel pharmacological targets. For instance, 4′-chlorodiazepam, a ligand of the mitochondrial benzodiazepine receptor, has proven effective in stabilizing the mitochondrial membrane potential primarily by blocking the mitochondrial inner membrane anion channel (IMAC) at the time of increased oxidative stress and protect against reperfusion arrhythmias and post-ischemic contractile impairment. Remarkably, unlike other pre-conditioning agents, 4′-chlorodiazepam, was effective when given as a single bolus upon reperfusion.101
Conclusion
It is apparent that there is an intimate relationship between energy metabolism and ion homeostasis. The varied ways in which altered metabolism can be arrhythmogenic provides valuable mechanistic insights and identifies potential molecular targets for pharmacological interventions to treat arrhythmias. Given the proarrhythmic risk associated with conventional, ion channel-targeted antiarrhythmic drug therapies, a new approach to arrhythmias is urgently needed. In this respect, correction of the cellular bioenergetic deficit associated with structural heart disease might represent a novel and promising antiarrhythmic strategy.
Acknowledgments
Funding Sources
The work and authors are supported by NIH NHLBI R33 HL087363 and PO1 HL077180.
Footnotes
Journal Subject Codes: [132] Arrhythmias-basic studies; [140] Energy metabolism; [152] Ion channels/membrane transport; [91] Oxidant stress
Disclosures
None. All authors have read and agree to the manuscript as written.
References
- 1.Cobb LA, Fahrenbruch CE, Olsufka M, Copass MK. Changing incidence of out-of-hospital ventricular fibrillation, 1980–2000. Jama. 2002;288:3008–3013. doi: 10.1001/jama.288.23.3008. [DOI] [PubMed] [Google Scholar]
- 2.Myerburg RJ. Sudden cardiac death: exploring the limits of our knowledge. J Cardiovasc Electrophysiol. 2001;12:369–381. doi: 10.1046/j.1540-8167.2001.00369.x. [DOI] [PubMed] [Google Scholar]
- 3.Zipes DP, Wellens HJ. Sudden cardiac death. Circulation. 1998;98:2334–2351. doi: 10.1161/01.cir.98.21.2334. [DOI] [PubMed] [Google Scholar]
- 4.Nass RD, Aiba T, Tomaselli GF, Akar FG. Mechanisms of disease: ion channel remodeling in the failing ventricle. Nat Clin Pract Cardiovasc Med. 2008;5:196–207. doi: 10.1038/ncpcardio1130. [DOI] [PubMed] [Google Scholar]
- 5.Shah M, Akar FG, Tomaselli GF. Molecular basis of arrhythmias. Circulation. 2005;112:2517–2529. doi: 10.1161/CIRCULATIONAHA.104.494476. [DOI] [PubMed] [Google Scholar]
- 6.Barth E, Stammler G, Speiser B, Schaper J. Ultrastructural quantitation of mitochondria and myofilaments in cardiac muscle from 10 different animal species including man. J Mol Cell Cardiol. 1992;24:669–681. doi: 10.1016/0022-2828(92)93381-s. [DOI] [PubMed] [Google Scholar]
- 7.O’Rourke B, Cortassa S, Aon MA. Mitochondrial ion channels: gatekeepers of life and death. Physiology (Bethesda) 2005;20:303–315. doi: 10.1152/physiol.00020.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murphy E, Steenbergen C. Ion transport and energetics during cell death and protection. Physiology (Bethesda) 2008;23:115–123. doi: 10.1152/physiol.00044.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.John SA, Kondo R, Wang SY, Goldhaber JI, Weiss JN. Connexin-43 hemichannels opened by metabolic inhibition. J Biol Chem. 1999;274:236–240. doi: 10.1074/jbc.274.1.236. [DOI] [PubMed] [Google Scholar]
- 10.Kondo RP, Wang SY, John SA, Weiss JN, Goldhaber JI. Metabolic inhibition activates a non-selective current through connexin hemichannels in isolated ventricular myocytes. J Mol Cell Cardiol. 2000;32:1859–1872. doi: 10.1006/jmcc.2000.1220. [DOI] [PubMed] [Google Scholar]
- 11.Manjunath CK, Page E. Cell biology and protein composition of cardiac gap junctions. Am J Physiol. 1985;248:H783–791. doi: 10.1152/ajpheart.1985.248.6.H783. [DOI] [PubMed] [Google Scholar]
- 12.Neubauer S, Newell JB, Ingwall JS. Metabolic consequences and predictability of ventricular fibrillation in hypoxia. A 31P- and 23Na-nuclear magnetic resonance study of the isolated rat heart. Circulation. 1992;86:302–310. doi: 10.1161/01.cir.86.1.302. [DOI] [PubMed] [Google Scholar]
- 13.Tomaselli GF, Beuckelmann DJ, Calkins HG, Berger RD, Kessler PD, Lawrence JH, Kass D, Feldman AM, Marban E. Sudden cardiac death in heart failure. The role of abnormal repolarization. Circulation. 1994;90:2534–2539. doi: 10.1161/01.cir.90.5.2534. [DOI] [PubMed] [Google Scholar]
- 14.Veglio M, Chinaglia A, Cavallo-Perin P. QT interval, cardiovascular risk factors and risk of death in diabetes. J Endocrinol Invest. 2004;27:175–181. doi: 10.1007/BF03346265. [DOI] [PubMed] [Google Scholar]
- 15.Tomaselli GF, Marban E. Electrophysiological remodeling in hypertrophy and heart failure. Cardiovasc Res. 1999;42:270–283. doi: 10.1016/s0008-6363(99)00017-6. [DOI] [PubMed] [Google Scholar]
- 16.Hansen PS, Clarke RJ, Buhagiar KA, Hamilton E, Garcia A, White C, Rasmussen HH. Alloxan-induced diabetes reduces sarcolemmal Na+-K+ pump function in rabbit ventricular myocytes. Am J Physiol Cell Physiol. 2007;292:C1070–1077. doi: 10.1152/ajpcell.00288.2006. [DOI] [PubMed] [Google Scholar]
- 17.Shivkumar K, Deutsch NA, Lamp ST, Khuu K, Goldhaber JI, Weiss JN. Mechanism of hypoxic K loss in rabbit ventricle. J Clin Invest. 1997;100:1782–1788. doi: 10.1172/JCI119705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Janse MJ, van Capelle FJ, Morsink H, Kleber AG, Wilms-Schopman F, Cardinal R, d’Alnoncourt CN, Durrer D. Flow of “injury” current and patterns of excitation during early ventricular arrhythmias in acute regional myocardial ischemia in isolated porcine and canine hearts. Evidence for two different arrhythmogenic mechanisms. Circ Res. 1980;47:151–165. doi: 10.1161/01.res.47.2.151. [DOI] [PubMed] [Google Scholar]
- 19.Liu T, O’Rourke B. Enhancing mitochondrial Ca2+ uptake in myocytes from failing hearts restores energy supply and demand matching. Circ Res. 2008;103:279–288. doi: 10.1161/CIRCRESAHA.108.175919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huser J, Blatter LA, Sheu SS. Mitochondrial calcium in heart cells: beat-to-beat oscillations or slow integration of cytosolic transients? J Bioenerg Biomembr. 2000;32:27–33. doi: 10.1023/a:1005556227425. [DOI] [PubMed] [Google Scholar]
- 21.Saks V, Dzeja P, Schlattner U, Vendelin M, Terzic A, Wallimann T. Cardiac system bioenergetics: metabolic basis of the Frank-Starling law. J Physiol. 2006;571:253–273. doi: 10.1113/jphysiol.2005.101444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maack C, Cortassa S, Aon MA, Ganesan AN, Liu T, O’Rourke B. Elevated cytosolic Na+ decreases mitochondrial Ca2+ uptake during excitation-contraction coupling and impairs energetic adaptation in cardiac myocytes. Circ Res. 2006;99:172–182. doi: 10.1161/01.RES.0000232546.92777.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sedova M, Dedkova EN, Blatter LA. Integration of rapid cytosolic Ca2+ signals by mitochondria in cat ventricular myocytes. Am J Physiol Cell Physiol. 2006;291:C840–850. doi: 10.1152/ajpcell.00619.2005. [DOI] [PubMed] [Google Scholar]
- 24.Goldhaber JI, Parker JM, Weiss JN. Mechanisms of excitation-contraction coupling failure during metabolic inhibition in guinea-pig ventricular myocytes. J Physiol. 1991;443:371–386. doi: 10.1113/jphysiol.1991.sp018838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hool LC. Hypoxia increases the sensitivity of the L-type Ca(2+) current to beta-adrenergic receptor stimulation via a C2 region-containing protein kinase C isoform. Circ Res. 2000;87:1164–1171. doi: 10.1161/01.res.87.12.1164. [DOI] [PubMed] [Google Scholar]
- 26.Lederer WJ, Nichols CG, Smith GL. The mechanism of early contractile failure of isolated rat ventricular myocytes subjected to complete metabolic inhibition. J Physiol. 1989;413:329–349. doi: 10.1113/jphysiol.1989.sp017657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Losito VA, Tsushima RG, Diaz RJ, Wilson GJ, Backx PH. Preferential regulation of rabbit cardiac L-type Ca2+ current by glycolytic derived ATP via a direct allosteric pathway. J Physiol. 1998;511 (Pt 1):67–78. doi: 10.1111/j.1469-7793.1998.067bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chantawansri C, Huynh N, Yamanaka J, Garfinkel A, Lamp ST, Inoue M, Bridge JH, Goldhaber JI. Effect of metabolic inhibition on couplon behavior in rabbit ventricular myocytes. Biophys J. 2008;94:1656–1666. doi: 10.1529/biophysj.107.114892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Verkerk AO, Veldkamp MW, van Ginneken AC, Bouman LN. Biphasic response of action potential duration to metabolic inhibition in rabbit and human ventricular myocytes: role of transient outward current and ATP-regulated potassium current. J Mol Cell Cardiol. 1996;28:2443–2456. doi: 10.1006/jmcc.1996.0237. [DOI] [PubMed] [Google Scholar]
- 30.Mohabir R, Lee HC, Kurz RW, Clusin WT. Effects of ischemia and hypercarbic acidosis on myocyte calcium transients, contraction, and pHi in perfused rabbit hearts. Circ Res. 1991;69:1525–1537. doi: 10.1161/01.res.69.6.1525. [DOI] [PubMed] [Google Scholar]
- 31.Overend CL, Eisner DA, O’Neill SC. Altered cardiac sarcoplasmic reticulum function of intact myocytes of rat ventricle during metabolic inhibition. Circ Res. 2001;88:181–187. doi: 10.1161/01.res.88.2.181. [DOI] [PubMed] [Google Scholar]
- 32.Xu L, Mann G, Meissner G. Regulation of cardiac Ca2+ release channel (ryanodine receptor) by Ca2+, H+, Mg2+, and adenine nucleotides under normal and simulated ischemic conditions. Circ Res. 1996;79:1100–1109. doi: 10.1161/01.res.79.6.1100. [DOI] [PubMed] [Google Scholar]
- 33.Meissner G, Henderson JS. Rapid calcium release from cardiac sarcoplasmic reticulum vesicles is dependent on Ca2+ and is modulated by Mg2+, adenine nucleotide, and calmodulin. J Biol Chem. 1987;262:3065–3073. [PubMed] [Google Scholar]
- 34.Fukumoto GH, Lamp ST, Motter C, Bridge JH, Garfinkel A, Goldhaber JI. Metabolic inhibition alters subcellular calcium release patterns in rat ventricular myocytes: implications for defective excitation-contraction coupling during cardiac ischemia and failure. Circ Res. 2005;96:551–557. doi: 10.1161/01.RES.0000159388.61313.47. [DOI] [PubMed] [Google Scholar]
- 35.Clusin WT. Mechanisms of calcium transient and action potential alternans in cardiac cells and tissues. Am J Physiol Heart Circ Physiol. 2008;294:H1–H10. doi: 10.1152/ajpheart.00802.2007. [DOI] [PubMed] [Google Scholar]
- 36.Qian YW, Clusin WT, Lin SF, Han J, Sung RJ. Spatial heterogeneity of calcium transient alternans during the early phase of myocardial ischemia in the blood-perfused rabbit heart. Circulation. 2001;104:2082–2087. doi: 10.1161/hc4201.097136. [DOI] [PubMed] [Google Scholar]
- 37.Lee HC, Mohabir R, Smith N, Franz MR, Clusin WT. Effect of ischemia on calcium-dependent fluorescence transients in rabbit hearts containing indo 1. Correlation with monophasic action potentials and contraction. Circulation. 1988;78:1047–1059. doi: 10.1161/01.cir.78.4.1047. [DOI] [PubMed] [Google Scholar]
- 38.Dilly SG, Lab MJ. Electrophysiological alternans and restitution during acute regional ischaemia in myocardium of anaesthetized pig. J Physiol. 1988;402:315–333. doi: 10.1113/jphysiol.1988.sp017206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gao Z, Barth AS, DiSilvestre D, Akar FG, Tian Y, Tanskanen A, Kass DA, Winslow RL, Tomaselli GF. Key pathways associated with heart failure development revealed by gene networks correlated with cardiac remodeling. Physiol Genomics. 2008;35:222–230. doi: 10.1152/physiolgenomics.00100.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scarpulla RC. Transcriptional paradigms in mammalian mitochondrial biogenesis and function. Physiol Rev. 2008;88:611–638. doi: 10.1152/physrev.00025.2007. [DOI] [PubMed] [Google Scholar]
- 41.Watanabe A, Arai M, Ohyama Y, Kurabayashi M. Mitochondrial Transcription Factors, Tfam and Tfb2m Regulate the SERCA2 Gene Transcription - A Novel Mechanism of the Coordinate Regulation of Energy Production and Expenditure. Circulation. 2008;118:S-517. [Google Scholar]
- 42.Ogbaghebriel A, Shrier A. Inhibition of metabolism abolishes transient outward current in rabbit atrial myocytes. Am J Physiol. 1994;266:H182–190. doi: 10.1152/ajpheart.1994.266.1.H182. [DOI] [PubMed] [Google Scholar]
- 43.Aksnes TA, Schmieder RE, Kjeldsen SE, Ghani S, Hua TA, Julius S. Impact of new-onset diabetes mellitus on development of atrial fibrillation and heart failure in high-risk hypertension (from the VALUE Trial) Am J Cardiol. 2008;101:634–638. doi: 10.1016/j.amjcard.2007.10.025. [DOI] [PubMed] [Google Scholar]
- 44.Xu Z, Patel KP, Lou MF, Rozanski GJ. Up-regulation of K(+) channels in diabetic rat ventricular myocytes by insulin and glutathione. Cardiovasc Res. 2002;53:80–88. doi: 10.1016/s0008-6363(01)00446-1. [DOI] [PubMed] [Google Scholar]
- 45.Xu Z, Patel KP, Rozanski GJ. Metabolic basis of decreased transient outward K+ current in ventricular myocytes from diabetic rats. Am J Physiol. 1996;271:H2190–2196. doi: 10.1152/ajpheart.1996.271.5.H2190. [DOI] [PubMed] [Google Scholar]
- 46.Shimoni Y, Firek L, Severson D, Giles W. Short-term diabetes alters K+ currents in rat ventricular myocytes. Circ Res. 1994;74:620–628. doi: 10.1161/01.res.74.4.620. [DOI] [PubMed] [Google Scholar]
- 47.Marionneau C, Aimond F, Brunet S, Niwa N, Finck B, Kelly DP, Nerbonne JM. PPARalpha-mediated remodeling of repolarizing voltage-gated K+ (Kv) channels in a mouse model of metabolic cardiomyopathy. J Mol Cell Cardiol. 2008;44:1002–1015. doi: 10.1016/j.yjmcc.2008.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu Z, Rozanski GJ. K+ current inhibition by amphiphilic fatty acid metabolites in rat ventricular myocytes. Am J Physiol. 1998;275:C1660–1667. doi: 10.1152/ajpcell.1998.275.6.C1660. [DOI] [PubMed] [Google Scholar]
- 49.Shimoni Y, Liu XF. Gender differences in ANG II levels and action on multiple K+ current modulation pathways in diabetic rats. Am J Physiol Heart Circ Physiol. 2004;287:H311–319. doi: 10.1152/ajpheart.01212.2003. [DOI] [PubMed] [Google Scholar]
- 50.Lengyel C, Virag L, Biro T, Jost N, Magyar J, Biliczki P, Kocsis E, Skoumal R, Nanasi PP, Toth M, Kecskemeti V, Papp JG, Varro A. Diabetes mellitus attenuates the repolarization reserve in mammalian heart. Cardiovasc Res. 2007;73:512–520. doi: 10.1016/j.cardiores.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 51.Inagaki N, Gonoi T, Clement JPt, Namba N, Inazawa J, Gonzalez G, Aguilar-Bryan L, Seino S, Bryan J. Reconstitution of IKATP: an inward rectifier subunit plus the sulfonylurea receptor. Science. 1995;270:1166–1170. doi: 10.1126/science.270.5239.1166. [DOI] [PubMed] [Google Scholar]
- 52.Noma A. ATP-regulated K+ channels in cardiac muscle. Nature. 1983;305:147–148. doi: 10.1038/305147a0. [DOI] [PubMed] [Google Scholar]
- 53.Zingman LV, Alekseev AE, Hodgson-Zingman DM, Terzic A. ATP-sensitive potassium channels: metabolic sensing and cardioprotection. J Appl Physiol. 2007;103:1888–1893. doi: 10.1152/japplphysiol.00747.2007. [DOI] [PubMed] [Google Scholar]
- 54.O’Rourke B, Ramza BM, Marban E. Oscillations of membrane current and excitability driven by metabolic oscillations in heart cells. Science. 1994;265:962–966. doi: 10.1126/science.8052856. [DOI] [PubMed] [Google Scholar]
- 55.Weiss JN, Lamp ST. Glycolysis preferentially inhibits ATP-sensitive K+ channels in isolated guinea pig cardiac myocytes. Science. 1987;238:67–69. doi: 10.1126/science.2443972. [DOI] [PubMed] [Google Scholar]
- 56.Dhar-Chowdhury P, Harrell MD, Han SY, Jankowska D, Parachuru L, Morrissey A, Srivastava S, Liu W, Malester B, Yoshida H, Coetzee WA. The glycolytic enzymes, glyceraldehyde-3-phosphate dehydrogenase, triose-phosphate isomerase, and pyruvate kinase are components of the K(ATP) channel macromolecular complex and regulate its function. J Biol Chem. 2005;280:38464–38470. doi: 10.1074/jbc.M508744200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jovanovic S, Du Q, Crawford RM, Budas GR, Stagljar I, Jovanovic A. Glyceraldehyde 3-phosphate dehydrogenase serves as an accessory protein of the cardiac sarcolemmal K(ATP) channel. EMBO Rep. 2005;6:848–852. doi: 10.1038/sj.embor.7400489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jovanovic S, Jovanovic A. High glucose regulates the activity of cardiac sarcolemmal ATP-sensitive K+ channels via 1,3-bisphosphoglycerate: a novel link between cardiac membrane excitability and glucose metabolism. Diabetes. 2005;54:383–393. doi: 10.2337/diabetes.54.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shimoni Y, Light PE, French RJ. Altered ATP sensitivity of ATP-dependent K+ channels in diabetic rat hearts. Am J Physiol. 1998;275:E568–576. doi: 10.1152/ajpendo.1998.275.4.E568. [DOI] [PubMed] [Google Scholar]
- 60.Smith JM, Wahler GM. ATP-sensitive potassium channels are altered in ventricular myocytes from diabetic rats. Mol Cell Biochem. 1996;158:43–51. doi: 10.1007/BF00225881. [DOI] [PubMed] [Google Scholar]
- 61.Li RA, Leppo M, Miki T, Seino S, Marban E. Molecular basis of electrocardiographic ST-segment elevation. Circ Res. 2000;87:837–839. doi: 10.1161/01.res.87.10.837. [DOI] [PubMed] [Google Scholar]
- 62.Zingman LV, Hodgson DM, Bast PH, Kane GC, Perez-Terzic C, Gumina RJ, Pucar D, Bienengraeber M, Dzeja PP, Miki T, Seino S, Alekseev AE, Terzic A. Kir6.2 is required for adaptation to stress. Proc Natl Acad Sci U S A. 2002;99:13278–13283. doi: 10.1073/pnas.212315199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bauer A, Becker R, Dreyhaupt J, Voss F, Kraft P, Kelemen K, Senges-Becker JC, Katus HA, Schoels W. Role of KATP channels in repetitive induction of ventricular fibrillation. Europace. 2007;9:154–161. doi: 10.1093/europace/eul146. [DOI] [PubMed] [Google Scholar]
- 64.Billman GE, Avendano CE, Halliwill JR, Burroughs JM. The effects of the ATP-dependent potassium channel antagonist, glyburide, on coronary blood flow and susceptibility to ventricular fibrillation in unanesthetized dogs. J Cardiovasc Pharmacol. 1993;21:197–204. doi: 10.1097/00005344-199302000-00003. [DOI] [PubMed] [Google Scholar]
- 65.Marin-Garcia J, Goldenthal MJ. Heart mitochondria signaling pathways: appraisal of an emerging field. J Mol Med. 2004;82:565–578. doi: 10.1007/s00109-004-0567-7. [DOI] [PubMed] [Google Scholar]
- 66.Turrens JF, Boveris A. Generation of superoxide anion by the NADH dehydrogenase of bovine heart mitochondria. Biochem J. 1980;191:421–427. doi: 10.1042/bj1910421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen Q, Vazquez EJ, Moghaddas S, Hoppel CL, Lesnefsky EJ. Production of reactive oxygen species by mitochondria: central role of complex III. J Biol Chem. 2003;278:36027–36031. doi: 10.1074/jbc.M304854200. [DOI] [PubMed] [Google Scholar]
- 68.Corbucci GG, Perrino C, Donato G, Ricchi A, Lettieri B, Troncone G, Indolfi C, Chiariello M, Avvedimento EV. Transient and reversible deoxyribonucleic acid damage in human left ventricle under controlled ischemia and reperfusion. J Am Coll Cardiol. 2004;43:1992–1999. doi: 10.1016/j.jacc.2004.01.040. [DOI] [PubMed] [Google Scholar]
- 69.Zorov DB, Filburn CR, Klotz LO, Zweier JL, Sollott SJ. Reactive oxygen species (ROS)-induced ROS release: a new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes. J Exp Med. 2000;192:1001–1014. doi: 10.1084/jem.192.7.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Aon MA, Cortassa S, Marban E, O’Rourke B. Synchronized whole cell oscillations in mitochondrial metabolism triggered by a local release of reactive oxygen species in cardiac myocytes. J Biol Chem. 2003;278:44735–44744. doi: 10.1074/jbc.M302673200. [DOI] [PubMed] [Google Scholar]
- 71.Aon MA, Cortassa S, O’Rourke B. Percolation and criticality in a mitochondrial network. Proc Natl Acad Sci U S A. 2004;101:4447–4452. doi: 10.1073/pnas.0307156101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cortassa S, Aon MA, Winslow RL, O’Rourke B. A mitochondrial oscillator dependent on reactive oxygen species. Biophys J. 2004;87:2060–2073. doi: 10.1529/biophysj.104.041749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Brady NR, Hamacher-Brady A, Westerhoff HV, Gottlieb RA. A wave of reactive oxygen species (ROS)-induced ROS release in a sea of excitable mitochondria. Antioxid Redox Signal. 2006;8:1651–1665. doi: 10.1089/ars.2006.8.1651. [DOI] [PubMed] [Google Scholar]
- 74.Aon MA, Cortassa S, Akar FG, O’Rourke B. Mitochondrial criticality: a new concept at the turning point of life or death. Biochim Biophys Acta. 2006;1762:232–240. doi: 10.1016/j.bbadis.2005.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Aon MA, Cortassa S, O’Rourke B. The fundamental organization of cardiac mitochondria as a network of coupled oscillators. Biophys J. 2006;91:4317–4327. doi: 10.1529/biophysj.106.087817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Akar FG, Aon MA, Tomaselli GF, O’Rourke B. The mitochondrial origin of postischemic arrhythmias. J Clin Invest. 2005;115:3527–3535. doi: 10.1172/JCI25371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cheung PC, Salt IP, Davies SP, Hardie DG, Carling D. Characterization of AMP-activated protein kinase gamma-subunit isoforms and their role in AMP binding. Biochem J. 2000;346 Pt 3:659–669. [PMC free article] [PubMed] [Google Scholar]
- 78.Davies SP, Hawley SA, Woods A, Carling D, Haystead TA, Hardie DG. Purification of the AMP-activated protein kinase on ATP-gamma-sepharose and analysis of its subunit structure. Eur J Biochem. 1994;223:351–357. doi: 10.1111/j.1432-1033.1994.tb19001.x. [DOI] [PubMed] [Google Scholar]
- 79.Hardie DG, Carling D. The AMP-activated protein kinase--fuel gauge of the mammalian cell? Eur J Biochem. 1997;246:259–273. doi: 10.1111/j.1432-1033.1997.00259.x. [DOI] [PubMed] [Google Scholar]
- 80.Hardie DG. AMP-activated protein kinase: the guardian of cardiac energy status. J Clin Invest. 2004;114:465–468. doi: 10.1172/JCI22683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pelletier A, Joly E, Prentki M, Coderre L. Adenosine 5′-monophosphate-activated protein kinase and p38 mitogen-activated protein kinase participate in the stimulation of glucose uptake by dinitrophenol in adult cardiomyocytes. Endocrinology. 2005;146:2285–2294. doi: 10.1210/en.2004-1565. [DOI] [PubMed] [Google Scholar]
- 82.Davies JK, Wells DJ, Liu K, Whitrow HR, Daniel TD, Grignani R, Lygate CA, Schneider JE, Noel G, Watkins H, Carling D. Characterization of the role of gamma2 R531G mutation in AMP-activated protein kinase in cardiac hypertrophy and Wolff-Parkinson-White syndrome. Am J Physiol Heart Circ Physiol. 2006;290:H1942–1951. doi: 10.1152/ajpheart.01020.2005. [DOI] [PubMed] [Google Scholar]
- 83.Sidhu JS, Rajawat YS, Rami TG, Gollob MH, Wang Z, Yuan R, Marian AJ, DeMayo FJ, Weilbacher D, Taffet GE, Davies JK, Carling D, Khoury DS, Roberts R. Transgenic mouse model of ventricular preexcitation and atrioventricular reentrant tachycardia induced by an AMP-activated protein kinase loss-of-function mutation responsible for Wolff-Parkinson-White syndrome. Circulation. 2005;111:21–29. doi: 10.1161/01.CIR.0000151291.32974.D5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zou L, Shen M, Arad M, He H, Lofgren B, Ingwall JS, Seidman CE, Seidman JG, Tian R. N488I mutation of the gamma2-subunit results in bidirectional changes in AMP-activated protein kinase activity. Circ Res. 2005;97:323–328. doi: 10.1161/01.RES.0000179035.20319.c2. [DOI] [PubMed] [Google Scholar]
- 85.Arad M, Benson DW, Perez-Atayde AR, McKenna WJ, Sparks EA, Kanter RJ, McGarry K, Seidman JG, Seidman CE. Constitutively active AMP kinase mutations cause glycogen storage disease mimicking hypertrophic cardiomyopathy. J Clin Invest. 2002;109:357–362. doi: 10.1172/JCI14571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gollob MH, Seger JJ, Gollob TN, Tapscott T, Gonzales O, Bachinski L, Roberts R. Novel PRKAG2 mutation responsible for the genetic syndrome of ventricular preexcitation and conduction system disease with childhood onset and absence of cardiac hypertrophy. Circulation. 2001;104:3030–3033. doi: 10.1161/hc5001.102111. [DOI] [PubMed] [Google Scholar]
- 87.Light PE, Wallace CH, Dyck JR. Constitutively active adenosine monophosphate-activated protein kinase regulates voltage-gated sodium channels in ventricular myocytes. Circulation. 2003;107:1962–1965. doi: 10.1161/01.CIR.0000069269.60167.02. [DOI] [PubMed] [Google Scholar]
- 88.Li J, Hu X, Selvakumar P, Russell RR, 3rd, Cushman SW, Holman GD, Young LH. Role of the nitric oxide pathway in AMPK-mediated glucose uptake and GLUT4 translocation in heart muscle. Am J Physiol Endocrinol Metab. 2004;287:E834–841. doi: 10.1152/ajpendo.00234.2004. [DOI] [PubMed] [Google Scholar]
- 89.Bonnet D, Martin D, Pascale De L, Villain E, Jouvet P, Rabier D, Brivet M, Saudubray JM. Arrhythmias and conduction defects as presenting symptoms of fatty acid oxidation disorders in children. Circulation. 1999;100:2248–2253. doi: 10.1161/01.cir.100.22.2248. [DOI] [PubMed] [Google Scholar]
- 90.Cha YM, Dzeja PP, Shen WK, Jahangir A, Hart CY, Terzic A, Redfield MM. Failing atrial myocardium: energetic deficits accompany structural remodeling and electrical instability. Am J Physiol Heart Circ Physiol. 2003;284:H1313–1320. doi: 10.1152/ajpheart.00337.2002. [DOI] [PubMed] [Google Scholar]
- 91.Mayr M, Yusuf S, Weir G, Chung YL, Mayr U, Yin X, Ladroue C, Madhu B, Roberts N, De Souza A, Fredericks S, Stubbs M, Griffiths JR, Jahangiri M, Xu Q, Camm AJ. Combined metabolomic and proteomic analysis of human atrial fibrillation. J Am Coll Cardiol. 2008;51:585–594. doi: 10.1016/j.jacc.2007.09.055. [DOI] [PubMed] [Google Scholar]
- 92.Barth AS, Merk S, Arnoldi E, Zwermann L, Kloos P, Gebauer M, Steinmeyer K, Bleich M, Kaab S, Hinterseer M, Kartmann H, Kreuzer E, Dugas M, Steinbeck G, Nabauer M. Reprogramming of the human atrial transcriptome in permanent atrial fibrillation: expression of a ventricular-like genomic signature. Circ Res. 2005;96:1022–1029. doi: 10.1161/01.RES.0000165480.82737.33. [DOI] [PubMed] [Google Scholar]
- 93.Ausma J, Coumans WA, Duimel H, Van der Vusse GJ, Allessie MA, Borgers M. Atrial high energy phosphate content and mitochondrial enzyme activity during chronic atrial fibrillation. Cardiovasc Res. 2000;47:788–796. doi: 10.1016/s0008-6363(00)00139-5. [DOI] [PubMed] [Google Scholar]
- 94.Barbey O, Pierre S, Duran MJ, Sennoune S, Levy S, Maixent JM. Specific up-regulation of mitochondrial F0F1-ATPase activity after short episodes of atrial fibrillation in sheep. J Cardiovasc Electrophysiol. 2000;11:432–438. doi: 10.1111/j.1540-8167.2000.tb00339.x. [DOI] [PubMed] [Google Scholar]
- 95.Echt DS, Liebson PR, Mitchell LB, Peters RW, Obias-Manno D, Barker AH, Arensberg D, Baker A, Friedman L, Greene HL. Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The Cardiac Arrhythmia Suppression Trial. N Engl J Med. 1991;324:781–788. doi: 10.1056/NEJM199103213241201. [DOI] [PubMed] [Google Scholar]
- 96.Waldo AL, Camm AJ, deRuyter H, Friedman PL, MacNeil DJ, Pauls JF, Pitt B, Pratt CM, Schwartz PJ, Veltri EP. Effect of d-sotalol on mortality in patients with left ventricular dysfunction after recent and remote myocardial infarction. The SWORD Investigators. Survival With Oral d-Sotalol. Lancet. 1996;348:7–12. doi: 10.1016/s0140-6736(96)02149-6. [DOI] [PubMed] [Google Scholar]
- 97.Freudenberger RS, Wilson AC, Kostis JB. Comparison of rate versus rhythm control for atrial fibrillation in patients with left ventricular dysfunction (from the AFFIRM Study) Am J Cardiol. 2007;100:247–252. doi: 10.1016/j.amjcard.2007.02.101. [DOI] [PubMed] [Google Scholar]
- 98.Van Gelder IC, Hagens VE, Bosker HA, Kingma JH, Kamp O, Kingma T, Said SA, Darmanata JI, Timmermans AJ, Tijssen JG, Crijns HJ. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N Engl J Med. 2002;347:1834–1840. doi: 10.1056/NEJMoa021375. [DOI] [PubMed] [Google Scholar]
- 99.Coplen SE, Antman EM, Berlin JA, Hewitt P, Chalmers TC. Efficacy and safety of quinidine therapy for maintenance of sinus rhythm after cardioversion. A meta-analysis of randomized control trials. Circulation. 1990;82:1106–1116. doi: 10.1161/01.cir.82.4.1106. [DOI] [PubMed] [Google Scholar]
- 100.Abozguia K, Clarke K, Lee L, Frenneaux M. Modification of myocardial substrate use as a therapy for heart failure. Nat Clin Pract Cardiovasc Med. 2006;3:490–498. doi: 10.1038/ncpcardio0583. [DOI] [PubMed] [Google Scholar]
- 101.Brown DA, Aon MA, Akar FG, Liu T, Sorarrain N, O’Rourke B. Effects of 4′-chlorodiazepam on cellular excitation-contraction coupling and ischaemia-reperfusion injury in rabbit heart. Cardiovasc Res. 2008;79:141–149. doi: 10.1093/cvr/cvn053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jupiter DC, VanBuren V. A visual data mining tool that facilitates reconstruction of transcription regulatory networks. PLoS ONE. 2008;3:e1717. doi: 10.1371/journal.pone.0001717. [DOI] [PMC free article] [PubMed] [Google Scholar]