Abstract
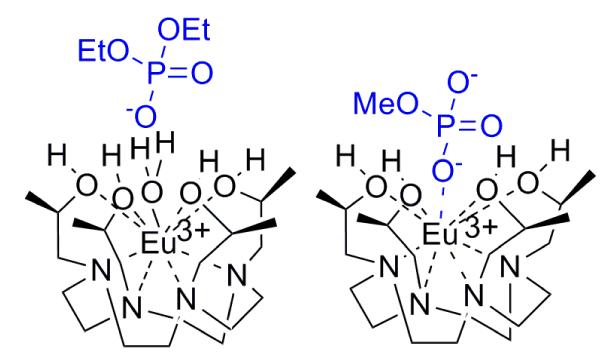
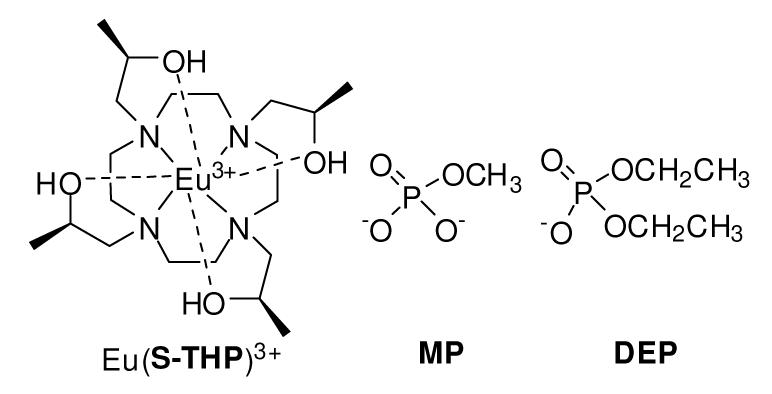
Eu(S-THP)3+ is the first PARACEST agent that functions through exchange of hydroxyl groups with water protons in aqueous solution. The CEST spectrum of this complex is highly pH dependent and is modulated by the presence of phosphate esters, as shown for diethyl phosphate which forms an outersphere complex and by methyl phosphate which forms an innersphere complex with Eu(S-THP)3+. The sensitivity of the alcohol proton environment to interactions with these anions shows that this complex is promising as a responsive PARACEST MRI contrast agent.
The development of magnetic resonance imaging (MRI) contrast agents that report on their environment through specific molecular recognition events is an active area of research.1,2 MRI contrast agents have shown the potential to sense pH,3 temperature,4 metabolite concentration,5-7 metal ions,8-10 proteins,11 or enzymes.12,13 A major goal in these studies is to prepare MRI contrast agents that respond to molecules that serve as early biomarkers of disease.1 Ln(III) complexes that function as contrast agents through PARACEST (paramagnetic chemical exchange saturation transfer) are especially promising for development as responsive MRI contrast agents. PARACEST agents have paramagnetically shifted mobile protons that are in slow exchange with bulk water protons. Application of a presaturation pulse to these mobile protons leads to a decrease in the intensity of the water signal.14 The environment of the mobile protons is influenced by interaction of Ln(III) PARACEST agents with macromolecules or with small molecule metabolites.7,11,15,16 The development of responsive PARACEST agents, however, is restricted by the limited number of ligand types with exchangeable protons.
To address this, we reported on Ln(III) macrocyclic complexes with alcohol exchangeable protons, but these complexes functioned as PARACEST agents only in water/acetonitrile mixtures.17 The alcohol proton exchange rate constant was predicted to be too large to observe a CEST effect. Here we show for the first time that a Eu(III) macrocyclic complex with alcohol groups, Eu(S-THP)3+ (Chart 1) acts as a PARACEST agent in pure water under controlled pH. In addition, the CEST spectrum of this complex is selectively responsive to two biologically important classes of phosphate esters. The modulation of the CEST effect is unexpectedly mediated by an outersphere phosphate diester or an innersphere phosphate monoester complex as shown by direct excitation Eu(III) luminescence spectroscopy. These differences may provide a basis for designing selectively responsive PARACEST agents.
Chart 1.
The CEST spectrum of Eu(S-THP)3+ as shown in Figure 1 was recorded by applying a presaturation pulse in 1 ppm increments. There is a CEST feature at about 6 ppm downfield of bulk water that arises from the alcohol group as shown by the corresponding alcohol proton resonance (Figure S1). A pronounced pH-dependence is observed for the CEST spectrum of Eu(S-THP)3+ over the pH range of 4.5 to 7.3 with an optimum pH of 5.9 (Figure S2). This pH dependence is characteristic of base catalyzed exchange with a low pH optimum due to the acidic alcohol protons.18 In addition, anionic ligands such as phosphate esters modulate the pH dependence of the CEST effect.
Figure 1.
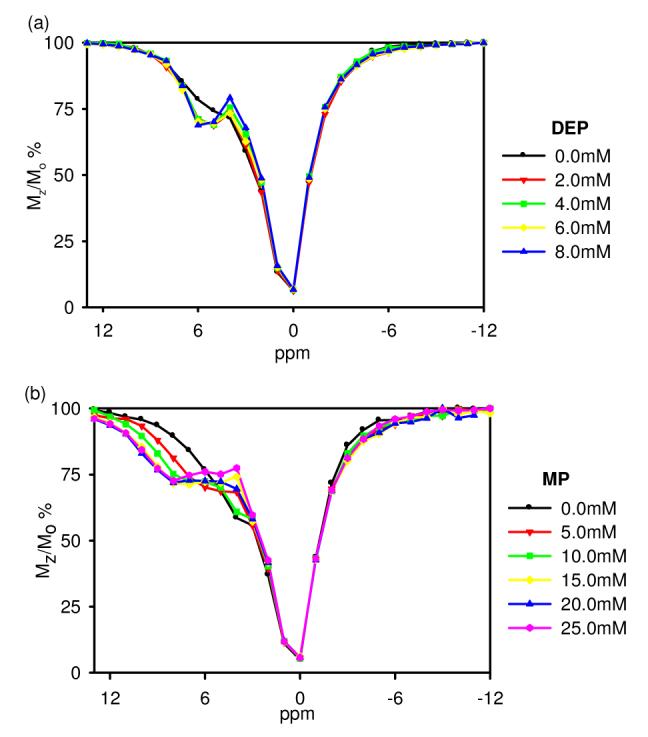
CEST spectra of 5.00 mM Eu(S-THP)3+ with 20.0 mM MES and 100 mM NaCl and (a) addition of diethyl phosphate at pH 6.6, (b) addition of methyl phosphate at pH 6.7. Mz is the water resonance intensity with saturation at the frequency shown and Mo is the water resonance intensity without selective saturation.
Titration of Eu(S-THP)3+ with diethylphosphate (DEP) in buffered solution, pH 6.6 and 100 mM NaCl increases the intensity of the existing CEST alcohol peak (Figure 1a). A plot of the Eu(S-THP)3+ CEST response as a function of DEP concentration (Figure S3a) shows that even one equivalent of DEP changes the CEST effect. By contrast, addition of methylphosphate (MP) to Eu(S-THP)3+ changes the CEST spectrum in two ways (Figure 1b). The alcohol CEST peak of Eu(S-THP)3+ decreases and a new CEST peak at about 8 ppm grows in, corresponding to a new alcohol proton resonance (Figure S4). A plot of the intensity of the new CEST peak as a function of MP is fit to a 1:1 binding curve (Figure S3b) with a dissociation constant of 10 mM. These phosphate ester complexes of Eu(S-THP)3+ have distinct pH dependent CEST spectra over the pH range of 5.5 to 7.0. In the presence of one equivalent of DEP, Eu(S-THP)3+ has an optimal CEST effect at pH 7.0, and with MP there is a less pronounced pH dependence with the strongest CEST effect from pH 5.5 to 6.5. (Figure S5-S8). In addition, MP and DEP modulate the CEST spectrum of Eu(S-THP)3+ in an interdependent way when one to two equivalents of both esters are added (Figure S9).
These studies show that binding of the two phosphate esters to Eu(S-THP)3+ gives rise to different CEST spectra and that DEP influences the CEST spectrum at lower concentrations than does MP, a surprising observation in view of previous work on relative binding strengths of phosphate esters to lanthanide(III) macrocyclic complexes.19 In order to study the nature of the phosphate ester interactions, direct excitation Eu(III) luminescence spectroscopy was used. Shown in Figure 2 are the 7Fo →5Do excitation spectra for Eu(S-THP)3+ as DEP or MP are added. The 7Fo →5Do transition is especially useful because both ground state and excited states are non-degenerate so that the number of observed peaks corresponds to the number of different Eu(III) species in solution.20 At pH 6.6, the Eu(S-THP)3+ complex has one excitation peak for the aqua complex, Eu(S-THP)(OH2)3+ (579.32 nm, Figure 2a).21 Addition of 10 equivalents of DEP to Eu(S-THP)3+ at pH 6.6 does not change the excitation peak intensity at 579.32 nm. Luminescence lifetime data in H2O and D2O show that the number of bound water ligands does not change upon addition of excess DEP (Table S1). Taken together, these data suggest that interaction of Eu(S-THP)3+ with DEP under these conditions does not involve direct interaction with Eu(III) through water ligand displacement. By contrast, addition of MP to Eu(S-THP)3+ leads to a decrease in the major excitation peak at 579.32 nm for Eu(S-THP)(OH2)3+ (Figure 2b). Binding occurs through displacement of a water ligand to give Eu(S-THP)(MP)+ as confirmed by luminescence lifetime data (Table S1). Data for luminescence intensity or lifetime versus MP concentration are plot and fit to a 1:1 binding isotherm to give Kd values of 22 or 7.0 mM, respectively (Figure S10-11) close to that measured in the CEST titration under similar conditions. Luminescence data show that the complex is stable over several days even in the presence of physiologically relevant concentrations of phosphate and carbonate (Figure S12-13).
Figure 2.
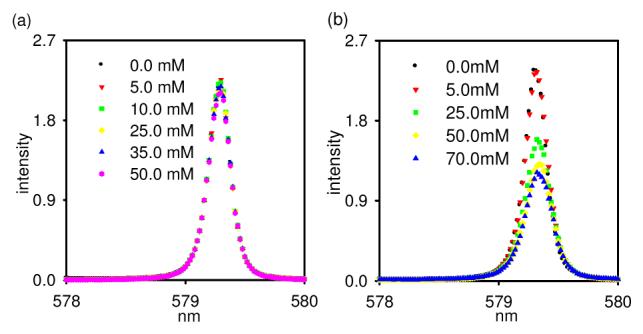
Luminescence excitation spectra (7Fo →5Do ), (emission at 628 nm ± 27 nm) of 5.00 mM Eu(S-THP)3+ in (a) 20.0 mM MES and 100 mM NaCl with addition of DEP at pH 6.6, (b) 20.0 mM MES and 100 mM NaCl with addition of MP at pH 6.6.
These results highlight the different binding modes of the two phosphate esters that lead to distinct CEST responses for Eu(S-THP)3+. The CEST spectrum modulation by outersphere DEP is mediated through a change in the rate constant for alcohol proton exchange as suggested by the sharpening of both the CEST alcohol peak (Figure 1a) and the 1H NMR alcohol resonance (Figure S1). This effect likely involves interaction of DEP with the alcohol protons of Eu(S-THP)3+ through an outersphere binding pocket.22 By contrast, the innersphere MP complex gives rise to a new CEST peak corresponding to a new alcohol resonance. The distinct pH dependence of the CEST effect of the MP complex is attributed to a change in the acidity of the hydroxyl groups.
In summary, Ln(III) macrocyclic complexes with alcohol pendent groups are a promising new class of responsive PARACEST agent. The innersphere/outersphere binding dichotomy for two different ligands suggests a mechanism for developing selectively responsive CEST agents. Good innersphere ligands such as MP replace a water ligand to give rise to a new pH dependent CEST peak that is characteristic of the new complex. Poor ligands such as DEP do not replace the water ligand under similar conditions and thus promote a distinct CEST response. Nonetheless, low concentrations of DEP modulate the CEST spectrum of Eu(S-THP)(OH2)3+ in high concentrations of NaCl and buffer, supporting a specific interaction that may be further tuned. Binding constants reported here suggest that it may be feasible to develop PARACEST agents for the detection of phosphate containing metabolites that are present in low millimolar concentrations.23
Supplementary Material
Acknowledgment
We thank the National Institutes of Health for support of this work (EB-04609) and the National Science Foundation for a major instrumentation award (CHE-0321058) to build the MOPO laser system.
Footnotes
Supporting Information Available: Luminescence excitation spectra, CEST and 1H NMR spectra, binding curves and experimental details. This material is available free of charge via the Internet at http://pubs.acs.org
References
- (1).Yoo B, Pagel MD. Front. Biosci. 2008;13:1733–1752. doi: 10.2741/2796. [DOI] [PubMed] [Google Scholar]
- (2).Sherry AD, Woods M. Annu. Rev. Biomed. Eng. 2008;10:391–411. doi: 10.1146/annurev.bioeng.9.060906.151929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Aime S, Barge A, Castelli DD, Fedeli F, Mortillaro A, Nielsen FU, Terreno E. Magn. Reson. Med. 2002;47:639–648. doi: 10.1002/mrm.10106. [DOI] [PubMed] [Google Scholar]
- (4).Li AX, Wojciechowski F, Suchy M, Jones CK, Hudson RHE, Menon RS, Bartha R. Magn. Reson. Med. 2008;59:374–381. doi: 10.1002/mrm.21482. [DOI] [PubMed] [Google Scholar]
- (5).Ren J, Trokowski R, Zhang S, Malloy CR, Sherry AD. Magn. Reson. Med. 2008;60:1047–1055. doi: 10.1002/mrm.21722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).van Zijl PCM, Jones CK, Ren J, Malloy CR, Sherry AD. Proc. Natl. Acad. Sci. U. S. A. 2007;104:4359–4364. doi: 10.1073/pnas.0700281104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Zhang S, Trokowski R, Sherry AD. J. Am. Chem. Soc. 2003;125:15288–15289. doi: 10.1021/ja038345f. [DOI] [PubMed] [Google Scholar]
- (8).Major JL, Parigi G, Luchinat C, Meade TJ. Proc. Natl. Acad. Sci. U. S. A. 2007;104:13881–13886. doi: 10.1073/pnas.0706247104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Que EL, Chang CJ. J. Am. Chem. Soc. 2006;128:15942–15943. doi: 10.1021/ja065264l. [DOI] [PubMed] [Google Scholar]
- (10).Zhang X-A, Lovejoy KS, Jasanoff A, Lippard SJ. Proc. Natl. Acad. Sci. U. S. A. 2007;104:10780–10785. doi: 10.1073/pnas.0702393104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Ali MM, Woods M, Suh EH, Kovacs Z, Tircso G, Zhao P, Kodibagkar VD, Sherry AD. J. Biol. Inorg. Chem. 2007;12:855–865. doi: 10.1007/s00775-007-0240-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Chauvin T, Durand P, Bernier M, Meudal H, Doan B-T, Noury F, Badet B, Beloeil J-C, Toth E. Angew. Chem., Int. Ed. 2008;47:4370–2. doi: 10.1002/anie.200800809. [DOI] [PubMed] [Google Scholar]
- (13).Yoo B, Raam MS, Rosenblum RM, Pagel MD. Contrast Media Mol. Imaging. 2007;2:189–198. doi: 10.1002/cmmi.145. [DOI] [PubMed] [Google Scholar]
- (14).Sherry AD, Woods M. Mol. Cell. MR Imaging. 2007:101–122. [Google Scholar]
- (15).Aime S, Castelli D. Delli, Fedeli F, Terreno E. J. Am. Chem. Soc. 2002;124:9364–9365. doi: 10.1021/ja0264044. [DOI] [PubMed] [Google Scholar]
- (16).Trokowski R, Ren J, Kalman FK, Sherry AD. Angew. Chem., Int. Ed. 2005;44:6920–6923. doi: 10.1002/anie.200502173. [DOI] [PubMed] [Google Scholar]
- (17).Woods M, Woessner DE, Zhao PY, Pasha A, Yang MY, Huang CH, Vasalitiy O, Morrow JR, Sherry AD. J. Am. Chem. Soc. 2006;128:10155–10162. doi: 10.1021/ja061498t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Chin KOA, Morrow JR. Inorg. Chem. 1994;33:5036–5041. [Google Scholar]
- (19).Nwe K, Andolina CM, Morrow JR. J. Am. Chem. Soc. 2008;130:14861–14861. doi: 10.1021/ja8037799. [DOI] [PubMed] [Google Scholar]
- (20).Horrocks WD, Jr., Sudnick DR. Acc. Chem. Res. 1981;14:384–392. [Google Scholar]
- (21).Chappell LL, Voss DA, Horrocks WD, Morrow JR. Inorg. Chem. 1998;37:3989–3998. doi: 10.1021/ic980191v. [DOI] [PubMed] [Google Scholar]
- (22).Smith CB, Buntine MA, Lincoln SF, Wainwright KP. Dalton Trans. 2003:3028–3033. [Google Scholar]
- (23).Wishart DS. Nucl. Acid. Res. 2007;35:D521–D526. doi: 10.1093/nar/gkl923. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


