We investigated enhanced fluorescence of Cyanine 5 dyes near silver island films (SIFs) by the use of single molecule spectroscopic method. We found that, on average, the Cy5 molecules are 18-fold brighter on SIFs than on a glass surface and that single-molecule lifetimes are 10-fold shorter on SIFs as compared to glass. We observed Cy5 molecules until photobleaching occurred and found that the molecules on the SIF emitted 20-fold more photons as compared to those on glass prior to photobleaching. These results demonstrate that the use of fluorophore–metal interactions can increase the brightness and photostability of fluorophores for single-molecule detection.
During the past decade, there has been considerable progress in the development of fluorescence probes. Many probes, such as the cyanine dyes, have high extinction coeffiecients and high quantum yields. Hence, it is unlikely that the future probe development will result in dramatic increases in detectability for small organic fluorphores. For this reason, there is an interest in quantum dots and polymeric probes. In the past years we have taken a different approach to create improved probes, this being the use of metallic nanoparticles [1–3]. Ensemble experiments have revealed that proximity to silver particles could increase the intensity and photostability of fluorphores [4–9]. This effect is due to the through-space near-field interaction between the fluorphore and surface plasmons on the metals. Thus, the use of metallic nanostructure to enhance fluorescence has great potential for applications in the fields of medical diagnostics and biotechnology.
The fluorescence spectral properties of fluorphores are especially important for sensitive detection. The photostability of the fluorphore resolves the time it can be observed prior to photobleaching. Hence, it is of interest to determine if the improved spectral properties observed at the ensemble level extends to the single-molecule level. The interactions of fluorophores with metallic particles have been studied theoretically and empirically at the single-molecule level in recent years [10–13]. In these reports, the major concern was the dependence of the emission rate on the distance between the dye layer and the metallic surface. The emission can be quenched due to radiation energy transfer to the metal as molecules adsorbed directly on the surface [12, 13]. Another consideration is the enhanced fluorescence due to increased electromagnetic field of surface plasmon and/or enhanced quantum yield [10, 11]. Therefore, we examined the widely used fluorescent probe Cy5 near SIFs. These films consist of sub-wavelength-size silver particles on a glass surface [4, 5]. Our single-molecule experiments of this system reveal both the spectral changes due to the metal particles and the heterogeniety due to a range of fluorophore–metal distances.
For these studies, the Cy5 molecules were spin-coated onto the SIFs from an aqueous solution containing 0.5% polyvinyl alcohol (PVA). Initially, we examined the ensemble emission spectra in which the Cy5 concentration is 20-fold higher than for the single-molecule experiments (Figure 1). Both Cy5 spectra exhibit emission maxima near 680 nm. A significant enhancement in fluorescence emission is clearly evident on silvered surfaces. The presence of silver nanostructure did not distort the Cy5 spectrum. Additionally, the SIF sample without Cy5 did not contribute significant background. This initial ensemble result indicates that the Cy5 dyes deposited on SIF display similar fluorescence characteristics compared to those in the absence of SIF.
Figure 1.
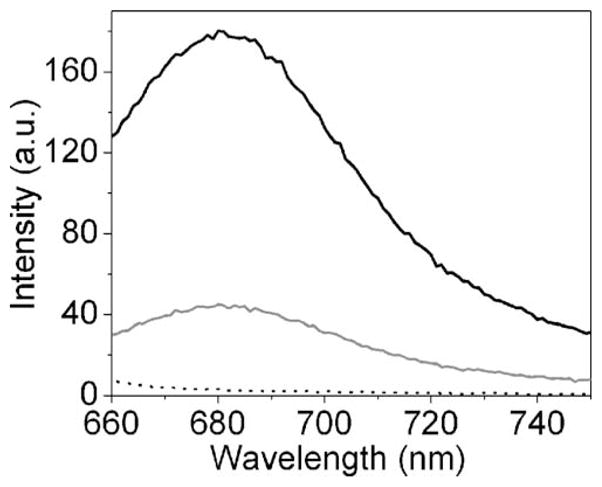
Fluorescence emission spectra of Cy5 dispersed on glass (gray line) and SIF (black line). The dot line represents the emission background from SIF. Emission spectra were collected in front-face geometry on a SLM 8000 spectrofluorometer with 635 nm excitation from a xenon lamp.
Next, we examined single Cy5 molecules spin-coated on glass and SIFs (Figure 2). The concentration of Cy5 in 0.5% PVA solution was adjusted to the nanomolar level to give an appropriate surface density for the observation of single molecule. The brightness on these images depicts the fluorescence intensity detected for each image pixel. An image obtained from an as-prepared SIF film without Cy5 probes is shown for comparison. The SIF surface alone is rather dim in marked contrast with Figure 2a, b. Hence, the silver particles did not interfere with our single-molecule experiments. We assume that most of the well-separated, bright round spots found in image a and image b are attributed to fluorescence from single Cy5 dye molecules. Proof of this assumption is drawn from several experimental criteria [14]. Most of the time traces collected in the single-molecule measurements show randomly “blinking” and clearly one-step photobleaching, corresponding to the typical behavior expected for a single Cy5 molecule (Figure 3), providing strong evidence that single molecules had been observed. This fact, plus the observation that the number of fluorescence spots per image linearly increased with increasing Cy5 concentration, confirms our observation of single molecules. These images provide initial, qualitative information on the molecular level information of metal-enhanced fluorescence. The significant difference in emission intensity of round spots on a silvered surface and on a bare glass surface is immediately noticeable. Additionally, the brightness of fluorescent spots on bare glass coverslips shows a fairly uniform intensity distribution. On the contrary, the emission intensity of single Cy5 molecules on SIF surface varies dramatically over an order of magnitude or more. The observed heterogeneity of brightness is likely due to site-to-site variations in local electromagnetic interaction between the fluorophore and the metallic nanoparticle. On an unsilvered surface, the homogeneous distribution of brightness can be ascribed simply to the intrinsic property of the immobilized probe.
Figure 2.
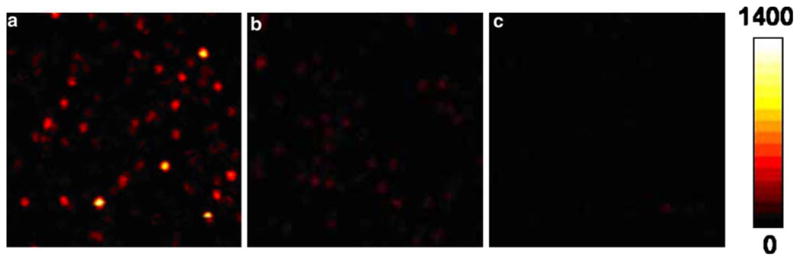
Fluorescence images of 10×10-μm2 sample regions recorded for Cy5 spin-cast on a SIF a and on a glass coverslip b at the subnanomolar concentration. c An as-prepared SIF in the absence of Cy5. Intensity scale unit: cps. Images were recorded by raster scanning the sample over a time-resolved confocal microscopy (MicroTime 200, PicoQuant). A single mode pulsed laser diode (635 nm, 100 ps, 40 MHz) (PDL800, PicoQuant) was used as the excitation light. An oil immersion objective (Olympus, 100×, 1.3 NA) was used.
Figure 3.
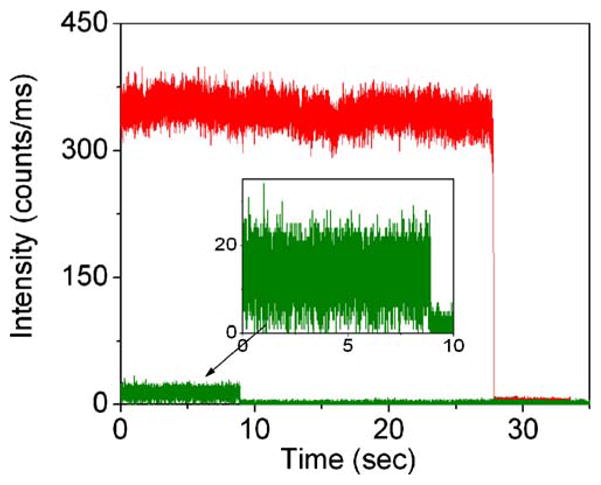
Representative fluorescence time traces recorded for Cy5 immobilized on a glass coverslip (green) and on a SIF surface (red). The excitation power into the microscope was maintained less than 2μW.
Figure 3 shows representative time–intensity traces for Cy5 on glass and SIFs. In general, the Cy5 molecules on the SIFs are about 10-fold brighter than on glass. However, we did notice considerable heterogeneity in the brightness of the Cy5 molecules on the SIF. We attribute this heterogeneity to a range of fluorophore–metal distances and orientations and to a range of silver particle sizes. In addition to increased brightness, the Cy5 molecules appear to emit for longer periods of time prior to photobleaching. The time profiles observed from sparse bright spots on an as-prepared SIF without Cy5 probes showed apparent different behaviors from Cy5 dye molecules, with “characteristic” blinking [15] different from the Cy5 molecule and the absence of a clearly defined photobleaching event.
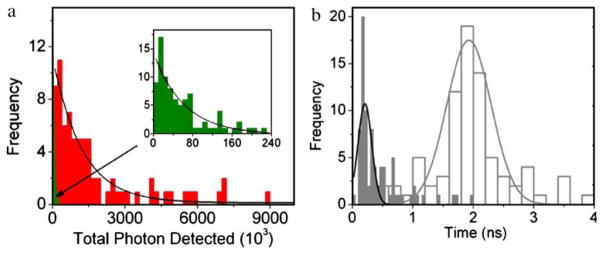
An important parameter for SMD is the total photons a fluorophore can emit prior to photobleaching. Hence, we observed 80 different Cy5 molecules on glass and the SIF until the fluorophores were photobleached. These traces revealed the total number of photons observed for each fluorophore until it stopped emitting. The histograms of these results are shown in Figure 4. The maxima counts observed are in the ~107 range. The histograms of total photon counts indicate a single population of fluorophores, as demonstrated by the single-exponential fit in the distribution (Figure 4a). The mean values (i.e., exponential decay constant) from 80 individual molecules are 69×103 and 1,270×103 for Cy5 dye adsorbed on glass and on SIF surface, respectively. These results show that, on average, a single Cy5 molecule emits 20-fold more photons on the SIF than on glass. The higher photon counting on the SIF indicates that the incident light intensities can be reduced for probes on the SIFs to thereby increase the observation times for the fluorophores.
Figure 4.
a Histograms of total photons detected prior to photobleaching from Cy5 molecules on glass (green) and SIFs (red). b Histograms of lifetimes from Cy5 molecules on glass (white) and SIFs (gray). The fluorescence lifetime of single molecules was measured by time- correlated single photon counting with the TimeHarp 200 PCI-board (PicoQuant). Lifetimes were estimated by fitting to a χ2 value of less than 1.2 and with a residuals trace that was fully symmetrical about the zero axis.
The intrinsic emission rate of a fluorophore is not changed in most fluorescence experiments. Changes in intensity and lifetimes are usually the result of changes in the rates of nonradiative decay. This situation is changed near metal particles, which can increase the intrinsic radiative rates of nearby probes [16]. If this effect occurs at the single-molecule level, we expect the Cy5 molecules to display shorter lifetimes on the SIFs. Using the same sets of 80 molecules, we constructed lifetime histograms on glass and silver (Figure 4b). On glass, the Cy5 lifetimes were distributed approximately with a mean value 2 ns (Figure 4b, white columns). On the SIF, we observed lifetime ranging from 0.2 to 1 ns, with a small number of molecules displaying lifetimes near 2 ns. We attribute these large lifetimes of Cy5 molecules that are distant from and unaffected by silver particles. The short lifetimes are presumed to be due to Cy5 molecules near the silver particles. Because shorter lifetimes allow less time for excited state reactions, we expect that the short lifetime molecules can be observed for longer periods of time prior to photobleaching. As the lifetime of the fluorophores decreases, the number of excitation cycles prior to photodecomposition increases.
Because the molecules with the shorter lifetimes had higher intensities, these results show that the radiative decay rate of the Cy5 molecules is much larger on the SIF than on glass. In single-molecule measurements, the brightness is limited by the rate of emission at optical saturation. A shorter fluorescence lifetime should allow a higher photon emission rate at optical saturation. We measured the photon count rate vs incident intensity for Cy5 (Figure 5). The maximum count rate is about 10-fold higher on the SIF, which is in agreement with the reciprocal lifetime on the two surfaces. At this time, we do not know if the half-saturation intensities occur at the same incident power. Enhanced excitation due to high fields around the particle is expected to decrease the half-saturation incident intensity, and decreased lifetimes are expected to increase the incident intensity needed for half-saturation. The shorter lifetime of fluorophores on SIF as compared to glass is most likely the reason for higher count rates at optical saturation, as shown in Figure 5. We expect such saturation experiments to provide useful information about fluorophore–metal interactions.
Figure 5.
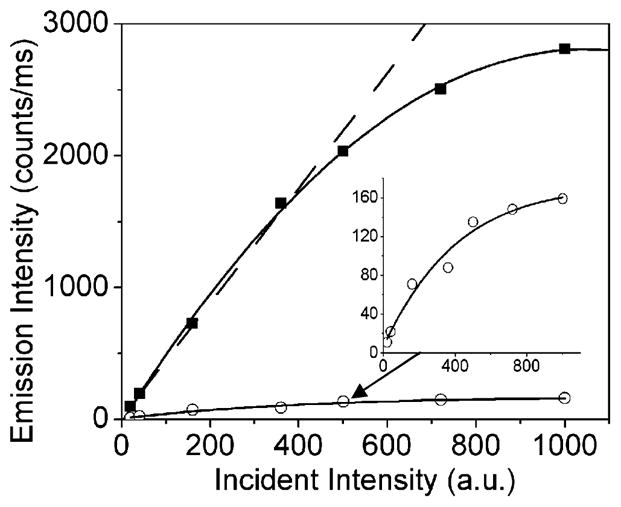
Optical saturation of Cy5 on Glass (open gray circle) and SIF (solid black circle).
In conclusion, single-molecule detection of fluorophores near silver particles allows direct observation of various fluorescence characteristics of individual molecules, such as intensity fluctuations, total photons emitted per fluorophore, and lifetime variations. These phenomena are generally hidden in ensemble measurements due to averaging. Time-dependent measurements show diverse behaviors for fluorophores near the SIF surfaces. These fluorophores display higher intensities and decreased lifetimes. It appears that the spread of lifetimes and intensities from molecule to molecule is due to variations in distance, orientation, and particle size. The detected emitted photons in the presence of metal nanostructure are relatively large. It is assumed that the fluorescence was enhanced by the surface plasmons. The strong interaction of the excited molecules with the metal nanostructures dramatically shortens the lifetime of the excited state, thus significantly increasing the number of excitation cycles a molecule can survive until photobleaching occurs. In total, our results demonstrate improved detectability of single molecules due to fluorophore interactions with nearby silver particles.
Acknowledgments
This research was supported by grants from NIH, HG-02655, EB-00682, and the National Center for Research Resources, RR-08119.
References
- 1.Aslan K, Gryczynski I, Malicka J, Matveeva E, Lakowicz JR, Geddes CC. Metal-enhanced fluorescence: an emerging tool in biotechnology. Curr Opin Biotechnol. 2005;16:55–62. doi: 10.1016/j.copbio.2005.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang J, Lakowicz JR. A model for DNA detection by metal-enhanced fluorescence from immobilized silver nanoparticles on solid substrate. J Phys Chem B. 2006;110:2387–2392. doi: 10.1021/jp055370u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang J, Gryczynski I, Cryszynski Z, Lakowicz JR. Dye-labeled silver nano-shell-bright particle. J Phys Chem B. 2006;110:8986–8991. doi: 10.1021/jp057032z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Malicka J, Gryczynski I, Gryczynski Z, Lakowicz JR. Increased resonance energy transfer between fluorophores bound to DNA in proximity to metallic silver particles. Anal Biochem. 2003;315:57–66. doi: 10.1016/S0003-2697(02)00710-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lakowicz JR, Shen Y, D’Auria S, Malicka J, Fang J, Gryczynski Z, Gryczynski I. Radiative decay engineering. 2. Effects of silver island films on fluorescence intensity, lifetimes, and resonance energy transfer. Anal Biochem. 2002;301:111–116. doi: 10.1006/abio.2001.5503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aussenegg FR, Leitner A, Lippotch ME, Reinisch H, Reigler M. Novel aspects of fluorescence lifetime for molecules positioned close to metal surface. Surf Sci. 1987;139:935–945. [Google Scholar]
- 7.Leitner A, Lippitch ME, Draxler S, Riegler M, Aussenegg FR. Fluorescence properties of dyes adsorbed to silver islands, investigated by picosecond techniques. Appl Phys B. 1985;36:105–109. [Google Scholar]
- 8.Sokolov K, Chumanov G, Cotton TM. Enhancement of molecular fluorescence near the surface of colloidal metal films. Anal Chem. 1998;70:3898–3905. doi: 10.1021/ac9712310. [DOI] [PubMed] [Google Scholar]
- 9.Malicka J, Gryczynski I, Fang J, Kusba J, Lakowicz JR. Fluorescence spectral properties of Cyanine dye-labeled DNA oligomers on surfaces coated with silver particles. Anal Biochem. 2003;317:136–146. doi: 10.1016/S0003-2697(03)00005-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Enderlein J. A theoretical investigation of single-molecule fluorescence detection on thin metallic layers. Biophys J. 2000;78:2151–2158. doi: 10.1016/S0006-3495(00)76761-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bouhelier A, Renger J, Beversluis MR, Novotny L. Plasmon-coupled tip-enhanced near-field optical microscopy. J Microsc. 2003;210:220–224. doi: 10.1046/j.1365-2818.2003.01108.x. [DOI] [PubMed] [Google Scholar]
- 12.Stefani FD, Vasilev K, Bocchio N, Stoyanova N, Kreiter M. Fluorescence through a thin metallic film. Phys Rev Lett. 2005;94:023005. doi: 10.1103/PhysRevLett.94.023005. [DOI] [PubMed] [Google Scholar]
- 13.Anger P, Bharadwaj P, Novotny L. Enhancement and quenching of single-molecule fluorescence. Phys Rev Lett. 2006;96:11302. doi: 10.1103/PhysRevLett.96.113002. [DOI] [PubMed] [Google Scholar]
- 14.Weston KD, Carson PJ, Metiu H, Buratto SK. Room-temperature fluorescence characteristics of single dye molecules adsorbed on a glass surface. J Chem Phys. 1998;109:7474–7485. [Google Scholar]
- 15.Peyser LA, Vinson AE, Bartko AP, Dickson RM. Photo-activated fluorescence from individual silver nanoclusters. Science. 2001;291:103–106. doi: 10.1126/science.291.5501.103. [DOI] [PubMed] [Google Scholar]
- 16.Lakowicz JR. Radiative decay engineering: biophysical and biomedical applications. Anal Biochem. 2001;298:1–24. doi: 10.1006/abio.2001.5377. [DOI] [PMC free article] [PubMed] [Google Scholar]