Abstract
Culture filtrate and cell extracts from Mycobacterium bovis cultures contain molecules which could promote protective immunity to tuberculosis in animals. Different protein fractions of M. bovis cultures were obtained by elution electrophoresis and were tested in experimentally infected cattle. The fractions that elicited gamma interferon (IFN-γ) responses were resolved by two-dimensional gel electrophoresis, and individual proteins were identified by matrix-assisted laser desorption ionization-time of flight mass spectrometry. The open reading frames were cloned, expressed as their recombinant forms, and retested with naturally and experimentally infected animals. Eleven protein fractions were highly reactive, from which the Rv1636, HspX, Rv0138, Rv2524, EsxI, and Rv3740 recombinant proteins were obtained. EsxI and HspX were the antigens most recognized by the IFN-γ release assay. In summary, a proteomic approach allowed the identification of novel antigens useful for the diagnosis of bovine tuberculosis.
Infection and disease with Mycobacterium bovis, the causative organism of bovine tuberculosis (TB), is an important health problem in cattle and other animal species. The disease presents a major barrier to animal-related trade and production, causing significant losses to farming economies worldwide. Furthermore, bovine TB has serious zoonotic implications, especially in developing regions. In North and South America, there are 420 million cattle, half of which are in countries with an incidence of bovine TB higher than 1% (13). The disease control programs carried out in most countries are based on a test and removal strategy utilizing the tuberculin skin test (TST) with purified protein derivative (PPD) (4). Accurate detection using the TST and removal of infected cattle form the base of control strategies for bovine TB. It is widely recognized that tests for cell-mediated immunity are far more sensitive than antibody tests in detecting bovine TB (38). Disease control based on the use of PPD can be facilitated by using the gamma interferon (IFN-γ) test for bovine TB (39). The PPDs are obtained by acid precipitation from heat-killed cultures of M. bovis (PPD-B) and Mycobacterium avium (PPD-A) and are poorly defined (27), containing many proteins, lipids, and sugars. Many of these compounds are shared by different mycobacterial species or even with other bacteria (22). The detection of false positives has been a significant part of the economic cost, particularly in areas where there is a relatively low incidence of the disease (28), and thus, there is an urgent need for more specific reagents for the diagnosis of M. bovis infection.
Many mycobacterial proteins have been isolated, and bovine T-cell epitopes have been characterized for several of them (2, 9, 26, 30, 32). Immunodominant peptides from M. tuberculosis Rv3873, Rv3879c, Rv3019c, Rv0288, ESAT-6, and CFP-10 genes were described as good T-cell inducers, and the diagnostic potential of a cocktail composed of these dominant peptides was demonstrated using naturally infected field animals (10). The Rv3615c epitope in combination with a previously described differential test based on ESAT-6 and CFP-10 has the potential to significantly increase diagnostic sensitivity without reducing specificity in M. bovis BCG-vaccinated populations (34). Other mycobacterial antigens (TPX, TRB-B, Rv3747, and L7/L12) were reported to have induced high stimulation indices in M. bovis-infected animals with the IFN-γ assay (3). Our group has previously participated in a multilaboratory study that assessed the sensitivity and specificity of the IFN-γ test using several antigens in cattle naturally infected with M. bovis. When the skin test-positive and -negative groups were compared, the recombinant antigens (ESAT-6, CFP-10, and PE13) which induced the highest levels of IFN-γ release in the cattle examined were all able to differentiate the positive and negative Argentinean groups (1). IFN-γ tests utilizing specific mycobacterial antigens have the advantage of enhanced specificity compared to that of the PPD-based IFN-γ test, but currently, the sensitivity of these tests needs to be improved. To improve the sensitivity, additional immunoreactive mycobacterial antigens need to be identified and added to a pool of the most reactive and specific mycobacterial antigens.
The aim of this work was to identify additional antigens through a screening of native protein fractions from a culture of M. bovis AN5. For this objective, we used blood from cattle experimentally infected with M. bovis to select native fractions as a source of antigens in IFN-γ tests. The selected antigenic fractions were further separated into individual proteins by two-dimensional (2D) gel electrophoresis (2D-E), and the spots were identified by matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS). Through this approach, we could recognize novel proteins with diagnostic potential.
MATERIALS AND METHODS
Bacterial cultures and sample preparation.
The M. bovis AN5 strain was used throughout the study. Mycobacterial cultures were grown as described previously (3). Cultures were prepared in Middlebrook 7H9 (BD, United States) liquid medium containing 0.4% pyruvic acid and 0.4% glucose. Mycobacterial cultures were incubated at 37°C and harvested at 21 days. To obtain the culture filtrate, the supernatant was separated from the cell extract by centrifugation for 30 min at 7,000 × g and then filtered using a 0.2-μm filter (Millipore). The proteins were precipitated with 50% ammonium sulfate for 18 h at 4°C. Following centrifugation at 10,000 × g for 1 h, the precipitate was dissolved in a minimal volume of phosphate-buffered saline (PBS) and then dialyzed against PBS for 18 h at 4°C. To obtain the cell extract, the culture supernatant was removed and the cell mass washed and resuspended in PBS. Mycobacteria were killed by heating for 40 min at 80°C, followed by sonication (Branson Sonifier 250) on ice five times for 1 min with rest intervals of 2 min. The protein contents of the supernatants and cell extracts were quantified by the bicinchonic acid method (Pierce, Rockford, IL).
Molecular mass fractions of CSF and cell extract.
Proteins were divided into narrow molecular mass fractions by continuous elution of polyacrylamide gels as described previously (27). In brief, culture supernatant filtrate (CSF) and cell extract (5 mg of protein) were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Antigen preparations resuspended in loading buffer (2% SDS, 0.125 M Tris HCl, pH 6.8, 1% 2-mercaptoethanol, 0.02% bromophenol blue, 10% glycerol) were heated for 4 min in boiling water. Thirty fractions were collected using a whole-gel elutor (Bio-Rad). The protein concentration of the different fractions was estimated by the micro-bicinchoninic acid method. The 30 fractions were pooled according to their molecular weights, and they were dialyzed against PBS. We obtained 15 fractions from the cell extract (1E to 15E, in decreasing order of molecular mass) and 13 fractions from the CSF (1S to 13S), and all were kept frozen at −80°C until used.
2D-E.
Protein separation by 2D-E was performed as described previously (18), with some modifications. Briefly, samples of cell extract or CSF ranging from 0.2 to 15 μg of protein were diluted in an appropriate volume of rehydration solution {7 M urea, 2 M thiourea, 2% 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate [CHAPS], 0.5% immobilized pH gradient [IPG] buffer, pH 3 to 10, 50 mM dithiothreitol} and incubated with shaking for 1 h at room temperature. Samples were applied by cup loading into rehydrated 11-cm IPG strips, pH 3 to 10 (GE Healthcare, Piscataway, NJ). Isoelectric focusing (IEF) was performed in an Ettan IPGphor IEF system (GE Healthcare, Piscataway, NJ). After IEF, strips were incubated for 20 min in equilibration buffer (50 mM Tris-HCl, pH 8, 6 M urea, 30% glycerol [vol/vol], 2% SDS, 0.005% bromophenol blue) containing 1% dithiothreitol, followed by a second 20-min incubation with 4% iodoacetamide in equilibration buffer. SDS-PAGE was performed on 10% polyacrylamide gels using a Mini Protean 3 system (Bio-Rad, Hercules, CA). IPG strip ends were clipped to fit gel width and loaded onto 8- by 7.3-cm SDS-polyacrylamide gels (100V for 1 h). After the gels were run, the proteins were detected using MS-compatible silver staining (20).
MALDI-TOF MS.
MS identification of 2D spots was performed with MALDI-TOF MS as a paid service by the Institut de Biotecnologia i Biomedicina, Universitat Autónoma de Barcelona, Barcelona, Spain. Proteins were identified from spectrometry data by using the MSFit (http://prospector.ucsf.edu/prospector/mshome.htm) on-line services.
Expression and purification of M. bovis Rv1636, HspX, Rv0138, Rv2524, EsxI, and Rv3740.
Gene sequences for the mature Rv1636, HspX, Rv0138, EsxI, and Rv3740 proteins were amplified by PCR using primers based on published sequences from M. tuberculosis H37Rv (Table 1). The Rv2524 gene, encoding a fatty acid synthase, is large in size, and in consequence, only a portion of the gene, corresponding to the fragment detected by MALDI, was cloned. Two restriction enzyme sites were introduced into primers to facilitate subsequent cloning in frame into the expression vector pRSET-A (Invitrogen BV, Leek, The Netherlands). Digested PCR products were ligated to pRSET-A. The pRSET-derived constructions were used to transform Escherichia coli BL21(DE3) competent cells. An inoculum (3 ml grown in LB-ampicillin for 16 h at 37°C) of E. coli BL21 harboring pRSET-A with Rv1636, HspX, Rv0138, Rv2524, EsxI, or Rv3740 gene inserts was diluted to an initial optical density (OD) at 650 nm of 0.01 and grown to the mid-logarithmic growth phase. A 0.5 mM concentration of isopropyl-β-d-thiogalactopyranoside (IPTG) was added to induce recombinant-gene expression over 3 h. Cells were then harvested by centrifugation and frozen at −20°C until further use. The cell pellet was lysed with 1 ml of lysis buffer (1 M Tris-HCl, pH 7.5) using a FastPrep FP120 (Bio101-Savant, Holbrook, NY). The expression of antigens was confirmed both by analysis of Coomassie blue-stained polyacrylamide gels and Western blot analysis using anti-His antibodies (GE Healthcare). The recombinant protein in the E. coli cell extract was purified by using a Pro-Bond resin column with immobilized nickel (Invitrogen Corp., CA) and eluted with increasing concentrations of 50 mM to 500 mM imidazole (Sigma, St. Louis, MO).
TABLE 1.
PCR primers used for cloning of Rv1636, Rv0138, Rv2524, esxI, Rv3740, and hspX
| Locus tag or gene | Primer sequence (5′-3′) | Restriction Site |
|---|---|---|
| Rv1636 | up-GGATCCATGAGCGCCTATAAGACCGTGG | BamHI |
| low-GAATTCCTAGGTGGTGTGCACGATCAGCA | EcoRI | |
| Rv3740 | up-GGATCCATGTCACCGATCGATGCGCT | BamHI |
| low-AAGCTTTCATAGCCCGACCGCGCGCT | HindIII | |
| esxI | up-GGATCCATGACCATCAACTATCAATT | BamHI |
| low-AAGCTTTTAGGCCCAGCTGGAGCCGA | HindIII | |
| Rv0138 | up-GGATCCGTGAGCGCTTCGGAGTTCTCC | BamHI |
| low-AAGCTTTTAAGGACCTCCATGCCGGCG | HindIII | |
| Rv2524 | up-GGATCCGTCGGCGGCCAGATCCCGAC | BamHI |
| low-AAGCTTTCACAGTTGAGCCTTGCCCAACCGGA | HindIII | |
| hspX | up-GGATCCGGATCCATGGCCACCACCCTTCCCGTTC | BamHI |
| low-AAGCTTAAGCTTTCAGTTGGTGGACCGGATCTGA | HindIII |
Experimentally infected animals.
Animal experimentation was performed inside INTA's biosecurity box facilities in compliance with regulations set by the Ethical Committees of the European Union and New Zealand. To test the different fractions, four castrated male Friesian cattle 6 months of age were obtained from herds with no history of M. bovis infection for at least 6 years. These animals were confirmed as bovine TB negative by in vitro testing of T-cell responses to mycobacterial antigens as determined by using a IFN-γ test. During the study, these animals were housed in isolation. They were infected by intranasal instillation of 106 CFU of M. bovis AN5 using previously described methods (27). Two animals of the same age and origin were used as uninfected controls. Before inoculation, blood samples were taken from the jugular vein and the IFN-γ assay was performed in all animals to set a control point. At different times after experimental infection, blood samples were collected from the jugular vein in heparinized vials to be used to test the antigenicity of protein fractions in the IFN-γ blood test (Bovigam; Pfizer Animal Health Ltd., Melbourne, Australia). Eight months after experimental infection, the animals were skin tested using the single intradermal comparative tuberculin test. The four experimentally infected animals were PPD positive, while the two uninfected controls remained negative. One year after experimental infection, the animals were slaughtered and necropsies were performed. A postmortem inspection of lymph nodes, liver, and lungs was performed. Samples of the tissues mentioned were taken, and M. bovis infection was confirmed by bacterial culture.
A second experimental inoculation study was carried out in order to evaluate immunoreactivity to the recombinant antigens. Ten calves were inoculated intratracheally with 103 CFU of M. bovis (strain WAg201) as described previously (36). All animals were positive in the single intradermal comparative tuberculin test at 14 weeks after challenge. Blood samples were taken at 16 weeks after challenge, and all cattle were necropsied 17 weeks after challenge. Samples from four thoracic lymph nodes (the left and right bronchial lymph nodes, as well as the anterior and posterior mediastinal ones) were collected from all of the animals for bacterial culture and histology. Additional samples were collected from all tuberculous lesions observed in the lungs, other lymph nodes, or organs. Procedures for the identification of macroscopic tuberculous lesions and processing for histopathology and bacterial counts have been described previously (36). Gross tuberculous lesions were observed in thoracic lymph nodes of all of the animals and in the lungs of 7 of the 10. M. bovis was isolated from lymph node samples from all of the animals.
Naturally infected animals.
To test the performance of recombinant antigens in naturally infected cattle, five dairy herds suspected of containing tuberculous animals (n = 79) and one TB-negative dairy herd (n = 22) were selected. The herds were located in different regions of Argentina, at least 200 km apart. The animals were skin tested using the single intradermal comparative tuberculin test. The 79 animals from the infected herds were all skin test positive, while the 22 animals from the negative herd were skin test negative. Blood samples were collected from the jugular vein into heparinized vials.
IFN-γ blood test.
A commercial bovine IFN-γ microplate enzyme-linked immunosorbent assay kit (Bovigam; Pfizer Animal Health Ltd., Melbourne, Australia) was used according to the manufacturer's instructions to measure the T-cell responses in whole-blood cultures after 16 h of in vitro stimulation with various antigens (PPD-A, PPD-B, protein fractions, or the recombinant antigens). Briefly, aliquots of 200 μl of blood were added in duplicate to wells in a 96-well microplate for culture with antigens in duplicate. The optimal antigen concentrations were determined by comparing the area-under-the-curve values obtained by compound symmetry analysis at eight dilution points for TB-infected and TB-free animals (1), and the final concentration was set at 4 μg/ml for all antigens tested, except for PPD-A and PPD-B, which were used at a final concentration of 20 μg/ml. Negative-control wells, to which only PBS was added, were included for each animal tested, as well as positive controls containing 1 μg/ml pokeweed mitogen (Sigma-Aldrich, United Kingdom). PPD-A, PPD-B, and an ESAT-6/CFP-10 combination were included as reference antigens. Blood and antigens were incubated in a humidified 5% CO2 incubator at 37°C for 16 h. Culture supernatants were pooled before the measurement of IFN-γ release by enzyme-linked immunosorbent assay. The OD for the PBS controls, which was usually approximately 0.1 OD units, was used to normalize individual readouts and to calculate OD indices (ODIs), where the results obtained by antigen stimulation were divided by the results for the PBS-stimulated cultures.
Polyclonal antibody production against recombinant antigens.
Two BALB/c mice for each antigen were inoculated intraperitoneally with purified recombinant antigen. They received two immunizations with 4 μg of each antigen in incomplete Freund's adjuvant (Sigma, United Kingdom) at days 1 and 8. At day 20, sera were obtained and the recognition of the specific protein was confirmed by Western blot analysis. Anti-His6 antibody (GE Healthcare) was used as a control in order to locate the band.
Western blot analysis.
Mycobacterial proteins were separated on SDS-12% polyacrylamide gels and transferred to nitrocellulose membrane (GE Healthcare). Blots were blocked with 5% nonfat dried milk-0.1% Tween 20 (vol/vol) in Tris-buffered saline (T-TBS), washed with T-TBS, and probed with mouse-specific polyclonal antibody at a 1:100 dilution. Alkaline phosphatase-conjugated anti-mouse immunoglobulin G (Sigma) was used at a dilution of 1:2,000. To evaluate the recognition of the recombinant antigens by the bovine sera, a 1:100 dilution of sera in 5% nonfat dried milk-T-TBS was employed. As a secondary antibody, alkaline phosphatase-conjugated anti-bovine immunoglobulin G (Sigma) was used at a dilution of 1:30,000. For detection, 5-bromo-4-chloro-3-indolylphosphate (BCIP)/nitroblue tetrazolium color development substrates (Promega, Madison, WI) were used.
Statistical analysis.
ODI values were modeled as dependent on the time postinfection, the fraction (supernatant or extracellular), and their interaction. The ODI measurements were undertaken on the same animal over time. A total of four animals were used in this assay. The animal effect was included as a random effect, creating a compose-symmetry structure of the correlation for the same fraction over time. The residual variance was also modeled as dependent on time because of the presence of heteroscedasticity. The model was estimated with the lme function of the nlme library of the R package, using InfoStat. The results of the post hoc test of mean difference were assessed by the Di Rienzo-Gnzman-Casa Moves procedure (35).
RESULTS
Separation of protein fractions by electroelution (SDS-PAGE) and evaluation by IFN-γ release tests in blood from experimentally infected animals.
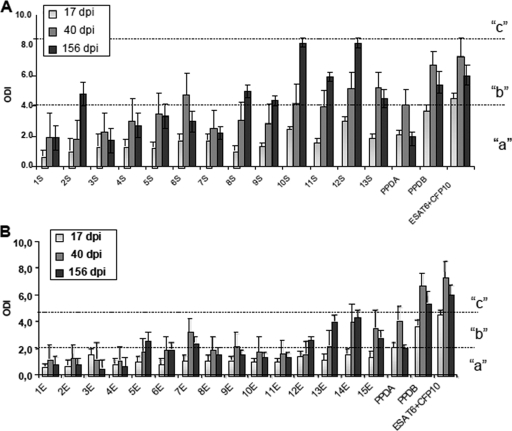
Thirty protein fractions were obtained in various concentrations from 17 to 80 μg/ml and with molecular masses ranging from 7 to 208 kDa in a 12.5%-SDS-PAGE, as shown in the supplemental figure. Pairs of consecutive fractions were pooled, and these were used to test blood from experimentally infected cattle in IFN-γ release tests. Blood was collected at 17, 40, and 156 days postinfection (dpi). We observed that, in general, fractions from culture supernatant were more antigenic than those from cell extract (Fig. 1). In both the culture supernatant and cell extract, the lower-molecular-mass fractions were more antigenic. We also observed that at longer times postinfection, the response was stronger for most fractions.
FIG. 1.
Results of IFN-γ whole-blood-based assay against the culture supernatant fractions (A) and cell extract fractions (B) in four cattle following intranasal infection with M. bovis. Reference and recombinant antigen results are also shown. Results are expressed as the ODI for each time point of blood extraction. There were three levels of ODI, “a,” “b,” and “c,” corresponding to significant differences among ODI means. Error bars show standard deviations.
Supernatant fraction.
For the supernatant fraction, the relationship between time postinfection and level of IFN-γ released was significant (P < 0.0001), indicating that ODI means differed between fractions depending on the evaluation time point (Fig. 1A). There were three levels of ODI: level “a,” low (ODI < 4.1); level “b,” intermediate (ODI 4.1 to 8.2); and level “c,” high (ODI > 8.2), corresponding to significant differences among ODI means between the different fractions. Combinations of supernatant fractions and times postinfection are identified in Fig. 1A with the appropriate label indicating their level.
Supernatant fractions 1, 3, 4, 5, and 7 remained at level “a” during all times in the evaluation. These fractions had statistically similar ODI means irrespective of whether the comparison was between or within the fractions. Fraction 6 showed a significant increase at 40 dpi, reaching level “b.” At 17 and 156 dpi, its level was “a.” Fractions 2, 8, and 9 showed significant increases at the end of the evaluation period (156 dpi), reaching level “b.” Fractions 11 and 13 had initial values corresponding to level “a” but then increased to level “b” for the last two evaluation times. Fractions 10 and 12 increased their ODI values according to the time postinfection; they began at level “a” and ended up at level “c.” These fractions had ODI levels higher than the PPD-B or ESAT-6/CFP-10 values at 156 dpi.
Cell extract fraction.
The comparison of cell extract fractions by time relationship was significant (P < 0.0209), indicating that the ODI means differed between fractions depending on the evaluation time point. The level “a” ODI (<2), level “b” ODI (2 to 4.5), and level “c” ODI (>4.5) corresponded to significant differences. The results are summarized in Fig. 1B.
Cell extract fractions 1, 2, 3, 4, 6, 8, 10, and 11 remained at level “a” during all of the evaluation times. These fractions had statistically similar ODI means irrespective of whether the comparison was between or within the fractions. Fractions 7, 9, 13, 14, and 15 showed significant increases at 40 dpi, reaching level “b.” Fractions 5 and 12 showed significant increases at the end of the evaluation period (156 dpi), reaching level “b,” whereas fractions 13, 14, and 15 showed significant increases at both 40 and 156 dpi. None of the fractions reached a IFN-γ level as high as that of PPD-B or the ESAT-6/CFP-10 combination at the corresponding times.
When naturally infected animals were tested with these fractions, a pattern similar to that seen for the experimentally infected cattle was observed, in that there were stronger responses for the culture supernatant antigens and higher antigenicity of low-molecular-weight fractions (data not shown).
Analysis of M. bovis fractions by preparative 2D-E.
Those fractions with high ODIs and enough protein concentration to perform the technique were selected for preparative 2D-E. Individual proteins from the selected fractions (2S, 8S, 12E, and 13E) were separated by using 2D-E with IEF as the first step, followed by separation according to size in SDS-PAGE gels. Between two and four spots were identified in each fraction (Fig. 2), excised, and submitted to MALDI-TOF MS.
FIG. 2.
Selection of spots in the 2D gels. Gels were stained with silver nitrate. Arrows indicate selected spots.
Identification and characterization of selected proteins.
The proteins identified by MS and bioinformatics screening of Mycobacterium tuberculosis complex genomes are listed in Table 2. In the cell extract fractions, Rv1636, HspX, and Rv0138 were identified, while Rv3740, EsxI, and a fragment of FasI were identified in the culture supernatant fractions. No signal sequences were observed in the primary sequences of the proteins found in the cell extract (data not shown). On the other hand, the presence of signal sequences in the culture supernatant proteins was varied. Rv3740 is the largest protein identified and shows an N-terminal sequence compatible with a signal sequence. EsxI belongs to the Esx protein family; EsxA, the founding member of the family, has no signal sequence and is secreted by an ad hoc secretion system (17). FasI (326 kDa) is the central enzyme in fatty acid synthesis and reportedly cytoplasmic. The finding of a fragment of FasI in culture supernatants may indicate cell lysis with subsequent proteolysis.
TABLE 2.
Characteristics of selected proteins
| Protein | Locus tags | Culture fraction | Molecular mass (kDa) | % Similarity in M. avium | % Similarity in M. smegmatis | MSFit score | Function (reference) |
|---|---|---|---|---|---|---|---|
| TB15.3 | Rv1636, Mb1662 | 12E | 15.31 | 95 | 88 | 55 | Unknown |
| HspX | Rv2031c, Mb2057c | 12E | 16 | 59 | 78 | 72 | Stress protein induced by anoxia (11, 14) |
| Rv3740 | Rv3740c, Mb3766c | 2S | 48 | 78 | 76 | 78 | Unknown |
| FasI (fragment) | Rv2524c, Mb2553c | 8S | 15 | 86 | 90 | 69 | Lipid synthesis (15) |
| EsxI | Rv1037c, Mb1066c | 8S | 10 | 91 | 0 | 79 | Unknown |
| Rv0138 | Rv0138, Mb0143 | 13E | 19 | 86 | 86 | 64 | Unknown |
Expression and purification of recombinant mycobacterial proteins.
The genes corresponding to the antigens selected, Rv1636, Rv0138, EsxI, HspX, Rv3740, and a fragment of Rv2524 (Table 2), were amplified by PCR and cloned in the pRSET-A expression vector in order to create a fusion to an N-terminal histidine tag. They were expressed in E. coli BL21 and purified under native conditions. As Rv2524c is a very large fatty acid synthase protein, we decided to clone just the 15-kDa fragment that comprised the region detected by MALDI-TOF MS. The levels of expression of recombinant proteins were determined both by analysis of Coomassie blue-stained polyacrylamide gels and Western blot analysis using antihistidine antibody (data not shown).
Localization.
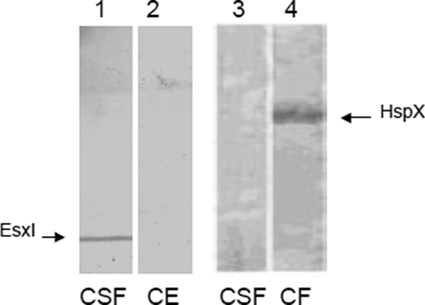
To determine the cellular localization of the antigens, specific sera obtained from mice were used in Western blot assays. EsxI was detected in the M. bovis (AN5) culture supernatant, while HspX was detected in the cell extract (Fig. 3). At the same time, Rv1636 and Rv0138 were localized in the cell extract, whereas Rv3740 and Rv2524c were detected in the culture supernatant, as expected (data not shown). All the proteins showed apparent molecular masses in SDS-PAGE that were expected from sequence analysis.
FIG. 3.
EsxI and HspX detection by Western blot analysis in CSF or cell extract of M. bovis AN5 culture. EsxI- and HspX-specific mouse antibodies were used for detection in blotted CFS and cell extract proteins.
Recombinant antigen recognition in experimentally infected animals.
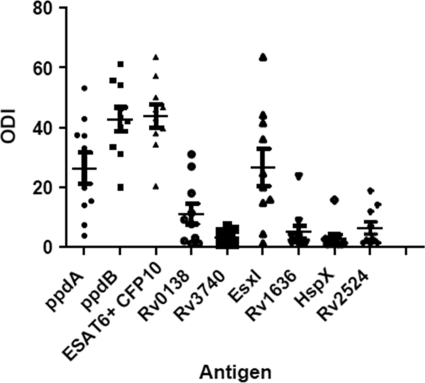
To further assess the antigenicity of the protein antigens identified herein, blood samples were collected from 10 experimentally infected animals. PPD-B and a combination of ESAT-6 and CFP-10 showed high ODIs in the IFN-γ test for all animals at 114 dpi. The average reactivity of EsxI was 26.6 and that of Rv0138 was 11.1 for the ODI. The reference antigens PPD-B and ESAT-6/CFP-10 had average values of 42 and 46 ODI, respectively (Fig. 4). Nine of 10 animals responded to EsxI, whereas 7 of 10 animals responded to Rv0138, based on an arbitrary ODI of 2.0 used as the cutoff. M. bovis infection was confirmed in all animals based upon the presence of tuberculous lesions and culture of M. bovis (data not shown).
FIG. 4.
Results of IFN-γ whole-blood-based assay against recombinant antigens in 10 cattle experimentally infected with M. bovis. Results are expressed as the scatter plot of the ODIs. ODIs (points) and means (horizontal bars) are shown. The IFN-γ data are for samples obtained at 114 dpi. Error bars show standard deviations.
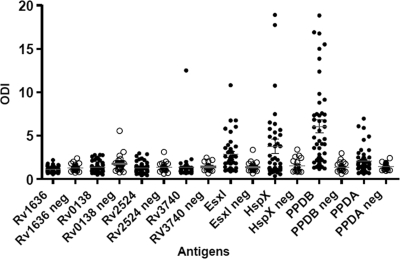
Recombinant antigen recognition in naturally infected animals.
To assess cell-mediated immune responses toward mycobacterial antigens in skin test-positive and -negative animals, blood samples were obtained from 79 skin test reactors from different herds (naturally infected animals) and 22 nonreactor cattle in TB-free herds. The six recombinant proteins, along with PPD-B (average ODI, 6.2) and PPD-A (average ODI, 2.4), were screened against samples from skin test-positive animals (Fig. 5). Based on the average of the IFN-γ responses, HspX (average ODI, 3.85) and EsxI (average ODI, 2.75) were the most antigenic of this set of novel antigens. The average ODI values for novel and reference antigens in TB-free herds were very low (1.15 for EsxI, 1.35 for HspX, 1.35 for PPD-B, and 1.25 for PPD-A). The other proteins did not show a significant level of immune reactivity in either group of animals.
FIG. 5.
Results of study of recombinant antigens by IFN-γ whole-blood-based assay in TST-positive (•; n = 79) and -negative (neg [○]; n = 22) animals. Results are expressed as the scatter plot of the ODIs. ODIs (points) and means (horizontal bars) for infected animals are shown. According to the recommendations of the Office des International Epizooties, in Argentina, the cutoff point for positivity of the TST is >5 mm (skin thickness), for suspected infection is 3 to 5 mm, and for negativity is <3 mm. Error bars show standard deviations.
DISCUSSION
The resolution of cell extract and culture supernatant proteins from several molecular weight fractions of M. bovis and the study of their immune cellular reactivities following an experimental infection have allowed us to identify various trends in immune response patterns. As observed by other authors (6), proteins from the culture supernatant, were in general, more reactive than those from the cell extract. In fact, many of the more strongly reacting antigens (Ag85 complex, ESAT-6, CFP-10, MPB70, MPB83, P38, etc.) of M. tuberculosis complex proteins are found in the supernatant (7, 8, 24, 27, 29, 33, 37).
The low-molecular-weight fractions (fractions 10 and greater) appeared to be more antigenic, especially those from the culture supernatant. It is possible that low-molecular-weight fractions are enriched in peptides resulting from the degradation of larger proteins. Bassey et al. (6) observed a predominance of low-molecular-weight fractions with immune reactivity when working with cell extracts. This observation could not be attributed only to the presence of strong antigens, such ESAT-6 and CFP-10, in low-molecular-weight fractions, because their predominance was also observed when the fraction were prepared from M. bovis BCG, where these antigens are absent. A similar predominance was reported by Lamb and Young (21), working with cell extracts. The possible explanations for this predominance of high reactivity in low-molecular-weight fractions have been discussed by Bassey et al. (6). One exception to this consistent pattern of predominance was the results of Gulle et al. (19). However, it must be noted that they inoculated animals with BCG and not with a virulent strain, although they did discriminate between the responses to culture filtrate and cell extracts.
We have also observed that in most cases, the reactivities of fractions increased with time postinfection for both the cell extract and culture supernatant fractions. This observation is in contrast to that of Aagaard et al. (2) using recombinant proteins. They observed that the level of IFN-γ decreased at the late stages of infection. Conversely and in concurrence with our observations, Fifis et al. (16), working with very late time points postinfection (27 months postinoculation) and native purified antigens, observed an increase of reactivity with time, although it was dependent on antigens and individual animals. At present, it is difficult to identify the reason for these differences.
Another common finding shared with other researchers was the high variability of the immune response at the individual level. This variability may reflect the polymorphism in the major histocompatibility complex genes (BoLA) of individual cattle.
Although we obtained 30 different fractions, not all of them reached the minimal concentration required for 2D-E analysis. This was the main reason for choosing just some of the pooled fractions.
2D-E identified different proteins in the fractions studied. The numbers of spots were reduced (two to four) compared to the numbers theoretically expected. The low resolution of 2D gel or the method of protein detection may have influenced this result. In addition, some spots could not be identified by MS (data not shown). This opens the possibility that some spots which were not identified by MS could be immunoreactive. One of the antigens identified, HspX (Rv2031c), also known as Acr, or 16-kDa protein, has been previously described. It is a stress protein induced by hypoxic culture conditions. Other evidence (11, 14, 23, 31) has led to the proposition that HspX has a central role in the viability of the bacilli during latent, asymptomatic infections in humans and in a mouse model of TB. In contrast, its role in bovine TB has been not studied. The other proteins have been reported as diagnostic antigens; Rv1636 has been indicated as a cell wall protein (5, 25, 32). There are no previous reports of Rv0138 as an antigen. Rv3740 has been postulated to be a possible triacylglycerol synthase (diacylglycerol acyltransferase), involved in the initiation of dormancy (12). EsxI is a member of the Esx protein family (17), all of which are secreted and of low molecular weight. The founding member of this group is EsxA (ESAT-6). In spite of being a member of this important family of antigens, there are no previous reports about its antigenicity. A special case is that of Rv2524, or Fas, the FasI fatty acid synthase of the M. tuberculosis complex. FAS was not previously reported as antigenic, although the amino acid sequence similarity of Fas to other enzymes involved in lipid synthesis (Pks and Mas) may contribute to a sort of cross-talk antigenicity (15).
The presence of EsxI in the cell extract or culture supernatant in low concentrations was confirmed, and the presence of HspX in the cell extract, as expected, was confirmed. In our study, EsxI and Rv0138 were the more reactive antigens when tested in experimentally infected animals, according to IFN-γ results at an advanced stage of infection. To our knowledge, this report is the first description of the antigenicity of EsxI and Rv0138. Among all antigens tested, the strongest reaction was that directed to CFP-10/ESAT-6, as previously observed (1). Rv3740, Rv1636, and Rv2524 had low or null antigenicity for cell-mediated immunity. One may suppose that not all of the proteins identified by the proteomic approach used in this study would be antigenic. In some cases, the true antigenic proteins in a given fraction would not have been identified in the 2D gels.
Cellular responses toward selected antigens were also tested in naturally infected animals. Based on the average of the IFN-γ responses, HspX discriminates PPD skin test-positive animals from PPD skin test-negative animals better than EsxI. HspX was described as a latency-associated protein. The time when these animals were infected is unknown, but we could assume that they were in an advanced stage of infection. To our knowledge, this is the first report about the antigenic potential of HspX in bovine TB. It could be assumed that HspX detects animals not identified by testing based on EsxI. In this case, the two proteins should be complementary as diagnostic antigens. Different antigen responses could show up in naturally and experimentally infected animals. This could be due to the differential virulence of the strains or to the stage of bovine TB infection in different animals.
In summary, we report novel antigens of Mycobacterium bovis, shown to react in IFN-γ assays in naturally and experimentally infected animals. Antigen-specific T-cell and IFN-γ responses to a panel of mycobacterial fractions were assessed at regular intervals throughout the infection period. Among the antigens identified here, HspX, EsxI, and Rv0138 were shown to be promising antigens for diagnosis, and even if the sensitivities and specificities of tests based on these antigens are not optimal, they may form part of a combination or cocktail for the diagnosis of bovine TB.
Supplementary Material
Acknowledgments
We thank Sergio Garbaccio for his help in the necropsy procedure and Julio Di Rienzo for his excellent technical help with the statistical analysis.
This work was supported by INCO-EC grant CT2000-30023. Angel Cataldi, Andrea Gioffre, and Andrea Peralta are CONICET fellows.
Footnotes
Published ahead of print on 29 July 2009.
Supplemental material for this article may be found at http://cvi.asm.org/.
REFERENCES
- 1.Aagaard, C., M. Govaerts, V. Meikle, A. J. Vallecillo, J. A. Gutierrez-Pabello, F. Suarez-Güemes, J. McNair, A. Cataldi, C. Espitia, P. Andersen, and J. M. Pollock. 2006. Optimizing antigen cocktails for detection of Mycobacterium bovis in herds with different prevalences of bovine tuberculosis: ESAT6-CFP10 mixture shows optimal sensitivity and specificity. J. Clin. Microbiol. 44:4326-4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aagaard, C., M. Govaerts, L. Meng Okkels, P. Andersen, and J. M. Pollock. 2003. Genomic approach to identification of Mycobacterium bovis diagnostic antigens in cattle. J. Clin. Microbiol. 41:3719-3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alito, A., J. McNair, M. Guirvin, M. Zumarraga, F. Bigi, M. I. Romano, J. Pollock, and A. Cataldi. 2003. Bovine T-cell responses to secreted antigen fractions of Mycobacterium bovis. Braz. J. Med. Biol. Res. 36:1523-1531. [DOI] [PubMed] [Google Scholar]
- 4.Amadori, M., S. Tagliabue, S. Lauzi, G. Finazzi, G. Lombardi, P. Telo, L. Pacciarini, and L. Bonizzi. 2002. Diagnosis of Mycobacterium bovis infection in calves sensitized by mycobacteria of the avium/intracellulare group. J. Vet. Med. B 49:89-96. [DOI] [PubMed] [Google Scholar]
- 5.Bahk, Y. Y., S. A. Kim, J. S. Kim, H. J. Euh, G. H. Bai, S. N. Cho, and Y. S. Kim. 2004. Antigens secreted from Mycobacterium tuberculosis: identification by proteomics approach and test for diagnostic marker. Proteomics 4:3299-3307. [DOI] [PubMed] [Google Scholar]
- 6.Bassey, E. O., P. F. Life, D. Catty, J. S. Gaston, and D. S. Kumararatne. 1996. T-cell response to mycobacterial proteins: a comparative study of tuberculous and control immunoblots of Mycobacterium tuberculosis and M. bovis BCG. Tuber. Lung Dis. 77:146-153. [DOI] [PubMed] [Google Scholar]
- 7.Carr, M. D., M. J. Bloemink, E. Dentten, A. O. Whelan, S. V. Gordon, G. Kelly, T. A. Frenkiel, R. G. Hewinson, and R. A. Williamson. 2003. Solution structure of the Mycobacterium tuberculosis complex protein MPB70: from tuberculosis pathogenesis to inherited human corneal disease. J. Biol. Chem. 278:43736-43743. [DOI] [PubMed] [Google Scholar]
- 8.Cataldi, A., M. I. Romano, and F. A. Bigi. 1994. Western blot characterization of Mycobacterium bovis antigens recognized by cattle sera. Res Microbiol. 145:689-698. [DOI] [PubMed] [Google Scholar]
- 9.Cockle, P. J., S. V. Gordon, A. Lalvani, B. M. Buddle, R. G. Hewinson, and H. M. Vordermeier. 2002. Identification of novel Mycobacterium tuberculosis antigens with potential as diagnostic reagents or subunit vaccine candidates by comparative genomics. Infect. Immun. 70:6996-7003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cockle, P. J., S. V. Gordon, R. G. Hewinson, and H. M. Vordermeier. 2006. Field evaluation of a novel differential diagnostic reagent for detection of Mycobacterium bovis in cattle. Clin. Vaccine Immunol. 13:1119-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cunningham, A. F., and C. L. Spreadbury. 1998. Mycobacterial stationary phase induced by low oxygen tension: cell wall thickening and localization of the 16-kilodalton alpha-crystallin homolog. J. Bacteriol. 180:801-808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daniel, J., C. Deb, V. S. Dubey, T. D. Sirakova, B. Abomoela, H. R. Morbidoni, and P. E. Kolattukudy. 2004. Induction of a novel class of diacylglycerol acyltransferases and triacylglycerol accumulation in Mycobacterium tuberculosis as it goes into a dormancy-like state in culture. J. Bacteriol. 186:5017-5030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Kantor, I. N., and V. Ritacco. 2006. An update on bovine tuberculosis programmes in Latin American and Caribbean countries. Vet. Microbiol. 112:111-118. [DOI] [PubMed] [Google Scholar]
- 14.Desjardin, L. E., L. G. Hayes, C. D. Sohaskey, L. G. Wayne, and K. D. Eisenach. 2001. Microaerophilic induction of the alpha-crystallin chaperone protein homologue (hspX) mRNA of Mycobacterium tuberculosis. J. Bacteriol. 183:5311-5316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fernandes, N. D., and P. E. Kolattukudy. 1996. Cloning, sequencing and characterization of a fatty acid synthase-encoding gene from Mycobacterium tuberculosis var. bovis BCG. Gene 170:95-99. [DOI] [PubMed] [Google Scholar]
- 16.Fifis, T., L. A. Corner, J. S. Rothel, and P. R. Wood. 1994. Cellular and humoral immune responses of cattle to purified Mycobacterium bovis antigens. Scand. J. Immunol. 39:267-274. [DOI] [PubMed] [Google Scholar]
- 17.Gey Van Pittius, N. C., J. Gamieldien, W. Hide, G. D. Brown, R. J. Siezen, and A. D. Beyers. 2001.The ESAT-6 gene cluster of Mycobacterium tuberculosis and other high G+C Gram-positive bacteria. Genome Biol. 2:RESEARCH0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Görg, A., C. Obermaier, G. Boguth, A. Harder, B. Scheibe, R. Wildgruber, and W. Weiss. 2000. The current state of two-dimensional electrophoresis with immobilized pH gradients. Electrophoresis 21:1037-1053. [DOI] [PubMed] [Google Scholar]
- 19.Gulle, H., L. M. Fray, E. P. Gormley, A. Murray, and K. M. Moriarty. 1995. Responses of bovine T cells to fractionated lysate and culture filtrate proteins of Mycobacterium bovis BCG. Vet. Immunol. Immunopathol. 48:183-190. [DOI] [PubMed] [Google Scholar]
- 20.Heukeshoven, J., and R. Dernick. 1988. Improved silver staining procedure for fast staining in PhastSystem Development Unit. I. Staining of sodium dodecyl sulfate gels. Electrophoresis 9:28-32. [DOI] [PubMed] [Google Scholar]
- 21.Lamb, J. R., and D. B. Young. 1987. A novel approach to the identification of T-cell epitopes in Mycobacterium tuberculosis using human T-lymphocyte clones. Immunology 60:1-5. [PMC free article] [PubMed] [Google Scholar]
- 22.Lyashchenko, K., C. Manca, R. Colangeli, A. Heijbel, A. Williams, and M. L. Gennaro. 1998. Use of Mycobacterium tuberculosis complex-specific antigen cocktails for a skin test specific for tuberculosis. Infect. Immun. 66:3606-3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mao, Q., D. Ke, X. Feng, and Z. Chang. 2001. Preheat treatment for Mycobacterium tuberculosis Hsp16.3: correlation between a structural phase change at 60 degrees C and a dramatic increase in chaperone-like activity. Biochem. Biophys. Res. Commun. 284:942-947. [DOI] [PubMed] [Google Scholar]
- 24.Michell, S. L., A. O. Whelan, P. R. Wheeler, M. Panico, R. L. Easton, A. T. Etienne, S. M. Haslam, A. Dell, H. R. Morris, A. J. Reason, J. L. Herrmann, D. B. Young, and R. G. Hewinson. 2003. The MPB83 antigen from Mycobacterium bovis contains O-linked mannose and (1->3)-mannobiose moieties. Biol. Chem. 278:16423-16432. [DOI] [PubMed] [Google Scholar]
- 25.Mollenkopf, H. J., P. R. Jungblut, B. Raupach, J. Mattow, S. Lamer, U. Zimny-Arndt, U. E. Schaible, and S. H. A. Kaufmann. 1999. Dynamic two-dimensional polyacrylamide gel electrophoresis database: the mycobacterial proteome via Internet. Electrophoresis 20:2172-2180. [DOI] [PubMed] [Google Scholar]
- 26.Mustafa, A. S., P. J. Cockle, F. Shaban, R. G. Hewinson, and H. M. Vordermeier. 2002. Immunogenicity of Mycobacterium tuberculosis RD1 region gene products in infected cattle, 2002. Clin. Exp. Immunol. 130:37-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pollock, J. M., and P. Andersen. 1997. Predominant recognition of the ESAT-6 protein in the first phase of interferon with Mycobacterium bovis in cattle. Infect. Immun. 65:2587-2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pollock, J. M., R. M. Girvin, K. A. Lightbody, R. A. Clements, S. D. Neill, B. M. Buddle, and P. Andersen. 2000. Assessment of defined antigens for the diagnosis of bovine tuberculosis in skin test-reactor cattle. Vet. Rec. 146:659-665. [DOI] [PubMed] [Google Scholar]
- 29.Radford, A. J., P. R. Wood, H. Billman-Jacobe, H. M. Geysen, T. J. Mason, and G. Tribbick. 1990. Epitope mapping of the Mycobacterium bovis secretory protein MPB70 using overlapping peptide analysis. Gen. Microbiol. 136:265-272. [DOI] [PubMed] [Google Scholar]
- 30.Rhodes, S. G., D. Gavier-Widen, B. M. Buddle, A. O. Whelan, M. Singh, R. G. Hewinson, and H. M. Vordermeier. 2000. Antigen specificity in experimental bovine tuberculosis. Infect. Immun. 68:2573-2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosenkrands, I., R. A. Slayden, J. Crawford, C. Aagaard, C. E. Barry III, and P. Andersen. 2002. Hypoxic response of Mycobacterium tuberculosis studied by metabolic labeling and proteome analysis of cellular and extracellular proteins. J. Bacteriol. 184:3485-3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosenkrands, I., K. Weldingh, S. Jacobsen, C. V. Hansen, W. Florio, I. Gianetri, and P. Andersen. 2000. Mapping and identification of Mycobacterium tuberculosis proteins by two-dimensional gel electrophoresis, microsequencing and immunodetection. Electrophoresis 21:935-948. [DOI] [PubMed] [Google Scholar]
- 33.Salata, R. A., A. J. Sanson, I. J. Malhotra, H. G. Wiker, M. Harboe, N. B. Phillips, and T. M. Daniel. 1991. Purification and characterization of the 30,000 dalton native antigen of Mycobacterium tuberculosis and characterization of six monoclonal antibodies reactive with a major epitope of this antigen. Lab. Clin. Med. 118:589-598. [PubMed] [Google Scholar]
- 34.Sidders, B., C. Pirson, P. J. Hogarth, R. G. Hewinson, N. G. Stoker, H. M. Vordermeier, and K. Ewer. 2008. Screening of highly expressed mycobacterial genes identifies Rv3615c as a useful differential diagnostic antigen for the Mycobacterium tuberculosis complex. Infect. Immun. 76:3932-3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Valdano, S., and J. Di Rienzo. 2007. Discovering meaningful groups in hierarchical cluster analysis. An extension to the multivariate case of a multiple comparison method based on cluster analysis. http://interstat.statjournals.net/YEAR/2007/abstracts/0704002.php
- 36.Wedlock, D. N., M. Denis, G. F. Painter, G. D. Ainge, H. M. Vordermeier, R. G. Hewinson, and B. M. Buddle. 2008. Enhanced protection against bovine tuberculosis after coadministration of Mycobacterium bovis BCG with a mycobacterial protein vaccine-adjuvant combination but not after coadministration of adjuvant alone. Clin. Vaccine Immunol. 15:765-772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wiker, H. G., K. P. Lyashchenko, A. M. Aksoy, K. A. Lightbody, J. M. Pollock, S. V. Komissarenko, S. O. Bobrovnik, I. N. Kolesnikova, L. O. Mykhalsky, M. L. Gennaro, and M. Harboe. 1998. Immunochemical characterization of the MPB70/80 and MPB83 proteins of Mycobacterium bovis. Infect. Immun. 66:1445-1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wood, P. R., and S. L. Jones. 2001. BOVIGAM: an in vitro cellular diagnostic test for bovine tuberculosis. Tuberculosis (Edinburgh) 81:147-155. [DOI] [PubMed] [Google Scholar]
- 39.Wood, P. R., L. A. Corner, J. S. Rothel, C. Baldock, S. L. Jones, D. B. Cousins, B. S. McCormick, B. R. Francis, J. Creeper, and N. E. Tweddle. 1991. Field comparison of the interferon-gamma assay and the intradermal tuberculin test for the diagnosis of bovine tuberculosis. Aust. Vet. J. 68:286-290. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.