Abstract
Worldwide, Streptococcus pneumoniae (pneumococcus) is a major cause of morbidity and mortality, especially in infants and elderly people. Pneumococcal capsular polysaccharides are well characterized, and more than 90 different serotypes have been identified. Serotype-specific antibodies against the capsular polysaccharide are produced during infection. Detection of antibodies against pneumococci by enzyme-linked immunosorbent assay (ELISA) is performed according to WHO guidelines, using antigens provided by ATCC. However, testing the ELISA for specificity is challenging due to the difficulty in obtaining human naïve serum with pneumococcal antibodies as well as human serum with antibodies against a single serotype. The application of well-defined serotype-specific sera produced in animals to evaluate the specificity of the ATCC antigens and the effect of adsorption with cell wall and 22F polysaccharides has not been performed before, to our knowledge. In this study, the specificity of ATCC antigens (serotypes 4, 6B, 9V, 14, 18C, 19F, and 23F) was tested by using commercial serotype-, serogroup-, and pool-specific pneumococcal rabbit antisera.
Worldwide, Streptococcus pneumoniae (pneumococcus) is a significant cause of morbidity and mortality, especially in infants and elderly people. Pneumococcal infections range from mild upper respiratory tract diseases and otitis media to pneumonia, bacteremia, and meningitis (4). Pneumococcal strains possess a polysaccharide capsule, and more than 90 different varieties (serotypes) have been identified (11, 18). Serotype-specific antibodies against the capsular polysaccharides provide protection against the corresponding serotypes. Four vaccines have been developed, two of which are currently used, including a 23-valent polysaccharide (Pneumovax) for the elderly and for children over the age of 2 years and a 7-valent protein-conjugated vaccine (Prevenar) for children under 2 years of age (9). In 2009, a PCV-10 vaccine (PhiD-CV; GlaxoSmithKline) and a PCV-13 vaccine (Wyeth) are expected to be licensed and used (9).
Detection of antibodies against pneumococci is performed by an enzyme-linked immunosorbent assay (ELISA) according to WHO guidelines. Antigens are provided by ATCC, with the exact compositions being unknown (16; www.vaccine.uab.edu).
The ATCC antigens used in the WHO ELISA are believed to be serotype specific; however, very few studies have been performed to investigate the actual specificity of the antigens (15, 17). These studies indicate that cross-reactions between serotypes occur, resulting in diagnostic challenges. Furthermore, the antibody responses in human sera seem to differ between immunized and naturally infected subjects (15, 17). Adsorbing the human sera by using pneumococcal cell wall polysaccharides (C-Ps) and 22F polysaccharide was shown to improve the specificity of the WHO ELISA (3). However, due to the widespread colonization/infection of humans by different pneumococcal serotypes, it is uncertain if individuals have been exposed to the bacterium and to what extent. Thus, it is almost impossible to obtain a human serum naïve of pneumococcal antibodies as well as human serum with antibodies against a single serotype. The use of alternative, well-defined serotype-specific sera produced in animals to evaluate the specificity of the ATCC antigens and the effect of adsorption with C-Ps and 22F polysaccharide has not been tested previously, to our knowledge. Thus, the specificity of the ATCC antigens was evaluated in this study by using commercial pneumococcal rabbit antisera specific to capsular polysaccharides (13). Furthermore, the effect of serum adsorption with C-Ps and/or 22F polysaccharide on the pneumococcal titer was determined.
MATERIALS AND METHODS
Antigens.
Pneumococcal polysaccharides (1 mg/ml) of serotypes 4 (lot 2097655), 6B (lot 2089390), 9V (lot 2046913), 14 (lot 2098654), 18C (lot 2082876), 19F (lot 2096923), and 23F (lot 2030189) (ATCC, Manassas, VA) were used.
Furthermore, other ATCC lot numbers for 19F (lot 2044325) and 23F (lot 2099298) were available, and we tested them to see the effect of using different ATCC lots and the effect of another person performing the test (data not presented).
Antisera.
The commercial pneumococcal rabbit sera used in this study were obtained from SSI Diagnostika (Hillerød, Denmark). The following types, groups, and pools were used: type 4, group 6 (6A, 6B, and 6C), group 9 (9A, 9L, 9N, and 9V), type 14, group 16 (16F and 16A), group 18 (18F, 18A, 18B, and 18C), group 19 (19F, 19A, 19B, and 19C), group 23 (23F, 23A, and 23B), pool B (group 19, group 6, type 3, and type 8), and pool P (type 1, group 19, group 7, and type 14).
The sera are produced by immunizing rabbits with a whole-cell vaccine, the specificity of the sera is tested, and cross-reactions are removed by adsorption (2) (Diagnostika, Hillerød, Denmark). The general vaccine/serum production procedure for rabbit serum has been described by Lund and Henrichsen (8).
Adsorption of rabbit sera.
The rabbit sera were adsorbed by adding both purified C-Ps (1 mg/ml) (SSI-Diagnostika) and 22F capsular polysaccharide (1 mg/ml) (ATCC lot 2045909) (10, 16). Briefly, 40 μl rabbit serum was mixed with 25 μl C-P solution, 25 μl 22F solution, and 910 μl dilution buffer (20% 20× phosphate-buffered saline [PBS1] plus 20% 20× NaCl plus 0.05% Tween 20 plus 10 g/liter bovine serum albumin plus Milli Q water [pH 7.2; Millipore]). The serum dilutions were incubated for 2 h at 37°C, and 15 ml dilution buffer was added with 50 μl to each well.
ELISA.
The ELISA procedure used was previously described by Slotved et al. (14). Briefly, MaxiSorp polystyrene microtiter plates (Nunc, Denmark) were coated with ATCC antigen (diluted with 20% 20× PBS1 plus 20% 20× NaCl plus Milli Q water [pH 7.2; Millipore] to a final concentration of 2.5 μg/ml). The plates were incubated overnight at 5°C and then washed with washing buffer (20× PBS plus 0.05% Tween 20 plus Milli Q water [pH 7.4; Millipore]). After washing of the plate, test sera (diluted 1:400 with dilution buffer) were added to the plate with the ATCC antigens and incubated at room temperature for 1 hour. Following incubation, the plates were washed and polyclonal secondary antibody conjugate was added to all wells. Horseradish peroxidase-conjugated polyclonal goat anti-rabbit immunoglobulin G serum (DakoA/S, Denmark) (diluted 1:1,000 in dilution buffer) was used as a secondary antibody.
The plates were incubated for another hour, followed by washing. The peroxidase-catalyzed color reaction was started by adding TMB substrate (TMB Plus; Kem-En-Tec Diagnostics A/S, Denmark) to all wells and leaving the plates for 15 min. The reaction was stopped by adding 1.2 M H2SO4 to all wells, and the color intensity was measured in an immunoreader (Tecan Schweiz AG, Sunrise, Switzerland) at 450 nm.
Statistics.
All tests in Fig. 1, 2, and 3 were carried out in triplicate determinations on the same plate. The data in Fig. 2 were compared using the Kruskal-Wallis test and Dunn's multiple comparison test.
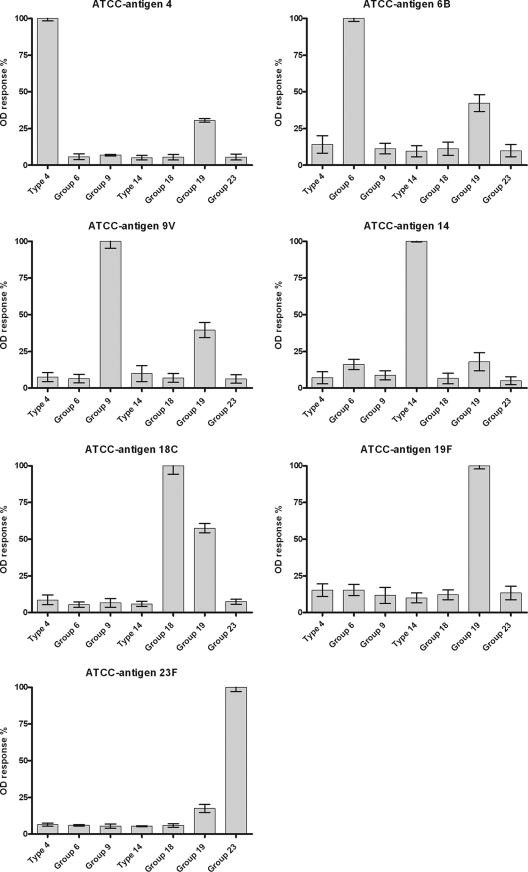
FIG. 1.
Serotype- and serogroup-specific rabbit pneumococcal antisera (adsorbed against C-Ps and 22F polysaccharide) tested against seven ATCC antigens. The OD values are presented as normalized mean data with standard deviations (SD). All tests were performed in triplicate.
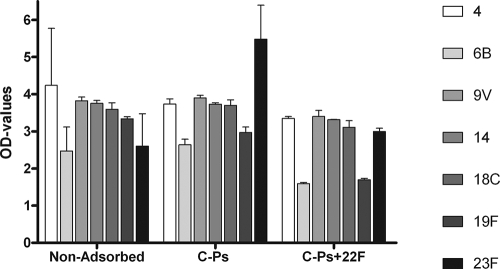
FIG. 2.
Comparison of specific reactions of ATCC antigens and corresponding unadsorbed or adsorbed type- and group-specific rabbit antisera. All tests were performed in triplicate, and the data are presented as mean OD values with SD. Comparing the effects of absorption on each serotype showed significant differences (P < 0.05) only for 19F (nonadsorbed group 19 rabbit serum and C-P-plus-22F-adsorbed group 19 rabbit serum).
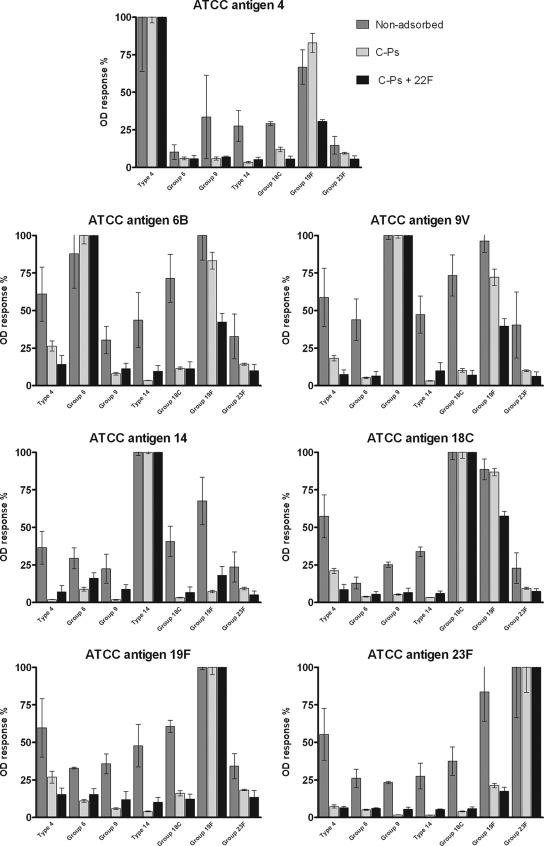
FIG. 3.
Comparison of effects of absorption on nonspecific cross-reactions, using group- and type-specific rabbit antisera. ATCC OD values are presented as mean normalized data with SD. All tests were performed in triplicate. In the graph presenting data for antigen 6B, a higher response against group 19 serum (nonadsorbed) than against group 6 serum (nonadsorbed) was observed; therefore, the normalized responses were determined against the group 19 value, not the group 6 value.
The normalized data in Fig. 1 were calculated by the formula (x optical density [OD] value/specific OD value) × 100%, as can be seen in Fig. 1 for antigen 4, where x represents the OD value for each serotype response and the specific OD value is always the serotype-specific OD response for serotype 4. The present data are therefore normalized data presented as percentages of the specific reaction. The data in Fig. 2 and 3 are also presented as normalized data, using the formula (x specific OD value/unadsorbed specific OD value) × 100% (Fig. 2) and the formula (x OD value/specific OD value) × 100% (Fig. 3) on each adsorption step (nonadsorbed, C-P adsorbed, and C-P and 22F polysaccharide adsorbed).
All statistical analyses were performed using the GraphPad Prism 5 software package.
RESULTS
Testing the specificity of ATCC antigens.
Figure 1 presents normalized data showing the specificity of the ATCC antigens. To evaluate the specificity of the antigens, rabbit pneumococcus-specific antisera (adsorbed with both C-Ps and 22F capsular polysaccharide) were used, and a cross-reaction board was set up using ATCC antigens (2.5 μg/ml). The seven ATCC antigens showed generally high specificities against the specific rabbit sera, although several of the ATCC antigens showed a high background response, especially toward rabbit serum specific for group 19 (Fig. 1).
Tests with serum pools B and P showed similar high percentages of nonspecific cross-reactions for ATCC antigens (data not shown).
Effect of adsorption with C-Ps and 22F polysaccharide, using rabbit antisera.
Figures 2 and 3 present data on ATCC antigens, showing the effect of adsorption with a combination of C-Ps and 22F polysaccharide. To evaluate the amounts of specific antibodies against C-Ps and 22F polysaccharide in the rabbit antisera, a cross-reaction board was performed using ATCC antigens (2.5 μg/ml) (Fig. 2). Comparison of the ATCC antigen response against the corresponding serotype-specific unadsorbed rabbit antiserum with the response against C-P- or C-P-plus-22F-adsorbed antiserum showed a relatively small effect. There were no significant differences (P > 0.05) between the positive responses of nonadsorbed, C-P-adsorbed, and C-P-plus-22F-adsorbed sera, except between nonadsorbed group 19 serum (antigen 19F) and C-P-plus-22F-adsorbed group 19 serum (antigen 19F), where a significant difference was found (P < 0.05) (Fig. 2). The effect of adsorption on nonspecific cross-reactions with the ATCC antigens showed a profound reduction with C-P adsorption (Fig. 3). Adding a further adsorption step including 22F polysaccharide, however, did not generally show a further decrease in the percentage of nonspecific cross-reactions, and in several cases, an increase in the percentage of nonspecific cross-reactions was observed (Fig. 3).
DISCUSSION
At present, reference laboratories generally use serological methods based on pneumococcal antigens from ATCC, as described in the WHO reference guideline (16).
Very few studies have performed tests on possible cross-reactions within pneumococcal serotypes when performing the WHO pneumococcal reference ELISA. One of the most recent studies to have addressed this problem is that of Soininen et al. (15). Due to the difficulty in obtaining human naïve serum without pneumococcal antibodies as well as human serum with antibodies against a single serotype, we decided to use commercial specific pneumococcal rabbit antisera. The rabbit sera provide well-described specific antisera not affected with unknown pneumococcal infections, thus making it possible to measure cross-reactions and the effect of adsorption with C-Ps and 22F polysaccharide, which to our knowledge has not previously been performed.
A previous study determined the specificity of the ATCC antigens in the WHO ELISA in comparison with an opsonophagocytic assay measuring opsonic antibody titers (1). Soininen et al. (15) performed a study in which cross-reactions were assessed, using inhibition of antipolysaccharide serum binding by homologous and heterologous polysaccharides. This, however, has the limitation of how similar the epitopes of the tested polysaccharides are (15), and it is an indirect measurement for estimating the specificity of an antigen.
By using rabbit antisera, the specificity of the antigens can be assessed and data extrapolated to human sera, although with the limitation of the unknown differences between rabbit and human sera. Figure 1 presents the specificities of ATCC antigens based on the seven antigens present in the protein-conjugated Prevenar vaccine (6). It was observed that the seven ATCC antigens showed specific responses against their corresponding rabbit sera and generally low cross-reactive responses against the six remaining rabbit sera (Fig. 1). There were, however, exceptions with regard to the specificity of the ATCC antigens, in particular when testing rabbit serum for group 19, where antigens showed cross-reactions between the rabbit serum and several of the type-specific antigens. Data from an identical test performed by another person showed similar results (data not presented). This observation was also described by Soininen et al. (15), who observed cross-reactivity, particularly with types 6B, 19F, and 23F, in sera from nonimmunized subjects, an observation also noted by Yu et al. (17). Furthermore, as previously described, ATCC antigen for serotype 14 showed a specific reaction in this study (Fig. 1), as observed in other studies (15, 17).
The antigens from ATCC (16) contain about 5% (by weight) unspecific C-Ps covalently linked to the serotype-specific polysaccharide (5, 12, 16). Studies have shown that adsorption increases the specificity of the antigens in cross-reaction tests by reducing the contents of the assumed nonspecific antibodies in the sera (7, 15). Wernette et al. (16) described C-P adsorption as improving the quantification of serotype-specific antibodies. However, cross-reactions still seem to occur, demonstrating the continuing presence of impurities. Concepcion and Frasch (1) showed that adsorption with a heterologous polysaccharide, such as 22F polysaccharide, further removed cross-reactions and contaminants in the sera. The effect of these adsorption steps on the specificity of a serotype-specific reaction, however, is based only on studies comparing the ELISA data with methods such as the opsonophagocytic assay, which estimates the “functional” phagocytic activity of human serum against S. pneumoniae isolates (1, 5). These comparisons do not show the effect of the adsorption steps on the ELISA by, e.g., nonspecific cross-reactions. This study provides data (Fig. 2 and 3) showing that adsorption with C-Ps improves the specificity of ATCC antigens in the ELISA. However, when adsorption was performed with 22F polysaccharide in addition to C-Ps (Fig. 3), it was observed that adsorption with 22F polysaccharide did not seem to further reduce the number of nonspecific cross-reactions concerning all seven serotypes, and in several cases, an increase in the number of nonspecific cross-reactions was observed. In the study by Concepcion and Frasch (1), the authors compared the ELISA data with results of the opsonophagocytic assay and found a better correlation, but they were not able to see the effect on nonspecific cross-reactions with other serotype-specific antigens.
In Fig. 2, an increase in OD values for group 23F is shown, and in repeating the test with a different lot and another operator at another time (data not presented), a similar difference was observed between nonadsorbed and C-P-adsorbed samples. In this test, however, the OD values of C-P-adsorbed and C-P-plus-22F-adsorbed samples were nearly identical. The reason that there seems to be an increase between nonadsorbed (23F) and adsorbed (23F) samples is not known. However, neither of the tests showed any significant differences in the OD values.
Based on the presented data, further studies on the effect of adsorption with 22F polysaccharide are probably needed. Furthermore, the study shows nonspecific reactions with the ATCC antigens which seem not to be removed completely by adsorption with C-Ps and 22F polysaccharide. Further purification of the ATCC antigens or the need for different pneumococcal antigens for improvement of the specificity might be needed in the future.
Acknowledgments
We thank Marian Jørgensen for editing the manuscript, and we thank Annie Kleis Nielsen, Suheil Nasim, Maja Hansen, and the Unit for Serology, Department of Bacteriology, Mycology and Parasitology, Statens Serum Institut, Denmark, for their helpful suggestions, advice, and support.
Footnotes
Published ahead of print on 8 July 2009.
REFERENCES
- 1.Concepcion, N. F., and C. E. Frasch. 2001. Pneumococcal type 22F polysaccharide absorption improves the specificity of a pneumococcal-polysaccharide enzyme-linked immunosorbent assay. Clin. Diagn. Lab. Immunol. 8:266-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Henrichsen, J. 1999. Typing of Streptococcus pneumoniae: past, present, and future. Am. J. Med. 107:50S-54S. [DOI] [PubMed] [Google Scholar]
- 3.Inostroza, J., S. Villanueva, K. Mason, L. E. Leiva, and R. U. Sorensen. 2005. Effects of absorption with pneumococcal type 22F polysaccharide on maternal, cord blood, and infant immunoglobulin G antipneumococcal polysaccharide antibodies. Clin. Diagn. Lab. Immunol. 12:722-726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaltoft, M. S., U. B. Sørensen, H.-C. Slotved, and H. B. Konradsen. 2008. An easy method for detection of nasopharyngeal carriage of multiple Streptococcus pneumoniae serotypes. J. Microbiol. Methods 75:540-544. [DOI] [PubMed] [Google Scholar]
- 5.Kolibab, K., S. L. Smithson, A. K. Shriner, S. Khuder, S. Romero-Steiner, G. M. Carlone, and M. A. J. Westerink. 2005. Immune response to pneumococcal polysaccharides 4 and 14 in elderly and young adults. I. Antibody concentrations, avidity and functional activity. Immun. Ageing 2:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Konradsen, H. B., and M. S. Kaltoft. 2002. Invasive pneumococcal infections in Denmark from 1995 to 1999: epidemiology, serotypes, and resistance. Clin. Diagn. Lab. Immunol. 9:358-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koskela, M. 1987. Serum antibodies to pneumococcal C polysaccharide in children: response to acute pneumococcal otitis media or to vaccination. Pediatr. Infect. Dis. 6:519-526. [DOI] [PubMed] [Google Scholar]
- 8.Lund, E., and J. Henrichsen. 1978. Laboratory diagnosis, serology and epidemiology of Streptococcus pneumoniae, p. 241-262. In T. Bergan and J. R. Norris (ed.), Methods in microbiology. Academic Press, London, United Kingdom.
- 9.Lynch, J. P., III, and G. G. Zhanel. 2009. Streptococcus pneumoniae: epidemiology, risk factors, and strategies for prevention. Semin. Respir. Crit. Care Med. 30:189-209. [DOI] [PubMed] [Google Scholar]
- 10.Marchese, R. D., N. T. Jain, J. Antonello, L. Mallette, K. L. Butterfield-Gerson, J. Raab, P. Burke, C. Schulman, H. Adgate, D. J. Sikkema, and N. Chirmule. 2006. Enzyme-linked immunosorbent assay for measuring antibodies to pneumococcal polysaccharides for the PNEUMOVAX 23 vaccine: assay operating characteristics and correlation to the WHO international assay. Clin. Vaccine Immunol. 13:905-912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park, I., D. G. Pritchard, R. Cartee, A. Brandao, M. C. C. Brandileone, and M. H. Nahm. 2007. Discovery of a new capsular serotype (6C) 1 within serogroup 6 of Streptococcus pneumoniae. J. Clin. Microbiol. 45:1225-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Skovsted, I. C., M. B. Kerrn, J. Sonne-Hansen, L. E. Sauer, A. K. Nielsen, H. B. Konradsen, B. O. Petersen, N. T. Nyberg, and J. Ø. Duus. 2007. Purification and structure characterization of the active component in the pneumococcal 22F polysaccharide capsule used for absorption in the pneumococcal enzyme-linked immunosorbent assay. Vaccine 25:6490-6500. [DOI] [PubMed] [Google Scholar]
- 13.Slotved, H. C., M. Kaltoft, I. C. Skovsted, M. B. Kerrn, and F. Espersen. 2004. Simple, rapid latex agglutination test for serotyping of pneumococci (pneumotest latex). J. Clin. Microbiol. 42:2518-2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Slotved, H. C., J. N. Jacobsen, H. K. Møller, and K. A. Krogfeldt. 2007. Developing an ELISA using serotype-specific antigens from selected pneumococcal strains of own selection. Vaccine 25:2513-2517. [DOI] [PubMed] [Google Scholar]
- 15.Soininen, A., G. Van den Dobbelsteen, L. Oomen, and H. Käythy. 2000. Are the enzyme immunoassays for antibodies to pneumococcal capsular polysaccharides serotype specific? Clin. Diagn. Lab. Immunol. 7:468-476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wernette, C. M., C. E. Frasch, D. Madore, G. Carlone, D. Goldblatt, B. Plikaytis, W. Benjamin, S. A. Quataert, S. Hildreth, D. J. Sikkema, H. Kayhty, I. Jonsdottir, and M. H. Nahm. 2003. Enzyme-linked immunosorbent assay for quantitation of human antibodies to pneumococcal polysaccharides. Clin. Diagn. Lab. Immunol. 10:514-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu, X., Y. Sun, C. Frasch, N. Conception, and M. H. Nahm. 1999. Pneumococcal capsular polysaccharide preparations may contain non-C-polysaccharide contaminants that are immunogenic. Clin. Diagn. Lab. Immunol. 6:519-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zartler, E. R., R. J. Porambo, C. L. Anderson, L. H. Chen, J. Yu, and M. H. Nahm. 2009. Structure of the capsular polysaccharide of pneumococcal serotype 11A reveals a novel acetylglycerol that is the structural basis for 11A subtypes. J. Biol. Chem. 13:7318-7329. [DOI] [PMC free article] [PubMed] [Google Scholar]