Abstract
For the diagnosis of vulvovaginal candidiasis, we developed a simple immunochromatographic method that enables the detection of vaginal Candida spp. within about 30 min. Overall, the sensitivity, specificity, positive predictive value, and negative predictive value of this method appeared to be 80.3, 99.3, 98.0, and 92.0%, respectively.
Vulvovaginitis is one of the most common infectious diseases in women's clinics. In the United States, 6 to10 million cases of gynecology clinic visits per year were estimated to be due to vaginitis (4, 7). The major etiologies of vaginitis are infection by bacteria, fungi, and trichomonads (10). Among vulvovaginitis cases caused by fungi, about 80 to 90% are due to infection with Candida albicans (8, 11).
In routine clinical practice, vulvovaginal infection is primarily diagnosed from the patient's complaints and clinical symptoms such as pruritus and increased vaginal discharge. Symptomatic vulvovaginitis may be readily diagnosed from microscopic examination of the vaginal discharge. However, this method can detect only 40 to 70% of Candida species compared with the culture method (5, 10) and requires experience and the use of expensive equipment. The culture method may be regarded as the standard; it requires skill, costly equipment, and several days to obtain a reliable result. Accordingly, a rapid and simple method to detect vulvovaginal Candida infection has been long awaited. We developed an immunochromatographic method that can detect vulvovaginal Candida infection within about 30 min.
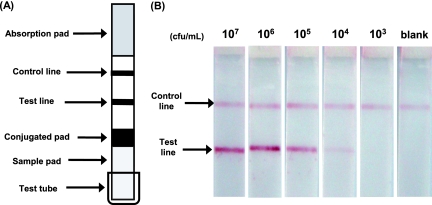
The immunochromatographic strip was prepared with anti-Candida mannan polyclonal antibody (anti-mannan immunoglobulin G [IgG]) and anti-rabbit IgG antibody serving as the test line and the control line, respectively (Fig. 1A; see the supplemental material). The colloidal gold-anti-mannan IgG conjugate was placed on a conjugated pad. The immunochromatography works as follows. A test strip (Fig. 1A) is kept standing in a small test tube containing 0.5 ml of the sample to be tested, and the chromatography is developed at ∼24°C for 20 min. The sample developed in the sample pad reaches the conjugated pad, and the Candida mannan in the solution reacts with the colloidal gold-anti-mannan IgG conjugate. This complex further migrates toward the distal end of the test strip (Fig. 1A). When this complex reaches the test line, immobilized anti-mannan IgG traps the complex and forms a sandwich-type immunocomplex. The product appears as a red line (Fig. 1A, test line). The colloidal gold-anti-mannan IgG conjugate that has not reacted with Candida mannan passes the test line and reaches the control line. The anti-rabbit IgG antibody immobilized at the control line traps the conjugate, forming a second red line (Fig. 1A, control line).
FIG. 1.
Immunochromatographic test strip. (A) Schematic representation of the strip. The nitrocellulose membrane strip consists of anti-mannan IgG and anti-rabbit IgG antibodies that are immobilized as a test line and a control line, respectively. The conjugated pad contains the colloidal gold-anti-mannan IgG conjugate. The proximal and distal ends of the nitrocellulose membrane are covered with the sample pad and the absorption pad, respectively. (B) Representative results. Suspensions of C. albicans ATCC 10231 at cell densities of 103 to 107 CFU/ml were heated in antigen extraction buffer and subjected to immunochromatography for 20 min. The sample with double red lines is Candida positive, and that with a single red line only at the control line is negative. Data for other Candida species are not shown. Only a portion of the strip is shown.
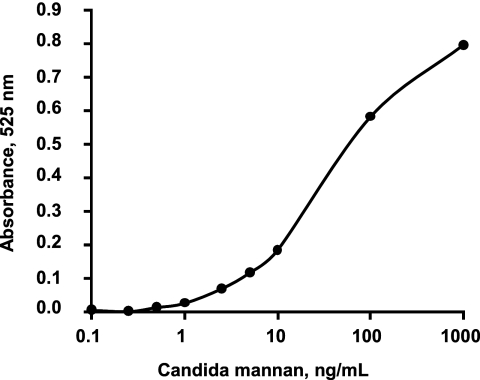
First we tested the sensitivity of immunochromatography with purified Candida mannan that was extracted from C. albicans ATCC 10231 and quantified as reported previously (3, 9). Extracts containing a Candida mannan level over the threshold of 0.5 ng/ml showed a positive reaction at the test line. The test line was scanned with a densitometer, and the absorbance was plotted against the amount of Candida mannan. The absorbance curve appeared as a pseudolinear line in a concentration-dependent manner (Fig. 2). Candida mannan at concentrations below about 0.5 ng/ml was undetectable macroscopically.
FIG. 2.
Correlation between the amount of Candida mannan and the intensity of the test line. Purified C. albicans mannan was serially diluted and then subjected to immunochromatography. The test line was traced by a densitometer at 525 nm. The absorbance was plotted against the amount of mannan quantified by the phenol sulfuric acid method with d(+)-mannose as the reference (3).
In the next experiment, the Candida suspension was adjusted to a cell density of 103 to 107 CFU/ml and Candida mannan was extracted by heating the cells in antigen extraction buffer (see the supplemental material). The extracts were subjected to immunochromatography. The results of these experiments revealed that the minimum cell densities detectable by this method were 104, 104, 104, 104, 105, and 105 CFU/ml of C. albicans, C. tropicalis, C. guilliermondii, C. parapsilosis, C. glabrata, and C. krusei, respectively (Fig. 1B [only results obtained with C. albicans are shown]). The amount of mannan extracted from 104 CFU/ml of C. albicans was equivalent to 3.2 ng/ml, as estimated from the above-described calibration curve. Therefore, the amount of mannan in 103 CFU/ml should be 0.32 ng/ml, which is below the detection limit of this test, yielding a negative result.
To test the specificity of this immunochromatographic method, bacterial cells at a density of 108 CFU/ml were heated and then tested as described above. None of the 28 bacterial species (Table 1) tested yielded a positive result. Similarly, eight fungal species listed in Table 1 were tested with extracts from 108 CFU/ml. Among these, only the extract from Saccharomyces cerevisiae gave positive results at cell densities over 105 CFU/ml but was negative at 104 CFU/ml. It was reported that anti-Candida antisera cross-reacted with S. cerevisiae (1).
TABLE 1.
Strains used in this study and their culture conditions
| Organism(s) | Culture conditions |
|---|---|
| Candida species: Candida albicans ATCC 10231, Candida tropicalis ATCC 750, Candida guilliermondii ATCC 6260, Candida parapsilosis ATCC 22019, Candida glabrata ATCC 2001, | |
| Candida krusei ATCC 6258 | Sabouraud dextrose agar, 35°C, 48 h |
| Other fungal species: Trichosporon cutaneum IFM40140, Trichosporon asahii IFM48575, Trichosporon montevideense IFM51966, Trichosporon mucoides IFM48611, Cryptococcus neoformans clinical isolate no. 365, Cryptococcus curvatus clinical isolate no. 334, | |
| Rhodotorula mucilaginosa IFM48529, Saccharomyces cerevisiae IFM40022 | Sabouraud dextrose agar, 35°C, 48 h |
| Bacterial species | |
| Staphylococcus aureus FDA209P, Staphylococcus epidermidis clinical isolate no. 1, Micrococcus luteus ATCC 9341, Enterococcus faecalis ATCC 29212, Enterococcus faecium NCTC12204, Escherichia coli NIHJ JC-2, Citrobacter freundii ATCC 8090, Klebsiella pneumoniae NCTN9632, Klebsiella oxytoca clinical isolate no. 1, Proteus mirabilis IFO3849, Proteus vulgaris OX-19, Morganella morganii IIDKono, Serratia marcescens IFO12648, Enterobacter cloacae IFO13535, Enterobacter aerogenes NCTC10006, Pseudomonas aeruginosa E-2, | |
| Acinetobacter calcoaceticus IFO12552 | Mueller-Hinton agar, 35°C, 24 h |
| Listeria monocytogenes clinical isolate no. 1, Corynebacterium sp. clinical isolate no. 1 | Trypticase soy agar with 5% sheep blood, 35°C, 24 h |
| Streptococcus pyogenes GTC262, Streptococcus agalactiae GTC1234 | Trypticase soy agar with 5% sheep blood, 35°C, 24 h, 5% CO2 |
| Neisseria gonorrhoeae clinical isolate no. 1 | Thayer-Martin selective agar, 35°C, 24 h, 5% CO2 |
| Bacteroides fragilis clinical isolate no. 4 | Gifu anaerobic medium agar, 35°C, 2 days, anaerobically |
| Lactobacillus casei ATCC 393, Lactobacillus gasseri JCM1017, Lactobacillus crispatus JCM1030, | |
| Lactobacillus acidophilus JCM1132 | de Man, Rogosa, and Sharpe agar, 35°C, 2 days, anaerobically |
| Chlamydia trachomatis serovar D/UW-3/Cx | Propagation by McCoy cell culture (6) |
To test the reliability of this immunochromatographic test, a total of 200 clinical vaginal swabs were examined and the results were compared with those obtained by the culture method. Of these 200 swabs, 50 were immunochromatography positive; of these 50, 49 were culture positive and 1 was culture negative. The culture method detected Candida in swabs from 61 subjects, of which 49 were immunochromatography positive and 12 were negative. Of the 200 subjects, 138 were negative by both immunochromatography and the culture method. Therefore, the total numbers of negative results obtained by immunochromatography and the culture method were 150 and 139, respectively. Immunochromatography yielded false-negative results with 12 samples whose viable Candida cell levels were below the limit of detection by the immunochromatography method. One sample showed a very faint line that was barely detectable macroscopically and was regarded as a false-positive result. The reason for this false-positive result is not known. Compared with the results of the culture method, the sensitivity, specificity, positive predictive value, and negative predictive value of the immunochromatography method were calculated to be 80.3 (49/61), 99.3 (138/139), 98.0 (49/50), and 92.0% (138/150), respectively.
To the best of our knowledge, there has been only one report on an immunochromatographic test for vaginal Candida diagnosis (2). Unfortunately, that paper reported only on the testing of clinical subjects without presenting basic study data. The sensitivity, specificity, positive predictive value, and negative predictive value of our method are superior to the values obtained by that method.
Newly developed immunochromatography can detect as few as 104 CFU/ml of Candida spp. in about 30 min without using expensive equipment or skill. Therefore, the method can be used even in small clinics, as well as large laboratories.
Supplementary Material
Acknowledgments
This work was supported by grants-in-aid from the Food Safety Commission Japan.
Footnotes
Published ahead of print on 5 August 2009.
Supplemental material for this article may be found at http://cvi.asm.org/.
REFERENCES
- 1.Ataoglu, H., J. Zueco, and R. Sentandreu. 1993. Characterization of epitopes recognized by Candida factor 1 and 9 antisera by use of Saccharomyces cerevisiae mnn mutants. Infect. Immun. 61:3313-3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chatwani, A. J., R. Mehta, S. Hassan, S. Rahimi, S. Jeronis, and V. Dandolu. 2007. Rapid testing for vaginal yeast detection: a prospective study. Am. J. Obstet. Gynecol. 169:309.e1-309.e4. [DOI] [PubMed] [Google Scholar]
- 3.Dubois, M., K. A. Gilles, J. K. Hamilton, P. A. Rebers, and F. Smith. 1956. Colorimetric method for detection of sugars and related substances. Anal. Chem. 28:350-356. [Google Scholar]
- 4.Eckert, L. O. 2006. Acute vulvovaginitis. N. Engl. J. Med. 355:1244-1252. [DOI] [PubMed] [Google Scholar]
- 5.Eckert, L. O., S. E. Hawes, C. E. Stevens, L. A. Koutsky, D. A. Eschenbach, and K. K. Holmes. 1998. Vulvovaginal candidiasis: clinical manifestations, risk factors, management algorithm. Obstet. Gynecol. 92:757-765. [DOI] [PubMed] [Google Scholar]
- 6.Ikeda-Dantsuji, Y., I. Konomi, and A. Nagayama. 2005. In vitro assessment of the APTIMA Combo 2 assay for the detection of Chlamydia trachomatis using highly purified elementary bodies. J. Med. Microbiol. 54:357-360. [DOI] [PubMed] [Google Scholar]
- 7.Kent, H. L. 1991. Epidemiology of vaginitis. Am. J. Obstet. Gynecol. 165:1168-1176. [DOI] [PubMed] [Google Scholar]
- 8.Linhares, I. M., S. S. Witkin, S. D. Miranda, A. M. Fonseca, J. A. Pinotti, and W. J. Ledger. 2001. Differentiation between women with vulvovaginal symptoms who are positive or negative for Candida species by culture. Infect. Dis. Obstet. Gynecol. 9:221-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reiss, E., L. de Repentigny, R. J. Kuykendall, A. W. Carter, R. Galindo, P. Auger, S. L. Bragg, and L. Kaufman. 1986. Monoclonal antibodies against Candida tropicalis mannan: antigen detection by enzyme immunoassay and immunofluorescence. J. Clin. Microbiol. 24:796-802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sobel, J. D. 1997. Vaginitis. N. Engl. J. Med. 337:1896-1903. [DOI] [PubMed] [Google Scholar]
- 11.Vermitsky, J. P., M. J. Self, S. G. Chadwick, J. P. Trama, M. E. Adelson, E. Mordechai, and S. E. Gygax. 2008. Survey of vaginal-flora Candida species isolates from women of different age groups by use of species-specific PCR detection. J. Clin. Microbiol. 46:1501-1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.