Abstract
The immunogenicity and protective efficacy of a recombinant subunit West Nile virus (WNV) vaccine was evaluated in rhesus macaques (Macaca mulatta). The vaccine consisted of a recombinant envelope (E) protein truncated at the C-terminal end, resulting in a polypeptide containing 80% of the N-terminal amino acids of the native WNV protein (WN-80E), mixed with an adjuvant (GPI-0100). WN-80E was produced in a Drosophila melanogaster expression system with high yield and purified by immunoaffinity chromatography using a monoclonal antibody specific for flavivirus E proteins. Groups of monkeys were vaccinated with formulations containing 1 or 25 μg of WN-80E antigen, and both humoral and cellular immunity were assessed after vaccination. The results demonstrated potent antibody responses to vaccination, as determined by both enzyme-linked immunosorbent assay and virus-neutralizing antibody assays. All vaccinated animals responded favorably, and there was little difference in response between animals immunized with 1 or 25 μg of WN-80E. Cellular immunity was determined by lymphocyte proliferation and cytokine production assays using peripheral blood mononuclear cells from vaccinated animals stimulated in vitro with WN-80E. Cell-mediated immune responses varied from animal to animal within each group. About half of the animals responded with lymphoproliferation, cytokine production, or both. Again, there was little difference in response between animals immunized with a 1- or 25-μg dose of WN-80E in the vaccine formulations. In a separate experiment, groups of monkeys were immunized with the WN-80E/GPI-0100 vaccine or an adjuvant-only control formulation. Animals were then challenged by inoculation of wild-type WNV, and the level of viremia in each animal was monitored daily for 10 days. The results showed that whereas all animals in the control group had detectable viremia for at least 3 days after challenge, all of the vaccinated animals were negative on all days after challenge. Thus, the WN-80E vaccine was 100% efficacious in protecting monkeys against infection with WNV.
West Nile virus (WNV) was first detected in North America in 1999 and spread rapidly across the continental United States (3, 32), as well as into Canada (8), Mexico (9), and Central and South America (17). The virus is transmitted via mosquitoes, primarily through the bite of Culex species but also by many other genera of mosquitoes (14). Birds are the natural hosts and serve as the zoonotic reservoir, while mammals and reptiles are considered to be incidental hosts from which, it is believed, further transmission generally does not occur (12). This is thought to be due to the relatively low levels of viremia that develop in these latter hosts, which may be insufficient to allow for secondary mosquito transmission (11). However, more recent studies (2, 38) have suggested that, in some mammals and reptiles, sufficient viremia may develop to yield at least a low competence for transmission.
Based on retrospective seroepidemiological surveys conducted after the initial discovery of this virus in North America, it was determined that about 20% of those individuals infected developed clinical disease (3). The large majority of clinical cases resulted in a self-limiting, influenza-like syndrome (3); however, about 1 in 150 infected patients developed neurological complications (28). These complications included cases of meningitis; encephalitis; meningoencephalitis; and an acute, flaccid paralytic, poliomyelitis-like syndrome (13). The cases with WNV-associated neurological complications tend to be severe, often resulting in permanent disabilities, with reported case fatality rates of 5 to 15% (3). However, in a recent study (4), it was reported that even in those cases of mild, nonneuropathological disease, after resolution of the infection had apparently occurred, residual defects in neuromotor and cognitive function could be measured for at least 1 year after the original diagnosis.
Moreover, the disease course tends to be much more severe in elderly individuals, with significantly higher case fatality rates of about 30% in neuroinvasive cases (5, 30, 33, 42). This may be due to declining immunocompetence concomitant with aging (“immunosenescence”). In addition to the elderly population, individuals whose immune systems have been compromised through primary immune deficiencies, acquired deficiencies, or immunosuppressive therapies are also at increased risk of severe disease caused by WNV infection (10, 18). Certain other chronic diseases, such as diabetes mellitus and hypertension, may also render individuals infected with WNV more susceptible to developing severe disease (16).
WNV is a member of the Flaviviridae family, genus Flavivirus. It is an enveloped, positive-strand RNA virus. The RNA genome comprises 10 genes, coding for three structural and seven nonstructural proteins (31). The structural proteins are the core or capsid protein (C); a premembrane protein (prM), which is cleaved to yield the membrane protein in the mature virion; and the envelope protein (E). The latter two are glycosylated. The E protein shares significant homology with the E proteins of other flaviviruses, particularly those of the other members of the Japanese encephalitis virus (JEV) serocomplex, JEV itself, St. Louis encephalitis virus (SLEV), and Murray Valley encephalitis virus. Antibodies directed against particular epitopes contained within the E protein are capable of virus neutralization. These epitopes have recently been mapped to at least two of three domains of the E protein, domains II and III, using sets of monoclonal antibodies for dengue virus (DENV) (6), as well as JEV (20) and WNV (1, 41). Neutralizing antibodies reacting with domain III are generally specific for each virus and do not cross-neutralize other viruses (or other serotypes of the same virus if multiple serotypes exist), while those targeting domain II are often cross-reactive. A high titer of virus-neutralizing antibodies is generally accepted as the best in vitro correlate of in vivo protection against virus infection or immunity to subsequent infection (23, 45). For this reason, the E protein was selected as the appropriate immunogen for use in the development of a WNV vaccine candidate.
In previous studies at Hawaii Biotech, Inc. (HBI), a proprietary method of expression was used successfully to produce recombinant E proteins from flaviviruses, such as DENV serotypes 1 to 4, JEV, hepatitis C virus, and WNV (7, 19, 25, 26, 35). These proteins are truncated at the C terminus, leaving 80% of the native E protein (80E). The truncation deletes the membrane anchor portion of the protein, thus allowing it to be secreted into the extracellular medium, facilitating recovery. Furthermore, the expressed DENV and WNV proteins have been shown to be properly glycosylated and to maintain native conformation as determined by reactivity with conformationally sensitive monoclonal antibodies 4G2 and 9D12 (B. -A. Coller, D. E. Clements, and G. S. Bignami, unpublished data) and X-ray crystallography structure determination (25, 26). The immunogenicity of the vaccine formulations using the truncated WNV E protein (WN-80E [amino acids 1 to 401]) was demonstrated in mice (19), and its protective efficacy documented in hamsters (39, 43) and geese (15). The present report for the first time documents the immunogenicity and protective efficacy of a WN-80E vaccine formulation in a nonhuman primate animal model.
MATERIALS AND METHODS
Animals.
Rhesus monkeys (Macaca mulatta) of either sex weighing 3 to 7 kg were used. Animals were screened by serology for exposure to herpes B virus, simian immunodeficiency virus, WNV, DENV (all serotypes), yellow fever virus, SLEV, and JEV. Only animals that were negative for the aforementioned viruses were used in this study. Animals were housed and maintained at the Southwest National Primate Research Center/Southwest Foundation for Biomedical Research (SNPRC/SFBR), San Antonio, TX, according to established principles of animal welfare under a protocol approved by the Institutional Animal Care and Use Committee.
Vaccines.
The vaccines used were comprised of recombinant E protein from WNV (NY 99 isolate) formulated with an adjuvant (GPI-0100). WN-80E was produced in a Drosophila melanogaster expression system and purified from transformed cell culture supernatants by immunoaffinity chromatography using a flavivirus group-specific monoclonal antibody. The preparation; purification; properties; and immunogenicity in mice, hamsters, and geese of the WN-80E protein have been previously described (15, 19, 39, 43). A “mock” antigen was also prepared for use as a control in antigen stimulation experiments. This material was prepared by subjecting culture supernatants from induced Drosophila cells transformed with plasmids lacking the genes encoding the specific antigens to the same purification scheme used for the WN-80E protein. The purpose of including this material with adjuvant was to control for any possible nonspecific immunostimulatory effects of potential contaminants from the cell cultures copurified with the antigens. GPI-0100 (HBI, Aiea, HI) is a semisynthetic saponin previously shown to enhance the immunogenicity of soluble protein antigens (22, 36). Vaccines were formulated at HBI and shipped to SFBR immediately prior to administration to the animals.
Vaccination and immunogenicity.
Twenty animals were divided into five groups of four animals in each group and administered vaccines formulated to contain 1 or 25 μg of WN-80E per dose and 0.5 or 2.0 mg of GPI-0100 adjuvant per dose, along with a group that was administered 25 μg of WN-80E without adjuvant (Table 1). Immunization was performed by the intramuscular route in the deltoid muscle with a 0.5-ml dose. Animals were given four doses of vaccine at intervals of approximately 3 weeks, and blood was collected pre- and postvaccination for analysis of the immune response. The vaccination regimen chosen was based on the results of previous studies of rhesus monkeys with a recombinant dengue vaccine (35; B.-A. Coller, M. M. Lieberman, and J. R. Putnak, unpublished data).
TABLE 1.
Experimental design for testing immunogenicity of WN-80E vaccine in rhesus monkeysa
| Group | WN-80E dose (μg) | Adjuvant dose (mg) |
|---|---|---|
| 1 | 1 | 2 |
| 2 | 1 | 0.5 |
| 3 | 25 | 2 |
| 4 | 25 | 0.5 |
| 5 | 25 | 0 |
Each group had four animals. There were four doses of vaccine, administered on days 0, 20, 41, and 59. Blood for serology was drawn prior to vaccination on the same days, as well as on day 77. Blood for CMI assays was drawn on days 0 and 77.
Challenge experiment.
The WNV challenge experiment was conducted within the animal biosafety level 3 (ABSL-3) facility at the SNPRC. Nine additional animals were divided into two groups of five and four animals in each group (Table 2) and administered either 5 μg of WN-80E plus 2.0 mg of GPI-0100 (group 1) or 2.0 mg of GPI-0100 without antigen (group 2). Three doses of vaccine were given at 3-week intervals. Animals were then rested for 6 months prior to the administration of a fourth dose of vaccine, followed by challenge (infection) with 2 × 105 PFU of the NY99 strain of WNV (37) by the subcutaneous route 2 months later. Animals were bled daily for 10 days after infection, and the level of viremia in each animal was determined by direct plaque assays on Vero cells as described below. The challenge dose was chosen based on results from a preliminary experiment which showed that challenge with 2 × 105 or 106 PFU yielded no significant difference in viremia (data not shown). These latter doses were selected based on similar doses used in previously published studies of WNV pathogenesis in nonhuman primates (37).
TABLE 2.
Experimental design for testing protective efficacy of WN-80E vaccine in rhesus monkeysa
| Group | No. of animals | WN-80E dose (μg) | Adjuvant dose (mg) |
|---|---|---|---|
| 1 | 5 | 5 | 2 |
| 2 | 4 | 0 | 2 |
Four doses of vaccine were administered, on days 0, 21, 42, and 230. Animals were challenged on day 290. One animal from group 1 was euthanized after completion of the vaccination schedule but prior to challenge due to chronic severe anemia of an unknown origin that was nonresponsive to nutritional supplementation, as well as abnormal behaviors.
WNV plaque assay.
Viremia in serum samples was determined by direct plaque assay on Vero cells. Vero cells were prepared at a density of 6 × 105 cells/well in M-199 growth medium in six-well plates. Plates were incubated at 37°C in 5% CO2 for 3 days to reach 90% confluence for the plaque assay. Serum samples were diluted 1:10 and 1:100 in M-199 growth medium, and 100 μl of undiluted serum and each dilution of serum were added onto Vero cells in triplicate and incubated for 1 h at 37°C. After incubation, the cells were overlaid with 3 ml of M-199 maintenance medium (M-199 with 2% fetal bovine serum) containing 1% agarose. For visualization of plaques, each culture was overlaid at 3 days postinfection with M-199 maintenance medium containing neutral red (0.004% final concentration). Plaques were counted at 5 days postinfection, and titers are expressed in PFU/ml.
Antibody assays.
Blood specimens were collected in clot tubes immediately prior to each vaccination (days 0, 20, 41, and 59) and after dose 4 (day 77). Serum was separated, frozen, and shipped to HBI or to the John A. Burns School of Medicine, University of Hawaii (JABSOM, UH), Honolulu, HI, for further analysis.
Enzyme-linked immunosorbent assays (ELISAs) were performed as described previously (19), using an anti-rhesus monkey immunoglobulin-specific secondary antibody. Briefly, 96-well microtiter plates were coated with WN-80E (15 μg/ml in phosphate-buffered saline [PBS], pH 7.2, 75 μl/well at 4°C overnight), blocked with 1% bovine serum albumin (BSA) in PBS, washed with PBS containing 0.05% Tween 20 (PBST), and incubated for 2 h at room temperature with monkey serum samples diluted in PBST containing 0.1% BSA (PBST-BSA). After incubation, wells were washed and incubated with horseradish peroxidase-conjugated anti-rhesus monkey immunoglobulin (Serotec Labs) diluted in PBST/BSA for 1 h at room temperature. Wells were then washed and incubated with 100 μl/well peroxidase substrate (o-phenylenediamine dihydrochloride; Sigma Chemical Co.) for 30 min. Reactions were stopped by the addition of 50 μl of 2 N H2SO4 and read at 450 nm in an automated ELISA plate reader. ELISA titers were reported as the serum dilution yielding 50% maximum absorbance in the assay using a sigmoidal dose response (variable slope) model, with the aid of a commercially available statistical program (GraphPad Prism).
Virus-neutralizing assays (plaque reduction neutralization tests [PRNT]) were conducted as described previously (40, 44) using wild-type WNV (Egypt 101 strain) or, alternatively, chimeric DENV serotype 4-WNV (34) and immunofocus visualization of plaques, using standard laboratory protocols. The chimeric-virus assay was developed as a surrogate assay which can be conducted in a BSL-2 laboratory rather than a BSL-3 laboratory. For the wild-type WNV assay, serum dilutions were incubated with ∼50 PFU of virus at 4°C for 16 h and then serum-virus mixtures were plated on Vero cells in six-well cell culture plates, incubated for 1 h at 37°C, overlaid with agarose, and further incubated for 4 to 5 days. Plaques were visualized after 5 days of growth at 35°C by using neutral red staining. The percent reduction in plaque numbers was calculated compared to the growth of the virus control, and the highest dilution of serum resulting in 90% reduction in the number of plaques compared to the growth of the virus control (PRNT90 titer) was determined.
For the chimeric-virus assay, serum dilutions were incubated with ∼50 PFU of virus at 37°C for 30 min. Serum-virus mixtures were plated on Vero cells in six-well cell culture plates, incubated for 1 h at 37°C, and overlaid with carboxymethyl cellulose. Infective foci were revealed after formalin fixation and immunostaining. The dilution of serum resulting in 50% reduction in the number of plaques compared to the growth of the virus control was calculated using GraphPad Prism sigmoidal dose response, variable slope (four-parameter logistic equation equivalent) curve fit and defined as the PRNT50 titer.
Cell-mediated immune (CMI) response assays.
Heparin-anticoagulated whole-blood specimens (20 ml) were collected from each animal prior to vaccination (day 0) and after dose 4 (day 77) and transported to HBI or to JABSOM, UH, for processing and performance of assays. Whole blood was centrifuged at 1,000 × g for 10 min, and the buffy coats (white blood cell interphase between the erythrocytes and plasma/platelets) were collected. Residual erythrocytes were lysed with an ammonium chloride-based lysis solution (0.15 M NH4Cl, 0.1 mM EDTA, 10 mM NaHCO3, pH 7.3), washed two times with culture medium (RPMI 1640 supplemented with 10% fetal bovine serum, 2 mM l-glutamine, 100 U of penicillin/ml, 100 μg of streptomycin/ml, and 50 μg of gentamicin/ml), and resuspended in culture medium.
Lymphocyte proliferation assays were performed using peripheral blood mononuclear cells (PBMC) from vaccinated monkeys. Cell suspensions were counted and adjusted to 2 × 106 PBMC/ml, and 100-μl aliquots of the suspensions were added to wells of a 96-well cell culture plate. Equal volumes of antigen (WN-80E, 10 μg/ml), “mock” antigen (equivalent to 10 μg/ml), mitogen (concanavalin A, 20 μg/ml), or culture medium were added to each well. Plates were incubated at 37°C and 5% CO2 in a humidified incubator for 3 days (with mitogen stimulation) or 7 days (with antigen stimulation). One microcurie of tritiated thymidine ([3H]methyl thymidine, 50 Ci/mmol; ICN Biomedicals) was then added to each well, and incubation continued for another 18 to 24 h. Plates were then harvested using a Filtermate 196 cell harvester system (PerkinElmer Corp.), and the amount of radioactivity incorporated was determined with a TopCount microplate scintillation system (PerkinElmer Corp.). Separate unstimulated control cultures were included for the mitogen-stimulated and antigen-stimulated assays.
Cytokine production assays were also performed using PBMC from vaccinated monkeys. Cell suspensions were counted and adjusted to 107 PBMC/ml, and aliquots (0.5 ml) added to wells of 24- or 48-well cell culture plates. Equal volumes of antigen (WN-80E, 10 μg/ml), “mock” antigen (equivalent to 10 μg/ml), mitogen (pokeweed mitogen, 10 μg/ml), or culture medium were added to each well. Plates were incubated at 37°C and 5% CO2 in a humidified incubator for 5 days, and then culture supernatants were harvested and frozen for later analysis. Gamma interferon (IFN-γ), tumor necrosis factor-α, interleukin-2 (IL-2), IL-4, IL-5, and IL-6 cytokine levels in culture supernatants were quantified simultaneously using a multiplexed flow cytometric bead array assay (Becton Dickinson Corp.) as directed by the manufacturer.
Statistical analysis.
Tests of significance (t tests), calculations of geometric mean titers, 95% confidence intervals, standard deviations, and correlation coefficients (r2) were performed using GraphPad Prism statistical programs.
RESULTS
Immunogenicity of WN-80E vaccine in rhesus monkeys.
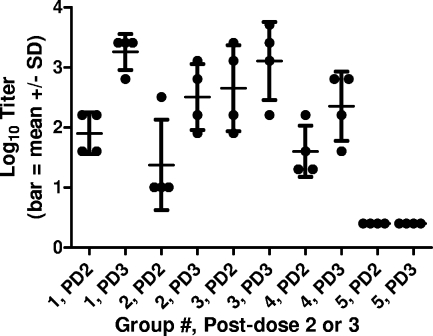
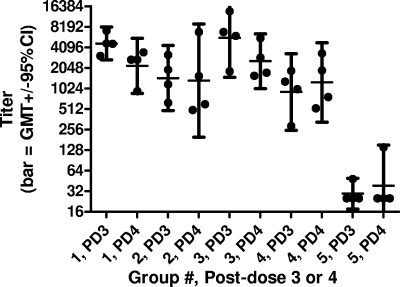
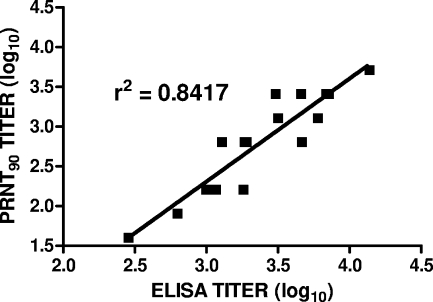
PRNT and ELISA antibody assays were performed on groups of vaccinated animals (Table 1). The results of PRNT antibody assays (using wild-type virus) after two and three doses of vaccine are shown in Fig. 1. Antibody titers increased with the number of vaccine doses given up through dose 3 (groups 1 to 4). The titers in sera collected prior to immunization (day 0) and after one immunization (day 20) were undetectable in all animals (data not shown). All group 5 monkeys had titers of <5 after the second and third immunizations (depicted as log10 0.4 in the figure), with P values of <0.05 (unpaired t test) for pair-wise comparisons of groups 1 to 4 with group 5 at each time point. There was little or no difference in titers (P > 0.1; unpaired t test) between groups administered 1 or 25 μg of WN-80E at the same dose of adjuvant (0.5 or 2.0 mg of GPI-0100) except after dose 2 with 2.0 mg of adjuvant, where a small increase in titer was observed with the higher dose of WN-80E compared to the titer with the lower dose. However, antibody titers were higher in the groups of animals receiving 2.0 mg of GPI-0100 than in the groups receiving 0.5 mg. The results of PRNTs performed with the chimeric virus were consistent with the results from assays with wild-type virus (data not shown). The results of ELISAs performed after three and four doses of vaccine demonstrate that no further increase in antibody titers occurs after three doses of vaccine (Fig. 2). All group 5 animals had titers of <100 at both time points except for one animal after dose 4. There were good correlations between the results of PRNTs performed with either virus (data not shown) and between the results of PRNTs and ELISAs (Fig. 3).
FIG. 1.
PRNT90 antibody titers (log10) in monkey sera collected after two and three doses of vaccine (days 41 and 59, respectively; see footnote a to Table 1). All group 5 monkeys had titers of <5 at both time points (depicted as log10 0.4 in the figure). SD, standard deviation.
FIG. 2.
ELISA antibody titers in monkey sera collected after three and four doses of vaccine (days 59 and 77; see footnote a to Table 1). Titers of <50 are depicted as 25 (group 5). GMT, geometric mean titer; CI, confidence interval.
FIG. 3.
Correlation of ELISA and PRNT antibody titers in monkey sera. Sera were obtained from animals in groups 1 through 4 after three vaccinations (day 59; see footnote a to Table 1).
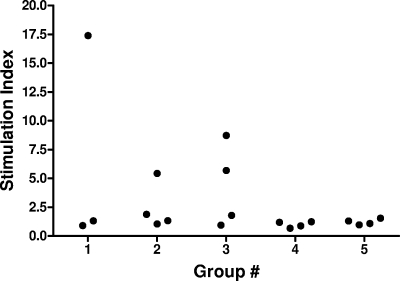
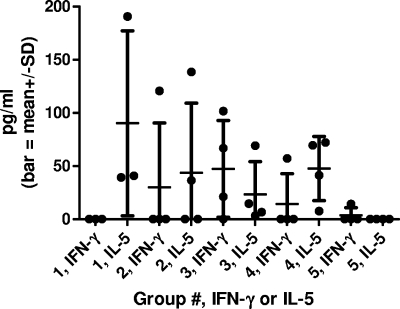
CMI responses were evaluated after four doses of vaccine and were found to vary between animals within each group. In groups 1 to 4, about half of the animals responded with either positive antigen-stimulated lymphocyte proliferation (Fig. 4) or antigen-stimulated cytokine production (Fig. 5). All group 5 animals, however, were negative for either measure of cellular immunity, with the exception of a small amount of IFN-γ produced by one monkey. Among animals in groups 1 to 4, the production of various cytokines, including IFN-γ, tumor necrosis factor-α, IL-4, IL-5, and IL-6, was also detected (data not shown). Vaccination with increased antigen (1 or 25 μg) and/or adjuvant (0.5 or 2.0 mg) did not appear to increase the cellular immune responses observed.
FIG. 4.
Antigen-stimulated lymphocyte proliferation after four doses of vaccine. Only three monkeys from group 1 were tested, as one animal was euthanized prior to sample collection due to unrelated pathology (suppurative synovitis of left knee, weight loss, and depression). Results were obtained from 2 × 105 PBMC/0.2 ml in 96-well cell culture plates, with antigen (WN-80E) at 5 μg/ml. “Mock” antigen stimulation indices were all <2, while mitogen (concanavalin A) stimulation indices were all within the range of 28 to 72 (data not shown).
FIG. 5.
Antigen-stimulated cytokine production in vitro after four doses of vaccine. Only three monkeys from group 1 were tested, as one animal was euthanized prior to sample collection due to unrelated pathology (see legend to Fig. 4). Results were obtained from 5 × 106 PBMC/ml in 24- or 48-well cell culture plates, with antigen (WN-80E) at 5 μg/ml. Values represent the difference between cytokine levels for WN-80E-stimulated animals and either “mock” antigen-stimulated or unstimulated controls, whichever was higher. For IFN-γ, this difference was at least 2.5-fold for all but one positive animal. For IL-5, this difference was at least threefold for each positive animal. Pokeweed mitogen-stimulated IFN-γ production was at least 2,000 pg/ml for all animals (data not shown). Pokeweed mitogen-stimulated IL-5 production was ≤20 pg/ml (data not shown). SD, standard deviation.
Protective efficacy of WN-80E vaccine in rhesus monkeys.
Protective efficacy was determined by prevention of viremia after WNV infection of vaccinated animals in a second experiment (Table 2). Antibody titers were determined after each vaccination, and peak antibody titers were observed 28 days after dose 3, with PRNT90 titers in the range of 320 to 1,280 for group 1 animals. Titers determined in sera collected from these monkeys 140 days after the third vaccination (about 1 month prior to the fourth vaccination and about 3 months prior to challenge) were in the range of 80 to 160 for all five animals, whereas monkeys receiving adjuvant alone (group 2) had no detectable antibody titers at any time. Lymphocyte proliferation responses were also observed in two of the vaccinated animals (stimulation indices, 7.5 and 5.6) but not in any of the animals receiving adjuvant alone (stimulation indices, <1.7) after dose 3.
The results of daily viremia determinations after infection of WN-80E- and control-vaccinated animals with wild-type WNV are given in Table 3 and demonstrate that while all control animals had detectable viremia on multiple days postchallenge, all vaccinated animals were negative for viremia on all days postchallenge. Thus, the WN-80E vaccine protected these animals against challenge with wild-type WNV.
TABLE 3.
Levels of viremia postchallenge in vaccinated and control rhesus monkeys
| Groupa | Monkey | Level of viremiab at indicated day postinfection
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | ||
| 1 | 28824 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 1 | 28828 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 1 | 28829 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 1 | 28831 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 2 | 28823 | 0.00 | 0.00 | 0.00 | 0.82 | 1.00 | 1.00 | 1.72 | 0.48 | 0.00 | 0.00 |
| 2 | 28825 | 1.54 | 0.00 | 1.60 | 1.72 | 1.48 | 1.48 | 0.00 | 0.00 | 0.00 | 0.00 |
| 2 | 28826 | 0.00 | 0.00 | 1.00 | 1.30 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 2 | 28830 | 1.40 | 0.70 | 1.12 | 1.70 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
One animal from group 1 was euthanized after completion of the vaccination schedule but prior to challenge due to chronic severe anemia of an unknown origin that was nonresponsive to nutritional supplementation, as well as abnormal behavior. Thus, only four animals from group 1 were challenged.
Viremia is expressed as log10PFU/ml of serum. The minimum detectable level was 0.48 log10PFU/ml.
DISCUSSION
The results reported herein describe the immunogenicity and protective efficacy of the WN-80E vaccine in nonhuman primates. The vaccine was shown to elicit a good humoral immune response, yielding high titers of antibodies as determined by an antigen-binding assay (ELISA), as well as a functional virus-neutralizing assay (PRNT). Virus-neutralizing antibodies are generally accepted as the best correlate of protective immunity to flavivirus infection (23, 45). Maximal antibody titers were raised after three immunizations, although good titers were observed after the second immunization. Thus, a vaccination regimen of only two immunizations could be adequate for effective protection. All animals in groups immunized with a vaccine formulated with adjuvant responded well, with high antibody titers.
The CMI response was found to vary from animal to animal, with some monkeys responding to vaccination by exhibiting good antigen-stimulated lymphocyte proliferation and/or cytokine production in vitro while others did not. The reason for this variability is not clear, but similar results have been observed with monkeys immunized with a DENV recombinant subunit vaccine (Coller et al., unpublished data). The monkeys used for these studies varied considerably in age, having been selected based on a weight range and seronegativity for a panel of viruses as described above. It may be possible that the CMI responses of older animals are less vigorous than those of younger animals. Previous data from studies of mice have demonstrated that aged mice do not yield good lymphocyte proliferative responses after vaccination (M. M. Lieberman and T. A. Wong, unpublished data). Nevertheless, aged animals have been shown to be completely protected against a lethal challenge with WNV in a hamster model of WN encephalitis (39).
In nonhuman primates, infection with WNV (and other flaviviruses) has generally not been reported to cause clinical disease as it does in humans (35, 37), although in one recent study (2), one of five experimentally infected rhesus monkeys developed neurologic disease. There has also been a reported case of naturally acquired WN encephalitis in a Barbary macaque housed in a zoo (29). However, regardless of clinical presentation, a low level of viremia develops postinfection, which can be monitored by serial blood collections. The prevention or inhibition of the development of viremia by prior vaccination is considered to be a demonstration of the protective efficacy of the vaccine. In a second experiment with rhesus monkeys, vaccinated and control animals were challenged with live WNV and monitored for viremia. The results showed that there was no detectable viremia in any of the vaccinated animals on any day postchallenge, while all control animals had multiple days of detectable viremia. Thus, the vaccine demonstrated protective efficacy in this nonhuman primate model of WNV infection.
These results extend and complement previously published data in which the preparation, properties, immunogenicity in mice (19), and protective efficacy in hamsters (39, 43) and geese (15) of different WN-80E vaccine formulations were described. The durability of immunization in hamsters was documented for at least 1 year postvaccination (43). The hamster model of lethal encephalitis has also been used to demonstrate protection in immunocompromised (leukopenic) animals, as well as young (weanling) and old (12 months old at initial vaccination) animals (39). These models target the particular populations of patients at highest risk of serious complications from WNV infection. This is particularly relevant in the case of elderly individuals, whose case fatality rate is double that of younger individuals (5, 33, 42). Other candidate WNV vaccines are based on a live virus (21, 24, 27, 34), which may be contraindicated in elderly (immunosenescent) and immunocompromised individuals. A noninfectious, yet effective WNV vaccine would provide a valuable addition to the available preventive measures against disease caused by this emerging, neurotropic, mosquito-borne virus.
Acknowledgments
We thank the following staff of the Southwest National Primate Research Center ABSL-3 facility for their technical contributions to this study: Stacey Perez, Tony Bowers, Laura Rumpf, Melissa Mann, George Villanueva, and Juan Zapata.
This work was supported in part by grant 9 R44 NS52139-02A1 from the National Institutes of Health (NIH). V. R. Nerurkar is partially supported by grants P20RR018727 and G12RR003061 from NCRR, NIH.
Footnotes
Published ahead of print on 29 July 2009.
REFERENCES
- 1.Beasley, D. W. C., and A. D. T. Barrett. 2002. Identification of neutralizing epitopes within structural domain III of the West Nile virus envelope protein. J. Virol. 76:13097-13100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bowen, R. A., and N. M. Nemeth. 2007. Experimental infections with West Nile virus. Curr. Opin. Infect. Dis. 20:293-297. [DOI] [PubMed] [Google Scholar]
- 3.Campbell, G. L., A. A. Marfin, R. S. Lanciotti, and D. J. Gubler. 2002. West Nile virus. Lancet Infect. Dis. 2:519-529. [DOI] [PubMed] [Google Scholar]
- 4.Carson, P. J., P. Konewko, K. S. Wold, P. Mariani, S. Goli, P. Bergloff, and R. D. Crosby. 2006. Long-term clinical and neuropsychological outcomes of West Nile virus infection. Clin. Infect. Dis. 43:723-730. [DOI] [PubMed] [Google Scholar]
- 5.Chowers, M. Y., R. Lang, F. Nassar, D. Ben-David, M. Giladi, E. Rubinshtein, A. Itzhaki, J. Mishal, Y. Siegman-Igra, R. Kitzes, N. Pick, Z. Landau, D. Wolf, H. Bin, E. Mendelson, S. D. Pitlik, and M. Weinberger. 2001. Clinical characteristics of the West Nile fever outbreak, Israel, 2000. Emerg. Infect. Dis. 7:675-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crill, W. D., and J. T. Roehrig. 2001. Monoclonal antibodies that bind to domain III of Dengue virus E glycoprotein are the most efficient blockers of virus adsorption to Vero cells. J. Virol. 75:7769-7773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cuzzubbo, A. J., T. P. Endy, A. Nisalak, S. Kalayanarooj, D. W. Vaughn, S. A. Ogata, D. E. Clements, and P. L. Devine. 2001. Use of recombinant envelope proteins for serological diagnosis of dengue virus infection in an immunochromatographic assay. Clin. Diagn. Lab. Immunol. 8:1150-1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drebot, M. A., L. R. Lindsay, I. K. Barker, P. A. Buck, M. Fearon, F. Hunter, P. Sockett, and H. Artsob. 2003. West Nile virus surveillance and diagnosis: a Canadian perspective. Can. J. Infect. Dis. 14:105-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Estrada-Franco, J. G., R. Navarro-Lopez, D. W. Beasley, L. Coffey, A. S. Carrara, A. Travassos da Rosa, T. Clements, E. Wang, G. V. Ludwig, A. C. Cortes, P. P. Ramírez, R. B. Tesh, A. D. Barrett, and S. C. Weaver. 2003. West Nile virus in Mexico: evidence of widespread circulation since July 2002. Emerg. Infect. Dis. 9:1604-1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guarner, J., W.-J. Shieh, S. Hunter, C. D. Paddock, T. Morken, G. L. Campbell, A. A. Marfin, and S. R. Zaki. 2004. Clinicopathologic study and laboratory diagnosis of 23 cases with West Nile virus encephalomyelitis. Hum. Pathol. 35:983-990. [DOI] [PubMed] [Google Scholar]
- 11.Hayes, E. B., and D. R. O'Leary. 2004. West Nile virus infection: a pediatric perspective. Pediatrics 113:1375-1381. [DOI] [PubMed] [Google Scholar]
- 12.Hayes, E. B., N. Komar, R. S. Nasci, S. P. Montgomery, D. R. O'Leary, and G. L. Campbell. 2005. Epidemiology and transmission dynamics of West Nile virus disease. Emerg. Infect. Dis. 11:1167-1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hayes, E. B., J. J. Sejvar, S. R. Zaki, R. S. Lanciotti, A. V. Bode, and G. L. Campbell. 2005. Virology, pathology, and clinical manifestations of West Nile virus disease. Emerg. Infect. Dis. 11:1174-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hubalek, Z., and J. Halouzka. 1999. West Nile fever—a reemerging mosquito-borne viral disease in Europe. Emerg. Infect. Dis. 5:643-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jarvi, S. I., M. M. Lieberman, E. Hofmeister, V. R. Nerurkar, T. Wong, and C. Weeks-Levy. 2008. Protective efficacy of a recombinant subunit West Nile virus vaccine in domestic geese (Anser anser). Vaccine 26:5338-5344. [DOI] [PubMed] [Google Scholar]
- 16.Jean, C. M., S. Honarmand, J. K. Louie, and C. A. Glasser. 2007. Risk factors for West Nile virus neuroinvasive disease, California, 2005. Emerg. Infect. Dis. 13:1918-1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Komar, N., and G. G. Clark. 2006. West Nile virus activity in Latin America and the Caribbean. Rev. Panam. Salud Publica 19:112-117. [DOI] [PubMed] [Google Scholar]
- 18.Kumar, D., G. V. R. Prasad, J. Zaltzman, G. A. Levy, and A. Humar. 2004. Community-acquired West Nile virus infection in solid-organ transplant recipients. Transplantation 77:399-402. [DOI] [PubMed] [Google Scholar]
- 19.Lieberman, M. M., D. E. Clements, S. Ogata, G. Wang, G. Corpuz, T. Wong, T. Martyak, L. Gilson, B.-A. Coller, J. Leung, D. M. Watts, R. B. Tesh, M. Siirin, A. Travassos da Rosa, T. Humphreys, and C. Weeks-Levy. 2007. Preparation and immunogenic properties of a recombinant West Nile subunit vaccine. Vaccine 25:414-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin, C.-W., and S.-C. Wu. 2003. A functional epitope determinant on domain III of the Japanese encephalitis virus envelope protein interacted with neutralizing-antibody combining sites. J. Virol. 77:2600-2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lustig, S., U. Olshevsky, D. Ben-Nathan, B. E. Lachmi, M. Malkinson, D. Kobiler, and M. Halevy. 2000. A live attenuated West Nile virus strain as a potential veterinary vaccine. Viral. Immunol. 13:401-410. [DOI] [PubMed] [Google Scholar]
- 22.Marciani, D. J., R. C. Reynolds, A. K. Pathak, K. Finley-Woodman, and R. D. May. 2003. Fractionation, structural studies, and immunological characterization of the semi-synthetic Quillaja saponins derivative GPI-0100. Vaccine 21:3961-3971. [DOI] [PubMed] [Google Scholar]
- 23.Markoff, L. 2000. Points to consider in the development of a surrogate for efficacy of novel Japanese encephalitis virus vaccines. Vaccine 18:26-32. [DOI] [PubMed] [Google Scholar]
- 24.Minke, J. M., L. Siger, K. Karaca, L. Austgen, P. Gordy, R. Bowen, R. W. Renshaw, S. Loosmore, J. C. Audonnet, and B. Nordgren. 2004. Recombinant canarypoxvirus vaccine carrying the prM/E genes of West Nile virus protects horses against a West Nile virus-mosquito challenge. Arch. Virol. Suppl. 18:221-230. [DOI] [PubMed] [Google Scholar]
- 25.Modis, Y., S. Ogata, D. Clements, and S. C. Harrison. 2003. A ligand-binding pocket in the dengue virus envelope glycoprotein. Proc. Natl. Acad. Sci. USA 100:6986-6991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Modis, Y., S. Ogata, D. Clements, and S. C. Harrison. 2004. Structure of the dengue virus envelope protein after membrane fusion. Nature 427:313-319. [DOI] [PubMed] [Google Scholar]
- 27.Monath, T. P., J. Arroyo, C. Miller, and F. Guirakhoo. 2001. West Nile virus vaccine. Curr. Drug Targets Infect. Disord. 1:37-50. [DOI] [PubMed] [Google Scholar]
- 28.Mostashari, F., M. L. Bunning, P. T. Kitsutani, D. A. Singer, D. Nash, M. J. Cooper, N. Katz, K. A. Liljebjelke, B. J. Biggerstaff, A. D. Fine, M. C. Layton, S. M. Mullin, A. J. Johnson, D. A. Martin, E. B. Hayes, and G. L. Campbell. 2001. Epidemic West Nile encephalitis, New York, 1999: results of a household-based seroepidemiological survey. Lancet 358:261-264. [DOI] [PubMed] [Google Scholar]
- 29.Olberg, R.-A., I. K. Barker, G. J. Crawshaw, M. F. Bertelsen, M. A. Drebot, and M. Andonova. 2004. West Nile virus encephalitis in a Barbary Macaque (Macaca sylvanus). Emerg. Infect. Dis. 10:712-714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O'Leary, D. R., A. A. Marfin, S. P. Montgomery, A. M. Kipp, J. A. Lehman, B. J. Biggerstaff, V. L. Elko, P. D. Collins, J. E. Jones, and G. L. Campbell. 2004. The epidemic of West Nile Virus in the United States, 2002. Vector Borne Zoonotic Dis. 4:61-70. [DOI] [PubMed] [Google Scholar]
- 31.Petersen, L. R., and J. T. Roehrig. 2001. West Nile virus: a reemerging global pathogen. Emerg. Infect. Dis. 7:611-614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Petersen, L. R., J. T. Roehrig, and J. M. Hughes. 2002. West Nile virus encephalitis. N. Engl. J. Med. 347:1225-1226. [DOI] [PubMed] [Google Scholar]
- 33.Platonov, A. E., G. A. Shipulin, O. Y. Shipulina, E. N. Tyutyunnik, T. I. Frolochkina, R. S. Lanciotti, S. Yazyshina, O. V. Platonova, I. L. Obukhov, A. N. Zhukov, Y. Y. Vengerov, and V. I. Pokrovskii. 2001. Outbreak of West Nile virus infection, Volgograd region, Russia, 1999. Emerg. Infect. Dis. 7:128-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pletnev, A. G., R. Putnak, J. Speicher, E. J. Wagar, and D. W. Vaughn. 2002. West Nile virus/dengue type 4 virus chimeras that are reduced in neurovirulence and peripheral virulence without loss of immunogenicity or protective efficacy. Proc. Natl. Acad. Sci. USA 99:3036-3041. (Erratum, 99:7184.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Putnak, J. R., B.-A. Coller, G. Voss, D. W. Vaughn, D. Clements, I. Peters, G. Bignami, H.-S. Houng, R. C.-M. Chen, D. A. Barvir, J. Seriwatana, S. Cayphas, N. Garçon, D. Gheysen, N. Kanesa-thasan, M. McDonell, T. Humphreys, K. H. Eckels, J.-P. Prieels, and B. L. Innis. 2005. An evaluation of dengue type-2 inactivated, recombinant subunit, and live attenuated vaccine candidates in the rhesus macaque model. Vaccine 23:4442-4452. [DOI] [PubMed] [Google Scholar]
- 36.Quenelle, D. C., D. J. Collins, D. J. Marciani, and E. R. Kern. 2006. Effect of immunization with herpes simplex virus type-1 (HSV-1) glycoprotein D (gD) plus the immune enhancer GPI-0100 on infection with HSV-1 or HSV-2. Vaccine 24:1515-1522. [DOI] [PubMed] [Google Scholar]
- 37.Ratterree, M. S., R. A. Gutierrez, A. P. Travassos da Rosa, B. J. Dille, D. W. Beasley, R. P. Bohm, S. M. Desai, P. J. Didier, L. G. Bikenmeyer, G. J. Dawson, T. P. Leary, G. Schochetman, K. Phillippi-Falkenstein, J. Arroyo, A. D. Barrett, and R. B. Tesh. 2004. Experimental infection of rhesus macaques with West Nile virus: level and duration of viremia and kinetics of the antibody response after infection. J. Infect. Dis. 189:669-676. [DOI] [PubMed] [Google Scholar]
- 38.Rodrigues, S. C., and J. E. Maruniak. 2006. Blood meal identification from mosquitoes collected at a commercial alligator farm. J. Am. Mosq. Control Assoc. 22:557-560. [DOI] [PubMed] [Google Scholar]
- 39.Siirin, M. T., A. P. A. Travassos da Rosa, P. Newman, C. Weeks-Levy, B.-A. Coller, S.-Y. Xiao, M. M. Lieberman, and D. M. Watts. 2008. Evaluation of the efficacy of a recombinant subunit West Nile vaccine in Syrian golden hamsters. Am. J. Trop. Med. Hyg. 79:955-962. [PMC free article] [PubMed] [Google Scholar]
- 40.Tesh, R. B., J. Arroyo, A. P. Travassos Da Rosa, H. Guzman, S. Y. Xiao, and T. P. Monath. 2002. Efficacy of killed virus vaccine, live attenuated chimeric virus vaccine, and passive immunization for prevention of West Nile virus encephalitis in hamster model. Emerg. Infect. Dis. 8:1392-1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Throsby, M., C. Geuijen, J. Goudsmit, A. Q. Bakker, J. Korimbocus, R. A. Kramer, M. Clijsters-van der Horst, M. de Jong, M. Jongeneelen, S. Thijsse, R. Smit, T. J. Visser, N. Bijl, W. E. Marissen, M. Loeb, D. J. Kelvin, W. Preiser, J. ter Meulen, and J. de Kruif. 2006. Isolation and characterization of human monoclonal antibodies from individuals infected with West Nile virus. J. Virol. 80:6982-6992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tsai, T. F., F. Popovici, C. Cernescu, G. L. Campbell, and N. I. Nedelcu. 1998. West Nile encephalitis epidemic in southeastern Romania. Lancet 352:767-771. [DOI] [PubMed] [Google Scholar]
- 43.Watts, D. M., R. B. Tesh, M. Siirin, A. Travassos da Rosa, P. C. Newman, D. E. Clements, S. Ogata, B.-A. Coller, C. Weeks-Levy, and M. M. Lieberman. 2007. Efficacy and durability of a recombinant subunit West Nile vaccine candidate in protecting hamsters from West Nile encephalitis. Vaccine 25:2913-2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xiao, S. Y., H. Guzman, H. Zhang, A. P. Travassos da Rosa, and R. B. Tesh. 2001. West Nile virus infection in the golden hamster (Mesocricetus auratus): a model for West Nile encephalitis. Emerg. Infect. Dis. 7:714-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zent, O., J. Beran., W. Jilg., T. Mach., and A. Banhoff. 2003. Clinical evaluation of a polygeline-free tick-borne encephalitis vaccine for adolescents and adults. Vaccine 21:738-741. [DOI] [PubMed] [Google Scholar]