Abstract
Preexisting immunity to adenovirus serotype 5 (Ad5) diminishes immune responses to vaccines using Ad5 as a vector. Alternate Ad serotypes as vaccine vectors might overcome Ad5-specific neutralizing antibodies and enhance immune responses in populations with a high prevalence of Ad5 immunity. To test this hypothesis, healthy human immunodeficiency virus (HIV)-seronegative adults were enrolled in a blinded, randomized, dose-escalating, placebo-controlled study. In part A, subjects with baseline Ad6 titers of ≤18 received the Merck Ad6 (MRKAd6) HIV type 1 (HIV-1) trigene vaccine at weeks 0, 4, and 26. In part B, subjects stratified by Ad5 titers (≤200 or >200) and Ad6 titers (≤18 or >18) received the MRKAd5-plus-MRKAd6 (MRKAd5+6) HIV-1 trigene vaccine at weeks 0, 4, and 26. Immunogenicity was assessed by an enzyme-linked immunospot (ELISPOT) assay at week 30. No serious adverse events occurred. MRKAd6 trigene vaccine recipients responded more often to Nef than to Gag or Pol. In part A, ELISPOT response rates to ≥2 vaccine antigens were 14%, 63%, and 71% at 109, 1010, and 1011 viral genomes (vg)/dose, respectively. All responders had positive Nef-specific ELISPOT results. In part B, Nef-ELISPOT response rates at 1010 vg/dose of the MRKAd5+6 trigene vaccine were 50% in the low-Ad5/low-Ad6 stratum (n = 8), 78% in the low-Ad5/high-Ad6 stratum (n = 9), 75% in the high-Ad5/low-Ad6 stratum (n = 8), and 44% in the high-Ad5/high-Ad6 stratum (n = 9). The MRKAd6 and MRKAd5+6 trigene vaccines elicited dose-dependent responses predominantly to Nef and were generally well tolerated, indicating that Ad6 should be considered a candidate vector for future vaccines. Although small sample sizes limit the conclusions that can be drawn from this exploratory study, combining two Ad vectors may be a useful vaccine strategy for circumventing isolated immunity to a single Ad serotype.
Adenovirus (Ad) vectors have been investigated as a vaccination strategy for inducing cell-mediated immunity (CMI) to several viral and bacterial pathogens (11, 13, 22, 24, 26). In preclinical and phase I studies, vaccination with attenuated Ad serotype 5 (Ad5) vectors expressing human immunodeficiency virus type 1 (HIV-1) gag elicited strong CMI responses in both macaques and humans (4, 5, 14, 20, 23). Although a similar Ad5-vectored trivalent HIV-1 vaccine did not prevent or modulate infection in the proof-of-concept STEP trial (2), adenoviruses remain attractive candidates as vectors for inducing CMI against a variety of common infections.
Diminished immune responses to transgenes carried by Ad5 vectors as a result of preexisting Ad5-specific immunity have been a concern from the advent of Ad5-based vaccine trials in humans (2, 5, 13, 16, 18, 25). High preexisting titers of neutralizing antibodies against Ad5 substantially diminished CMI responses to HIV-1 vaccines using Ad5 vectors (2, 5, 16, 18). Most North American adults have demonstrable neutralizing antibody against Ad5, and nearly one-third have relatively high titers (21, 25, 26). The frequency and magnitude of Ad5 titers are even higher in other parts of the world (8, 21). Neutralizing antibody against Ad6 is present less frequently and in lower titers (8, 21). Relatively few individuals would be expected to have high titers of antibodies against both Ad5 and Ad6.
Strategies for overcoming preexisting Ad5 immunity include increasing the dose of Ad5-based vaccines, employing heterologous prime-boost regimens, or using different vectors, such as alternative adenovirus serotypes (3, 15, 26). The current trial was designed to explore the use of Ad6 with or without Ad5 as a vaccine vector for delivering HIV-1 gag, nef, and pol transgenes.
(These data have been presented in part at the AIDS Vaccine 2007 Conference, Seattle, WA, August 2007 [12a, 12b].)
MATERIALS AND METHODS
Objectives.
The primary objectives of the study were (i) to assess the safety and tolerability of the administration of a three-dose regimen of the Merck Ad6 (MRKAd6) and MRKAd5-plus-MRKAd6 (MRKAd5+6) HIV-1 trigene vaccines and (ii) to evaluate the immunogenicity of a three-dose regimen of these vaccines. The secondary objective was to evaluate the immunogenicity of a three-dose regimen of the MRKAd5+6 HIV-1 trigene vaccine in subjects with preexisting antibodies to either Ad5 (titers, >200) or Ad6 (titers, >18).
Vaccine composition.
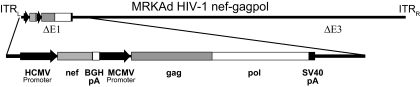
Trigene vaccines were constructed using two recombinant adenovirus vectors (MRKAd5nef-gag/pol and MRKAd6nef-gag/pol), which were subsequently combined to make the MRKAd5+6 HIV-1 trigene vaccine (Fig. 1). The same near-consensus clade B gag, pol, and nef genes in the trivalent vaccine (18) were utilized to construct the trigene vaccines. The E1 region of the wild-type adenovirus was deleted and replaced with the transgene containing the nef and gag/pol expression cassettes. The nef expression cassette consisted of (i) the immediate-early gene promoter from human cytomegalovirus (HCMV) (6), (ii) the coding sequence of the HIV-1 nef (strain JR-FL) gene, and (iii) the bovine growth hormone polyadenylation signal sequence (12). The nef cassette was directly followed by the gag/pol expression cassette, consisting of (i) the immediate-early gene promoter from mouse cytomegalovirus (MCMV), (ii) the coding sequence of the HIV-1 gag (strain CAM-1) gene fused to the coding sequence of the HIV-1 pol (genes for the reverse transcriptase and integrase from strain IIIB) gene, and (iii) the simian virus 40 polyadenylation signal sequence. The amino acid sequences of the Gag, Pol, and Nef proteins closely resembled the clade B consensus amino acid sequences (17).
FIG. 1.
MRKAd5 and MRKAd6 trigene vaccine constructs. The HIV nef-gag/pol expression cassette (lower diagram) was added to both the MRKAd5 and MRKAd6 vectors. ITRL, inverted terminal repeat (left); ITRR, inverted terminal repeat (right); BGH, bovine growth hormone; pA, polyadenylation signal sequence; SV40, simian virus 40.
The Ad5-based trivalent vaccine was composed of an equimolar mixture of the MRKAd5gag, MRKAd5pol, and MRKAd5nef vectors (2, 18). The transgene in each case contained an expression cassette containing the immediate-early gene promoter from HCMV, the coding sequence of the HIV-1 gag, pol, or nef gene, and the bovine growth hormone polyadenylation signal sequence.
The vaccine doses (expressed in units of [adeno]viral genomes [vg]) administered in this study were 0.5 × 109 Ad6 vg, 0.5 × 1010 Ad6 vg, or 0.5 × 1011 Ad6 vg for the MRKAd6 trigene vaccine; 0.5 × 1010 Ad5 vg for the MRKAd5 trigene vaccine; 1.5 × 1010 Ad5 vg for the MRKAd5 trivalent vaccine; and 0.5 × 109 Ad5 vg plus 0.5 × 109 Ad6 vg (total dose, 1.0 × 109 Ad vg) or 0.5 × 1010 Ad5 vg plus 0.5 × 1010 Ad6 vg (total dose, 1.0 × 1010 Ad vg) for the MRKAd5+6 trigene vaccine.
Study design.
This study was designed as a multicenter, blinded, randomized, dose-escalating, placebo-controlled trial of a three-dose prime-boost regimen for HIV-seronegative adults to be followed for as long as 52 weeks for immunogenicity and 260 weeks for safety. Healthy subjects between the ages of 18 and 50 years without hepatitis B or C and with no evidence of HIV infection were assessed for eligibility at 17 sites in the United States. Individuals were excluded if they were considered by the investigator to be at high behavioral risk of HIV exposure during the study based on risk factor assessment. The protocol was approved by the institutional review boards at participating centers. Written informed consent was obtained from all subjects. Ongoing HIV risk assessments and preventative counseling were offered to the study participants during the trial.
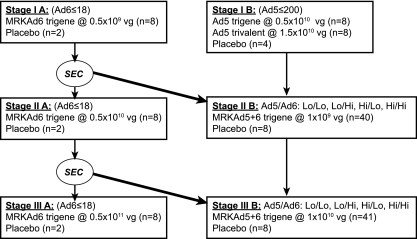
The study had two parts (Fig. 2), each with three stages. Stages IA, IIA, and IIIA evaluated the MRKAd6 HIV-1 trigene vaccine. Stage IB evaluated the MRKAd5 HIV-1 trigene and MRKAd5 HIV-1 trivalent vaccines. Stages IIB and IIIB evaluated the MRKAd5+6 HIV-1 trigene vaccine. Randomization was stratified by titers of neutralizing antibody against Ad5 (≤200 or >200) and/or Ad6 (≤18 or >18) (1). Subjects were to receive three 0.5-ml intramuscular injections of vaccine or placebo, one each at day 1, week 4, and week 26.
FIG. 2.
Study schema. The Safety Evaluation Committee (SEC) examined adverse-event reports from completed stages where indicated before subjects were vaccinated in later stages. For stages IIB and IIIB, randomization was stratified by high versus low titers of neutralizing antibodies against Ad5 (≤200 versus >200) and by high versus low titers of neutralizing antibodies against Ad6 (≤18 versus >18) at baseline (1).
Safety measurements.
Safety endpoints included the rate of vaccine-related serious adverse events throughout the duration of the study, injection site reactions for 5 days following each injection, and systemic adverse events and non-vaccine-related serious adverse events for 15 days following each injection. Samples for Ad5 and Ad6 shedding were to be collected on day 3 and at subsequent visits from subjects with clinical symptoms suggestive of adenovirus infection. Urinalysis, complete blood counts, determination of prothrombin and partial thromboplastin times, liver enzyme tests, and measurement of serum phosphorus and creatinine levels were performed at screening and at 1 and 2 weeks following each injection.
Subjects were tested for the presence of anti-HIV antibody at entry and at weeks 30, 52, 104, and 260, or upon early discontinuation. Positive anti-HIV antibody test results were further evaluated in order to distinguish HIV infection from vaccine-induced seroconversion (19).
Immunological measurements.
Immunogenicity was measured by an unfractionated gamma interferon (IFN-γ) enzyme-linked immunospot (ELISPOT) assay to 15-mer clade B Gag, Pol, and Nef peptide pools (7, 9, 10). At screening and weeks 4, 8, 30, and 52, blood was collected for immunogenicity and serological testing. For each antigen, a positive ELISPOT response was defined as ≥55 spot-forming cells/106 peripheral blood mononuclear cells and ≥4-fold over the level for the medium control (9). Geometric mean ELISOPT responses were calculated using all subjects in a given group, not just subjects with positive responses.
Statistical analyses (i) Safety.
All subjects receiving ≥1 dose were included in the safety assessment.
(ii) Immunogenicity.
A subject with a positive response in at least one of the Gag-, Pol-, or Nef-specific IFN-γ ELISPOT assays was considered an ELISPOT responder. Due to the planned small sample size, no formal hypothesis testing was specified per protocol; only descriptive data were to be provided.
RESULTS
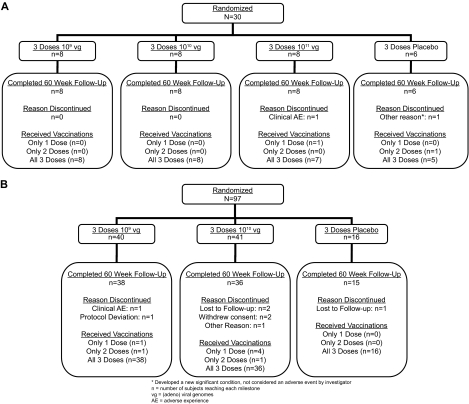
From April 2005 through January 2006, 118 (93.7%) of the 126 subjects randomized to the vaccine groups received all three doses. The placebo and vaccine groups were generally comparable in age and race, but the placebo group included a higher proportion of females (Table 1). A total of 97 subjects were randomized to stages IIB and IIIB, with ∼10 subjects in each of the four baseline Ad5/Ad6 strata for each of the two vaccine dose groups (Fig. 3). Through week 60, 10 subjects (7.9%) discontinued the study: 3 were lost to follow-up, 2 withdrew consent, 2 discontinued due to nonserious clinical adverse events, 1 discontinued because of a new condition not considered an adverse event by the investigator, 1 discontinued due to a protocol deviation, and 1 dropped out of the study after the week 60 visit.
TABLE 1.
Demographic characteristics of subjects by treatment group
| Characteristic | Value for the following vaccine vector and construct at the indicated stage:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Placebo, all stages combined | MRKAd6 trigene
|
MRKAd5 trigene, stage IB | MRKAd5 trivalent, stage IB | MRKAd5+6 trigene
|
||||
| IA | IIA | IIIA | IIB | IIIB | ||||
| Dose (vg) | 0.5 × 109 | 0.5 × 1010 | 0.5 × 1011 | 0.5 × 1010 | 1.5 × 1010 | 1.0 × 109 | 1.0 × 1010 | |
| No. of subjects | 26 | 8 | 8 | 8 | 8 | 8 | 40 | 41 |
| Gender | ||||||||
| No. (%) male | 10 (38) | 4 (50) | 4 (50) | 5 (62) | 4 (50) | 3 (38) | 20 (50) | 19 (49) |
| No. (%) female | 16 (62) | 4 (50) | 4 (50) | 3 (38) | 4 (50) | 5 (62) | 20 (50) | 21 (51) |
| Age | ||||||||
| Mean | 30.1 | 36.3 | 34.6 | 37.3 | 29.9 | 31.3 | 34.8 | 33.9 |
| SD | 8.8 | 7.2 | 9.9 | 7.6 | 9.3 | 11.0 | 9.5 | 9.7 |
| Median | 26.5 | 37 | 40 | 37.5 | 28.5 | 26.5 | 37.5 | 31 |
| Range | 20-47 | 22-46 | 20-45 | 26-45 | 20-45 | 20-49 | 19-48 | 21-50 |
| No. (%) of the following race/ethnicity: | ||||||||
| Asian/Pacific | 3 (12) | 0 (0) | 0 (0) | 0 (0) | 1 (13) | 0 (0) | 0 (0) | 3 (7) |
| Black | 4 (15) | 1 (13) | 5 (62) | 0 (0) | 2 (25) | 2 (25) | 6 (15) | 7 (17) |
| Caucasian | 18 (69) | 7 (87) | 3 (38) | 7 (87) | 4 (50) | 6 (75) | 30 (75) | 27 (66) |
| Hispanic | 0 (0) | 0 (0) | 0 (0) | 1 (13) | 1 (13) | 0 (0) | 4 (10) | 2 (5) |
| Other | 1 (4) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (5) |
FIG. 3.
Subject accounting. (A) Stages IA, IIA, and IIIA; (B) stages IIB and IIIB. (Stage IB, which only used the Ad5 vector, is not displayed for clarity.)
Safety and tolerability.
Safety data through week 30 were available for all 30 subjects (6 placebo and 24 vaccine recipients) in stage A and for 109 (94%; 19 placebo and 90 vaccine recipients) subjects in stage B (Table 2). No serious adverse events occurred. Injection site reactions were the most commonly observed adverse events, increasing in frequency with the vaccine dose. The types and frequencies of systemic adverse events were generally comparable among vaccine and placebo recipients and were mild to moderate in severity. Overall, the most commonly reported systemic adverse events were headache and fatigue. No consistent laboratory abnormalities were observed. No shedding of the Ad5 or Ad6 vaccine strain was identified.
TABLE 2.
Percentage of subjects experiencing common adverse events after any injection of the MRKAd5 or MRKAd6 vaccine
| Adverse event | % of subjects receiving the following vaccine vector and construct at the indicated stage (dose [vg])a:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Placebo, all stages combined | MRKAd6 trigene
|
MRKAd5 trigene, stage IB (0.5 × 1010) | MRKAd5 trivalent, stage IB (1.5 × 1010) | MRKAd5+6 trigene
|
||||
| IA (0.5 × 109) | IIA (0.5 × 1010) | IIIA (0.5 × 1011) | IIB (1 × 109) | IIIB (1 × 1010) | ||||
| Local (injection site reactions) | 38.5 | 50.0 | 87.5 | 100.0 | 62.5 | 87.5 | 65.0 | 85.4 |
| Systemic | ||||||||
| Headache | 42.3 | 12.5 | 37.5 | 62.5 | 25.0 | 75.0 | 35.0 | 26.8 |
| Fatigue | 7.7 | 25.0 | 12.5 | 25.0 | 37.5 | 37.5 | 15.0 | 22.0 |
| Sore throat | 11.5 | 37.5 | 0.0 | 25.0 | 25.0 | 25.0 | 15.0 | 9.8 |
| Myalgia | 11.5 | 12.5 | 0.0 | 12.5 | 37.5 | 12.5 | 15.0 | 7.3 |
| Nausea | 7.7 | 12.5 | 12.5 | 12.5 | 25.0 | 25.0 | 7.5 | 14.6 |
| Pyrexia | 3.8 | 12.5 | 12.5 | 12.5 | 25.0 | 37.5 | 7.5 | 4.9 |
| Diarrhea | 3.8 | 37.5 | 0.0 | 0.0 | 12.5 | 0.0 | 7.5 | 12.2 |
| Cough | 11.5 | 0.0 | 0.0 | 0.0 | 12.5 | 0.0 | 15.0 | 4.9 |
| Upper respiratory infection | 11.5 | 0.0 | 12.5 | 12.5 | 0.0 | 12.5 | 2.5 | 9.8 |
The total numbers of subjects were as follows: 26 for the placebo, 8 at each stage for the MRKAd6 trigene vaccine, 8 each for the MRKAd5 trigene and MRKAd5 trivalent vaccines, and 40 and 41 for the MRKAd5+6 trigene vaccine at stages IIB and IIIB, respectively.
Immunogenicity.
Table 3 displays the ELISPOT responses to dose escalation of the MRKAd6 trigene vaccine in subjects with baseline Ad6 titers of ≤18 (stage A). Trigene vaccine recipients responded more frequently to Nef than to Gag or Pol peptide pools. No subject responded to Gag or Pol who did not also respond to Nef. Geometric mean responses and the percentage of responders increased with the dose, especially for the Gag and Pol peptides, but responses to Nef remained highest regardless of dose. Responses persisted up to at least 52 weeks.
TABLE 3.
Summaries of responses to 15-mer ELISPOT assays in subjects with baseline Ad6 titers of ≤18 who received the MRKAd6 trigene vaccine during the dose escalation stages (IA, IIA, and IIIA)
| Peptide pool antigen(s), vaccine, and regimen (vg/dose) | Wk 4
|
Wk 8
|
Wk 30
|
Wk 52
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of subjects | % Responding | GMa | No. of subjects | % Responding | GM | No. of subjects | % Responding | GM | No. of subjects | % Responding | GM | |
| Gag | ||||||||||||
| Placebo | 6 | 0 | 27 | 6 | 0 | 15 | 5 | 0 | 33 | 5 | 0 | 38 |
| MRKAd6 trigene | ||||||||||||
| 0.5 × 109 | 5 | 20 | 37 | 8 | 13 | 23 | 7 | 0 | 14 | 8 | 13 | 23 |
| 0.5 × 1010 | 8 | 38 | 86 | 8 | 50 | 73 | 8 | 38 | 98 | 8 | 38 | 95 |
| 0.5 × 1011 | 7 | 57 | 136 | 7 | 43 | 134 | 6 | 67 | 180 | 7 | 43 | 100 |
| Pol | ||||||||||||
| Placebo | 6 | 0 | 71 | 6 | 0 | 46 | 5 | 0 | 55 | 5 | 0 | 97 |
| MRKAd6 trigene | ||||||||||||
| 0.5 × 109 | 5 | 20 | 114 | 8 | 13 | 65 | 7 | 0 | 35 | 8 | 13 | 54 |
| 0.5 × 1010 | 8 | 63 | 236 | 8 | 75 | 238 | 8 | 50 | 299 | 8 | 63 | 248 |
| 0.5 × 1011 | 7 | 57 | 282 | 7 | 57 | 247 | 6 | 50 | 390 | 7 | 43 | 218 |
| Nef | ||||||||||||
| Placebo | 6 | 0 | 21 | 6 | 0 | 19 | 5 | 0 | 31 | 5 | 0 | 35 |
| MRKAd6 trigene | ||||||||||||
| 0.5 × 109 | 5 | 40 | 105 | 8 | 88 | 205 | 7 | 43 | 112 | 8 | 63 | 144 |
| 0.5 × 1010 | 8 | 100 | 432 | 8 | 88 | 279 | 8 | 88 | 270 | 8 | 100 | 253 |
| 0.5 × 1011 | 7 | 71 | 311 | 7 | 71 | 329 | 6 | 83 | 315 | 7 | 71 | 184 |
| ≥1 peptide pool | ||||||||||||
| Placebo | 6 | 0 | 6 | 0 | 5 | 0 | 5 | 0 | ||||
| MRKAd6 trigene | ||||||||||||
| 0.5 × 109 | 5 | 40 | 8 | 88 | 7 | 43 | 8 | 63 | ||||
| 0.5 × 1010 | 8 | 100 | 8 | 88 | 8 | 88 | 8 | 100 | ||||
| 0.5 × 1011 | 7 | 86 | 7 | 86 | 6 | 83 | 7 | 86 | ||||
| ≥2 peptide pools | ||||||||||||
| Placebo | 6 | 0 | 6 | 0 | 5 | 0 | 5 | 0 | ||||
| MRKAd6 trigene | ||||||||||||
| 0.5 × 109 | 5 | 20 | 8 | 13 | 7 | 0 | 8 | 13 | ||||
| 0.5 × 1010 | 8 | 63 | 8 | 75 | 8 | 63 | 8 | 75 | ||||
| 0.5 × 1011 | 7 | 71 | 7 | 57 | 6 | 83 | 7 | 43 | ||||
| All 3 peptide pools | ||||||||||||
| Placebo | 6 | 0 | 6 | 0 | 5 | 0 | 5 | 0 | ||||
| MRKAd6 trigene | ||||||||||||
| 0.5 × 109 | 5 | 20 | 8 | 13 | 7 | 0 | 8 | 13 | ||||
| 0.5 × 1010 | 8 | 38 | 8 | 50 | 8 | 25 | 8 | 25 | ||||
| 0.5 × 1011 | 7 | 29 | 7 | 29 | 6 | 33 | 7 | 29 | ||||
GM, geometric mean ELISPOT response, expressed as the number of spot-forming cells per 106 peripheral blood mononuclear cells.
Table 4 compares the ELISPOT responses to the MRKAd5 trigene and MRKAd5 trivalent vaccines by subjects with baseline anti-Ad5 antibody titers of ≤200 (stage IB). At weeks 30 and 52, the MRKAd5 trivalent vaccine recipients consistently responded more often to Gag than to Nef; however, the responses at weeks 4 and 8 were numerically the same to both antigens. At weeks 30 and 52, MRKAd5 trigene vaccine recipients responded equally to Gag and Nef; however, at the earlier time points, responses to Nef were seen more often than responses to Gag.
TABLE 4.
Responses to 15-mer ELISPOT assays in subjects with baseline anti-Ad5 titers of ≤200 receiving the MRKAd5 trigene or MRKAd5 trivalent vaccine (stage IB)
| Peptide pool antigen(s) and vaccinea | Wk 4
|
Wk 8
|
Wk 30
|
Wk 52
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of subjects | % Responding | GMb | No. of subjects | % Responding | GM | No. of subjects | % Responding | GM | No. of subjects | % Responding | GM | |
| Gag | ||||||||||||
| Placebo | 4 | 0 | 12 | 4 | 0 | 27 | 3 | 0 | 27 | 3 | 0 | 42 |
| MRKAd5 trigene | 6 | 50 | 78 | 7 | 43 | 71 | 4 | 75 | 367 | 7 | 71 | 129 |
| MRKAd5 trivalent | 8 | 88 | 244 | 8 | 75 | 247 | 7 | 100 | 302 | 6 | 83 | 348 |
| Pol | ||||||||||||
| Placebo | 4 | 0 | 26 | 4 | 0 | 51 | 3 | 0 | 66 | 3 | 0 | 111 |
| MRKAd5 trigene | 6 | 50 | 135 | 7 | 57 | 105 | 4 | 75 | 282 | 7 | 14 | 131 |
| MRKAd5 trivalent | 8 | 63 | 350 | 8 | 75 | 427 | 7 | 71 | 435 | 6 | 67 | 555 |
| Nef | ||||||||||||
| Placebo | 4 | 0 | 9 | 4 | 0 | 23 | 3 | 0 | 24 | 3 | 0 | 46 |
| MRKAd5 trigene | 6 | 100 | 199 | 7 | 86 | 172 | 4 | 75 | 385 | 7 | 71 | 233 |
| MRKAd5 trivalent | 8 | 88 | 257 | 8 | 75 | 188 | 7 | 57 | 221 | 6 | 67 | 239 |
| ≥1 peptide pool | ||||||||||||
| Placebo | 4 | 0 | 4 | 0 | 3 | 0 | 3 | 0 | ||||
| MRKAd5 trigene | 6 | 100 | 7 | 86 | 4 | 75 | 7 | 71 | ||||
| MRKAd5 trivalent | 8 | 88 | 8 | 88 | 7 | 100 | 6 | 83 | ||||
| ≥2 peptide pools | ||||||||||||
| Placebo | 4 | 0 | 4 | 0 | 3 | 0 | 3 | 0 | ||||
| MRKAd5 trigene | 6 | 67 | 7 | 71 | 4 | 75 | 7 | 71 | ||||
| MRKAd5 trivalent | 8 | 88 | 8 | 75 | 7 | 71 | 6 | 67 | ||||
| All 3 peptide pools | ||||||||||||
| Placebo | 4 | 0 | 4 | 0 | 3 | 0 | 3 | 0 | ||||
| MRKAd5 trigene | 6 | 33 | 7 | 29 | 4 | 75 | 7 | 14 | ||||
| MRKAd5 trivalent | 8 | 63 | 8 | 63 | 7 | 57 | 6 | 67 | ||||
The MRKAd5 trigene vaccine was given at 0.5×1010 vg/dose, and the MRKAd5 trivalent vaccine was given at 1.5×1010 vg/dose.
GM, geometric mean ELISPOT response, expressed as the number of spot-forming cells per 106 peripheral blood mononuclear cells.
Table 5 illustrates the ELISPOT results at week 30 for the MRKAd5+6 trigene dose escalation portion of this study (stages IIB and IIIB). Rates of response to Nef were generally higher than those to Gag or Pol peptides. Response rates were numerically higher in subjects with baseline immunity to either Ad5 or Ad6 exclusively than in subjects immune to both Ad serotypes at entry.
TABLE 5.
Responses to 15-mer ELISPOT assays at week 30 in subjects receiving the MRKAd5+6 trigene vaccine during the dose escalation stages (IIB and IIIB)
| Baseline Ad5 and Ad6 titers | Regimen (vg/dose) | Peptide
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Gag
|
Pol
|
Nef
|
||||||||
| No. of subjects | % Responding | GMa | No. of subjects | % Responding | GM | No. of subjects | % Responding | GM | ||
| ≤200, ≤18 | Placebo | 4 | 0 | 45 | 4 | 0 | 105 | 4 | 25b | 54 |
| MRKAd5+6 trigene (1 × 109) | 10 | 30 | 61 | 10 | 30 | 117 | 10 | 70 | 135 | |
| MRKAd5+6 trigene (1 × 1010) | 8 | 38 | 104 | 8 | 25 | 185 | 8 | 50 | 178 | |
| ≤200, >18 | Placebo | 4 | 0 | 32 | 4 | 0 | 52 | 4 | 0 | 17 |
| MRKAd5+6 trigene (1 × 109) | 7 | 43 | 79 | 7 | 43 | 164 | 7 | 71 | 319 | |
| MRKAd5+6 trigene (1 × 1010) | 9 | 44 | 117 | 9 | 56 | 402 | 9 | 78 | 333 | |
| >200, ≤18 | Placebo | 4 | 0 | 21 | 4 | 0 | 46 | 4 | 0 | 23 |
| MRKAd5+6 trigene (1 × 109) | 10 | 10 | 47 | 10 | 10 | 88 | 10 | 30 | 117 | |
| MRKAd5+6 trigene (1 × 1010) | 8 | 25 | 86 | 8 | 13 | 146 | 8 | 63 | 226 | |
| >200, >18 | Placebo | 3 | 0 | 58 | 3 | 0 | 99 | 3 | 0 | 38 |
| MRKAd5+6 trigene (1 × 109) | 9 | 0 | 44 | 9 | 0 | 98 | 9 | 11 | 70 | |
| MRKAd5+6 trigene (1 × 1010) | 10 | 20 | 40 | 10 | 10 | 64 | 10 | 40 | 65 | |
GM, geometric mean ELISPOT response, expressed as the number of spot-forming cells/106 peripheral blood mononuclear cells.
One placebo recipient had a positive ELISPOT response of 210 spot-forming cells/106 peripheral blood mononuclear cells to Nef peptides.
DISCUSSION
This exploratory phase I trial was the first study to test the safety and immunogenicity of an Ad6-vectored vaccine, using a novel trigene construct not previously evaluated in clinical trials. Prior to the availability of the STEP trial results (2), we assessed the immunogenicity of a combined MRKAd5+6 trigene vaccine in subjects with high titers of antibody against Ad5 and/or Ad6 at baseline. Despite the discouraging results of the STEP trial, testing an Ad5-vectored HIV-1 trivalent vaccine (which failed to prevent or modulate HIV infection) on uninfected volunteers, our findings remain pertinent to the development of any vaccine delivered by attenuated viral vectors.
The trigene vaccines with the MRKAd5 and/or MRKAd6 vectors appeared to be well tolerated at all doses studied. Adverse events were generally dose related, with injection site reactions being the most common. The types and frequencies of systemic adverse events were generally comparable among vaccine and placebo recipients, and systemic adverse events were typically mild to moderate. No serious adverse events were reported.
All three ascending doses of the MRKAd6 HIV-1 trigene vaccine were immunogenic in Ad6-naïve subjects relative to placebo recipients, and the ELISPOT responses were durable for at least 52 weeks. The percentage of responders and the magnitude of responses were comparable in the 0.5 × 1010-vg and 0.5 × 1011-vg dose groups. Both of these doses appeared more immunogenic than the 0.5 × 109-vg dose. Responses to the MRKAd5 HIV-1 trigene vaccine at a dose of 0.5 × 1010 vg were similar to responses seen for recipients of the two higher doses of the MRKAd6 HIV-1 trigene vaccine. However, unlike responses to the trivalent Ad5 vaccine (2, 16, 18), antigen-specific ELISPOT responses in subjects receiving the MRKAd6 or MRKAd5+6 trigene vaccine were consistently higher to Nef than to either Gag or Pol peptides in all dose groups. Differences between rates of response to Gag and Nef were not as apparent with the MRKAd5 trigene vaccine, especially at later time points.
The disparity in responses to different peptide pools between the trivalent and trigene constructs could be explained by different expression of separate gag and pol transgenes in the trivalent construct compared to the gag/pol fusion transgene expression in the trigene construct. The trivalent vaccine consisted of a mixture of three different monovalent constructs, and gene expression in each construct was driven by the HCMV immediate-early gene promoter. The trigene vaccine contained a tri-antigen transgene beginning with the nef expression cassette driven by the HCMV immediate-early gene promoter, followed directly by the fused gag/pol expression cassette driven by the MCMV early gene promoter. The purpose of using two different promoters was to mitigate the risk of vector recombination events, but diminished gag/pol expression may have resulted from inferior MCMV promoter activity relative to nef expression driven by the HCMV promoter. The individual properties of the MRKAd5 and MRKAd6 vectors may also have contributed to differences in rates of response to various peptides. Despite relatively poor immunogenicity to the Gag and Pol antigens, the MRKAd6 trigene construct appeared to be immunogenic in subjects with low preexisting Ad6 immunity, based on Nef responses, and it remains a viable candidate for future Ad-based vaccines.
Interpretation of the CMI responses to the combination MRKAd5+6 HIV-1 trigene vaccine was hindered by sample size constraints and the unanticipated apparent antigen expression issues. For both doses of the MRKAd5+6 HIV-1 trigene vaccine tested in our study, rates of ELISPOT response to Nef were uniformly higher than those to Gag and Pol, regardless of baseline anti-Ad5 or anti-Ad6 titers. Nef ELISPOT responses in the MRKAd5+6 HIV-1 trigene vaccine recipients in the low-Ad5 and low-Ad6 strata were generally similar to the responses observed in the MRKAd5 or MRKAd6 trigene vaccine recipients with low baseline anti-Ad5 or low anti-Ad6 titers, indicating that combining the MRKAd5 and MRKAd6 vectors did not adversely impact the immunogenicity of either vaccine. Although the MRKAd5+6 HIV-1 trigene vaccine produced relatively high rates of response to Nef peptides in subjects with immunity to only one of the two Ad vectors, the responses in subjects with high preexisting immunity to both vectors appeared to be diminished. Direct comparisons of immune responses to the MRKAd5 and MRKAd6 trigene vaccines separately in participants with the four baseline seroprofiles detailed in Table 5 would have been instructive but were beyond the scope of our hypothesis-generating study.
Our data cannot address the quality of the immune response to the vaccine constructs used in this study, because we measured responses by an unfractionated IFN-γ ELISPOT assay. For example, it is plausible that the proportions of CD4 and CD8 cells elicited by the different vaccines differed. Comparison of immune responses in individuals with the four baseline seroprofiles vaccinated with either an Ad5-vectored or an Ad6-vectored vaccine alone would be an instructive next step.
Combining Ad5 and Ad6 vectors may be a useful strategy for overcoming preexisting Ad serotype-specific immunity. We found comparable ELISPOT responses to the MRKAd5+6 trigene vaccine in the high-Ad5/low-Ad6 stratum and the two low-Ad5 strata. Admittedly, the small sample sizes in the individual strata limit confidence in the trends observed in the present hypothesis-generating study. Larger trials, possibly evaluating additional transgene designs that incorporate novel promoter combinations for enhanced antigen expression, are required before definitive conclusions can be advanced. Our results were generated in the context of an HIV-vaccine program. Nevertheless, even in the face of the STEP trial results suggesting that administering an Ad-vectored HIV-1 vaccine to individuals with preexisting vector-specific immunity might enhance susceptibility to HIV infection (2, 16), these data will hopefully contribute to a better understanding of any vaccine using Ad vectors to generate protective CMI responses.
Acknowledgments
We thank the study participants and staff at the study sites for their dedication to the development of a preventive HIV-1 vaccine. At Merck Research Laboratories, we gratefully acknowledge the sage advice of Sheri Dubey, Robin Isaacs, Lisa Kiersted, Lori O'Neill, and Michael Robertson as well as the expert assistance of Karyn Davis and Joann DiLullo.
The V526-001 principal investigators are as follows: C. del Rio and F. Priddy, Emory Vaccine Research Center; T. Friel, Lehigh Valley Hospital; M. Gaitanis, Miriam Hospital; A. Myers, Body Positive; S. Brown, AIDS Research Alliance; F. Judson, Colorado Prevention Center; I. Frank, University of Pennsylvania; M. Keefer, University of Rochester; R. Poblete, North Jersey Community Research Initiative; S. Buchbinder, San Francisco Department of Public Health; C. Harro, Johns Hopkins University; D. Asmuth, University of California, Davis; P. Wright and P. Spearman, Vanderbilt University; L. Baden, Brigham and Women's Hospital; R. Novak, Project WISH; P. Rogge, Dow Pharmaceutical Sciences; S. Santiago, Care Resource Center.
Individual authors contributed to this work as follows: C.H., enrollment of subjects and data collection, analysis and interpretation of data, and manuscript preparation; J.E.S., X.S., and M.J.D., analysis and interpretation of data and manuscript preparation; F.W., A.J.B., D.R.C., and J.W.S., design and development of the trigene vaccine and critical review of the manuscript; R.Y.L., D.V.M., and E.Q., study concept and design, analysis and interpretation of data, and manuscript preparation.
Merck & Co., Inc., sponsored and funded this study. Employees of the sponsor (indicated on the title page) may own stock or stock options in the company. C.H. has served as an investigator and consultant for Merck related to its influenza, HIV-1, and Staphylococcus aureus vaccine programs and has previously received fees for service on safety advisory committees for HIV-1 vaccine trials and for review of clinical safety reports for S. aureus vaccine trials. The report was drafted primarily by C.H., E.Q., J.E.S., and M.J.D. The sponsor formally reviewed a penultimate draft. All coauthors approved the final version of the manuscript.
Footnotes
Published ahead of print on 15 July 2009.
REFERENCES
- 1.Aste-Amézaga, M., A. J. Bett, F. Wang, D. R. Casimiro, J. M. Antonello, D. K. Patel, E. C. Dell, L. L. Franlin, N. M. Dougherty, P. S. Bennett, H. C. Perry, M. E. Davies, J. W. Shiver, P. M. Keller, and M. D. Yeager. 2004. Quantitative adenovirus neutralization assays based on the secreted alkaline phosphatase reporter gene: application in epidemiologic studies and in the design of adenovector vaccines. Hum. Gene Ther. 15:293-304. [DOI] [PubMed] [Google Scholar]
- 2.Buchbinder, S. P., D. V. Mehrotra, A. Duerr, D. W. Fitzgerald, R. Mogg, D. Li, P. B. Gilbert, J. R. Lama, M. Marmor, C. del Rio, M. J. McElrath, D. R. Casimiro, K. M. Gottesdiener, J. A. Chodakewitz, L. Corey, M. N. Robertson, and the Step Study Protocol Team. 2008. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet 372:1881-1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Capone, S., A. Meola, B. B. Ercole, et al. 2006. A novel adenovirus type 6 (Ad6)-based hepatitis C virus vector that overcomes preexisting anti-Ad5 immunity and induces potent and broad cellular immune responses in rhesus macaques. J. Virol. 80:1688-1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casimiro, D. R., L. Chen, T. M. Fu, R. K. Evans, M. J. Caulfield, M. E. Davies, A. Tang, M. Chen, L. Huang, V. Harris, D. C. Freed, K. A. Wilson, S. Dubey, D. M. Zhu, D. Nawrocki, H. Mach, R. Troutman, L. Isopi, D. Williams, W. Hurni, Z. Xu, J. G. Smith, S. Wang, X. Liu, L. Guan, R. Long, W. Trigona, G. J. Heidecker, H. C. Perry, N. Persaud, T. J. Toner, Q. Su, X. Liang, R. Youil, M. Chastain, A. J. Bett, D. B. Volkin, E. A. Emini, and J. W. Shiver. 2003. Comparative immunogenicity in rhesus monkeys of DNA plasmid, recombinant vaccinia virus, and replication-defective adenovirus vectors expressing a human immunodeficiency virus type 1 gag gene. J. Virol. 77:6305-6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Catanzaro, A. T., R. A. Koup, M. Roederer, R. T. Bailer, M. E. Enama, Z. Moodie, L. Gu, J. E. Martin, L. Novik, B. K. Chakrabarti, B. T. Butman, J. G. Gall, C. R. King, C. A. Andrews, R. Sheets, P. L. Gomez, J. R. Mascola, G. J. Nabel, B. S. Graham, and the Vaccine Research Center 006 Study Team. 2006. Phase 1 safety and immunogenicity evaluation of a multiclade HIV-1 candidate vaccine delivered by a replication-defective recombinant adenovirus vector. J. Infect. Dis. 194:1638-1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chapman, B. S., R. M. Thayer, K. A. Vincent, and N. L. Haigwood. 1991. Effect of intron A from human cytomegalovirus (Towne) immediate-early gene on heterologous expression in mammalian cells. Nucleic Acids Res. 19:3979-3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coplan, P. M., S. B. Gupta, S. A. Dubey, P. Pitisuttithum, A. Nikas, B. Mbewe, E. Vardas, M. Schechter, E. G. Kallas, D. C. Freed, T. M. Fu, C. T. Mast, P. Puthavathana, J. Kublin, K. Brown-Collins, J. Chisi, R. Pendame, S. J. Thaler, G. Gray, J. Mcintyre, W. L. Straus, J. H. Condra, D. V. Mehrotra, H. A. Guess, E. A. Emini, and J. W. Shiver. 2005. Cross-reactivity of anti-HIV-1 T cell immune responses among the major HIV-1 clades in HIV-1-positive individuals from 4 continents. J. Infect. Dis. 191:1427-1434. [DOI] [PubMed] [Google Scholar]
- 8.D'Ambrosio, E., N. Del Grosso, A. Chicca, and M. Midulla. 1982. Neutralizing antibodies against 33 human adenoviruses in normal children in Rome. J. Hyg. 89:155-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dubey, S., J. Clair, T. M. Fu, L. Guan, R. Long, R. Mogg, K. Anderson, K. B. Collins, C. Gaunt, V. R. Fernandez, L. Zhu, L. Kierstead, S. Thaler, S. B. Gupta, W. Straus, D. Mehrotra, T. W. Tobery, D. R. Casimiro, and J. W. Shiver. 2007. Detection of HIV vaccine-induced cell-mediated immunity in HIV-seronegative clinical trial participants using an optimized and validated enzyme-linked immunospot assay. J. Acquir. Immune Defic. Syndr. 45:20-27. [DOI] [PubMed] [Google Scholar]
- 10.Fu, T. M., S. A. Dubey, D. V. Mehrotra, D. C. Freed, W. L. Trigona, L. Adams-Muhler, J. H. Clair, T. G. Evans, R. Steigbigel, J. M. Jacobson, P. A. Goepfert, M. J. Mulligan, S. A. Kalams, C. Rinaldo, L. Zhu, K. S. Cox, L. Guan, R. Long, N. Persaud, M. J. Caulfield, J. C. Sadoff, E. A. Emini, S. Thaler, and J. W. Shiver. 2007. Evaluation of cellular immune responses in subjects chronically infected with HIV type 1. AIDS Res. Hum. Retrovir. 23:67-76. [DOI] [PubMed] [Google Scholar]
- 11.Gabitzsch, E. S., Y. Xu, L. H. Yoshida, J. Balint, R. B. Gayle, A. Amalfitano, and F. R. Jones. 2009. A preliminary and comparative evaluation of a novel Ad5 [E1-, E2b-] recombinant-based vaccine used to induce cell mediated immune responses. Immunol. Lett. 122:44-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goodwin, E. C., and F. M. Rottman. 1992. The 3′-flanking sequence of the bovine growth hormone gene contains novel elements required for efficient and accurate polyadenylation. J. Biol. Chem. 267:16330-16334. [PubMed] [Google Scholar]
- 12a.Harro, C., X. Sun, R. Y. Leavitt, D. V. Mehrotra, J. E. Stek, M. J. DiNubile, and E. K. Quirk. 2007. Safety and immunogenicity of an adenovirus type-6 (MRKAd6) HIV-1 gag/pol/nef trigene vaccine dose escalation study, abstr. OA08-05. Abstr. AIDS Vaccine 2007 Conf., Seattle, WA, August 2007. http://www.hivvaccineenterprise.org/_dwn/Oral_Sessions.pdf.
- 12b.Harro, C., X. Sun, R. Y. Leavitt, D. V. Mehrotra, J. E. Stek, M. J. DiNubile, and E. K. Quirk. 2007. Safety and immunogenicity of the MRKAd5 HIV-1 trigene and MRKAd6 HIV-1 trigene vaccines in combination (MRKAd5+6 trigene vaccine) in healthy adults, abstr. OA08-06. Abstr. AIDS Vaccine 2007 Conf., Seattle, WA, August 2007. http://www.hivvaccineenterprise.org/_dwn/Oral_Sessions.pdf.
- 13.Harro, C. D., M. N. Robertson, M. A. Lally, L. D. O'Neill, S. Edupuganti, P. A. Goepfert, M. J. Mulligan, F. H. Priddy, S. A. Dubey, L. S. Kierstead, X. Sun, D. R. Casimiro, M. J. DiNubile, J. W. Shiver, R. Y. Leavitt, and D. V. Mehrotra. 2009. Safety and immunogenicity of adenovirus-vectored near-consensus HIV type 1 clade B gag vaccines in healthy adults. AIDS Res. Hum. Retrovir. 25:103-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harvey, B. G., J. Maroni, K. A. O'Donoghue, K. W. Chu, J. C. Muscat, A. L. Pippo, C. E. Wright, C. Hollmann, J. P. Wisnivesky, P. D. Kessler, H. S. Rasmussen, T. K. Rosengart, and R. G. Crystal. 2002. Safety of local delivery of low- and intermediate-dose adenovirus gene transfer vectors to individuals with a spectrum of morbid conditions. Hum. Gene Ther. 13:15-63. [DOI] [PubMed] [Google Scholar]
- 15.Mack, C. A., W. R. Song, H. Carpenter, et al. 1997. Circumvention of anti-adenovirus neutralizing immunity by administration of an adenoviral vector of an alternate serotype. Hum. Gene Ther. 8:99-109. [DOI] [PubMed] [Google Scholar]
- 16.McElrath, M. J., S. C. De Rosa, Z. Moodie, S. Dubey, L. Kierstead, H. Janes, O. D. Defawe, D. K. Carter, J. Hural, R. Akondy, S. P. Buchbinder, M. N. Robertson, D. V. Mehrotra, S. G. Self, L. Corey, J. W. Shiver, D. R. Casimiro, and the Step Study Protocol Team. 2008. HIV-1 vaccine-induced immunity in the test-of-concept Step Study: a case-cohort analysis. Lancet 372:1894-1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Myers, G., B. Korber, B. H. Hahn, K. T. Jeang, J. W. Mellors, F. E. McCutchan, L. E. Henderson, and G. N. Pavlakis (ed.). 1995. Human retroviruses and AIDS 1995: a compilation and analysis of nucleic acid and amino acid sequences. Theoretical Biology and Biophysics Group T-10, Los Alamos National Laboratory, Los Alamos, NM.
- 18.Priddy, F. H., D. Brown, J. Kublin, K. Monahan, D. P. Wright, J. Lalezari, S. Santiago, M. Marmor, M. Lally, R. M. Novak, S. J. Brown, P. Kulkarni, S. A. Dubey, L. S. Kierstead, D. R. Casimiro, R. Mogg, M. J. DiNubile, J. W. Shiver, R. Y. Leavitt, M. N. Robertson, D. V. Mehrotra, E. Quirk, and the Merck V520-016 Study Group. 2008. Safety and immunogenicity of a replication-incompetent adenovirus type 5 HIV-1 clade B gag/pol/nef vaccine in healthy adults. Clin. Infect. Dis. 46:1769-1781. [DOI] [PubMed] [Google Scholar]
- 19.Quirk, E. K., R. Mogg, D. D. Brown, M. A. Lally, D. V. Mehrotra, M. J. DiNubile, and M. N. Robertson. 2008. HIV seroconversion without infection after receipt of adenovirus-vectored HIV type 1 vaccine. Clin. Infect. Dis. 47:1593-1599. [DOI] [PubMed] [Google Scholar]
- 20.Santra, S., M. S. Seaman, L. Xu, D. H. Barouch, C. I. Lord, M. A. Lifton, D. A. Gorgone, K. R. Beaudry, K. Svehla, B. Welcher, B. K. Chakrabarti, Y. Huang, Z. Y. Yang, J. R. Mascola, G. J. Nabel, and N. L. Letvin. 2005. Replication-defective adenovirus serotype 5 vectors elicit durable cellular and humoral immune responses in nonhuman primates. J. Virol. 79:6516-6522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmitz, H., R. Wigand, and W. Heinrich. 1983. Worldwide epidemiology of human adenovirus infections. Am. J. Epidemiol. 117:455-466. [DOI] [PubMed] [Google Scholar]
- 22.Shiver, J. W., and E. A. Emini. 2004. Recent advances in the development of HIV-1 vaccines using replication-incompetent adenovirus vectors. Annu. Rev. Med. 55:355-372. [DOI] [PubMed] [Google Scholar]
- 23.Shiver, J. W., T. M. Fu, L. Chen, D. R. Casimiro, M. E. Davies, R. K. Evans, Z. Q. Zhang, A. J. Simon, W. L. Trigona, S. A. Dubey, L. Huang, V. A. Harris, R. S. Long, X. Liang, L. Handt, W. A. Schleif, L. Zhu, D. C. Freed, N. V. Persaud, L. Guan, K. S. Punt, A. Tang, M. Chen, K. A. Wilson, K. B. Collins, G. J. Heidecker, V. R. Fernandez, H. C. Perry, J. G. Joyce, K. M. Grimm, J. C. Cook, P. M. Keller, D. S. Kresock, H. Mach, R. D. Troutman, L. A. Isopi, D. M. Williams, Z. Xu, K. E. Bohannon, D. B. Volkin, D. C. Montefiori, A. Miura, G. R. Krivulka, M. A. Lifton, M. J. Kuroda, J. E. Schmitz, N. L. Letvin, M. J. Caulfield, A. J. Bett, R. Youil, D. C. Kaslow, and E. A. Emini. 2002. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature 415:331-335. [DOI] [PubMed] [Google Scholar]
- 24.Singh, N., A. Pandey, L. Jayashankar, and S. K. Mittal. 2008. Bovine adenoviral vector-based H5N1 influenza vaccine overcomes exceptionally high levels of pre-existing immunity against human adenovirus. Mol. Ther. 16:965-971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sprangers, M. C., W. Lakhai, W. Koudstaal, M. Verhoeven, B. F. Koel, R. Vogels, J. Goudsmit, M. J. Havenga, and S. Kostense. 2003. Quantifying adenovirus-neutralizing antibodies by luciferase transgene detection: addressing pre-existing immunity to vaccine and gene therapy vectors. J. Clin. Microbiol. 41:5046-5052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tatsis, N., and C. J. Hildegund. 2004. Adenovirus as vaccine vectors. Mol. Ther. 10:616-629. [DOI] [PMC free article] [PubMed] [Google Scholar]