Abstract
The immune response elicited by LC16m8, a candidate smallpox vaccine that was developed in Japan by cold selection during serial passage of the Lister vaccine virus in primary rabbit kidney cells, was compared to Dryvax in a mouse model. LC16m8 carries a mutation resulting in the truncation of the B5 protein, an important neutralizing target of the extracellular envelope form of vaccinia virus (EV). LC16m8 elicited a broad-spectrum immunoglobulin G (IgG) response that neutralized both EV and the intracellular mature form of vaccinia virus and provoked cell-mediated immune responses, including the activation of CD4+ and CD8+ cells, similarly to Dryvax. Mice inoculated with LC16m8 had detectable but low levels of anti-B5 IgG compared to Dryvax, but both Dryvax and LC16m8 sera neutralized vaccinia virus EV in vitro. A truncated B5 protein (∼8 kDa) was expressed abundantly in LC16m8-infected cells, and both murine immune sera and human vaccinia virus immunoglobulin recognized the truncated recombinant B5 protein in antigen-specific enzyme-linked immunosorbent assays. At a high-dose intranasal challenge (100 or 250 50% lethal doses), LC16m8 and Dryvax conferred similar levels of protection against vaccinia virus strain WR postvaccination. Taken together, the results extend our current understanding of the protective immune responses elicited by LC16m8 and indicate that the relative efficacy in a mouse model rivals that of previously licensed smallpox vaccines.
The last naturally occurring case of smallpox was reported in 1977. Following the successful containment of a laboratory-acquired case in Birmingham, England, in 1978, smallpox was officially declared eradicated by the World Health Organization in 1980, after the disease had claimed millions of human lives across the globe during a pandemic that lasted several millennia. Despite the eradication of smallpox, the potential for a reemergence of smallpox and a prevailing threat of clinical infections by other poxviruses associated with smallpox-like symptoms in humans make a sustained supply of smallpox vaccines imperative. For example, outbreaks of zoonotic monkeypox virus infection of humans have been reported in different parts of the world in the past four decades (4, 17), including a 2003 outbreak in the United States (5, 31).
Adverse events associated with traditional smallpox vaccines necessitated the quest for new vaccines with less reactogenicity. Two attenuated smallpox vaccines, modified vaccinia virus Ankara (MVA) (25) and a Lister virus derivative (LC16m8), underwent considerable development near the end of the smallpox eradication campaign. LC16m8 was developed as an attenuated smallpox vaccine in Japan in the early 1970s by about 45 serial passages and cold selection of the Lister/Elstree vaccine virus in primary rabbit kidney cells (13). Unlike MVA, LC16m8 does not carry large deletions of gene blocks in its genome and can replicate productively in mammalian cells. Similar to its parent virus strain (Lister/Elstree), for instance, inoculation of LC16m8 by scarification on the skin surface results in a “take” that manifests as a pock lesion that is characteristic of replication-competent smallpox vaccines. In a number of animal models (mice, rabbits, and monkeys), vaccination with LC16m8 conferred protection against lethal poxvirus challenge (7, 32; reviewed in reference 20). LC16m8 was used as a smallpox vaccine in Japanese children at the end of the smallpox eradication campaign in the 1970s, but its efficacy against smallpox is unknown, since smallpox was no longer endemic at the time of its use. A major genetic difference between LC16m8 and the parent Lister vaccine strain used in the campaign to eradicate smallpox has been mapped to a single-base-pair deletion within the open reading frame that encodes the vaccinia virus B5 protein (28, 38). This mutation creates a stop codon in B5R that theoretically would encode a 91-amino-acid protein instead of the 317-amino-acid full-length B5 protein. In addition, if this 91-amino-acid protein were processed correctly, it should be secreted as a 73-amino-acid protein. The B5R mutation in LC16m8 has been shown to be responsible for the smaller-plaque phenotype, smaller pock size on chorioallantoic membranes, and restricted growth in Vero cells relative to that of the Lister strain (37, 38). B5 is a 42- to 45-kDa protein component of the extracellular enveloped virus form of vaccinia virus (EV), one of two major mature infectious forms of vaccinia virus. These two infectious forms of vaccinia virus, intracellular mature virus (MV) and EV, are antigenically distinct. MV is thought to be primarily responsible for host-to-host spread, whereas the EV form, which contains an additional membrane and several EV-specific proteins, facilitates the spread of vaccinia virus in animal hosts (reviewed in reference 36). A robust antibody response to B5 is elicited in humans following vaccination with Dryvax (18, 23), and B5 was identified as being the major target for vaccinia virus EV-neutralizing antibody (3, 30), suggesting its importance for the protective immune response to vaccinia virus-based smallpox vaccines. In addition, immunization with subunit B5 vaccines in animal models elicited robust antibody responses and protected animals from severe disease symptoms (2, 8, 9, 11, 14) following challenge with lethal doses of poxviruses.
The evaluation of new-generation smallpox vaccines such as LC16m8 is complicated by an inadequate understanding of the correlates of immune protection conferred by traditional smallpox virus vaccines (e.g., Dryvax and Lister/Elstree) that were used during the smallpox eradication campaign. Thus, rigorous and detailed analyses of protective efficacy and the immune responses elicited by new-generation smallpox vaccines in appropriate animal models, complemented with controlled safety and immunogenicity clinical trials with humans, are critical for the evaluation of the immunogenicity of new-generation smallpox vaccines. To date, only a limited number of studies characterizing the protective immune response to LC16m8 in animal models have been reported (reviewed in reference 20), and they include various models and techniques that make generalizations about the relative immunogenicity and protective capacity of LC16m8 difficult. We have undertaken a detailed comparison of a variety of immune responses to LC16m8 and Dryvax in a mouse model of vaccination and challenge. Dryvax was chosen for this comparison because it was used during the smallpox eradication campaign and was the only available licensed smallpox vaccine in the United States for many years. We show here that when administered by tail scarification, LC16m8 elicited antibody and cell-mediated immune responses similar to those of Dryvax. Because of the known mutation in the LC16m8 EV gene encoding B5, we focused particular attention on the EV antibody response. We find that the EV-neutralizing activities induced by LC16m8 and Dryvax are similar and, interestingly, that LC16m8 also elicits an antibody response to B5. We demonstrate that a truncated B5 protein (∼8 kDa) predicted by the LC16m8 sequence is the predominant form of B5 expressed in whole-cell lysates and infected-cell supernatants of cells infected with LC16m8 and that immune sera from immunized mice as well as human vaccinia virus immunoglobulin (VIG) recognize truncated B5. The results also suggest that LC16m8 retains EV-neutralizing activity in spite of the truncation in B5. Finally, using a rigorous intranasal vaccinia virus challenge animal model, we find that vaccination with LC16m8 and Dryvax conferred similar levels of protection in mice. Taken together, the results extend our current understanding of the protective immune responses elicited by LC16m8 and indicate that the relative efficacy in a mouse model rivals that of previously licensed smallpox vaccines.
MATERIALS AND METHODS
Viruses.
Lyophilized LC16m8 (obtained from Kaketsuken, Kumamoto, Japan) was reconstituted in LC16m8 diluent supplied by the manufacturer and stored at 4°C. The titer of LC16m8 was determined using RK-13 cells (ATCC CCL-37; ATCC, Manassas, VA). The Dryvax virus seed stock was derived from a reconstituted vial of the licensed smallpox vaccine, Dryvax (lot number 321401), manufactured by Wyeth, and was prepared from infected BSC-1 cells as previously described (18), and titers were determined using BSC-40 cells. Vaccinia virus strain WR and vaccinia virus strain IHD-J were prepared from infected BSC-40 cells as previously described (27), and titers were determined using BSC-40 cells. For EV production, vaccinia virus strain IHD-J was propagated in RK-13 cells, culture supernatant was harvested after 48 h of infection, and titers were determined using BSC-40 cells. Vaccinia virus NYCBH strain (Dryvax and WR) suspensions for inoculation were prepared in sterile endotoxin-free phosphate-buffered saline (PBS).
Immunizations.
In all experiments, 4- to 5-week-old male BALB/cByJ and nude (CByJ.Cg-Foxn1<nu>/J-hom [nu/nu]) strains of mice were obtained from the Jackson Laboratory, Bar Harbor, ME, housed, and cared for at a CBER animal facility according to guidelines of the Animal Research Advisory Committee of the National Institutes of Health. Intramuscular and subcutaneous inoculations were performed as previously described (27). For tail scarification, viruses were diluted to 2.5 × 108 PFU/ml, and mice were anesthetized with Avertin (2,2,2,-tribromoethanol dissolved in tertiary amyl alcohol) that was diluted in sterile PBS. The anesthesia was administered (20 μl [308 μg] per gram body weight) by intraperitoneal injection. Animals were pricked/scratched approximately 15 to 20 times with a 25-gauge needle at the base of the tail, and 4 μl (106 PFU) of the appropriate virus suspension (LC16m8 or Dryvax) was applied to the scarified skin surface.
Inactivated vaccinia virus antibody ELISA.
MV antibody enzyme-linked immunosorbent assay (ELISA) for the quantitation of total immunoglobulin G (IgG) and IgG isotype distribution was performed using psoralen/UV irradiation-inactivated vaccinia virus (Dryvax) MV particles as previously described (27).
Preparation of vaccinia virus EV proteins and vaccinia virus EV antibody ELISA.
Previously, we described the development of an ELISA for the quantitative measurement of anti-A33 antibody using a recombinant A33 protein as an antigen (27). The construction and purification of a recombinant vaccinia virus B5 protein were described previously (10). A similar method was used to construct a recombinant truncated B5 protein that includes only the 91 amino acids N terminal of the B5 polypeptide, designated B5del91. Briefly, the nucleotide sequence encoding B5del91 was amplified by PCR using primers 5′-CACCATGAAAACGATTTCCGTTGTTACGTTG and 5′-TTATTTGCCGTCAATAAGACGTGGATCAACCTGATCTTCTTCGTATAGCGGTTTATTATATAGTTC and plasmid pSFV-B5R-prC, containing the B5R open reading frame from Dryvax (10), as a template. The amplified PCR product was cloned into Semliki Forest virus expression vector pSFV to generate plasmid pSFV-B5 del91. The B5del91 product was expressed in BHK-21 cells as described previously (33, 34), and the recombinant protein was partially purified from infected-cell lysates by affinity chromatography as previously described (10). Immulon 2HB plates were coated with optimal concentrations of the A33 or B5 protein or the truncated B5del91 protein and used for ELISAs as described previously (27).
Detection of B5 in virus-infected cells.
Confluent monolayers of RK-13 cells in a six-well tissue culture plate were infected with LC16m8 or Dryvax at a multiplicity of infection of 2.0. After 2 h of virus adsorption, infection medium was removed from cells, and cells were rinsed with fresh culture medium. Each well received 1 ml growth medium, and the plate was reincubated for 48 h. The culture supernatant (100 μl) from each well was sampled. The infected cells and uninfected control cells were scraped, harvested, and pelleted at 2,000 rpm at 4°C for 5 min. Cell pellets were lysed in radioimmunoprecipitation assay buffer (Thermo Scientific, Rockford, IL), and the lysate was clarified by centrifugation at 13,000 rpm at 4°C for 1 min. The sampled supernatants and clarified cell lysates were boiled and resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Detection of proteins was done by standard Western blotting by using a mouse anti-B5 monoclonal antibody, NR-561 (1) (BEI Resources, Manassas, VA), at a 1:1,000 dilution as a primary antibody and ECL anti-mouse peroxidase at 1:2,500 as a detection antibody. Following the addition of the ECL West Duran Super Signal peroxidase substrate (Thermo Scientific, Rockford, IL), images of protein bands were captured by chemiluminescence using Image Reader LAS-3000 software (Fuji Medical Systems, Stamford, CT).
EV neutralization assay.
Vaccinia virus EV was produced from RK-13 cells infected with the IHD-J strain of vaccinia virus as previously described (41), with slight modifications. Briefly, for each fresh EV production, a confluent T-75 flask monolayer of RK-13 cells was infected with vaccinia virus strain IHD-J at a multiplicity of infection of 3.0. After 2 h of virus adsorption to cells, the infection medium was aspirated, and cells were rinsed with 10 ml of culture medium. Fresh cell culture medium (10 ml) was added to the cells, and the culture flask was reincubated for 48 h. The culture supernatant was harvested and centrifuged at 800 rpm for 10 min at 4°C. The clarified supernatant was consistently about 2 × 108 PFU/ml when titrated in the presence of a mixture of vaccinia virus MV-neutralizing antibodies: an in-house purified monoclonal antibody,10F5, that binds the vaccinia virus MV L1 protein and an affinity-purified rabbit anti-vaccinia virus A27 IgG, which binds the vaccinia virus MV A27 protein. Monoclonal antibody 10F5 was purified from mouse hybridomas (a gift from Bernard Moss, NIAID, NIH), and anti-A27 antibody was a generous gift of Yong He (CBER, FDA). Serum samples from mice treated with LC16m8, Dryvax, or PBS were pooled by treatment group, and each pool was diluted 1:50 and tested for EV neutralization. Freshly prepared EV (300 PFU) was incubated for 1.5 h at 37°C with a 1:50 dilution of each serum pool in the presence of 10F5 (1:100 dilution of a 1.4-mg/ml stock) and anti-A27 (1:50 dilution of a ∼0.4-mg/ml stock) antibodies. Antibody-treated EV was used to infect confluent monolayers of BSC-40 cells (duplicate wells per test sample) at an input of 100 PFU/well in a 12-well tissue culture plate. After 2 h, infection medium was removed, and medium containing 5% methylcellulose was added. The plate was reincubated for 24 to 48 h, and cells were fixed and stained with crystal violet solution. The number of plaques was counted, and the percent EV neutralization was calculated relative to the number of plaques in control wells where EV was not treated with antibodies (except with anti-MV antibodies 10F5 and anti-A27).
EV neutralization using serum samples depleted of B5-specific IgG was performed as described above except that a 1:50 dilution of each test antiserum (or control sera) was preincubated (in PBS at 37°C for 1.5 h) with 20 μg of affinity-purified B5 protein (NR-2624; BEI Resources, Manassas, VA) prior to the EV neutralization assay.
Intracellular cytokine staining.
Splenocytes from animals inoculated with PBS, LC16m8, or Dryvax and from naïve mice were prepared as previously described (26). Spleen cells were plated in 24-well tissue culture plates at 5 × 106 cells in 1 ml of medium per well and were stimulated either with live vaccinia virus strain WR at a multiplicity of infection of 1.0 or without virus. Control (naïve) splenocytes were plated in wells that had been precoated (2.5 μg per well in bicarbonate buffer) overnight with purified hamster anti-mouse CD3e monoclonal antibody (BD Pharmingen). After approximately 16 h of incubation, 10 μg of brefeldin A was added to each well, and plates were incubated for an additional 2.5 h. Splenocytes were harvested, washed in ice-cold PBS containing 2% fetal bovine serum, and resuspended in the same buffer. Cells were dispensed at 106 cells per tube in 5-ml polystyrene round-bottomed fluorescence-activated cell sorter tubes (Becton Dickinson Labware, Franklin Lakes, NJ) and blocked with Fc block (except for “unstained” cells) for 10 min. Cells were incubated with fluorescein isothiocyanate (FITC)-conjugated rat anti-mouse CD8a (CD8-FITC) or phycoerythrin (PE)-conjugated rat anti-mouse CD4 (CD4-PE) on ice for 30 min in the dark. All labeling antibodies were obtained from BD Pharmingen. After three washes in PBS-2% fetal bovine serum (FBS), cells were resuspended in wash buffer and fixed in 1% paraformaldehyde for 20 min in the dark. The fixative was removed by centrifugation at 1,000 rpm for 10 min, and cells were washed once in PBS-2% FBS and resuspended in wash buffer. Cell pellets in tubes that were to be labeled with allophycocyanin (APC)-conjugated rat anti-mouse gamma interferon (IFN-γ) (IFN-γ-APC) or with combinations of CD8-FITC and IFN-γ-APC or CD4-PE and IFN-γ-APC were permeabilized in buffer containing 0.11% saponin, and IFN-γ-APC was added to each tube. After 30 min of incubation on ice, cells were washed once in permeabilization buffer and twice in PBS-2% FBS and resuspended in the latter. Data (20,000 events per sample) were acquired using a FACS Diva flow cytometer (BD Biosciences) and analyzed with FlowJo fluorescence-activated cell sorter analysis software (Tree Star, Inc., Ashland, OR).
Lethal intranasal challenge.
Intranasal challenge of animals with vaccinia virus strain WR was performed as previously described (26). Briefly, mice were weighed, anesthetized with Avertin, and inoculated intranasally with 100 50% lethal doses (LD50) (3.2 × 106 PFU) or 250 LD50 (8 × 106 PFU) of vaccinia virus strain WR on day 0. Mice were weighed over a 2-week period postchallenge, and animals that had lost 25% of their initial body weight were euthanized.
Statistical analysis.
The significance of the difference (P < 0.05) between pairs of treatment groups was determined by an unpaired two-tailed Student's t test by using InStat software (GraphPad Software, Inc.).
RESULTS
Vaccinia virus antibody response to LC16m8.
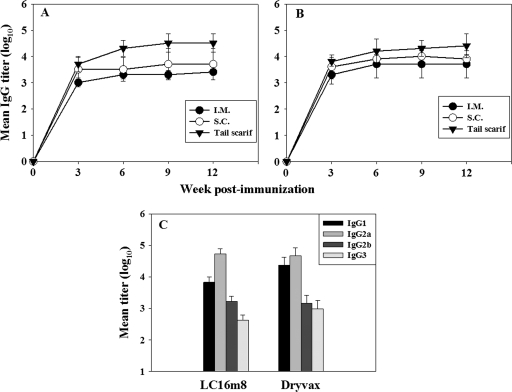
In order to compare the antibody responses to LC16m8 and Dryvax, groups of mice were vaccinated with 106 PFU of LC16m8 or Dryvax by tail scarification. In the first set of experiments, groups of three mice were inoculated with LC16m8 or Dryvax via three different routes: intramuscular, subcutaneous, and tail scarification. A control group was treated with PBS by tail scarification. Serum samples were collected at 3-week intervals for 12 weeks following immunization and analyzed for total anti-vaccinia virus IgG by ELISA by using inactivated Dryvax as an antigen. There was no detectable anti-vaccinia virus IgG in sera obtained from the PBS control group. The mean (log10) IgG titers for the LC16m8 and Dryvax groups are shown in Fig. 1 (A and B, respectively). Serum samples obtained from LC16m8- and Dryvax-immunized mice had measurable levels of anti-vaccinia virus IgG that peaked at about 6 weeks and remained high up to 12 weeks postimmunization irrespective of the route of inoculation. For both LC16m8 and Dryvax, the intramuscular route of inoculation provoked the lowest level of antibody response, with week 6 mean titers (log10) of 3.31 and 3.71, respectively. For both LC16m8 and Dryvax immune sera, inoculation by tail scarification elicited the strongest IgG response, with week 6 IgG titers (log10) of 4.31 and 4.21, respectively. Antibody responses were higher in sera from subcutaneously immunized mice than from intramuscularly immunized mice. In two-tailed unpaired Student t tests, the differences in antibody titers between sera derived from mice vaccinated via the intramuscular and subcutaneous routes (LC16m8 and Dryvax), subcutaneous and tail scarification routes (LC16m8 and Dryvax), and intramuscular and tail scarification routes (Dryvax) were not statistically significant. The high level of antibodies induced by LC16m8 via tail scarification was, however, statistically significant (P < 0.05) compared to the intramuscular route. Inoculation by tail scarification was used in all subsequent experiments both because it elicited the highest level of antibody responses (Fig. 1) and because this percutaneous route is the traditional method of smallpox vaccine immunization of humans. Although the level of the total IgG response to LC16m8 was slightly higher than the level of response to Dryvax, the difference is not statistically significant, suggesting that the total vaccinia virus antibody response to LC16m8, as measured by ELISA using Dryvax antigen, is similar to that elicited by Dryvax immunization.
FIG. 1.
Antibody responses to LC16m8 and Dryvax. (A and B) Groups of mice (three per group) were inoculated with 106 PFU of LC16m8 (A) or Dryvax (B) by the intramuscular (I.M.), subcutaneous (S.C.), or percutaneous/tail scarification (Tail scarif) route. Serum samples were collected at intervals of 3 weeks and analyzed for vaccinia virus MV-specific IgG by ELISA by using inactivated Dryvax as an antigen. (C) Immune sera were tested for IgG isotype distribution. Error bars represent standard deviations.
To further characterize the antibody response to LC16m8, immune sera obtained from mice 6 weeks after immunization were analyzed for levels of IgG isotypes by ELISA. Both LC16m8 and Dryvax immune sera showed broad IgG isotype distribution, including high titers of IgG1 (log10 mean titers of 3.83 and 4.37, respectively) and IgG2a (log10 mean titers of 4.73 and 4.67, respectively) (Fig. 1C). Both IgG2b and IgG3 were also detectable but at lower titers than IgG1 and IgG2a. Thus, LC16m8 elicited broad antibody isotype profiles indicative of mixed Th1/Th2-type immune responses, as does Dryvax.
Sera from mice immunized with either LC16m8 or Dryvax by tail scarification were evaluated for the presence of neutralizing antibody using a vaccinia virus WR recombinant expressing β-galactosidase, as previously described (24, 27). Since purified vaccinia virus is utilized in this assay, the neutralization antibody measured is predominantly that directed to the MV form of the virus. Serum pools from PBS-treated mice had no detectable vaccinia virus-neutralizing activity. Sera from both LC16m8- and Dryvax-vaccinated mice neutralized vaccinia virus, with mean 50% neutralization titers (log10) of 2.29 (±0.09 standard deviations) and 2.26 (±0.12 standard deviations), respectively. Taken together, the results indicate that LC16m8 elicits a vaccinia virus-specific antibody response, including an anti-vaccinia virus MV-neutralizing response that is comparable to that elicited by an equivalent dose of the licensed smallpox vaccine, Dryvax.
EV-specific antibody response to LC16m8.
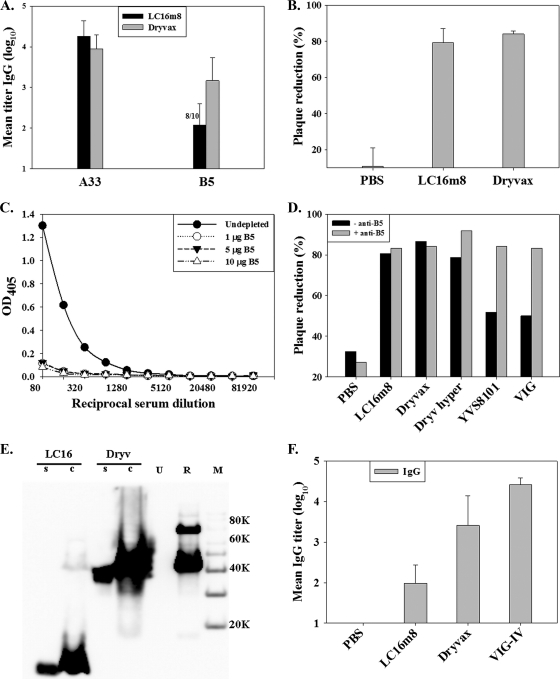
Vaccinia virus EV facilitates the dissemination of virus in an infected host. The B5 protein, which is responsible for the LC16m8 attenuation/small-plaque phenotype and whose open reading frame is truncated in LC16m8 (38), is critical for EV formation and was previously shown to be the major EV-neutralizing target (3, 30) in human VIG. To evaluate the antibody response to vaccinia virus EV, antigen-specific ELISAs were used to measure the antibody response to the EV A33 and B5 proteins. Serum samples obtained (6 weeks postimmunization) from mice inoculated with LC16m8 or Dryvax were tested for antibodies specific to the affinity-purified A33 and B5 proteins (10), respectively. Animals immunized with PBS had no detectable anti-A33 or anti-B5 antibodies (<1:20) (data not shown). ELISA data from one of two identical experiments (n = 10 each for LC16m8 and Dryvax) are presented in Fig. 2A. Both LC16m8 and Dryvax immune sera contained high titers (mean log10 titers of 4.26 and 3.95, respectively) of anti-A33 IgG, suggesting that a robust antibody response to A33 was elicited by either virus. All Dryvax-immunized mice (10/10 mice) had detectable anti-B5 IgG titers (titer range, 2.51 to 4.01 logs), with a mean (log10) titer of 3.17. Interestingly, 8 out of 10 (80%) immune sera from LC16m8-vaccinated mice had detectable levels (titer range, 1.90 to 2.81 logs) of anti-B5 IgG, with a mean titer (log10) of 2.08. Statistical analyses (unpaired Student t tests) of the LC16m8 and Dryvax antibody responses to the EV proteins showed that while the difference in anti-A33 antibody levels was not significant, the observed difference in anti-B5 antibody levels was significant (two-tailed P value of <0.05 for anti-B5 antibody comparison).
FIG. 2.
Antibody response to vaccinia virus EV proteins. Groups of five mice were vaccinated by tail scarification. (A) Serum samples were obtained from mice 6 weeks after vaccination and tested for A33-specific and B5-specific IgG. (B) Serum pools were diluted 1:50 and tested for neutralization of vaccinia virus EV by plaque reduction. (C) Depletion of B5-specific IgG from Dryvax hyperimmune serum was verified by ELISA. (D) Serum pools from mice inoculated with PBS, LC16m8, Dryvax, Dryvax hyperimmune serum (Dryv hyper), rabbit anti-vaccinia virus antibody (YVS8101), and human VIG were depleted (− anti-B5) or undepleted (+ anti-B5) of B5-specific IgG and tested for the neutralization of vaccinia virus EV. OD405, optical density at 405 nm. (E) Expression of the truncated B5 protein in LC16m8 (LC16)-infected or Dryvax (Dryv)-infected RK-13 cell supernatant (s) or cell lysate (c) was detected by Western blotting using a monoclonal antibody, NR-561 (1). Lane U represents uninfected-cell lysate, lane R represents affinity-purified recombinant B5, and lane M is the protein molecular weight marker. (F) Immune serum sample pools were tested by ELISA for levels of IgG to the truncated B5 using a recombinant truncated B5 protein as an antigen. Error bars represent standard deviations.
In order to determine whether the antibody response to LC16m8 included a neutralizing antibody response to vaccinia virus EV, pools of immune sera from mice that had been vaccinated with Dryvax or LC16m8 were tested for the ability to neutralize EV prepared from the culture supernatant of RK-13 cells infected with the IHD-J strain of vaccinia virus. Serum samples from LC16m8- and Dryvax-immunized mice showed a significant reduction in levels of EV plaque formation compared to those of the PBS-treated group (Fig. 2B), and the measured EV plaque reductions were similar for the two virus-immunized groups. The results indicate that LC16m8 induces B5-specific IgG in mice and that LC16m8 immune sera neutralized vaccinia virus EV despite the truncation in the B5R gene.
In order to determine whether the anti-B5 response in LC16m8 sera contributed to EV neutralization, mouse serum pools were depleted of B5-specific IgG by preincubation with affinity-purified B5 protein that was prepared in baculovirus prior to the EV neutralization assay. The depletion of B5-specific IgG was verified by B5-specific IgG ELISA (Fig. 2C). Serum samples (both LC16m8 and Dryvax sera) depleted of anti-B5 IgG retained the capacity to neutralize vaccinia virus EV (Fig. 2D). Similarly, a hyperimmune Dryvax mouse serum that was raised by multiple tail scarification with Dryvax retained EV neutralization, but a commercially available rabbit anti-vaccinia virus (Lister) antibody (YVS8101; Accurate Chemicals, Westbury, NY) and human VIG showed significant reductions in EV neutralization after the depletion of anti-B5 antibodies (Fig. 2D). The retention of EV neutralization in LC16m8 and Dryvax immune antisera after the depletion of B5-specific IgG suggests the possibility of B5-independent EV neutralization.
Expression and secretion of an LC16m8 8-kDa B5 protein.
The antibody response against B5 elicited by LC16m8 may arise due to the restoration of full-length B5R by mutation during the course of infection or may be a response to the truncated N-terminal 91-amino-acid polypeptide predicted from the DNA sequence of the LC16m8 B5R gene (21). To investigate these possibilities and to determine whether a truncated B5 protein is actually produced during LC16m8 infection, RK-13 cell monolayers were infected with LC16m8 or Dryvax, and infected-cell culture supernatants and whole-cell lysates were probed by Western blotting using a B5-specific monoclonal antibody (NR-561) (1) that recognizes an epitope between residues 56 and 75 of the B5 protein as the primary antibody (Fig. 2E). No bands were detected in either culture supernatant or whole-cell lysate of uninfected RK-13 cells. A band corresponding to the full-length B5 protein (∼42 to 45 kDa) was the only band identified in Dryvax-infected-cell supernatants or cell lysates. In contrast, culture supernatant and whole-cell lysates of LC16m8-infected cells had a prominent band at approximately 8 kDa, corresponding to the predicted size of a ∼73- to 91-amino-acid N-terminal polypeptide, demonstrating that the predicted truncated B5 was indeed expressed in LC16m8-infected RK-13 cells. However, low but discernible levels of full-length B5 were also detected in LC16m8-infected cells. Thus, although the truncated B5 protein appeared to be much more prominent than the full-length B5 protein, it is possible that the antibody response to B5 in LC16m8-treated mice could also have been provoked by full-length B5 expressed at relatively low levels. Although it is difficult to rule out this possibility, we verified that the truncated B5 protein could be recognized by antibodies made during infection with LC16m8 and Dryvax by developing an ELISA for truncated B5 using the same approach as that used for full-length B5. The first 91 amino acids of the B5 protein were expressed as a recombinant protein in Semliki Forest fever virus and purified by affinity chromatography as previously described (10). Sera from mice immunized with LC16m8 or Dryvax were analyzed by ELISA using the truncated B5 protein as the capture antigen (Fig. 2F). Similar to the results obtained using full-length B5 as an ELISA antigen, animals immunized with PBS had no detectable truncated B5 antibodies (<1:20), whereas both LC16m8 and Dryvax immune sera contained antibody that recognized the N-terminal polypeptide of B5. In addition, VIG contained antibodies to the truncated B5. Truncated B5 titers were lower in LC16m8- than in Dryvax-immunized mice (P < 0.05). The data indicate that the truncated B5R gene in LC16m8 results in the production of a truncated B5 protein and suggest that this protein may be responsible for the B5 antibody response following LC16m8 infection.
Cell-mediated immune response to LC16m8.
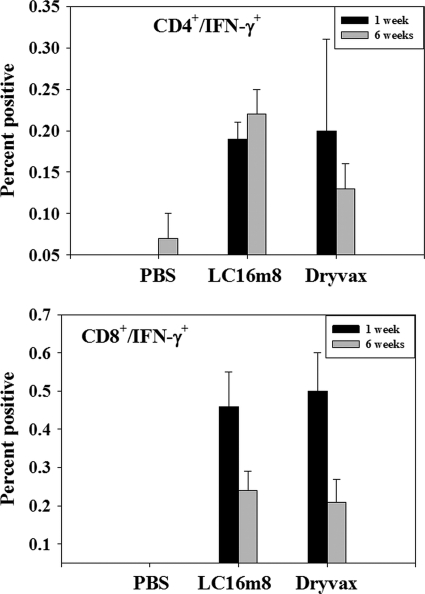
The cell-mediated immune response is believed to be an important component of the protective immune response against poxvirus infections. The cellular immune response to LC16m8 and Dryvax was assessed by intracellular cytokine staining of splenocytes obtained in mice 1 week and 6 weeks postimmunization. Spleen cells from treatment groups were harvested at either 1 week or 6 weeks postinoculation and restimulated in vitro with live vaccinia virus strain WR at a multiplicity of infection of 1.0. The restimulated spleen cells were stained for intracellular IFN-γ and analyzed for IFN-γ-secreting CD4+ and CD8+ cells. PBS-treated mice had no detectable CD4+/IFN-γ+ or CD8+/IFN-γ+ cells. Both Dryvax- and LC16m8-treated mice had detectable CD4+/IFN-γ+ and CD8+/IFN-γ+ cells, suggesting the activation of CD4+ and CD8+ lymphocytes in these mice. The mean percentages of CD4+/IFN-γ+ and CD8+/IFN-γ+ cells are presented in Fig. 3 for both the 1-week and 6-week analysis time points. The mean percentage of CD4+/IFN-γ+ remained high after 6 weeks for the LC16m8 group but dropped from 0.2% (week 1) to 0.13% (week 6) in the Dryvax group. The percentage of CD8+/IFN-γ+ cells was higher after vaccination, with mean percentages of week 1 positive cells of 0.46% and 0.50% for the LC16m8 and Dryvax groups, respectively. However, the percentage of CD8+/IFN-γ+-positive cells dropped to 0.24% (LC16m8) and 0.21% (Dryvax) by week 6. Furthermore, similar levels of IFN-γ-secreting cells were detected in spleen cells from LC16m8- and Dryvax-treated mice when tested by enzyme-linked immunospot assay (data not shown). Taken together, this set of data indicates that LC16m8, as well as Dryvax, effectively primed cells of the cellular arm of the immune response.
FIG. 3.
Cell-mediated immune response to LC16m8 and Dryvax. Groups of three mice were inoculated with PBS, LC16m8, or Dryvax, and splenocytes were obtained 1 week or 6 weeks later and restimulated in vitro with vaccinia virus strain WR at a multiplicity of infection of 1.0. Cells were harvested about 18.5 h poststimulation (including a 2.5-h incubation in the presence of brefeldin A) and stained for primed CD4+ and CD8+ cells expressing IFN-γ. The percentages of primed CD4+/IFN-γ+ and CD8+/IFN-γ+ spleen cells at 1 week and 6 weeks postimmunization for three mice in each group were averaged. Error bars represent standard deviations.
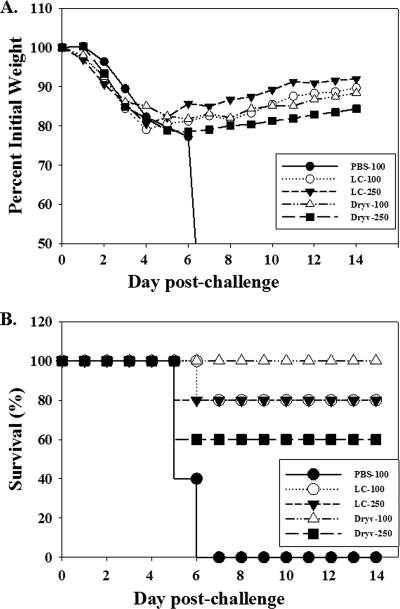
LC16m8 protection against lethal intranasal challenge with vaccinia virus.
The immune response data described above suggest that LC16m8 elicits levels of antibody and cellular immune responses comparable to those elicited by Dryvax in mice save for the significantly lower level of anti-B5 response provoked by LC16m8. The efficacy of LC16m8 in eliciting a protective immune response was evaluated in a series of experiments using the intranasal challenge model. Groups of mice were treated with LC16m8 or Dryvax and were challenged with vaccinia virus strain WR via the intranasal route and at 6 weeks postimmunization. In a preliminary experiment, all mice treated with LC16m8 or Dryvax survived a 25-LD50 intranasal challenge with vaccinia virus strain WR, while none in the PBS control group survived (data not shown). In subsequent experiments, mice were immunized with LC16m8 or Dryvax and challenged at high dosages of 100 LD50 and 250 LD50 of vaccinia virus strain WR. Figure 4 shows representative plots of disease progression measured by weight loss (A) and survival (B) from one of two identical experiments. Irrespective of the immunizing virus (LC16m8 or Dryvax), the majority of mice lost weight between day 3 and day 6 postchallenge, similar to PBS-treated mice (challenged with 100 LD50). However, some mice in the LC16m8 and Dryvax groups, but none in the PBS group, regained weight or survived beyond day 6. Among mice vaccinated with LC16m8, 80% survived either the 100-LD50 or the 250-LD50 challenge. All Dryvax-treated mice (100%) survived the 100-LD50 and 60% survived the 250-LD50 challenge. Thus, as with immune responses elicited by LC16m8 and Dryvax, the two vaccine viruses conferred similar levels of protection against stringent intranasal challenge with vaccinia virus strain WR.
FIG. 4.
Immune protection from lethal intranasal vaccinia virus challenge. Groups of five mice were inoculated with PBS (PBS-100), LC16m8 (LC-100 and LC-250), or Dryvax (Dryv-100 and Dryv-250). After 6 weeks, mice were challenged intranasally with vaccinia virus strain WR at 100 LD50 (“-100” groups) (3.2 × 106 PFU) or 250 LD50 (“-250” groups) (8.0 × 106 PFU) and weighed daily over a 2-week period. Changes in body weight postchallenge (A) and the percentage of surviving animals in each group after the 2-week monitoring period (B) are shown. Data are representative of two identical experiments.
DISCUSSION
Evaluation of new-generation smallpox vaccines will be complex. As in the case of most vaccines, safety will be evaluated both with preclinical animal models and in clinical trials. Because of the well-documented safety issues of traditional smallpox vaccines, there is a need for smallpox vaccines that produce less vaccine-related adverse events. LC16m8 was developed in Japan toward the end of the smallpox eradication campaign and is an attenuated virus derived by cold selection upon passage of the Lister vaccine strain in primary kidney cells (13). Although attenuated, LC16m8 is a replicating virus, and it is not yet clear in which target populations it would provide safety advantages over less-attenuated smallpox vaccines such as Dryvax. For example, all replicating vaccinia viruses might pose unacceptable risks for immunocompromised individuals. A direct comparison of LC16m8 with Dryvax for lethality upon intracranial inoculation has not been reported. However, initial studies of the characterization of LC16m8 compared the neurovirulence of LC16m8 with that of its parent virus (Lister strain) by intracerebral inoculations into rabbits and cynomolgus monkeys (13). The data suggested that LC16m8, relative to Lister, was attenuated. In preliminary experiments, we evaluated LC16m8 in BALB/cByJ and nu/nu mice in comparison to Dryvax by tail scarification (data not shown). All mice inoculated with vaccinia virus developed a typical vaccine “take” between days 8 and 15 after inoculation. While the inoculation site lesions healed in immunocompetent BALB/cByJ mice, about 4 weeks after the inoculations, the vaccinia virus-treated nu/nu mice developed pock lesions on their tails at sites distal from the inoculation site, with some having pock lesions on their eyes and trunk (data not shown). No noticeable difference in attenuation was seen between LC16m8- and Dryvax-treated nu/nu mice, suggesting that the reactogenicity of LC16m8 in immunocompromised animals is not different from that of Dryvax in the mouse model. Nevertheless, LC16m8 may have safety advantages over nonattenuated vaccinia virus vaccines in certain situations.
The efficacy evaluation of new-generation smallpox vaccines will require a combination of immunogenicity and protection studies of animal models complemented by immunogenicity studies of human clinical trials. Vaccination with LC16m8 was shown to elicit a broad immune responses and to protect animals from lethal poxvirus challenge in a number of animal models (7, 32) and was used to inoculate more than 50,000 children in Japan in the 1970s (reviewed in reference 20). However, the efficacy of LC16m8 against smallpox has never been demonstrated, since smallpox was no longer endemic at the time of its use in Japan. In the work described here, a comparative characterization of the immune responses to LC16m8 and Dryvax, a vaccine used in the campaign to eradicate smallpox, with particular emphasis on the antibody response to the vaccinia virus B5 protein, a dominant target for vaccinia virus EV neutralization (30) that is truncated in LC16m8, was undertaken.
Initially, we investigated the effect of dosage and route of inoculation on antibody responses to LC16m8 and Dryvax. Although vaccinia virus-based live smallpox vaccines were administered by the percutaneous route, the influence of the route of inoculation using attenuated live viral vaccines has not been studied. In a preliminary dose-ranging experiment in which groups of mice were inoculated subcutaneously with LC16m8, there was a direct correlation between vaccine dose and antibody levels (data not shown). This observation is similar to data from dose-ranging studies using MVA (27). Both LC16m8 and Dryvax induced a strong and persistent antibody response after a single inoculation. When the viruses were inoculated by the intramuscular, subcutaneous, and percutaneous (tail scarification) routes, the temporal antibody response patterns were similar (Fig. 1). For both viruses, the absolute intensity of the antibody reaction was similar for each route of inoculation, although the titer was always highest after tail scarification. This difference was more pronounced and statistically significant for LC16m8. This enhanced response to tail scarification could be the result of the proximity of the inoculating virus to the important first messengers of the immune response, ostensibly Langerhans cells, or it may reflect the propensity of LC16m8 to grow on skin, since it was selected for growth at reduced temperatures (13). A reduced immune response was also observed for clinical trials in which vaccinia virus was administered by alternatives to the percutaneous route (12).
The composition of the MV and EV forms of vaccinia virus has been well investigated (35; reviewed in reference 36), and the two forms mediate virus dissemination. Thus, it is likely that a potent smallpox vaccine must target both the MV and EV forms of vaccinia virus. Previous work with subunit vaccines have shown that maximum protection occurs when components of both the MV and EV forms are present in the vaccine (8, 15, 16, 29). LC16m8 encodes the complete set of MV and EV proteins, save the truncated form of B5 (21).
In our studies, similar broad-spectrum antibody responses were elicited by both LC16m8 and Dryvax, as determined by ELISA using Dryvax as the capture antigen (Fig. 1). A similar antibody analysis reported previously suggested that LC16m8 elicited higher total anti-vaccinia virus antibody titers than did Dryvax following immunization of mice (7). Although the assay in that study utilized vaccinia virus WR as a capture antigen rather than Dryvax, that difference seems unlikely to explain the conflicting results since both Dryvax and WR are NYCBH virus derivatives. We suspect that the different results are more likely explained by slight differences in immunizing virus titers used in the experiments. In our studies, we independently determined the titer of each virus stock at the same time before they were used in the reported experiments. Regardless, over the course of many independent experiments, we did not observe obvious differences in total anti-vaccinia virus antibody titers elicited by LC16m8 and Dryvax.
We also found that LC16m8 and Dryvax immunization elicited similar titers of neutralizing antibody, as determined by a quantitative reporter gene assay. As in a traditional plaque neutralization assay, the neutralizing antibody measured in the reporter gene assay is that which neutralizes the MV form of the virus. Somewhat conflicting results were previously reported regarding the relative levels of neutralizing antibody elicited by LC16m8 and nonattenuated vaccinia viruses. The results reported in one study suggested that LC16m8 elicited higher rabbitpox-neutralizing antibody titers than did Dryvax following immunization of rabbits (7). In a second study, similar levels of monkeypox-neutralizing antibody were induced by LC16m8 and the parent Lister virus (32). In a third study using a traditional plaque neutralization assay against vaccinia virus WR, similar levels of neutralizing antibody were observed in sera from mice immunized with either LC16m8 or Dryvax (21). Our results using a reporter gene assay appear to confirm that equivalent doses of LC16m8 and Dryvax elicit similar MV-neutralizing antibody titers in mice.
Galmiche et al. (9) previously demonstrated that immunization with A33 protected mice from lethal vaccinia virus challenge, indicating the importance of this EV antigen in protection. Similarly, sera from animals immunized with B5 neutralized EV infection in vitro, animals were protected from lethal vaccinia virus challenge, and the majority of EV-neutralizing antibody was shown to target B5 (3, 9, 30). A single-base deletion in the B5R gene in LC16m8 results in a truncated B5R gene such that the putative translational product is limited to the 91 N-terminal amino acids (38), including the N-terminal signal sequence, the full-length short consensus repeat 1 domain, and approximately 33% of the sequence of the short consensus repeat 2 domain (1).
We used multiple approaches to evaluate the comparative EV responses to LC16m8 and Dryvax, including EV-specific ELISAs and EV-neutralizing antibody assays. By ELISA, both LC16m8 and Dryvax provoked similarly high titers of anti-A33 antibody (Fig. 2A). Somewhat unexpectedly, sera from LC16m8 mice also contained B5-specific antibody, although the anti-B5 antibody level in LC16m8 immune sera was statistically significantly lower than that in Dryvax immune sera (two-tailed P value of <0.05), and a measurable anti-B5 response was not detected in every animal serum sample (Fig. 2A). In one other study, there was no detectable B5-specific antibody response following LC16m8 immunization (28). However, there was also no detectable anti-B5 response to Lister immunization in those experiments either, suggesting that the assay employed was not as sensitive as the one that we have developed and used here. In addition, we found that immune sera from mice vaccinated with LC16m8 neutralized EV as well as sera from mice vaccinated with Dryvax in vitro (Fig. 2B). Although EV neutralization following LC16m8 immunization of mice was not observed in a previous report (28), no EV neutralization was measurable in sera from Lister-immunized mice, again suggesting difficulties in assay sensitivity. In a separate report, similar levels of rabbitpox EV neutralization were measured in sera from LC16m8- and Dryvax-immunized rabbits (7). Taken together, the results indicated that LC16m8 induces an EV-neutralizing response similar to that of Dryvax.
In order to determine whether the observed EV neutralization activity in mouse immune sera reported here is directed against B5, B5-specific antibodies in LC16m8 and Dryvax antisera were depleted by preincubation with the B5 protein and tested for EV neutralization. Both Dryvax and LC16m8 immune sera depleted of B5-specific antibodies retained the capacity to neutralize vaccinia virus EV (Fig. 2D), suggesting the possibility of a B5-independent mechanism for EV neutralization. The difference between our data and those reported previously (3, 30) may in part be due to differences in antibody responses elicited in different host species. In this regard, it was observed that a rabbit anti-vaccinia virus polyclonal antibody neutralized EV that was produced from a mutant vaccinia virus that was deleted of the B5R gene (22). In our hands, a rabbit anti-vaccinia virus antibody and human VIG showed significant reductions in levels of EV neutralization following anti-B5 antibody depletion compared to mouse anti-Dryvax hyperimmune serum (Fig. 2D). Although a recent report noted that a baculovirus vector-expressed truncated B5 protein was not protective by itself (28), the contribution of the truncated B5 protein to LC16m8-mediated protection remains to be determined.
While it may seem counterintuitive that an appreciable anti-B5 titer could be generated by LC16m8 since the virus encodes only a truncated B5 protein, there are at least two plausible explanations that could account for this observation. First, the intact B5R gene can be restored by a single-base insertion generating full-length B5R, and such a virus has been shown to replicate much better than LC16m8 (21). The titer of anti-B5 antibody for each mouse vaccinated with LC16m8 may be reflective of how quickly the conversion of the replicating LC16m8 to a B5-expressing variant occurs. Consistent with this hypothesis, we observed that hyperimmune mouse serum raised by multiple tail scarification with LC16m8 recognized B5 by Western blot analysis, whereas LC16m8 hyperimmune serum raised by intraperitoneal inoculation did not despite containing high levels of anti-vaccinia virus IgG against the MV form detected by ELISA (data not shown). Limited replication at the elevated temperature in the peritoneum compared to the dermis might result in a limited amount of B5R reversion. The second possibility that might account for the generation of an appreciable antibody response to B5 in LC16m8-immunized animals is that the truncated B5 protein that is predicted from the sequence actually results in a protein that is immunogenic. We used several means to investigate this possibility. We found that the truncated form of B5 was abundantly expressed in RK-13 cells infected with LC16m8 and was detected with a monoclonal antibody, VMC-30 (NR 561) (1), that recognizes amino acids 56 to 75 of the B5 polypeptide (Fig. 2E). Here, we demonstrate, unambiguously, that the B5R lesion in LC16m8 results in the production of a truncated B5 protein. In addition, we expressed a recombinant version of the truncated B5 protein in a Semliki Forest virus vector system and used this purified antigen to set up a truncated B5-specific ELISA. The results demonstrated that serum samples from mice that were immunized with either LC16m8 or Dryvax recognized this truncated B5 antigen. High-titer human antibody (VIG) also recognizes the truncated B5 (Fig. 2F). Although the results cannot formally establish that the truncated B5 antigen produced by LC16m8 is responsible for the EV-neutralizing ability of sera from LC16m8-immunized mice, they strongly suggest this possibility. Additional studies to produce a high-titer antiserum that is specific to the truncated B5 protein may be able to help resolve this question.
Several studies indicated that cell-mediated immunity is also important for protection against orthopoxviruses (6, 19, 39, 40); however, there have been no previous comparative studies of the cell-mediated response to LC16m8. We showed that splenocytes from mice immunized with LC16m8 elicited specific T-cell responses (Fig. 3), including the activation of vaccinia virus-specific CD4+ and CD8+ cells similarly to Dryvax-treated mice, indicating that LC16m8 primes naïve T cells for a cellular response.
Since efficacy studies using humans are not feasible, animal protection studies are a critical component of the efficacy evaluation for candidate smallpox vaccines. However, there are a variety of animal models for assessing the protective capacity of smallpox vaccines that have been described. In addition, comparison of protective capacities is oftentimes not straightforward because of differences in experimental setups. In two previously reported studies using a mouse intranasal challenge model, LC16m8 appeared to provide protection similar to that of either vaccinia virus Lister or Dryvax when mice were challenged with relatively low doses of vaccinia virus WR (106 PFU [10 LD50]) (21, 28). In the experiments reported here, we used a similar mouse intranasal challenge model and observed that inoculation with equivalent doses of either LC16m8 or Dryvax protected mice against a high-dose challenge with vaccinia virus WR (100 or 250 LD50) (Fig. 4). These results confirm the previously reported results using this model and provide additional supportive data to indicate that LC16m8 may be similar to Dryvax in its ability to provide protection against an orthopoxvirus challenge. LC16m8 was also shown to be comparable to Dryvax for protection of rabbits against rabbitpox challenge and protection of mice against ectromelia virus challenge, although those experiments employed relatively low doses of orthopoxvirus challenge (7). Similarly, LC16m8 was shown to be comparable to vaccinia virus Lister for protection of monkeys against monkeypox challenge, although the rigor of the challenge doses used is not clear (32). Thus, although the total database is still limited, the data to date indicate a favorable comparison between the protective capacity of LC16m8 and those of other nonattenuated vaccinia viruses in several relevant animal models.
In summary, the data presented in this work suggest that, overall, LC16m8 and Dryvax elicited similar levels of antibody and cellular responses and conferred similar levels of protection in mice. However, a major difference in the immune responses to LC16m8 and Dryvax lies in the level of IgG against the B5 protein. Although B5 is a dominant target for the vaccinia virus EV-neutralizing antibody response, and a weaker antibody response against B5 is elicited by LC16m8 than by Dryvax, both vaccines seem to elicit similar levels of protection and, indeed, neutralization of both MV and EV. The results suggest that the level of the response against B5, albeit lower than that elicited by Dryvax, may contribute to protective immunity, and B5-independent mechanisms may be involved in EV neutralization in the mouse model. Alternatively, a redundant immune response that targets many other virus proteins may be sufficient to compensate for the loss of any particular protective antigen. Studies which selectively address the role of individual vaccine antigens in the protective response should be able to address this question and provide a sound basis for evaluating new-generation smallpox vaccines.
Acknowledgments
We thank Karen Elkins, CBER/FDA, for advice on developing the intracellular cytokine staining assay and Kaketsuken (Kumamoto, Japan) for the LC16m8 vaccine. We thank Bernard Moss, NIAID/NIH, for the 10F5 hybridomas and Yong He, CBER/FDA, for the anti-A27 antibody. Anti-B5 antibody NR-561 (VMC-30) and B5 protein (NR-2624) were obtained from the Biodefense and Emerging Infections Research Resources Repository (BEI Resources), Manassas, VA. We are grateful to Joan Adamo and Carol Weiss, CBER/FDA, for critically reviewing the manuscript.
Footnotes
Published ahead of print on 15 July 2009.
REFERENCES
- 1.Aldaz-Carroll, L., J. C. Whitbeck, M. Ponce de Leon, H. Lou, L. Hirao, S. N. Isaacs, B. Moss, R. J. Eisenberg, and G. H. Cohen. 2005. Epitope-mapping studies define two major neutralization sites on the vaccinia virus extracellular enveloped virus glycoprotein B5R. J. Virol. 79:6260-6271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barefoot, B., N. J. Thornburg, D. H. Barouch, J.-S. Yu, C. Sample, R. E. Johnston, H. X. Liao, T. B. Kepler, B. F. Haynes, and E. Ramsburg. 2008. Comparison of multiple vaccine vectors in a single heterologous prime-boost trial. Vaccine 26:6108-6118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bell, E., M. Shamim, J. C. Whitbeck, G. Sfyroera, J. D. Lambris, and S. N. Isaacs. 2004. Antibodies against the extracellular enveloped virus B5R protein are mainly responsible for the EEV neutralizing capacity of vaccinia immune globulin. Virology 325:425-431. [DOI] [PubMed] [Google Scholar]
- 4.Breman, J. G., R. Kalisa, M. V. Steniowski, E. Zanotto, A. I. Gromyko, and I. Arita. 1980. Human monkeypox, 1970-79. Bull. W. H. O. 58:165-182. [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. 2003. Multistate outbreak of monkeypox—Illinois, Indiana, and Wisconsin. MMWR Morb. Mortal. Wkly. Rep. 52:537-540. [PubMed] [Google Scholar]
- 6.Drexler, I., C. Staib, W. Kastenmuller, S. Stevanovic, B. Schmidt, F. A. Lemonnier, H. G. Rammensee, D. H. Busch, H. Bernhard, V. Erfle, and G. Sutter. 2003. Identification of vaccinia virus epitope-specific HLA-A*0201-restricted T cells and comparative analysis of smallpox vaccines. Proc. Natl. Acad. Sci. USA 100:217-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Empig, C., J. R. Kenner, M. Perret-Gentil, B. E. Youree, E. Bell, A. Chen, M. Gurwith, K. Higgins, M. Lock, A. D. Rice, J. Schriewer, F. Sinangil, E. White, R. M. Buller, T. S. Dermody, S. N. Isaacs, and R. W. Moyer. 2006. Highly attenuated smallpox vaccine protects rabbits and mice against pathogenic orthopoxvirus challenge. Vaccine 24:3686-3694. [DOI] [PubMed] [Google Scholar]
- 8.Fogg, C., S. Lustig, J. C. Whitbeck, R. J. Eisenberg, G. H. Cohen, and B. Moss. 2004. Protective immunity to vaccinia virus induced by vaccination with multiple recombinant outer membrane proteins of intracellular and extracellular virions. J. Virol. 78:10230-10237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galmiche, M. C., J. Goenaga, R. Wittek, and L. Rindisbacher. 1999. Neutralizing and protective antibodies directed against vaccinia virus envelope antigens. Virology 254:71-80. [DOI] [PubMed] [Google Scholar]
- 10.Garcia, A. D., C. A. Meseda, A. E. Mayer, A. Kumar, M. Merchlinsky, and J. P. Weir. 2007. Characterization and use of mammalian-expressed vaccinia virus extracellular membrane proteins for quantification of the humoral immune response to smallpox vaccines. Clin. Vaccine Immunol. 14:1032-1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Golovkin, M., S. Spitsin, V. Andrianov, Y. Smirnov, Y. Xiao, N. Pogrebnyak, K. Markley, R. Brodzik, Y. Gleba, S. N. Isaacs, and H. Koprowski. 2007. Smallpox subunit vaccine produced in planta confers protection in mice. Proc. Natl. Acad. Sci. USA 104:6864-6869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greenberg, R. N., J. S. Kennedy, D. J. Clanton, E. A. Plummer, L. Hague, J. Cruz, F. A. Ennis, W. C. Blackwelder, and R. J. Hopkins. 2005. Safety and immunogenicity of new cell-cultured smallpox vaccine compared with calf-lymph derived vaccine: a blind, single-centre, randomised controlled trial. Lancet 365:398-409. [DOI] [PubMed] [Google Scholar]
- 13.Hashizume, S., H. Yoshizawa, M. Morita, and K. Suzuki. 1985. Properties of attenuated mutant of vaccinia virus LC16m8, derived from Lister strain, p. 87-99. In G. V. Quinnan, Jr. (ed.), Vaccinia viruses as vectors for vaccine antigens. Elsevier Science Publishing Co., Inc., San Diego, CA.
- 14.Heraud, J. M., Y. Edghill-Smith, V. Ayala, I. Kalisz, J. Parrino, V. S. Kalyanaraman, J. Manischewitz, L. R. King, A. Hryniewicz, C. J. Trindade, M. Hassett, W. P. Tsai, D. Venzon, A. Nalca, M. Vaccari, P. Silvera, M. Bray, B. S. Graham, H. Golding, J. W. Hooper, and G. Franchini. 2006. Subunit recombinant vaccine protects against monkeypox. J. Immunol. 177:2552-2564. [DOI] [PubMed] [Google Scholar]
- 15.Hooper, J. W., D. M. Custer, C. S. Schmaljohn, and A. L. Schmaljohn. 2000. DNA vaccination with vaccinia virus L1R and A33R genes protects mice against a lethal poxvirus challenge. Virology 266:329-339. [DOI] [PubMed] [Google Scholar]
- 16.Hooper, J. W., E. Thompson, C. Wilhelmsen, M. Zimmerman, M. A. Ichou, S. E. Steffen, C. S. Schmaljohn, A. L. Schmaljohn, and P. B. Jahrling. 2004. Smallpox DNA vaccine protects nonhuman primates against lethal monkeypox. J. Virol. 78:4433-4443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jezek, Z., S. S. Marennikova, M. Mutumbo, J. H. Nakano, K. M. Paluku, and M. Szczeniowski. 1986. Human monkeypox: a study of 2,510 contacts of 214 patients. J. Infect. Dis. 154:551-555. [DOI] [PubMed] [Google Scholar]
- 18.Jones-Trower, A., A. Garcia, C. A. Meseda, Y. He, C. Weiss, A. Kumar, J. P. Weir, and M. Merchlinsky. 2005. Identification and preliminary characterization of vaccinia virus (Dryvax) antigens recognized by vaccinia immune globulin. Virology 343:128-140. [DOI] [PubMed] [Google Scholar]
- 19.Karupiah, G., V. Panchanathan, I. Sakala, and G. Chaudhri. 2007. Genetic resistance to smallpox; lessons from mouse. Novartis Found. Symp. 281:129-140. [DOI] [PubMed] [Google Scholar]
- 20.Kenner, J., F. Cameron, C. Empig, D. V. Jobes, and M. Gurwith. 2006. LC16m8: an attenuated smallpox vaccine. Vaccine 24:7009-7022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kidokoro, M., M. Tashiro, and H. Shida. 2005. Genetically stable and fully effective smallpox vaccine strain constructed from highly attenuated vaccinia LC16m8. Proc. Natl. Acad. Sci. USA 102:4152-4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Law, M., and G. L. Smith. 2001. Antibody neutralization of the extracellular enveloped form of vaccinia virus. Virology 280:132-142. [DOI] [PubMed] [Google Scholar]
- 23.Lawrence, S. J., K. R. Lottenbach, F. K. Newman, R. M. Buller, C. J. Bellone, J. J. Chen, G. H. Cohen, R. J. Eisenberg, R. B. Belshe, S. L. Stanley, Jr., and S. E. Frey. 2007. Antibody responses to vaccinia membrane proteins after smallpox vaccination. J. Infect. Dis. 196:220-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manischewitz, J., L. R. King, N. A. Bleckwenn, J. Shiloach, R. Taffs, M. Merchlinsky, N. Eller, M. G. Mikolajczyk, D. J. Clanton, T. Monath, R. A. Weltzin, D. E. Scott, and H. Golding. 2003. Development of a novel vaccinia-neutralization assay based on reporter-gene expression. J. Infect. Dis. 188:440-448. [DOI] [PubMed] [Google Scholar]
- 25.Mayr, A., H. Stickl, H. K. Muller, K. Danner, and H. Singer. 1978. The smallpox vaccination strain MVA: marker, genetic structure, experience gained with the parenteral vaccination and behavior in organisms with a debilitated defence mechanism. Zentralbl. Bakteriol. B 167:375-390. (In German.) [PubMed] [Google Scholar]
- 26.Meseda, C. A., K. L. Elkins, M. J. Merchlinsky, and J. P. Weir. 2002. Prime-boost immunization with DNA and modified vaccinia virus Ankara vectors expressing herpes simplex virus-2 glycoprotein D elicits greater specific antibody and cytokine responses than DNA vaccine alone. J. Infect. Dis. 186:1065-1073. [DOI] [PubMed] [Google Scholar]
- 27.Meseda, C. A., A. D. Garcia, A. Kumar, A. E. Mayer, J. Manischewitz, L. R. King, H. Golding, M. Merchlinsky, and J. P. Weir. 2005. Enhanced immunogenicity and protective effect conferred by vaccination with combinations of modified vaccinia virus Ankara and licensed smallpox vaccine Dryvax in a mouse model. Virology 339:164-175. [DOI] [PubMed] [Google Scholar]
- 28.Morikawa, S., T. Sakiyama, H. Hasegawa, M. Saijo, A. Maeda, I. Kurane, G. Maeno, J. Kimura, C. Hirama, T. Yoshida, Y. Asahi-Ozaki, T. Sata, T. Kurata, and A. Kojima. 2005. An attenuated LC16m8 smallpox vaccine: analysis of full-genome sequence and induction of immune protection. J. Virol. 79:11873-11891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pulford, D. J., A. Gates, S. H. Bridge, J. H. Robinson, and D. Ulaeto. 2004. Differential efficacy of vaccinia virus envelope proteins administered by DNA immunisation in protection of BALB/c mice from a lethal intranasal poxvirus challenge. Vaccine 22:3358-3366. [DOI] [PubMed] [Google Scholar]
- 30.Putz, M. M., C. M. Midgley, M. Law, and G. L. Smith. 2006. Quantification of antibody responses against multiple antigens of the two infectious forms of vaccinia virus provides a benchmark for smallpox vaccination. Nat. Med. 12:1310-1315. [DOI] [PubMed] [Google Scholar]
- 31.Reed, K. D., J. W. Melski, M. B. Graham, R. L. Regnery, M. J. Sotir, M. V. Wegner, J. J. Kazmierczak, E. J. Stratman, Y. Li, J. A. Fairley, G. R. Swain, V. A. Olson, E. K. Sargent, S. C. Kehl, M. A. Frace, R. Kline, S. L. Foldy, J. P. Davis, and I. K. Damon. 2004. The detection of monkeypox in humans in the Western Hemisphere. N. Engl. J. Med. 350:342-350. [DOI] [PubMed] [Google Scholar]
- 32.Saijo, M., Y. Ami, Y. Suzaki, N. Nagata, N. Iwata, H. Hasegawa, M. Ogata, S. Fukushi, T. Mizutani, T. Sata, T. Kurata, I. Kurane, and S. Morikawa. 2006. LC16m8, a highly attenuated vaccinia virus vaccine lacking expression of the membrane protein B5R, protects monkeys from monkeypox. J. Virol. 80:5179-5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smerdou, C., and P. Liljestrom. 1999. Non-viral amplification systems for gene transfer: vectors based on alphaviruses. Curr. Opin. Mol. Ther. 1:244-251. [PubMed] [Google Scholar]
- 34.Smerdou, C., and P. Liljestrom. 1999. Two-helper RNA system for production of recombinant Semliki forest virus particles. J. Virol. 73:1092-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith, G. L., and A. Vanderplasschen. 1998. Extracellular enveloped vaccinia virus. Entry, egress, and evasion. Adv. Exp. Med. Biol. 440:395-414. [PubMed] [Google Scholar]
- 36.Smith, G. L., A. Vanderplasschen, and M. Law. 2002. The formation and function of extracellular enveloped vaccinia virus. J. Gen. Virol. 83:2915-2931. [DOI] [PubMed] [Google Scholar]
- 37.Sugimoto, M., and K. Yamanouchi. 1994. Characteristics of an attenuated vaccinia virus strain, LC16m0, and its recombinant virus vaccines. Vaccine 12:675-681. [DOI] [PubMed] [Google Scholar]
- 38.Takahashi-Nishimaki, F., S. Funahashi, K. Miki, S. Hashizume, and M. Sugimoto. 1991. Regulation of plaque size and host range by a vaccinia virus gene related to complement system proteins. Virology 181:158-164. [DOI] [PubMed] [Google Scholar]
- 39.Tang, J., M. Murtadha, M. Schnell, L. C. Eisenlohr, J. Hooper, and P. Flomenberg. 2006. Human T-cell responses to vaccinia virus envelope proteins. J. Virol. 80:10010-10020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tscharke, D. C., G. Karupiah, J. Zhou, T. Palmore, K. R. Irvine, S. M. Haeryfar, S. Williams, J. Sidney, A. Sette, J. R. Bennink, and J. W. Yewdell. 2005. Identification of poxvirus CD8+ T cell determinants to enable rational design and characterization of smallpox vaccines. J. Exp. Med. 201:95-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Viner, K. M., and S. N. Isaacs. 2005. Activity of vaccinia virus-neutralizing antibody in the sera of smallpox vaccinees. Microbes Infect. 7:579-583. [DOI] [PubMed] [Google Scholar]