Abstract
Previous studies have shown that the synthetic peptide GK1, derived from Taenia crassiceps cysticerci, enhances the immunogenicity of the commercial inactivated influenza vaccine Fluzone in both young and aged mice. In particular, antibody responses were much improved. Since GK1 is a peptide and is rapidly cleared from the body, it offers the possibility to improve vaccine performance without undesirable effects. This study was therefore designed to understand the mechanisms of action involved in the adjuvant properties of GK1. For this, transgenic mice expressing a T-cell receptor specific for an epitope from the influenza virus hemagglutinin (HA) protein were employed. The GK1 peptide significantly increased the in vivo proliferative response of HA-specific CD4+ T cells when it was coimmunized with the HA epitope. Dendritic cells treated in vitro with GK1 were capable of enhancing T-cell activation. Furthermore, in synergy with lipopolysaccharide, GK1 enhanced the expression of major histocompatibility complex class II and costimulatory molecules of dendritic cells and promoted the secretion of proinflammatory cytokines and chemokines upon antigen-driven T-cell interaction. These data provide important insights into the mechanism that underlies the GK1 adjuvant capacity observed previously and underline the feasibility of using the transgenic mouse model described herein as a tool for investigation of the modes of action of different influenza vaccine adjuvants.
Dendritic cells (DCs) are the main antigen-presenting cells regulating adaptive immune responses in vivo. In order to induce effective cellular and humoral responses, DCs must be activated by pathogen-associated molecular patterns (6) that drive their migration toward secondary lymphoid organs and increase their capacity to prime naïve T cells. Over the last 10 years, various classes of pattern recognition receptors have been described; and many of them are expressed on DCs, such as Toll-like receptors (TLRs), nucleotide oligomerization domain-like receptors, and some members of the C-type lectin family (for a review, see reference 6). The development of noninfectious subunit vaccines greatly increases the safety of prophylactic immunization, but it also reinforces the need for a new generation of immunostimulatory adjuvants.
Since the first use of aluminum compounds in humans in 1926 (12), much effort has been focused on the development of novel adjuvants to improve the immunogenicity of vaccines. However, because adverse effects are a paramount concern, few new adjuvants have received approval for use in the developed world. This endeavor has been relevant to the improvement of the immunity induced by the influenza vaccine (9). In particular, several adjuvants are being designed and developed to overcome the reduced efficacy of the influenza vaccine in elderly individuals (1). The oil-in-water adjuvant emulsion MF59 is the first new adjuvant to have recently been licensed for use by humans (2). Its adjuvant effect was first tested in young and aged mice vaccinated against influenza, and its use resulted in an increase in the immunity induced by the vaccine (5). Although MF59 improved the immunogenicity of the human influenza vaccine in elderly individuals (3, 18), the vaccine continues to have a low degree of efficacy in this population (2). The majority of commercially available influenza vaccines (Fluzone [Sanofi Pasteur Inc.], Flulaval [ID Biomedical Corporation of Quebec, Quebec, Canada], Fluvirin [Chiron Vaccines Limited, United Kingdom], Fluarix [GlaxoSmithkline Biologicals, Germany]) include the hemagglutinins (HAs) of three different strains to which thimerosal is added as a preservative. MF59 is included as adjuvant in only one of the new versions of the commercially available vaccine (Fluad). Clinical trials are now in process to determine whether the addition of aluminum hydroxide as an adjuvant can improve the immunogenicity of the A/H5N1 flu vaccine. A new potent adjuvant, AS04, based on 3-O-desacyl-4′-monophosphoryl lipid A adsorbed on aluminum salt, was recently submitted for licensing in the United States, but its efficacy remains to be determined (7). Thus, the enhancement of the influenza vaccine remains a clear challenge due to the influenza-related morbidity and mortality, particularly among high-risk populations such as elderly individuals.
An 18-amino-acid peptide named GK1, first identified in a cDNA library of Taenia crassiceps cysticerci (11), has previously been described to induce a high level of protection against cysticercosis (21) even without the need for an adjuvant. Further studies showed that the subcutaneous coadministration of GK1 with the influenza vaccine (Fluzone) increased the levels of anti-influenza virus antibodies in aged mice before and after infection, reducing the local level of inflammation that accompanied the influenza vaccination itself and favoring virus clearance after infection in both young and aged mice (16). Since GK1 is a peptide and is rapidly cleared from the body, it offers the possible ability to improve the performance of a vaccine without undesirable effects.
These results prompted us to further investigate the cellular mechanisms by which GK1 exerts its effect as an adjuvant in a vaccine against influenza. In order to do so, we used T cells from mice that express a transgenic T-cell receptor (TCR) specific for a peptide of the influenza virus HA and is restricted to major histocompatibility complex (MHC) class II molecules (8, 14). This permitted us to monitor the level of HA-specific CD4+ T-cell priming upon influenza immunization with or without the GK1 peptide. The present report presents evidence supporting the capacity of GK1 to enhance CD4+ T-cell proliferation in vivo and in vitro by upregulating the expression of costimulatory molecules on DCs and inducing the secretion of proinflammatory cytokines and chemokines.
MATERIALS AND METHODS
Mice.
Pathogen-free, inbred Thy1.1. BALB/c mice (females; ages, 6 to 8 weeks) were originally purchased from Taconic Laboratories (Germantown, NY). TCR-HA transgenic mice, first reported by Kirberg et al. (8), have the BALB/c mouse background and express an αβ TCR specific for the HA protein from positions 111 to 119 (peptide HA111-119) from the influenza virus HA presented by the MHC class II molecule I-Ed. All mice were housed according to the standards of the American Association for Accreditation of Laboratory Animal Care. All procedures performed with animals in this study were conducted in accordance with institutional guidelines following the protocol approved by the Bioethical Committee.
Reagents.
The influenza vaccine Fluzone (formulated in 2005 to 2006; Sanofi Pasteur Inc.) contains 45 μg of HA (15 μg of HA from influenza A virus H1N1, 15 μg of HA from influenza A virus H3N2, and 15 μg of HA from influenza B virus). The GK1 peptide consists of 18 amino acids (GYYYPSDPNTFYAPPYSA) and was purchased from AnaSpec, Inc. The immunodominant peptide HA111-119 was synthesized by adding four amino acids to increase its binding affinity to the MHC (SVSSFERFEIFPK) and was purchased from Genosys. Endotoxin was removed from the synthesized peptides by using polymyxin B-agarose (Sigma-Aldrich), as reported previously (15), to abolish uncontrolled effects over DCs. Lipopolysaccharide (LPS) from Escherichia coli serotype O111:B4 was purchased from Sigma-Aldrich.
Adoptive transfer experiments and immunization.
For the adoptive transfer experiments, HA-positive T cells from TCR transgenic mice were positively selected by magnetic sorting with a clonotypic antibody (antibody 6.5), as described previously (19, 20). Briefly, cell suspensions obtained from the lymph nodes of transgenic mice were incubated with biotinylated antibody 6.5, washed, and incubated with magnetic beads coupled to streptavidin (Miltenyi Biotec). After the beads were washed, they were loaded onto a magnetic column. The positive fraction corresponded to CD4+ antibody 6.5-positive (6.5+) cells, and the purity varied from 90 to 95%, according to flow cytometry analysis (data not shown). Positive selection by the clonotypic antibody does not result in activation of the cells, since the entire procedure was performed at 4°C. Before transfer, the CD4+ 6.5+ T cells were labeled with 5 μM carboxyfluorescein succinimidyl ester (CFSE; Molecular Probes), according to the manufacturer's instructions, and extensively washed. The cells were then transferred intravenously into the recipient Thy1.1 BALB/c mice (1 × 106 cells per mouse).
One day after the adoptive transfer, the recipient mice were immunized at the base of the tail with 1.0 μg of the influenza vaccine or 1.0 μg of peptide HA111-119 with or without peptide GK1 (10 μg per mouse). Control mice were injected with phosphate-buffered saline (PBS). Three days after immunization, the lymph nodes draining the immunization site (the popliteal and inguinal lymph nodes) were recovered. Cell suspensions from the draining lymph nodes were obtained and stained for flow cytometry analysis.
In each experiment, three mice per immunization group were used, and two independent experiments were performed.
Flow cytometry.
For flow cytometry, the cell suspensions were stained at 4°C with the following antibodies, purchased from Pharmingen (BD Biosciences): CD40-fluorescein isothiocyanate, CD80-fluorescein isothiocyanate, Ia/Ie-biotin, CD11c-biotin, CD4-cychrome, and Thy1.2-phycoerythrin. The 6.5 clonotypic antibody has been described previously (10) and was produced and coupled to biotin in our laboratory. Acquisition was conducted on a FACScalibur cytometer (BD Biosciences).
Recovery and stimulation of DCs.
Bone marrow-derived DCs (BMDCs) were prepared by culturing bone marrow cells obtained from the femur and tibia of BALB/c mice in RPMI 1640 and 10% fetal calf serum supplemented with 5 to 10% of a supernatant from a granulocyte-macrophage colony-stimulating factor-expressing cell line (provided by Laura Bonifaz, Centro Médico Nacional Siglo XXI, Mexico). The cells were washed and recultured with fresh RPMI 1640-10% fetal calf serum containing 10% of the supernatant from the cells expressing the granulocyte-macrophage colony-stimulating factor cell every 3 days for a period of 6 days. On day 6, the immature DCs (purity, >80% CD11c+ cells, as assessed by flow cytometry) were nonstimulated or treated with LPS (100 ng/ml) and/or GK1 (10 μg/ml) for 24 h. The cells were then recovered, extensively washed, and used for in vitro assays or flow cytometry.
In vitro assays.
In order to monitor in vitro the priming of influenza virus-specific T cells, two different types of cells were used: the T-cell line 6.5-NFAT (which expresses the αβ TCR specific for peptide HA111-119 and the gene reporter [LacZ] under the control of the interleukin-2 [IL-2] promoter) (15) or 6.5+ CD4+ T cells from TCR-HA transgenic mice. As antigen-presenting cells, BMDCs obtained and treated as described above were used.
Nontreated BMDCs or BMDCs treated overnight with LPS or GK1 (1 or 10 μg), or both, were cultured in 96-well round-bottom plates at 2.5 × 104 cells per well. A total of 5 × 104 6.5-NFAT cells were added to each well, together with the HA peptide. Four to 5 h later, the optical density at 595 nm was measured to quantify the expression of β-galactosidase by the cell line after the cells were lysed in a buffer containing the detergent NP-40 and the β-galactosidase substrate chlorophenol red-β-d-galactopyranoside (Fluka, Germany).
When the 6.5+ CD4+ T cells (isolated as described above and labeled with CFSE) were employed, the cells were cultured in triplicate in flat-bottom 96-well plates at 5 × 104 T cells per well, together with 2 × 104 treated or untreated BMDCs, in the absence or presence of different doses of the HA peptide. Four days later, the cells were recovered and stained for CD4+. The levels of CFSE among the CD4+ cells were determined by fluorescent-activated cell sorting. The supernatants from the cocultures were recovered for cytokine measurement by flow cytometry with a cytometric bead array mouse inflammation kit (BD Biosciences). In each experiment, triplicate wells were used for each condition. Each type of experiment was performed two to three times.
Statistical analysis.
The means for the different groups tested were compared by the nonparametric unpaired t test to identify differences in the different parameters measured between groups. For multiple comparisons, analysis of variance was used. Differences were considered significant if the P value was <0.05. Statistical calculations were performed with the Statistica computer program (Statsoft Inc., 1993).
RESULTS
GK1 enhances priming of antigen-specific CD4+ T cells in vivo.
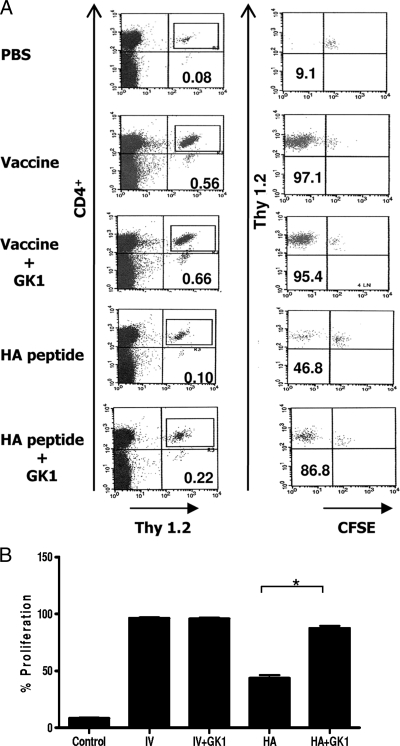
In order to monitor the priming of influenza virus-specific CD4+ T cells in vivo, we used T cells expressing a transgenic TCR specific for the I-Ed-restricted, immunodominant epitope (HA111-119) of the influenza virus HA protein. These cells were labeled with CFSE and adoptively transferred into immunocompetent Thy1.1 BALB/c mice in order to study the HA-specific activation and proliferation under conditions that have been widely used to study the priming of immune responses under physiological conditions (13). One day after transfer, the recipient mice were immunized with influenza vaccine (Fluzone) or with the HA peptide in the absence or the presence of GK1. As controls, a group of mice received PBS or GK1 alone. Three days later, the mice were killed and popliteal and inguinal draining lymph nodes were recovered. Cell suspensions from the lymph nodes were then stained with CD4 and Thy1.2 antibodies and analyzed by fluorescent-activated cell sorting. As Fig. 1A shows, no proliferation was observed in control mice that did not receive any stimulus (PBS), since the majority of the transferred cells retained high levels of the CFSE label. In contrast, in mice that received the influenza vaccine, a very strong proliferation of HA-specific T cells occurred in the lymph nodes, with more than 95% of cells completely losing their CFSE label (Fig. 1A, left column, and Fig. 1B). The proliferative response induced by the vaccine alone was maximal and was not enhanced by the GK1 peptide. However, when mice received the HA peptide, which is much less efficiently captured and presented than the whole HA protein (10), a twofold increase in the percentage of CD4+ cells in the lymph node (0.1% ± 0.01% in the absence of the GK1 peptide versus 0.22% ± 0.03% in the presence of the GK1 peptide), as well as in their proliferation (46.8% in the absence of the GK1 peptide versus 86.6% in the presence of the GK1 peptide), was observed when the HA peptide was coadministered with GK1 compared to the findings obtained by administration of the HA peptide alone.
FIG. 1.
In vivo enhancement of HA-specific T-cell responses by GK1. Recipient Thy1.1 BALB/c mice were injected intravenously with 1 × 106 CFSE-labeled 6.5+ CD4+ T cells isolated from the lymph nodes of TCR-HA transgenic mice and were immunized 1 day later with influenza vaccine (IV) or HA peptide with or without GK1 peptide. (A) Percentage and CFSE label of Thy1.2 CD4+ T cells in the draining lymph nodes of mice that received the different immunization stimuli. One representative dot plot for each condition is shown. In each experiment, three mice per immunization group were used, and two independent experiments were performed. (B) Percentage of cells that lost their CFSE label. Shown are the results of one of three independent experiments in which similar results were obtained in each experiment. *, P = 0.002.
These results show that GK1 enhances specific CD4 T-cell proliferation in vivo and that this enhancement can be appreciated when antigen presentation is suboptimal.
GK1 acts directly on DCs, enhancing their priming capacity.
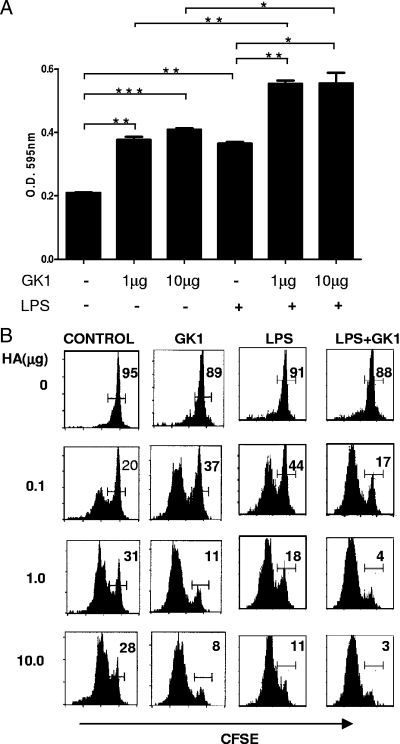
To determine whether the enhanced proliferative response of CD4+ T cells observed in vivo could be the consequence of a direct adjuvant-like effect of GK1 on DCs, the following experiments were performed. BMDCs were stimulated in vitro with GK1 or LPS, or both, and their capacity to activate a cell line that expresses the transgenic TCR-HA at the cell surface and a lacZ reporter gene under control of the IL-2 promoter (6.5-NFAT cells) was analyzed. This system permits the quantification of T-cell activation in terms of IL-2 gene transcription, as described previously (23). As can be seen in Fig. 2A, the amount of activation induced by DCs treated with GK1 was significantly higher than that induced by untreated DCs and similar to that induced by DCs matured with LPS. However, the highest level of activation was achieved when both LPS and GK1 were used together. In order to determine whether this was also true for the priming of naïve CD4+ T lymphocytes, a similar in vitro experiment was performed in which the 6.5-NFAT cells were replaced by naïve 6.5+ CD4+ T cells isolated from the TCR-HA transgenic mice. As Fig. 2B shows, DCs matured in the presence of GK1 alone induced stronger proliferative responses than untreated DCs, and the responses were similar to those induced by LPS-treated DCs. Once again, the highest proliferative response was induced by DCs treated with both LPS and GK1.
FIG. 2.
GK1-treated DCs display enhanced priming capacity. (A) β-Galactosidase production (by determination of the optical density [O.D.] at 595 nm) by the 6.5-NFAT cell line measured after coincubation with DCs that were previously treated with GK1 or LPS, or both, and loaded with HA peptide (50 μg/ml). Bars indicate the means + standard deviations obtained from three independent experiments, *, P < 0.05; **, P < 0.01; ***, P < 0.001. (B) Percentage of CFSE-positive cells among CD4+ T cells, as determined by flow cytometry. DCs were stimulated overnight with nothing (medium), GK1, LPS, or LPS and GK1; washed; and coincubated with naïve CFSE-labeled 6.5+ CD4+ T cells obtained from TCR-HA mice in the presence of different doses of the HA peptide. After 3 days, the cells were stained for CD4 and analyzed by flow cytometry. The results of one representative experiment are shown. Two independent experiments gave similar results.
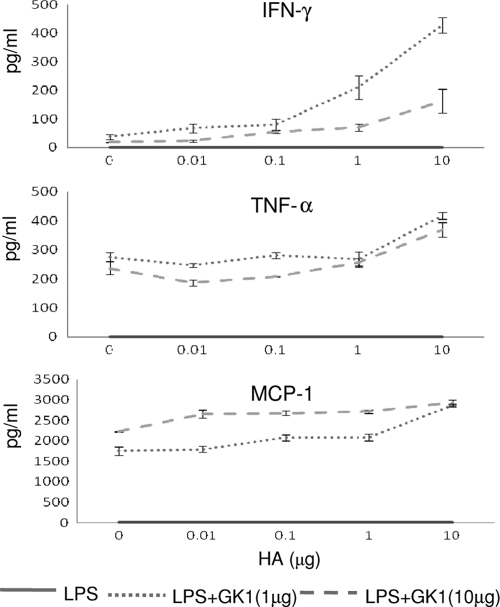
In order to determine whether the synergistic effect of LPS and GK1 on T-cell activation and proliferation that was observed (Fig. 2B) also affected cytokine production, the cytokine levels in the supernatants of the cocultures were measured. Figure 3 shows the levels of tumor necrosis factor alpha (TNF-α), gamma interferon (IFN-γ), and the inflammatory chemokine CCL2 (MCP-1) determined by flow cytometry by the cytometric bead assay. The effect of GK1 was quite striking, in that under the experimental conditions used in this study, only the addition of GK1 stimulated the production of the proinflammatory cytokines IFN-γ and TNF-α (P < 0.0001), as well as that of the inflammatory chemokine CCL2 (MCP-1) (P < 0.0001), and did so in a GK1 dose-dependent manner. Furthermore, while the level of IFN-γ production clearly increased with the dose of the HA peptide, indicating that the production of the cytokine was dependent on the magnitude of the antigen-specific T-cell response, TNF-α and, more particularly, CCL2 were readily detectable even without the addition of the HA peptide. These results indicate that GK1 in combination with LPS could be inducing the secretion of TNF-α and CCL2 directly by the DCs independently of T-cell activation.
FIG. 3.
GK1 enhances the secretion of proinflammatory cytokines resulting from cognate interactions between activated DCs and T cells. Supernatants from the cocultures of LPS-treated DCs or LPS- and GK1-treated DCs obtained from the experiment whose results are shown in Fig. 2B were recovered, and the cytokine levels were determined by flow cytometry with a cytometric bead array mouse inflammation kit (BD Biosciences). The results represent the mean of two independent experiments.
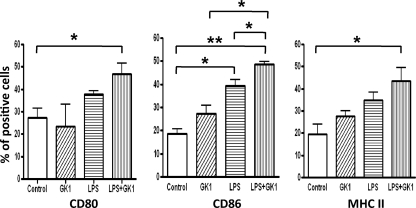
GK1 induces upregulation of costimulatory molecules by DCs.
Bacterial LPS is a prototypical pathogen-associated molecular pattern recognized by TLR4, which activates a variety of cells, including DCs, leading to the upregulation of costimulatory molecules and the efficient initiation of T-cell responses. Considering the effects of GK1 on the priming capacity of DCs described above, it was determined whether GK1 was also capable of upregulating the level of costimulatory molecules on DCs. The levels of expression of CD80, CD86, and MHC class II by BMDCs after incubation with GK1 or LPS, or both, were measured by flow cytometry. As Fig. 4 shows, GK1 induced CD86 and MHC class II molecule upregulation in DCs, although the difference did not reach statistical significance and the level of upregulation was lower than that induced by LPS. In accordance with the data obtained and presented in Fig. 3, the maximal upregulation was observed when DCs were treated with LPS plus GK1.
FIG. 4.
GK1 enhances the upregulation of costimulatory molecules CD86 and MHC class II on DCs. Cell surface levels of costimulatory molecules and MHC class II on CD11c+ determined by flow cytometry in BMDCs incubated overnight with medium alone, GK1, LPS, or both GK1 and LPS. The results are representative of those from two independent experiments. *, P < 0.05; **, P < 0.01.
These results show that GK1 alone can partially enhance CD86 and MHC class II upregulation by DCs, which would explain the adjuvant effect of GK1 when the HA peptide was used as the immunogen (Fig. 1). Nevertheless, the upregulation of costimulatory molecules is much more efficient when GK1 acts in synergy with LPS.
DISCUSSION
Our group has previously demonstrated that the coadministration of the synthetic parasite-derived peptide GK1 with the influenza vaccine increased the levels of anti-influenza antibodies in aged mice before and after infection, reduced the local inflammation that accompanied influenza vaccination itself, and favored virus clearance after infection in both young and aged mice (1, 6).
In this study, we show that the synthetic parasite-derived peptide GK1 effectively enhances antigen-specific CD4+ T-cell priming in vivo and in vitro. We also demonstrate that GK1 acts directly on DCs and leads to their upregulation of costimulatory molecules. Moreover, this effect is much more striking when GK1 is used in combination with LPS. However, it is possible that GK1 may be inducing other changes in the DCs, apart from the upregulation of costimulatory molecules, since in the absence of LPS, its effect on T-cell activation and proliferation (Fig. 2) was much more striking than its effect on the upregulation of costimulatory molecules by the DCs (Fig. 4). Our results also suggest that GK1 used in combination with LPS, which results in synergistic activity, can boost the immune response by inducing the secretion of CCL2, a proinflammatory chemokine that attracts monocytes, NK cells, immature DCs, and activated T cells to the lymph nodes draining the site of immunization (17). This, together with other proinflammatory cytokines, would result in increased antigen presentation and amplification of the immune response. Indeed, in this study the proliferative response of T cells that specifically recognize an influenza vaccine epitope is improved by GK1, an effect that could be extended to the different antigen components of the vaccine when the whole influenza vaccine is used as the immunogen.
The mechanism by which GK1 activates DCs as well as its synergistic effect with LPS remains to be determined. Ongoing research in our laboratory points to the capacity of GK1 to activate presenting cells through the participation of TLR4 and TLR3 (Fernando Esquivel, personal communication). This synergistic action could be feasible, since it has been reported that NOD1 (a pattern recognition receptor specific for gram-negative peptidoglycan) synergistically increases cytokine secretion upon the ligation of TLR4 (4). Alternatively, GK1 could be increasing LPS sensing or LPS-mediated signaling in a more specific manner, for example, by binding to the MD2 protein, which is required for TLR4 signal transduction (22).
Overall, this study provides two major conclusions: GK1 exerts a direct adjuvant-like effect on DCs that leads to the upregulation of costimulatory molecules and enhanced T-cell activation both in vitro and in vivo; and LPS plus GK1 have a synergistic effect on the induction of T-cell proliferation and, even more strikingly, on the induction of proinflammatory cytokines. We are currently investigating whether GK1 can act in synergy with other adjuvants already used in vaccines.
Since GK1 is an 18-amino-acid peptide that can be rapidly cleared from the body, it offers the possible ability to improve vaccine performance without producing undesirable effects. The results obtained in this study and in our previous studies indicate that GK1 can be considered a promising adjuvant to be tested for its ability to optimize immune responses upon vaccination against influenza virus or other pathogens, particularly in cases in which a low level of efficacy is expected, such as in elderly people and immunocompromised patients.
Acknowledgments
We are grateful to Gerardo Arrellin and Georgina Diaz Herrera for excellent animal breeding and care.
This work was funded by Consejo Nacional de Ciencia y Tecnologia (CONACyT) grants 44212 and 62471-M and by the Dirección General de Personal Académico de la Universidad Nacional Autónoma de México (grant IN-217908-3). A.S. was funded by the Institut National de la Santé et la Recherche Médicale (INSERM, France).
Footnotes
Published ahead of print on 15 July 2009.
REFERENCES
- 1.Aguilar, J. C., and E. G. Rodríguez. 2007. Vaccine adjuvants revisited. Vaccine 25:3752-3762. [DOI] [PubMed] [Google Scholar]
- 2.Banzhoff, A., P. Nacci, and A. Podda. 2003. A new MF59-adjuvanted influenza vaccine enhances the immune response in the elderly with chronic diseases: results from an immunogenicity meta-analysis. Gerontology 49:177-184. [DOI] [PubMed] [Google Scholar]
- 3.De Donato, S., D. Granoff, M. Minutello, G. Lecchi, M. Faccini, M. Agnello, F. Senatore, P. Verweij, B. Fritzell, and A. Podda. 1999. Safety and immunogenicity of MF59-adjuvanted influenza vaccine in the elderly. Vaccine 17:3094-3101. [DOI] [PubMed] [Google Scholar]
- 4.He, W., A. Casadevall, S. Lee, and D. Goldman. 2003. Phagocytic activity and monocyte chemotactic protein expression by pulmonary macrophages in persistent pulmonary cryptococcosis. Infect. Immun. 71:930-936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Higgins, D. A., J. R. Carlson, and G. Van Nest. 1996. MF59 adjuvant enhances the immunogenicity of influenza vaccine in both young and old mice. Vaccine 14:478-484. [DOI] [PubMed] [Google Scholar]
- 6.Joffre, O., M. A. Nolte, R. Spörri, and C. Reis e Sousa. 2009. Inflammatory signals in dendritic cell activation and the induction of adaptive immunity. Immunol. Rev. 227:234-247. [DOI] [PubMed] [Google Scholar]
- 7.Keam, S. J., and D. M. Harper. 2008. Human papillomavirus types 16 and 18 vaccine (recombinant AS04 adjuvanted, adsorbed). Drugs 68:359-372. [DOI] [PubMed] [Google Scholar]
- 8.Kirberg, B., A. Baron, S. Jakob, A. Rolink, K. Karjalainen, and H. von Boehmer. 1994. Thymic selection of CD8+ single positive cells with a class II major histocompatibility complex-restricted receptor. J. Exp. Med. 180:25-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumar, R., and E. A. Burns. 2008. Age-related decline in immunity: implications for vaccine responsiveness. Expert Rev. Vaccines 7:467-479. [DOI] [PubMed] [Google Scholar]
- 10.Lanoue, A., C. Bona, H. von Boehmer, and A. Sarukhan. 1997. Conditions that induce tolerance in mature CD4+ T cells. J. Exp. Med. 185:405-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manoutcharian, K., G. Rosas, M. Hernández, G. Fragoso, A. Aluja, N. Villalobos, L. F. Rodarte, and E. Sciutto. 1996. Cisticercosis: identification and cloning of protective recombinant antigens. J. Parasitol. 82:250-254. [PubMed] [Google Scholar]
- 12.Marciani, D. 2003. Vaccine adjuvants: role and mechanisms of action in vaccine immunogenicity. Drug Discov. Today 8:934-943. [DOI] [PubMed] [Google Scholar]
- 13.Reinhardt, R. L., and M. K. Jenkins. 2003. Whole-body analysis of T cell responses. Curr. Opin. Immunol. 15:366-371. [DOI] [PubMed] [Google Scholar]
- 14.Sarukhan, A., A. Lanoue, A. Franzke, F. Brousse, J. Buer, and H. von Boehmer. 1998. Changes in function of antigen-specific lymphocytes correlating with progression towards diabetes in a transgenic model. EMBO J. 17:71-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sarukhan, A., C. Soudais, O. Danos, and K. Jooss. 2001. Factors influencing cross-presentation of non-self antigens expressed from recombinant adeno-associated virus vectors. J. Gene Med. 3:260-270. [DOI] [PubMed] [Google Scholar]
- 16.Segura-Velazquez, R., A. Perez-Torres, G. Rosas, A. Toledo, M. Restelli, E. Acosta, R. Corral, F. Rosetti, G. Fragoso, S. Grinstein, and E. Sciutto. 2006. A novel synthetic adjuvant effectively enhances the immunogenicity of the influenza vaccine. Vaccine 24:1073-1080. [DOI] [PubMed] [Google Scholar]
- 17.Serbina, N. V., T. Jia, T. M. Hohl, and E. G. Pamer. 2008. Monocyte-mediated defense against microbial pathogens. Annu. Rev. Immunol. 26:421-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Squarcione, S., S. Sgricia, L. R. Biasio, and E. Perinetti. 2003. Comparison of the reactogenicity and immunogenicity of a split and a subunit-adjuvanted influenza vaccine in elderly subjects. Vaccine 21:1268-1274. [DOI] [PubMed] [Google Scholar]
- 19.Sumoza-Toledo, A., A. D. Eaton, and A. Sarukhan. 2006. Regulatory T cells inhibit protein kinase C theta recruitment to the immune synapse of naive T cells with the same antigen specificity. J. Immunol. 176:5779-5787. [DOI] [PubMed] [Google Scholar]
- 20.Tanchot, C., F. Vasseur, C. Pontoux, C. Garcia, and A. Sarukhan. 2004. Immune regulation by self-reactive T cells is antigen specific. J. Immunol. 172:4285-4291. [DOI] [PubMed] [Google Scholar]
- 21.Toledo, A., C. Larralde, G. Fragoso, K. Gevorkian G. Manoutcharian, M. Hernández, G. Acero, G. Rosas, F. Lopez-Casillas, C. Kubli-Garfias, R. Vazquez, X. Terrazas, and E. Sciutto. 1999. Towards Taenia solium cysticercosis vaccine: an epitope shared by Taenia crassiceps and Taenia solium protects mice against experimental cysticercosis. Infect. Immun. 67:2522-2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Heel, D., S. Ghosh, M. Butler, K. Hunt, B. M. Foxwell, D. Mengin-Lecreulx, and R. Playford. 2005. Synergistic enhancement of Toll-like receptor responses by NOD1 activation. Eur. J. Immunol. 35:2471-2476. [DOI] [PubMed] [Google Scholar]
- 23.Wilder, J. A., J. S. Cowdery, and R. F. Ashman. 1988. The influence of lipopolysaccharide content on the apparent B cell stimulating activity of anti-mu preparations. J. Immunol. Methods 110:63-68. [DOI] [PubMed] [Google Scholar]