Abstract
In the search for better tools to control bovine tuberculosis, the development of diagnostic tests with improved specificity and sensitivity has a high priority. We chose to search for novel immunodiagnostic reagents. In this study, Rv0899 (outer membrane protein A of Mycobacterium tuberculosis [OmpATb]) was evaluated as a stimulation antigen in a gamma interferon (IFN-γ) release assay to diagnose bovine tuberculosis. OmpATb induced IFN-γ responses in cattle experimentally infected with M. bovis as early and as persistently as ESAT-6 and CFP-10, the current lead diagnostic antigens. In naturally infected cattle, OmpATb stimulated IFN-γ production in 22 of 26 animals (85%). Importantly, OmpATb detected a portion of M. bovis-infected cattle which did not respond to ESAT-6 and CFP-10 (five of six cattle). The combined diagnostic sensitivity of OmpATb, ESAT-6, and CFP-10 for a preselected group consisting of naturally infected cattle with an overrepresentation of ESAT-6/CFP-10 nonresponders was 96% (25 of 26 animals). The specificity of OmpATb for uninfected cattle was 100% (27 cattle were tested; 12 of them gave false-positive results with tuberculins). In summary, our results indicate that OmpATb has the potential to enhance the sensitivity of previously described diagnostic tests based on ESAT-6 and CFP-10 and that the combined use of OmpATb, ESAT-6, CFP-10, and other proteins may achieve at least equal sensitivity to that obtained with purified protein derivative, but at a higher specificity. Further studies evaluating the diagnostic performance of OmpATb in combination with other proteins are ongoing.
Mycobacterium bovis, the causative agent of bovine tuberculosis (bTB), is also responsible for a proportion of human TB cases. Thus, infection of cattle with M. bovis constitutes both a human health hazard and an animal welfare problem, with economic implications in terms of trade restrictions, productivity losses, and massive annual expenditure on bTB eradication programs. The control of bTB is based mainly on a policy of test and slaughter. The persistence of this zoonotic disease combined with the loss of trade and the exponential costs for control justify a need not only for more sensitive but also for more specific diagnostic assays. The occurrence of false-positive results can be attributed, at least in part, to the fact that immune responses to purified protein derivative (PPD) are present not only in animals with TB but also in animals exposed to environmental mycobacteria (reviewed in reference 29). PPDs are prepared by precipitation from heat-killed cultures of mycobacteria and are poorly defined, complex antigens containing many proteins, some of which are shared by different mycobacterial species or even other bacteria. Coinfection of cattle with M. bovis and M. avium subsp. paratuberculosis has been reported to coincide not only with increased numbers of false-positive results in PPD-based diagnostic assays but also with an increased frequency of false-negative results due to a general depression of cell-mediated immune responses to PPDs in advanced paratuberculosis (4, 5, 6, 20).
The Bovigam gamma interferon (IFN-γ) assay (47) is being incorporated into bTB eradication programs in many countries (42). Increased sensitivity compared to the skin test, the possibility of more rapid repeat testing, no need for a second visit to the farm, and a more objective test procedure and interpretation are the main benefits of the IFN-γ assay recognized by authorities, veterinarians, and farmers (34, 42). In areas of low TB prevalence, concerns exist about Bovigam specificity, as well as that of the skin test (22, 25, 27, 29). Study results are divergent; Cagiola and colleagues (11) found a lower specificity of a single comparative cervical skin test than that of the IFN-γ assay. Regardless, the identification of individual mycobacterial antigens which allow the replacement of PPDs in diagnostic tests has been a long-standing research goal.
Attempts to identify candidate antigens have led to the purification and characterization of many proteins from M. bovis and M. tuberculosis. Based on a genomic approach, a great variety of M. tuberculosis complex antigens have been screened for immunogenicity. ESAT-6 and CFP-10 have been shown to be outstanding diagnostic target proteins in the whole-blood IFN-γ assay for cattle (1, 2, 10, 14, 18, 29, 40, 41, 45) and for humans (3, 8, 15, 17). These proteins are considered particularly interesting because their genes are absent from M. bovis BCG, thus allowing differentiation between infected and vaccinated individuals. Cockle and colleagues (14) identified a peptide cocktail comprising peptides from ESAT-6 and CFP-10 together with peptides from Rv3873, Rv3879c, Rv0288, and Rv3019c which was significantly better than tuberculin for identifying skin test-negative animals with confirmed bTB. In addition, the specificity of this cocktail was not compromised by M. bovis BCG vaccination. Rv3615c represents another antigen stimulating an IFN-γ response in a significant proportion of M. bovis-infected cattle (37%) but not in BCG-vaccinated animals (37). Vaccination has particular application in countries which cannot afford the traditional test-and-slaughter control approach. However, a precondition for the use of TB vaccines in cattle would be the availability of diagnostic tests to differentiate M. bovis-infected from vaccinated cattle (reviewed in reference 30).
The use of individual mycobacterial antigens has enhanced the specificity of the IFN-γ assay but reduced its sensitivity (10, 29; reviewed in reference 42). A wider range of antigens is therefore needed to improve specificity while keeping the sensitivity of the standard (PPD-based) IFN-γ assay.
The objective of this study was to evaluate the potential of Rv0899 (outer membrane protein A of M. tuberculosis [OmpATb]), and thus Mb0923, an M. bovis-related protein with the identical sequence, as a diagnostic target for bTB. OmpATb belongs to the OmpA family of outer membrane proteins. OmpATb is a pore-forming protein (36) with the functional properties of a porin which enables bacteria to respond to reduced environmental pH (32). In chlamydiae, which are gram-negative intracellular bacteria, the major outer membrane protein (MOMP) also functions as a porin (38), and MOMP possesses serotype-, subspecies-, species-, and genus-specific antigenic determinants (16, 35). Its gene, ompA, has been used as the basis of a taxonomic classification system for chlamydiae (21). MOMP of chlamydiae is known to elicit both humoral and cell-mediated immune responses: the protein provides a useful species-specific serodiagnostic antigen (19), and it has been shown to act successfully as a vaccine (23). To our knowledge, the usefulness of OmpATb for bTB diagnostics has not yet been evaluated.
We describe herein the assessment of OmpATb as a novel antigen in the whole-blood IFN-γ assay for detection of tuberculous cattle. Our data show that OmpATb is immunogenic and elicits an IFN-γ response in M. bovis-infected cattle as early and as persistently as ESAT-6 and CFP-10 do. Importantly, OmpATb detected a proportion of M. bovis-infected cattle which did not respond to ESAT-6 and CFP-10. We suggest that this antigen has the potential to enhance the sensitivity of previously described diagnostic tests based on ESAT-6 and CFP-10.
MATERIALS AND METHODS
Antigens.
Bovine (PPD B) and avian (PPD A) tuberculins were supplied by Prionics AG, Schlieren, Switzerland, and by the Tuberculin Production Unit at the Veterinary Laboratories Agency (VLA), Weybridge, Surrey, United Kingdom. They were used to stimulate whole blood at 20 μg/ml (Prionics PPDs) and 10 μg/ml (Weybridge PPDs).
OmpATb and maltose binding protein (MBP) were obtained from a commercial source (Proteix, Prague, Czech Republic). A truncated OmpATb protein lacking the N-terminal 72 residues (OmpATb73-326) was produced according to the method of Senaratne et al. (36) as a fusion protein with MBP. After synthetic gene synthesis, DNA fragments were inserted into the BstBI site of the pET28b-MaIE expression vector at the C-terminal coding region for the MaIE protein. Large-scale production was performed with Escherichia coli BL24 λ(DE3). A recombinant ESAT-6:CFP-10 fusion protein was received as a kind gift from F. C. Minion, Iowa State University, and produced as described by Waters et al. (45). All recombinant proteins were used at a concentration of 5 μg/ml in whole-blood culture.
A set of 34 overlapping peptides, which were 20 residues long, overlapped by 12 residues, and covered the complete sequence of Rv0899 (OmpATb) except its amino-terminal signal sequence, were designed (Fig. 1), and single peptides were used at a final concentration of 25 μg/ml, whereas in a pool, each peptide had a concentration of 10 μg/ml. Other peptides with lengths between 16 and 20 amino acids were synthesized and formulated into an ESAT-6-CFP-10 peptide cocktail (E/C) as described previously (13), and the cocktail was used at a concentration of 5 μg/ml/peptide. All peptides were produced by a commercial manufacturer (Pepscan Systems BV, Lelystad, The Netherlands). Pokeweed mitogen (PWM; Sigma) and staphylococcal enterotoxin B from Staphylococcus aureus (SEB; Sigma) were included as positive controls at 5 μg/ml (PWM) and 1 μg/ml (SEB).
FIG. 1.
Sequence of truncated Rv0899 (lacking its amino-terminal signal sequence) and synthetic peptides. The positions of the synthetic peptides are shown in relation to the amino acid sequence of Rv0899. Amino acid residues are given in one-letter code.
Cattle.
Male, TB-free Holstein-Friesian calves were housed according to institutional guidelines at the National Animal Disease Center (NADC), Ames, IA, in a biosafety level 3 facility. All animal care and use procedures were reviewed and approved by the NADC Animal Care and Use Committee. Calves received M. bovis strain 95-1315 by aerosol at 6 months of age as described previously (44). Blood samples were taken repeatedly during the 4 months postinfection. Cattle naturally infected with M. bovis were obtained from herds with a history of bTB, as determined by the State Veterinary Service, United Kingdom. Single intradermal comparative cervical tuberculin test-positive reactors were kept at the VLA, Weybridge Animal Units, United Kingdom. Animal experiments at the VLA were undertaken under a license granted by the UK Home Office that was obtained after approval by the local ethical review committee. The animals were predominantly Holstein-Friesians, and all were older than 12 months of age. TB was confirmed in all animals by postmortem analysis, including culture.
Uninfected control animals were obtained from herds free of bTB in Great Britain and Switzerland. Please refer to Table 1 for an overview of the animal groups and experiments performed during this study.
TABLE 1.
Summary of experiments and cattle groups
| Expt | Animal group | Antigens in IFN-γ assay | Group size (n) | Location | Cattle breed | Age |
|---|---|---|---|---|---|---|
| Immunoreactivity of rOmpATb | Cattle experimentally infected with M. bovis; blood sampling was performed at 9, 14, and 29 dpi and 2, 3, and 4 mo postinfection | PPDs (Prionics), recombinant ESAT-6: CFP-10, rOmpATb | 5 | NADC | Holstein-Friesians | 6 mo (at time of infection) |
| Cattle naturally infected with M. bovis | PPDs (Weybridge), E/C peptides, rOmpAtb | 26 | VLA | Holstein-Friesians | Older than 12 mo | |
| Uninfected cattle | PPDs (Weybridge), E/C, rOmpATb | 7 | VLA | Holstein-Friesians | Older than 12 mo | |
| Immunoreactivity of individual | Cattle naturally infected with M. bovis | PPDs (Weybridge), OmpATb peptides | 10 | VLA | Holstein-Friesians | Older than 12 mo |
| OmpATb peptides | Uninfected cattle | PPDs (Weybridge), OmpATb peptides | 10 | Prionics | Holstein-Friesians and Swiss Brown | From 2 to 9 yr |
| Immunoreactivity of OmpATb | Cattle naturally infected with M. bovis | PPDs (Weybridge), OmpATb peptides | 18 | VLA | Holstein-Friesians | Older than 12 mo |
| peptide pool | Uninfected cattle | PPDs (Weybridge), OmpATb peptides | 20 | Prionics | Holstein-Friesians and Swiss Brown | From 2 to 9 yr |
IFN-γ assay.
Whole-blood cultures were performed in 96-well plates by mixing 0.25 ml of heparinized blood with 25 μl of antigen-containing solution. For OmpATb peptide experiments, aliquots of 0.1 ml of blood were mixed with an equal volume of antigen-containing solution. Supernatants were harvested after 24 h of culture at 37°C and 5% CO2. OmpATb peptide-stimulated cultures were incubated for 40 h, and the IFN-γ concentration was determined using a Bovigam enzyme-linked immunosorbent assay kit (Prionics AG, Schlieren, Switzerland). The optical density at 450 nm (OD450) was also determined. Only samples with an unstimulated (nil) OD450 of <0.2 or a PWM/SEB-stimulated OD450 of >0.5 were considered valid for analysis. A result was considered positive if the PPD B OD450 minus the PPD A OD450 was ≥0.1 and the PPD B OD450 minus the unstimulated OD450 was ≥0.1. For recombinant antigens, if the stimulated OD450 minus the unstimulated OD450 was ≥0.1, the result was considered positive.
RESULTS
Immunoreactivity of rOmpATb.
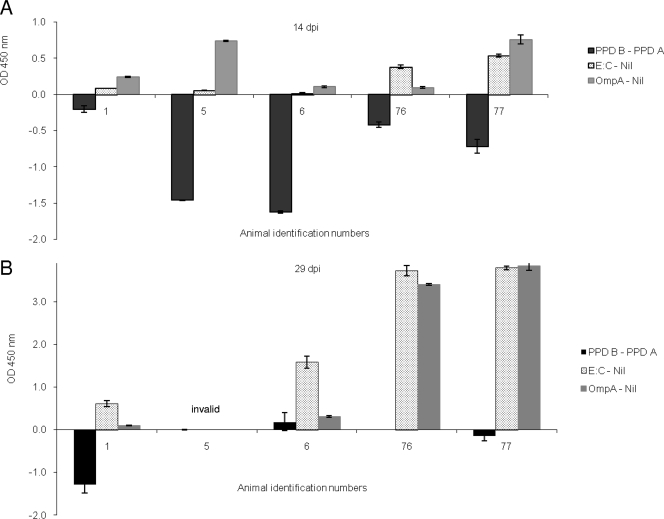
Whole blood of experimentally infected animals was stimulated with PPDs, recombinant OmpA (rOmpATb), or a recombinant fusion protein of ESAT-6 and CFP-10 at 9, 14, or 29 days postinfection (dpi). Positive diagnoses in response to rOmpATb and recombinant ESAT-6:CFP-10 started at day 14 (five of five animals with rOmpATb and two of five animals with recombinant ESAT-6:CFP-10). At 29 dpi, all animals were detected with both rOmpATb and recombinant ESAT-6:CFP-10 (at that time point, samples from only four animals were valid for analysis, as one animal had a markedly elevated background value [OD450] of 2.5). The IFN-γ response to these antigens persisted at all time points tested later (2 to 4 months postinoculation) (results not shown). In contrast to the defined antigens, PPD stimulation resulted in only one positive diagnosis at day 29 (animal number 6) (Fig. 2), while all animals were negative at 9 and 14 dpi. Detailed analysis of IFN-γ production showed that the animals also responded to PPDs starting at day 14, but the response to PPD A was mostly stronger than that to PPD B and therefore resulted in a negative diagnosis.
FIG. 2.
IFN-γ responses in cattle experimentally infected with M. bovis. Data are mean (± standard error of the mean) ΔOD450 values (PPD B OD450 minus PPD A OD450 at 20 μg/ml, recombinant ESAT-6:CFP-10 OD450 minus nil OD450, or rOmpATb OD450 minus nil OD450) at 14 dpi (A) and 29 dpi (B). Samples from animal 5 were invalid at 29 dpi due to a markedly elevated nil value (OD of 2.5), indicating an increased level of circulating IFN-γ.
IFN-γ responses in cattle naturally infected with M. bovis and in uninfected cattle are summarized in Table 2. OmpATb elicited IFN-γ production in 22 of 26 M. bovis-infected cattle (85%); 5 of these 22 animals did not respond to E/C. Twenty of 26 M. bovis-infected cattle (77%) responded to E/C; 3 of these 20 cattle did not respond to rOmpATb. The combined diagnostic sensitivity of E/C and rOmpATb for this preselected group was 96% (25 of 26 animals). This experiment does not represent sensitivity under normal field conditions, as the data for the group of animals in this experiment were biased by the goal to test a large portion of E/C nonresponders. In fact, field studies have shown that E/C alone has a sensitivity of 81% (H. M. Vordermeier and K. Ewer, unpublished data), as opposed to 77% in our study. Similarly, the diagnostic field sensitivity of E/C combined with OmpATb would be expected to be higher than the sensitivity achieved with our preselected group.
TABLE 2.
Stimulation of IFN-γ responses with rOmpATb and other antigens in cattle infected naturally with M. bovis and in uninfected cattle
| Antigen | No. of positive cattle in infected group (n = 26) | No. of false-positive cattle in uninfected group (n = 7) |
|---|---|---|
| PPDs | 26 | 3 |
| E/C | 20 (3 negative with rOmpATb) | 0 |
| rOmpATb | 22 (5 negative with E/C) | 0 |
| E/C or rOmpATb | 25 | 0 |
Three of seven uninfected animals were falsely positive with PPD, compared to no false-positive results with E/C or rOmpATb, indicating the potential for increased diagnostic specificity of these antigens compared to PPDs.
rOmpATb was used as a fusion protein with MBP. As a control, we included whole-blood stimulation with MBP alone. MBP induced no IFN-γ production in all but one assay, in which one of the experimentally infected animals had an elevated reaction to MBP at 29 dpi (OD = 0.919). The corresponding OD value for rOmpATb was 3.842, suggesting additional reactivity to the OmpATb part of the fusion protein.
Immunoreactivity of OmpATb peptides.
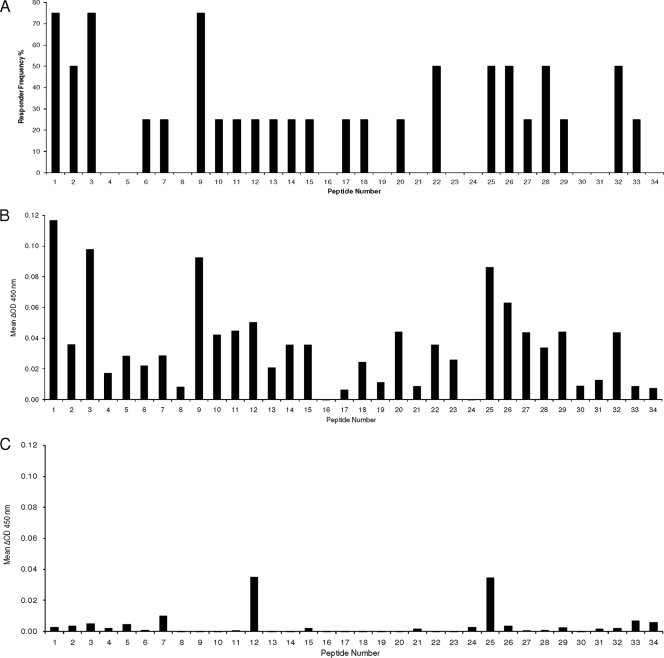
Peptide mapping for the selection of immunoreactive OmpATb peptides was done by screening responses to a panel of overlapping peptides in 10 animals naturally infected with M. bovis. IFN-γ production in response to individual peptides was found in 4 of the 10 animals. Figure 3 shows a comparison of responder frequencies and mean relative IFN-γ responses (ΔOD450 = OD450 with peptide minus OD450 without peptide) for the peptides in these four animals. The criterion used for the determination of responder frequency was as follows: a response was indicated when the ΔOD450 was >0.05. Peptides 1, 3, and 9 were recognized by 75% of these animals, and peptides 2, 22, 25, 26, 28, and 32 were recognized by 50%. A lower response frequency, 25%, was found after stimulation with peptides 6, 7, 10 to 15, 17, 18, 20, 27, 29, and 33. We did not detect any IFN-γ responses to OmpA peptides in the uninfected cattle, except with peptides 12 and 25, in one animal each. Based on the responses with individual peptides, a peptide pool consisting of peptides 1, 3, 9, 27, and 32 was formulated and used to stimulate blood from 18 naturally infected and 20 uninfected cattle. Peptides were chosen based on the following criteria: a responder frequency of at least 75% and/or an IFN-γ response of at least 0.1 (ΔOD450) in individual animals and no reaction in negative animals. Responses to the peptide pool were detected in 5 of 18 animals (28%) based on a cutoff ΔOD450 of >0.1 and in 9 of 18 animals (50%) based on a cutoff ΔOD450 of >0.05. None of the 20 uninfected cattle showed a response to the OmpATb peptide pool (with either cutoff), as opposed to 9 of the 20 animals which were falsely positive with PPD stimulation (Table 3).
FIG. 3.
Identification of immunoreactive OmpATb peptides recognized by T cells from M. bovis-infected cattle selected for reaction with OmpATb. (A) Responder frequencies in M. bovis-infected cattle (n = 4; data are percentages of cattle tested that recognized a particular peptide). (B) Mean IFN-γ responses after peptide stimulation in M. bovis-infected cattle (n = 4; data are ΔOD450 values [OD450 with peptide minus OD450 without peptide]). (C) Mean IFN-γ responses after peptide stimulation in uninfected cattle (n = 10; data are ΔOD450 values [OD450 with peptide minus OD450 without peptide]).
TABLE 3.
Stimulation of IFN-γ responses with OmpATb peptides and PPDs in cattle infected naturally with M. bovis and in uninfected cattle
| Antigen and group (n) | No. of positive or false-positive (for uninfected cattle) results |
|---|---|
| Infected cattle (18) | |
| PPDs | 18 |
| OmpATb peptide pool, with cutoff ΔOD450 of >0.1 | 5 |
| OmpATb peptide pool, with cutoff ΔOD450 of >0.05 | 9 |
| Uninfected cattle (20) | |
| PPDs | 9 |
| OmpATb peptide pool, with cutoff ΔOD450 of >0.1 | 0 |
| OmpATb peptide pool, with cutoff ΔOD450 of >0.05 | 0 |
DISCUSSION
This paper shows that OmpATb specifically induces an IFN-γ response in M. bovis-infected cattle. The antigen represents a novel immunodiagnostic reagent which has the potential to complement ESAT-6 and CFP-10 in a diagnostic assay by markedly enhancing sensitivity. In fact, this increase of sensitivity by OmpATb indicates that alternative antigens (ESAT-6/CFP-10 combined with OmpATb and further specific antigens) have the potential to achieve a diagnostic sensitivity at least equal to that obtained with PPDs.
For a preselected group consisting of naturally TB-infected cattle with an overrepresentation of E/C nonresponders, OmpATb combined with E/C had a sensitivity of 96%. OmpATb detected 83% of cattle not responding to E/C peptides, suggesting that OmpATb will complement the use of ESAT-6 and CFP-10.
Data obtained from experimentally infected animals showed that IFN-γ production induced by OmpATb started at 14 dpi, as early as that elicited by ESAT-6 and CFP-10. Early after infection, the sensitivity obtained with the defined antigens was clearly higher than that obtained with PPDs due to the fact that responses to avian tuberculin mostly exceeded those to bovine tuberculin. In a further study, we saw that this effect was less evident with a lower PPD concentration (10 μg/ml), showing that the relative PPD response may be influenced by the protein concentration and indicating the necessity to use balanced tuberculins (data not shown). Regardless, increased sensitivity with alternative antigens compared to that with PPD might be useful not only early after infection but also for other conditions such as superinfection with M. avium subsp. paratuberculosis or other non-M. tuberculosis complex mycobacteria.
For bTB eradication programs, especially when they progress toward completion, highly specific diagnostic tests are needed to result in fewer false-positive reactors being culled from herds and in fewer herds being identified falsely as bTB suspects. In addition, high sensitivity is also crucial in order to identify residual infections. The IFN-γ assay is accepted to be more sensitive than the skin test (12, 34), thus indicating that it would be beneficial to use the assay as a primary screening test. Its level of specificity in regions with a low incidence of bTB has been reported to be too low with the standard PPD-based assay; however, by use of specific antigens, the assay may be adapted to provide a highly specific and sensitive screening test, which could then be used as a stand-alone test or in conjunction with other tests (9, 29). There has been a long search for antigens having potential as diagnostic markers of bTB (1, 10, 14, 29). In a recent study, Rv3615c stimulated responses in M. bovis-infected animals not responding to ESAT-6 and CFP-10 (four of seven animals). Diagnostics for bTB require a panel of antigens; so far, no single antigen has been identified which could be used as an efficient diagnostic immunoreagent. The cellular response to individual antigens is known to vary between animals and to change over time during the course of infection. These findings may be related to the turnover of mycobacterial growth in vivo (data suggest that there are intervals of 5 to 7 weeks between peaks of cellular activity) or to the sequestration of reactive clones, resulting in periodical recognition in peripheral blood (33). Similar mechanisms may also explain the one bTB-positive animal which was not detected with the combination of OmpATb, ESAT-6, and CFP-10 in our study. This animal had been tested repeatedly with the IFN-γ assay before our study: 5 months before our sampling, it was positive with PPD and OmpATb but negative with ESAT-6 and CFP-10, and 8 months before our sampling, it was negative with the PPD-based IFN-γ assay (alternative antigens were not tested at that time point).
In total, 85% (22 of 26 animals) of naturally infected cattle reacted to rOmpATb. Unfortunately, these animals were no longer available for testing with OmpATb peptides. With a different group of naturally infected cattle, 28% (5 of 18 animals; based on a cutoff OD450 of 0.1) or 50% (9 of 18 animals; based on a cutoff OD450 of 0.05) were positive with an OmpATb peptide pool. Changing immunoreactivity to certain antigens over the course of an infection may be an explanation for the lower percentage of animals detected with the OmpATb peptides than with the OmpATb recombinant protein. In addition, BoLA class II recognition of some OmpATb peptides restricted to certain BoLA haplotypes cannot be excluded and needs further evaluation. Generally, synthetic peptides are not only strong inducers of cellular immune responses in vivo but also suitable as in vitro diagnostic reagents for bTB (14, 28, 39, 40). Compared to recombinant proteins, peptides offer the advantage that M. bovis-specific immunogenic regions of a protein can be used selectively and regions containing epitopes cross-reacting with other bacteria can be omitted.
The specificity of the rOmpATb assay with cattle free of bTB was 100% (7 cattle tested with rOmpATb and 20 cattle tested with OmpATb peptide pool). These animals were preselected on the basis that a proportion of them had false-positive reactions in a previous PPD-based IFN-γ test. During our study, 12 of these 27 animals were falsely positive with PPDs, indicating the superior specificity of OmpATb compared to that of tuberculin.
rOmpATb was constructed as a fusion protein with MBP. This tag system has some advantages compared to six-His-tagged systems for the expression and purification of recombinant proteins, such as increased solubility and no need for harsh chemicals, such as urea, used for solubilization of eluted proteins. Disadvantages reported are that the MBP affinity tag is more immunogenic and much larger (42 kDa) than the polyhistidine tag (7). MBP may also have an impact on specificity, as the protein was produced in an expression vector carrying a gene encoding bacterial MBP from E. coli K-12, which is homologous to wild-type E. coli. For this reason, we added an additional control in each assay by using MBP alone for stimulation. All animals but one (at one time point during experimental infection) were negative with MBP. Further examination of MBP reactivities in healthy cows kept at a Swiss farm showed that 43 of 69 cattle were MBP positive in the IFN-γ assay (data not shown). As indicated, this frequent reactivity of MBP is most likely caused by cross-reaction with homologous E. coli proteins and clearly limits the use of the fusion protein for routine field diagnostics.
Specificity remains a great challenge in bTB diagnostics, as genes of M. tuberculosis antigens also occur in some nontuberculous mycobacteria: genes for ESAT-6 and CFP-10 homologues are present in M. kansasii, M. gordonae, M. marinum, M. szulgai, M. flavescens, and M. gastri, and genes for MPB83, TB10.4, and TB10.3 are found in M. kansasii. Cattle infected/sensitized with M. kansasii may respond false-positively to ESAT-6 and CFP-10 recombinant proteins and peptides (43, 46). Western blot analysis has suggested that the presence of OmpATb is restricted to certain pathogenic species (M. tuberculosis, M. bovis, and M. microti) and that it is not present in nonpathogenic mycobacteria, such as M. smegmatis, M. chelonae, M. scrofulaceum, M. thermoresistibile, M. malmoense, and M. kansasii (24). BLAST search analysis showed that OmpATb shares 100% sequence identity with the M. bovis homologue and 65% identity with the M. ulcerans homologue but no identity with other mycobacterial homologues. Significant sequence similarities with other bacteria are restricted to the carboxy-terminal region. M. ulcerans causes Buruli ulcer in humans, which is closely associated with tropical wetlands, especially in central and west Africa. Natural infections in animals have been detected in Australian koalas, ringtail possums, and captive alpacas (31). Bioinformatics can prioritize target genes for subsequent immunological assays, but comparing sequence identity/homology alone is not sufficient to predict species specificity. Biological evaluations are needed to characterize species specificity, taking into account that some cross-reactivity may be restricted to certain BoLA haplotypes (18, 43). The same holds true for cross-reactive responses to BCG, which cannot be predicted from sequence data alone but require biological evaluation (14). The fact that peptide sequences are derived from an open reading frame within RD1 is not the only criterion deciding cross-reactivity with BCG: other factors, such as less appropriate presentation of proteins, may be relevant for specificity and also for observed differences between humans and cattle (26). In our study, rOmpATb and the OmpATb peptide pool were 100% specific with uninfected animals, but more data are needed to confirm the diagnostic specificity. OmpATb is present in BCG (24), but it remains to be tested whether vaccination with BCG sensitizes cattle to OmpATb and would interfere with OmpATb as a diagnostic antigen.
Taking all the data together, we suggest that OmpATb has the potential to enhance diagnosis by increasing the sensitivity of diagnostic tests based on ESAT-6 and CFP-10. More importantly, a substantial gain in sensitivity may be achieved by the addition of OmpATb. We are confident that using combinations with further antigens, it will be feasible to create a peptide cocktail leading to at least the same sensitivity as that obtained with PPDs. Further studies evaluating the diagnostic performance of OmpATb in combination with other proteins are ongoing.
Acknowledgments
We thank Jessica Pollock, Rachel Huegel, Bart Olthof, and Mike Howard for excellent technical support. We also express our appreciation to the staffs of the Animal Service Unit at the VLA, the NADC, and the ETH Forschungsanstalt Chamau.
Footnotes
Published ahead of print on 8 July 2009.
REFERENCES
- 1.Aagaard, C., M. Govaerts, O. L. Meng, P. Andersen, and J. M. Pollock. 2003. Genomic approach to identification of Mycobacterium bovis diagnostic antigens in cattle. J. Clin. Microbiol. 41:3719-3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aagaard, C., M. Govaerts, V. Meikle, A. J. Vallecillo, J. A. Gutierrez-Pabello, F. Suarez-Guemes, J. McNair, A. Cataldi, C. Espitia, P. Andersen, and J. M. Pollock. 2006. Optimizing antigen cocktails for detection of Mycobacterium bovis in herds with different prevalences of bovine tuberculosis: ESAT6-CFP10 mixture shows optimal sensitivity and specificity. J. Clin. Microbiol. 44:4326-4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agger, E. M., I. Brock, L. M. Okkels, S. M. Arend, C. S. Aagaard, K. N. Weldingh, and P. Andersen. 2003. Human T-cell responses to the RD1-encoded protein TB27.4 (Rv3878) from Mycobacterium tuberculosis. Immunology 110:507-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alvarez, M., J. M. Bielsa, L. Santos, and B. Makoschey. 2007. Compatibility of a live infectious bovine rhinotracheitis (IBR) marker vaccine and an inactivated bovine viral diarrhoea virus (BVDV) vaccine. Vaccine 25:6613-6617. [DOI] [PubMed] [Google Scholar]
- 5.Amadori, M., K. P. Lyashchenko, M. L. Gennaro, J. M. Pollock, and I. Zerbini. 2002. Use of recombinant proteins in antibody tests for bovine tuberculosis. Vet. Microbiol. 85:379-389. [DOI] [PubMed] [Google Scholar]
- 6.Aranaz, A., L. De Juan, J. Bezos, J. Alvarez, B. Romero, F. Lozano, J. L. Paramio, J. Lopez-Sanchez, A. Mateos, and L. Dominguez. 2006. Assessment of diagnostic tools for eradication of bovine tuberculosis in cattle co-infected with Mycobacterium bovis and M. avium subsp. paratuberculosis. Vet. Res. 37:593-606. [DOI] [PubMed] [Google Scholar]
- 7.Bannantine, J. P., V. Rosu, S. Zanetti, S. Rocca, N. Ahmed, and L. A. Sechi. 2008. Antigenic profiles of recombinant proteins from Mycobacterium avium subsp. paratuberculosis in sheep with Johne's disease. Vet. Immunol. Immunopathol. 122:116-125. [DOI] [PubMed] [Google Scholar]
- 8.Billeskov, R., C. Vingsbo-Lundberg, P. Andersen, and J. Dietrich. 2007. Induction of CD8 T cells against a novel epitope in TB10.4: correlation with mycobacterial virulence and the presence of a functional region of difference-1. J. Immunol. 179:3973-3981. [DOI] [PubMed] [Google Scholar]
- 9.Buddle, B. M., T. J. Ryan, J. M. Pollock, J. M. Andersen, and G. W. de Lisle. 2001. Use of ESAT-6 in the interferon-γ test for diagnosis of bovine tuberculosis following skin testing. Vet. Microbiol. 80:37-46. [DOI] [PubMed] [Google Scholar]
- 10.Buddle, B. M., A. R. McCarthy, T. J. Ryan, J. M. Pollock, H. M. Vordermeier, R. G. Hewinson, P. Andersen, and G. W. de Lisle. 2003. Use of mycobacterial peptides and recombinant proteins for the diagnosis of bovine tuberculosis in skin test-positive cattle. Vet. Rec. 153:615-620. [DOI] [PubMed] [Google Scholar]
- 11.Cagiola, M., F. Feliziani, G. Severi, P. Pasquali, and D. Rutili. 2004. Analysis of possible factors affecting the specificity of the gamma interferon test in tuberculosis-free cattle herds. Clin. Diagn. Lab. Immunol. 11:952-956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coad, M., S. H. Downs, P. A. Durr, R. S. Clifton-Hadley, R. G. Hewinson, H. M. Vordermeier, and A. O. Whelan. 2008. Blood-based assays to detect Mycobacterium bovis-infected cattle missed by tuberculin skin testing. Vet. Rec. 162:382-384. [DOI] [PubMed] [Google Scholar]
- 13.Cockle, P. J., S. V. Gordon, A. Lalvani, B. M. Buddle, R. G. Hewinson, and H. M. Vordermeier. 2002. Identification of novel Mycobacterium tuberculosis antigens with potential as diagnostic reagents or subunit vaccine candidates by comparative genomics. Infect. Immun. 70:6996-7003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cockle, P. J., S. V. Gordon, R. G. Hewinson, and H. M. Vordermeier. 2006. Field evaluation of a novel differential diagnostic reagent for detection of Mycobacterium bovis in cattle. Clin. Vaccine Immunol. 13:1119-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Codecasa, L., P. Mantegani, L. Galli, A. Lazzarin, P. Scarpellini, and C. Fortis. 2006. An in-house RD1-based enzyme-linked immunospot-gamma interferon assay instead of the tuberculin skin test for diagnosis of latent Mycobacterium tuberculosis infection. J. Clin. Microbiol. 44:1944-1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Conlan, J. W., I. N. Clarke, and M. E. Ward. 1988. Epitope mapping with solid-phase peptides: identification of type-, subspecies-, species- and genus-reactive antibody binding domains on the major outer membrane protein of Chlamydia trachomatis. Mol. Microbiol. 2:673-679. [DOI] [PubMed] [Google Scholar]
- 17.Cosgrove, C. A., R. R. Castello-Branco Luiz, T. Hussell, A. Sexton, R. Giemza, R. Phillips, A. Williams, G. E. Griffin, G. Dougan, and J. M. Lewis David. 2006. Boosting of cellular immunity against Mycobacterium tuberculosis and modulation of skin cytokine responses in healthy human volunteers by Mycobacterium bovis BCG substrain Moreau Rio de Janeiro oral vaccine. Infect. Immun. 74:2449-2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ewer, K., P. Cockle, S. Gordon, H. Mansoor, M. Govaerts, K. Walravens, S. Marche, G. Hewinson, and M. Vordermeier. 2006. Antigen mining with iterative genome screens identifies novel diagnostics for the Mycobacterium tuberculosis complex. Clin. Vaccine Immunol. 13:90-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoelzle, L. E., K. Hoelzle, and M. M. Wittenbrink. 2004. Recombinant major outer membrane protein (MOMP) of Chlamydophila abortus, Chlamydophila pecorum, and Chlamydia suis as antigens to distinguish chlamydial species-specific antibodies in animal sera. Vet. Microbiol. 103:85-90. [DOI] [PubMed] [Google Scholar]
- 20.Hope, J. C., and B. Villarreal-Ramos. 2008. Bovine TB and the development of new vaccines. Comp. Immunol. Microbiol. Infect. Dis. 31:77-100. [DOI] [PubMed] [Google Scholar]
- 21.Kaltenboeck, B., K. G. Kousoulas, and J. Storz. 1993. Structures of and allelic diversity and relationships among the major outer membrane protein (ompA) genes of the four chlamydial species. J. Bacteriol. 75:487-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lauzi, S., D. Pasotto, M. Amadori, I. L. Archetti, G. Poli, and L. Bonizzi. 2000. Evaluation of the specificity of the interferon-γ test in Italian bovine tuberculosis-free herds. Vet. J. 160:17-24. [DOI] [PubMed] [Google Scholar]
- 23.Longbottom, D., and M. Livingstone. 2006. Vaccination against chlamydial infections of man and animals. Vet. J. 171:263-275. [DOI] [PubMed] [Google Scholar]
- 24.Molle, V., N. Saint, S. Campagna, L. Kremer, E. Lea, P. Draper, and G. Molle. 2006. pH-dependent pore-forming activity of OmpATb from Mycobacterium tuberculosis and characterization of the channel by peptidic dissection. Mol. Microbiol. 61:826-837. [DOI] [PubMed] [Google Scholar]
- 25.Monaghan, M., P. J. Quinn, A. P. Kelly, K. McGill, C. McMurray, K. O'Crowley, H. F. Bassett, E. Costello, F. Quigley, J. S. Rothel, P. R. Wood, and J. D. Collins. 1997. A pilot trial to evaluate the g-interferon assay for the detection of Mycobacterium bovis-infected cattle under Irish conditions. Irish Vet. J. 50:229-232. [Google Scholar]
- 26.Mustafa, A. S., R. Al-Attiyah, S. N. Hanif, and F. A. Shaban. 2008. Efficient testing of large pools of Mycobacterium tuberculosis RD1 peptides and identification of major antigens and immunodominant peptides recognized by human Th1 cells. Clin. Vaccine Immunol. 15:916-924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palmer, M. V., W. R. Waters, T. C. Thacker, R. Greenwald, J. Esfandiari, and K. P. Lyashchenko. 2006. Effects of different tuberculin skin-testing regimens on gamma interferon and antibody responses in cattle experimentally infected with Mycobacterium bovis. Clin. Vaccine Immunol. 13:387-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pollock, J. M., A. J. Douglas, D. P. Mackie, and S. D. Neill. 1995. Peptide mapping of bovine T-cell epitopes for the 38 kDa tuberculosis antigen. Scand. J. Immunol. 41:85-93. [DOI] [PubMed] [Google Scholar]
- 29.Pollock, J. M., R. M. Girvin, K. A. Lightbody, R. A. Clements, S. D. Neill, B. M. Buddle, and P. Andersen. 2000. Assessment of defined antigens for the diagnosis of bovine tuberculosis in skin test-reactor cattle. Vet. Rec. 146:659-665. [DOI] [PubMed] [Google Scholar]
- 30.Pollock, J. M., B. M. Buddle, and P. Andersen. 2001. Towards more accurate diagnosis of bovine tuberculosis using defined antigens. Tuberculosis (Edinburgh) 81:65-69. [DOI] [PubMed] [Google Scholar]
- 31.Portaels, F., K. Chemlal, P. Elsen, P. D. Johnson, J. A. Hayman, J. Hibble, R. Kirkwood, and W. M. Meyers. 2001. Mycobacterium ulcerans in wild animals. Rev. Sci. Tech. 20:252-264. [DOI] [PubMed] [Google Scholar]
- 32.Raynaud, C., K. G. Papavinasasundaram, R. A. Speight, B. Springer, P. Sander, E. C. Bottger, M. J. Colston, and P. Draper. 2002. The functions of OmpATb, a pore-forming protein of Mycobacterium tuberculosis. Mol. Microbiol. 46:191-201. [DOI] [PubMed] [Google Scholar]
- 33.Rhodes, S. G., B. M. Buddle, R. G. Hewinson, and H. M. Vordermeier. 2000. Bovine tuberculosis: immune responses in the peripheral blood and at the site of active disease. Immunology 99:195-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rua-Domenech, R., A. T. Goodchild, H. M. Vordermeier, R. G. Hewinson, K. H. Christiansen, and R. S. Clifton-Hadley. 2006. Ante mortem diagnosis of tuberculosis in cattle: a review of the tuberculin tests, gamma-interferon assay and other ancillary diagnostic techniques. Res. Vet. Sci. 81:190-210. [DOI] [PubMed] [Google Scholar]
- 35.Salti-Montesanto, V., E. Tsoli, P. Papavassiliou, E. Psarrou, B. K. Markey, G. E. Jones, and E. Vretou. 1997. Diagnosis of ovine enzootic abortion, using a competitive ELISA based on monoclonal antibodies against variable segments 1 and 2 of the major outer membrane protein of Chlamydia psittaci serotype 1. Am. J. Vet. Res. 58:228-235. [PubMed] [Google Scholar]
- 36.Senaratne, R. H., H. Mobasheri, K. G. Papavinasasundaram, P. Jenner, E. J. Lea, and P. Draper. 1998. Expression of a gene for a porin-like protein of the OmpA family from Mycobacterium tuberculosis H37Rv. J. Bacteriol. 180:3541-3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sidders, B., C. Pirson, P. J. Hogarth, R. G. Hewinson, N. G. Stoker, and H. M. Vordermeier. 2008. Screening of highly expressed mycobacterial genes identifies Rv3615c as a useful differential diagnostic antigen for the Mycobacterium tuberculosis complex. Infect. Immun. 76:3932-3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun, G., S. Pal, A. K. Sarcon, S. Kim, E. Sugawara, H. Nikaido, M. J. Cocco, E. M. Peterson, and L. M. de la Maza. 2007. Structural and functional analyses of the major outer membrane protein of Chlamydia trachomatis. J. Bacteriol. 189:6222-6235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vordermeier, H. M., P. Cockle, A. Whelan, S. Rhodes, N. Palmer, D. Bakker, and R. G. Hewinson. 1999. Development of diagnostic reagents to differentiate between Mycobacterium bovis BCG vaccination and M. bovis infection in cattle. Clin. Diagn. Lab. Immunol. 65:675-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vordermeier, H. M., A. Whelan, P. J. Cockle, L. Farrant, N. Palmer, and R. G. Hewinson. 2001. Use of synthetic peptides derived from the antigens ESAT-6 and CFP-10 for differential diagnosis of bovine tuberculosis in cattle. Clin. Diagn. Lab. Immunol. 8:571-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vordermeier, H. M., R. Pontarollo, B. Karvonen, P. Cockle, R. Hecker, M. Singh, L. A. Babiuk, R. G. Hewinson, and S. van Drunen Littel-van den Hurk. 2005. Synthetic peptide vaccination in cattle: induction of strong cellular immune responses against peptides derived from the Mycobacterium bovis antigen Rv3019c. Vaccine 23:4375-4384. [DOI] [PubMed] [Google Scholar]
- 42.Vordermeier, H. M., A. Whelan, K. Ewer, T. Goodchild, R. Clifton-Hadley, J. Williams, and R. G. Hewinson. 2006. The BOVIGAM assay as ancillary test to the tuberculin skin test. Gov. Vet. J. 16:72-80. [Google Scholar]
- 43.Vordermeier, H. M., J. Brown, P. J. Cockle, W. P. Franken, S. M. Arend, T. H. Ottenhoff, K. Jahans, and R. G. Hewinson. 2007. Assessment of cross-reactivity between Mycobacterium bovis and M. kansasii ESAT-6 and CFP-10 at the T-cell epitope level. Clin. Vaccine Immunol. 14:1203-1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Waters, W. R., M. V. Palmer, D. L. Whipple, M. P. Carlson, and B. J. Nonnecke. 2003. Diagnostic implications of antigen-induced gamma interferon, nitric oxide, and tumor necrosis factor alpha production by peripheral blood mononuclear cells from Mycobacterium bovis-infected cattle. Clin. Diagn. Lab. Immunol. 10:960-966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Waters, W. R., B. J. Nonnecke, M. V. Palmer, S. Robbe-Austermann, J. P. Bannantine, J. R. Stabel, D. L. Whipple, J. B. Payeur, D. M. Estes, J. E. Pitzer, and F. C. Minion. 2004. Use of recombinant ESAT-6:CFP-10 fusion protein for differentiation of infections of cattle by Mycobacterium bovis and by M. avium subsp. avium and M. avium subsp. paratuberculosis. Clin. Diagn. Lab. Immunol. 11:729-735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Waters, W. R., M. V. Palmer, T. C. Thacker, J. B. Payeur, N. B. Harris, F. C. Minion, R. Greenwald, J. Esfandiari, P. Andersen, J. McNair, J. M. Pollock, and K. P. Lyashchenko. 2006. Immune responses to defined antigens of Mycobacterium bovis in cattle experimentally infected with Mycobacterium kansasii. Clin. Vaccine Immunol. 13:611-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wood, P. R., L. A. Corner, and P. Plackett. 1990. Development of a simple, rapid in vitro cellular assay for bovine tuberculosis based on the production of gamma interferon. Res. Vet. Sci. 49:46-49. [PubMed] [Google Scholar]