Abstract
Common variable immunodeficiency (CVID) is a B cell immunodeficiency disorder characterized frequently by failure of memory B cell development and antibody secretion. A unifying cellular pathogenesis for CVID has not been forthcoming, but given the immunoregulatory role of invariant NK (iNK) T cells and their absence in several other immunodeficiencies, we quantified these cells in the blood of 58 CVID patients. There was a marked decrease in the proportion of iNK T cells in CVID patients compared with controls. This was particularly notable in those with low isotype-switched memory B cells, but subset analysis demonstrated no difference when stratified by specific clinical features. We propose that the decreased proportion of iNK T cells in CVID might be linked to the failure of memory B cell generation, which may contribute to reduced antibody production in these patients.
Keywords: B cells, common variable immunodeficiency, NK T cells
Introduction
Invariant NK (iNK) T cells are thymic-derived T lymphocytes that express Vα24-Jα18/Vβ11 T-cell receptor (TCR) chains in humans, and are selected to recognize foreign and self-derived lipid antigens presented on CD1d [1,2]. Their ability to produce rapidly a variety of T helper type 1 (Th1) and Th2 cytokines [1–4], as well as interleukin (IL)-21 [5], suggests an important but as yet poorly defined role in immunoregulation. Indeed, iNK T cells have been implicated in the development and prevention of numerous autoimmune and inflammatory conditions, as well as in tumour/immune surveillance [1,2]. A critical function of iNK T cells in immune regulation and homeostasis is supported further by the findings of a near-absence of iNK T cells in patients with X-linked lymphoproliferative disease (XLP) due to mutations in SH2D1A and XIAP[6–9], and reduced frequencies of iNK T cells in Omenn's syndrome [10], Wiskott–Aldrich syndrome [11], human immunodeficiency virus (HIV)-1 infection [12,13] and possibly hyper-immunoglobulin (Ig)M syndrome [14]. Thus, a lack of iNK T cells in these conditions may contribute to infection susceptibility in affected patients. Common variable immunodeficiency (CVID) has an incidence of approximately 1:25 000, and is manifested primarily by hypogammaglobulinaemia associated with recurrent sinopulmonary and gastrointestinal infections. The most common cellular abnormality that accompanies the loss of Ig production is a paucity of isotype-switched memory B cells [15,16]. Despite this, little is known about the regulatory abnormalities underlying this defect. We report that patients with CVID have low proportions of iNK T cells and speculate that this abnormality may underlie some of the humoral irregularities in this condition.
Methods
Fifty-eight CVID patients and 30 healthy controls were recruited from Westmead, Concord and Royal Prince Alfred Hospitals in Sydney, Australia, following informed consent, according to protocols approved by local ethics review boards. CVID was diagnosed based on a decreased serum IgG level along with a decrease in either serum IgA or IgM, and exclusion of secondary causes of hypogammaglobulinaemia. The clinical phenotype was determined as described by Berglund et al.[15]. Peripheral blood mononuclear cells (PBMC) were isolated by Ficoll-Hypaque centrifugation; in the initial study commenced in 2005, cells were cryopreserved until used, whereas in the confirmatory study of 2008 cells were stained immediately upon isolation. iNK T cells were identified by staining with anti-CD3 (UCHT1) conjugated to either Pacific Blue (eBioscience, San Diego, CA, USA) or allophycocyanin (APC) (BD Biosciences Pharmingen, San Diego, USA), anti-T cell receptor (TCR)-Vα24-fluorescein isothiocyanate (FITC) (C15; Immunotech, Marseille, France) and anti-TCR-Vβ11-phycoerythrin (PE) (C21; Immunotech). The percentage of CD3+ T cells co-expressing TCR-Vα24/Vβ11 was determined by flow cytometry (Fig. 1), following the collection of 3–10 × 105 events. Isotype-switched memory B cells were quantified by staining either cryopreserved cells with anti-CD20-FITC (L27; BD), anti-IgD-PE (IA6-2; BD) and anti-CD27-biotin (0323; eBioscience); or fresh cells with anti-CD20-PE-Cy5 (B9E9; Immunotech), anti-IgD-FITC (IA6-2; BD) and anti-CD27-PE (1A4C27; Immunotech). Proportions of isotype-switched (CD20+/CD27+/IgD-) B cells of < 0·4% of gated lymphocytes were allocated to Freiburg group I and the remainder to group II, whereas patients with total B cell proportions below 1% were excluded from subdivision analysis [16]. The samples were fixed in 1% formaldehyde; data were acquired on a fluorescence activated cell sorter (FACS)Calibur flow cytometer (BD Biosciences Pharmingen) and analysed using FlowJo (Tree Star, Inc., Ashland, OR, USA). PBMCs were stained with PE-labelled α-GalCer-loaded CD1d tetramers (a kind gift of Dale Godfrey, Department Microbiology and Immunology, University of Melbourne) at a concentration of 0·82 ug/ml, along with anti-CD3-Pacific Blue (as above).
Fig. 1.
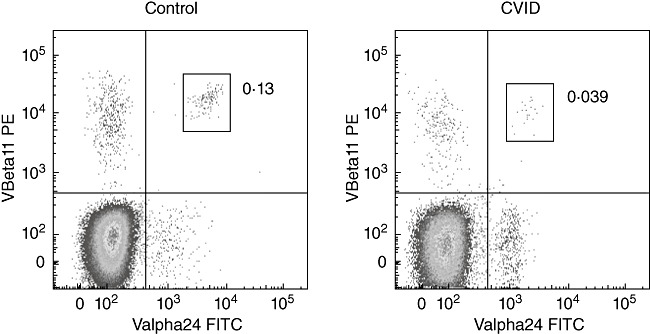
Quantification of invariant natural killer (iNK) T cells by co-expression of T cell receptor (TCR)-Vα24 and TCR-Vβ11 chains by flow cytometry. Initial gating on lymphocytes by forward and side light scatter was followed by gating on CD3+ cells. Plots represent the median values from the cohort of control subjects (left), and common variable immunodeficiency (CVID) patients (middle); the right-hand plot depicts a CVID patient with the highest iNK T cell proportion.
Statistical analysis was performed using Prism version 4·0c for Macintosh (GraphPad Software Inc., La Jolla, CA, USA). The mean difference between continuous variables was analysed using the Mann–Whitney U-test, with P-values < 0·05 considered significant. For analysis of iNK T cell proportion differences over time, the two-tailed paired Wilcoxon signed-rank test was used.
Results
CD3+ T cells present in cryopreserved PBMCs from a cohort of 47 CVID patients and 14 controls were analysed for co-expression of the Vα24 and Vβ11 TCR chains (Fig. 1). There was a significant reduction in the proportion of iNK T cells within the CD3+ T cell population in the CVID cohort [median 0·01%, interquartile range (IQR) 0·0–0·04] compared with controls (median 0·07%, IQR 0·015–0·165) (Fig. 2a). This reduction was most striking in patients with decreased frequencies of isotype-switched memory B cells, referred to commonly as Freiburg group I [16] (Fig. 2b).
Fig. 2.
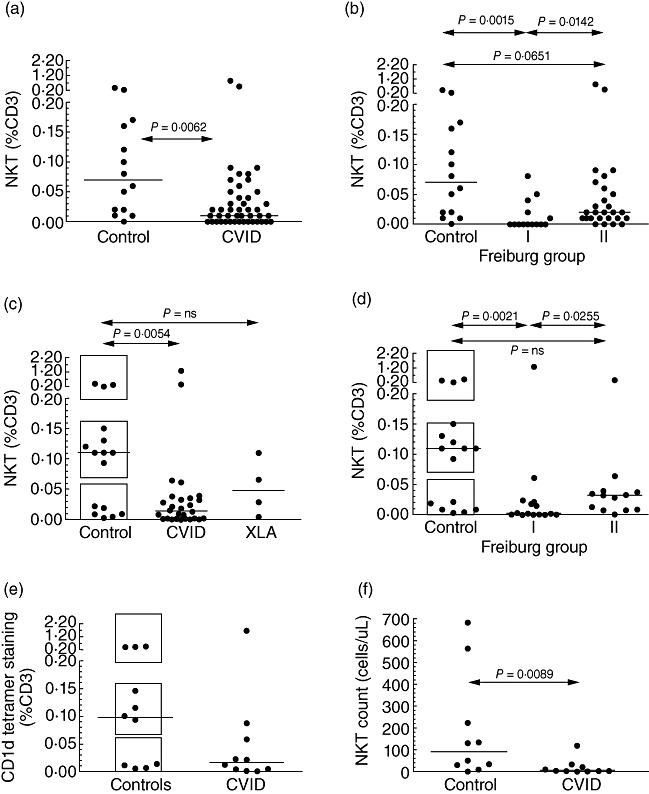
Invariant natural killer (iNK) T cell proportions expressed as a percentage of CD3+ cells in control subjects and common variable immunodeficiency (CVID) patients, based on staining of cryopreserved (a, b) cells from the first cohort, or fresh (c–e) peripheral blood mononuclear cells from the second. Graphs represent CVID patients versus control subjects (a, c) or the breakdown of CVID patients according to the proportion of isotype-switched memory B cells (b, d), with low proportions designated Freiburg group I and normal proportions, group II. In (c), analysis of four X-linked agammaglobulinaemia (XLA) patients is shown for comparison. In (c, d), boxes have been drawn to indicate the three apparent tiers of iNK T proportions in control subjects. (e) Proportions of iNK T cells as detected by CD1d tetramer staining in 10 CVID patients and 10 controls. (f) Absolute counts of iNK T cells in the peripheral blood of 10 CVID patients and 10 controls.
To confirm these findings, and correlate with clinical features, proportions of iNK T cells were then determined in fresh PBMCs from an overlapping cohort of 28 fully phenotyped CVID patients (aged median 46 years, range 23–75) and 16 controls (aged median 41 years, range 22–62). The data from these patients were analysed as a separate group because, in 17 CVID patients analysed by both collection methods, iNK T cell proportions in cryopreserved samples were slightly but significantly higher than in freshly collected samples (Supplementary Fig. S1). As expected, iNK T cell proportions were again decreased markedly in the CVID cohort irrespective of whether the data were expressed as a proportion of total T cells (CVID: median 0·013%, IQR 0·001–0·034, versus control: 0·110%, IQR 0·014–0·140) (Fig. 2c) or of PBMCs (data not shown), and again patients from Freiburg group I showed the lowest proportions (Fig. 2d). There was no relationship between iNK T cell proportions and age (data not shown). Interestingly, the proportions of iNK T cells in normal donors seemed to partition into three separate groups – low (< 0·06%), moderate (0·07–0·16%) and high (> 0·2%; Fig. 2c,d) – which would be consistent with the finding that the production of iNK T cells is controlled genetically [17,18]. Unlike CVID patients, there was no difference in the percentage of switched memory B cells in controls with low NK T cells compared with those who had higher NK T cell proportions (data not shown). On the other hand, the great majority of CVID patients fell within the low group (< 0·06%), except for two patients who had unexpectedly high proportions (Fig. 2c). To exclude the possibility that intravenous gammaglobulin replacement therapy itself might be contributing to a reduction in NK T cell numbers, four patients with X-linked agammaglobulinaemia (XLA) were analysed, but had iNK T proportions not different to controls (Fig. 2c). When the second cohort of CVID patients was stratified on the basis of clinical characteristics (splenomegaly, lymphadenopathy, autoimmune disease), there were no significant differences in iNK T cell proportions in those subsets (data not shown).
The stability of the iNK T cell proportion over time was examined by reanalysing a subset of 10 CVID patients and 10 controls from the second cohort 8 months after the first analysis. Values were very similar to baseline, with no significant differences in iNK T proportions between the two time-points (Fig. 3). To validate the Vα24 and Vβ11 TCR expression, the same subset of 20 subjects was also stained with CD1d tetramers, with similar findings (Fig. 2e) and very close correlation between the two methods (Fig. 4). Finally, when these data were analysed in terms of absolute NK T cell counts, there was again a marked reduction in CVID patients compared with controls (Fig. 2f).
Fig. 3.
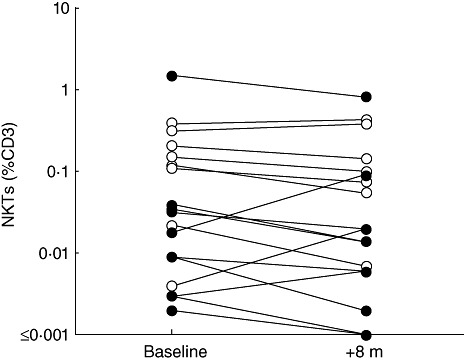
Invariant natural killer (iNK) T cell proportions expressed as a percentage of CD3+ cells in 10 control subjects (open circles) and 10 common variable immunodeficiency (CVID) patients (filled circles), analysed at two time-points, 8 months apart (P = 0·22).
Fig. 4.
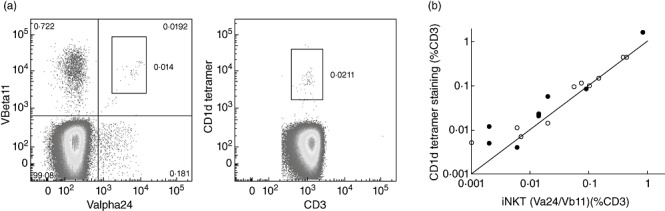
CD1d tetramer staining in comparison to staining with T cell receptor (TCR)-Vα24 and TCR-Vβ11. (a) Dot plots from a representative common variable immunodeficiency (CVID) patient stained by the two methods; (b) comparison between the two methods in 10 controls (open circles) and 10 CVID patients (filled circles). The line of equivalence is shown.
Discussion
We report the finding that CVID is another immunodeficiency disease characterized by depressed or absent iNK T cells, suggesting that iNK T deficiency may be a common factor in the pathogenesis of humoral immunodeficiencies. The low iNK T cell phenotype was demonstrated in a large cohort of CVID patients, was reproducible using two distinct identification reagents, was shown to be a stable finding over time and was not associated with intravenous gammaglobulin itself, as it was not noted in patients with XLA. A paucity of iNK T cells was noted particularly in those patients with low or absent switched-memory B cells, suggesting a link between iNK T cells and B cell function, making an intriguing parallel to XLP in which failure of antibody production and absence of both iNK T cells and memory B cells are common characteristics [7,19]. Further support for an association between iNK T cells and humoral immunity comes from two cardinal observations: first, iNK T cells can express CD40L [1,20] and have the capacity to secrete large concentrations of IL-4, IL-10, IL-13 and IL-21 [1–5], which are all capable of inducing isotype switching and antibody secretion by CD40L-stimulated human B cells [21]; and secondly, iNK T cells can provide cognate help to stimulate B cell activation and enhance antigen-specific responses, both in vivo in mouse models [22,23] and in vitro in co-culture systems utilizing human B cells and autologous iNK T cells [20]. Therefore, it is highly likely that iNK T cells make a significant contribution to basal Ig production in vivo, and an absence of iNK T cells can result in the development of hypogammaglobulinaemia as observed in XLP and CVID. On the other hand, it is unlikely that decreased iNK T cell proportions alone are responsible for the entirety of the CVID phenotype, as a subset of healthy control donors also had proportions of iNK T cells that were as low as CVID patients (Fig. 2) and, similarly, some patients with CVID had normal or even high proportions; rather, this characteristic may be a contributing factor in a multi-factorial process.
Reduced iNK T cell proportions in CVID may relate to defective thymic development, similar to other immunodeficiency disorders. Thus, the failure of iNK T cells to develop normally in XLP patients relates presumably to the loss of signalling lymphocyte activation molecule (SLAM)-associated protein (SAP)-mediated signalling downstream of SLAM and NK T–B antigen (NTB-A) (murine Ly108) following homotypic interactions between these molecules on CD4+/CD8+ thymocytes and thymic epithelium [24], thereby preventing positive selection. In Omenn's syndrome, due to hypomorphic mutations in Rag-1 and Rag-2, defective ability to make appropriate VDJ recombinations might prevent the specific rearrangements required to express the invariant TCR. No clear parallel exists for CVID, because a genetic explanation is apparent in < 10% of cases, yet our findings point towards the thymus in the pathogenesis of this condition, in which multiple other defects in conventional T cell biology have been described [25]. However, we cannot exclude the alternate possibility that our findings represent a deficiency in peripheral blood iNK T cells in CVID due to their sequestering to sites outside the circulation.
One further observation in this study was that iNK T cell values in cryopreserved samples were generally higher than in fresh samples taken 3 years later (Supplementary Fig. S1). It is likely that this finding is methodological, as iNK T cell proportions appeared to be constant in CVID patients and controls, at least over an 8-month period (Fig. 3).
In conclusion, deficiency of iNK T cells may have implications for our understanding of antibody failure in CVID and may suggest a common link with other immunodeficiency disorders.
Acknowledgments
We would like to acknowledge with thanks the help of Marjorie Bennett, Judy Pointing and Philippa Kirkpatrick in the collection of samples for this study, and the contributions of the patients and controls in supporting this work. We also thank Dr Kim Nichols for helpful discussion and suggestions and critical review of this manuscript, and Dr Dale Godfrey for providing the CD1 tetramers. This work was supported by the National Health and Medical Research Council of Australia and Cancer Council NSW.
Disclosure
The authors have no conflicts of interest to declare.
Supporting information
Additional Supporting Information may be found in the online version of this article:
Fig. S1. Comparison of inducible natural killer(iNK) T cell proportions as derived from cryopreserved cells(y-axis) in comparison to fresh cells (x-axis) in asubset of 18 common variable immunodeficiency (CVID) patientsanalysed by both methods at different time-points. Line of best fitis dashed (r2 = 0·9962, slope =1·27), while the y = x line is shown in grey for comparison.
Please note: Wiley-Blackwell is not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 2.Kronenberg M, Gapin L. The unconventional lifestyle of NKT cells. Nat Rev Immunol. 2002;2:557–68. doi: 10.1038/nri854. [DOI] [PubMed] [Google Scholar]
- 3.Kadowaki N, Antonenko S, Ho S, et al. Distinct cytokine profiles of neonatal natural killer T cells after expansion with subsets of dendritic cells. J Exp Med. 2001;193:1221–6. doi: 10.1084/jem.193.10.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee PT, Benlagha K, Teyton L, Bendelac A. Distinct functional lineages of human V(alpha)24 natural killer T cells. J Exp Med. 2002;195:637–41. doi: 10.1084/jem.20011908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coquet JM, Kyparissoudis K, Pellicci DG, et al. IL-21 is produced by NKT cells and modulates NKT cell activation and cytokine production. J Immunol. 2007;178:2827–34. doi: 10.4049/jimmunol.178.5.2827. [DOI] [PubMed] [Google Scholar]
- 6.Chung B, Aoukaty A, Dutz J, Terhorst C, Tan R. Signaling lymphocytic activation molecule-associated protein controls NKT cell functions. J Immunol. 2005;174:3153–7. doi: 10.4049/jimmunol.174.6.3153. [DOI] [PubMed] [Google Scholar]
- 7.Nichols KE, Hom J, Gong S-Y, et al. Regulation of NKT cell development by SAP, the protein defective in XLP [see comment] Nat Med. 2005;11:340–5. doi: 10.1038/nm1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pasquier B, Yin L, Fondaneche M-C, et al. Defective NKT cell development in mice and humans lacking the adapter SAP, the X-linked lymphoproliferative syndrome gene product. J Exp Med. 2005;201:695–701. doi: 10.1084/jem.20042432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rigaud S, Fondaneche M-C, Lambert N, et al. XIAP deficiency in humans causes an X-linked lymphoproliferative syndrome. Nature. 2006;444:110–14. doi: 10.1038/nature05257. [DOI] [PubMed] [Google Scholar]
- 10.Matangkasombut P, Pichavant M, Saez DE, et al. Lack of iNKT cells in patients with combined immune deficiency due to hypomorphic RAG mutations. Blood. 2008;111:271–4. doi: 10.1182/blood-2007-06-096487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Locci M, Draghici E, Marangoni F, et al. The Wiskott–Aldrich syndrome protein is required for iNKT cell maturation and function. J Exp Med. 2009;206:735–42. doi: 10.1084/jem.20081773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li D, X-N X. NKT cells in HIV-1 infection. Cell Res. 2008;18:817–22. doi: 10.1038/cr.2008.85. [DOI] [PubMed] [Google Scholar]
- 13.Sandberg JK, Fast NM, Palacios EH, et al. Selective loss of innate CD4(+) V alpha 24 natural killer T cells in human immunodeficiency virus infection. J Virol. 2002;76:7528–34. doi: 10.1128/JVI.76.15.7528-7534.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herve M, Isnardi I, Ng Y-S, et al. CD40 ligand and MHC class II expression are essential for human peripheral B cell tolerance. J Exp Med. 2007;204:1583–93. doi: 10.1084/jem.20062287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berglund LJ, Wong SWJ, Fulcher DA. B-cell maturation defects in common variable immunodeficiency and association with clinical features. Pathology. 2008;40:288–94. doi: 10.1080/00313020801911470. [DOI] [PubMed] [Google Scholar]
- 16.Warnatz K, Denz A, Drager R, et al. Severe deficiency of switched memory B cells (CD27(+)IgM(-)IgD(-)) in subgroups of patients with common variable immunodeficiency: a new approach to classify a heterogeneous disease. Blood. 2002;99:1544–51. doi: 10.1182/blood.v99.5.1544. [DOI] [PubMed] [Google Scholar]
- 17.Lee PT, Putnam A, Benlagha K, Teyton L, Gottlieb PA, Bendelac A. Testing the NKT cell hypothesis of human IDDM pathogenesis. J Clin Invest. 2002;110:793–800. doi: 10.1172/JCI15832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jordan MA, Baxter AG. The genetics of immunoregulatory T cells. J Autoimmun. 2008;31:237–44. doi: 10.1016/j.jaut.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 19.Ma CS, Nichols KE, Tangye SG. Regulation of cellular and humoral immune responses by the SLAM and SAP families of molecules. Ann Rev Immunol. 2007;25:337–79. doi: 10.1146/annurev.immunol.25.022106.141651. [DOI] [PubMed] [Google Scholar]
- 20.Galli G, Nuti S, Tavarini S, et al. Innate immune responses support adaptive immunity: NKT cells induce B cell activation. Vaccine. 2003;21(Suppl. 2):S48–54. doi: 10.1016/s0264-410x(03)00200-7. [DOI] [PubMed] [Google Scholar]
- 21.Bryant VL, Ma CS, Avery DT, et al. Cytokine-mediated regulation of human B cell differentiation into Ig-secreting cells: predominant role of IL-21 produced by CXCR5+ T follicular helper cells. J Immunol. 2007;179:8180–90. doi: 10.4049/jimmunol.179.12.8180. [DOI] [PubMed] [Google Scholar]
- 22.Barral P, Eckl-Dorna J, Harwood NE, et al. B cell receptor-mediated uptake of CD1d-restricted antigen augments antibody responses by recruiting invariant NKT cell help in vivo. Proc Natl Acad Sci USA. 2008;105:8345–50. doi: 10.1073/pnas.0802968105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leadbetter EA, Brigl M, Illarionov P, et al. T cells provide lipid antigen-specific cognate help for B cells. Proc Natl Acad Sci USA. 2008;105:8339–44. doi: 10.1073/pnas.0801375105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Griewank K, Borowski C, Rietdijk S, et al. Homotypic interactions mediated by Slamf1 and Slamf6 receptors control NKT cell lineage development. Immunity. 2007;27:751–62. doi: 10.1016/j.immuni.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giovannetti A, Pierdominici M, Mazzetta F, et al. Unravelling the complexity of T cell abnormalities in common variable immunodeficiency. J Immunol. 2007;178:3932–43. doi: 10.4049/jimmunol.178.6.3932. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Comparison of inducible natural killer(iNK) T cell proportions as derived from cryopreserved cells(y-axis) in comparison to fresh cells (x-axis) in asubset of 18 common variable immunodeficiency (CVID) patientsanalysed by both methods at different time-points. Line of best fitis dashed (r2 = 0·9962, slope =1·27), while the y = x line is shown in grey for comparison.


