Abstract
Lower extremity peripheral vein bypass grafts (LE-PVBG) imaged with high-resolution black blood 3D inner-volume (IV) FSE MRI at 1.5T possess a two layer appearance in T1W images while only the inner layer appears visible in the corresponding T2W images. This study quantifies this difference in six patients imaged six months post-implantation, and attributes the difference to the T2 relaxation rates of vessel wall tissues measured ex vivo in two specimens with histologic correlation. The visual observation of two LEPVBG vessel wall components imaged in vivo is confirmed to be significant (p<0.0001), with a mean vessel wall area difference of 6.8±2.7 mm2 between contrasts, and a ratio of T1W to T2W vessel wall area of 1.67±0.28. The difference is attributed to a significantly (p<0.0001) shorter T2 relaxation in the adventitia (T2=52.6±3.5ms) compared to the neointima/media (T2=174.7±12.1ms). Notably, adventitial tissue exhibits bi-exponential T2 signal decay (p<0.0001 vs mono-exponential). Our results suggest that high-resoultion black blood 3D IV-FSE can be useful for studying the biology of bypass graft wall maturation and pathophysiology in vivo, by enabling independent visualization of the relative remodeling of the neo-intima/media and adventitia.
Keywords: Ultra-high resolution magnetic resonance imaging; Vessel wall imaging; Peripheral vein bypass graft MRI; Multi-contrast MRI; black blood Inner-Volume Imaging, 3D FSE
Introduction
Lower extremity peripheral vein bypass graft (LE-PVBG) failure is an important complication in patients who undergo surgery for critical limb ischemia. Intermediate-term vein graft failure (3-24 months) occurs in up to 30-50% of vein grafts and is typically attributed to over-exuberant neo-intimal hyperplasia causing hemodynamically significant stenosis and ultimately thrombosis of the bypass graft (1-3). Despite its clinical importance, the pathophysiology of vein graft failure and the local hemodynamic mechanisms underlying the development of disease remain largely uncharacterized (1,4). Specifically, while some wall thickening is universal as vein grafts adapt to the high pressure arterial environment, the balance between physiologic and pathologic thickening and the biological signal that triggers pathological thickening is unclear (2). Typically, thickening of the intima begins within a few weeks post-operatively, and is characterized by an increase in cellularity (migration and proliferation of smooth muscle cells), which subsequently deposit extracellular matrix (1,2). With respect to pathophysiology, it has been postulated that excessive wall thickening, manifested primarily as intimal hyperplasia, as opposed to medial hypertrophy, is a critical step towards intermediate-term vein graft failure (1,3).
Routine clinical graft assessment uses ultrasound (US), a modality well suited to measure the lumen and thus identify hemodynamically significant graft stenosis, but lacking the spatial resolution and soft tissue contrast to characterize changes in the vessel wall that occur in early graft disease (5). Magnetic resonance imaging (MRI) can potentially overcome these limitations and better characterize the vessel wall, given the improved spatial resolution and diverse tissue contrast it provides (6-8). Resolutions currently available for routine vessel wall MR imaging applications (400-500 μm in-plane resolution and 3-4 mm section thickness (8)), are suitable to readily quantify disease in large vessels (9,10), but pose a limitation for imaging vein grafts (typical 3.5 - 5 mm lumen diameter (5,11,12) and 300 - 500 μm wall thickness at implantation).
Recent advances in vessel wall MRI have enhanced imaging efficiency in order to increase spatial resolution while maintaining suitable signal-to-noise ratio (SNR) and scan times (6,13-16). Our laboratory developed a high sampling efficiency high-resolution black blood inner-volume 3D volumetric encoding FSE approach with multi-contrast capabilities (6,17) to study the LE-PVBG lumen and vessel wall changes over time (18). At 1.5 T this method achieves a nominal voxel resolution of 312 μm × 312 μm × 2 mm in under 10 min per contrast (6). Initial studies of LE-PVBGs using this technique demonstrated that the outer graft wall boundary is significantly larger in T1-weighted (T1W) images compared to T2-weighted (T2W) images. Furthermore, the difference visually appears to stem from a second layer of the graft wall that is visible in T1W images and that is absent in T2W images. The purpose of this work is to quantify this difference, and demonstrate that using high resolution 3D IV-FSE MRI at 1.5 T, the media and adventitia of the vein graft vessel wall can be separated non-invasively, much as native artery vessel wall layers can be separated with ultra-high field MRI (19) and intravascular MRI methods (20).
Methods
Subjects and specimens
The institutional human research committee approved this study and written informed consent was obtained from 6 patients (mean age: 64.8±12.7 yrs, mean weight: 87.3±19.2 kg, 1 female) who underwent LE-PVBG MRI at 6 months (mean: 189±21 days) post-implantation. Four patients had diabetes mellitus. The indication for arterial reconstruction was either critical limb ischemia (n=3) or popliteal artery aneurysm. The source of inflow was either the superficial femoral artery (n=4) or the common femoral artery, while the outflow consisted of either popliteal (n=3) or tibial vessels. In all cases the bypass was constructed with a single-segment greater saphenous vein (1 reversed).
Informed consent was also obtained in two additional patients whose LE-PVBG specimens underwent MR transverse T2 relaxation studies. Both specimens were obtained at clinically indicated surgical revision due to graft stenosis. The first specimen (denoted S1 below) was 3 cm long and was obtained 174 days post-implantation from a diabetic 68 yr old male with a single intragraft lesion in a single-segment reversed greater saphenous vein graft (common femoral artery inflow and popliteal artery outflow). The second specimen (denoted S2 below) was 9 cm long and was obtained 247 days post-implantation from a 70 yr old male with two intragraft lesions separated by approximately 4 cm. The graft had been constructed from a spliced greater and lesser saphenous vein (superficial femoral artery inflow and posterior tibial artery outflow).
Magnetic resonance imaging
All MRI experiments were performed on a 1.5 T GE HDx MR imager (General Electric, Milwaukee, WI), equipped with 40 mT/m, 150 T/m/s gradients. A 5” circular surface coil was used for signal reception unless otherwise noted, and the body resonator was used for radio-frequency (RF) excitation.
In Vivo protocol
In vivo imaging of the six LE-PVBG patients followed a previously developed protocol (18). The LE-PVBG was identified using a bright-blood 2D time-of-flight (TOF) sequence (spoiled gradient recalled-echo, 33/6.9 ms TR/TE, 50 FA, 1 NEX, 16 kHz BW, 1.4 × 1.4 × 4 mm resolution). A 3D IV-FSE sequence (17) was then used to obtain high-resolution cross-sectional black blood images, with the section-select dimension orthogonal to the orientation of the LE-PVBG as determined by the TOF projection images.
The 3D IV-FSE sequence resembles a standard 3D FSE sequence with two modifications: first, a small volume is selected for imaging at the intersection of the first two slab-selective RF pulses (the 90-degree tip-down pulse and the first 180-degree refocusing pulse) by applying them with their associated gradients so as to excite slabs that are situated at a 90° angle relative to each other (17,21). The intersection of these two slabs defines the inner volume to be imaged. To minimize scan time, a long tip-down 90-degree slab-selective RF pulse is used for IV selection (6.2 ms, 19.2 ms-kHz time-bandwidth product), designed to produce a sharp excitation profile. This reduces the section oversampling that is necessary to avoid wrap-around aliasing artifact to 11% of the imaged sections (6). The second modification involves the use of non-selective refocusing RF pulses of 500 μ-s duration to form the second and subsequent echoes. This optimizes sampling efficiency by minimizing the FSE inter-echo spacing from the second echo on (13,17,22).
Imaging of the LE-PVBGs with the 3D IV-FSE sequence used standard double-inversion recovery for blood signal nulling (23), fat-resonance selective saturation for T2W imaging but not for T1W imaging, and, cardiac gating for both sequences. Spatial parameters included a 3 × 3 × 3.6 cm inner volume and 96 (frequency) × 96 (phase) × 18 (section) matrix (312 μm × 312 μm × 2 mm resolution, in-plane zero-fill interpolated to 117 μm × 117 μm). T1W images were acquired with 12 ETL, ±16 kHz BW, 17 ms TE and 1 R-R TR. T2W images were acquired with 18 ETL, ±10 kHz BW, 60 ms TE and 2 R-R TR. The first echo occurred at 17 ms for both contrasts, and echo spacing for subsequent echoes was 7.6 ms for T1W imaging and 11.7 ms for T2W imaging. Total imaging time was 9.5 min per contrast with 4 NEX at 60 bpm.
Ex Vivo protocol
Ex vivo imaging included the in vivo protocol described above, plus a Carr-Purcell-Meiboom-Gill (CPMG) inner-volume 3D MRI pulse sequence (3D IV-CPMG) based on the 3D IV-FSE but modified in order to access the T2-induced signal decay of the tissue. This was achieved by producing separate images from each echo. Specifically, rather than encoding each echo with a different phase encode so as to speed up the acquisition, each echo is encoded with the same phase encode so as to produce a separate volumetric image. In order to measure the T2-induced signal decay alone, indirect and stimulated echo pathways (24) were suppressed using additional modifications to the 3D IV-FSE as recommended by Poon and Henkelman for accurate T2 decay measurements (25). Specifically, the non-selective refocusing RF pulses are replaced with non-selective composite 90x-180y-90x pulses; both the in-plane and section phase encoding are performed once, immediately following the tip-down RF excitation (i.e., no phase encode wind/rewind steps between refocusing pulses); and, the amplitude of the dephasing gradients surrounding each refocusing pulse is alternated and stepped down between each successive echo and pair of echoes, respectively.
Both specimens were immersed in saline and imaged at room temperature within 4 hrs after excision using a 3” circular receive coil. A total of 16 echo times were collected using the 3D IV-CPMG, with the first echo at 19.4 ms TE and subsequent echoes at intervals of either 12.9 ms (S1) or 12.2 ms (S2). Fat-selective saturation and DIR blood suppression pulses were not used. The following imaging parameters were common to both specimens: 2 s TR, ±7.81 kHz BW, 96 (frequency) × 72 (phase) matrix, (312 μm × 312 μm resolution) and 2 mm section thickness. Sixteen section encodes were used for S1 (4 NEX) and 34 section encodes were used for S2 (1 NEX), in order to cover the length of the respective specimen (3.2 cm and 6.8 cm, respectively) and maintain equivalent SNR.
Matching of MR and histology images
Following ex vivo imaging, Masson's trichrome stain histology was obtained from both specimens. Histological sections obtained every 4 mm were matched to MRI sections based on the distance from the proximal end of the specimens. The matching of MR images and histology slides was visually confirmed based on vessel wall shape and morphological features.
Image analysis
Measurements of the vessel lumen and wall areas from the in vivo images were performed using direct planimetry (OsiriX version 3.0) by consensus of two dedicated cardiovascular imagers with 4 and 6 years experience. The readers were blinded to the patient identity and T1W images were presented separately from T2W images to minimize bias. The first and last sections in each volume acquired by the 3D IV-FSE are oversamples and were thus discarded. Of the remaining 96 sections (16 per patient), eight were discarded due to limited SNR because of suboptimal receiver coil placement.
Ex vivo T2 relaxation studies were performed in ten regions-of-interest (ROI) in each layer of each specimen (40 ROIs total, mean ROI area: 0.71 mm2). Care was taken to select ROIs away from layer interfaces, as determined by histology and the latest echo MR images, so as to avoid partial voluming of different vessel wall tissue components. In addition, ROIs were chosen in different sections of each volumetric acquisition, in order to avoid any per-section bias. Signal decays extracted from each ROI were analyzed in Matlab (Mathworks, Natick, MA) with mono- and bi-exponential decay model fitting (26) using a Levenberg-Marquardt non-linear optimization routine employing a non-negativity constraint.
Statistical Analysis
Analysis of the in vivo images tested the hypotheses that a) the area of the lumen measured from T1W images is not significantly different than that measured from T2W images, and, b) that the vessel wall area measured from T1W images is not significantly different than its counterpart measured from T2W images. Both hypotheses were tested using the exact Wilcoxon signed rank test to determine whether the median difference between the corresponding areas is zero.
Analysis of the ex vivo T2 data was based on the reduced chi-squared (r.χ2) values calculated for the mono- and bi-exponential fits. F-tests were then performed to determine if any reduction in χ2, relative to the differences in degrees of freedom for each type of fit, was statistically significant. The ratio of variances F (F-ratio) was converted by the F-cumulative distribution function to yield the probability p of type I error (rejection of a true null hypothesis). A p value less than 0.05 indicated a significant difference, in this case implying that a bi-exponential fit was statistically more appropriate than a mono-exponential fit (27). Inter- and intra-specimen comparison of the fitted T2 decay time constants was performed using the unpaired Student t-test.
The ratio of signal intensities of the vessel wall layers observed in T1W images was correlated to the T2 relaxation rates of the different vessel wall tissue components determined by histology. Specifically, the ratio of signal intensities in the two layers was compared to its expected value based on the ex vivo T2 rates measured for the media and adventitia for the effective TE of the T1W images. Signal intensities for each layer were obtained by assuming that only the inner vessel wall layer is apparent in T2W images. Thus, superimposing the outer wall boundary determined from a T2W image to its corresponding T1W image separated the two wall layers in that image; the annular ROI defined by the original lumen boundary in the T1W image and the outer wall boundary copied from the T2W image was assumed to delineate the neo-intima and media in T1W images. Similarly, the annular ROI defined by the outer wall boundary copied from the T2W image and the outer vessel wall boundary originally determined in the corresponding T1W image was assumed to delineate the adventitia. The ratio of the mean T1W signal intensities in the two ROIs, RT1W, was measured for each T1W image and subsequently averaged over all in vivo images collected. The media-to-adventitia contrast-to-noise ratio (CNR) is given by the inverse of this ratio, CNRT1W = 1/RT1W.
Wall layer signal intensities were used to measure SNR in both layers for a) the T1W images ( and ) and b) the inner layer for T2W images (). The outer layer could not be delineated in the T2W images. SNR was measured as the ratio of the mean signal intensity in the corresponding vessel wall layer to the mean signal intensity measured in an ROI placed in a tissue-free “noise” region of the corresponding image. This measurement was only possible in a subset of two patients in which the small inner volume field-of-view included a volume free of tissue.
Results
All 88 in vivo cross-sectional 3D IV-FSE images analyzed were of high quality, although occasionally demonstrated some artifact due to incomplete blood nulling, likely from slow-flowing blood. Nonetheless, sufficient contrast was available to identify the lumen/wall interface in all sections analyzed. Selected sections from 4 patients are shown in Figure 1. The lumen of the LE-PVBG in one patient was significantly narrowed (Figure 1, 4th row), apparently due to myointimal hyperplasia.
Figure 1.
Example T1W (1st and 3rd columns) and T2W (2nd and 4th columns) images from 4 patients. White arrow in third T2W image from top indicates incomplete blood nulling artifact. The two far right columns show the lumen and outer vessel wall boundary traces in red lines, a 1 mm scale denoted by a black (T1W) or white (T2W) horizontal line, and the lumen and vessel wall area measurements superposed on the lumen and above the vessel respectively.
LE-PVBG morphological characteristics measured in this patient cohort, including lumen and wall area as well as lumen diameter and wall thickness are provided in Table 1. Diameter and thickness measurements were derived by treating the lumen and total wall areas in each cross-sectional image as if corresponding to concentric disks. There was a significant (Wilcoxon p < 0.0001) difference of 0.8 ± 0.6 mm2 (95% CI: 0.7 - 0.9 mm2) in mean lumen area between contrast weightings. However, lumen areas were significantly correlated between contrast weightings (Pearson correlation coefficient r2=0.9953, Figure 2a), and Bland-Altman analysis (Figure 2b) indicated the difference was a systematic absolute bias. There was a significant (Wilcoxon p < 0.0001) difference of 6.8 ± 2.7 mm2 (95% CI: 6.2 - 7.4 mm2) in mean vessel wall area measured between contrasts, with the area measured in T1W images on average 1.67 ± 0.28 times larger than its T2W counterpart (95% CI: 1.61 - 1.72). Vessel wall area measurements between contrast weightings were moderately correlated (r2=0.6595), indicating this difference was not a systematic bias.
Table 1.
Lumen and wall area direct planimetry measurements in T1W and T2W images, and differences between contrast weightings in 88 LE-PVBG sections imaged in 6 patients at 6 months post-implantation.
| Area (mm2) |
Average Diameter/Thickness** (mm) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Mean | Range | Excluding lesion*** |
Mean | Range | Excluding lesion*** |
|||
| Mean | Range | Mean | Range | |||||
| T1W Lumen | 18.4 ± 8.9 | 1.6 - 43.4 | 21.1 ± 7.0 | 14.4 - 43.4 | 4.7 ± 1.3 | 1.4 - 7.4 | 5.1 ± 0.8 | 4.3 - 7.4 |
|
| ||||||||
| T2W Lumen | 17.5 ± 8.9 | 1.5 - 43.3 | 20.2 ± 7.2 | 12.1 - 43.3 | 4.5 ± 1.3 | 1.4 - 7.4 | 5.0 ± 0.8 | 3.9 - 7.4 |
|
| ||||||||
| T1-T2 Lumen | 0.8 ± 0.6 | -0.6 - 2.4 | 0.9 ± 0.6 | -0.6 - 2.4 | 0.11 ± 0.09 | -0.12 - 0.36 | 0.12 ± 0.09 | -0.05 - 0.36 |
|
| ||||||||
| T1W Wall* | 17.5 ± 4.5 | 9.6 - 30.8 | 18.3 ± 4.3 | 11.6 - 30.8 | 1.0 ± 0.2 | 0.7 - 1.5 | 1.0 ± 0.1 | 0.7 - 1.2 |
|
| ||||||||
| T2W Wall* | 10.7 ± 2.9 | 5.9 - 19.7 | 10.8 ± 3.1 | 5.9 - 19.7 | 0.7 ± 0.2 | 0.4 - 1.5 | 0.6 ± 0.1 | 0.4 - 0.9 |
|
| ||||||||
| T1-T2 Wall | 6.8 ± 2.7 | 0.8 - 13.3 | 7.5 ± 2.3 | 3.0 - 13.3 | 0.32 ± 0.12 | 0.01 - 0.59 | 0.35 ± 0.10 | 0.14 - 0.59 |
|
| ||||||||
Wall area is the total vessel wall area minus the lumen area measured.
Average diameter (lumen) and thickness (vessel wall) were obtained from the diameter of idealized disks filling the measured areas; wall thickness is the radius of the disk corresponding to the total vessel wall area minus that of the lumen.
Respective measurements for the 5 LE-PVBG patients excluding that with apparent myointimal hyperplasia.
Figure 2.
Correlation of the lumen area measured from T1W and T2W images (a), and, Bland-Altman plot of lumen area difference as measured from T1W vs. T2W images (b). Plotted measurements correspond to 88 sections obtained in vivo from 6 LE-PVBG patients.
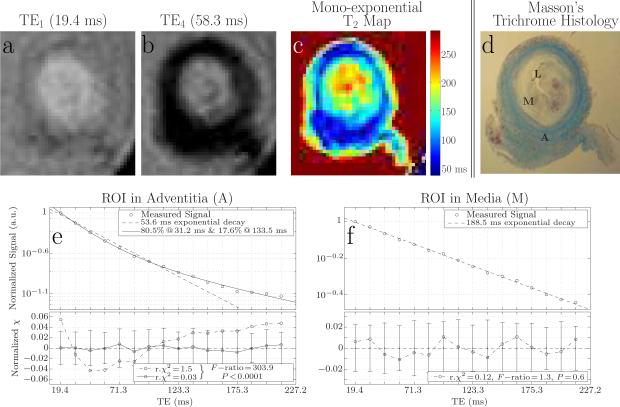
The mean characteristic T2 decay constant fitted to the signal observed ex vivo in ROIs placed in the adventitia, composed of collagenous material as determined by correlative histology (Figures 3c, d) was 52.6 ± 3.5 ms (Table 2, Figure 3e). A significantly (t-test p < 0.0001) longer mean characteristic T2 decay constant of 174.7 ± 12.1 ms (Table 2, Figure 3f) was fitted to signal observed in ROIs placed in the neo-intima/media, composed of cells and proteoglycan matrix (Figures 3c, d). Fitted decay constants were not significantly different between specimens in either the adventitia (t-test p > 0.18, Table 2) or the neo-intima/media (t-test p > 0.06, Table 2). Signal decays observed in all ROIs placed in adventitial tissue were better fitted by a bi-exponential decay model rather than a mono-exponential decay model (F-ratio range: 20.9 - 310.3, F-test 0 < p < 0.001). Nonetheless, the mono-exponential model suffices for bulk comparison of T2 relaxation rates in the adventitia versus in the neo-intima/media with respect to the in vivo results, since the bulk of the bi-exponential decay (83.0 ± 4.9%) was characterized by a short T2 (35.5 ± 3.0 ms, Table 2). Thus, the overall signal magnitude is not significantly different between the mono- and bi-exponential decay models at the TEs used for in vivo T1W and T2W imaging (Figure 3e).
Figure 3.
Example section of an LE-PVBG specimen at two of the 16 echoes acquired by the 3D IV-CPMG sequence (a,b). Map of decay times fitted in each voxel of the same section using the mono-exponential decay model (c). Masson's trichrome histology of matched section (d). Example signal decay curve observed in that section in a ROI located in the adventitia (as determined by histology) exhibiting bi-exponential decay (e), and, at a ROI located in the neoinima/media of the same section, exhibiting mono-exponential decay (f). Note the error bars shown in the residual plots (bottom plots in panels e, f) represent the mean signal observed in an empty space “noise” ROI.
Table 2.
T2 characteristic decay times of tissues observed in two LE-PVBG specimens.
| Specimen | F-ratio | F-test | Mono-exp. T2fit (ms) |
t-test* (p) |
Bi-exponential T2 fit |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Short component (ms) |
t-test* (p) |
Long Component (ms) |
t-test* (p) |
Short Component (%) |
||||||
| Adventitia | S1 (n=10) |
37.1 - 310.3 |
p < 0.0001 |
51.5 ± 3.5 |
>0.18 |
35.1 ± 3.4 |
>0.58 |
136.5 ± 21.0 |
>0.66 |
83.8 ± 5.2 |
| S2 (n=10) |
20.9 - 222.7 |
p < 0.003 |
53.5 ± 3.3 |
35.8 ± 2.8 |
133.3 ± 13.6 |
82.4 ± 4.7 |
||||
| Combined | 20.9 - 310.3 | p < 0.003 | 52.6 ± 3.5 | 35.5 ± 3.0 | 134.8 ± 17.3 | 83.0 ± 4.9 | ||||
|
| ||||||||||
|
Neo-intima/media
|
S1 (n=10) |
2.0 - 4.7 |
p > 0.07 |
179.8 ± 9.8 |
>0.06 |
|||||
| S2 (n=10) |
0.0 - 4.3 |
p > 0.08 |
170.5 ± 12.4 |
|||||||
|
Combined
|
0.0 - 4.6 |
p > 0.07 |
174.7 ± 12.1 |
|||||||
Unpaired t-test of mean T2 observed in between specimens.
Given the T2 relaxation rates observed, the ratio of signal intensities between the two vessel wall layers at the TE of the T1W images is roughly . This is based on ignoring proton density and T1 relaxation rate differences, and assuming that in vivo T2 rates are not significantly different than ex vivo. In agreement with this predicted value, the average ratio of mean signal intensities between the two layers measured in the in vivo T1W images was RT1W = 0.84 ± 0.09. In the two patients with noise measurements, the mean vessel wall layer SNRs were and in the T1W images, and, in the T2W images.
Discussion
High resolution 3D IV-FSE enables differences between T1W and T2W images to be observed in LE-PVBG wall area in vivo as early as 1 month post-implantation (6,18). Furthermore, follow-up imaging in one patient has demonstrated changes of the wall area between 1 and 6 months post-implantation with either contrast weighting (18). The current study quantified those differences in LE-PVBGs imaged six months after implantation, in six patients with no clinical evidence of graft failure. The observed mean difference in vessel wall area was found to be significant and corresponded to a difference of 0.32 ± 0.12 mm in average vessel wall thickness (Table 1). Furthermore, the difference was entirely attributable to an enlarged outer vessel wall boundary in all T1W images compared to their T2W counterparts. This result remained significant even after accounting for a systematic negative bias in the lumen area measured from T2W images.
Prior studies of native arteries, specifically in carotid endarterectomy specimens (7,28-32), indicate that at 9.4 - 11.7 T the media has a longer T2 (70 - 76 ms (28,30)) than fibrous tissue (40 - 60 ms), and that relative differences in T1 relaxation rates between these components are less pronounced (28,30,32). This led us to believe that the difference in wall area observed in vivo between the two contrasts in LE-PVBGs is primarily due to the differing T2 properties of the neo-intima/media versus the adventitia. While T2 rates are expected to be longer at lower field strengths, conflicting measurements have been reported for the carotid media (40 - 60 ms) at 0.5 - 1.5 T (7,33). This is also confounded by the fact that LE-PVBGs have a different tissue composition than native arteries, and T2 rates of wall tissue may also vary during graft maturation due to changes in composition. We previously described early lumen dilatation (0-1 month) followed later by stiffening of the vein graft wall (3-6 months) (5,34). This suggests significant deposition of fibrous protein during the later phase of vein graft maturation, which is likely to be accompanied by changes in T2 rates.
To directly assess whether wall area differences observed in vivo may indeed be driven by differing T2 rates in the two LE-PVBG wall layers, ex vivo T2 relaxometry studies were performed in two fresh LE-PVBG specimens immersed in saline at room temperature. Care was taken to match all other experimental conditions, and both specimens were excised at similar post-implantation times as those grafts imaged in vivo. In agreement with previous studies we also found a significant mean difference of 122.1 ms between the T2 relaxation rate of the neo-intimal/medial (T2 of 174.7 ± 12.1 ms) versus the adventitial (T2 of 52.6 ± 3.5 ms) tissue, as determined by correlative histology. We believe this difference sufficiently accounts for the in vivo observation of differing vessel wall area; the slow decay of signal from tissue in the neo-intima/media may render it hyper-intense in either contrast weighting. In contradistinction, signal from the adventitia may persist at the short TE of the T1W images (17 ms) but would vanish at the longer TE of the T2W images (60 ms).
Accordingly, we hypothesized that the outer wall boundary determined from in vivo T2W images extends only to the neo-intima/media, while that identified in T1W images includes the adventitia. This would explain the significant difference in vessel wall area between contrasts. To indirectly test this hypothesis, we compared the signal intensities of the two vessel wall layers in T1W images. Under idealized assumptions, we found that the signal intensity ratio between these two layers was RT1W = 0.84 ± 0.09 (CNRT1W = 1/RT1W = 1.19 ± 0.13) in the in vivo T1W images, in excellent agreement with its predicted value of 0.8 based on the ex vivo T2 rates and the effective TE used for T1W imaging. In comparison, for the in vivo T2W images the expected signal loss is predicted to be relatively larger with a ratio of , thereby rendering the outer layer less likely to be perceived as part of the vessel wall.
This work presents a number of methodological limitations. The significance of the in vivo observation of differing wall areas necessitated accounting for the small bias in lumen area observed between contrasts. Ideally, one would expect lumen area to be independent of contrast weighting. However, at the spatial resolutions achieved here there are two likely explanations for such a bias. First, the media may be more blurred into the lumen due to the longer echo train duration used for T2W as opposed to T1W imaging (ETL of 18 vs 12, echo train duration of 210.6 ms vs 91.2 ms) (35), thereby reducing the perceived area of the lumen. Second, it is possible that differences in blood nulling between contrasts can lead to this discrepancy. A combination of these effects is also possible.
Another potential limitation stems from the assumptions used to correlate the in vivo T1W wall layer signal intensities ratio with that predicted from the ex vivo T2 measurements. The assumption of similar proton density appears reasonable given the small signal intensity differences throughout the vessel wall at a short TE (c.f., Figure 3a). It is also known that T2 rates of vessel wall tissues become slightly elongated (5%-20%) at room temperature as opposed to body temperature (33). Hence, one would expect a slightly lower signal intensity ratio in vivo than that predicted using ex vivo T2 measurements Additionally, T1 weighting effects in LE-PVBGs have not been studied, although previously reported differences in T1 rates are relatively small between layers (28) and their effect is expected to be limited.
It may also be argued that the use of fat saturation renders the outer boundary necessarily smaller in T2W images than that available from the high contrast between the outer vessel wall boundary and surrounding fat in the T1W images. The media-to-adventitia CNR in T1W images suggests that the two tissues are likely to be perceived as a single component. For the in vivo T2W images the higher CNR between the two layers suggests that they can be separated. However, while the SNR of both layers in T1W images is similar to that of the media in T2W images (approximately 9), the SNR for the adventitia given its faster relaxation rate is expected to be significantly lower in T2W images, approximately SNRAT2W = SNRMT2W × RT2W ≈ 3.9. In conjunction with the increased contrast, delineation of the outer wall would thus be much more problematic in T2W images than in T1W images with the current protocols. Thus, although the lack of fat saturation may have contributed to the perception of a larger vessel wall area in T1W images, the loss of outer wall layer signal in T2W images is an independent effect and should be detected even if fat saturation is applied to T1W images as well.
A final limitation is the lack of ex vivo measurements and histology for those LE-PVBGs imaged in vivo. While the segment imaged in one patient enrolled in this study exhibited significant intimal hyperplasia, it was not excised at revision, a common surgical practice. This limitation is exacerbated in the comparison of in vivo observations based on the ex vivo measurements. One of the two specimens was excised at 247 days postimplantation, 58 days longer than the mean graft maturation time for those patients imaged in vivo. However, the fact that measured T2 rates were statistically similar between specimens for both layers (Table 2) supports this analysis.
Further enhancements in resolution will enhance our ability to differentiate the LE-PVBG wall layers, given the small thickness attributed to the adventitia. The method can be readily applied at 3 T to increase resolution. Additionally, the ETL can be extended to increase resolution while avoiding signal-to-noise reduction or scan time increase. However, in our experience, a longer echo train introduces significant blurring at the resolutions achieved here. This can be avoided by the use of variable flip angle refocusing, based on the expected tissue T1 and T2 relaxation rates (36,37). At present, the influence of vein graft maturation on T2 is not known, nor is it known if differences would be significant in this context. Increased longitudinal graft coverage will also be important for future clinical studies. This can be achieved by trading the number of signal averages (NEX) used in this study for longitudinal coverage. However, we have found that the use of DIR blood-nulling limits our ability to image a segment longer than the 3.6 cm used here. Methods based on flow-induced dephasing (15) can overcome this limitation.
In conjunction with prior studies encompassing earlier time-points and follow-up imaging (6,18), the present data suggests that larger studies concentrating on different time points post-implantation using high-resolution multi-contrast 3D IV-FSE are likely to yield significant results regarding the relative remodeling of lower extremity vein graft wall layers during their adaptation to the arterial environment. Most of what is currently known about vein graft pathology is limited to specimens acquired either post-mortem (38) or in animal models (39). The ability to sequentially assess the relative remodeling of the neo-intima/media and the adventitia of the vein graft in vivo would be of great value in understanding the biology of vein graft maturation, and the relationship with disturbances in biomechanics, and state of inflammation (34).
Acknowledgments
Supported in part by NIH K23-EB00882 and NIH R01-HL075771
References
- 1.Davies MG, Hagen PO. Pathophysiology of vein graft failure: a review. Eur J Vasc Endovasc Surg. 1995;9(1):7–18. doi: 10.1016/s1078-5884(05)80218-7. [DOI] [PubMed] [Google Scholar]
- 2.Cox JL, Chiasson DA, Gotlieb AI. Stranger in a strange land: the pathogenesis of saphenous vein graft stenosis with emphasis on structural and functional differences between veins and arteries. Prog Cardiovasc Dis. 1991;34(1):45–68. doi: 10.1016/0033-0620(91)90019-i. [DOI] [PubMed] [Google Scholar]
- 3.Lau GT, Lowe HC, Kritharides L. Cardiac saphenous vein bypass graft disease. Semin Vasc Med. 2004;4(2):153–159. doi: 10.1055/s-2004-835373. [DOI] [PubMed] [Google Scholar]
- 4.Mills JL., Sr. Infrainguinal vein graft surveillance: how and when. Semin Vasc Surg. 2001;14(3):169–176. doi: 10.1053/svas.2001.25489. [DOI] [PubMed] [Google Scholar]
- 5.Owens CD, Wake N, Jacot JG, Gerhard-Herman M, Gaccione P, Belkin M, Creager MA, Conte MS. Early biomechanical changes in lower extremity vein grafts--distinct temporal phases of remodeling and wall stiffness. J Vasc Surg. 2006;44(4):740–746. doi: 10.1016/j.jvs.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 6.Mitsouras D, Mulkern RV, Owens CD, Conte MS, Ersoy H, Luu TM, Whitmore AG, Creager MA, Rybicki FJ. High resolution peripheral vein bypass graft wall studies using high sampling efficiency inner volume 3D FSE. Magn Reson Med. 2008;59(3):650–654. doi: 10.1002/mrm.21359. [DOI] [PubMed] [Google Scholar]
- 7.Toussaint JF, LaMuraglia GM, Southern JF, Fuster V, Kantor HL. Magnetic resonance images lipid, fibrous, calcified, hemorrhagic, and thrombotic components of human atherosclerosis in vivo. Circulation. 1996;94(5):932–938. doi: 10.1161/01.cir.94.5.932. [DOI] [PubMed] [Google Scholar]
- 8.Yuan C, Kerwin WS, Yarnykh VL, Cai J, Saam T, Chu B, Takaya N, Ferguson MS, Underhill H, Xu D, Liu F, Hatsukami TS. MRI of atherosclerosis in clinical trials. NMR Biomed. 2006;19(6):636–654. doi: 10.1002/nbm.1065. [DOI] [PubMed] [Google Scholar]
- 9.Clarke SE, Hammond RR, Mitchell JR, Rutt BK. Quantitative assessment of carotid plaque composition using multicontrast MRI and registered histology. Magn Reson Med. 2003;50(6):1199–1208. doi: 10.1002/mrm.10618. [DOI] [PubMed] [Google Scholar]
- 10.Varghese A, Merrifield RD, Crowe LA, Collins SA, Keenan NG, Firmin DN, Yang GZ, Pennell DJ. Evaluation of carotid artery wall volume measurement using novel semiautomated analysis software. J Magn Reson Imaging. 2006;24(6):1401–1408. doi: 10.1002/jmri.20798. [DOI] [PubMed] [Google Scholar]
- 11.Fillinger MF, Cronenwett JL, Besso S, Walsh DB, Zwolak RM. Vein adaptation to the hemodynamic environment of infrainguinal grafts. J Vasc Surg. 1994;19(6):970–978. doi: 10.1016/s0741-5214(94)70208-x. discussion 978-979. [DOI] [PubMed] [Google Scholar]
- 12.Idu MM, Buth J, Hop WC, Cuypers P, van de Pavoordt ED, Tordoir JM. Factors influencing the development of vein-graft stenosis and their significance for clinical management. Eur J Vasc Endovasc Surg. 1999;17(1):15–21. doi: 10.1053/ejvs.1998.0676. [DOI] [PubMed] [Google Scholar]
- 13.Luk-Pat GT, Gold GE, Olcott EW, Hu BS, Nishimura DG. High-resolution three-dimensional in vivo imaging of atherosclerotic plaque. Magn Reson Med. 1999;42(4):762–771. doi: 10.1002/(sici)1522-2594(199910)42:4<762::aid-mrm19>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 14.Crowe LA, Gatehouse P, Yang GZ, Mohiaddin RH, Varghese A, Charrier C, Keegan J, Firmin DN. Volume-selective 3D turbo spin echo imaging for vascular wall imaging and distensibility measurement. J Magn Reson Imaging. 2003;17(5):572–580. doi: 10.1002/jmri.10294. [DOI] [PubMed] [Google Scholar]
- 15.Koktzoglou I, Chung YC, Carroll TJ, Simonetti OP, Morasch MD, Li D. Three-dimensional black-blood MR imaging of carotid arteries with segmented steady-state free precession: initial experience. Radiology. 2007;243(1):220–228. doi: 10.1148/radiol.2431060310. [DOI] [PubMed] [Google Scholar]
- 16.Fayad ZA. The assessment of the vulnerable atherosclerotic plaque using MR imaging: a brief review. Int J Cardiovasc Imaging. 2001;17(3):165–177. doi: 10.1023/a:1010611530845. [DOI] [PubMed] [Google Scholar]
- 17.Mitsouras D, Mulkern RV, Rybicki FJ. Strategies for inner volume 3D fast spin echo magnetic resonance imaging using nonselective refocusing radio frequency pulses. Med Phys. 2006;33(1):173–186. doi: 10.1118/1.2148331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rybicki FJ, Mitsouras D, Owens CD, Whitmore AG, Ersoy H, Mulkern RV, Creager MA, Conte MS. Lower extremity peripheral vein bypass graft wall thickness changes demonstrated at 1 and 6 months after surgery with ultra-high spatial resolution black blood inner volume three-dimensional fast spin echo magnetic resonance imaging. Int J Cardiovasc Imaging. 2008;24(5):529–533. doi: 10.1007/s10554-007-9287-8. [DOI] [PubMed] [Google Scholar]
- 19.Jahnke C, Dietrich T, Paetsch I, Koehler U, Preetz K, Schnackenburg B, Fleck E, Graf K, Nagel E. Experimental evaluation of the detectability of submillimeter atherosclerotic lesions in ex vivo human iliac arteries with ultrahigh-field (7.0 T) magnetic resonance imaging. Int J Cardiovasc Imaging. 2007;23(4):519–527. doi: 10.1007/s10554-006-9185-5. [DOI] [PubMed] [Google Scholar]
- 20.Zimmermann GG, Erhart P, Schneider J, von Schulthess GK, Schmidt M, Debatin JF. Intravascular MR imaging of atherosclerotic plaque: ex vivo analysis of human femoral arteries with histologic correlation. Radiology. 1997;204(3):769–774. doi: 10.1148/radiology.204.3.9280257. [DOI] [PubMed] [Google Scholar]
- 21.Feinberg DA, Hoenninger JC, Crooks LE, Kaufman L, Watts JC, Arakawa M. Inner volume MR imaging: technical concepts and their application. Radiology. 1985;156(3):743–747. doi: 10.1148/radiology.156.3.4023236. [DOI] [PubMed] [Google Scholar]
- 22.Mugler JP, 3rd, Bao S, Mulkern RV, Guttmann CR, Robertson RL, Jolesz FA, Brookeman JR. Optimized single-slab three-dimensional spin-echo MR imaging of the brain. Radiology. 2000;216(3):891–899. doi: 10.1148/radiology.216.3.r00au46891. [DOI] [PubMed] [Google Scholar]
- 23.Edelman RR, Chien D, Kim D. Fast selective black blood MR imaging. Radiology. 1991;181(3):655–660. doi: 10.1148/radiology.181.3.1947077. [DOI] [PubMed] [Google Scholar]
- 24.Hennig J. Echoes -- how to generate, recognize, use or avoid them in MR-imaging sequences part I: fundamental and not so fundamental properties of spin echoes. Concepts in Magn Reson. 1991;3:125–143. [Google Scholar]
- 25.Poon CS, Henkelman RM. Practical T2 quantitation for clinical applications. J Magn Reson Imaging. 1992;2(5):541–553. doi: 10.1002/jmri.1880020512. [DOI] [PubMed] [Google Scholar]
- 26.Whittall KP, MacKay A. Quantitative interpretation of NMR relaxation data. J Magn Reson. 1989;84:134–152. [Google Scholar]
- 27.Clark PR, Chua-anusorn W, Pierre TG. Bi-exponential proton transverse relaxation rate (R2) image analysis using RF field intensity-weighted spin density projection: potential for R2 measurement of iron-loaded liver. Magn Reson Imaging. 2003;21(5):519–530. doi: 10.1016/s0730-725x(03)00080-8. [DOI] [PubMed] [Google Scholar]
- 28.Sharma R. MR imaging in carotid artery atherosclerosis plaque characterization. Magn Reson Med Sci. 2002;1(4):217–232. doi: 10.2463/mrms.1.217. [DOI] [PubMed] [Google Scholar]
- 29.Rogers WJ, Prichard JW, Hu YL, Olson PR, Benckart DH, Kramer CM, Vido DA, Reichek N. Characterization of signal properties in atherosclerotic plaque components by intravascular MRI. Arterioscler Thromb Vasc Biol. 2000;20(7):1824–1830. doi: 10.1161/01.atv.20.7.1824. [DOI] [PubMed] [Google Scholar]
- 30.Morrisett J, Vick W, Sharma R, Lawrie G, Reardon M, Ezell E, Schwartz J, Hunter G, Gorenstein D. Discrimination of components in atherosclerotic plaques from human carotid endarterectomy specimens by magnetic resonance imaging ex vivo. Magn Reson Imaging. 2003;21(5):465–474. doi: 10.1016/s0730-725x(02)00643-4. [DOI] [PubMed] [Google Scholar]
- 31.Pessanha BS, Potter K, Kolodgie FD, Farb A, Kutys R, Mont EK, Burke AP, O'Leary TJ, Virmani R. Characterization of intimal changes in coronary artery specimens with MR microscopy. Radiology. 2006;241(1):107–115. doi: 10.1148/radiol.2411042201. [DOI] [PubMed] [Google Scholar]
- 32.Qiao Y, Ronen I, Viereck J, Ruberg FL, Hamilton JA. Identification of atherosclerotic lipid deposits by diffusion-weighted imaging. Arterioscler Thromb Vasc Biol. 2007;27(6):1440–1446. doi: 10.1161/ATVBAHA.107.141028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dalager-Pedersen S, Falk E, Ringgaard S, Kristensen IB, Pedersen EM. Effects of temperature and histopathologic preparation on the size and morphology of atherosclerotic carotid arteries as imaged by MRI. J Magn Reson Imaging. 1999;10(5):876–885. doi: 10.1002/(sici)1522-2586(199911)10:5<876::aid-jmri37>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 34.Owens CD, Rybicki FJ, Wake N, Schanzer A, Mitsouras D, Gerhard-Herman MD, Conte MS. Early remodeling of lower extremity vein grafts: Inflammation influences biomechanical adaptation. J Vasc Surg. 2008;47(6):1235–1242. doi: 10.1016/j.jvs.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mulkern RV, Melki PS, Jakab P, Higuchi N, Jolesz FA. Phase-encode order and its effect on contrast and artifact in single-shot RARE sequences. Med Phys. 1991;18(5):1032–1037. doi: 10.1118/1.596644. [DOI] [PubMed] [Google Scholar]
- 36.Park J, Mugler JP, 3rd, Horger W, Kiefer B. Optimized T1-weighted contrast for single-slab 3D turbo spin-echo imaging with long echo trains: application to whole-brain imaging. Magn Reson Med. 2007;58(5):982–992. doi: 10.1002/mrm.21386. [DOI] [PubMed] [Google Scholar]
- 37.Gold GE, Busse RF, Beehler C, Han E, Brau AC, Beatty PJ, Beaulieu CF. Isotropic MRI of the knee with 3D fast spin-echo extended echo-train acquisition (XETA): initial experience. AJR Am J Roentgenol. 2007;188(5):1287–1293. doi: 10.2214/AJR.06.1208. [DOI] [PubMed] [Google Scholar]
- 38.Barboriak JJ, Pintar K, Van Horn DL, Batayias GE, Korns ME. Pathologic findings in the aortocoronary vein grafts. A scanning electron microscope study. Atherosclerosis. 1978;29(1):69–80. doi: 10.1016/0021-9150(78)90095-3. [DOI] [PubMed] [Google Scholar]
- 39.Dobrin PB, Littooy FN, Golan J, Blakeman B, Fareed J. Mechanical and histologic changes in canine vein grafts. J Surg Res. 1988;44(3):259–265. doi: 10.1016/0022-4804(88)90056-x. [DOI] [PubMed] [Google Scholar]