Abstract
Background and Purpose
Docosahexaenoic acid (DHA 22:6n-3), an omega-3 essential fatty acid family member, is the precursor of neuroprotectin D1, which downregulates apoptosis and, in turn, promotes cell survival. This study was conducted to assess whether DHA would show neuroprotective efficacy when systemically administered in different doses after middle cerebral artery occlusion (MCAo) in rats.
Methods
Sprague-Dawley rats were anesthetized with isoflurane and subjected to 2 h of MCAo. Animals were treated with either DHA (low doses=3.5 or 7 mg/kg; medium doses= 16 or 35 mg/kg; and high dose= 70 mg/kg) or an equivalent volume of saline, IV, at 3 h after MCAo onset. The neurological status was evaluated during occlusion (60 min), and on day 1, 2, 3 and 7 after MCAo. Seven days after MCAo, brains were perfusion-fixed, and infarct areas and volumes were determined.
Results
Only the low and medium doses of DHA significantly improved the neurological score compared to vehicle rats at 24 h, 48 h, 72 h and 7 days. DHA markedly reduced total corrected infarct volume in all treated groups compared to vehicle rats (3.5 mg/kg: 26±9, 7 mg/kg: 46±12, 16 mg/kg: 37±5, and 35 mg/kg: 34±15 vs. vehicle: 94±12 mm3). Cortical and striatal infarct volumes were also significantly reduced by treatment with DHA. No neuroprotective effects were observed with 70 mg/kg of DHA.
Conclusions
We conclude that DHA experimental therapy in low and medium doses improves neurological and histological outcome following focal cerebral ischemia and might provide benefits in patients suffering ischemic stroke.
Keywords: focal ischemia, lipids, neuroprotection, animal models
Introduction
Stroke is the third leading cause of death and the first cause of adult disability in the industrial countries. Despite progress made in understanding the pathophysiology of stroke, today the only efficacious treatment approved for ischemic stroke is thrombolysis. Unfortunately, only 3-5% of patients can be elected to undergo this treatment. Therefore, the need for developing an effective treatment for stroke remains vital.
Cerebral ischemia results in rapid accumulation of free fatty acids, including arachidonic and docosahexaenoic acids, which are released from membrane phospholipids. Both fatty acids are derived from dietary essential fatty acids; however, only docosahexaenoic acid (DHA, 22:6n-3), the omega-3 polyunsaturated fatty acyl chain, is concentrated in phospholipids of various cells of the central nervous system.1 After ingestion, DHA is processed in the liver and transported by the bloodstream to various tissues. The release of DHA from cell membranes occurs mainly under conditions of excessive oxidative stress.1 DHA, as an acyl chain of membrane phospholipids, is necessary for ion channels, receptors, and transporters to maintain their proper physical conformation,1 and is involved in memory formation,2 excitable membrane function,3 and neuroprotection.4,5
DHA is the precursor of neuroprotectin D1 (NPD1) that, in turn, downregulates apoptosis and promotes cell survival.6,7,8 We have recently shown that NPD1 inhibits brain ischemia-reperfusion-mediated leukocyte infiltration and proinflammatory gene expression, promotes neurogenesis both in vitro and in vivo, and has been implicated in neuroprotection.5,9,10 This study was conducted to assess whether DHA would show neuroprotective efficacy when systemically administered in different doses after middle cerebral artery occlusion (MCAo) in rats.
Materials and Methods
Animal Preparation
All studies were approved by the Institutional Animal Care and Use Committee of the Louisiana State University Health Sciences Center. Male Sprague-Dawley rats (Charles River Lab., Wilmington, MA) weighing 279-329 g were fasted overnight but allowed free access to water. Rats were anesthetized and maintained with 1-3% isoflurane in 70% N2O and 30% O2, intubated and mechanically ventilated. The right femoral artery and vein were catheterized for blood sampling for arterial gases, pH, plasma glucose, and drug infusion. Rectal (CMA/Microdialysis AB, Stockholm) and cranial (left temporalis muscle) (Omega Engineering, Stamford, CT) temperatures were maintained at 36-37°C during surgical procedure.
Middle Cerebral Artery Occlusion
The right middle cerebral artery was temporarily occluded for 2 h by an intraluminal-filament, coated with poly-L-lysine.11 A 4-cm length of 3-0 monofilament nylon suture was inserted via the proximal external carotid artery into the internal carotid artery and middle cerebral artery, a distance of 20-22 mm from the common carotid artery bifurcation. Animals were allowed to awaken from anesthesia and, at 60 min of MCAo, were tested on a standardized neurobehavioral battery to confirm the presence of a high-grade neurological deficit. After 2 h of MCAo, rats were reanesthetized with the same anesthetic combination and intraluminal sutures were removed.
Behavioral Tests
Behavioral tests were performed by an observer blinded to the treatment groups at 60 min (during MCAo) and then on days 1, 2, 3 and 7 after MCAo. The battery consisted of two tests that have been used previously to evaluate various aspects of neurologic function: (1) the postural reflex test, to examine upper body posture while the animal is suspended by the tail, and (2) the forelimb placing test, to examine sensorimotor integration in forelimb placing responses to visual, tactile and proprioceptive stimuli. Neurological function was graded on a scale of 0-12 (normal score = 0, maximal score = 12), as previously described.11
Treatment and Experimental Groups
At 3 h after onset of stroke, the agent (DHA; 3.5, 7, 14, 35, 70 mg/kg) or vehicle (Saline, 5 ml/kg) was administered into the femoral vein over 3 min using an infusion pump. Rats were randomly assigned to the following groups: (1) Vehicle: Saline (n=14); (2) DHA, 3.5 mg/kg (n=9); (3) DHA, 7 mg/kg (n=9), (4) DHA, 14 mg/kg (n=8), (5) DHA, 35 mg/kg (n=8) and (6) DHA, 70 mg/kg (n=5).
Histopathology
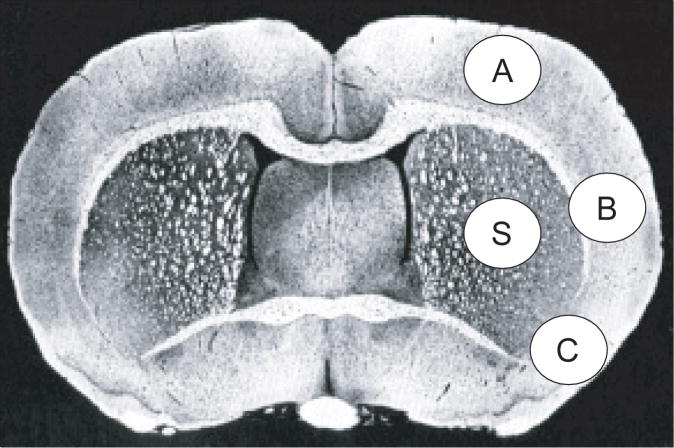
Animals were allowed to survive for 7 days, reanesthetized with the same anesthetic combination and transcardially perfused with saline followed by 4% paraformaldehyde. Brains were removed and embedded in paraffin. Histological sections were cut, and stained with Thionine (Nissl) and digitized at 9 standardized coronal levels. The area of infarction was measured with an MCID image-analysis system (MCID™ Core imaging software, InterFocus Imaging Ltd, Linton, Cambridge).11 An investigator blinded to the experimental groups then outlined the zones of infarction as well as the outlines of the left and right hemisphere on each section. Infarct volume was calculated as the integrated product of the cross-sectional area and the intersection distance and corrected for brain swelling. Adjacent sections in saline and DHA (14 mg/kg) groups were set aside for immunohistochemistry with anti-glial fibrillary acidic protein (GFAP), as previously described.12 GFAP and Nissl positive cell counts were conducted in the cortex (A, B and C) and striatum (S) at the level of the central lesion (bregma level -0.3 mm; Figure 1). Data were expressed as numbers of GFAP and Nissl positive cells per high-power microscopic field (magnification × 40).
Figure 1.
Schematic brain diagram showing locations of regions for GFAP and Nissl positive cell counts in the cortex (A, B and C) and striatum (S).
Statistical Analysis
Data are presented as mean values ± SEM. Repeated measures analysis of variance (ANOVA), followed by Bonferroni procedures to correct for multiple comparisons, was used for intergroup comparisons of neurobehavioral scores over time and infarct areas across coronal levels. Two-tailed Student's t tests were used for two-group comparisons. Differences at P<0.05 were considered statistically significant.
Results
Physiological Variables
Rectal and cranial (temporalis muscle) temperatures, plasma glucose and blood gases showed no significant differences among groups (Table 1).
Table 1. Physiological variables.
| Saline group | DHA groups | |||||
|---|---|---|---|---|---|---|
| (3.5mg/kg) | (7 mg/kg) | (14 mg/kg) | (35 mg/kg) | (70 mg/kg) | ||
| Before MCAo (15 min) | ||||||
| Rectal temperature (°C) | 36.7 ± 0.1 | 36.5 ± 0.1 | 36.7 ± 0.2 | 36.9 ± 0.1 | 36.6 ± 0.1 | 36.6 ± 0.2 |
| Cranial temperature (°C) | 36.7±0.2 | 36.9 ± 0.1 | 37.0 ± 0.1 | 36.8 ± 0.2 | 37.0 ± 0.1 | 37.0 ± 0.2 |
| pH | 7.4 ± 0.0 | 7.5 ± 0.0 | 7.4 ± 0.0 | 7.4 ± 0.0 | 7.5 ± 0.0 | 7.5 ± 0.1 |
| pO2, mm Hg | 112 ± 6 | 118 ± 5 | 125 ± 7 | 109 ± 6 | 127 ± 7 | 135 ± 8 |
| pCO2, mm Hg | 38 ± 1 | 38 ± 1 | 41 ± 1 | 39 ± 1 | 39 ± 1 | 35 ± 0 |
| Plasma glucose, mg/dL | 159 ± 8 | 152 ± 3 | 163 ± 10 | 132 ± 10 | 146 ± 9 | 148 ± 15 |
| Body weight (g) | 329 ± 8 | 294 ± 12 | 301 ± 12 | 302 ± 4 | 298 ± 9 | 279 ± 4 |
| During MCAo (15 min) | ||||||
| Rectal temperature (°C) | 37.0 ± 0.1 | 36.6 ± 0.1 | 36.9 ± 0.1 | 36.9 ± 0.1 | 36.7 ± 0.2 | 37.1 ± 0.1 |
| Cranial temperature (°C) | 37.2 ± 0.2 | 36.8 ± 0.1 | 37.1 ± 0.1 | 37. ± 0.0 | 37.1 ± 0.1 | 37.5 ± 0.3 |
| pH | 7.4 ± 0.0 | 7.4 ± 0.0 | 7.4 ± 0.0 | 7.4 ± 0.0 | 7.4 ± 0.0 | 7.4 ± 0.1 |
| pO2, mm Hg | 99 ± 3 | 108 ± 5 | 104 ± 6 | 98 ± 4 | 97 ± 3 | 106 ± 7 |
| pCO2, mm Hg | 42 ± 1 | 40 ± 1 | 41 ± 1 | 39 ± 1 | 41 ± 1 | 38 ± 1 |
| Plasma glucose, mg/dL | 144 ± 7 | 146 ± 8 | 153 ± 8 | 148 ± 15 | 132 ± 6 | 144 ± 11 |
| After MCAo (15 min) | ||||||
| Rectal temperature (°C) | 37.3 ± 0.1 | 37.0 ± 0.2 | 37.3 ± 0.2 | 38.0 ± 0.2 | 37.0 ± 0.2 | 36.9 ± 0.4 |
| Cranial temperature (°C) | 36.7 ± 0.2 | 36.5 ± 0.3 | 36.3 ± 0.2 | 36.6 ± 0.2 | 36.5 ± 0.3 | 36.0 ± 0.1 |
| After treatment (24 h) | ||||||
| Rectal temperature (°C) | 38.0 ± 0.2 | 37.6 ± 0.2 | 37.7 ± 0.1 | 38.0 ± 0.2 | 37.4 ± 0.3 | 38.0 ± 0.3 |
| Body weight (g) | 293 ± 7 | 281 ± 13 | 277 ± 11 | 274 ± 7 | 283 ± 10 | 252 ± 7 |
| After treatment (48 h) | ||||||
| Rectal temperature (°C) | 37.7 ± 0.1 | 37.2 ± 0.4 | 37.5 ± 0.2 | 37.5 ± 0.2 | 37.5 ± 0.2 | 38.3 |
| Body weight (g) | 282 ± 11 | 291 ± 15 | 274 ± 15 | 266 ± 10 | 284 ± 16 | 250 ± 12 |
| After treatment (72 h) | ||||||
| Rectal temperature (°C) | 37.5 ± 0.2 | 37.9 ± 0.1 | 37.3 ± 0.2 | 37.1 ± 0.2 | 37.8 ± 0.2 | 37.4 ± 0.2 |
| Body weight (g) | 283 ± 11 | 291 ± 11 | 276 ± 13 | 261 ± 13 | 292± 11 | 254 ± 15 |
| After treatment (1 week) | ||||||
| Rectal temperature (°C) | 37.2 ± 0.5 | 37.7 ± 0.1 | 37.3 ± 0.2 | 37.5 ± 0.2 | 37.5 ± 0.2 | 37.6 ± 0.2 |
| Body weight (g) | 314 ± 14 | 330 ± 11 | 313 ± 19 | 297 ± 16 | 321 ± 13 | 300 ± 11 |
Values are mean ± SEM
MCAo, middle cerebral artery occlusion
Neurobehavioral Assessment
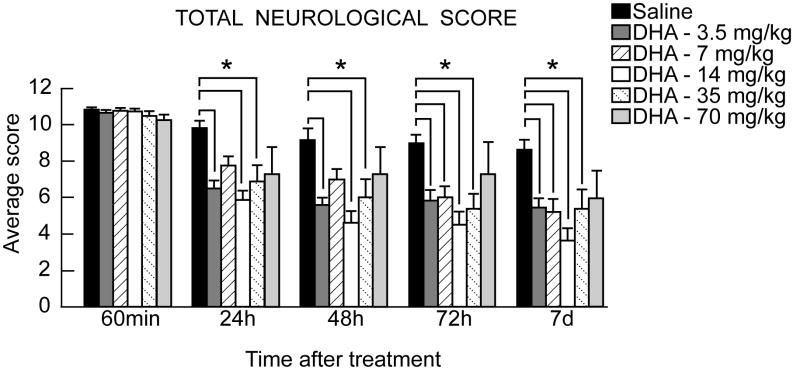
Before MCAo, neurological score was normal (score=0) in all animals. High-grade behavioral deficits (score=10 to 11) were present in all animals when tested at 60 min of MCAo (Figure 2). Saline-treated animals continued to exhibit severe behavioral impairments throughout the 7-day survival period. Only the low and medium doses of DHA (3.5, 7, 14 and 35mg/kg) significantly improved the neurological score compared to vehicle rats at 24 h, 48 h, 72 h and 7 days (Figure 2). Rats treated with DHA (70 mg/kg) tended to have a slightly improved neurological score, but this did not reach statistical significance (Figure 2).
Figure 2.
Total neurological score (normal score=0, maximal score=12) during MCAo and at various times after treatment. DHA or vehicle treatment was administered at 3 h after onset of ischemia. Values shown are means ± SEM. *, significantly different from corresponding vehicle group (p<0.05; repeated-measures ANOVA followed by Bonferroni tests).
Histopathology
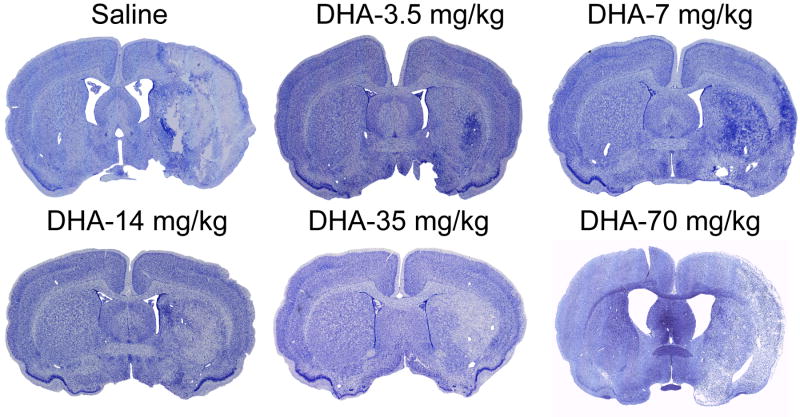
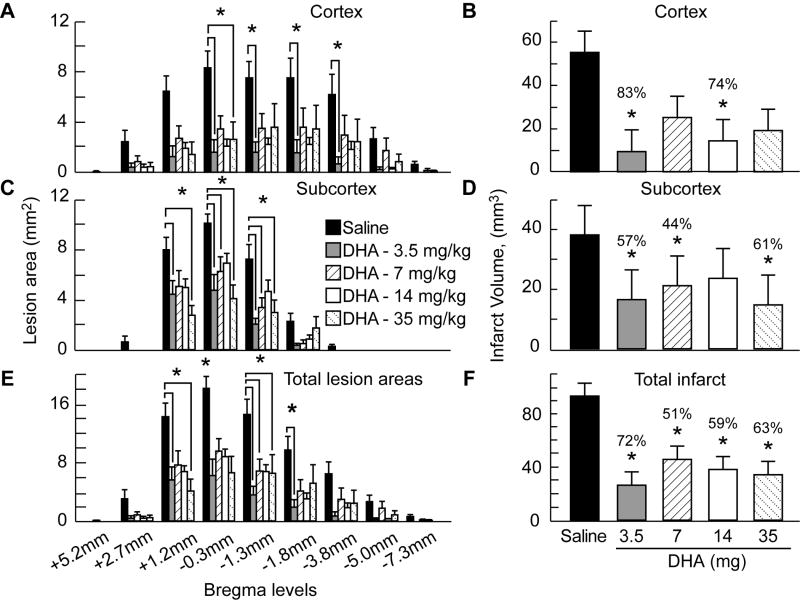
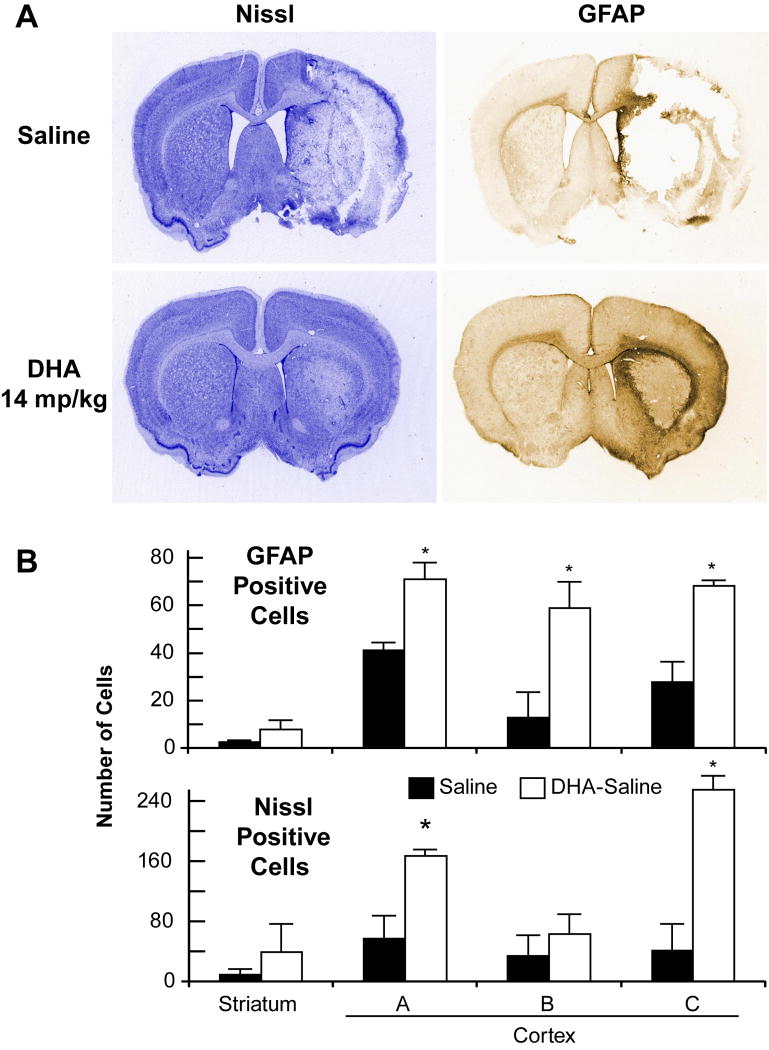
The brains of saline-treated animals with MCAo exhibited a consistent pannecrotic lesion involving both cortical and subcortical regions of the right hemisphere, characterized microscopically by destruction of neuronal, glial, and vascular elements. By contrast, infarct size was dramatically reduced by DHA therapy in the low and medium doses treatment groups, but not in the high dose group (Figure 3). The dose-response study revealed that the extent of neuroprotection was profound in the neocortex (mean tissue salvage, 83% and 74%, respectively, in the 3.5 and 35 mg/kg DHA treated groups) and extended across multiple coronal levels (Figure 4A-B). The subcortex was also significantly protected by DHA in 3.5, 7 and 35 mg/kg treated groups (by 57%, 44% and 61%, respectively; Figure 4C-D). Total infarct volume corrected for brain swelling was reduced by a mean of 72%, 51%, 59% and 63%, respectively, by treatment with 3.5, 7, 14 and 35 mg/kg DHA (Figure 4E-F). No protection was observed with 70 mg/kg of DHA compared to saline treated rats (cortex: 82±40 vs. 55±11 mm3; subcortex: 37±11 vs. 38±19 mm3; total infarct volume: 119±52 vs. 93±12 mm3, respectively; repeated-measures ANOVA followed by Bonferroni tests). Figure 5A presents Nissl and GFAP stained brain sections from saline and DHA (14 mg/kg) treated rats. Saline-treated rats showed large cortical and subcortical infarction. In contrast, rats treated with DHA showed less extensive damage, mostly in the subcortical area. GFAP and Nissl positive cell counts are shown in Figure 5B. Treatment with DHA (14 mg/kg) significantly increased GFAP and Nissl positive cells compared to the saline-treated rats.
Figure 3.
Computer-generated MosaiX processed images (Zeiss Axio Imager.M1; AxioVision Release 4.6.3) of Nissl stained paraffin-embedded brain sections from rats treated with saline, DHA-3.5 mg/kg, DHA-7 mg/kg, DHA-14 mg/kg, DHA-35 mg/kg and DHA-70 mg/kg. Saline-treated rat shows large cortical and subcortical infarction. In contrast, rats treated with low and medium doses of DHA show less extensive damage, mostly in the subcortical area. Rat treated with high dose of DHA (70 mg/kg) shows a large infarct involving cortical and subcortical regions.
Figure 4.
Cortical, subcortical and total infarct areas measured at 9 coronal levels and integrated infarct volumes in rats with 2-h MCAo and on day 7 of survival. Rostrocaudal distribution of cortical infarct area and cortical infarct volume (Panels A and B), subcortical infarct area and subcortical infarct volume (Panels C and D) and total infarct area and total corrected infarct volume (Panels E and F) in all groups. Data are presented as mean ± SEM. *, significantly different from corresponding vehicle group (p <0.05; repeated-measures ANOVA followed by Bonferroni tests).
Figure 5.
Panel A: Representative Nissl and GFAP stained brain sections of rats treated with vehicle and DHA (14 mg/kg). Saline-treated rats show large cortical and subcortical infarction. In contrast, rats treated with DHA show less extensive damage and increased GFAP staining. Panel B: GFAP and Nissl positive cell counts in cortex (A, B and C) and striatum (S) (see Figure 1 for details). Treatment with DHA (14 mg/kg) significantly increased GFAP and Nissl positive cells compared to the saline-treated rats. Values shown are means ± SEM. *, significantly different from corresponding vehicle group (p<0.05; repeated-measures ANOVA followed by Bonferroni tests).
Twelve animals died during the experiment: five rats in the saline group (died on days 2, 3, 4, 6 and 7), one rat in DHA – 3.5 mg group (died on day 7), three rats in DHA – 7 mg group (died on days 2, 5 and 7), one rat in DHA – 35 mg group (died on day 7), and two rats in DHA – 70 mg group (both died during drug administration). Three animals in the DHA - 70 mg group developed transient hematuria, which disappeared after 24 h.
Discussion
The goal of our study was to determine whether DHA was efficacious in protecting the brain when systemically administered after transient focal cerebral ischemia. Our results clearly demonstrate that this treatment, when applied at 3 h after MCAo onset, improves the outcome as measured by neurological score and by pathological estimation of the size of infarction.
Docosahexaenoic acid (DHA, 22:6, n-3), an omega-3 fatty acid and the main polyunsaturated fatty acid in the mammalian brain, plays a crucial role in the development and function of brain neurons.13 Although every cell contains DHA, its highest concentrations are found in the brain and retina. Once in the nervous system, it is incorporated into phospholipids in neuronal and photoreceptor membranes, where it supports neural activity and vision.14 Synaptic membranes and photoreceptors share the highest content of DHA of all cell membranes.15 In vivo, the active DHA supply to the brain is necessary for normal brain function. It also may play a critical role in conditions where, due to enhanced oxidative stress, the polyunsaturated fatty acyl chains of membrane phospholipids are decreased as a consequence of lipid peroxidation, as occurs in aging, retinal degenerations, and neurodegenerations such as Alzheimer's disease.14
The neuroprotective effect of DHA was also evaluated in animal models of cerebral ischemia. Long-term fish oil treatment was able to improve learning/memory function in rats subjected to global cerebral ischemia.16 Pre-treatment with DHA (100 and 500 nmol/kg at 1h and 3 days or daily for 6 weeks before ischemia, ip) was accompanied by decreases in blood-brain barrier disruption, brain edema, malondialdehyde production, inflammatory cell infiltration, interleukin-6 expression and caspase-3 activity in rats, subjected to 90 min of MCAo.17 However, acute post treatment with DHA [500 nmoles per rat (0.16 mg), at 1h after reperfusion, ip] after 90 min of MCAo remarkably exacerbated cerebral ischemia-reperfusion injury.18 Recently we have demonstrated that administration of DHA complexed to human serum albumin (DHA-Alb; 2 mmol DHA per ml of albumin) provides improvement of behavioral function and reduction of brain infarction and edema (1.25 or 0.63 g/kg, at 2h after onset ischemia, iv) after 120 min of MCAo.5 Lipidomic analysis of DHA-Alb treated brains revealed formation of the neuroprotective DHA-derived mediator, NPD1, in the ipsilateral hemisphere.5
In the present study, we have found that much lower concentrations of DHA were required to elicit protection (3.5 mg/kg to 35 mg/kg). These relatively lower DHA concentrations may be carried by endogenous proteins (e.g., albumin, lipoproteins). The reason higher concentrations of DHA were used in the albumin/DHA studies may be due to the fact that DHA may be tightly retained by added human serum albumin, and only a fraction of DHA may be delivered to the brain.
Recently we discovered that brain ischemia-reperfusion generates stereospecific DHA pathways that lead to the formation of novel messengers. One of them is NPD1, a stereospecific derivative of DHA formed through a lipoxygenase enzyme that acts on free DHA.10 The synthesis of NPD1 after 1 h of MCAo in mouse coincides with free DHA availability that results from phospholipase A2 activation. NPD1 was formed in the ipsilateral hippocampus and peaks at 8 h of reperfusion; and it was still elevated 25 h later.9 In that study, NPD1 was formed from endogenous DHA. NPD1 was found to serve an endogenous neuroprotective role by inhibiting apoptotic DNA damage, upregulating antiapoptotic and downregulating proapoptotic proteins, and also binding toxic peroxides, particularly in hemorrhagic stroke.14,15 The relatively large ischemic insult may overcome the ability of endogenously generated docosanoids to elicit neuroprotection. Thus, when NPD1 (400 ng) was infused into the third ventricle during 48 h after 60 min of MCAo, a reduced infarct size (by 50%) and 80% inhibition of leukocyte infiltration in mouse hippocampus and neocortex was detected.9 It is of interest that ischemia-reperfusion-induced NFκB activation and pro-inflammatory COX-2 expression, but not COX-1, were downregulated by this docosanoid.9
The results of this study established efficacy and dose-response relationship for neuroprotective effects of DHA in a rat model of focal cerebral ischemia. The most remarkable effect was found in the lowest (3.5 and 7 mg/kg) and medium (14 and 35 mg/kg) doses of DHA-treated groups. Vehicle-treated rats showed a large cortical and subcortical infarct at multiple coronal levels. By contrast, DHA-treated animals significantly reduced cortical (by 74-83%), subcortical (by 38-61%) and total (by 51-72%) infarct volumes compared to the vehicle-treated group. No significant neuroprotective effects were observed at the highest dose (70 mg/kg) of DHA. A previous study had shown that high doses of DHA induced brain edema in slices of rat brain cortex.19 Our study shows that 70 mg/kg of DHA caused toxic effect as well. All animals developed hematuria and 40% of the animals died during drug injection. Three animals survived but had very extensive damage, mostly in the cortical area. Thus, we would like to emphasize that the low-to-medium doses of DHA will be considered in the future as therapeutic doses for treatment of ischemic stroke.
Our data show that after 7 days of MCAo, GFAP expression was found in the boundary zone to the infarct or in the areas of selective incomplete ischemic necrosis. The expression was localized to the same areas where neurons are destined to survive the ischemic insult (detected by Nissl positive cells count). Glial cells are activated after brain ischemia. It has been demonstrated that microglia secretes neurotoxic agents and astrocytes that produce neurotrophic factors.20 Astrocytes can release a number of growth factors for neurons.21 Some of these, such as nerve growth factor, may stimulate the neuron as a whole as well as promote neurite growth. Thus, GFAP expression may be related to a possible “protective” effect on the adjacent neurons within regions of the brain that remain viable following focal cerebral ischemia.
Neurological deficits are an important also indicator of stroke development in the clinic as well as in experimental cerebral ischemia models. Stroke in humans is commonly associated with impaired sensorimotor and cognitive function, and approximately 80% of all patients experience hemiparesis after the insult. Rodents that experience focal ischemia also exhibit a neurological deficit characterized by sensorimotor dysfunction. In the present study, animals treated with low and medium doses of DHA showed neurological improvements within the first day after insult that continued for 7 days. Rats treated with higher doses of DHA tended to have a slightly improved neurological score, but this did not reach statistical significance.
The timing of DHA administration is another important factor. In several previous studies, the DHA is given before or shortly after experimental cerebral ischemia. During this time, it is logistically difficult to institute therapy in most patients with acute stroke. Other focal ischemia models have shown post-treatment successes even when treatment is delayed longer than 1 h after onset of ischemia. However, in most models, benefit is lost if treatment is delayed more than 2 or 3 h. It is therefore important that a significant neuroprotective effect of DHA in our study was achieved when therapy was initiated 3 h after the onset of temporary MCAo. We demonstrated that DHA did not have direct effects on brain temperature or arterial blood gases because these variables were carefully controlled and did not differ among groups.
The beneficial effect of DHA has been shown in a well-controlled animal model of MCAo. In the present study and in recently published observations5, 11 we employed a poly-L-lysine-coated suture and have found that this technique produces substantial, consistent cortical-plus-subcortical infarction. This infarction closely mimics, in extent and severity, the large hemispheric infarcts resulting from proximal MCA and internal carotid artery occlusion in patients.
Summary
Our results show that DHA is markedly neuroprotective in acute focal cerebral ischemia in rats, improving neurological function and reducing the extent of histological damage with a therapeutic window of at least 3 h after MCAo onset. We therefore suggest that this agent offers great promise in developing therapies for cerebral ischemia in patients with acute ischemic stroke.
Acknowledgments
The authors thank Hilary W. Thompson, Ph.D. for statistical analysis and Neuroscience Associates, Inc., for histology service.
Source of Funding: This investigation was supported by NIH Grant R01 NS046741 (NGB).
Footnotes
Disclosures: NGB is a consultant for Resolvyx Pharmaceuticals, Inc., Bedford, MA.
References
- 1.Bazan NG. Synaptic lipid signaling: significance of polyunsaturated fatty acids and platelet-activating factor. J Lipid Res. 2003;44:2221–2233. doi: 10.1194/jlr.R300013-JLR200. [DOI] [PubMed] [Google Scholar]
- 2.Gamoh S, Hashimoto M, Sugioka K, Shahdat Hossain M, Hata N, Misawa Y, Masumura S. Chronic administration of docosahexaenoic acid improves reference memory-related learning ability in young rats. Neuroscience. 1999;93:237–241. doi: 10.1016/s0306-4522(99)00107-4. [DOI] [PubMed] [Google Scholar]
- 3.McGahon BM, Martin DS, Horrobin DF, Lynch MA. Age-related changes in synaptic function: analysis of the effect of dietary supplementation with omega-3 fatty acids. Neuroscience. 1999;94:305–314. doi: 10.1016/s0306-4522(99)00219-5. [DOI] [PubMed] [Google Scholar]
- 4.Rodriguez de Turco EB, Belayev L, Liu Y, Busto R, Parkins N, Bazan NG, Ginsberg MD. Systemic fatty acid responses to transient focal cerebral ischemia: influence of neuroprotectant therapy with human albumin. J Neurochem. 2002;83:515–524. doi: 10.1046/j.1471-4159.2002.01121.x. [DOI] [PubMed] [Google Scholar]
- 5.Belayev L, Marcheselli VL, Khoutorova L, Rodriguez de Turco EB, Busto R, Ginsberg MD, Bazan NG. Docosahexaenoic acid complexed to albumin elicits high-grade ischemic neuroprotection. Stroke. 2005;36:118–123. doi: 10.1161/01.STR.0000149620.74770.2e. [DOI] [PubMed] [Google Scholar]
- 6.Serhan CN, Gotlinger K, Hong S, Lu Y, Siegelman J, Baer T, Yang R, Colgan SP, Petasis NA. Anti-inflammatory actions of neuroprotectin D1/protectin D1 and its natural stereoisomers: assignments of dihydroxy-containing docosatrienes. J Immunol. 2006;176:1848–1859. doi: 10.4049/jimmunol.176.3.1848. [DOI] [PubMed] [Google Scholar]
- 7.Mukherjee PK, Marcheselli VL, Serhan CN, Bazan NG. Neuroprotectin D1: a docosahexaenoic acid-derived docosatriene protects human retinal pigment epithelial cells from oxidative stress. Proc Natl Acad Sci U S A. 2004;101:8491–8496. doi: 10.1073/pnas.0402531101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mukherjee PK, Chawla A, Loayza MS, Bazan NG. Docosanoids are multifunctional regulators of neural cell integrity and fate: significance in aging and disease. Prostaglandins Leukot Essent Fatty Acids. 2007;77:233–238. doi: 10.1016/j.plefa.2007.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marcheselli VL, Hong S, Lukiw WJ, Tian XH, Gronert K, Musto A, Hardy M, Gimenez JM, Chiang N, Serhan CN, Bazan NG. Novel docosanoids inhibit brain ischemia-reperfusion-mediated leukocyte infiltration and pro-inflammatory gene expression. J Biol Chem. 2003;278:43807–43817. doi: 10.1074/jbc.M305841200. [DOI] [PubMed] [Google Scholar]
- 10.Niemoller TD, Stark DT, Bazan NG. Omega-3 fatty acid docosahexaenoic acid is the precursor of neuroprotectin D1 in the nervous system. In: Simopoulos AP, Bazan NG, editors. Omega-3 fatty acids, the brain and retina. Vol. 99. 2009. pp. 46–54. [DOI] [PubMed] [Google Scholar]
- 11.Belayev L, Alonso OF, Busto R, Zhao W, Ginsberg MD. Middle cerebral artery occlusion in the rat by intraluminal suture. Neurological and pathological evaluation of an improved model. Stroke. 1996;27:1616–1622. doi: 10.1161/01.str.27.9.1616. [DOI] [PubMed] [Google Scholar]
- 12.Lin B, Ginsberg MD, Busto R, Dietrich WD. Sequential analysis of subacute and chronic neuronal, astrocytic and microglial alterations after transient global ischemia in rats. Acta Neuropathol. 1998;95:511–523. doi: 10.1007/s004010050832. [DOI] [PubMed] [Google Scholar]
- 13.Scott BL, Bazan NG. Membrane docosahexaenoate is supplied to the developing brain and retina by the liver. Proc Natl Acad Sci U S A. 1989;86:2903–2907. doi: 10.1073/pnas.86.8.2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bazan NG. Omega-3 fatty acids, pro-inflammatory signaling and neuroprotection. Curr Opin Clin Nutr Metab Care. 2007;10:136–141. doi: 10.1097/MCO.0b013e32802b7030. [DOI] [PubMed] [Google Scholar]
- 15.Bazan NG. Cell survival matters: docosahexaenoic acid signaling, neuroprotection and photoreceptors. Trends Neurosci. 2006;29:263–271. doi: 10.1016/j.tins.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 16.Fernandes JS, Mori MA, Ekuni R, Oliveira RM, Milani H. Long-term treatment with fish oil prevents memory impairments but not hippocampal damage in rats subjected to transient, global cerebral ischemia. Nutr Res. 2008;28:798–808. doi: 10.1016/j.nutres.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 17.Pan HC, Kao TK, Ou YC, Yang DY, Yen YJ, Wang CC, Chuang YH, Liao SL, Raung SL, Wu CW, Chiang AN, Chen CJ. Protective effect of docosahexaenoic acid against brain injury in ischemic rats. J Nutr Biochem. 2008 doi: 10.1016/j.jnutbio.2008.06.014. in press. [DOI] [PubMed] [Google Scholar]
- 18.Yang DY, Pan HC, Yen YJ, Wang CC, Chuang YH, Chen SY, Lin SY, Liao SL, Raung SL, Wu CW, Chou MC, Chiang AN, Chen CJ. Detrimental effects of post-treatment with fatty acids on brain injury in ischemic rats. Neurotoxicology. 2007;28:1220–1229. doi: 10.1016/j.neuro.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 19.Chan PH, Fishman RA. Brain edema: induction in cortical slices by polyunsaturated fatty acids. Science. 1978;201:358–360. doi: 10.1126/science.663662. [DOI] [PubMed] [Google Scholar]
- 20.Eddleston M, Mucke L. Molecular profile of reactive astrocytes--implications for their role in neurologic disease. Neuroscience. 1993;54:15–36. doi: 10.1016/0306-4522(93)90380-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kimelberg HK, Norenberg MD. Astrocytes. Sci Am. 1989;260:66–72. doi: 10.1038/scientificamerican0489-66. [DOI] [PubMed] [Google Scholar]