Abstract
Background
The implementation of disease-specific research or service programs may have an ancillary beneficial or harmful impact on routine clinical services.
Methods
We reviewed the records of 5801 first visits to 22 antenatal clinics from 1997 to 2004 in Lusaka, Zambia and examined documented syphilis rapid plasma reagin (RPR) screening and syphilis treatment before and after implementation of research and/or service programs in prevention of mother-to-child (PMTCT) HIV transmission.
Findings
Compared with before PMTCT program implementation, the prevalence odds ratios (PORs) and 95% confidence intervals (CIs) for documented RPR screening were 0.9 (0.7 to 1.1) after implementation of research, 0.7 (0.6 to 0.8) after service, and 2.5 (2.1 to 3.0) after research and service programs.
Conclusions
Documented RPR screening was improved after implementation of PMTCT research and service were operating simultaneously and not with research or service alone. Health policy makers and researchers should plan explicitly for how the targeted HIV programs, service, and/or research can have a broader primary care impact.
Keywords: antenatal syphilis, health care system, PMTCT, HIV, program evaluation, Zambia
Externally funded research and service delivery programs are often implemented for the purpose of reducing the impact of a specific disease or outcome. Monitoring and evaluation of such programs are of interest to developing country policy makers, public health leaders, and international donors.1 Such data are often provided without contextual data on the potential impact of programs on ancillary services and conditions that may or may not be affected by the disease-specific programs.
Single-focus (vertical) service programs may be assumed to be beneficial to a severely underresourced health system, whereas research programs may be perceived as less relevant to immediate health care improvements per se; evidence for either view is scarce. Because research in developing countries is often externally initiated, funded, and/or managed,2–4 local officials and advocates may express concerns that donor country researchers may act as “research imperialists,” attending to narrow research needs and neglecting infrastructure development or training of national expertise.2,5–8 In contrast to this view, a study of the effect of clinical trials on public health and the general health of the population reported that apparent health gains were frequently seen as beyond the scope of each individual trial.9 If such gains occurred in resource-limited settings through vertical service programs or topic-specific research activities that catalyze the scaling up of health systems and filling of resource gaps, millennium development goals (MDGs) could be better achieved.10,11 It is especially important now to study the extent to which research or service delivery in one arena might bring ancillary measurable benefits (presumably attributable to enhancements in training and resources) or harms (presumably attributable to resource reallocation toward the research or the single targeted condition) in the context of huge new HIV-related investments in sub-Saharan Africa.12 We assessed whether 7 years of staged implementation of prevention of mother-to-child transmission (PMTCT) of HIV research and/or service programs in Lusaka, Zambia improved antenatal care in clinics in which they were implemented, as defined by a marker of good antenatal care, namely, documented rapid plasma reagin (RPR) screening, and, among those with a positive RPR result, documented syphilis treatment.
Methods
Study Population
Our study was conducted in Zambia's capital city, Lusaka, whose population is estimated to be 1.5 to 2.0 million persons. In Lusaka, >90% of available allopathic health care is provided by a network of satellite public clinics and a referral teaching hospital (MS, estimated from unpublished data). Twenty-six Lusaka District clinics provide antenatal care, 8 have small inpatient units, and 12 have labor wards. Twenty-two of the 26 clinics were under the management of the Lusaka District during the study period and are included in this study, 9 of which had labor wards. Two of the 4 clinics excluded from analysis were not under the administration of the Lusaka District during the study period, a third did not provide antenatal care services, and the fourth served a highly transient female prisoner population.
Routine Antenatal Clinic and RPR Screening Procedures in Lusaka District Clinics
The general antenatal clinic (ANC) routine is for nurse midwives to interview pregnant women at their first clinic “booking” visits to obtain demographic information and obstetric histories, perform physical examinations, and collect blood for routine RPR screening for same-day results and treatment for RPR-seropositive cases (without confirmatory syphilis testing). The midwife records the assessments and test results in the antenatal record book given to each woman on booking and documentation of treatment with penicillin. Since the 1990s, syphilis testing has been a part of the government antenatal care package in Zambia.
HIV Program Implementation
In the Lusaka District clinics, PMTCT-related research studies with varying staffing levels and funding were introduced in 9 of 22 clinics at different time points from the year 2000 until 2003. PMTCT services with antiretroviral prophylaxis were first introduced into 1 Lusaka District clinic in year 2000 with United Nations Children's Fund (UNICEF) support. Beginning in the year 2001, all clinics in the Lusaka District implemented nevirapine-based PMTCT services (ie, implementation at several clinics simultaneously from 2001 until all were covered by the year 2004), with financial support provided by the Elizabeth Glaser Pediatric AIDS Foundation.13,14 In this study, 3 interventions are studied: implementation of PMTCT-related research, service, or both simultaneously into an ANC. There were no research or service programs unrelated to PMTCT introduced into antenatal care during the time of this study, from July 1997 through July 2004.
Research Programs
Clinics were often selected for research because of logistic considerations such as the number of patients available for recruitment or geographic location to minimize the possibility of nonindependence if clinics were the unit of randomization. PMTCT research supported study staff training in the protocol, clinical care related to study outcomes, HIV counseling, and data collection/record keeping; supply procurement systems for study supplies and RPR testing and treatment; and community sensitization regarding research, blood draws, and/or HIV testing. Some research studies supported RPR test kits and/or benzathine penicillin.
Service Programs
PMTCT services for HIV were introduced first into clinics with labor wards and then into other clinics, without geographic preference. The PMTCT service program consisted of universal counseling and voluntary HIV testing with same-day test results and single-dose nevirapine (NVP) offered to HIV-infected pregnant women and their infants.13 At program introduction, the antenatal nurse-midwives received HIV counseling and testing training and record keeping training. For pregnant women who agreed to HIV testing, the nurse-midwife used a clinic-based laboratory slip to request an HIV test using the same blood specimen as for RPR testing. Thus, once introduced as a program, PMTCT service operations were incorporated as a new standard into the already-existing package of antenatal care that included RPR testing. PMTCT funds were sometimes used to purchase supplies of RPR test kits or penicillin if government supplies of these items were unavailable (MS, personal observation). The PMTCT service program established procurement systems for HIV testing supplies.
Study Design and Data Collection
We used a quasiexperimental research design to take advantage of the introduction of PMTCT research and/or service programs into various clinics at different times. The study outcomes included documented RPR testing for syphilis and, in RPR-positive women, penicillin treatment for syphilis. As is typical in resource-limited settings of sub-Saharan Africa, women with positive RPR screening results do not routinely receive a confirmatory treponeme-specific test before treatment.15
Systematic chart sampling and review of documented antenatal syphilis screening and/or treatment at booking in the 22 district clinics were conducted for time points before and after initiation of PMTCT research or service. In each clinic, 50 records from antenatal booking months of January and June were sampled “before” and “after” research and/or service implementation for each clinic. In cases in which the number of records was <50 for the targeted months, as was occasionally the case with the smaller facilities, records were sampled first from months before the targeted month (eg, December or May for January or June samples, respectively) and, if unavailable, after the targeted month (eg, February or July). For clinics where more than 1 implementation had occurred in a short time frame such that only 1 of our targeted months occurred between program implementation, records were sampled for whichever targeted month fell between the programs implemented (in other words, January or June but not both). Records were sampled from 3 January and June time points in the clinics with both program implementations, with a postimplementation assessment for research or service serving as a preimplementation assessment for the other program (Fig. 1). Based on this inclusion algorithm, a total of 5801 records were reviewed. All data were entered into an MS Access 2002 (Microsoft Corporation, Redmond, WA) database. This study was approved by the University of Alabama at Birmingham Institutional Review Board and the University of Zambia Research Ethics Committee.
Figure 1.
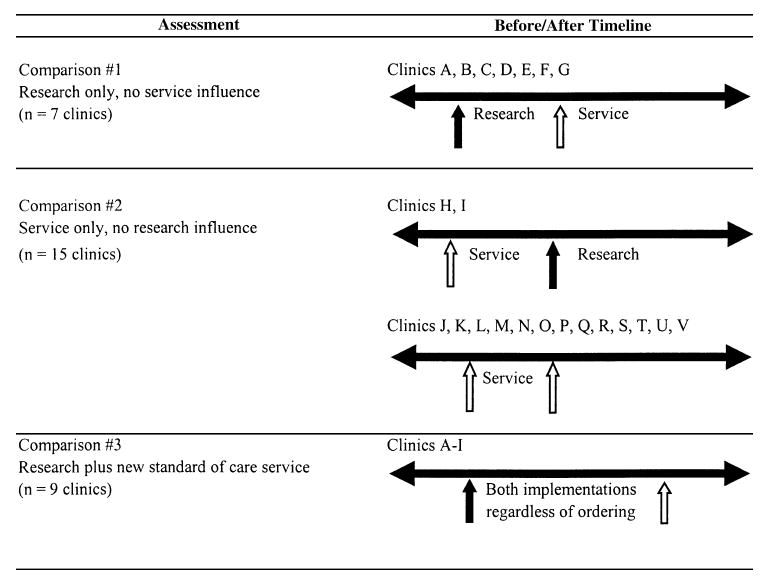
Timeline depicting before and after assessments of PMTCT research, new standard-of-care service, or both implementations in clinics in Lusaka, Zambia.
Statistical Analyses
Within-clinic comparisons were performed using logistic regression analyses to determine documented RPR and treatment coverage before and after implementation, with prevalence odds ratios (PORs) and 95% confidence intervals (CIs) as the point estimates of interest. Three comparisons were made to examine PMTCT research alone, service alone, or both in relation to subsequently documented RPR screening and treatment of seropositive cases. We first examined RPR screening and syphilis treatment after the implementation of PMTCT research alone in the 7 clinics where research was implemented before service (comparison 1; see Fig. 1). We then examined RPR screening and syphilis treatment after the implementation of PMTCT service alone in the 15 clinics where the service was implemented before or without any research (comparison 2; see Fig. 1). Finally, we assessed outcomes before and after when research and service were operating simultaneously in all 9 clinics (comparison 3). Using a 2-tailed α-value of 0.05, with 5801 records available, we had 82.5% power for comparison 1 and >99% power for comparisons 2 and 3 to detect a 20% difference in the proportion of documented RPR screening before as compared with after program implementation. Findings are presented as RPR screening and syphilis treatment frequencies, proportions, PORs, and 95% CIs. All data were analyzed in SAS version 9.1 (SAS Institute, Cary, NC). No correction for multiple comparisons was used.
Results
Clinic Characteristics and Records Assessed
Clinics differed by the number of monthly antenatal bookings and, among the 9 clinics with labor wards, by the number of monthly deliveries (Table 1). Average monthly antenatal bookings ranged from 24 (clinic V) to 638 (clinic F), whereas the number of monthly deliveries ranged from 91 (clinic E) to 654 (clinic F). Only 75% of antenatal records had documented RPR screening, whereas 77% of documented RPR seropositive records had documented treatment. The overall RPR seropositivity among records reviewed ranged from 4% to 32% in the 22 clinics. Clinic V, with an RPR seropositivity rate of 32%, differs from the other clinics in that it serves military personnel and their families.
Table 1.
Clinic Characteristics, Total Records Assessed (n = 5801) by Clinic and Clinic-Specific Record Counts for Assessments of Documented RPR Screening Before and After Implemented PMTCT Research, New Standard-of-Care Service, or Both Programs Among 22 Lusaka Urban District Clinics, Where All 22 Clinics Had Implemented PMTCT New Standard-of-Care Service and 9 of 22 Clinics Had Research Studies in Addition to the Service Programs, 1997 to 2004
| Clinic | Clinic Characteristics | |||
|---|---|---|---|---|
| Average Monthly Antenatal Bookings (%)* | Monthly Deliveries (%)† | RPR-Positive (%)‡ | Records Assessed (%) | |
| Total | 5333 | 2905 | 345 (6%) | 5801 |
| Clinics with research studies and PMTCT new standard-of-care service but where research was implemented before PMTCT new service (n = 7 clinics) | ||||
| A | 471 (9%) | 620 (21%) | 13 (4%) | 367 (6%) |
| B | 236 (4%) | 190 (7%) | 19 (4%) | 489 (8%) |
| C | 450 (8%) | 395 (14%) | 21 (6%) | 365 (6%) |
| D | 437 (8%) | 363 (12%) | 13 (4%) | 326 (6%) |
| E | 176 (3%) | 91 (3%) | 12 (5%) | 255 (4%) |
| F | 638 (12%) | 654 (23%) | 15 (5%) | 327 (6%) |
| G | 410 (8%) | 294 (10%) | 18 (6%) | 295 (5%) |
| Clinics with research studies and PMTCT new standard-of-care service but where new PMTCT service was implemented before research (n = 2 clinics) | ||||
| H | 248 (5%) | § | 14 (4%) | 327 (6%) |
| I | 316 (6%) | 198 (7%) | 14 (4%) | 354 (6%) |
| Clinics with PMTCT new standard-of-care service only, no research (n = 15 clinics) | ||||
| J | 185 (3%) | § | 19 (9%) | 222 (4%) |
| K | 127 (2%) | § | 15 (6%) | 248 (4%) |
| L | 108 (2%) | § | 12 (7%) | 168 (3%) |
| M | 179 (3%) | § | 8 (4%) | 213 (4%) |
| N | 289 (5%) | § | 21 (8%) | 267 (5%) |
| O | 108 (2%) | § | 14 (5%) | 273 (5%) |
| P | 53 (1%) | § | 11 (6%) | 180 (3%) |
| Q | 210 (4%) | § | 9 (5%) | 189 (3%) |
| R | 203 (4%) | § | 41 (19%) | 219 (4%) |
| S | 175 (3%) | § | 9 (4%) | 219 (4%) |
| T | 143 (3%) | 100 (3%) | 10 (4%) | 260 (4%) |
| U | 147 (3%) | § | 14 (8%) | 166 (3%) |
| V | 24 (1%) | § | 23 (32%) | 72 (1%) |
Average July 2001 and January 2002 ANC new booking attendees.
January 2004 deliveries.
RPR seropositivity percentage for assessed records.
ANC without delivery facility.
Documented RPR Screening Coverage
Documented RPR screening after research implementation was similar to that before implementation, (comparison 1, n = 7 clinics), with a POR of 0.9 (95% CI: 0.7 to 1.1) for research (Table 2). The POR of documented RPR screening among women attending clinics after the implementation of a PMTCT service program was associated with a lower likelihood of RPR screening (comparison 2, n = 15 clinics; POR = 0.7, 95% CI: 0.5 to 0.8). For the assessments of combined research and service programs, the PORs indicated improved testing after program intervention (POR = 2.5, 95% CI: 2.1 to 3.0) for the 9 clinics that had implementation of both programs irrespective of order of program introduction (comparison 3). The time frame and total months assessed for the comparisons are presented in Table 2.
Table 2.
Documented RPR Screening Coverage Among Records Sampled Before and After PMTCT Research, New Standard-of-Care Service, or Both Programs Were Implemented (n = 22 Clinics), Lusaka, Zambia, 1997 to 2004
| Assessment | Documented RPR Coverage Before Program Implementation | Documented RPR Coverage After Program Implementation | POR for Documented RPR Screening | |||
|---|---|---|---|---|---|---|
| Total Records | Documented Screening (%) | Total Records | Documented Screening (%) | Odds Ratio (95% CI) | P | |
| Comparison 1, PMTCT research only, July 1997 to July 2002, 21 mo assessed | ||||||
| Clinics A to G, n = 7 clinics | 807 | 524 (65%) | 693 | 433 (62%) | 0.9 (0.7 to 1.1) | 0.33 |
| Comparison 2, PMTCT service as new standard of care only, July 2000 to July 2004, 38 mo assessed | ||||||
| Clinics H to V, n = 15 clinics | 1689 | 1364 (81%) | 1406 | 1045 (74%) | 0.7 (0.6 to 0.8) | <0.0001 |
| Comparison 3, PMTCT research plus service as new standard of care, July 1997 to February 2004, 25 mo assessed | ||||||
| Clinics A to I, n = 9 clinics | 1099 | 692 (63%) | 1206 | 975 (81%) | 2.5 (2.1 to 3.0) | <0.0001 |
Referencing Table 1, a breakdown of the 5801 total records was assessed for the 22 clinics as follows: comparison 1: 807, 693 (1500 records); comparison 2: 1689, 1406 (3095 records); and comparison 3: 1206.
The 1099 “before implementation records” for comparison 3 are the same 807 records assessed for comparison 1.
Documented Treatment Coverage of RPR-Positive Cases
The POR of documented treatment for women attending clinics after the introduction of PMTCT research (n = 15 clinics, comparison 1) was 2.8 (95% CI: 0.8 to 9.7; Table 3). The POR of documented treatment for women attending clinics after introduction of PMTCT service (n = 15 clinics, comparison 2) was 0.7 (95% CI: 0.4 to 1.3). The PORs were: 1.7 (95% CI: 0.7 to 3.6) in the 9 clinics where both programs were implemented, regardless of order of implementations (comparison 3). The time frame and total months assessed for the comparisons are presented in Table 3.
Table 3.
Documented Treatment Coverage Among Documented RPR-Positive Screened Women Among Records Sampled Before and After PMTCT Research, New Standard-of-Care Service, or Both Programs Were Implemented (n = 22 Clinics), Lusaka, Zambia, 1997 to 2004
| Assessment | Documented Treatment Coverage Before Program Implementation | Documented Treatment Coverage After Program Implementation | POR for Documented Treatment of RPR-Positive Cases | |||
|---|---|---|---|---|---|---|
| Total Records | Documented Treatment (%) | Total Records | Documented Treatment (%) | Odds Ratio (95% CI) | P | |
| Comparison 1, PMTCT research only, July 1997 to July 2002, 21 mo assessed | ||||||
| Clinics A to G, n = 7 clinics | 42 | 26 (62%) | 22 | 18 (82%) | 2.8 (0.8 to 9.7) | 0.11 |
| Comparison 2, PMTCT service as new standard of care only, July 2000 to July 2004, 38 mo assessed | ||||||
| Clinics H to V, n = 15 clinics | 117 | 92 (79%) | 105 | 75 (71%) | 0.7 (0.4 to 1.3) | 0.22 |
| Comparison 3, PMTCT research plus service as new standard of care, July 1997 to February 2004, 25 mo assessed | ||||||
| Clinics A to I, n = 9 clinics | 50 | 29 (58%) | 59 | 41 (69%) | 1.7 (0.8 to 3.6) | 0.21 |
Referencing Table 1, the 345 documented RPR seroreactive cases are counted in the comparisons as follows: comparison 1: 42 and 22 (64 records); comparison 2: 117 and 105 (22 records); and comparison 3: 59 records.
The 50 “before program implementation” records for comparison 3 are counted in the “before program implementation records” for comparison 1.
The 42 and 8 “before program implementation” records for comparisons 4 and 5 are counted in the “before program implementation records” for comparisons 1 and 2.
Discussion
Our study suggests that the simultaneous operation of PMTCT research and service programs in ANCs was associated with improved antenatal RPR screening coverage. We expected but did not find that the implementation of PMTCT research or service independently would improve on important and routine components of antenatal care, namely, RPR screening and treatment.
PMTCT research and service programs continue to evolve in resource-limited settings as elsewhere in sub-Saharan Africa.16 The available evidence on the association between maternal syphilis with mother-to-child HIV transmission makes universal antenatal syphilis screening and treatment an urgent task in PMTCT efforts.17 As PMTCT programs are being scaled up, now is the time to coordinate resources to achieve economies of scale in extending PMTCT and integrated antenatal care. To our knowledge, this is the first study to assess the benefit or harms of PMTCT research or service programs on ancillary antenatal care in a resource-limited setting.
At the end of 2001, approximately 15.6% of the adult Zambian population was infected with HIV;18 up to 30% of antenatal attendees were HIV-seropositive in the urban antenatal clinics.19 In women aged 20 to 24 years, the 2004 Lusaka Urban District Clinic syphilis prevalence ranged from 3.7% to 9.7% by RPR testing, with confirmation by the treponemal hemaglutination assay.19
Several factors may be associated with changes observed over time in documented RPR and syphilis treatment coverage in clinics where PMTCT research and service programs were implemented simultaneously. Improved RPR screening and treatment could have been attributed to PMTCT-related and/or general antenatal care staff retraining; clinic supply procurement support; laboratory upgrading; additional human resources for research; stocking of reagents, syringes, and needles by PMTCT research and service programs beyond routine services; and, often, pharmaceutic agents for patients even if they were not included in research programs per se.20 Improved RPR screening and treatment, which were seen in the 9 clinics with research and service programs, could have been attributable to more highly skilled nurse managers being assigned to work in the clinics that had research and service programs to manage. Alternately, the nurse-midwife PMTCT service training in the 9 clinics with both programs could have been focused more on maintaining antenatal services at the same time that PMTCT service was being implemented, as compared with the training in the 15 clinics that were never selected for research activities. It is possible that the clinics with both research and service were selected for these programs because of higher performance and staff capability to begin with. Our finding of less RPR screening in clinics with only PMTCT service may be attributable to stressed staff taking on more HIV-related screening work, with consequent neglect of RPR coverage. After PMTCT service expansion, blood drawn from women was used for HIV and RPR testing; community suspicions regarding blood collection for the HIV test could have resulted in lower RPR screening after PMTCT program implementation.
A strength of our study lies in the large number of historic antenatal records available for examination through systematic sampling. Other strengths were that all 22 clinics studied had a research and/or service program during the study time frame and that there was likely little or no self-selection bias in the pregnant women seeking antenatal care with regard to RPR screening. Study limitations are also evident. We could not test our hypothesis of ancillary benefit or harm from PMTCT programs in a randomized trial. All data were obtained from clinic records and were an imperfect representation of what might have occurred at an antenatal booking visit. It is possible that in some cases, RPR screening or treatment was provided but not documented. We recognize that there may have been nonindependence among nurses if they transferred among clinics. Likewise, there could have been nonindependence among women attending antenatal clinics over time or by clinic if they delivered multiple times within the district system during the study time frame. Missing records, particularly for clinic E, was a study limitation. Our inability to document the irregular availability of clinic stocks of penicillin because of procurement of the medication from the local government central stores was a limitation. Sustainability of the combined programs after the completion of research studies was not assessed, because several of these studies were still in progress at the end of our study time frame. Finally, some clinic characteristics that could have been potential confounders were not collected by the programs and were not measured, such as staffing-to-patient ratios by clinic, training, experience, and capacity of clinic staff; clinic-based procurement of government stocks of RPR testing and treatment supplies; clustering at the clinic level; or time. Such potential covariates should be measured and controlled for in any future work of this type.
We believe that the present study has broad implications for clinical services and public health policy in resource-limited settings. In sub-Saharan Africa, shortages of human resources, social capital, and health care expenditures may undermine implementation of health care provision.21–26 The ability of developing country health care leaders to provide quality care when operating under severe financial and human resource constraints may depend in part on external funding for research or service programs to meet health system needs.27 One lesson learned from our study is that combined program and research implementations may be associated with unexpected routine health care gains, although further research should be conducted to determine the extent and type of benefit.
We recommend that health policy makers in resource-limited settings, and donor country research and service implementers, plan explicitly for how targeted programs can have a broader programmatic impact. Ways to maximize ancillary benefits while minimizing potential health system drains are warranted.28–31 Full integration of PMTCT services (with opt-out testing for HIV and syphilis, which was implemented in Lusaka after this study time frame) into antenatal care is a desirable long-term goal for current global initiatives.32–37
Acknowledgments
The authors thank the following individuals: Dr. Rosemary Kumwenda for her review and comments pertaining to historic research programs operating within the Lusaka District and Alexandria Mwale, the late Maclean Ukwimi, Stanley Mwale, Lavender Chalabesa, Susanna Mwachanda, Miriam Nyambe, Hilda Banda, Cathy Mulenga, Tatulini Musukuma, Gift Chola Mulenga, and Lawrence Phiri for their comments and/or assistance with the study.
Supported in part by National Institutes of Health grants 1 F31 HD 046415-01, U01 AI47972-05 (HIV Prevention Trials Network), and D43 TW01035-07 (AIDS International Training and Research Program). Service programs were supported by the Call to Action program of the Elizabeth Glaser Pediatric AIDS Foundation, with added financial support from the US Government, the Mother and Child HIV Prevention Initiative, and the Bill and Melinda Gates Foundation.
Footnotes
Author Contributions: D. Potter played a role in study design, performed data collection, data analyses, literature searches, and drafted the manuscript. R. L. Goldenberg contributed the study idea and to study design and writing. A. Chao contributed to epidemiologic methods, analyses guidance, and writing. M. Sinkala facilitated data accessibility and reviewed the write-up for important content. A. Degroot contributed to analyses. J. Stringer contributed to guidance regarding the issues raised in the manuscript and to writing. M. Bulterys contributed literature suggestions, writing, and thoughtful content suggestions, particularly in relation to issues raised from the study outcomes. S. H. Vermund contributed to the study idea, epidemiologic design, literature suggestions, and writing.
References
- 1.Stringer JS, Sinkala M, Goldenberg RL, et al. Monitoring nevirapine-based programmes for prevention of mother-to-child transmission of HIV-1. Lancet. 2003;362:667. doi: 10.1016/S0140-6736(03)14172-4. [DOI] [PubMed] [Google Scholar]
- 2.Finau SA, Finau E, Ofanoa M. Research imperialism in Pacific health: the case of Tonga (1966–1997) Pac Health Dialog. 2000;7:109–114. [PubMed] [Google Scholar]
- 3.Hyder AA, Wali SA, Khan AN, et al. Ethical review of health research: a perspective from developing country researchers. J Med Ethics. 2004;30:68–72. doi: 10.1136/jme.2002.001933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gonzalez-Block MA. Health policy and systems research agendas in developing countries. Health Res Policy Syst. 2004;2:6. doi: 10.1186/1478-4505-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilmshurst P. Scientific imperialism. BMJ. 1997;314:840–841. doi: 10.1136/bmj.314.7084.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Macklin R. International research: ethical imperialism or ethical pluralism? Account Res. 1999;7:59–83. doi: 10.1080/08989629908573942. [DOI] [PubMed] [Google Scholar]
- 7.de Zulueta P. Randomised placebo-controlled trials and HIV-infected pregnant women in developing countries. Ethical imperialism or unethical exploitation? Bioethics. 2001;15:289–311. doi: 10.1111/1467-8519.00240. [DOI] [PubMed] [Google Scholar]
- 8.Kopelman LM, van Niekerk AA. AIDS and Africa. Introduction. J Med Philos. 2002;27:139–142. doi: 10.1076/jmep.27.2.139.2991. [DOI] [PubMed] [Google Scholar]
- 9.Johnston SC, Rootenberg JD, Katrak S, et al. Effect of a US National Institutes of Health programme of clinical trials on public health and costs. Lancet. 2006;367:1319–1327. doi: 10.1016/S0140-6736(06)68578-4. [DOI] [PubMed] [Google Scholar]
- 10.Borghi J, Ensor T, Somanathan A, et al. Mobilising financial resources for maternal health. Lancet. 2006;368:1457–1465. doi: 10.1016/S0140-6736(06)69383-5. [DOI] [PubMed] [Google Scholar]
- 11.Travis P, Bennett S, Haines A, et al. Overcoming health-systems constraints to achieve the Millennium Development Goals. Lancet. 2004;364:900–906. doi: 10.1016/S0140-6736(04)16987-0. [DOI] [PubMed] [Google Scholar]
- 12.Bush to seek expansion of AIDS effort. [July 21, 2007];Washington Post. Available at: http://www.washingtonpost.com/wp-dyn/content/article/2007/05/30/AR2007053000012.html.
- 13.Stringer EM, Sinkala M, Stringer JS, et al. Prevention of mother-to-child transmission of HIV in Africa: successes and challenges in scaling-up a nevirapine-based program in Lusaka, Zambia. AIDS. 2003;17:1377–1382. doi: 10.1097/01.aids.0000060395.18106.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Online Presidential Initiatives Network (OPIN) Status of presidential initiatives FY 2003, Mother and Child HIV Prevention Initiative, U.S. Agency for International Development, Bureau for Policy and Program Coordination. [February 23, 2007]; Available at: http://www.usaid.gov/about_usaid/presidential_initiative/status_fy2003.pdf.
- 15.Gloyd S, Chai S, Mercer MA. Antenatal syphilis in sub-Saharan Africa: missed opportunities for mortality reduction. Health Policy Plan. 2001;16:29–34. doi: 10.1093/heapol/16.1.29. [DOI] [PubMed] [Google Scholar]
- 16.Bulterys M. Use of antiretroviral drugs to prevent mother-to-child HIV-1 transmission in high-prevalence, resource-poor settings. In: Butera ST, editor. HIV Chemotherapy: A Critical Review. Norfolk, UK: Caister Academic Press; 2005. pp. 159–194. [Google Scholar]
- 17.Mwapasa V, Rogerson SJ, Kwiek JJ, et al. Maternal syphilis infection is associated with increased risk of mother-to-child transmission of HIV in Malawi. AIDS. 2006;20:1869–1877. doi: 10.1097/01.aids.0000244206.41500.27. [DOI] [PubMed] [Google Scholar]
- 18.Central Statistical Office (Zambia), Central Board of Health (Zambia), and ORC Marco. Zambia Demographic and Health Survey 2001–2002. Calverton, MD: Central Statistical Office, Central Board of Health, and ORC Marco; 2003. [Google Scholar]
- 19.Ministry of Health, Central Board of Health, Government of Republic of Zambia (GRZ) Zambia Antenatal Clinic Sentinel Surveillance Report, 1994–2004. Lusaka, Zambia: Nov, 2005. [Google Scholar]
- 20.Chi BH, Sinkala M, Stringer EM, et al. Employment of off-duty staff: a strategy to meet the human resource needs for a large PMTCT program in Zambia. J Acquir Immune Defic Syndr. 2005;40:381–382. doi: 10.1097/01.qai.0000159515.39982.c0. [DOI] [PubMed] [Google Scholar]
- 21.Dovlo D. Wastage in the health workforce: some perspectives from African countries. Hum Resour Health. 2005;3:6. doi: 10.1186/1478-4491-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van der Geest S, Macwan'gi M, Kamwanga J, et al. User fees and drugs: what did the health reforms in Zambia achieve? Health Policy Plan. 2000;15:59–65. doi: 10.1093/heapol/15.1.59. [DOI] [PubMed] [Google Scholar]
- 23.Nullis-Kapp C. Health worker shortage could derail development goals. Bull World Health Organ. 2005;83:5–6. [PMC free article] [PubMed] [Google Scholar]
- 24.Chen L, Evans T, Anand S, et al. Human resources for health: overcoming the crisis. Lancet. 2004;364:1984–1990. doi: 10.1016/S0140-6736(04)17482-5. [DOI] [PubMed] [Google Scholar]
- 25.Kajula PW, Kintu F, Barugahare J, et al. Political analysis of rapid change in Uganda's health financing policy and consequences on service delivery for malaria control. Int J Health Plann Manage. 2004;19(Suppl 1):S133–S153. doi: 10.1002/hpm.772. [DOI] [PubMed] [Google Scholar]
- 26.Borghi J, Ensor T, Somanathan A, et al. Lancet Maternal Survival Series Steering Group. Mobilising financial resources for maternal health. Lancet. 2006;368:1457–1465. doi: 10.1016/S0140-6736(06)69383-5. [DOI] [PubMed] [Google Scholar]
- 27.Prata N, Greig F, Walsh J, et al. Ability to pay for maternal health services. What will it take to meet WHO standards? Health Policy (New York) 2004;70:163–174. doi: 10.1016/j.healthpol.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 28.Flanagin A, Winker MA. Global health-targeting problems and achieving solutions: a call for papers. JAMA. 2003;290:1382–1384. doi: 10.1001/jama.290.10.1382. [DOI] [PubMed] [Google Scholar]
- 29.Global Forum Update on Research for Health 2005. Health research to achieve the millennium development goals. [November 30, 2006]; Available at: http://www.globalforumhealth.org/filesupld/global_update1/GlobalUpdate1.pdf.
- 30.Potter D, Goldenberg RL, Read JS, et al. Correlates of syphilis seroreactivity among pregnant women: the HIVNET 024 trial in Malawi, Tanzania, and Zambia. Sex Transm Dis. 2006;33:604–609. doi: 10.1097/01.olq.0000216029.00424.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gloyd S, Montoya P, Floriano F, et al. Scaling up antenatal syphilis screening in Mozambique: transforming policy to action. Sex Transm Dis. 2007;34(Suppl):S31–S36. doi: 10.1097/01.olq.0000264586.49616.72. [DOI] [PubMed] [Google Scholar]
- 32.Stringer JS, Zulu I, Levy J, et al. Rapid scale-up of antiretroviral therapy at primary care sites in Zambia: feasibility and early outcomes. JAMA. 2006;296:782–793. doi: 10.1001/jama.296.7.782. [DOI] [PubMed] [Google Scholar]
- 33.Ferradini L, Jeannin A, Pinoges L, et al. Scaling up of highly active antiretroviral therapy in a rural district of Malawi: an effectiveness assessment. Lancet. 2006;367:1335–1342. doi: 10.1016/S0140-6736(06)68580-2. [DOI] [PubMed] [Google Scholar]
- 34.Welty TK, Bulterys M, Welty ER, et al. Integrating prevention of mother-to-child HIV transmission into routine antenatal care: the key to program expansion in Cameroon. J Acquir Immune Defic Syndr. 2005;40:486–493. doi: 10.1097/01.qai.0000163196.36199.89. [DOI] [PubMed] [Google Scholar]
- 35.Reithinger R, Megazzini K, Durako SJ, et al. Prevention of mother-to-child transmission of HIV in Africa: monitoring and evaluation of operational programmes. BMJ. 2007;334:1143–1146. doi: 10.1136/bmj.39211.527488.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brugha R. Evaluation of HIV programmes. BMJ. 2007;334:1123–1124. doi: 10.1136/bmj.39223.583773.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Creek TL, Ntumy R, Seipone K, et al. Successful introduction of routine opt-out HIV testing in antenatal care in Botswana. J Acquir Immune Defic Syndr. 2007;45:102–107. doi: 10.1097/QAI.0b013e318047df88. [DOI] [PubMed] [Google Scholar]


