Abstract
Elimination of the costimulatory molecule B7-2 prevents autoimmune diabetes in NOD mice, but leads to the development of a spontaneous autoimmune polyneuropathy (SAP), which resembles the human disease chronic inflammatory demyelinating polyradiculoneuropathy (CIDP). In this study, we examined the immunopathogenic mechanisms in this model, including identification of SAP antigen/s. We found that B7-2 deficient NOD mice exhibit changes in cytokine and chemokine gene expression in spleens over time. There was an increase in IL-17 and a decrease in IL-10 transcript levels at 4 mo (preclinical phase), while interferon-γ expression peaked at 8 mo (clinical phase). There was also an increase in transcript levels of Th1 cytokines, CXCL10, and RANTES in sciatic nerves of mice that developed SAP. Splenocytes from SAP mice exhibited proliferative and Th1 cytokine responses to myelin P0 (180–199), but not to other P0 peptides or P2 (53–78). Adoptive transfer of P0-reactive T cells generated from SAP mice induced neuropathy in 4 of 6 NOD.SCID mice. Data from intravenous tolerance studies indicate that myelin P0 is one of the autoantigens targeted by T cells in SAP in this model. The expression of P0 by peri-islet Schwann cells provides a potential mechanism linking islet autoimmunity and inflammatory neuropathy.
Keywords: Autoimmunity, Th1/Th2 cells, costimulation, CIDP, neuropathy
Introduction
The non-obese diabetic (NOD) mouse spontaneously develops polyendocrine autoimmunity and is used as a model for type 1 diabetes, thyroiditis, and Sjögren’s syndrome, as reviewed previously (1). Manifestations of autoimmunity in NOD mice are regulated by the cytokine milieu, and by co-stimulatory molecules such as B7-1 and B7-2. Neutralization or deficiency of B7-1 causes exacerbation of diabetes. In contrast, elimination of B7-2 leads to protection against diabetes, although peri-insulitis is present in some mice (2–4). Interestingly, B7-2 elimination triggers the development of a spontaneous autoimmune polyneuropathy (SAP) that mimics human chronic inflammatory demyelinating polyneuropathy (CIDP) clinically, histologically and electrophysiologically, albeit not in all aspects (3). Compared to SAP, CIDP is more heterogenous with regard to disease onset, and the course can be relapsing-remitting or chronic progressive. Histologically, CIDP in humans is characterized by the presence of segmental demyelination in peripheral nerves and nerve roots, axonal loss of variable degree, and an immune-mediated pathophysiology (5). NOD mice can develop both type 1 diabetes and SAP, or exclusively one but not the other, thought to depend on the balance between effector T cells and regulatory T cells (Tregs) (6).
Based on the cytokine profile, CD4+ effector T cells are classified into IFN-γ-producing Th1 cells, IL-4-producing Th2 cells, and IL-17-producing Th17 cells (7, 8). Both Th1 and Th17 cells are capable of inducing autoimmunity (9). The recruitment of effector T cells and macrophages into the target organ of autoimmunity is directed by chemokines and their receptors. For example, CXCL10 (IP-10), a chemoattractant for T cells, has been implicated in the pathogenesis of CIDP (10, 11). The goal of this study was to elucidate the molecular mechanisms underlying the pathogenesis of SAP in B7-2 KO NOD mice, focusing on the characterization of cytokine and chemokine profile, and the identification of potential SAP antigens. Candidate SAP antigens include but are not limited to known proteins of peripheral nerve myelin such as P0, P2 which can induce experimental allergic neuritis (EAN), a model of human Guillain-Barré syndrome (GBS)(12–15). We chose to focus on myelin protein zero (Mpz), also abbreviated as P0, in this study. It should be noted that P0+/− mice develop an inflammatory neuropathy spontaneously that is attributed to impaired central tolerance to P0 (16–18). In CIDP, proliferative responses to P0 or P2 as well as autoantibodies against P0, P2, and glycolipids have been reported in some patients (15.6% to 46%) but none has been shown to be highly sensitive and specific (19–21). This may reflect epitope spreading of variable extent or more likely the heterogeneity of this disease. Given the development of inflammatory neuropathy in NOD mice lacking B7-2, it is plausible that CIDP-like illness occurring on the background of type 1diabetes is a unique subset characterized by autoreactivity to a specific PNS antigen that is shared by pancreatic islets.
Materials and Methods
Clinical and electrophysiology assessment
All animal use procedures were conducted in strict accordance to the National Institutes of Health and University of Chicago institutional guidelines. For clinical assessment, the following nominal scale was used: 0- normal; 1- flaccid tail; 2- mild paraparesis; 3- severe paraparesis; 4- tetraparesis; 5- death. Grip strength testing consisted of five separate measurements in each of two trials per session with a grip strength meter (Columbus Instruments, OH). Results of two trials were averaged for each mouse per session. After the last grip strength measurement, electrophysiologic studies of sciatic nerves were performed as described in our previous publications (3, 22). Latencies, conduction velocities and peak to peak amplitudes were measured.
Histologic studies of sciatic nerves
Segments of the sciatic nerves were fixed in 0.5–4% paraformaldehyde, embedded in OCT compound, and snap frozen. Longitudinal sections (10 µm slices) of sciatic nerves were stained with haematoxylin–eosin (H&E) for evaluation of mononuclear cell infiltration, and used for immunohistochemistry with rat anti-CD3 Ab (1:100; Southern Biotech, Birmingham, AL) using the peroxidase method.
Splenocyte culture, proliferation and cytokine production assays
For proliferation assay, splenocytes were cultured at a density of 5 × 105 cells/well in HL-1™ medium plus non-essential amino acids (Biowhittaker), L-glutamine (2 mM), sodium pyruvate (1 mM), β-mercaptopurine (55 µM) in flat-bottom 96 well plates. Cells were then stimulated with Con A (2.5 µg/ml), PNS myelin (100 µg/ml), P0 peptide (20µg/ml), P2 peptide (20 µg/ml), or Schwann cell (SC) lysates (100 µg/ml). On day 3, these cultures were pulsed for 16 h with 1 µCi methyl-[3H]thymidine, and then harvested on glass fiber filter. The amount of incorporated methyl-[3H]thymidine was measured using Beckman liquid scintillation counter. A stimulation index was defined by cpm in the presence of antigen divided by cpm in the absence of the antigen. Supernatants collected at 48 hr from replicate cultures were assayed for IFN-γ (Endogen, Rockford, IL), IL-2, IL-10 (BD Biosciences, San Diego, CA) and IL-17 (eBioscience, San Diego, CA) using ELISA Minikits following the manufacturer’s instructions. The binding of peroxidase-conjugated secondary Ab was detected by TMB substrate reagent set (BD Biosciences, San Diego, CA).
Antigens used in in vitro T cells activation include P0 and P2 peptides, which were synthesized at the University of Chicago Protein-Peptide Core Facility. The following peptides were used: 1) P0 (180–199) (SSKRGRQTPVLYAMLDHSRS); 2) P0 (41–60) (PEGGRDAISIFHYAKGQPYI); 3) P0 (106–125) (IVGKTSQVTLYVFEKVPTRY); 4) P2 (53–78) (TESPFKNTEISFKLGQEFEETTADNR); 5) OVA (323–339) (ISQAVHAAHAEINEAGR) (GenScript Corporation, Piscataway, NJ). Myelin was prepared from mouse sciatic nerves as described by other investigators (23, 24). SC lysates were prepared by 5 cycles of rapid freeze (− 80°C) and thaw (37°C) without detergents. Cultured neonatal rat SCs were established as previously described (25).
Preparation of T cell lines and adoptive transfer studies
Lymphocytes were isolated from spleens, axillary and inguinal lymph nodes of B7-2 KO and wild type (WT) NOD mice using the Lympholyte-M gradient (Cedarlane, Hornby, Ontario). Purified CD4+ T cells were obtained using Dynal Mouse CD4 negative isolation kit (Invitrogen, Carlsbad, CA). To generate P0-specific T cell lines, CD4+T cells (1.5× 106 /well) were cultured with 3 × 106 irradiated (3000 R) syngeneic splenic APCs in the presence of P0 (180–199) (20 µg/ml) plus rIL-2 (25 U/ml) for 5 d followed by a 5 day resting period without antigen but with rIL-2 (25 U/ml). After 3 cycles of stimulation and rest, the specificity of the cell line was examined by proliferation assay in response to P0 (180–199) and control peptides. For adoptive transfer studies, P0-specific T cell lines were activated in-vitro with P0 (180–199) for 3 days prior to injection of 6.5 ×106 cells into the tail vein of NOD.SCID mice. Ovalbumin (OVA)-reactive T cell lines were generated from pooled splenocytes and lymph node cells of mice immunized with OVA (100 µg) in complete Freund’s adjuvant by subcutaneous injections over 4 sites.
Real-time PCR
The total RNA was isolated using a Trizol reagent (Invitrogen), followed by Qiagen column purification (Qiagen, Valencia, CA, USA). Reverse transcription was performed from 1 µg total RNA and the complementary DNA (cDNA) was used for SYBR green real-time PCR. Amplification was performed with forward and reverse primers for transcripts of interest, which was designed using Primer3 software. The expression of each cytokine or chemokine gene was normalized by corresponding amount of GAPDH mRNA for each condition. The relative amounts of each product were calculated by the comparative CT (2−ΔΔCT) method as described in the user manual #2 of ABI Prism 7900 Sequence Detection System (Applied Biosystems). Primer sequences for real-time PCR studies are listed in Table 1 (Supplementary data).
Intracellular cytokine analysis
Splenocytes (10 ×106 /well) in 6-well plates were stimulated at 37°C in a humidified CO2 incubator for 4 hr with Leukocyte Activation Cocktail containing PMA, ionomycin, brefeldin A and BD Golgiplug™. After culture, cells (1× 106) were stained for cell surface CD4 and intracellular IFN-γ and IL-17, using the Intracellular Cytokine Staining Starter Kit (BD Pharmingen, San Diego, CA). The number of IFN-γ and IL-17 producing CD4+ T cells was analyzed by FACScan (Becton Dickinson) and FlowJo software.
Western blot analysis and immunofluorescence studies
Samples (equal amount/lane) were resolved by 12% SDS-PAGE and electroblotted to PVDF membranes. Blots were blocked with 5% nonfat milk in 10 mM Tris (pH 7.5), 100 mM NaCl, 0.1% Tween 20 for 1 h at room temperature. Blots were incubated with mouse Ab α P0 (1:3000; Astexx, Graz, Austria) or sera (1:200) overnight at 4°C, washed 3× and then incubated for 1 hr with goat anti-mouse IgG HRP-conjugated secondary Abs (1:5000; Calbiochem, San Diego, CA). In some experiments, isotype-specific HRP-conjugated secondary Abs (Sta. Cruz Biotechnol., Sta. Cruz, CA) were used at 1:2000 to 1:10,000. Abs used for immunofluoresence studies include: mAb α P0 (1:500) (Axtexx, Graz, Austria); rabbit Ab α glial fibrillary acid protein (GFAP)(1:100–1:200)(Dako, Carpinteria, CA); Alexa Fluor 488- and Alexa Fluor 598-conjugated secondary Abs (1:500)(Molecular Probes, Eugene, OR).
Data analysis
Results from real-time PCR experiments, immunologic studies, grip strength measurements and electrophysiology are expressed as mean ± SEM. Statistical significance was determined by analysis of variance (ANOVA) followed by Student’s t test and the Bonferroni method for multiple group experiments. Significance levels were set at p < 0.05.
Results
Cytokine and chemokine profile in spleens and sciatic nerves
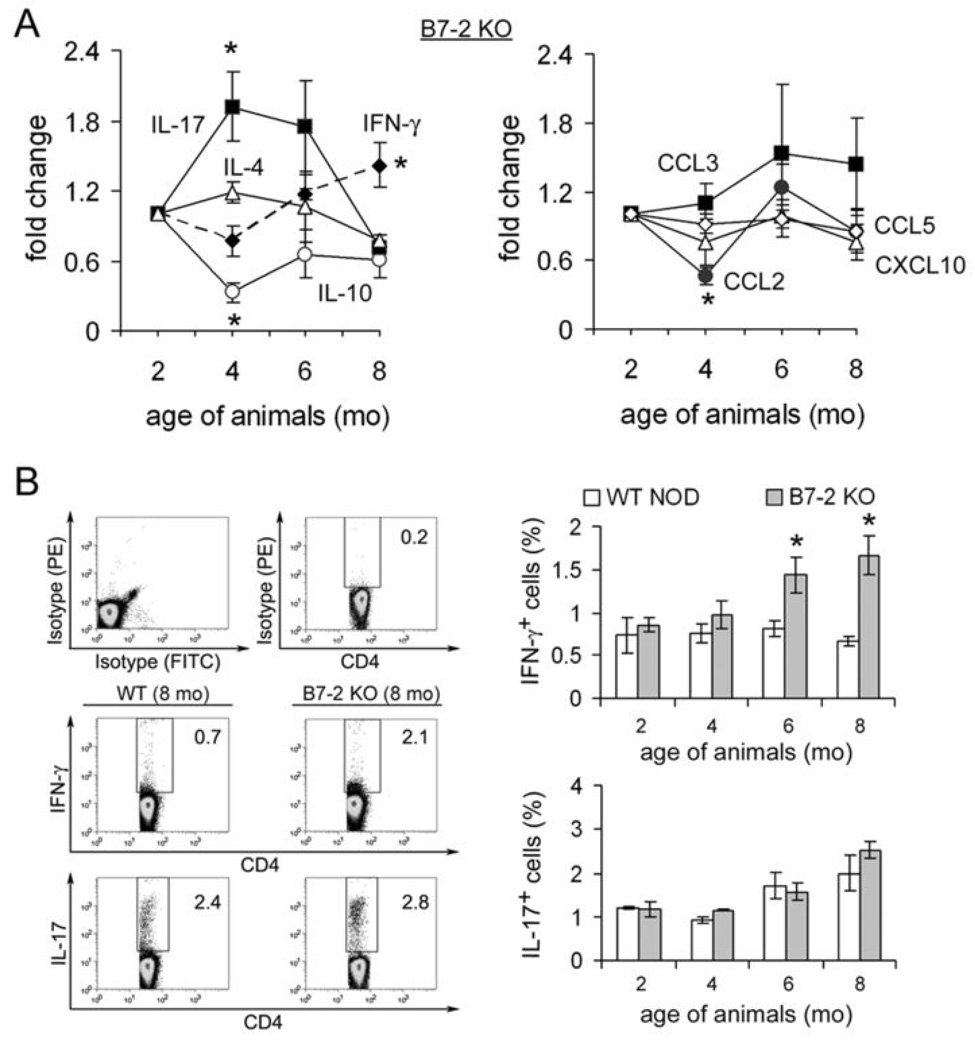
B7-2 knockout (KO) NOD mice begin to exhibit hindlimb weakness at 6–7 months, progressing to generalized paralysis with time (3). To examine the cytokine and chemokine profile in spleens, real-time PCR studies were performed on splenic RNA from female B7-2 KO NOD mice at age 2 mo, 4 mo, 6 mo and 8 mo (n=3 each). Results are expressed as fold change in gene expression compared to 2 mo data (calculated by the 2−ΔΔCT method). As shown in Fig.1A, there was a decrease in interleukin-10 (IL-10) transcript levels in spleens of female B7-2 KO NOD mice starting at 4 mo of age (preclinical phase). IL-17 transcripts peaked at 4 mo but declined towards baseline at 8 mo, while interferon-γ (IFN-γ) transcripts peaked at 8 mo (clinical phase) (At 4 mo, p <0.04 for IL-17 vs IL-10; p < 0.037 for IL-17 vs IFN-γ, p <0.002 for IL-10 vs IFN- γ. At 8 mo, p <0.003 for IFN-γ vs IL-17 and p < 0.01 for IFN-γ vs IL-10). With regards to chemokines, there was a decrease in CCL2 transcript levels at 4 mo (p <0.004 for CCL2 vs CCL3). There was no change in the levels of TNF-α, LT-β, IL-4, CCL3, CCL5 (RANTES), CXCL10 (IP-10), CXCL16 transcripts at any timepoints. For clarity, only 4 graphs are shown in each panel of Fig.1A. To determine whether changes in IFN-γ and IL-17 transcript levels correlate with functional polarization of T helper cells, we examined the percentage of IFN-γ-and IL-17-producing CD4+ T cells from the spleens of these mice by flow cytometry. Upon stimulation with PMA (10 ng/ml) and ionomycin (500 ng/ml) for 4 hrs, there was an increase in the percentage of CD4+ IFN-γ+ cells but not in CD4+IL-17+ cells in splenocytes from B7-2 KO mice when compared to those from WT NOD mice at 6 mo and 8 mo (Fig. 1B).
Figure 1.
Cytokine and chemokine perturbations in the spleens of B7-2 KO NOD mice. A. Time course of cytokine and chemokine transcripts in the spleens of B7-2 KO NOD. At 4 mo (preclinical phase), there was an increase in IL-17, a decrease in IL-10 and no change in IFN-γ transcript levels (*p <0.04 for IL-17 vs IL-10; p < 0.037 for IL-17 vs IFN-γ, p <0.002 for IL-10 vs IFN-γ). At 8 mo (clinical phase), there was evidence for Th1 bias (*p <0.003 for IFN-γ vs IL-17 and p < 0.01 for IFN-γ vs IL-10). There was a decrease in CCL2 transcript levels at 4 mo (*p <0.004 for CCL2 vs CCL3). Data shown represent an average from 3 independent experiments. The relative amounts of each product were calculated by the CT (2−ΔΔCT) method. Data were normalized to that of 2 mo old B7-2 KO NOD mice. B. Examples of intracellular cytokine analysis and summary showing an increase in IFN-γ- producing, but not in IL-17 producing CD4+ T cells in spleens of 6 mo and 8 mo old B7-2 KO mice when compared to age-matched WT NOD mice (n=3 each) (*CD4+ IFN-γ+ T cells: p < 0.026 for 8 mo and p <0.036 for 6 mo data, B7-2 KO vs WT NOD). Splenocytes were incubated with PMA (10 ng/ml) and ionomycin (500 ng/ml) for 4 hrs.
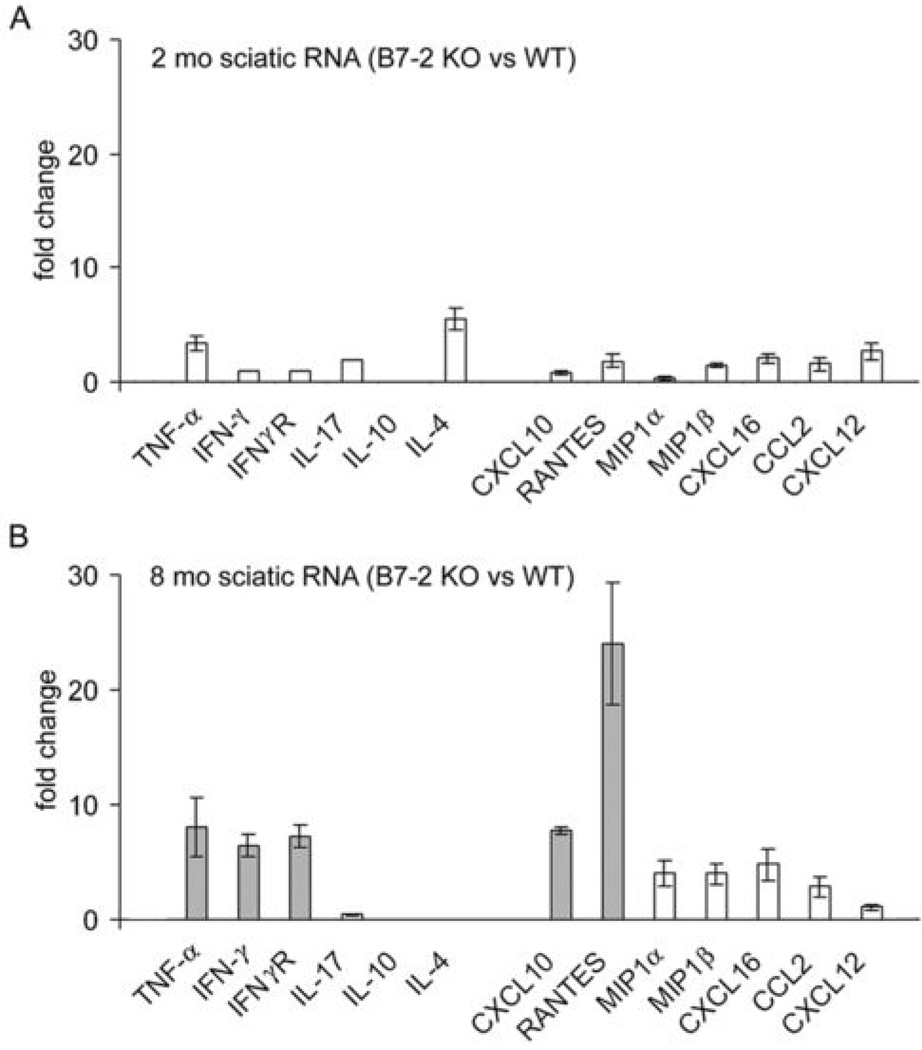
For cytokine and chemokine profile in sciatic nerves, real-time PCR studies were performed focusing on two timepoints only (2 mo and 8 mo). Three independent experiments were carried out, each sample consisted of pooled sciatic nerve RNA from 3 mice. TNF-α, IFN-γ, IFN-γR, CXCL10, and RANTES mRNAs were highly upregulated in sciatic nerves of B7-2 KO mice at 8 mo compared to those from age-matched WT NOD mice, or to those from 2 mo old B7-2 KO NOD mice (Fig. 2A, B). A more modest increase in MIP1α, MIPβ, CXCL16, and CCL2 was also observed at 8 mo. There was no increase in IL-17 transcript levels in sciatic nerves of 8 mo old B7-2 KO NOD mice when compared to WT NOD mice. These data suggest that Th1 cytokines, CXCL10 and RANTES are important mediators during the effector phase of SAP.
Figure 2.
Comparison of cytokine and chemokine transcripts in sciatic nerves of B7-2 KO NOD mice vs. WT NOD mice. A. 2 mo; B. 8 mo. Data shown represent an average from 3 independent experiments except for 2 mo data on IFN-γ, IFN-γR and IL-17 (n=2 each). There was a significant increase in the expression of TNF-α, IFN-γ, IFN γR, CXCL10, and RANTES in sciatic nerves of B7-2 KO NOD mice at 8 mo, when compared to age-matched WT NOD mice, or 2 mo old B7-2 KO NOD mice. Other chemokines were increased to a lesser extent. There was no increase in IL-17 transcripts in sciatic nerves at 8 mo. IL-10 and IL-4 transcripts were not detected.
Autoreactivity to PNS antigens in SAP
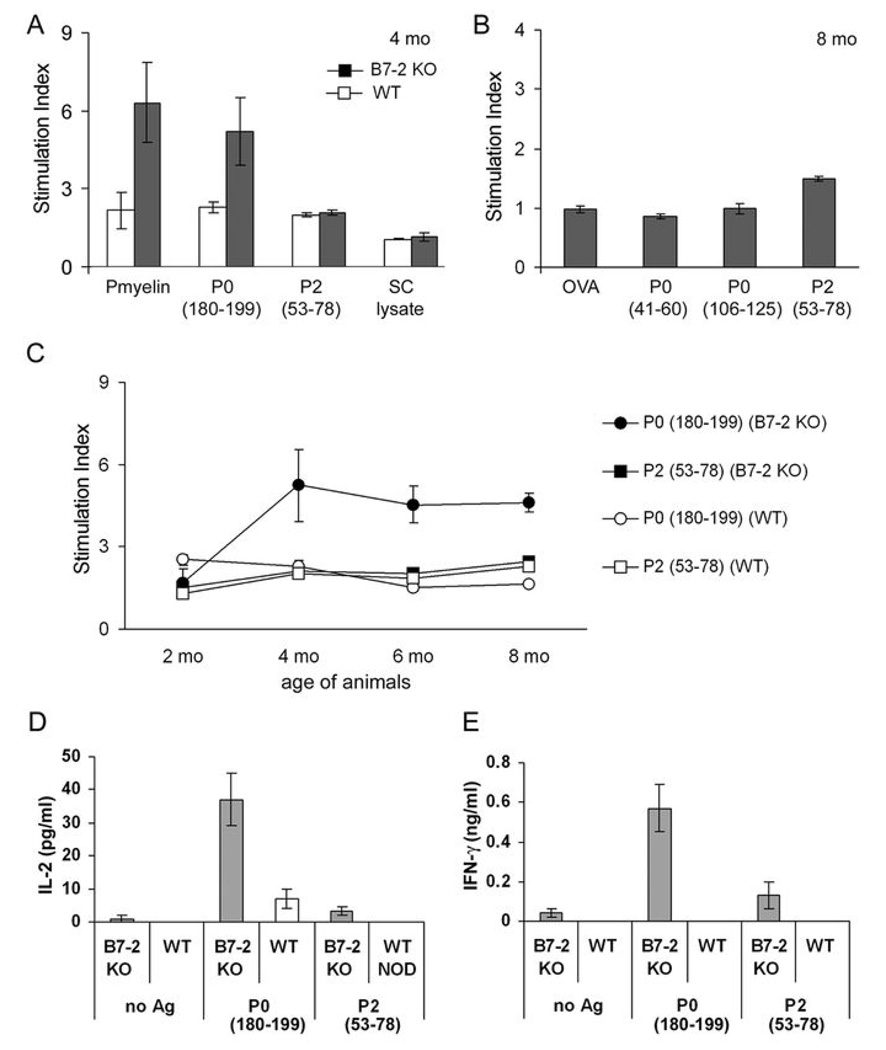
We characterized the autoreactive T cell repertoire responsible for SAP by examining the proliferative response of B7-2 KO NOD splenocytes to PNS myelin (100 µg/ml), lysates of cultured neonatal SCs (100 µg/ml), and two known neuritogens myelin P0 (180–199) (20 µg/ml) and P2 (57–81) (20 µg/ml). A stimulation index of >2.2 in the thymidine incorporation assay was considered positive. We observed a proliferative response to PNS myelin but not to lysates of neonatal SCs (non-myelinating), indicating that an autoantigen resides in peripheral nerve myelin or myelinating SCs. Autoreactivity to P0 (180–199) was not detected at 2 mo, but was detected at 4, 6 and 8 mo (Fig. 3A–C). Each data point represents the average from 3–4 independent experiments, each in triplicate wells. P2 (53–78), P0 (41–60) and P0 (106–125) did not induce a proliferative response (n = 3–5). Similarly, P0 (180–199) but not P2 (53–78) stimulates the secretion of IL-2 and IFN-γ by SAP splenocytes, as measured by ELISA (n = 3–4) (Fig. 3D,E). There was no significant change in IL-10 or IL-17 secretion (data not shown). In contrast to B7-2 KO NOD mice, splenocytes from WT NOD mice exhibit minimal proliferative and cytokine responses to P0 (180–199) or P2 (53–78).
Figure 3.
Splenocyte proliferative and cytokine responses to candidate SAP antigens. A, B. Splenocyte proliferation induced by PNS myelin (100 µg/ml), various P0 peptides (20 µg/ml), P2 (53–78) (20 µg/ml), SC lysate (100 µg/ml) and OVA (20 µg/ml). Unfilled bars: WT NOD; filled bars: B7-2 KO NOD (n=3–4 each in A, n=3–5 in B). Results are expressed as stimulation index, with values >2.2 considered positive. Untreated: 1110–2468 cpm. C. Time course of splenocyte proliferative response to P0 (180–199) and P2 (53–78) (n=3–4). D. P0 (180–199) but not P2 (53–78) stimulates the secretion of IL-2 and IFN-γ by splenocytes from B7-2 KO mice (n=3–4). Treatment duration was 72 hr for proliferative response and 48 hr for cytokine assays.
Myelin P0-reactive T cells are involved in the pathogenesis of SAP
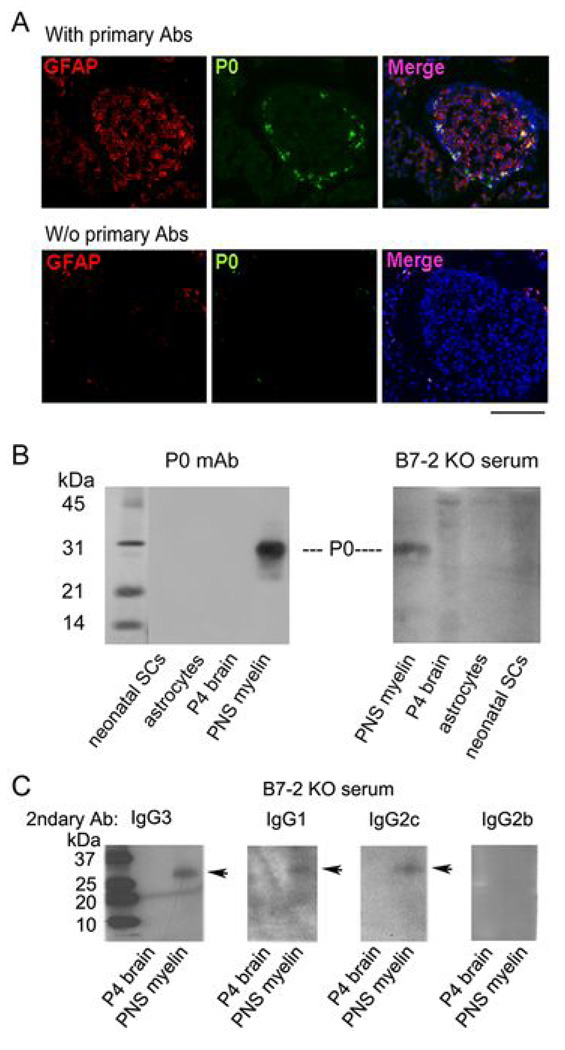
Given that peri-islet SCs, which express GFAP and S100β, are target of autoimmune attack in early insulitis (26), we examined whether these cells express P0 protein. Whereas the immunoreactivity against GFAP was observed in both peri-islet SCs and SCs infiltrating the islets of WT NOD mice, P0-reactive SCs were restricted to the peri-islet region (Fig. 4A). Western blot analysis using a mAb α P0 identified a band at 28 kDa in the lane loaded with PNS myelin extract, but not in lanes loaded with lysates of cultured SC (non-myelinating), cultured astrocytes or postnatal day4 brains (Fig. 4B). The same band was labeled by sera from B7-2 KO mice, albeit more frequently by sera obtained from 6–8 mo old mice than those from 2–4 mo old mice [8/8 (100%) vs 1/6 (16.7%)]. Immunoreactivity was also detected occasionally by WT NOD sera [1/7 (14.3%)]. Further studies revealed that serum Abs α P0 were of either Th1 or Th2-predominant IgG isotypes, with the frequency of IgG3 > IgG1 > IgG2c > IgG2b (Fig. 4C). It should be noted that IgG2a gene is deleted in NOD mice (27).
Figure 4.
Expression of P0 by peri-islet SCs and PNS, and autoAbs to P0 in SAP sera. A. Immunofluorescence studies demonstrating that P0 expression was restricted to peri-islet SCs, while GFAP was expressed by both peri-islet SCs and SCs infiltrating the islets. Bar represents 100 µm. B. Western blot analysis showing that the mAb α P0 used in (A) labeled a band at 28 kDa, the expected molecular weight of P0, in the lane loaded with PNS myelin. A similar band was labeled by B7-2 KO serum. C. Isotyping of anti-P0 Abs present in SAP sera. Serum Abs α P0 were of either Th1 or Th2 predominant IgG isotypes. Serum dilution was 1:200 in B & C.
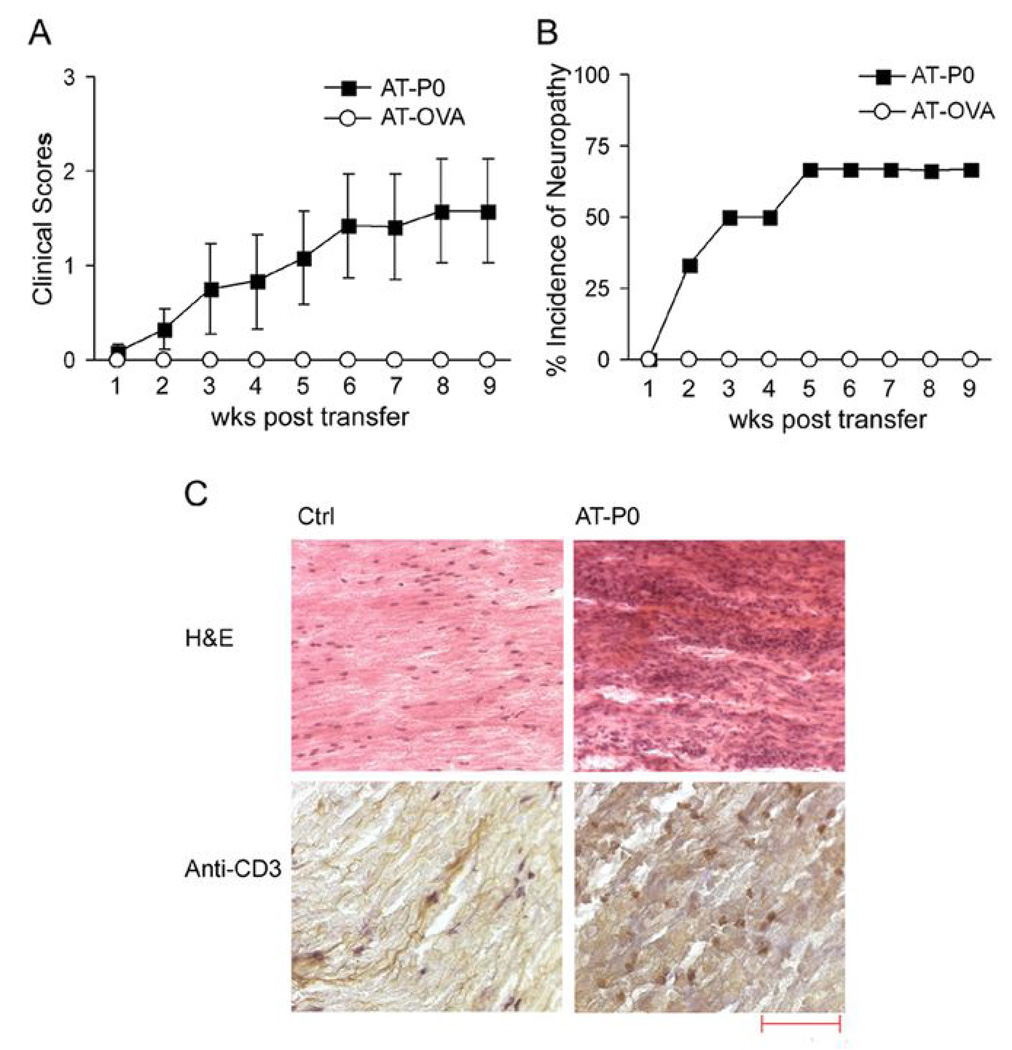
CD4+ T cells but not sera from SAP mice are sufficient to transfer disease to NOD.SCID mice (3). To further examine whether P0-reactive T cells are pathogenic in SAP, we generated P0-reactive T cell lines from pooled splenocytes and lymph node cells isolated from inguinal and axillary lymph nodes of SAP mice (7–8 mo old). These cell lines secrete greater amounts of IFN-γ (14.7–15.4 ng/ml) than IL-17 (0.5–0.67 ng/ml) upon exposure to P0 (180–199), but only IFN-γ secretion was inhibited by Ab α MHC class II (I-A/I-E) (10 µg/ml)(not shown). Adoptive transfer of 6.5 ×106 P0-reactive CD4+ T cell lines (AT-P0) led to the development of peripheral neuropathy in 4/6 (66.7%) of NOD.SCID mice, as shown in Fig. 5A, B. By comparison, transfer of purified CD4+ T cells from WT NOD mice or OVA-reactive T cell lines (AT-OVA) did not induce neuropathy (n=3 each). Because we were not able to generate OVA-specific T cell lines from SAP mice, these cell lines were derived from WT NOD mice immunized with OVA. Results were confirmed with grip strength measurements at peak disease or at the end of 9 wks. Forelimb grip strength (in Newtons) was 2.37 ± 0.26 and 3.2 ± 0.01 in AT-P0 and AT-OVA mice, respectively (p < 0.01); while hindlimb grip strength was 0.38 ± 0.11, and 1.02 ± 0.01, respectively (p <0.0003)(not shown). Histologic studies revealed the presence of T cell infiltrates in sciatic nerves of symptomatic AT-P0 mice (Fig. 5C).
Figure 5.
Adoptive transfer (AT) of P0 (180–199)-specific and OVA-reactive CD4+ T cell lines into 8–10 wk old female NOD.SCID mice. A. Summary of clinical scores (n=6 for AT-P0; n=3 for AT-OVA). P0-specific T cell lines were generated from SAP mice, while OVA-specific T cells lines were generated from WT NOD mice immunized with OVA. B. Incidence of neuropathy in AT-P0 mice and AT-OVA mice. C. Examples of sciatic nerve sections showing T cell infiltrates (CD3+ cells) in nerves from AT-P0 mice. Hematoxylin was used for counterstaining in immunohistochemical studies. Bar represents 100 µm for H&E and 50 µm for anti-CD3.
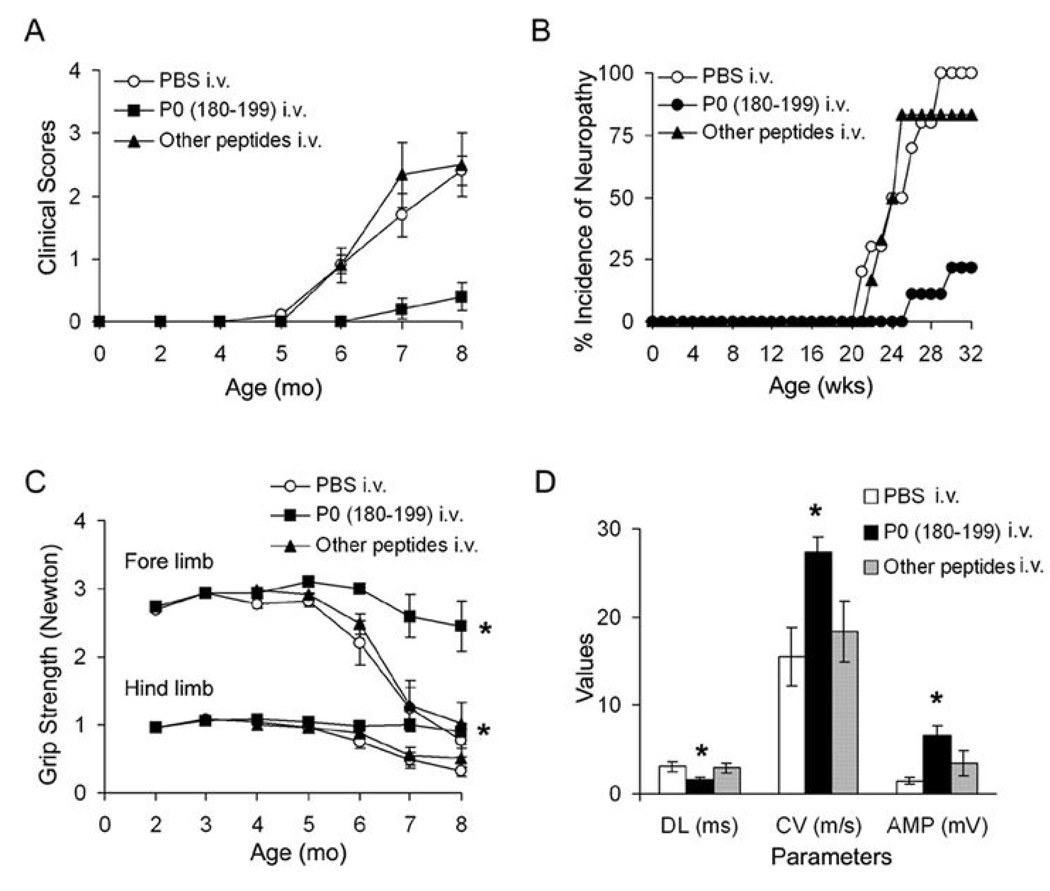
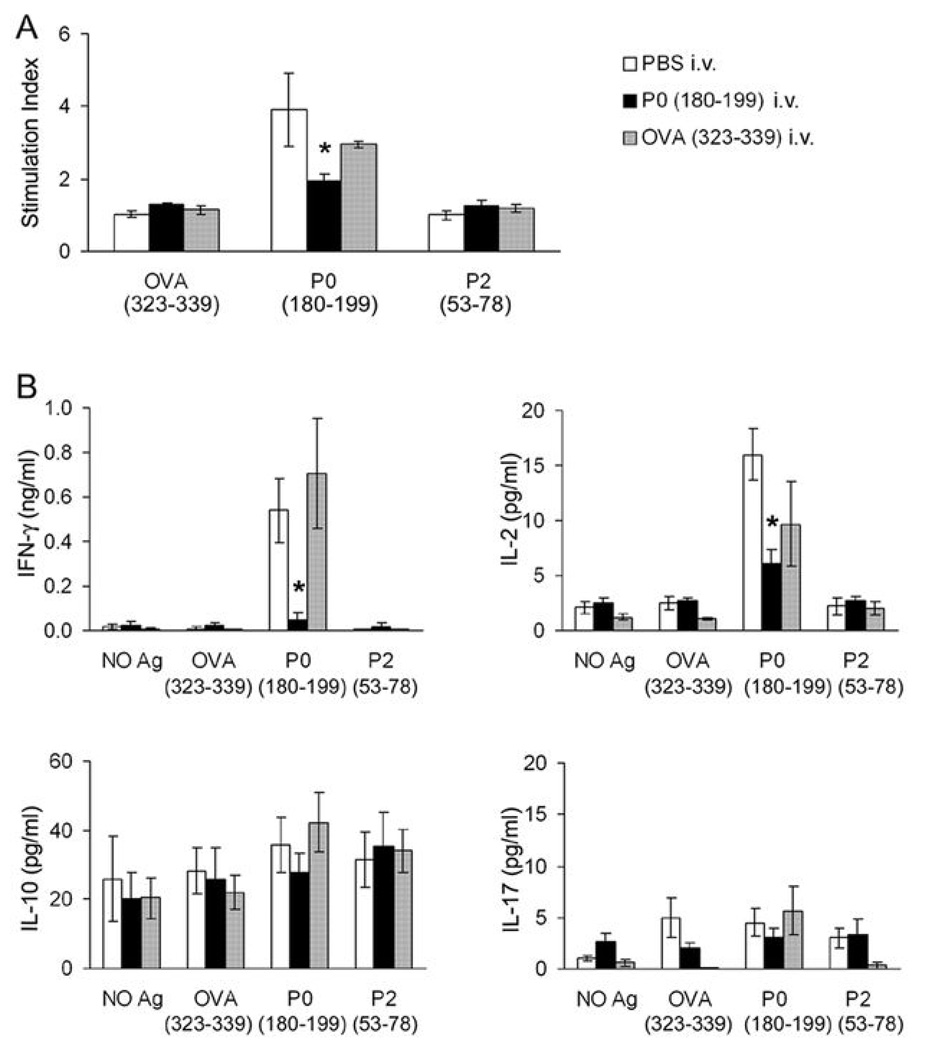
Lastly, we investigated whether SAP can be prevented in B7-2 KO NOD mice by a single intravenous injection of 200 µg P0 (180–199) at 2–4 mo of age. As depicted in Fig. 6A & B, a very mild neuropathy was observed at 8 mo in only 2/9 (22.2%) of B7-2 KO mice injected with P0 (180–199), as compared to a more severe neuropathy in 10/10 (100%) of mice receiving PBS, and in 5/6 (83.3%) of those given other peptides [OVA (323–339) or P2 (53–78)]. In P0-injected mice, there was no decline in grip strength over time, in contrast to grip strength measurements in other groups of animals (Fig. 6C). Furthermore, sciatic nerve conduction studies revealed a significant difference in distal latency, conduction velocity and amplitude of the motor response in P0-injected mice compared to those from other groups of animals (Fig. 6D). No improvement in clinical severity was observed when P0 (180–199) was given at 8 mo (after disease onset) (n= 2, not shown). Immunologic studies done at 8 mo revealed that there was significant attenuation of the proliferative response to P0 (180–199), and a decline in IL-2 and IFN-γ secretion in splenocytes from animals injected with P0 at 2–4 mo. No significant effect on IL-10 or IL-17 levels was noted (Fig. 7A, B). Taken together, these results indicate that myelin P0 (180–199) is one of the autoantigens involved in the development of SAP.
Figure 6.
Prevention of SAP by a single intravenous injection of P0 (180–199), but not by injection of PBS or other peptides in B7-2 KO NOD mice at 2 or 4 mo. A. Clinical scores. Three groups of animals were compared [n = 9 for P0 (180–199); n = 10 for PBS; other peptides include P2 (53–78), OVA (323–339), n = 3 each; p <0.0001 for P0 (180–199) vs PBS or other peptides at 8 mo]. Peptide dose injected: 200 µg. B. Incidence of neuropathy in these 3 groups of mice. C. Grip strength measurements (* At 8 mo, p < 0.0006 for P0 (180–199) vs PBS and other peptides in forelimb and p <0.0001 in hindlimb grip strength). D. Sciatic nerve electrophysiology at 8 mo. Distal latencies (DL), conduction velocities (CV) and amplitudes (AMP) of sciatic motor responses were measured. * DL: p < 0.009 for P0 (180–199) vs PBS or other peptides; CV: p< 0.003; Amp: p < 0.001.
Figure 7.
Suppression of splenocyte proliferation and Th1 cytokine secretion in B7-2 KO NOD mice given intravenous injection of P0 (180–199) at 2 or 4 mo. Three groups of mice were sacrificed at 8 mo [PBS group, n = 4; P0 group, n = 6; OVA group, n = 3]. A. Proliferative responses of splenocytes to 20 µg/ml OVA (323–339), P0 (180–199) and P2 (53–78). Treatment duration: 72 hr. The proliferative response to P0 (180–199) was decreased in splenocytes from P0-tolerized animals (*p < 0.004 for P0 vs PBS group, and p <0.008 for P0 vs OVA group). B. Cytokine responses of splenocytes to the same antigens in replicate cultures. Treatment duration: 48 hr. * IFN-γ: p <0.03 for P0 vs PBS group, and p <0.003 for P0 vs OVA group; IL-2: p <0.002 for P0 vs PBS group, but p > 0.05 for P0 vs OVA group of animals. There was no significant difference in IL-10 and IL-17 production in response to P0 (180–199) amongst 3 groups of animals.
Discussion
NOD mice exhibit a number of immune system defects that contribute to their susceptibility to develop multi-organ autoimmunity (1). Similar to other models of autoimmunity, functional polarization of T helper (Th) subsets is one of the crucial factors in the pathogenesis of type 1 diabetes. Th1 effector response (high IFN-γ/IL-4 ratio) is associated with destructive insulitis, although a dual pathogenic and protective role for IFN-γ has been demonstrated by other investigators (28–31). Recently, evidence has been introduced to indicate that IL-17 may contribute to the development of type 1 diabetes (31, 32).
We observed changes in cytokine and chemokine microenvironment in the spleens of B7-2 KO NOD mice, with IL-17 and IFN-γ predominating during the preclinical phase and clinical phase, respectively. That IFN-γ is a key effector mediating nerve damage in SAP mice is supported by the following: 1) IFN-γ and not IL-17 transcripts predominate in sciatic nerves of symptomatic mice, 2) splenocytes from symptomatic mice produce IFN-γ and not IL-17 upon stimulation with P0 (180–199); and 3) there was an increase in IFN-γ-producing, but not in IL-17-producing CD4+ T cells in spleens of SAP mice at 6–8 mo when compared to age-matched WT NOD mice. Furthermore, disease severity in both SAP and EAN is attenuated in mice deficient in IFN-γ or its receptor, respectively (33, 34). While there is evidence supporting a Th1 bias in SAP, the role of Th17 cells and the source of increased IL-17 transcripts in spleens of 4–6 mo old B7-2 KO mice remain to be clarified. Both Th1 and Th17 cells have been implicated in EAE, though recent data indicate that they induce distinct types of CNS inflammatory disease characterized by macrophage-rich infiltrates and neutrophil-rich infiltrates, respectively (8, 35–37). It has also been reported that increased Th17:Th1 ratio is more critical in the induction of CNS inflammation than in spinal cord inflammation (38).
The decline in IL-10 expression in spleens and its absence in sciatic nerves may contribute to disease development and progression in SAP. IL-10 expression correlates with spontaneous recovery in EAN, and exogenous IL-10 suppresses EAN by downregulating Th1 responses (39, 40). The role of TNF-α in inflammatory neuropathies is more complex, as both anti-inflammatory and pro-inflammatory actions of TNF-α have been reported (41, 42). With regards to chemokines, the transient dip in CCL2 transcript levels in spleens is interesting. CCL2 KO mice have diminished Th2 cytokines supporting a role for CCL2 in the regulation of Th2 polarization, in addition to its role as a monocyte chemoattractant (43). Cytokines and chemokines that are upregulated in sciatic nerves of SAP mice are the same ones found in EAN and CIDP nerves with prominent increase in TNF-α, IFN-γ, CXCL10, and RANTES expression (10, 44–46).
Aside from cytokine perturbations, another crucial determinant in the development of autoimmune diseases is the delicate balance between autoreactive T cells and Tregs, which is controlled by B7-2 and other co-stimulatory molecules (4, 6, 47). Though both B7-1 and B7-2 bind to their receptors CD28 and CTLA-4, signaling through CD28 promotes T cell activation whereas signaling through CTLA-4 downregulates T cell responses (48, 49). CD28−/− C57BL/6 mice are resistant to EAN induction and CTLA-4 blockade enhances the severity of EAN (50, 51). The requirement for B7-1 vs B7-2 in autoimmunity is more complex and is influenced by genetic background, and the target organ involved. Absence of either B7-1 or B7-2 leads to attenuation of EAE induced in NOD mice but not in C57BL/6 mice (52, 53). B7-2 elimination triggers the development of SAP in NOD mice but not in C57BL/6 mice. In SAP nerves, high levels of B7-1 expression were observed in infiltrating CD11b+ and CD11c+ cells (3). Similarly, there is a preferential upregulation of B7-1 in CIDP nerves (54). Yet, neuropathy is accelerated by treatment with anti-B7-1 Ab in B7-2 KO NOD mice (6, 33). Collectively, these findings suggest a dominant role of B7-1 in the development of autoimmune neuropathies, but its requirement can be bypassed in the absence of functional Treg compartment. It is possible that other costimulatory molecules also play a role in inflammatory neuropathies. Inducible costimulator (ICOS) and its unique ligand (ICOS-L) mRNA are upregulated in infiltrating T cells and macrophages, respectively, in nerve samples from CIDP and other inflammatory neuropathies (55). Other investigators found upregulation of a costimulatory molecule in SCs of CIDP nerves that was detected by anti-BB-1 Ab, but not by anti-B7-1 or anti-B7-2 Abs (56).
It is intriguing that elimination of a costimulatory molecule such as B7-2 would shift the autoimmunity from pancreatic islets to peripheral nervous tissue. Due to a generalized defect in central tolerance induction, NOD mice contain a diverse repertoire of T cells reactive against multiple tissue antigens including several nervous system antigens such as myelin basic protein (MBP) and GFAP (26, 57). Yet, B7-2 KO NOD mice develop neuropathy spontaneously but not encephalomyelitis. Perhaps, the above dichotomy is partially due to a lesser ability of the blood nerve barrier than the blood brain barrier to impede lymphocytes access to the tissues. A second explanation could be derived from our data. Splenocyte proliferative responses indicate that an autoantigen resides in peripheral myelin and / or myelinating SCs. Further studies revealed that P0 (180–199) but not P0 (106–125) or P0 (41–60) elicited splenocyte proliferative and Th1 cytokine responses. Two P0 peptides used in the current study, P0 (180–199) and P0 (106–125), have been used to induce EAN in resistant C57BL/6 mice (34, 58, 59). Mice are generally more resistant to EAN induction than Lewis rats. The third peptide, P0 (41–60), has been reported to elicit a strong immune response in P0 knockout mice but not in WT mice immunized with peripheral myelin (60). Interestingly, P0 is expressed by peri-islet SCs, which would not be expected of typical nonmyelinating SCs with rare exceptions such as peri-synaptic SCs (61). These findings imply that the initiation and amplification of autoreactivity to P0 can occur either in pancreatic islets or in peripheral nerves, and taken together with the imperfect blood nerve barrier, would account for the propensity of NOD mice to develop SAP rather than encephalomyelitis.
We found that P0 (180–199)-reactive T cells are pathogenic, based on our data from adoptive transfer and intravenous P0 experiments. Intravenous injection of soluble proteins such as MBP induces tolerance in EAE by clonal deletion, anergy or induction of Th1 /Treg cells depending on the timing of administration (62–64). The effect of soluble antigen treatment on effector T cell mobility and cytokine upregulation can be extremely rapid (within minutes) associated with trapping of these cells within lymphoid organs and later activation-induced cell death (65). In our study, a single intravenous injection of P0 peptide at 2 or 4 mo was sufficient to prevent SAP, which was associated with decreased activation of Th1 cells in response to P0 but was not accompanied by a shift to Th2 polarization. That myelin P0 is one of the SAP antigens has been demonstrated recently by Bour-Jourdan and colleagues using a different approach. Oligoclonal Ab responses to a 30 kDa protein were detected in accelerated models of autoimmune neuropathy, and the targeted protein was subsequently identified as myelin P0 by mass spectrometry (66). We found that Ab responses to P0 are more frequent in 6–8 mo old than in younger B7-2 KO NOD mice or WT NOD mice. Further studies are required to determine whether P0 Abs contribute to the pathogenesis of SAP, or simply act as markers of peripheral nerve damage.
That a CIDP-like illness can develop on a background of diabetes is interesting, given similar observations in humans; however, not all patients reported in the literature had type 1 diabetes (67–70). There are other findings demonstrating a link between islet and nervous system autoimmunity, or supporting a role of neurons and glial cells in type 1 diabetes. Islet inflammation and insulin resistance are controlled by TRPV+ pancreatic sensory neurons (71). Furthermore, GFAP-reactive T cell lines can transfer insulitis to NOD.SCID mice (26). It would be interesting to determine whether induction of tolerance to P0 would lead to suppression of islet autoimmunity in future studies. It is also recognized that some islet autoantigens are constituents of the nervous system such as glutamic acid decarboxylase (GAD65, GAD67); Ab α GAD is associated with specific neurologic syndromes such as stiff person syndrome (72).
In summary, we found that: 1) elimination of B7-2 leads to altered cytokine and chemokine milieu in lymphoid organs; 2) SAP is primarily a Th1-mediated disease; the cytokine and chemokine profile mimics that observed in CIDP nerves; and 3) myelin P0 is one of the autoantigens in SAP. These findings do not exclude the possibility of intramolecular spreading involving other P0 epitopes, intermolecular spreading to other PNS antigens, or a possible role for autoAbs / B cells in the progression of SAP. The expression of P0 by peri-islet SCs provides a potential mechanism linking islet autoimmunity and inflammatory neuropathy.
Supplementary Material
Acknowledgement
B7-2−/− NOD mice were generously provided by Dr. J.A. Bluestone. We also thank Shawna Cook for maintenance of mouse colony, Dr. Y.X. Fu for helpful discussions and Dr. Barry Arnason for critical review of the manuscript.
Grant support: This work was supported by National Institute of Health Grant R21 NS049014, a pilot grant from the GBS / CIDP Foundation International, Miller Group Charitable Trust Fund (Mr. M.P. Miller III), and Jack Miller Center for Peripheral Neuropathy.
Nonstandard abbreviations
- CIDP
chronic inflammatory demyelinating polyradiculoneuropathy
- EAE
Experimental autoimmune encephalomyelitis
- EAN
experimental autoimmune neuritis
- GFAP
glial fibrillary acid protein
- KO
knockout
- MBP
myelin basic protein
- P0
myelin protein zero
- Tregs
regulatory T cells
- SAP
spontaneous autoimmune polyneuropathy
- SCs
Schwann cells
References
- 1.Anderson MS, Bluestone JA. The NOD mouse: a model of immune dysregulation. Annu. Rev. Immunol. 2005;23:447–485. doi: 10.1146/annurev.immunol.23.021704.115643. [DOI] [PubMed] [Google Scholar]
- 2.Lenschow DJ, Ho SC, Sattar H, Rhee L, Gray G, Nabavi N, Herold KC, Bluestone JA. Differential effects of anti-B7-1 and anti-B7-2 monoclonal antibody treatment on the development of diabetes in the nonobese diabetic mouse. J. Exp. Med. 1995;181:1145–1155. doi: 10.1084/jem.181.3.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salomon B, Rhee L, Bour-Jordan H, Hsin H, Montag A, Soliven B, Arcella J, Girvin AM, Padilla J, Miller SD, Bluestone JA. Development of spontaneous autoimmune peripheral polyneuropathy in B7-2-deficient NOD mice. J. Exp. Med. 2001;194:677–684. doi: 10.1084/jem.194.5.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yadav D, Judkowski V, Flodstrom-Tullberg M, Sterling L, Redmond WL, Sherman L, Sarvetnick N. B7-2 (CD86) controls the priming of autoreactive CD4 T cell response against pancreatic islets. J. Immunol. 2004;173:3631–3639. doi: 10.4049/jimmunol.173.6.3631. [DOI] [PubMed] [Google Scholar]
- 5.Koller H, Kieseier BC, Jander S, Hartung HP. Chronic inflammatory demyelinating polyneuropathy. N. Engl. J. Med. 2005;352:1343–1356. doi: 10.1056/NEJMra041347. [DOI] [PubMed] [Google Scholar]
- 6.Bour-Jordan H, Salomon BL, Thompson HL, Szot GL, Bernhard MR, Bluestone JA. Costimulation controls diabetes by altering the balance of pathogenic and regulatory T cells. J. Clin. Invest. 2004;114:979–987. doi: 10.1172/JCI20483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu. Rev. Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 8.Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bettelli E, Oukka M, Kuchroo VK. T(H)-17 cells in the circle of immunity and autoimmunity. Nat. Immunol. 2007;8:345–350. doi: 10.1038/ni0407-345. [DOI] [PubMed] [Google Scholar]
- 10.Kieseier BC, Tani M, Mahad D, Oka N, Ho T, Woodroofe N, Griffin JW, Toyka KV, Ransohoff RM, Hartung HP. Chemokines and chemokine receptors in inflammatory demyelinating neuropathies: a central role for IP-10. Brain. 2002;125:823–834. doi: 10.1093/brain/awf070. [DOI] [PubMed] [Google Scholar]
- 11.Press R, Pashenkov M, Jin JP, Link H. Aberrated levels of cerebrospinal fluid chemokines in Guillain-Barre syndrome and chronic inflammatory demyelinating polyradiculoneuropathy. J. Clin. Immunol. 2003;23:259–267. doi: 10.1023/a:1024532715775. [DOI] [PubMed] [Google Scholar]
- 12.Brostoff SW, Levit S, Powers JM. Induction of experimental allergic neuritis with a peptide from myelin P2 basic protein. Nature. 1977;268:752–753. doi: 10.1038/268752a0. [DOI] [PubMed] [Google Scholar]
- 13.Milner P, Lovelidge CA, Taylor WA, Hughes RA. P0 myelin protein produces experimental allergic neuritis in Lewis rats. J. Neurol. Sci. 1987;79:275–285. doi: 10.1016/0022-510x(87)90235-8. [DOI] [PubMed] [Google Scholar]
- 14.Hahn AF, Feasby TE, Wilkie L, Lovgren D. P2-peptide induced experimental allergic neuritis: a model to study axonal degeneration. Acta Neuropathol. (Berl) 1991;82:60–65. doi: 10.1007/BF00310924. [DOI] [PubMed] [Google Scholar]
- 15.Rostami A, Brown MJ, Lisak RP, Sumner AJ, Zweiman B, Pleasure DE. The role of myelin P2 protein in the production of experimental allergic neuritis. Ann. Neurol. 1984;16:680–685. doi: 10.1002/ana.410160610. [DOI] [PubMed] [Google Scholar]
- 16.Shy ME, Arroyo E, Sladky J, Menichella D, Jiang H, Xu W, Kamholz J, Scherer SS. Heterozygous P0 knockout mice develop a peripheral neuropathy that resembles chronic inflammatory demyelinating polyneuropathy (CIDP) J. Neuropathol. Exp. Neurol. 1997;56:811–821. [PubMed] [Google Scholar]
- 17.Carenini S, Maurer M, Werner A, Blazyca H, Toyka KV, Schmid CD, Raivich G, Martini R. The role of macrophages in demyelinating peripheral nervous system of mice heterozygously deficient in p0. J. Cell. Biol. 2001;152:301–308. doi: 10.1083/jcb.152.2.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miyamoto K, Miyake S, Schachner M, Yamamura T. Heterozygous null mutation of myelin P0 protein enhances susceptibility to autoimmune neuritis targeting P0 peptide. Eur. J. Immunol. 2003;33:656–665. doi: 10.1002/eji.200323677. [DOI] [PubMed] [Google Scholar]
- 19.Khalili-Shirazi A, Atkinson P, Gregson N, Hughes RA. Antibody responses to P0 and P2 myelin proteins in Guillain-Barre syndrome and chronic idiopathic demyelinating polyradiculoneuropathy. J. Neuroimmunol. 1993;46:245–251. doi: 10.1016/0165-5728(93)90255-w. [DOI] [PubMed] [Google Scholar]
- 20.Ilyas AA, Mithen FA, Dalakas MC, Chen ZW, Cook SD. Antibodies to acidic glycolipids in Guillain-Barre syndrome and chronic inflammatory demyelinating polyneuropathy. J. Neurol. Sci. 1992;107:111–121. doi: 10.1016/0022-510x(92)90217-9. [DOI] [PubMed] [Google Scholar]
- 21.Yan WX, Archelos JJ, Hartung HP, Pollard JD. P0 protein is a target antigen in chronic inflammatory demyelinating polyradiculoneuropathy. Ann. Neurol. 2001;50:286–292. doi: 10.1002/ana.1129. [DOI] [PubMed] [Google Scholar]
- 22.Kim DH, Muthyala S, Soliven B, Wiegmann K, Wollmann R, Chelmicka-Schorr E. The beta 2-adrenergic agonist terbutaline suppresses experimental allergic neuritis in Lewis rats. J. Neuroimmunol. 1994;51:177–183. doi: 10.1016/0165-5728(94)90079-5. [DOI] [PubMed] [Google Scholar]
- 23.Hasse B, Bosse F, Muller HW. Proteins of peripheral myelin are associated with glycosphingolipid/cholesterol-enriched membranes. J. Neurosci. Res. 2002;69:227–232. doi: 10.1002/jnr.10287. [DOI] [PubMed] [Google Scholar]
- 24.Caroni P, Schwab ME. Two membrane protein fractions from rat central myelin with inhibitory properties for neurite growth and fibroblast spreading. J. Cell. Biol. 1988;106:1281–1288. doi: 10.1083/jcb.106.4.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iwase T, Jung CG, Bae H, Zhang M, Soliven B. Glial cell line-derived neurotrophic factor-induced signaling in Schwann cells. J. Neurochem. 2005;94:1488–1499. doi: 10.1111/j.1471-4159.2005.03290.x. [DOI] [PubMed] [Google Scholar]
- 26.Winer S, Tsui H, Lau A, Song A, Li X, Cheung RK, Sampson A, Afifiyan F, Elford A, Jackowski G, Becker DJ, Santamaria P, Ohashi P, Dosch HM. Autoimmune islet destruction in spontaneous type 1 diabetes is not beta-cell exclusive. Nat. Med. 2003;9:198–205. doi: 10.1038/nm818. [DOI] [PubMed] [Google Scholar]
- 27.Martin RM, Brady JL, Lew AM. The need for IgG2c specific antiserum when isotyping antibodies from C57BL/6 and NOD mice. J. Immunol. Methods. 1998;212:187–192. doi: 10.1016/s0022-1759(98)00015-5. [DOI] [PubMed] [Google Scholar]
- 28.Rabinovitch A, Suarez-Pinzon WL, Sorensen O, Bleackley RC, Power RF. IFN-gamma gene expression in pancreatic islet-infiltrating mononuclear cells correlates with autoimmune diabetes in nonobese diabetic mice. J. Immunol. 1995;154:4874–4882. [PubMed] [Google Scholar]
- 29.Kolb H. Benign versus destructive insulitis. Diabetes Metab. Rev. 1997;13:139–146. doi: 10.1002/(sici)1099-0895(199709)13:3<139::aid-dmr190>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 30.Trembleau S, Penna G, Gregori S, Giarratana N, Adorini L. IL-12 administration accelerates autoimmune diabetes in both wild-type and IFN-gamma-deficient nonobese diabetic mice, revealing pathogenic and protective effects of IL-12-induced IFN-gamma. J. Immunol. 2003;170:5491–5501. doi: 10.4049/jimmunol.170.11.5491. [DOI] [PubMed] [Google Scholar]
- 31.Jain R, Tartar DM, Gregg RK, Divekar RD, Bell JJ, Lee HH, Yu P, Ellis JS, Hoeman CM, Franklin CL, Zaghouani H. Innocuous IFNgamma induced by adjuvant-free antigen restores normoglycemia in NOD mice through inhibition of IL-17 production. J. Exp. Med. 2008;205:207–218. doi: 10.1084/jem.20071878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Srinivasan S, Bolick DT, Lukashev D, Lappas C, Sitkovsky M, Lynch KR, Hedrick CC. Sphingosine-1-phosphate reduces CD4+ T-cell activation in type 1 diabetes through regulation of hypoxia-inducible factor short isoform I.1 and CD69. Diabetes. 2008;57:484–493. doi: 10.2337/db07-0855. [DOI] [PubMed] [Google Scholar]
- 33.Bour-Jordan H, Thompson HL, Bluestone JA. Distinct effector mechanisms in the development of autoimmune neuropathy versus diabetes in nonobese diabetic mice. J. Immunol. 2005;175:5649–5655. doi: 10.4049/jimmunol.175.9.5649. [DOI] [PubMed] [Google Scholar]
- 34.Zhu Y, Ljunggren HG, Mix E, Li HL, van der Meide P, Elhassan AM, Winblad B, Zhu J. Suppression of autoimmune neuritis in IFN-gamma receptor-deficient mice. Exp. Neurol. 2001;169:472–478. doi: 10.1006/exnr.2001.7662. [DOI] [PubMed] [Google Scholar]
- 35.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat. Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bailey SL, Schreiner B, McMahon EJ, Miller SD. CNS myeloid DCs presenting endogenous myelin peptides 'preferentially' polarize CD4+ T(H)-17 cells in relapsing EAE. Nat. Immunol. 2007;8:172–180. doi: 10.1038/ni1430. [DOI] [PubMed] [Google Scholar]
- 37.Kroenke MA, Carlson TJ, Andjelkovic AV, Segal BM. IL-12- and IL-23-modulated T cells induce distinct types of EAE based on histology, CNS chemokine profile, and response to cytokine inhibition. J. Exp. Med. 2008;205:1535–1541. doi: 10.1084/jem.20080159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stromnes IM, Cerretti LM, Liggitt D, Harris RA, Goverman JM. Differential regulation of central nervous system autoimmunity by T(H)1 and T(H)17 cells. Nat. Med. 2008;14:337–342. doi: 10.1038/nm1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bai XF, Zhu J, Zhang GX, Kaponides G, Hojeberg B, van der Meide PH, Link H. IL-10 suppresses experimental autoimmune neuritis and down-regulates TH1-type immune responses. Clin. Immunol. Immunopathol. 1997;83:117–126. doi: 10.1006/clin.1997.4331. [DOI] [PubMed] [Google Scholar]
- 40.Jander S, Pohl J, Gillen C, Stoll G. Differential expression of interleukin-10 mRNA in Wallerian degeneration and immune-mediated inflammation of the rat peripheral nervous system. J. Neurosci. Res. 1996;43:254–259. doi: 10.1002/(SICI)1097-4547(19960115)43:2<254::AID-JNR13>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 41.Lu MO, Duan RS, Quezada HC, Chen ZG, Mix E, Jin T, Yang X, Ljunggren HG, Zhu J. Aggravation of experimental autoimmune neuritis in TNF-alpha receptor 1 deficient mice. J. Neuroimmunol. 2007;186:19–26. doi: 10.1016/j.jneuroim.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 42.Taylor JM, Pollard JD. Soluble TNFR1 inhibits the development of experimental autoimmune neuritis by modulating blood-nerve-barrier permeability and inflammation. J. Neuroimmunol. 2007;183:118–124. doi: 10.1016/j.jneuroim.2006.11.027. [DOI] [PubMed] [Google Scholar]
- 43.Gu L, Tseng S, Horner RM, Tam C, Loda M, Rollins BJ. Control of TH2 polarization by the chemokine monocyte chemoattractant protein-1. Nature. 2000;404:407–411. doi: 10.1038/35006097. [DOI] [PubMed] [Google Scholar]
- 44.Kieseier BC, Krivacic K, Jung S, Pischel H, Toyka KV, Ransohoff RM, Hartung HP. Sequential expression of chemokines in experimental autoimmune neuritis. J. Neuroimmunol. 2000;110:121–129. doi: 10.1016/s0165-5728(00)00323-4. [DOI] [PubMed] [Google Scholar]
- 45.Gold R, Archelos JJ, Hartung HP. Mechanisms of immune regulation in the peripheral nervous system. Brain Pathol. 1999;9:343–360. doi: 10.1111/j.1750-3639.1999.tb00231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mathey EK, Pollard JD, Armati PJ. TNF alpha, IFN gamma and IL-2 mRNA expression in CIDP sural nerve biopsies. J. Neurol. Sci. 1999;163:47–52. doi: 10.1016/s0022-510x(99)00009-x. [DOI] [PubMed] [Google Scholar]
- 47.Chen Z, Herman AE, Matos M, Mathis D, Benoist C. Where CD4+CD25+T reg cells impinge on autoimmune diabetes. J. Exp. Med. 2005;202:1387–1397. doi: 10.1084/jem.20051409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Karandikar NJ, Vanderlugt CL, Bluestone JA, Miller SD. Targeting the B7/CD28:CTLA-4 costimulatory system in CNS autoimmune disease. J. Neuroimmunol. 1998;89:10–18. doi: 10.1016/s0165-5728(98)00058-7. [DOI] [PubMed] [Google Scholar]
- 49.Carreno BM, Collins M. The B7 family of ligands and its receptors: new pathways for costimulation and inhibition of immune responses. Annu. Rev. Immunol. 2002;20:29–53. doi: 10.1146/annurev.immunol.20.091101.091806. [DOI] [PubMed] [Google Scholar]
- 50.Zhu Y, Ljunggren H, Mix E, Li HL, van der Meide P, Elhassan AM, Winblad B, Zhu J. CD28-B7 costimulation: a critical role for initiation and development of experimental autoimmune neuritis in C57BL/6 mice. J. Neuroimmunol. 2001;114:114–121. doi: 10.1016/s0165-5728(01)00241-7. [DOI] [PubMed] [Google Scholar]
- 51.Zhu J, Zou L, Zhu S, Mix E, Shi F, Wang H, Volkmann I, Winblad B, Schalling M, Ljunggren H. Cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) blockade enhances incidence and severity of experimental autoimmune neuritis in resistant mice. J. Neuroimmunol. 2001;115:111–117. doi: 10.1016/s0165-5728(01)00255-7. [DOI] [PubMed] [Google Scholar]
- 52.Chang TT, Jabs C, Sobel RA, Kuchroo VK, Sharpe AH. Studies in B7-deficient mice reveal a critical role for B7 costimulation in both induction and effector phases of experimental autoimmune encephalomyelitis. J. Exp. Med. 1999;190:733–740. doi: 10.1084/jem.190.5.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Girvin AM, Dal Canto MC, Rhee L, Salomon B, Sharpe A, Bluestone JA, Miller SD. A critical role for B7/CD28 costimulation in experimental autoimmune encephalomyelitis: a comparative study using costimulatory molecule-deficient mice and monoclonal antibody blockade. J. Immunol. 2000;164:136–143. doi: 10.4049/jimmunol.164.1.136. [DOI] [PubMed] [Google Scholar]
- 54.Kiefer R, Dangond F, Mueller M, Toyka KV, Hafler DA, Hartung HP. Enhanced B7 costimulatory molecule expression in inflammatory human sural nerve biopsies. J. Neurol. Neurosurg. Psych. 2000;69:362–368. doi: 10.1136/jnnp.69.3.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hu W, Janke A, Ortler S, Hartung HP, Leder C, Kieseier BC, Wiendl H. Expression of CD28-related costimulatory molecule and its ligand in inflammatory neuropathies. Neurol. 2007;68:277–282. doi: 10.1212/01.wnl.0000250240.99311.9d. [DOI] [PubMed] [Google Scholar]
- 56.Murata K, Dalakas MC. Expression of the co-stimulatory molecule BB-1, the ligands CTLA-4 and CD28 and their mRNAs in chronic inflammatory demyelinating polyneuropathy. Brain. 2000;123(Pt 8):1660–1666. doi: 10.1093/brain/123.8.1660. [DOI] [PubMed] [Google Scholar]
- 57.Winer S, Astsaturov I, Cheung R, Gunaratnam L, Kubiak V, Cortez MA, Moscarello M, O'Connor PW, McKerlie C, Becker DJ, Dosch HM. Type I diabetes and multiple sclerosis patients target islet plus central nervous system autoantigens; nonimmunized nonobese diabetic mice can develop autoimmune encephalitis. J. Immunol. 2001;166:2831–2841. doi: 10.4049/jimmunol.166.4.2831. [DOI] [PubMed] [Google Scholar]
- 58.Miletic H, Utermohlen O, Wedekind C, Hermann M, Stenzel W, Lassmann H, Schluter D, Deckert M. P0(106–125) is a neuritogenic epitope of the peripheral myelin protein P0 and induces autoimmune neuritis in C57BL/6 mice. J. Neuropathol. Exp. Neurol. 2005;64:66–73. doi: 10.1093/jnen/64.1.66. [DOI] [PubMed] [Google Scholar]
- 59.Zou LP, Ljunggren HG, Levi M, Nennesmo I, Wahren B, Mix E, Winblad B, Schalling M, Zhu J. P0 protein peptide 180–199 together with pertussis toxin induces experimental autoimmune neuritis in resistant C57BL/6 mice. J. Neurosci. Res. 2000;62:717–721. doi: 10.1002/1097-4547(20001201)62:5<717::AID-JNR11>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 60.Visan L, Visan IA, Weishaupt A, Hofstetter HH, Toyka KV, Hunig T, Gold R. Tolerance induction by intrathymic expression of P0. J. Immunol. 2004;172:1364–1370. doi: 10.4049/jimmunol.172.3.1364. [DOI] [PubMed] [Google Scholar]
- 61.Georgiou J, Charlton MP. Non-myelin-forming perisynaptic schwann cells express protein zero and myelin-associated glycoprotein. Glia. 1999;27:101–109. doi: 10.1002/(sici)1098-1136(199908)27:2<101::aid-glia1>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 62.Levine S, Hoenig EM, Kies MW. Allergic Encephalomyelitis: Passive Transfer Prevented by Encephalitogen. Science. 1968;161:1155–1157. doi: 10.1126/science.161.3846.1155. [DOI] [PubMed] [Google Scholar]
- 63.Critchfield JM, Racke MK, Zuniga-Pflucker JC, Cannella B, Raine CS, Goverman J, Lenardo MJ. T cell deletion in high antigen dose therapy of autoimmune encephalomyelitis. Science. 1994;263:1139–1143. doi: 10.1126/science.7509084. [DOI] [PubMed] [Google Scholar]
- 64.Hilliard BA, Kamoun M, Ventura E, Rostami A. Mechanisms of suppression of experimental autoimmune encephalomyelitis by intravenous administration of myelin basic protein: role of regulatory spleen cells. Exp. Mol. Pathol. 2000;68:29–37. doi: 10.1006/exmp.1999.2290. [DOI] [PubMed] [Google Scholar]
- 65.Odoardi F, Kawakami N, Li Z, Cordiglieri C, Streyl K, Nosov M, Klinkert WE, Ellwart JW, Bauer J, Lassmann H, Wekerle H, Flugel A. Instant effect of soluble antigen on effector T cells in peripheral immune organs during immunotherapy of autoimmune encephalomyelitis. Proc. Natl Acad. Sci. U S A. 2007;104:920–925. doi: 10.1073/pnas.0608383104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Su MA, Davini D, Jr, Giang K, Anderson MS, Bour-Jordan H. Specificity of autoantibody responses against neural proteins in a mouse model of autoimmune peripheral neuropathy. J. PNS. 2008;13:183. (Abstract). [Google Scholar]
- 67.Haq RU, Pendlebury WW, Fries TJ, Tandan R. Chronic inflammatory demyelinating polyradiculoneuropathy in diabetic patients. Muscle Nerve. 2003;27:465–470. doi: 10.1002/mus.1250. [DOI] [PubMed] [Google Scholar]
- 68.Sharma KR, Cross J, Farronay O, Ayyar DR, Shebert RT, Bradley WG. Demyelinating neuropathy in diabetes mellitus. Arch. Neurol. 2002;59:758–765. doi: 10.1001/archneur.59.5.758. [DOI] [PubMed] [Google Scholar]
- 69.Krendel DA, Costigan DA, Hopkins LC. Successful treatment of neuropathies in patients with diabetes mellitus. Arch. Neurol. 1995;52:1053–1061. doi: 10.1001/archneur.1995.00540350039015. [DOI] [PubMed] [Google Scholar]
- 70.Gorson KC, Ropper AH, Adelman LS, Weinberg DH. Influence of diabetes mellitus on chronic inflammatory demyelinating polyneuropathy. Muscle Nerve. 2000;23:37–43. doi: 10.1002/(sici)1097-4598(200001)23:1<37::aid-mus5>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 71.Razavi R, Chan Y, Afifiyan FN, Liu XJ, Wan X, Yantha J, Tsui H, Tang L, Tsai S, Santamaria P, Driver JP, Serreze D, Salter MW, Dosch HM. TRPV1+ sensory neurons control beta cell stress and islet inflammation in autoimmune diabetes. Cell. 2006;127:1123–1135. doi: 10.1016/j.cell.2006.10.038. [DOI] [PubMed] [Google Scholar]
- 72.Dalakas MC, Fujii M, Li M, McElroy B. The clinical spectrum of anti-AD antibody-positive patients with stiff-person syndrome. Neurol. 2000;55:1531–1535. doi: 10.1212/wnl.55.10.1531. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.