Abstract
Background
Chronic transplant dysfunction is characterized by renal function decline and proteinuria. Kidney injury molecule (KIM)-1, a transmembrane tubular protein with unknown function, is undetectable in normal kidneys, but markedly induced after injury. Urinary KIM-1 excretion has been quantified as biomarker of renal damage. We prospectively studied whether urinary KIM-1 predicts graft loss, independent of renal function and proteinuria.
Methods
Renal transplant recipients (n=145) visiting our outpatient clinic between August 2001 and July 2003 collected 24-hour urine samples for assessment of baseline urinary KIM-1 excretion (microsphere-based Luminex technology), and were followed for graft loss.
Results
Recipients participated at a median (interquartile range) of 6.0 (2.5–12.0) years posttransplant in baseline measurements. Follow-up beyond baseline was 4.0 (3.2–4.5) years. Urinary KIM-1 excretion was 0.72 (0.42–1.37) ng per 24 hours. Occurrence of graft loss increased over tertiles of KIM-1 excretion: 3 (6.3%), 11 (22.4%), and 17 cases (35.4%; P=0.001), respectively. High KIM-1 excretion was associated with proteinuria, low creatinine clearance, and high donor age (all P<0.01). In multivariate Cox regression analyses, prediction of graft loss by KIM-1 appeared independent of creatinine clearance, proteinuria, and donorage. Hazard ratios (95% CI) for the second and third tertile of KIM-1 excretion were 3.6 (0.9–13.5) and 5.1 (1.5–17.8) in the final model.
Conclusions
Urinary excretion of KIM-1 is an independent predictor of long-term graft loss and therefore a promising new biomarker in early prediction of graft loss.
Keywords: Chronic transplant dysfunction, Renal transplantation, Kidney injury molecule-1, Biomarker
Although the incidence of acute renal allograft rejection has decreased progressively during the last decades, there has been little improvement in long-term graft survival (1). Chronic transplant dysfunction (CTD), one of the leading causes of late allograft loss (2), is characterized clinically by a gradual decline in renal function with proteinuria and hypertension (3, 4). This is accompanied by histological changes, such as interstitial fibrosis, tubular atrophy, vascular occlusion, and glomerulosclerosis (5–7). Noninvasively, estimates of glomerular filtration rate (e.g., creatinine clearance or plasma creatinine) and proteinuria are used for identification of transplant recipients at increased risk for late renal allograft loss (8–11). However, once serum creatinine starts to rise or proteinuria develops, chronic structural lesions are already present and it is usually too late for intervention (12, 13). Thus, there is a great need for noninvasive markers of CTD that can predict graft loss in an earlier stage (14).
Kidney injury molecule-1 (KIM-1) is a recently discovered transmembrane protein with immunoglobulin (Ig)-like and mucin domains in its ectodomain, which is undetectable in normal kidneys but markedly upregulated in damaged tubular epithelial cells after different types of renal injury (15–19). KIM-1 ectodomain can be shed into the urine; it was previously shown that urinary excretion of KIM-1 relates to the extent of renal damage in experimental renal disease (19, 20) and in various inflammatory and fibrotic human renal diseases (21).
We hypothesized that urinary KIM-1 excretion predicts long-term graft loss in renal transplant patients. Therefore, in this prospective study we investigated whether urinary KIM-1 excretion is a predictor of graft loss independent of creatinine clearance and proteinuria in renal transplant recipients.
METHODS
Study Design and Patients
The current prospective study was part of a larger study and incorporated the Groningen Renal Transplant Outpatient Program, details of which have been published previously (22–24). Between August 2001 and July 2003, all adult renal allograft recipients who had a functioning graft for at least 1 year were eligible to participate. Patients with known or apparent systemic illnesses (e.g., malignancies or opportunistic infections) were considered ineligible. A total of 606 out of 847 (72%) eligible renal transplant recipients signed written informed consent. Mortality and graft loss were recorded for all renal transplant recipients until June 27, 2006. Graft loss was censored for death and defined as return to dialysis or retransplantation. For the current study, data processing and analyses are based on a case-cohort design: a random sample of the cohort (n=118) was enriched with remaining cases of graft loss (n=27) in the entire cohort. For patients who died with a functioning graft (n=15 in our study population), duration of follow-up was calculated until date of death. For patients with graft loss (n=31), duration of follow-up was calculated using date of start of dialysis or retransplantation.
The Institutional Review Board approved the study protocol (METc 01/039). Funding sources had neither a role in the collection and analyses of data, nor in publication of the manuscript.
Renal Transplant Characteristics
Relevant transplant characteristics were taken from the Groningen Renal Transplant Database. This database contains information on all renal transplantations performed at our center since 1968. Current medication was taken from the medical record. Standard immunosuppressive treatment was described previously (22–24).
Processing and Storage of Urinary Samples
After collection, fresh 24-hour urinary samples were centrifuged. An aliquot was used for routine measurements such as creatinine and protein, and was immediately stored (-80°C) until KIM-1 measurement.
Baseline Measurements
Body mass index and blood pressure were measured as described previously (23). Blood was drawn after an 8- to 12-hour overnight fasting period. Serum total cholesterol, high-density lipoprotein cholesterol (HDL), and non-HDL cholesterol were assessed as described previously (23, 24). Serum creatinine levels were determined using a modified version of the Jaffé method (MEGA AU 510, Merck Diagnostica, Darmstadt, Germany). Creatinine clearance was calculated from 24-hour urinary creatinine excretion and serum creatinine. Estimated glomerular filtration rate (eGFR) was calculated with the Modification of Diet in Renal Disease (MDRD) formula (25). Total urinary protein concentration was analyzed using the Biuret reaction (MEGA AU 510, Merck Diagnostica, Darmstadt, Germany) and proteinuria was defined as urinary protein excretion >0.5 g per 24 hours.
Measurement of Urinary KIM-1 Level
Urinary KIM-1 measurements were performed using microsphere-based Luminex xMAP technology with polyclonal antibodies raised against the human KIM-1 ectodomain, as described previously (26). This technique is an adaptation of the sandwich enzyme-linked immunosorbent assay, as described previously (16, 20). For measurements, 30 μL of urine samples were analyzed in duplicate. The lowest limit of detection for this assay is 0.125 ng/mL. The inter- and intra-assay variability is <10%. The urinary KIM-1 level was expressed in absolute terms (ng/mL). Urinary KIM-1 excretion was calculated by multiplication with the volume of the 24-hour urine collection, resulting in KIM-1 excretion expressed in ng per 24 hours.
Statistical Analyses
Analyses were performed with SPSS version 14.0 (SPSS Inc., Chicago, IL). Parametric variables are given as means±SD. Nonparametric variables are given as median (interquartile range). Subjects were divided in tertiles based on urinary KIM-1 excretion; differences between the tertiles were tested for statistical significance with one-way analysis of variance in case of a parametric variable or the Kruskal-Wallis test in case of a nonparametric variable; the chi-square test was used in case of a categorical variable. Kaplan-Meier survival analysis with log-rank testing was performed for prospective analysis of graft loss. We then proceeded with univariate and multivariate cox-regression analyses of urinary excretion of KIM-1, creatinine clearance, proteinuria, and other potential determinants of graft loss. In the final multivariate model, we included urinary excretion of KIM-1, donor age, creatinine clearance, and proteinuria. To further compare the predictive performance of urinary KIM-1 excretion with creatinine clearance, proteinuria, and donor age, we also generated receiver operator characteristic (ROC) curves. The areas under the ROC curve of KIM-1 and creatinine clearance, proteinuria, and donor age were compared nonparametrically by the method of DeLong et al. (27). A two-sided P value of <0.05 was considered to be statistically significant.
RESULTS
Recipients participated at a median (interquartile range) of 6.0 (2.5–12.0) years posttransplant in baseline measurements. Follow-up for graft loss beyond baseline was 4.0 (3.2–4.5) years. KIM-1 excretion was 0.72 (0.42–1.37) ng per 24 hours. Minimum and maximum values were 0.01 and 10.0 ng per 24 hours, respectively. Recipient-related baseline characteristics according to tertiles of KIM-1 excretion are shown in Table 1. There were no significant differences in recipient-related characteristics across the tertiles.
TABLE 1.
Recipient-related baseline parameters of the population subdivided by tertiles of KIM-1
| Tertiles of KIM-1 (ng/24 h) |
||||
|---|---|---|---|---|
| 0.30 (0.01–0.48) | 0.72 (0.49–1.09) | 1.69 (1.15–10.04) | P value | |
| N | 48 | 49 | 48 | |
| Recipient characteristics | ||||
| Tx until baseline (years) | 5.6 (2.5–11.6) | 6.1 (2.0–14.4) | 7.4 (3.0–11.2) | 0.85 |
| Dialysis prior Tx (months) | 28 (18–46) | 24 (11–33) | 30 (10–57) | 0.18 |
| Age (years) | 52.7±12.4 | 52.9±11.4 | 52.7±13.6 | 1.00 |
| Male gender, n (%) | 24 (50) | 30 (61) | 26 (54) | 0.53 |
| Body mass index (kg/m2) | 26.3±4.4 | 25.6±3.8 | 26.6±3.7 | 0.44 |
| Blood pressure | ||||
| MAP (mm Hg) | 110±13 | 112±14 | 116±14 | 0.13 |
| ACE inhibitor, n (%) | 14 (29) | 15 (31) | 22 (46) | 0.17 |
| β-blocker, n (%) | 34 (71) | 24 (49) | 25 (52) | 0.06 |
| Current smoking, n (%) | 12 (25.0) | 12 (24.5) | 14 (29.2) | 0.85 |
| Glycemia | ||||
| Fasting plasma glucose (mmol/L) | 4.8±1.3 | 4.9±1.2 | 5.0±2.0 | 0.85 |
| Posttransplant diabetes, n (%) | 13 (27) | 9 (18) | 7 (15) | 0.29 |
| Lipids | ||||
| Cholesterol (mmol/L) | 5.6 (5.2–6.0) | 5.5 (4.9–6.3) | 5.6 (4.8–6.7) | 0.90 |
| HDL cholesterol (mmol/L) | 1.2 (0.9–1.3) | 1.1 (0.9–1.3) | 1.0 (0.8–1.4) | 0.31 |
| Non-HDL cholesterol (mmol/L) | 4.4 (4.2–4.6) | 4.5 (4.1–4.8) | 4.6 (4.3–44.9) | 0.72 |
| Statin, n (%) | 26 (54) | 26 (53) | 23 (48) | 0.81 |
Parametric variables are expressed as mean±SD, whereas nonparametric variables are given as median (interquartile range), unless otherwise noted. Statistical analyses were performed with one-way analysis of variance or the Kruskal-Wallis test in case of a nonparametric variable. The χ2 test was used in case of a categorical variable.
Tx, transplantation; MAP, mean arterial pressure; ACE, angiotensin-converting enzyme; HDL, high-density lipoprotein.
Transplant-related characteristics are shown in Table 2. Donor age, serum creatinine, and proteinuria increased significantly with increasing KIM-1 excretion. Creatinine clearance, eGFR, and duration of follow-up beyond baseline measurements decreased significantly with increasing KIM-1 excretion. Occurrence of graft loss resulting in return to dialysis or retransplantation (death-censored graft loss) increased significantly with increasing urinary KIM-1 excretion at baseline: 3 (6.3%), 11 (22.4%), and 17 (35.4%) for the consecutive tertiles, respectively (P=0.001). A corresponding Kaplan-Meier curve for the tertiles of urinary KIM-1 excretion is shown in Figure 1.
TABLE 2.
Transplant-related parameters of the population subdivided by tertiles of KIM-1
| Tertiles of KIM-1 (ng/24 h) |
||||
|---|---|---|---|---|
| 0.30 (0.01–0.48) | 0.72 (0.49–1.09) | 1.69 (1.15–10.04) | P value | |
| N | 48 | 49 | 48 | |
| Donor characteristics | ||||
| Age (years) | 34.3±15.2 | 37.5±15.5 | 44.4±16.0 | 0.006 |
| Male gender, n (%) | 28 (58) | 30 (64) | 21 (44) | 0.13 |
| Deceased donor transplant, n (%) | 42 (88) | 43 (88) | 40 (83) | 0.78 |
| Time | ||||
| Warm ischemia time (min) | 36.0 (32.0–44.5) | 36.0 (30.0–45.0) | 35.0 (30.0–48.8) | 0.86 |
| Cold ischemia time (h) | 20.0 (13.5–26.8) | 22.0 (14.0–25.5) | 22.5 (10.3–27.0) | 0.80 |
| Acute rejection treatment | ||||
| High dose corticosteroids, n (%) | 13 (27) | 20 (41) | 17 (35) | 0.36 |
| Other rejection therapy, n (%) | 4 (8) | 8 (16) | 3 (6) | 0.23 |
| Immunosuppression | ||||
| Prednisolone dose (mg/day) | 10 (7.5–10.0) | 10 (7.5–10) | 10 (7.5–10) | 0.90 |
| Cyclosporine, n (%) | 29 (60) | 23 (47) | 31 (65) | 0.19 |
| Tacrolimus, n (%) | 8 (17) | 14 (29) | 6 (13) | 0.11 |
| Mycophenolate mofetil, n (%) | 23 (48) | 18 (37) | 15 (31) | 0.23 |
| Azathioprine, n (%) | 15 (31) | 18 (37) | 18 (38) | 0.78 |
| Renal allograft function | ||||
| Serum creatinine (μmol/L) | 138±60 | 157±65 | 195±86 | <0.001 |
| Creatinine clearance (mL/min) | 64±22 | 60±24 | 49±21 | 0.004 |
| eGFR (mL/min) | 49.1±14.3 | 44.7±15.8 | 35.7±15.3 | <0.001 |
| Proteinuria (g/24 h) | 0.1 (0.0–0.3) | 0.2 (0.0–0.5) | 0.5 (0.2–1.1) | <0.001 |
| Proteinuria, n (%) | 8 (17) | 12 (25) | 24 (50) | 0.001 |
| Follow up since baseline (years) | 4.2 (3.6–4.5) | 3.8 (3.0–4.3) | 3.7 (2.1–4.5) | 0.03 |
| Death-censored graft loss, n (%) | 3 (6.3) | 11 (22.4) | 17 (35.4) | 0.001 |
Parametric variables are expressed as mean±SD, whereas nonparametric variables are given as median (interquartile range), unless otherwise noted. Statistical analyses were performed with one-way analysis of variance or the Kruskal-Wallis test in case of a nonparametric variable. The χ2 test was used in case of a categorical variable.
FIGURE 1.
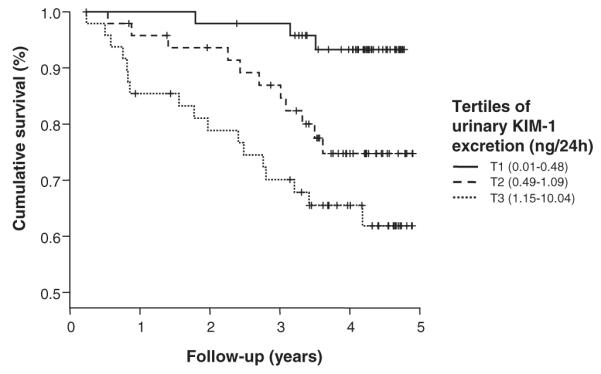
Kaplan-Meier survival curve of tertiles of urinary KIM-1 excretion (in ng/24 hr) at baseline for death-censored graft survival. P=0.001 according to log-rank test.
We subsequently investigated whether KIM-1 excretion is an independent predictor of graft loss (Table 3). After adjustment for donor age and creatinine clearance, hazard ratios of the second and third tertiles of KIM-1 excretion for graft loss decreased, but remained significant. Further adjustment for proteinuria in addition to donor age and creatinine clearance did not materially change the results. Hazard ratios for graft loss of the KIM-1 tertiles in the final model were 3.6 (0.9–13.5) and 5.1 (1.5–17.8), with P=0.06 and P=0.01, respectively. These hazard ratios are comparable to hazard ratios for proteinuria: 3.1 (1.0–9.2, P=0.04) and 6.0 (2.3–15.8, P<0.001) for urinary protein excretion >0.5 or ≤1 g per 24 hours and >1 g per 24 hours, respectively, in the final model. Adjustment for eGFR and serum creatinine instead of creatinine clearance did not materially change the results. In univariate analyses, donor age was a significant predictor of graft loss, but this association was no longer significant after adjustment for creatinine clearance, serum creatinine, or eGFR in the Cox-regression models (data not shown). Subsequently, we investigated whether the relationship between KIM-1 excretion and graft survival persists if analyzed in relation to time since transplantation rather than time since baseline measurements. We first investigated whether KIM-1 excretion at baseline is related to time since transplantation. It appeared that there is no significant correlation between KIM-1 excretion and time since transplantation (r=0.05; P=0.53). Also, the relationship between KIM-1 excretion and graft survival persists if analyzed in relation to time since transplantation rather than time since baseline measurements.
TABLE 3.
Univariate and multivariate Cox regression analyses of determinants of death-censored graft loss in renal transplant recipients
| Unadjusted model |
Adjusted for donor age |
Adjusted for donor age and creatinine clearance |
Adjusted for donor age, creatinine clearance and proteinuria |
|||||
|---|---|---|---|---|---|---|---|---|
| KIM-1 tertile | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value |
| I | 1.0 | 1.0 | 1.0 | 1.0 | ||||
| II | 4.2 (1.2–15.0) | 0.03 | 3.9 (1.1–13.9) | 0.04 | 3.2 (0.9–11.8) | 0.08 | 3.6 (0.9–13.5) | 0.06 |
| III | 7.2 (2.1–24.6) | 0.002 | 5.9 (1.7–20.7) | 0.005 | 4.3 (1.2–14.9) | 0.02 | 5.1 (1.5–17.8) | 0.01 |
Proteinuria was divided into three groups: I,≤0.5 g/24; II, >0.5 and ≤1.0 g/24; III, >1.0 g/24 h.
In addition, we performed a multivariate analysis with continuous variables. In this model creatinine clearance (hazard ratio [HR]=0.92 per mL/min, P<0.001), proteinuria (HR=1.34 per g/24h, P=0.0001) and KIM-1 (HR=1.31 per ng/24h, P=0.02) appeared independent predictors of graft loss. Donor age (HR=1.01 per year, P=0.40) was not an independent predictor of graft loss in this model. These results for continuous variables did not materially differ from the analyses with categorical variables.
Finally, we compared the predictive performance of urinary KIM-1 excretion with creatinine clearance, proteinuria, and donor age by generating ROC curves (Fig. 2). ROC analysis of prediction of graft loss by KIM-1 excretion revealed a mean (SE) area under the curve of 0.71 (0.05; P<0.001). The area under the curve for creatinine clearance was 0.89 (0.03; P<0.001), which was significantly higher than for KIM-1 (P=0.002 for creatinine clearance versus KIM-1). The area under the curve for proteinuria was 0.82 (0.05; P<0.001) and for donor age 0.65 (0.05; P<0.001). Proteinuria and donor age were equal predictors of graft loss when compared to KIM-1 (P=NS).
FIGURE 2.
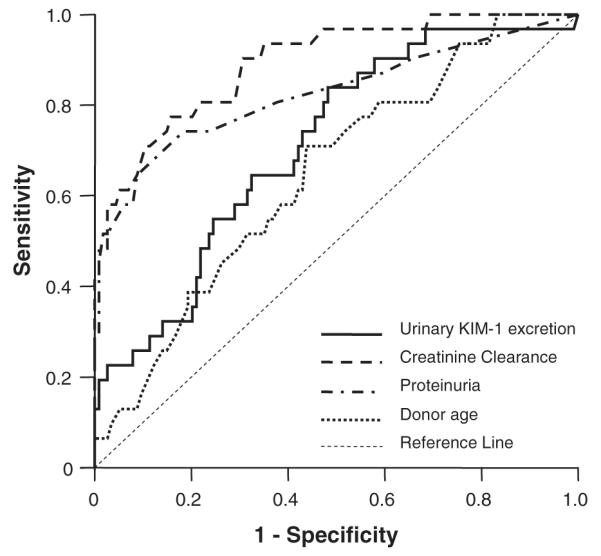
Areas under the receiver operating curves (ROC) with standard error (SE) of KIM-1, creatinine clearance, proteinuria and donor age at baseline for graft loss were: 0.71 (0.05; P<0.001), 0.89 (0.03; P<0.001), 0.82 (0.05; P<0.001), and 0.65 (0.05; P<0.001), respectively. The areas under the ROC curve were compared nonparametrically by the method of DeLong et al (27). The area under the curve for creatinine clearance was significantly higher than for KIM-1 (P=0.002), for proteinuria and donor age this did not differ (P=ns).
DISCUSSION
In this longitudinal prospective study, we investigated whether urinary KIM-1 excretion could be of use in the prediction of graft loss in renal transplant recipients in addition to established markers as creatinine clearance and proteinuria. We found that high KIM-1 excretion is a predictor of graft loss, independent of donor age, creatinine clearance, and proteinuria, with hazard ratios of similar magnitude as proteinuria.
To the best of our knowledge, this study is the first prospective study on urinary KIM-1 excretion as potential predictor of graft loss in renal transplant recipients. The only other prospective study concerning KIM-1 to date is by Liangos et al. (26). They have shown in a cohort of 201 hospitalized patients with established acute renal failure that urinary KIM-1 excretion was predictive for adverse clinical outcomes, such that patients in the highest KIM-1 quartile had 3.2-fold higher odds for dialysis requirement or hospital death than patients in the lowest quartile.
For the association between KIM-1 and progressive decline in renal function, several potential explanations are possible. KIM-1 is a type 1 transmembrane glycoprotein that is expressed in humans and rodents when injured renal tubules adopt a dedifferentiated phenotype (17, 21). Expression of KIM-1 is also found in renal tumor cells and in tubular cells adjacent to carcinoma cells; these cells may be compressed by the tumor cells and consequently adopt a dedifferentiated phenotype (28, 29). Cleavage of the KIM-1 ectodomain by matrix metalloproteinases results in KIM-1 excretion into the urine (30). Urinary excretion of KIM-1 is therefore a reflection of the degree of histopathological tubular damage that exists at a certain timepoint (19–21). Since renal damage and tubular dedifferentiation have been shown to predict renal function decline and graft loss (31, 32), KIM-1 may be a noninvasively assessable marker of these two entities.
Currently, the function of KIM-1 is unclear. Based on its protein structure, with Ig- and mucin-domains in the extracellular part, and its close resemblance to adhesion molecules, KIM-1 could function in cel/cel or cel/matrix interactions. KIM-1 is also known as T-cell immunoglobulin mucin-like domain 1 (TIM-1), and as such it is expressed on T cells. Meyers et al. (33) have demonstrated that Tim-4, which is exclusively expressed on antigen-presenting cells in the mouse, is the natural ligand for Tim-1. This suggests that KIM-1 can interact with other proteins. KIM-1 expression is induced after different types of renal injury, but we can only speculate whether tubular KIM-1 expression is a harmful or protective mechanism. From ischemia/reperfusion studies, it is known that KIM-1 expression persists during the first recovery phase, indicating that KIM-1 might not always be associated with a deleterious outcome. Furthermore, when renal damage is reduced with renoprotective interventions, KIM-1 expression is also reduced (34, 35).
In our Cox regression analysis model, we included—besides donor age, proteinuria, and KIM-1—creatinine clearance, which was also an independent predictor of graft loss. However, as 24-hour urine samples are not readily available in many clinics and/or studies, we also tested the regression model with the eGFR (calculated with the MDRD formula) that can be calculated from serum creatinine. When substituting creatinine clearance with eGFR, KIM-1 was still an independent predictor of graft loss.
Donor age is a commonly accepted predictor of renal function decline, and also in our study we found that higher KIM-1 levels were associated with a higher donor age. However, donor age did not independently predict graft loss in the multivariate analysis. Renal function, such as creatinine clearance, is highly associated with age; because renal function is a strong risk factor for graft failure, this may explain why donor age did not significantly contribute to the multivariate model for prediction of graft loss. This is in concordance with other studies showing that donor age alone is not a risk factor for worse long-term outcomes, as long as there was no history of hypertension and kidney function was normal (36–38).
The present study has some limitations. First, the present study is a single-center study and the predictive value of KIM-1 excretion needs to be confirmed in other centra and/or multicenter studies. Second, the renal transplant recipients were included at different timepoints after transplantation; this could possibly induce a healthy survivor bias and therefore it is advisable to study the predictive value of urinary KIM-1 excretion on graft survival at a fixed timepoint (e.g., 1 year) after transplantation. Furthermore, urinary KIM-1 excretion should be analyzed in relation to the histological diagnosis. Also in our study, creatinine clearance, proteinuria, and KIM-1 excretion were assessed from samples taken at one timepoint in each patient. It would be interesting to investigate in a future study whether sequential measurements of KIM-1 excretion could be used as an even earlier marker, and can be used to predict development of proteinuria and rises in plasma creatinine.
In conclusion, elevated urinary levels of KIM-1 independently predict long-term graft loss in renal transplant recipients. Therefore, measuring urinary KIM-1 excretion is of additional value, next to measurement of creatinine clearance and proteinuria, for identification of subjects at risk for renal allograft failure who may benefit from intervention.
Acknowledgments
This work was supported by grants from the Dutch Kidney Foundation (Nierstichting Nederland C00.1877), the Netherlands Organization for Scientific Research (NWO-AGIKO 920-03-181), the Graduate School Groningen University Institute for Drug Exploration (GUIDE), and the J. K. de Cock Foundation (Groningen, the Netherlands).
REFERENCES
- 1.Tantravahi J, Womer KL, Kaplan B. Why hasn’t eliminating acute rejection improved graft survival? Annu Rev Med. 2007;58:369. doi: 10.1146/annurev.med.58.061705.145143. [DOI] [PubMed] [Google Scholar]
- 2.Kreis HA, Ponticelli C. Causes of late renal allograft loss: Chronic allograft dysfunction, death, and other factors. Transplantation. 2001;71:SS5–SS9. [PubMed] [Google Scholar]
- 3.Massy ZA, Guijarro C, Wiederkehr MR, et al. Chronic renal allograft rejection: Immunologic and nonimmunologic risk factors. Kidney Int. 1996;49:518. doi: 10.1038/ki.1996.74. [DOI] [PubMed] [Google Scholar]
- 4.Joosten SA, Sijpkens YW, van Kooten C, Paul LC. Chronic renal allograft rejection: Pathophysiologic considerations. Kidney Int. 2005;68:1. doi: 10.1111/j.1523-1755.2005.00376.x. [DOI] [PubMed] [Google Scholar]
- 5.Nankivell BJ, Borrows RJ, Fung CL, et al. The natural history of chronic allograft nephropathy. N Engl J Med. 2003;349:2326. doi: 10.1056/NEJMoa020009. [DOI] [PubMed] [Google Scholar]
- 6.Racusen LC, Solez K, Colvin RB, et al. The Banff 97 working classification of renal allograft pathology. Kidney Int. 1999;55:713. doi: 10.1046/j.1523-1755.1999.00299.x. [DOI] [PubMed] [Google Scholar]
- 7.Solez K, Axelsen RA, Benediktsson H, et al. International standardization of criteria for the histologic diagnosis of renal allograft rejection: The Banff working classification of kidney transplant pathology. Kidney Int. 1993;44:411. doi: 10.1038/ki.1993.259. [DOI] [PubMed] [Google Scholar]
- 8.Lachenbruch PA, Rosenberg AS, Bonvini E, et al. Biomarkers and surrogate endpoints in renal transplantation: Present status and considerations for clinical trial design. Am J Transplant. 2004;4:451. doi: 10.1111/j.1600-6143.2004.00386.x. [DOI] [PubMed] [Google Scholar]
- 9.Kaplan B, Schold J, Meier-Kriesche HU. Poor predictive value of serum creatinine for renal allograft loss. Am J Transplant. 2003;3:1560. doi: 10.1046/j.1600-6135.2003.00275.x. [DOI] [PubMed] [Google Scholar]
- 10.Kasiske BL, Andany MA, Danielson B. A thirty percent chronic decline in inverse serum creatinine is an excellent predictor of late renal allograft failure. Am J Kidney Dis. 2002;39:762. doi: 10.1053/ajkd.2002.31996. [DOI] [PubMed] [Google Scholar]
- 11.Roodnat JI, Mulder PG, Rischen-Vos J, et al. Proteinuria after renal transplantation affects not only graft survival but also patient survival. Transplantation. 2001;72:438. doi: 10.1097/00007890-200108150-00014. [DOI] [PubMed] [Google Scholar]
- 12.Merville P. Combating chronic renal allograft dysfunction: Optimal immunosuppressive regimens. Drugs. 2005;65:615. doi: 10.2165/00003495-200565050-00004. [DOI] [PubMed] [Google Scholar]
- 13.Chapman JR, O’Connell PJ, Nankivell BJ. Chronic renal allograft dysfunction. J Am Soc Nephrol. 2005;16:3015. doi: 10.1681/ASN.2005050463. [DOI] [PubMed] [Google Scholar]
- 14.Marsden PA. Predicting outcomes after renal transplantation—new tools and old tools. N Engl J Med. 2003;349:182. doi: 10.1056/NEJMe030096. [DOI] [PubMed] [Google Scholar]
- 15.Ichimura T, Hung CC, Yang SA, et al. Kidney Injury Molecule —1 (Kim-1): A tissue and urinary biomarker for nephrotoxicant-induced renal injury. Am J Physiol Renal Physiol. 2003;286:F552. doi: 10.1152/ajprenal.00285.2002. [DOI] [PubMed] [Google Scholar]
- 16.Han WK, Bailly V, Abichandani R, et al. Kidney Injury Molecule-1 (KIM-1): A novel biomarker for human renal proximal tubule injury. Kidney Int. 2002;62:237. doi: 10.1046/j.1523-1755.2002.00433.x. [DOI] [PubMed] [Google Scholar]
- 17.Ichimura T, Bonventre JV, Bailly V, et al. Kidney injury molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. J Biol Chem. 1998;273:4135. doi: 10.1074/jbc.273.7.4135. [DOI] [PubMed] [Google Scholar]
- 18.Kuehn EW, Park KM, Somlo S, Bonventre JV. Kidney injury molecule-1 expression in murine polycystic kidney disease. Am J Physiol Renal Physiol. 2002;283:F1326. doi: 10.1152/ajprenal.00166.2002. [DOI] [PubMed] [Google Scholar]
- 19.Van Timmeren MM, Bakker SJ, Vaidya VS, et al. Tubular kidney injury molecule-1 in protein-overload nephropathy. Am J Physiol Renal Physiol. 2006;291:F456. doi: 10.1152/ajprenal.00403.2005. [DOI] [PubMed] [Google Scholar]
- 20.Vaidya VS, Ramirez V, Ichimura T, et al. Urinary kidney injury molecule-1: A sensitive quantitative biomarker for early detection of kidney tubular injury. Am J Physiol Renal Physiol. 2006;290:F517. doi: 10.1152/ajprenal.00291.2005. [DOI] [PubMed] [Google Scholar]
- 21.Van Timmeren MM, van den Heuvel MC, Bailly V, et al. Tubular kidney injury molecule-1 (KIM-1) in human renal disease. J Pathol. 2007;212:209. doi: 10.1002/path.2175. [DOI] [PubMed] [Google Scholar]
- 22.de Vries AP, Bakker SJ, van Son WJ, et al. Metabolic syndrome is associated with impaired long-term renal allograft function; Not all component criteria contribute equally. Am J Transplant. 2004;4:1675. doi: 10.1111/j.1600-6143.2004.00558.x. [DOI] [PubMed] [Google Scholar]
- 23.van Ree RM, Oterdoom LH, de Vries AP, et al. Elevated levels of Creactive protein independently predict accelerated deterioration of graft function in renal transplant recipients. Nephrol Dial Transplant. 2007;22:246. doi: 10.1093/ndt/gfl511. [DOI] [PubMed] [Google Scholar]
- 24.de Vries AP, van Son WJ, van der Heide JJ, et al. The predictive value of renal vascular resistance for late renal allograft loss. Am J Transplant. 2006;6:364. doi: 10.1111/j.1600-6143.2005.01192.x. [DOI] [PubMed] [Google Scholar]
- 25.Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 26.Liangos O, Perianayagam MC, Vaidya VS, et al. Urinary N-acetyl-beta-(D)-glucosaminidase activity and kidney injury molecule-1 level are associated with adverse outcomes in acute renal failure. J Am Soc Nephrol. 2007;18:904. doi: 10.1681/ASN.2006030221. [DOI] [PubMed] [Google Scholar]
- 27.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics. 1988;44:837. [PubMed] [Google Scholar]
- 28.Han WK, Alinani A, Wu CL, et al. Human kidney injury molecule-1 is a tissue and urinary tumor marker of renal cell carcinoma. J Am Soc Nephrol. 2005;16:1126. doi: 10.1681/ASN.2004070530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin F, Zhang PL, Yang XJ, et al. Human kidney injury molecule-1 (hKIM-1): A useful immunohistochemical marker for diagnosing renal cell carcinoma and ovarian clear cell carcinoma. Am J Surg Pathol. 2007;31:371. doi: 10.1097/01.pas.0000213353.95508.67. [DOI] [PubMed] [Google Scholar]
- 30.Bailly V, Zhang Z, Meier W, et al. Shedding of kidney injury molecule-1, a putative adhesion protein involved in renal regeneration. J Biol Chem. 2002;277:39739. doi: 10.1074/jbc.M200562200. [DOI] [PubMed] [Google Scholar]
- 31.Muramatsu M, Miyagi M, Ishikawa Y, et al. Estimation of damaged tubular epithelium in renal allografts by determination of vimentin expression. Int J Urol. 2004;11:954. doi: 10.1111/j.1442-2042.2004.00938.x. [DOI] [PubMed] [Google Scholar]
- 32.Grimm PC, Nickerson P, Gough J, et al. Quantitation of allograft fibrosis and chronic allograft nephropathy. Pediatr Transplant. 1999;3:257. doi: 10.1034/j.1399-3046.1999.00044.x. [DOI] [PubMed] [Google Scholar]
- 33.Meyers JH, Chakravarti S, Schlesinger D, et al. TIM-4 is the ligand for TIM-1, and the TIM-1-TIM-4 interaction regulates T cell proliferation. Nat Immunol. 2005;6:455. doi: 10.1038/ni1185. [DOI] [PubMed] [Google Scholar]
- 34.De Borst MH, Van Timmeren MM, Vaidya VS, et al. Induction of kidney injury molecule-1 in homozygous Ren2 rats is attenuated by blockade of the renin-angiotensin system or p38 MAP kinase. Am J Physiol Renal Physiol. 2007;292:F313. doi: 10.1152/ajprenal.00180.2006. [DOI] [PubMed] [Google Scholar]
- 35.Kramer AB, Van Timmeren MM, Schuurs TA, et al. De novo induction of Kidney Injury Molecule-1 (Kim-1) in proteinuria-induced tubular injury is attenuated by RAS blockade [abstract] J Am Soc Nephrol. 2005;16:430A. [Google Scholar]
- 36.Oien CM, Reisaeter AV, Leivestad T, et al. Living donor kidney transplantation: The effects of donor age and gender on short- and long-term outcomes. Transplantation. 2007;83:600. doi: 10.1097/01.tp.0000255583.34329.dd. [DOI] [PubMed] [Google Scholar]
- 37.Pessione F, Cohen S, Durand D, et al. Multivariate analysis of donor risk factors for graft survival in kidney transplantation. Transplantation. 2003;75:361. doi: 10.1097/01.TP.0000044171.97375.61. [DOI] [PubMed] [Google Scholar]
- 38.Cosio FG, Henry M, Pesavento TE, et al. The relationship between donor age and cadaveric renal allograft survival is modified by the recipient’s blood pressure. Am J Transplant. 2003;3:340. doi: 10.1034/j.1600-6143.2003.00064.x. [DOI] [PubMed] [Google Scholar]


