Abstract
Recent studies have identified adaptations of intracellular signaling pathways and target genes that could contribute or modulate the action of antidepressant drugs, as well as exercise-mediated antidepressant responses. Understanding these adaptations, particularly those changes that are common to diverse antidepressant treatments, is important for the development of more potent and specific treatments of depression. There is growing evidence that growth factors may be important mediators of antidepressant responses. Now, VGF (not an acronym), a neuropeptide that has previously been shown to be involved in maintaining organismal energy balance, as well as in mediating hippocampal synaptic plasticity, may be involved in mediating antidepressant responses. These studies use in vivo approaches to link VGF to the antidepressant-like behavioral effects produced by antidepressant drugs and exercise.
Mood disorders are prevalent forms of mental illness. Major depression is one of the most common mental illnesses that affects up to 20% of the general U.S. population (1–3). Major depression is characterized by anhedonia, which is the inability to experience pleasure from normally pleasurable events, alterations in cognition, intense feelings of sadness and despair, agitation, and self-deprecation, as well as abnormalities in psychomotor activity as set forth in the Diagnostic and Statistical Manual of Mental Disorders (4).
The first antidepressant, iproniazid, was originally developed in the early 1950s to treat tuberculosis when it was discovered to have mood-elevating effects in patients (5). Subsequent research showed that iproniazid inhibits an enzyme that breaks down monoamine neurotransmitters, such as serotonin and norepinephrine, resulting in an increase of these neurotransmitters in the synapse. This discovery influenced the field of depression for several decades and was the basis for the monoamine hypothesis of depression, which suggests that depression arises from a decrease of monoamine neurotransmission, particularly serotonin and norepinephrine. Thus, most treatments for depression, including the latest generation of antidepressants, have focused on elevating these two neurotransmitters in the brain. These diverse treatments include serotonin and norepinephrine reuptake inhibitors, monoamine oxidase inhibitors (which block degradation of monoamines and includes iproniazid), and tricyclic antidepressants (which inhibit the reuptake of monamines by neurons).
Despite the wide use of these compounds, up to one-third of patients are treatment-resistant and do not achieve clinical efficacy with these drugs (6, 7). Further, direct evidence for the monoamine hypothesis of depression has not been consistent. In particular, although changes in neurotransmission occur rather quickly following antidepressant administration, the clinical effects may take 3 to 6 weeks to manifest. This delay between the start of antidepressant treatment and clinical efficacy suggests that long-term alterations in brain function mediate the therapeutic benefit of these treatments (8, 9). Understanding these adaptations, particularly those changes that are common to diverse antidepressant treatment, is important for the development of more potent and specific treatments of depression.
Signaling Pathways Implicated in Antidepressant Responses
Recent studies have identified intracellular signaling proteins and target genes that could contribute to or modulate antidepressant action, as well as exercise-mediated antidepressant responses (9). To this end, converging lines of evidence have implicated neurotrophic factors, most notably brain-derived neurotrophic factor (BDNF), as a putative mediator of antidepressant responses, along with the MEK-ERK (mitogen-activated protein kinase kinase and its target extraceullular signal-regulated protein kinase) signaling pathway (10–13). Several studies have also suggested the involvement of other growth factors, such as IGF-1 (insulin-like growth factor–1), VEGF (vascular endothelial growth factor), and FGF2 (fibroblast growth factor 2), as important mediators of antidepressant responses (14). The potential involvement of multiple different growth factors in mediating antidepressant responses suggests that these factors, through their trophic actions and activation of signaling pathways, may be correcting cellular alteration produced in the disease state.
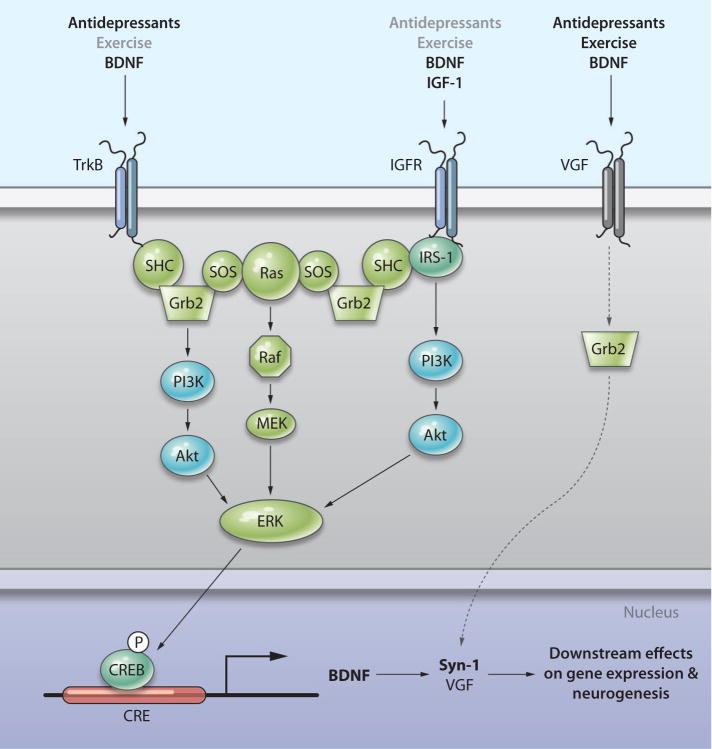
It is intriguing that some of the growth factors, such as BDNF and IGF-1, share common and overlapping features with exercise-mediated effects on signal transduction pathways (Fig. 1) (15, 16). This suggests that certain signaling pathways common to these neurotrophins or exercise may provide novel targets for the development of more potent and specific treatments of depression. Two studies suggest that VGF (not an acronym), a neuropeptide, which is encoded by a gene that is responsive to BDNF and exercise, may be an important mediator of antidepressant responses (17, 18).
Fig. 1.
Converging pathways underlie the actions of antidepressants and exercise. Multiple classes of antidepressant compounds stimulate the expression of the gene encoding BDNF, which activates the receptor TrkB, which is coupled to the MEK-ERK pathway and the PI3 kinase pathway. Antidepressants have also been shown to increase expression of the gene encoding IGF, which signals primarily though the IGF-1 receptor (IGF-1 R), and activates both the MEK-ERK pathway and PI3K pathway. Exercise also activates the MEK-ERK and PI3K pathways and increases the expression of the genes encoding BDNF and IGF-1. The hypothesized pathway of VGF is shown in gray arrows. Exercise stimulates expression of the gene encoding VGF, and both exercise and VGF result in increased expression of the genes encoding Grb2 and synapsin-1 (Syn-1), as well as (putatively) the MEK-ERK pathway. Akt and Raf, serine-threonine protein kinases; SHC, adaptor protein; SOS, a guanine nucleotide exchange factor; Ras, a small guanosine triphosphatase.
Discovery of VGF as a Mediator of Antidepressant Responses
A putative role for VGF in mediating antidepressant actions was independently discovered by both research groups through expression profiling in microarray studies. Hunsberger et al. (17) examined changes in gene expression in hippocampal samples of rats after exercise, whereas Thakker-Varia et al. (18) focused on common alterations in gene expression in hippocampal neuronal cultures following treatment with BDNF or 5-HT (5-hydroxytryptamine, also known as serotonin). Although one group was identifying downstream targets of exercise, and the other was focused on gene expression changes that were common following elevation of two putative mediators of antidepressant efficacy, the identification of VGF by both suggests a point of convergence between exercise and antidepressant signaling. Note that VGF is widely expressed in neurons in the brain and is involved in maintaining organismal energy balance, as well as in mediating hippocampal synaptic activity (19–21).
There is evidence that VGF is both an upstream regulator and a downstream target of the MEK-ERK signaling pathway. Hunsberger et al. found that either exercise or VGF stimulation of cells increases the expression of several genes, such as those encoding Egr2, Grb2, and Syn-1, which are part of the growth factor–stimulated MEK-ERK signaling pathway. Expression of these genes is stimulated both in vitro in PC 12 cells treated with VGF and in vivo in hippocampus after exercise. Therefore, Hunsberger et al. hypothesize that the MEK-ERK signaling cascade may be the common signaling pathway activated by both VGF and exercise. In contrast, Thakker-Varia’s discovery of the gene encoding VGF as a target of BDNF and, further, their finding that the antidepressant imipramine increases VGF protein in vivo suggest the involvement of the phosphatidylinositol 3-kinase (PI3K)-Akt pathway, the MEK-ERK pathway, or both.
VGF as a Mediator of Antidepressant Responses
To investigate whether VGF has antidepressant-like effects in vivo, both groups examined the behavioral effect of this neuropeptide in rodent models of depression. Local infusions of VGF into the midbrain or the hippocampus produced antidepressant effects in the learned helplessness paradigm, the tail suspension test, and forced swim test, which are three animal models of depression-like behavior that are used to measure antidepressant action (17, 18). In these paradigms, a rodent is exposed to an inescapable stress and its ability to attempt to escape is determined. VGF infusions into the brain altered the behavior of the animals similar to effects observed following exercise or administration of antidepressants.
To further explore the link between exercise and VGF, Hunsberger et al. examined heterozygous VGF+/− mice in the forced swim test. The VGF+/− mice displayed alterations in behavior that are suggestive of an increase in depression-like behavior. The VGF+/− mice and wild-type littermate controls were then subjected to an exercise paradigm. In the wild-type control animals, exercise increased VGF in the hippocampus, and these animals demonstrated an antidepressant-like response in the forced swim test. The heterozygous mice also demonstrated an increase in VGF after exercise, although this did not reach the level seen in the wild-type mice. Despite the increase in VGF in the heterozygous mice, there was no behavioral improvement in the forced swim test. Collectively, these results suggest that an exercise-induced increase in VGF per se is not sufficient to produce antidepressant-like effects but rather an intact VGF system may be necessary, or there may be a minimum threshold of VGF that is required, to mediate the exercise-induced antidepressant effect.
To elucidate the mechanism by which VGF may be mediating antidepressant-like effects, Thakker-Varia et al. investigated VGF effects on hippocampal cell proliferation and neurogenesis. Previous work suggested that clinically effective antidepressants increase hippocampal neurogenesis by increasing the number of newly born cells in the dentate gyrus, the majority of which mature into neurons (22). Multiple classes of antidepressant compounds, as well as nonpharmacological antidepressant therapies, such as electroconvulsive shock and exercise, increase neurogenesis, and it is thought that increasing neurogenesis may be a common mechanism of action by which antidepressant produce their therapeutic effects (11, 22). Hippocampal neurogenesis is increased after long-term but not short-term treatment with chemical antidepressants. Thakker-Varia et al. found that, in agreement with the antidepressant-like behavioral effects of VGF, this neuropeptide increases hippocampal neurogenesis, both in vivo and in vitro. Although the link between depression and neurogenesis is still an active area of investigation, the VGF-induced increase in cell proliferation may be one way that it produces the antidepressant effects. It is interesting to note that the effects of VGF on hippocampal neurogenesis were primarily on cellular differentiation, whereas the effects of BDNF, which regulates expression of the gene encoding VGF, have been suggested to be due to changes in cellular survival rather then differentiation (23).
Although these studies provide evidence that the gene encoding VGF may be an important target of exercise, as well as 5-HT and BDNF, both putative mediators of antidepressant actions, the actual link with chemical antidepressants is somewhat unclear. In one study, chronic imipramine, a tricyclic antidepressant, was shown to increase expression of the gene VGF in the hippocampus (18). In contrast, in the other study, expression of the gene encoding VGF was unaltered following administration of different chemical classes of antidepressants, including selective serotonin and norepinephrine reuptake inhibitors (17). Collectively, these data suggest that although some chemical antidepressants may stimulate expression of the VGF gene, it is not a common mechanism of action for chemical antidepressants in general. Thus, there may be additional ways that antidepressants can influence VGF signaling besides stimulation of the gene.
The studies by Hunsberger et al. and Thakker-Varia et al. suggest that VGF may be an important regulator of antidepressant responses. However, one caveat to these studies is the time course of drug administration used in these behavioral models compared to the time necessary to achieve clinical effects of these drugs in patients (24). As stated above, the therapeutic effects of compounds require long-term (3 to 6 weeks) treatment before clinical efficacy is seen, which is thought to be related to the time needed for stimulation of gene targets and subsequent changes in synaptic activity. However, the majority of preclinical depression tests, as well as the tests used in these studies, measure a single behavioral end point after 1 or 2 days of drug administration. Although these preclinical behavioral tests have been validated as “predictive” models of antidepressant action, these tests have limitations as true models for depression or antidepressant action. Therefore, it will be important in future studies to delineate the role of VGF in mediating antidepressant responses in behavioral models that are sensitive to the long-term effects of antidepressant action. It will also be important to delineate the pathway by which VGF is mediating its effect on antidepressant responses. In particular, because VGF is a downstream target of BDNF, why is the gene encoding VGF not regulated by the chemical antidepressants that stimulate BDNF activity?
It is clear from this discussion that a better understanding of the common signaling pathways in the brain that underlie mood disorders and mediate the responses to diverse antidepressant treatments are important areas for research into depression. These advances will hopefully result in the development of more rapid and effective treatments for depression.
References and Notes
- 1.Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, Rush AJ, Walters EE, Wang PS. The epidemiology of major depressive disorder: Results from the National Comorbidity Survey Replication (NCS-R) JAMA. 2003;289:3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- 2.Manji HK, Drevets WC, Charney DS. The cellular neurobiology of depression. Nat Med. 2001;7:541–547. doi: 10.1038/87865. [DOI] [PubMed] [Google Scholar]
- 3.Nestler EJ, Barrot M, Dileone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron. 2002;34:13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- 4.Diagnostic and Statistical Manual of Mental Disorders. 4. American Psychiatric Publishing, Inc; Washington, DC: 2000. Text Revision (DSM-IV-TR) [Google Scholar]
- 5.Kline NS. Clinical experience with iproniazid (Marsilid) J Clin Exp Psychopathol. 1958;19:72–78. [PubMed] [Google Scholar]
- 6.Rosenzweig-Lipson S, Beyer CE, Hughes ZA, Khawaja X, Rajarao SJ, Malberg JE, Rahman Z, Ring RH, Schechter LE. Differentiating antidepressants of the future: Efficacy and safety. Pharmacol Ther. 2007;113:134–153. doi: 10.1016/j.pharmthera.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 7.Schechter LE, Ring RH, Beyer CE, Hughes ZA, Khawaja X, Malberg JE, Rosenzweig-Lipson R. Innovative approaches for the development of antidepressant drugs: Current and future strategies. NeuroRx. 2005;2:590–611. doi: 10.1602/neurorx.2.4.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duman RS, Heninger GR, Nestler EJ. A molecular and cellular theory of depression. Arch Gen Psychiatry. 1997;54:597–606. doi: 10.1001/archpsyc.1997.01830190015002. [DOI] [PubMed] [Google Scholar]
- 9.Manji HK, Moore GJ, Rajkowska G, Chen G. Neuroplasticity and cellular resilience in mood disorders. Mol Psychiatry. 2000;5:578–593. doi: 10.1038/sj.mp.4000811. [DOI] [PubMed] [Google Scholar]
- 10.Adachi M, Barrot M, Autry AE, Theobald D, Monteggia LM. Selective loss of brain-derived neurotrophic factor in the dentate gyrus attenuates antidepressant efficacy. Biol Psychiatry. 2008;63:642–649. doi: 10.1016/j.biopsych.2007.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59:1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 12.Malberg JE, Blendy JA. Antidepressant action: To the nucleus and beyond. Trends Pharmacol Sci. 2005;26:631–638. doi: 10.1016/j.tips.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 13.Monteggia LM, Barrot M, Powell CM, Berton O, Galanis V, Gemelli T, Meuth S, Nagy A, Greene RW, Nestler EJ. Essential role of brain-derived neurotrophic factor in adult hippocampal function. Proc Natl Acad Sci USA. 2004;101:10827–10832. doi: 10.1073/pnas.0402141101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tanis KQ, Duman RS. Intracellular signaling pathways pave roads to recovery for mood disorders. Ann Med. 2007;39:531–544. doi: 10.1080/07853890701483270. [DOI] [PubMed] [Google Scholar]
- 15.Chen MJ, Russo-Neustadt AA. Running exercise- and antidepressant-induced increases in growth and survival-associated signaling molecules are IGF-dependent. Growth Factors. 2007;25:118–131. doi: 10.1080/08977190701602329. [DOI] [PubMed] [Google Scholar]
- 16.Mattson MP, Maudsley S, Martin B. A neural signaling triumvirate that influences ageing and age-related disease: lnsulin/IGF-1, BDNF and serotonin. Ageing Res Rev. 2004;3:445–464. doi: 10.1016/j.arr.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 17.Hunsberger JG, Newton SS, Bennett AH, Duman CH, Russell DS, Salton SR, Duman RS. Antidepressant actions of the exercise-regulated gene VGF. Nat Med. 2007;13:1476–1482. doi: 10.1038/nm1669. [DOI] [PubMed] [Google Scholar]
- 18.Thakker-Varia S, Krol JJ, Nettleton J, Bilimoria PM, Bangasser DA, Shors TJ, Black IB, Alder J. The neuropeptide VGF produces antidepressant-like behavioral effects and enhances proliferation in the hippocampus. J Neurosci. 2007;27:12156–12167. doi: 10.1523/JNEUROSCI.1898-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alder J, Thakker-Varia S, Bangasser DA, Kuroiwa M, Plummer MR, Shors TJ, Black IB. Brain-derived neurotrophic factor-induced gene expression reveals novel actions of VGF in hippocampal synaptic plasticity. J Neurosci. 2003;23:10800–10808. doi: 10.1523/JNEUROSCI.23-34-10800.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salton SR, Ferri GL, Hahm S, Snyder SE, Wilson AJ, Posssenti R, Levi A. VGF: A novel role for this neuronal and neuroendocrine polypeptide in the regulation of energy balance. Front Neuroendocrinol. 2000;21:199–219. doi: 10.1006/frne.2000.0199. [DOI] [PubMed] [Google Scholar]
- 21.Snyder SE, Pintar JE, Salton SR. Developmental expression of VGF mRNA in the prenatal and postnatal rat. J Comp Neurol. 1998;394:64–90. [PubMed] [Google Scholar]
- 22.Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saarelainen T, Hendolin R, Lucas G, Koponen E, Sairanen M, MacDonald E, Agerman K, Haapasalo A, Nawa H, Aloyz R, Ernfors P, Castren E. Activation of the TrkB neurotrophin receptor is induced by antidepressant drugs and is required for antidepressant-induced behavioral effects. J Neurosci. 2003;23:349–357. doi: 10.1523/JNEUROSCI.23-01-00349.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cryan JF, Markou A, Lucki I. Assessing antidepressant activity in rodents: Recent developments and future needs. Trends Pharmacol Sci. 2002;23:238–245. doi: 10.1016/s0165-6147(02)02017-5. [DOI] [PubMed] [Google Scholar]
- 25.J.E.M. is employed by PsychoGenics, Inc., a company that applies high-throughput behavioral testing platforms to discover drugs for CNS disorders.