Abstract
Ankyrins are critical components of ion channel and transporter signaling complexes in the cardiovascular system. Over the past five years, ankyrin dysfunction has been linked with abnormal ion channel and transporter membrane organization and fatal human arrhythmias. Loss-of-function variants in the ankyrin-B gene (ANK2) cause “ankyrin-B syndrome” (previously called type 4 long QT syndrome), manifested by a complex cardiac phenotype including ventricular arrhythmias and sudden cardiac death. More recently, dysfunction in the ankyrin-B-based targeting pathway has been linked with a highly penetrant and severe form of human sinus node disease. Ankyrin-G (a second ankyrin gene product) is required for normal expression, membrane localization, and biophysical function of the primary cardiac voltage-gated sodium channel, Nav1.5. Loss of the ankyrin-G/Nav1.5 interaction is associated with human cardiac arrhythmia (Brugada syndrome). Finally, in the past year ankyrin dysfunction has been associated with more common arrhythmia and cardiovascular disease phenotypes. Specifically, large animal studies reveal striking remodeling of ankyrin-B and associated proteins following myocardial infarction. Additionally, the ANK2 locus has been linked with QTc interval variability in the general human population. Together, these findings identify a host of unanticipated and exciting roles for ankyrin polypeptides in cardiac function. More broadly, these findings illustrate the importance of local membrane organization for normal cardiac physiology.
1. Introduction
Ankyrin polypeptides, once thought to solely serve structural roles in the erythrocyte, are now recognized as multifunctional proteins involved in targeting and stabilization of ion channels and transporters in diverse tissues and cell types including skeletal and cardiac myocytes, neurons, photoreceptors, and epithelial cells [1–4]. Ankyrin-R (ANK1 gene) was originally identified in the late 1970s as a critical structural protein of the erythrocyte plasma membrane [5]. In the mid 1990s, human gene mutations in ANK1 were discovered to cause erythrocyte spectrin-deficiency, resulting in hereditary spherocytosis and anemia [6]. Over the past decade, advances in ankyrin biology have generated new insights into the molecular mechanisms underlying a diverse range of human cardiovascular disorders. Ankyrin-B and ankyrin-G are now recognized to play essential roles in the targeting and membrane stabilization of ion channels and transporters in cardiomyocytes [7–16]. Inherited loss-of-function variants in the ankyrin-B gene (ANK2) cause “ankyrin-B syndrome” ventricular arrhythmias [10]. Moreover, ankyrin-B dysfunction was recently linked with human sinus node disease [17]. Ankyrin-G, a second ankyrin gene product, is required for targeting and membrane regulation of the primary voltage-gated sodium channel (Nav1.5) in human heart [12, 16]. A human mutation in the Nav1.5 ankyrin-G-binding motif is associated with Brugada syndrome, an arrhythmia syndrome associated with risk of sudden death [12]. Thus, the identification of genetic variants in ankyrins and associated proteins (e.g. Nav 1.5) has provided new information on the cellular and molecular basis of cardiac arrhythmias. This review will primarily focus on the emerging roles of ankyrin-B and ankyrin-G in cardiac function and cardiovascular disease.
2. Ankyrin genes
Ankyrin genes ANK1 (human chromosome 8p11), ANK2 (human chromosome 4q25–27), and ANK3 (human chromosome 10q21) encode three canonical ankyrins: 210 kD ankyrin-R, 220 kD ankyrin-B, and 190 kD ankyrin-G, respectively [4, 18]. Ankyrin-R expression is restricted to erythrocytes, neurons, and skeletal muscle, whereas ankyrin-B and ankyrin-G are ubiquitously expressed [4, 18]. In addition to canonical ankyrins, alternative splicing of ankyrin genes produces a series of gene products with distinct subcellular distributions and functional properties [4, 18, 19]. For example, alternative splicing of ANK2 results in differential distribution of 440 kD ankyrin-B (found at unmyelinated axons in neonatal brain) and 220 kD ankyrin-B (found in neural cell bodies, dendrites, and glial cells of adult brain) in the nervous system [20]. Likewise, alternative splicing of ANK1 and ANK2 genes in muscle results in differential subcellular location and function of ankyrin-R and ankyrin-B polypeptides [19, 21–27]. While all three ankyrin gene products have been identified in ventricular cardiomyocytes [7, 8, 12, 28], the differential expression and subcellular distribution patterns of individual ankyrin gene products throughout the heart is currently unknown.
3. Structure and function of ankyrin polypeptides
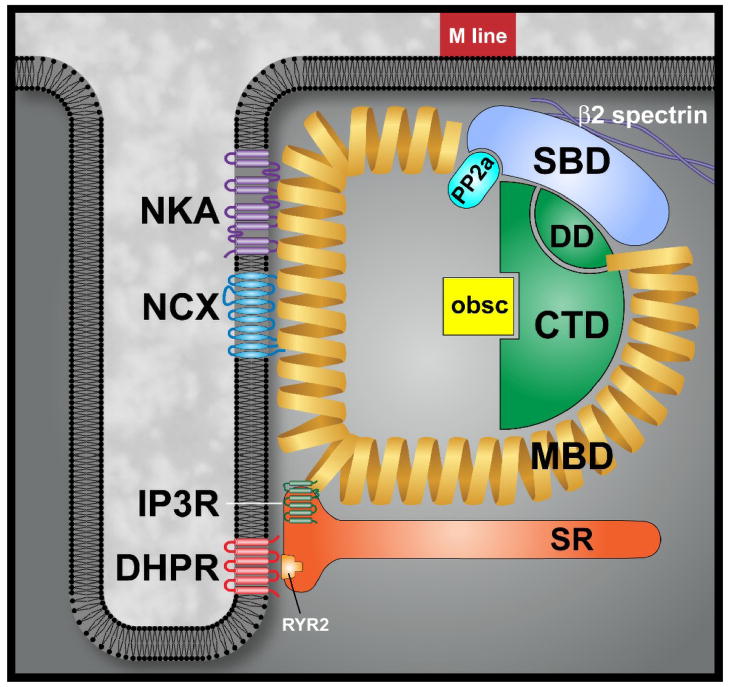
Canonical ankyrins consist of four major domains: a membrane-binding domain (MBD), a spectrin-binding domain (SBD), a death domain (DD), and a C-terminal domain (CTD, Figure 1) [29]. The ankyrin membrane-binding domain (24 ANK repeats) interacts with a host of diverse membrane proteins, with each ankyrin polypeptide linked to a unique set of associated proteins (Table 1). Specifically, the ankyrin MBD interacts with ion channels, transporters, and pumps including the Na/K ATPase, voltage-gated Na+ and K+ channels, the Na/Ca exchanger, the ammonium transporter, the inositol 1,4,5 trisphosphate receptor (IP3R), and the anion exchanger [7, 10, 12, 30–34]. Additionally, ankyrin MBD interacts with cell adhesion molecules (CAMs) including CD44, E-cadherin, and L1 CAMs [4]. Interestingly, based on the ability of the MBD to interact simultaneously with multiple membrane proteins [13, 35, 36], a single ankyrin may orchestrate the formation of large homomeric or heteromeric protein complexes in vivo. This multivalent nature of ankyrin is clearly illustrated in the erythrocyte, where a single ankyrin-R polypeptide can organize homomeric protein complexes (coupling two dimers of the anion exchanger) as well as heteromeric complexes (coupling the anion exchanger with L1CAM) [35, 36]. In cardiomyocytes, a single ankyrin-B polypeptide can form a ternary membrane protein complex of Na/Ca exchanger, Na+/K+ ATPase, and IP3 receptor (Figure 1) [13].
Figure 1. Cardiac ankyrin-B membrane-associated protein complex.
Ankyrins are comprised of four distinct folding domains including a membrane-binding domain (MBD), spectrin-binding domain (SBD), C-terminal domain (CTD) and death domain (DD). Together, the CTD and DD comprise the regulatory domain. In heart, ankyrin-B targets ion channels and transporters including Na/K ATPase (NKA), Na/Ca exchanger (NCX) and IP3 receptor (IP3R) in cardiomyocytes. Thorugh interactions with obscurin (obs), ankyrin-B also targets protein phosphatase 2A (PP2A) via the PP2A B56α subunit.
Table 1.
Ankyrin-interacting proteins
| Protein Class | Protein | Ankyrin(s) | Domain |
|---|---|---|---|
| Channel/Transporter/Pumps | Anion Exchanger | R,G,B | MBD |
| Na/K ATPase | R,B,G | MBD/SBD | |
| Na/Ca exchanger | R, B | MBD | |
| Nav channels | G | MBD | |
| Ammonium | G | MBD | |
| Transporter | |||
| InsP3 receptor | B | MBD | |
| Kv3.1 | G | ? | |
| Kv7.2, Kv7.3 | G | MBD | |
| Cyclic nucleotide- gated channel | G | ? | |
| Cell adhesion Molecules | L1CAMs | R,B,G | MBD |
| E-cadherin | G | ? | |
| dystrophin | B,G | ? | |
| Beta-dystroglycan | G | ? | |
| CD44 | R,B | MBD | |
| Nav channel β subunit | R,B,G | MBD | |
| Cell Transport Proteins | Clathrin | R | MBD |
| Dynactin-4 | B | ? | |
| Signaling Proteins | B56α subunit of | B | SBD |
| PP2A | |||
| Hdj-1 | B | CTD | |
| Fas | G | DD | |
| Cytoskeletal Proteins | Obscurin | R,B | CTD |
| Spectrin | R,B,G | SBD | |
| Tubulin | B,R | ? |
Abbreviations: R, ankyrin-R; B, ankyrin-B, G, ankyrin-G; MBD, membrane-binding domain, SBD, spectrin-binding domain; DD, death domain; CTD, C-terminal domain.
The central spectrin-binding domain (SBD) links ankyrin to the actin-based cytoskeleton via β-spectrin isoforms [37]. This link between ankyrin and spectrin is critical for normal erythrocyte physiology, as mutations in ankyrin-R produce cell membrane instability and hemolytic anemia [38]. The mechanism by which ankyrin-R stabilizes the red blood cell membrane was initially thought to be solely through vertical association of the ankyrin/spectrin-associated cytoskeleton with the lipid bilayer [39]. However, ankyrins likely have additional key roles in formation and/or maintenance of spectrin/actin cytoskeleton as evidenced by reduced spectrin levels in erythrocytes of humans and mice with ankyrin-R gene mutations [6, 40]. Beyond the erythrocyte, coordinate expression of ankyrin and spectrin have been implicated in normal neuronal and epithelial cell membrane formation and function [41–44]. Importantly, the ankyrin SBD also interacts with downstream protein partners including B56α, the regulatory subunit of protein phosphatase 2A (Figure 1, Table 1, discussed below).
The ankyrin death and C-terminal domains comprise the ankyrin ‘regulatory domain’ that confers specificity of function for ankyrin gene products [10, 45–47]. In cardiomyocytes, the ankyrin-B C-terminal domain interacts with the MBD and is important for driving ankyrin-B-specific functions [48]. Most importantly, the ankyrin-B C-terminal domain is the location of the majority of human ANK2 arrhythmia loss-of-function variants [28, 49]. Identified ankyrin regulatory domain interacting proteins include obscurin [21, 23, 27, 50], Hdj1 [47], and Fas [51] (Table 1).
Finally, while specific binding sites on the ankyrin have yet to be mapped, recent work from Bennett and colleagues has identified new interactions of ankyrin polypeptides with cell adhesion molecules and ion channels. Specifically, in the past year ankyrin-G has been found to target cyclic nucleotide-gated channels in rod photoreceptors [2]. Moreover, ankyrin-G has been linked with the adherens junction protein E-cadherin in early embryos and epithelial cells [52]. Finally, ankyrin-B and ankyrin-G have been shown to associate with dystrophin and beta-dystroglycan in skeletal muscle [1]. Interestingly, a human dystrophin mutation associated with Becker muscular dystrophy blocks ankyrin-B/dystrophin interactions, resulting in mis-localization of dystrophin-Dp71 in skeletal muscle[1].
4. Ankyrin-B Syndrome
The importance of ankyrins for normal heart physiology is highlighted by the growing number of cardiac arrhythmia syndromes linked to ankyrin dysfunction. An early example may be found in the long QT (LQT) syndrome, a heterogenous group of inherited arrhythmogenic diseases characterized by prolonged corrected QT interval (QTc) on the electrocardiogram (ECG) and susceptibility to life-threatening arrhythmias [53, 54]. To date, nearly a dozen LQT syndromes have been identified. However, variants in three LQT genes account for the majority of clinical LQT syndrome cases. Specifically, LQT1-3 are associated with human variants in either potassium (LQT1,2) or sodium channel (LQT3) genes, leading to either decreased outward currents or increased inward currents, thereby delaying repolarization and prolonging the QT interval [54–57].
Type 4 long QT syndrome (LQTS4) was first described in 1995, and further defined in 2003 [10, 58]. Linkage analysis studies on a four-generation French family with two consecutive cases of sudden death revealed a new LQTS locus on chromosome 4q25–27 [58]. Electrocardiographic screening of 56 members in the family revealed prolonged QTc in 21 individuals. Further clinical evaluation of the 21 affected family members identified a unique clinical phenotype characterized by severe sinus node dysfunction (all 21 affected patients), atrial fibrillation (12 of 21 adult patients) and notched biphasic T wave morphology (19 of 21 patients), in addition to the more common LQT features of prolonged QTc, syncope (4 of 21 patients) and sudden death (2 cases) [58].
In 2003, the human ANK2 gene encoding ankyrin-B was identified as the affected gene in the large French kindred [10], serving as the first example of an LQT syndrome gene that encoded a protein other than an ion channel or channel subunit. Sequencing of the ANK2 gene identified an A-to-G missense mutation at position 4274 in exon 36, resulting in the substitution of glycine for a glutamic acid at amino acid residue 1425 (E1425G) in the ankyrin-B spectrin-binding domain [10]. Twenty-two of 24 affected members who carried the E1425G variant displayed prolonged QTc. This variant was not found in unaffected family members or control subjects with normal ECGs [10].
Ankyrin-B+/− mice and human ANK2 E1425G carriers display a number of common cardiac phenotypes including severe bradycardia, prolonged QT interval, heart rate variability, and episodes of isorhythmic atrio-ventricular dissociation [10, 17]. Furthermore, similar to “ankyrin-B syndrome” patients, ankyrin-B+/− mice display polymorphic ventricular tachycardia, syncope, and sudden cardiac death in response to exercise and catecholamine injection [10]. Ankyrin-B localizes to both the M-line and Z-line in adult ventricular myocytes, and at M-lines in primary neonatal cardiomyocytes (Z-line/T-tubule domains not developed) [8, 10, 13, 28]. Interestingly, ankyrin-B+/− cardiomyocytes show selective reduction of ankyrin-B levels at transverse-tubule/sarcoplasmic reticulum sites [10]. This reduction is associated with aberrant expression and membrane localization of ankyrin-B-associated proteins Na/Ca exchanger, Na/K ATPase, and IP3R [10]. Importantly, these phenotypes are rescued by exogenous expression of ankyrin-B in cardiomyocytes, but not by an ankyrin-B variant containing the E1425G mutation [10].
At the level of the single cell, ankyrin-B+/− ventricular cardiomyocytes display elevated sarcoplasmic reticulum Ca2+ load and catecholamine-induced afterdepolarizations [10]. Cellular afterdepolarizations are likely caused by coordinate loss of membrane Na/Ca exchanger and Na/K ATPase due to disruption of ankyrin-based targeting. Similar to the activity of digitalis, decreased expression of membrane Na/K ATPase and Na/Ca exchanger in ankyrin-B+/− myocytes results in elevated cytosolic Na+ concentrations, that in turn decreases Ca2+ extrusion by the Na/Ca exchanger [10, 13, 15]. Ultimately, ankyrin-B+/− myocytes develop sarcoplasmic reticulum Ca2+ overload and increased likelihood for catecholamine-induced afterdepolarizations [10]. These events may result in triggered arrhythmias in vivo [59–61].
Since the discovery of the first ANK2 loss-of-function variant (E1425G), analysis of human populations has identified a select number of additional ANK2 human loss-of-function variants [28, 49, 62, 63], as well as a host of benign polymorphisms [49]. Loss-of-function gene variants are associated with a wide spectrum of phenotypes including sinus node dysfunction, atrial fibrillation, conduction defects, and ventricular arrhythmias [10, 28, 49]. Interestingly, despite its presence in the original French kindred [10], prolonged QT interval is not a consistent phenotype of many carriers with specific ANK2 variants [28, 49].
ANK2 variants expressed in primary cardiomyocytes demonstrate a range of functional perturbations [49]. In fact, based on both clinical and in vitro phenotypes, ANK2 loss-of-function variants are categorized into three distinct functional classes. Variants in the first class (G1406C, R1450W, and L1503V) are primarily associated with less severe clinical phenotypes (or asymptomatic individuals) and display only mild in vitro cellular phenotypes compared with wild-type neonatal cardiomyocytes [28, 49]. Variants in the second class (T1404I, T1552N, and V1777M) are generally associated with more severe human ventricular phenotypes and behave as loss-of-function alleles in the context of a primary myocyte [28, 49]. The most severe ankyrin-B loss-of-function (dominant-negative) activity in vitro is associated with the third class of variants (E1425G, V1516D, and R1788W) [28, 49], which are also linked with the most severe clinical phenotypes [28, 49]. Together, these findings demonstrate that ANK2 variants produce a spectrum of in vitro defects corresponding to a wide range of clinical phenotypes. However, additional cellular studies are necessary to determine the molecular mechanism underlying phenotypic variability of each human ANK2 variant.
5. Ankyrin-B is essential for sinoatrial node automaticity
While early studies focused on the role of ankyrin-B in ventricular cardiomyocytes and arrhythmias, it is now clear that ankyrin-B is expressed throughout the heart. In fact, recent studies have identified an important role for ankyrin-B in sinoatrial node pacemaking. Sinus node dysfunction (SND), or “sick sinus syndrome” is considered a disease of the elderly with an exponential increase in its frequency with age [64, 65]. In fact, SND affects one in every 600 individuals over age 65 years [66]. Furthermore, SND is a common cause of bradycardia and syncope and accounts for 50% of pacemaker implantations in the United States [66]. Despite its impact, the etiology of SND is heterogenous and not fully understood at this time. At the cellular level, replacement of nodal tissue by “fibrous tissue” has remained a prevailing theory [65, 67]. However, in recent years, discovery of single gene defects associated with ion channels has expanded our understanding of sinus node dysfunction to include “channelopathies” and “familial” forms of the disease that can manifest at significantly younger ages [68–70].
Our group recently mapped two families with a severe and highly penetrant sinus node dysfunction to ANK2 [17]. In the first family, 25 of 74 members were affected. Thirteen members had atrial fibrillation and 14 patients underwent permanent pacemaker implantation. Gene sequencing of the available family members revealed all 25 affected members as carriers of ANK2 mutation (E1425G), whereas none of the unaffected subjects were carriers. In the second family, 13 of 44 screened individuals had SND. Three members manifested atrial fibrillation and six patients required pacemaker implantation for SND. Twelve members presented with an abnormal sinusoidal T and prominent U waves, and QTc prolongation, similar to the first family. Echocardiographic screening revealed atrial septal defect in five cases. Out of 36 members of this family who underwent genotype testing, 20 were carriers of a common haplotype at the ANK2 locus, while the other 16 were non-carriers [17]. All thirteen affected members were carriers of the ANK2 disease haplotype. The maximum LOD score for marker D4S1616 showed evidence for strong linkage (Zmax = 5.9). Immunoblot analysis of a muscle biopsy revealed a striking decrease in ankyrin-B expression in one of the affected patients compared with samples from two unaffected individuals [17]. Collectively, these data demonstrate that the ANK2 variants with decreased ankyrin-B expression are strongly associated with a familial form of SND that may occur at much younger age than typical SND.
Similar to patients with ankyrin-B-associated SND, mice lacking ankyrin-B display severe SND. Moreover, immunoblots of sinoatrial node (SAN) cells from ankyrin-B+/− mice show reduced expression of Na/K ATPase, Na/Ca exchanger, and IP3 receptor [17]. Interestingly, the distribution of one L-type calcium channel isoform, Cav1.3, was also affected in ankyrin-B+/− SAN cells [17]. Moreover, L-type Ca2+ current was significantly reduced in isolated ankyrin-B+/− SAN cells. Finally, ankyrin-B+/− SAN cells display abnormal calcium handling and automaticity [17]. Collectively, these data indicate that a full complement of ankyrin-B is required for proper expression and membrane localization of Cav1.3, Na/K ATPase, Na/Ca exchanger, and IP3 receptor in SAN cells. These findings provide a new cellular mechanism underlying human sinus node dysfunction and further highlight the importance of ankyrin-based targeting pathways in ion channel physiology. Finally, as noted above, individuals harboring ANK2 variants may display atrial fibrillation in addition to SND [10, 17]. To date, the role of ankyrin-B in the vertebrate atria has not been defined. However, based on findings in other excitable cardiac cell types, it is likely that ankyrin-B dysfunction will result in defects in normal atrial excitability due to abnormal ion channel and transporter targeting.
6. Ankyrin-B and obscurin target cardiac protein phosphatase 2A
Ankyrin-B plays a critical role in cardiomyocyte signaling pathways. Specifically, ankyrin-B was recently identified as a binding partner in ventricular myocytes for B56α, the regulatory subunit of protein phosphatase 2A (PP2A) [14]. PP2A is a multifunctional serine/threonine phosphatase linked with cardiac β-adrenergic signaling [71–73]. Furthermore, PP2A regulates a number of ion channels and transporters including L-type Ca2+-channels [71, 74], ryanodine receptor [75], IP3 receptor [76], and Na/K ATPase [77]. Ankyrin-B and B56α are both localized over the M-line in neonatal cardiomyocytes, as well as at M-lines and Z-lines in adult cardiomyocytes [14]. Work from Bhasin et al. recently demonstrated that cardiomyocytes lacking ankyrin-B expression display a striking loss of B56α [14]. In a related study, Cunha et al. determined that the localization of ankyrin-B and PP2A is dependent on interaction of ankyrin-B with the large Rho-GEF obscurin [27] (previously identified as binding partner of small ankyrin-R isoforms in skeletal muscle [21, 23]). While these data demonstrate that ankyrin-B, obscurin and PP2A are physiological binding partners in cardiomyocytes, the targets of ankyrin-B-associated PP2A are currently unknown. Additionally, future studies are needed to define the role for ankyrin-B/obscurin interactions. Elegant work from Bloch and colleagues in skeletal muscle, suggest ankyrin-B may play a role in obscurin-dependent regulation of myocyte myofibrillogenesis [50, 78, 79].
More recently, Terentyev and colleagues showed that inhibition of B56α in myocytes (by overexpression of miR-1) resulted in RyR2 hyperphosphorylation (by CaMKII) and myocyte electrical instability [80]. Interestingly, B56α loss was associated with spontaneous SR calcium release and afterdepolarizations [80], similar to phenotypes observed in ankyrin-B+/− myocytes lacking normal B56α targeting [10, 14]. It is therefore intriguing to speculate that defects in CaMKII-based local signaling pathways may contribute to human ankyrin-B-linked catecholamine-induced arrhythmias.
7. Ankyrin-B in acquired arrhythmias
Recent large animal studies suggest that ankyrin-B dysfunction is associated with common acquired forms of heart disease [81]. Electrical remodeling in the peri-infarct zone creates a substrate favorable to the initiation and maintenance of reentrant arrhythmias following myocardial infarction [82, 83]. An important component of this remodeling process involves the redistribution of ion channels in surviving myocytes near the infarct [84, 85]. Recently, ankyrin-B levels were shown to be significantly affected following myocardial infarction in a large animal model [81]. Moreover, abnormal expression of ankyrin-B at both mRNA and protein levels was associated with reduced expression, and/or abnormal distribution of ankyrin-B associated membrane proteins (IP3R, NCX1, Na+/K+ ATPase, and PP2A) [81]. These findings provide the first data on the role of ankyrin-B in acquired arrhythmias, and suggest that ankyrin-B may represent a central player in the genesis of electrical remodeling and arrhythmias following myocardial infarction.
8. Common genetic variants in ANK2 are associated with QT interval variability
Recent data from large human population studies have raised the exciting prospect that variability in ankyrin-B function may influence arrhythmia susceptibility in the general human population. Prolongation of QT interval causes susceptibility to polymorphic ventricular tachycardia and sudden death [86, 87]. Conversely, shortening of QT interval may facilitate life-threatening reentrant arrhythmias in vulnerable patients [88]. In the general population, the QT interval is distributed normally, and may be influenced by many parameters including congenital factors, heart rate, age, sex, electrolyte levels, and many medications [89]. The rare genetic variants affecting ion channel subunits or ionic currents have long been recognized to cause repolarization abnormalities and long QT syndrome [10, 53, 55, 56, 90]. Recently, through linkage disequilibrium (LD) mapping analysis, investigators of the large human KORA study explored the association between common genetic variants and QT interval variability in the general population [91, 92]. Among other common genetic variants affecting repolarization [91, 93, 94], the study identified new common genetic variants in the 5′ genomic region of ANK2 associated with variations in QT interval [92]. In particular, one single nucleotide polymorphism (SNP), rs6850768, was found to be associated with slightly shorter QT intervals [92]. These findings suggest a link between common genetic variants in ANK2 and QT interval duration irrespective of confounding factors such as heart rate, age, sex and medication.
9. Ankyrin-G is required for targeting of cardiac Nav1.5
Voltage-gated sodium channels (Nav) are required for normal vertebrate cardiac function. Dysfunction in cardiac Nav1.5 may result in type 3 long QT syndrome, conduction defects, sick sinus syndrome, and Brugada syndrome [90, 95–97]. While the mechanisms underlying Nav1.5 channel biophysics are well described, little is known regarding the pathways required for targeting and regulation of Nav1.5 at cardiac membrane domains.
In the nervous system, ankyrin-G is required for Nav channel targeting to excitable membrane domains [41, 98, 99]. Nav channels co-localize with ankyrin-G throughout the brain (axon initial segments, nodes of Ranvier) and at the neuromuscular junction [100–102]. Moreover, Nav channels and ankyrin-G co-purify from brain [103, 104]. Mice lacking ankyrin-G (cerebellar-specific knock-out) display striking reduction in Nav channel targeting to axon initial segments and nodes of Ranvier [98]. Additionally, mice with cerebellar-knockout of ankyrin-G display aberrant distribution of β4-spectrin, and neurofascin [41]. Ankyrin-G cerebellar-specific knockout mice display abnormal action potentials and ataxia, likely due to abnormal inter-neuronal circuitry [98]. Finally, recent work from Cooper and colleagues demonstrates a role of ankyrin-G in targeting potassium channels (KCNQ2/KCNQ3, encode subthreshold M-currents required for stabilizing neuronal resting potential and preventing repetitive action potential firing) to specific membrane domains [105, 106]. Together, these studies conclusively link ankyrin-G function with the targeting of critical ion channels in excitable cells.
In 2003, two independent groups identified the structural requirements on brain Nav channels (Nav1.2) for ankyrin-G association as a highly conserved motif in the Nav channel DII-DIII cytoplasmic domain [107, 108]. Based on the conservation of the ankyrin-G-binding motif across Nav channel gene products (including Nav1.5), our group hypothesized that ankyrin-G regulates Nav1.5 channel targeting in excitable myocytes. Ankyrin-G co-immunoprecipitates with Nav1.5 from detergent-soluble lysates of heart, and directly interacts with Nav1.5 using purified proteins [12]. Consistent with an in vivo interaction, ankyrin-G and Nav1.5 are co-expressed at cardiac excitable membrane domains including the intercalated disc and transverse-tubule [12, 16]. Finally, as predicted from previous studies, Nav1.5 lacking the putative ankyrin-G-binding motif (nine residues) fails to associate with ankyrin-G [12].
More recently, our group utilized viral expression of ankyrin-G shRNAs to assess the requirement of ankyrin-G expression for Nav1.5 targeting and function in myocytes [16]. Reduction of ankyrin-G at the level of the single myocyte results in a marked reduction in Nav1.5 channel expression, targeting, and INa [16]. These phenotypes were specific for the ankyrin-G/Nav1.5 pathway as Na/Ca exchanger and Cav1.2 targeting were unaffected by the ankyrin-G shRNA [16]. Finally abnormal Nav1.5 phenotypes were rescued by exogenous expression of ankyrin-G, but not an ankyrin-G mutant lacking Nav1.5-binding activity [16]. Together, these data support the role of a direct Nav1.5/ankyrin-G interaction for normal expression, targeting, and function of membrane Nav1.5 in cardiomyocytes.
10. Dysfunction in the ankyrin-G-based Nav1.5 channel targeting pathway is associated with human Brugada syndrome arrhythmia
The Brugada syndrome is an autosomal-dominant, potentially-fatal cardiac arrhythmia syndrome characterized by ST segment elevation in the precordial leads associated with right bundle branch block and T-wave inversion [109]. In affected individuals, sudden death is most prevalent at night, and is the result of ventricular fibrillation [110, 111]. Gene variants in SCN5A (encodes Nav1.5) account for up to ~30% of Brugada syndrome cases [112], and are associated with Nav1.5 biophysical defects that disrupt inward (depolarizing) sodium current [113]. The vast majority of human Nav1.5 gene mutations affect channel biophysical properties.
We hypothesized that defects in normal Nav1.5 membrane expression may result in an alternative mechanism for the Brugada syndrome. In 2002, Priori and colleagues identified a G3157A SCN5A variant in a proband with Brugada syndrome [113]. Subsequent analysis revealed that this variant (resulting in Nav1.5 E1053K) is located in the ankyrin-binding motif of Nav1.5 DII-DIII[12]. Interestingly, Nav1.5 E1053K lacks in vitro ankyrin–binding activity [12]. Moreover, Nav1.5 E1053K displays abnormal intercalated disc and transverse-tubule targeting when introduced into adult rat cardiomyocytes [12]. Thus, the human Brugada syndrome is associated with an SCN5A mutation that affects normal Nav1.5 membrane targeting (consistent with Nav1.5 loss-of-function). Ankyrin-G may play an unexpected secondary role in Nav1.5 channel biophysical properties. Specifically, Nav1.5 E1053K lacking ankyrin-binding displays a more negative threshold for activation, faster inactivation, and slower recovery than wild-type channel when analyzed in HEK293 cells [12]. Clearly, additional experiments in primary myocytes will be necessary to further define a potential role of ankyrin-G for Nav channel biophysical regulation.
Thus, ankyrin-G is critical for the cellular machinery required for Nav channel targeting in cardiomyocytes. As Nav channel function is essential for excitable cell function, future experiments to define additional components of the ankyrin-dependent targeting pathway will be critical for our understanding of myocyte cell biology, as well as for defining potential future targets for the treatment of excitable cell disease.
11. Summary
Exciting discoveries over the past decade have established the importance of local signaling domains for excitable cell function. While key myocyte membrane receptors, transporters, and ion channels remain the primary mediators of cardiomyocyte signaling, the proteins responsible for local organization of ion channels and transporters with signaling and effector proteins are now recognized as critical determinants of normal myocyte function. Over the past five years, ankyrin-B and ankyrin-G have been recognized as essential cellular components for targeting ion channels and transporters to specialized membrane micro-domains in the heart. Dysfunction in cardiac ankyrin polypeptides results in several congenital arrhythmia syndromes including ankyrin-B syndrome, congenital sinus node dysfunction, and Brugada syndrome. Furthermore, recent large animal studies have linked ankyrin-B dysfunction to more common acquired arrhythmias (e.g. after myocardial infarction). Moreover, ankyrin-B expression has been linked to QT interval variability in the general population suggesting a broader role for ankyrins in regulating heart function. In conclusion, recent developments in ankyrin biology have expanded our understanding of the molecular bases of human arrhythmias. At the same time, many questions remain and further studies are needed to unravel the cellular pathways underlying ankyrin function in diverse excitable cardiac tissue.
Acknowledgments
Funding
We acknowledge financial support from the NIH (HL084583 and HL083422 to PJM; T32 HL07121 (PI: Abboud) to SMH); the Pew Scholars Trust (PJM), and the American Heart Association (0930378N to TJH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ayalon G, Davis JQ, Scotland PB, Bennett V. An ankyrin-based mechanism for functional organization of dystrophin and dystroglycan. Cell. 2008;135(7):1189–200. doi: 10.1016/j.cell.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 2.Kizhatil K, Baker SA, Arshavsky VY, Bennett V. Ankyrin–G promotes cyclic nucleotide-gated channel transport to rod photoreceptor sensory cilia. Science. 2009;323(5921):1614–7. doi: 10.1126/science.1169789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kizhatil K, Sandhu NK, Peachey NS, Bennett V. Ankyrin-B is required for coordinated expression of beta-2-spectrin, the Na/K-ATPase and the Na/Ca exchanger in the inner segment of rod photoreceptors. Experimental eye research. 2009;88(1):57–64. doi: 10.1016/j.exer.2008.09.022. [DOI] [PubMed] [Google Scholar]
- 4.Bennett V, Baines AJ. Spectrin and ankyrin-based pathways: metazoan inventions for integrating cells into tissues. Physiol Rev. 2001;81(3):1353–92. doi: 10.1152/physrev.2001.81.3.1353. [DOI] [PubMed] [Google Scholar]
- 5.Bennett V. Purification of an active proteolytic fragment of the membrane attachment site for human erythrocyte spectrin. J Biol Chem. 1978;253(7):2292–9. [PubMed] [Google Scholar]
- 6.Eber SW, Gonzalez JM, Lux ML, Scarpa AL, Tse WT, Dornwell M, et al. Ankyrin-1 mutations are a major cause of dominant and recessive hereditary spherocytosis. Nat Genet. 1996;13(2):214–8. doi: 10.1038/ng0696-214. [DOI] [PubMed] [Google Scholar]
- 7.Li ZP, Burke EP, Frank JS, Bennett V, Philipson KD. The cardiac Na+-Ca2+ exchanger binds to the cytoskeletal protein ankyrin. J Biol Chem. 1993;268(16):11489–91. [PubMed] [Google Scholar]
- 8.Mohler PJ, Gramolini AO, Bennett V. The Ankyrin-B C-terminal Domain Determines Activity of Ankyrin-B/G Chimeras in Rescue of Abnormal Inositol 1,4,5-Trisphosphate and Ryanodine Receptor Distribution in Ankyrin-B (−/−) Neonatal Cardiomyocytes. J Biol Chem. 2002;277(12):10599–607. doi: 10.1074/jbc.M110958200. [DOI] [PubMed] [Google Scholar]
- 9.Bourguignon LY, Jin H. Identification of the ankyrin-binding domain of the mouse T-lymphoma cell inositol 1,4,5-trisphosphate (IP3) receptor and its role in the regulation of IP3-mediated internal Ca2+ release. J Biol Chem. 1995;270(13):7257–60. doi: 10.1074/jbc.270.13.7257. [DOI] [PubMed] [Google Scholar]
- 10.Mohler PJ, Schott JJ, Gramolini AO, Dilly KW, Guatimosim S, duBell WH, et al. Ankyrin-B mutation causes type 4 long-QT cardiac arrhythmia and sudden cardiac death. Nature. 2003;421(6923):634–9. doi: 10.1038/nature01335. [DOI] [PubMed] [Google Scholar]
- 11.Mohler PJ, Davis JQ, Davis LH, Hoffman JA, Michaely P, Bennett V. Inositol 1,4,5-trisphosphate receptor localization and stability in neonatal cardiomyocytes requires interaction with ankyrin-B. J Biol Chem. 2004;279(13):12980–7. doi: 10.1074/jbc.M313979200. [DOI] [PubMed] [Google Scholar]
- 12.Mohler PJ, Rivolta I, Napolitano C, Lemaillet G, Lambert S, Priori SG, et al. Nav1.5 E1053K mutation causing Brugada syndrome blocks binding to ankyrin-G and expression of Nav1.5 on the surface of cardiomyocytes. Proc Natl Acad Sci U S A. 2004;101(50):17533–8. doi: 10.1073/pnas.0403711101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mohler PJ, Davis JQ, Bennett V. Ankyrin-B Coordinates the Na/K ATPase, Na/Ca Exchanger, and InsP(3) Receptor in a Cardiac T-Tubule/SR Microdomain. PLoS Biol. 2005;3(12):e423. doi: 10.1371/journal.pbio.0030423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhasin N, Cunha SR, Mudannayake M, Gigena MS, Rogers TB, Mohler PJ. Molecular basis for PP2A regulatory subunit B56{alpha} targeting in cardiomyocytes. Am J Physiol Heart Circ Physiol. 2007;293(1):H109–19. doi: 10.1152/ajpheart.00059.2007. [DOI] [PubMed] [Google Scholar]
- 15.Cunha SR, Bhasin N, Mohler PJ. Targeting and stability of na/ca exchanger 1 in cardiomyocytes requires direct interaction with the membrane adaptor ankyrin-B. J Biol Chem. 2007;282(7):4875–83. doi: 10.1074/jbc.M607096200. [DOI] [PubMed] [Google Scholar]
- 16.Lowe JS, Palygin O, Bhasin N, Hund TJ, Boyden PA, Shibata E, et al. Voltage-gated Nav channel targeting in the heart requires an ankyrin-G dependent cellular pathway. J Cell Biol. 2008;180(1):173–86. doi: 10.1083/jcb.200710107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Le Scouarnec S, Bhasin N, Vieyres C, Hund TJ, Cunha SR, Koval O, et al. Dysfunction in ankyrin-B-dependent ion channel and transporter targeting causes human sinus node disease. Proc Natl Acad Sci U S A. 2008;105(40):15617–22. doi: 10.1073/pnas.0805500105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cunha SR, Mohler PJ. Cardiac ankyrins: Essential components for development and maintenance of excitable membrane domains in heart. Cardiovasc Res. 2006;71(1):22–9. doi: 10.1016/j.cardiores.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 19.Cunha SR, Le Scouarnec S, Schott JJ, Mohler PJ. Exon organization and novel alternative splicing of the human ANK2 gene: Implications for cardiac function and human cardiac disease. J Mol Cell Cardiol. 2008;45(6):724–34. doi: 10.1016/j.yjmcc.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kunimoto M. A neuron-specific isoform of brain ankyrin, 440-kD ankyrinB, is targeted to the axons of rat cerebellar neurons. J Cell Biol. 1995;131(6 Pt 2):1821–9. doi: 10.1083/jcb.131.6.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kontrogianni-Konstantopoulos A, Jones EM, Van Rossum DB, Bloch RJ. Obscurin is a ligand for small ankyrin 1 in skeletal muscle. Mol Biol Cell. 2003;14(3):1138–48. doi: 10.1091/mbc.E02-07-0411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kontrogianni-Konstantopoulos A, Bloch RJ. The hydrophilic domain of small ankyrin-1 interacts with the two N-terminal immunoglobulin domains of titin. J Biol Chem. 2003;278(6):3985–91. doi: 10.1074/jbc.M209012200. [DOI] [PubMed] [Google Scholar]
- 23.Bagnato P, Barone V, Giacomello E, Rossi D, Sorrentino V. Binding of an ankyrin-1 isoform to obscurin suggests a molecular link between the sarcoplasmic reticulum and myofibrils in striated muscles. J Cell Biol. 2003;160(2):245–53. doi: 10.1083/jcb.200208109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou D, Birkenmeier CS, Williams MW, Sharp JJ, Barker JE, Bloch RJ. Small, membrane-bound, alternatively spliced forms of ankyrin 1 associated with the sarcoplasmic reticulum of mammalian skeletal muscle. J Cell Biol. 1997;136(3):621–31. doi: 10.1083/jcb.136.3.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Birkenmeier CS, Sharp JJ, Gifford EJ, Deveau SA, Barker JE. An alternative first exon in the distal end of the erythroid ankyrin gene leads to production of a small isoform containing an NH2-terminal membrane anchor. Genomics. 1998;50(1):79–88. doi: 10.1006/geno.1998.5305. [DOI] [PubMed] [Google Scholar]
- 26.Gallagher PG, Forget BG. An alternate promoter directs expression of a truncated, muscle-specific isoform of the human ankyrin 1 gene. J Biol Chem. 1998;273(3):1339–48. doi: 10.1074/jbc.273.3.1339. [DOI] [PubMed] [Google Scholar]
- 27.Cunha SR, Mohler PJ. Obscurin Targets Ankyrin-B and Protein Phosphatase 2A to the Cardiac M-line. J Biol Chem. 2008;283(46):31968–80. doi: 10.1074/jbc.M806050200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mohler PJ, Splawski I, Napolitano C, Bottelli G, Sharpe L, Timothy K, et al. A cardiac arrhythmia syndrome caused by loss of ankyrin-B function. Proc Natl Acad Sci U S A. 2004;101(24):9137–42. doi: 10.1073/pnas.0402546101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mohler PJ, Gramolini AO, Bennett V. Ankyrins. J Cell Sci. 2002;115(Pt 8):1565–6. doi: 10.1242/jcs.115.8.1565. [DOI] [PubMed] [Google Scholar]
- 30.Nelson WJ, Veshnock PJ. Ankyrin binding to (Na+ + K+)ATPase and implications for the organization of membrane domains in polarized cells. Nature. 1987;328(6130):533–6. doi: 10.1038/328533a0. [DOI] [PubMed] [Google Scholar]
- 31.Koob R, Zimmermann M, Schoner W, Drenckhahn D. Colocalization and coprecipitation of ankyrin and Na+,K+-ATPase in kidney epithelial cells. Eur J Cell Biol. 1988;45(2):230–7. [PubMed] [Google Scholar]
- 32.Morrow JS, Cianci CD, Ardito T, Mann AS, Kashgarian M. Ankyrin links fodrin to the alpha subunit of Na,K-ATPase in Madin-Darby canine kidney cells and in intact renal tubule cells. J Cell Biol. 1989;108(2):455–65. doi: 10.1083/jcb.108.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Devaux J, Alcaraz G, Grinspan J, Bennett V, Joho R, Crest M, et al. Kv3.1b is a novel component of CNS nodes. J Neurosci. 2003;23(11):4509–18. doi: 10.1523/JNEUROSCI.23-11-04509.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lopez C, Metral S, Eladari D, Drevensek S, Gane P, Chambrey R, et al. The ammonium transporter RhBG: requirement of a tyrosine-based signal and ankyrin-G for basolateral targeting and membrane anchorage in polarized kidney epithelial cells. J Biol Chem. 2005;280(9):8221–8. doi: 10.1074/jbc.M413351200. [DOI] [PubMed] [Google Scholar]
- 35.Michaely P, Bennett V. Mechanism for binding site diversity on ankyrin. Comparison of binding sites on ankyrin for neurofascin and the Cl-/HCO3- anion exchanger. J Biol Chem. 1995;270(52):31298–302. doi: 10.1074/jbc.270.52.31298. [DOI] [PubMed] [Google Scholar]
- 36.Michaely P, Bennett V. The ANK repeats of erythrocyte ankyrin form two distinct but cooperative binding sites for the erythrocyte anion exchanger. J Biol Chem. 1995;270(37):22050–7. doi: 10.1074/jbc.270.37.22050. [DOI] [PubMed] [Google Scholar]
- 37.Bennett V, Stenbuck PJ. Identification and partial purification of ankyrin, the high affinity membrane attachment site for human erythrocyte spectrin. J Biol Chem. 1979;254(7):2533–41. [PubMed] [Google Scholar]
- 38.Bodine DMt, Birkenmeier CS, Barker JE. Spectrin deficient inherited hemolytic anemias in the mouse: characterization by spectrin synthesis and mRNA activity in reticulocytes. Cell. 1984;37(3):721–9. doi: 10.1016/0092-8674(84)90408-2. [DOI] [PubMed] [Google Scholar]
- 39.Liu SC, Derick LH, Agre P, Palek J. Alteration of the erythrocyte membrane skeletal ultrastructure in hereditary spherocytosis, hereditary elliptocytosis, and pyropoikilocytosis. Blood. 1990;76(1):198–205. [PubMed] [Google Scholar]
- 40.Hanspal M, Yoon SH, Yu H, Hanspal JS, Lambert S, Palek J, et al. Molecular basis of spectrin and ankyrin deficiencies in severe hereditary spherocytosis: evidence implicating a primary defect of ankyrin. Blood. 1991;77(1):165–73. [PubMed] [Google Scholar]
- 41.Jenkins SM, Bennett V. Ankyrin-G coordinates assembly of the spectrin-based membrane skeleton, voltage-gated sodium channels, and L1 CAMs at Purkinje neuron initial segments. J Cell Biol. 2001;155(5):739–46. doi: 10.1083/jcb.200109026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kizhatil K, Bennett V. Lateral Membrane Biogenesis in Human Bronchial Epithelial Cells Requires 190-kDa Ankyrin-G. J Biol Chem. 2004;279(16):16706–14. doi: 10.1074/jbc.M314296200. [DOI] [PubMed] [Google Scholar]
- 43.Yang Y, Ogawa Y, Hedstrom KL, Rasband MN. betaIV spectrin is recruited to axon initial segments and nodes of Ranvier by ankyrinG. J Cell Biol. 2007;176(4):509–19. doi: 10.1083/jcb.200610128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang Y, Lacas-Gervais S, Morest DK, Solimena M, Rasband MN. BetaIV spectrins are essential for membrane stability and the molecular organization of nodes of Ranvier. J Neurosci. 2004;24(33):7230–40. doi: 10.1523/JNEUROSCI.2125-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Davis LH, Davis JQ, Bennett V. Ankyrin regulation: an alternatively spliced segment of the regulatory domain functions as an intramolecular modulator. J Biol Chem. 1992;267(26):18966–72. [PubMed] [Google Scholar]
- 46.Hall TG, Bennett V. Regulatory domains of erythrocyte ankyrin. J Biol Chem. 1987;262(22):10537–45. [PubMed] [Google Scholar]
- 47.Mohler PJ, Hoffman JA, Davis JQ, Abdi KM, Kim CR, Jones SK, et al. Isoform Specificity among Ankyrins: An amphipathic alpha-helix in the divergent regulatory domain of ankyrin-B interacts with the molecular co-chaperone Hdj1/Hsp40. J Biol Chem. 2004;279(24):25798–804. doi: 10.1074/jbc.M401296200. [DOI] [PubMed] [Google Scholar]
- 48.Abdi KM, Mohler PJ, Davis JQ, Bennett V. Isoform Specificity of Ankyrin-B: A SITE IN THE DIVERGENT C-TERMINAL DOMAIN IS REQUIRED FOR INTRAMOLECULAR ASSOCIATION. J Biol Chem. 2006;281(9):5741–9. doi: 10.1074/jbc.M506697200. [DOI] [PubMed] [Google Scholar]
- 49.Mohler PJ, Le Scouarnec S, Denjoy I, Lowe JS, Guicheney P, Caron L, et al. Defining the cellular phenotype of "ankyrin-B syndrome" variants: human ANK2 variants associated with clinical phenotypes display a spectrum of activities in cardiomyocytes. Circulation. 2007;115(4):432–41. doi: 10.1161/CIRCULATIONAHA.106.656512. [DOI] [PubMed] [Google Scholar]
- 50.Borzok MA, Catino DH, Nicholson JD, Kontrogianni-Konstantopoulos A, Bloch RJ. Mapping the binding site on small ankyrin 1 for obscurin. J Biol Chem. 2007;282(44):32384–96. doi: 10.1074/jbc.M704089200. [DOI] [PubMed] [Google Scholar]
- 51.Del Rio M, Imam A, DeLeon M, Gomez G, Mishra J, Ma Q, et al. The death domain of kidney ankyrin interacts with Fas and promotes Fas-mediated cell death in renal epithelia. J Am Soc Nephrol. 2004;15(1):41–51. doi: 10.1097/01.asn.0000104840.04124.5c. [DOI] [PubMed] [Google Scholar]
- 52.Kizhatil K, Davis JQ, Yoon W, Hogan BLM, Bennett V. American Society of Cell Biology; 2006. San Diego, CA: 2006. Polarized targeting of E-cadherin to sites of cell-cell contact in early embryos and epithelial cells requires ankyrin-G and beta-2-spectrin. [Google Scholar]
- 53.Splawski I, Shen J, Timothy KW, Lehmann MH, Priori S, Robinson JL, et al. Spectrum of mutations in long-QT syndrome genes. KVLQT1, HERG, SCN5A, KCNE1, and KCNE2. Circulation. 2000;102(10):1178–85. doi: 10.1161/01.cir.102.10.1178. [DOI] [PubMed] [Google Scholar]
- 54.Schwartz PJ, Priori SG, Locati EH, Napolitano C, Cantu F, Towbin JA, et al. Long QT syndrome patients with mutations of the SCN5A and HERG genes have differential responses to Na+ channel blockade and to increases in heart rate. Implications for gene-specific therapy. Circulation. 1995;92(12):3381–6. doi: 10.1161/01.cir.92.12.3381. [DOI] [PubMed] [Google Scholar]
- 55.Curran ME, Splawski I, Timothy KW, Vincent GM, Green ED, Keating MT. A molecular basis for cardiac arrhythmia: HERG mutations cause long QT syndrome. Cell. 1995;80(5):795–803. doi: 10.1016/0092-8674(95)90358-5. [DOI] [PubMed] [Google Scholar]
- 56.Wang Q, Curran ME, Splawski I, Burn TC, Millholland JM, VanRaay TJ, et al. Positional cloning of a novel potassium channel gene: KVLQT1 mutations cause cardiac arrhythmias. Nat Genet. 1996;12(1):17–23. doi: 10.1038/ng0196-17. [DOI] [PubMed] [Google Scholar]
- 57.Wang Q, Shen J, Li Z, Timothy K, Vincent GM, Priori SG, et al. Cardiac sodium channel mutations in patients with long QT syndrome, an inherited cardiac arrhythmia. Hum Mol Genet. 1995;4(9):1603–7. doi: 10.1093/hmg/4.9.1603. [DOI] [PubMed] [Google Scholar]
- 58.Schott JJ, Charpentier F, Peltier S, Foley P, Drouin E, Bouhour JB, et al. Mapping of a gene for long QT syndrome to chromosome 4q25–27. American journal of human genetics. 1995;57(5):1114–22. [PMC free article] [PubMed] [Google Scholar]
- 59.Berlin JR, Cannell MB, Lederer WJ. Cellular origins of the transient inward current in cardiac myocytes. Role of fluctuations and waves of elevated intracellular calcium. Circ Res. 1989;65(1):115–26. doi: 10.1161/01.res.65.1.115. [DOI] [PubMed] [Google Scholar]
- 60.Schlotthauer K, Bers DM. Sarcoplasmic reticulum Ca(2+) release causes myocyte depolarization. Underlying mechanism and threshold for triggered action potentials. Circ Res. 2000;87(9):774–80. doi: 10.1161/01.res.87.9.774. [DOI] [PubMed] [Google Scholar]
- 61.Egdell RM, De Souza AI, Macleod KT. Relative importance of SR load and cytoplasmic calcium concentration in the genesis of aftercontractions in cardiac myocytes. Cardiovasc Res. 2000;47(4):769–77. doi: 10.1016/s0008-6363(00)00147-4. [DOI] [PubMed] [Google Scholar]
- 62.Mank-Seymour AR, Richmond JL, Wood LS, Reynolds JM, Fan YT, Warnes GR, et al. Association of torsades de pointes with novel and known single nucleotide polymorphisms in long QT syndrome genes. Am Heart J. 2006;152(6):1116–22. doi: 10.1016/j.ahj.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 63.Sherman J, Tester DJ, Ackerman MJ. Targeted mutational analysis of ankyrin-B in 541 consecutive, unrelated patients referred for long QT syndrome genetic testing and 200 healthy subjects. Heart Rhythm. 2005;2(11):1218–23. doi: 10.1016/j.hrthm.2005.07.026. [DOI] [PubMed] [Google Scholar]
- 64.Mangrum JM, DiMarco JP. The evaluation and management of bradycardia. N Engl J Med. 2000;342(10):703–9. doi: 10.1056/NEJM200003093421006. [DOI] [PubMed] [Google Scholar]
- 65.Lev M. Aging changes in the human sinoatrial node. Journal of gerontology. 1954;9(1):1–9. doi: 10.1093/geronj/9.1.1. [DOI] [PubMed] [Google Scholar]
- 66.Bernstein AD, Parsonnet V. Survey of cardiac pacing in the United States in 1989. The American journal of cardiology. 1992;69(4):331–8. doi: 10.1016/0002-9149(92)90229-r. [DOI] [PubMed] [Google Scholar]
- 67.Demoulin JC, Kulbertus HE. Histopathological correlates of sinoatrial disease. British heart journal. 1978;40(12):1384–9. doi: 10.1136/hrt.40.12.1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Benson DW, Wang DW, Dyment M, Knilans TK, Fish FA, Strieper MJ, et al. Congenital sick sinus syndrome caused by recessive mutations in the cardiac sodium channel gene (SCN5A) J Clin Invest. 2003;112(7):1019–28. doi: 10.1172/JCI18062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Smits JP, Koopmann TT, Wilders R, Veldkamp MW, Opthof T, Bhuiyan ZA, et al. A mutation in the human cardiac sodium channel (E161K) contributes to sick sinus syndrome, conduction disease and Brugada syndrome in two families. J Mol Cell Cardiol. 2005;38(6):969–81. doi: 10.1016/j.yjmcc.2005.02.024. [DOI] [PubMed] [Google Scholar]
- 70.Ueda K, Nakamura K, Hayashi T, Inagaki N, Takahashi M, Arimura T, et al. Functional characterization of a trafficking-defective HCN4 mutation, D553N, associated with cardiac arrhythmia. J Biol Chem. 2004;279(26):27194–8. doi: 10.1074/jbc.M311953200. [DOI] [PubMed] [Google Scholar]
- 71.Klein G, Schroder F, Vogler D, Schaefer A, Haverich A, Schieffer B, et al. Increased open probability of single cardiac L-type calcium channels in patients with chronic atrial fibrillation. role of phosphatase 2A. Cardiovasc Res. 2003;59(1):37–45. doi: 10.1016/s0008-6363(03)00357-2. [DOI] [PubMed] [Google Scholar]
- 72.De Arcangelis V, Soto D, Xiang Y. Phosphodiesterase 4 and phosphatase 2A differentially regulate cAMP/protein kinase a signaling for cardiac myocyte contraction under stimulation of beta1 adrenergic receptor. Mol Pharmacol. 2008;74(5):1453–62. doi: 10.1124/mol.108.049718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Davare MA, Avdonin V, Hall DD, Peden EM, Burette A, Weinberg RJ, et al. A beta2 adrenergic receptor signaling complex assembled with the Ca2+ channel Cav1.2. Science. 2001;293(5527):98–101. doi: 10.1126/science.293.5527.98. [DOI] [PubMed] [Google Scholar]
- 74.Davare MA, Horne MC, Hell JW. Protein phosphatase 2A is associated with class C L-type calcium channels (Cav1.2) and antagonizes channel phosphorylation by cAMP-dependent protein kinase. J Biol Chem. 2000;275(50):39710–7. doi: 10.1074/jbc.M005462200. [DOI] [PubMed] [Google Scholar]
- 75.Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, et al. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell. 2000;101(4):365–76. doi: 10.1016/s0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- 76.DeSouza N, Reiken S, Ondrias K, Yang YM, Matkovich S, Marks AR. Protein kinase A and two phosphatases are components of the inositol 1,4,5-trisphosphate receptor macromolecular signaling complex. J Biol Chem. 2002;277(42):39397–400. doi: 10.1074/jbc.M207059200. [DOI] [PubMed] [Google Scholar]
- 77.Lecuona E, Dada LA, Sun H, Butti ML, Zhou G, Chew TL, et al. Na,K-ATPase alpha1-subunit dephosphorylation by protein phosphatase 2A is necessary for its recruitment to the plasma membrane. Faseb J. 2006;20(14):2618–20. doi: 10.1096/fj.06-6503fje. [DOI] [PubMed] [Google Scholar]
- 78.Borisov AB, Raeker MO, Kontrogianni-Konstantopoulos A, Yang K, Kurnit DM, Bloch RJ, et al. Rapid response of cardiac obscurin gene cluster to aortic stenosis: differential activation of Rho-GEF and MLCK and involvement in hypertrophic growth. Biochem Biophys Res Commun. 2003;310(3):910–8. doi: 10.1016/j.bbrc.2003.09.035. [DOI] [PubMed] [Google Scholar]
- 79.Borisov AB, Sutter SB, Kontrogianni-Konstantopoulos A, Bloch RJ, Westfall MV, Russell MW. Essential role of obscurin in cardiac myofibrillogenesis and hypertrophic response: evidence from small interfering RNA-mediated gene silencing. Histochemistry and cell biology. 2006;125(3):227–38. doi: 10.1007/s00418-005-0069-x. [DOI] [PubMed] [Google Scholar]
- 80.Terentyev D, Belevych AE, Terentyeva R, Martin MM, Malana GE, Kuhn DE, et al. miR-1 Overexpression Enhances Ca2+ Release and Promotes Cardiac Arrhythmogenesis by Targeting PP2A Regulatory Subunit B56{alpha} and Causing CaMKII-Dependent Hyperphosphorylation of RyR2. Circ Res. 2009 doi: 10.1161/CIRCRESAHA.108.181651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hund TJ, Wright PJ, Dun W, Snyder JS, Boyden PA, Mohler PJ. Regulation of the ankyrin-B-based targeting pathway following myocardial infarction. Cardiovasc Res. 2009;81(4):742–9. doi: 10.1093/cvr/cvn348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Janse MJ, Wit AL. Electrophysiological mechanisms of ventricular arrhythmias resulting from myocardial ischemia and infarction. Physiol Rev. 1989;69(4):1049–169. doi: 10.1152/physrev.1989.69.4.1049. [DOI] [PubMed] [Google Scholar]
- 83.Pinto JM, Boyden PA. Electrical remodeling in ischemia and infarction. Cardiovasc Res. 1999;42(2):284–97. doi: 10.1016/s0008-6363(99)00013-9. [DOI] [PubMed] [Google Scholar]
- 84.Baba S, Dun W, Cabo C, Boyden PA. Remodeling in cells from different regions of the reentrant circuit during ventricular tachycardia. Circulation. 2005;112(16):2386–96. doi: 10.1161/CIRCULATIONAHA.105.534784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pu J, Boyden PA. Alterations of Na+ currents in myocytes from epicardial border zone of the infarcted heart. A possible ionic mechanism for reduced excitability and postrepolarization refractoriness. Circ Res. 1997;81(1):110–9. doi: 10.1161/01.res.81.1.110. [DOI] [PubMed] [Google Scholar]
- 86.Priori SG, Bloise R, Crotti L. The long QT syndrome. Europace. 2001;3(1):16–27. doi: 10.1053/eupc.2000.0141. [DOI] [PubMed] [Google Scholar]
- 87.Priori SG, Napolitano C, Schwartz PJ. Electrophysiologic mechanisms involved in the development of torsades de pointes. Cardiovascular drugs and therapy/sponsored by the International Society of Cardiovascular Pharmacotherapy. 1991;5(1):203–12. doi: 10.1007/BF03029822. [DOI] [PubMed] [Google Scholar]
- 88.Gaita F, Giustetto C, Bianchi F, Wolpert C, Schimpf R, Riccardi R, et al. Short QT Syndrome: a familial cause of sudden death. Circulation. 2003;108(8):965–70. doi: 10.1161/01.CIR.0000085071.28695.C4. [DOI] [PubMed] [Google Scholar]
- 89.Keating MT, Sanguinetti MC. Molecular and cellular mechanisms of cardiac arrhythmias. Cell. 2001;104(4):569–80. doi: 10.1016/s0092-8674(01)00243-4. [DOI] [PubMed] [Google Scholar]
- 90.Wang Q, Shen J, Splawski I, Atkinson D, Li Z, Robinson JL, et al. SCN5A mutations associated with an inherited cardiac arrhythmia, long QT syndrome. Cell. 1995;80(5):805–11. doi: 10.1016/0092-8674(95)90359-3. [DOI] [PubMed] [Google Scholar]
- 91.Pfeufer A, Jalilzadeh S, Perz S, Mueller JC, Hinterseer M, Illig T, et al. Common variants in myocardial ion channel genes modify the QT interval in the general population: results from the KORA study. Circ Res. 2005;96(6):693–701. doi: 10.1161/01.RES.0000161077.53751.e6. [DOI] [PubMed] [Google Scholar]
- 92.Sedlacek K, Stark K, Cunha SR, Pfeufer A, Weber S, Berger I, et al. Common Genetic Variants in ANK2 Modulate QT Interval: Results from the KORA Study. Circulation: Cardiovascular Genetics. 2008;1(2):93–9. doi: 10.1161/CIRCGENETICS.108.792192. [DOI] [PubMed] [Google Scholar]
- 93.Akyol M, Jalilzadeh S, Sinner MF, Perz S, Beckmann BM, Gieger C, et al. The common non-synonymous variant G38S of the KCNE1-(minK)-gene is not associated to QT interval in Central European Caucasians: results from the KORA study. European heart journal. 2007;28(3):305–9. doi: 10.1093/eurheartj/ehl460. [DOI] [PubMed] [Google Scholar]
- 94.Arking DE, Pfeufer A, Post W, Kao WH, Newton-Cheh C, Ikeda M, et al. A common genetic variant in the NOS1 regulator NOS1AP modulates cardiac repolarization. Nat Genet. 2006;38(6):644–51. doi: 10.1038/ng1790. [DOI] [PubMed] [Google Scholar]
- 95.Bennett PB, Yazawa K, Makita N, George AL., Jr Molecular mechanism for an inherited cardiac arrhythmia. Nature. 1995;376(6542):683–5. doi: 10.1038/376683a0. [DOI] [PubMed] [Google Scholar]
- 96.Chen Q, Kirsch GE, Zhang D, Brugada R, Brugada J, Brugada P, et al. Genetic basis and molecular mechanism for idiopathic ventricular fibrillation. Nature. 1998;392(6673):293–6. doi: 10.1038/32675. [DOI] [PubMed] [Google Scholar]
- 97.Schott JJ, Alshinawi C, Kyndt F, Probst V, Hoorntje TM, Hulsbeek M, et al. Cardiac conduction defects associate with mutations in SCN5A. Nat Genet. 1999;23(1):20–1. doi: 10.1038/12618. [DOI] [PubMed] [Google Scholar]
- 98.Zhou D, Lambert S, Malen PL, Carpenter S, Boland LM, Bennett V. AnkyrinG is required for clustering of voltage-gated Na channels at axon initial segments and for normal action potential firing. J Cell Biol. 1998;143(5):1295–304. doi: 10.1083/jcb.143.5.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jenkins SM, Bennett V. Developing nodes of Ranvier are defined by ankyrin-G clustering and are independent of paranodal axoglial adhesion. Proc Natl Acad Sci U S A. 2002;99(4):2303–8. doi: 10.1073/pnas.042601799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Flucher BE, Daniels MP. Distribution of Na+ channels and ankyrin in neuromuscular junctions is complementary to that of acetylcholine receptors and the 43 kd protein. Neuron. 1989;3(2):163–75. doi: 10.1016/0896-6273(89)90029-9. [DOI] [PubMed] [Google Scholar]
- 101.Kordeli E, Ludosky MA, Deprette C, Frappier T, Cartaud J. AnkyrinG is associated with the postsynaptic membrane and the sarcoplasmic reticulum in the skeletal muscle fiber. J Cell Sci. 1998;111(Pt 15):2197–207. doi: 10.1242/jcs.111.15.2197. [DOI] [PubMed] [Google Scholar]
- 102.Wood SJ, Slater CR. beta-Spectrin is colocalized with both voltage-gated sodium channels and ankyrinG at the adult rat neuromuscular junction. J Cell Biol. 1998;140(3):675–84. doi: 10.1083/jcb.140.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kordeli E, Lambert S, Bennett V, Ankyrin G. A new ankyrin gene with neural-specific isoforms localized at the axonal initial segment and node of Ranvier. J Biol Chem. 1995;270(5):2352–9. doi: 10.1074/jbc.270.5.2352. [DOI] [PubMed] [Google Scholar]
- 104.Davis JQ, Lambert S, Bennett V. Molecular composition of the node of Ranvier: identification of ankyrin- binding cell adhesion molecules neurofascin (mucin+/third FNIII domain- ) and NrCAM at nodal axon segments. J Cell Biol. 1996;135(5):1355–67. doi: 10.1083/jcb.135.5.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pan Z, Kao T, Horvath Z, Lemos J, Sul JY, Cranstoun SD, et al. A common ankyrin-G-based mechanism retains KCNQ and NaV channels at electrically active domains of the axon. J Neurosci. 2006;26(10):2599–613. doi: 10.1523/JNEUROSCI.4314-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hill AS, Nishino A, Nakajo K, Zhang G, Fineman JR, Selzer ME, et al. Ion channel clustering at the axon initial segment and node of ranvier evolved sequentially in early chordates. PLoS genetics. 2008;4(12):e1000317. doi: 10.1371/journal.pgen.1000317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Garrido JJ, Giraud P, Carlier E, Fernandes F, Moussif A, Fache MP, et al. A targeting motif involved in sodium channel clustering at the axonal initial segment. Science. 2003;300(5628):2091–4. doi: 10.1126/science.1085167. [DOI] [PubMed] [Google Scholar]
- 108.Lemaillet G, Walker B, Lambert S. Identification of a conserved ankyrin-binding motif in the family of sodium channel alpha subunits. J Biol Chem. 2003;278(30):27333–9. doi: 10.1074/jbc.M303327200. [DOI] [PubMed] [Google Scholar]
- 109.Antzelevitch C, Brugada P, Borggrefe M, Brugada J, Brugada R, Corrado D, et al. Brugada syndrome: report of the second consensus conference. Heart Rhythm. 2005;2(4):429–40. doi: 10.1016/j.hrthm.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 110.Priori SG. Inherited arrhythmogenic diseases: the complexity beyond monogenic disorders. Circ Res. 2004;94(2):140–5. doi: 10.1161/01.RES.0000115750.12807.7E. [DOI] [PubMed] [Google Scholar]
- 111.Priori SG, Napolitano C. Genetics of cardiac arrhythmias and sudden cardiac death. Ann N Y Acad Sci. 2004;1015:96–110. doi: 10.1196/annals.1302.008. [DOI] [PubMed] [Google Scholar]
- 112.Smits JP, Eckardt L, Probst V, Bezzina CR, Schott JJ, Remme CA, et al. Genotype-phenotype relationship in Brugada syndrome: electrocardiographic features differentiate SCN5A-related patients from non-SCN5A-related patients. J Am Coll Cardiol. 2002;40(2):350–6. doi: 10.1016/s0735-1097(02)01962-9. [DOI] [PubMed] [Google Scholar]
- 113.Priori SG, Napolitano C, Gasparini M, Pappone C, Della Bella P, Giordano U, et al. Natural history of Brugada syndrome: insights for risk stratification and management. Circulation. 2002;105(11):1342–7. doi: 10.1161/hc1102.105288. [DOI] [PubMed] [Google Scholar]