Abstract
Objective:
This study explores the efficacy of an experimental orthodontic amorphous calcium phosphate (ACP) composite to remineralize in vitro subsurface enamel lesions microradiographically similar to those seen in early caries.
Methods:
Lesions were artificially created in extracted human molars. Single tooth sections a minimum of 120 μm thick were cut and individually placed in holders exposing only the carious enamel surface. The exposed surfaces were either left untreated (control) or coated with a 1 mm thick layer of the experimental ACP composite (mass fraction 40 % zirconiahybridized ACP and 60 % photo-activated resin), or a commercial fluoride-releasing orthodontic cement. The composite-coated sections were then photo-cured and microradiographic images were taken of all three groups of specimens before the treatment. Specimens were then cyclically immersed in demineralizing and remineralizing solutions for one month at 37 °C to simulate the pH changes occurring in the oral environment. Microradiographs of all specimens were taken before and after treatment.
Results:
Quantitative digital image analysis of matched areas from the contact microradiographs taken before and after treatment indicated higher mineral recovery with ACP composites compared to the commercial orthodontic F-releasing cement (14.4 % vs. 4.3 %, respectively), while the control specimens showed an average of 55.4 % further demineralization.
Significance:
Experimental ACP composite efficiently established mineral ion transfer throughout the body of the lesions and restored the mineral lost due to acid attack. It can be considered a useful adjuvant for the control of caries in orthodontic applications.
Keywords: amorphous calcium phosphate, caries, enamel, polymeric composite, remineralization
Introduction
Teeth are constantly going through cycles of mineral loss (oral pH is below or at the point at which tooth mineral begins to dissolve) and repair (conditions favor the redeposition of mineral). The net loss or gain in mineral over time ultimately determines whether tooth decay will advance, stabilize or regress. The ultimate goal of clinical intervention is the preservation of tooth structure and the prevention of lesion progression to the point where restoration is required.
Incorporation of fluoride (F) into dental mineral has been the scientific keystone for caries prevention [1-3]. The effectiveness of fluoride released from various delivery vehicles in promoting remineralization and inhibiting demineralization of enamel has been widely demonstrated [4-9]. It is generally accepted that the beneficial effects of F arise from its incorporation in tooth mineral as fluoroapatite or F-enriched hydroxyapatite, both leading to the decreased solubility of tooth enamel [10]. The remineralization and arrest of dentin lesions, however, has received less attention [11]. It was found that collagen has no effect on the remineralization of dentin [12, 13] but it can serve as a substrate for apatite formation when in the presence of specific proteins [14].
Fluoridation, however, is not the only mechanism for creating favorable remineralizing or anti-demineralizing conditions in the oral environment. Mineral growth can also be stimulated by increasing solution calcium and phosphate concentrations within the lesion to levels that exceed those existing in ambient oral fluids. Remineralization of enamel subsurface lesions has been successfully achieved by casein phosphopeptide-stabilized calcium phosphate solutions [15, 16] and an amorphous calcium phosphate (ACP) polymeric sealant [17]. Casein phosphopeptide-ACP complex delivered in a sugar-free chewing gum has the potential to control dental caries via the combination of active remineralization and salivary stimulation. In the case of ACP sealants, solution solubility of ACP [18] enabled the release of supersaturating levels of calcium and phosphate ions intralesionally and shifted the solution thermodynamic driving forces toward the formation of apatite. ACP can sustain these supersaturation conditions over extended periods of time [19, 20].
The present study was designed to assess whether ACP polymeric material, formulated for use as an orthodontic composite and applied to the surface of caries-like lesions created in human enamel, can efficiently establish mineral ion transfer into the body of the lesions and restore the mineral lost due to acid attack. To examine this hypothesis, ACP composites were tested against a commercial F-releasing orthodontic cement under identical pH-cycling regimen using the single section technique with lesions created before tooth sectioning. The changes in mineral content of the lesions were quantified by digital image analysis of their contact microradiographs before and after pH-cycling.
Materials and Methods
Resin formulation
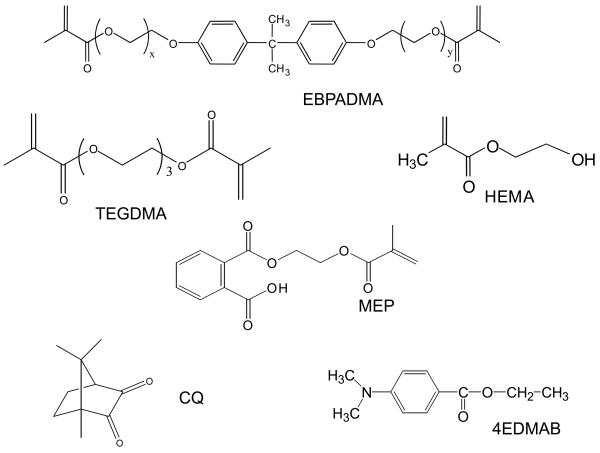
The resin was formulated from ethoxylated bisphenol A dimethacrylate (EBPADMA; mass fraction 62.8 %), triethylene glycol dimethacrylate (TEGDMA; mass fraction 23.2 %), 2-hydroxyethyl methacrylate (HEMA; mass fraction 10.4 %) and methacryloyloxyethyl phthalate (MEP; mass fraction 2.6 %) [ETHM resin]. It was photo-activated by the inclusion of camphorquinone (CQ; mass fraction 0.2 %) and ethyl-4,4-N,N-dimethylaminobenzoate (4E; mass fraction 0.8 %). The chemical structure of the monomers and the components of the photo-initiator system are shown in Fig. 1.
Fig. 1.
Chemical structure of the monomers and photo-initiator system employed in formulation of ETHM resin.
ACP filler
Zirconia hybridized ACP (Zr-ACP) was prepared according to our previously published protocol [19, 20]. The amorphous state of the filler was verified by X-ray diffractometry (Rigaku DMAX 2000 X-ray diffractometer, Rigaku/USA Inc., Danvers, MA, USA) and Fourier-transform infrared spectroscopy (Nicolet Magna-IR FTIR 550 spectrophotometer, Nicolet Instrumentations Inc., Madison, WI, USA). Morphology of the filler was examined by scanning electron microscopy (SEM; JEOL 35C instrument, JEOL Inc., Peabody, MA, USA) after the specimens were sputter-coated with gold. The particle size distribution of Zr-ACP particle size distribution was determined using laser obscuration concurrently with a computerized inspection system (CIS-100 Particle Size Analyzer, Ankersmid Ltd., Yokneam, Israel).
ACP composite preparation
Composite paste was prepared in 2 g batches by handmixing ETHM resin (mass fraction 60 %) and Zr-ACP (mass fraction 40 %). The paste was mixed until a uniform consistency was achieved, with no remaining visible particulates. Prior to photo-curing, the homogenized pastes were spread thinly on a dental slab (flat glass block) and kept under moderate vacuum (2.7 kPa) to eliminate the air entrained during mixing.
Human tooth specimens
The project received an Institutional Review Board exemption, since the teeth were collected from local dentists and have no associated patient identification. Teeth were initially soaked for 4 h in a 0.005 % promodyne disinfectant solution, after which they were mechanically scraped to remove any soft tissue and stored in refrigerated distilled water until use.
Tooth sections with caries-like lesions
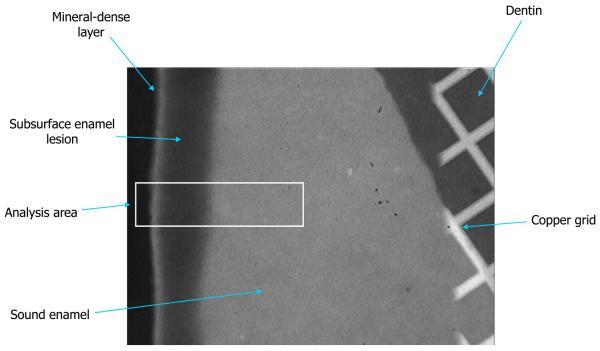
The subsurface enamel lesions were created by immersing varnish-coated tooth specimens, with 3 mm by 3 mm window on their labial surfaces exposed, into an acidic calcium phosphate solution of the following composition: 8.7 mmol/L CaCl2, 8.7 mmol/L KH2PO4, 0.05 ppm NaF, 75 mmol/L acetic acid, pH = 4.0 (adjusted with KOH). Each individual tooth was immersed in 40 mL solution for 72 h, at 37 °C with no stirring. After demineralization, sagittal sections were cut from the teeth with a circular diamond saw (Isomet, Buehler Ltd., Lake Bluff, II, USA) to a thicknesses ranging from 120 μm to 180 μm. In order to aid in the alignment of ‘before’ and ‘after’ microradiographic images, copper grids were adhered to the sound enamel portion of each tooth slice.
Microradiography
Contact microradiographs of the tooth sections were produced on holographic film (Integraf LLC, Kirkland, WA, USA) exposed for 30 min to CuKα radiation (40 kV, 3 mA; Faxitron Model #43855A, Hewlett Packard, McMinnville, OR, USA) and developed according to manufacturer's recommendations. An Al step wedge exposed along with the tooth sections was used to estimate the enamel mineral density [21].
Application of composites to tooth section specimens
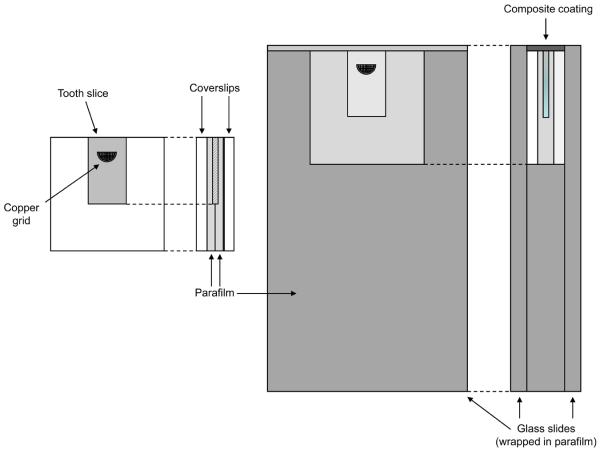
After the initial contact microradiographs were taken, the sections were randomly grouped and then sandwiched between parafilm and glass cover slips in the following order: cover slip, parafilm, tooth slice, parafilm, cover slip (keeping the demineralized edge of the slice even with one side of the sandwich). This assembly was wrapped and embedded in strips of parafilm to seal around the edges, leaving only the demineralized edge exposed. It was then sandwiched between two glass slides, with the exposed edge of the tooth section positioned approximately 1 mm below the slide edges. The demineralized surface was coated with a (1.0 ± 0.1) mm thick layer of the ACP composite paste or commercial adhesive. A schematic drawing of the specimen assembly is provided in Fig. 2. Both ACP composites and the commercial composites were irradiated with visible light for 60 s (Triad 2000, Dentsply International, York, PA, USA). Uncoated control sections required no curing and were assembled in the same manner.
Fig. 2.
Schematic diagram containing both the assembly which isolates the tooth slice (left) and the glass slide mount which holds the assembly and composite during cycling (right).
Cycling demineralization/remineralization treatment
The demineralizing solution (DS; [22]) used to mimic demineralizing oral fluid conditions has the following composition: 3.0 mmol/L CaCl2, 1.8 mmol/L K2HPO4, 0.1 mol/L lactic acid, mass fraction 1 % carboxymethylcellulose, and a pH of 4.0 (adjusted by the addition of KOH). The remineralizing solution (RS; [23]) used to simulate remineralizing oral fluid conditions contained 1.2 mmol/L CaCl2, 0.72 mmol/L K2HPO4, 2.6 μmol/L F, 50 mmol/L HEPES buffer, and had a pH equal to 7.0 (adjusted by the addition of KOH). The assemblies were alternately immersed in DS (1 h) and RS (23 h) at 37 °C for 30 d excluding weekends, when specimens were stored in distilled water. Twenty mL of fresh DS or RS was used per specimen for each immersion, with continuous magnetic stirring provided (38 rad/s). At every solution exchange, the assembly and exposed (composite) side of each specimen were rinsed with distilled water.
Mineral content of the lesions
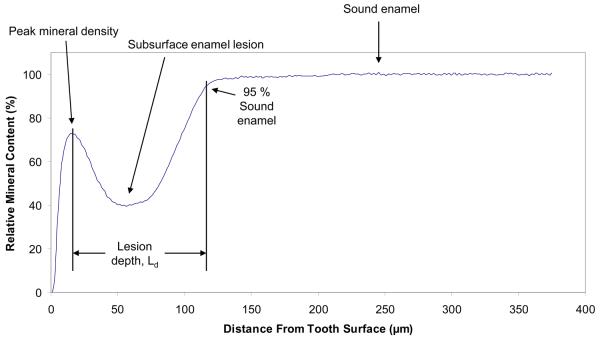
The mineral profiles of each specimen before and after demineralization/remineralization regimen were determined by quantitative analysis of contact microradiographs taken before and after treatment using the commercial digital-image-analysis system (Fig. 3a; Scion Image – release Alpha 4.0.3.2; National Institute of Health, Bethesda, MD, USA) interfaced with an optical microscope (Olympus BX50F; Olympus Optical Co., Ltd., Japan) and digital camera (RGB/YC/NTSC; Microimage Video Systems, Boyerstown, PA, USA). Digital imaging captured the gray levels (brightness) of a rectangular area (approx. 375 μm × 88 μm) of the radiographic images using an intensity resolution of 256 gray levels and a horizontal spatial resolution of 1.25 μm per pixel [22]. Changes in lesion depth (Ld, defined as the distance in μm from the point of peak mineral density in the surface layer to the point where mineral content reaches 95 % of the sound enamel value at the opposite side of the lesion; Fig. 3b), mineral loss (ΔZ; volume fraction % × 1.25 μm) were compared for each imaged area before and after treatment, as previously described in detail [17, 21, 22]. The difference in summed ΔZ values across the depth of each lesion before and after the pH-cycling regimen, i.e., the relative change in mineral content, Δ(ΔZ) in %, was calculated according to the following equation:
| (1) |
The mean Δ(ΔZ) values obtained for all image areas of ACP composite, F-orthodontic adhesive and uncoated control specimens were used to indicate remineralization (positive (+) Δ(ΔZ) values) or further demineralization of the lesions (negative (−) Δ(ΔZ) values) as a result of pH-cycling treatment.
Fig. 3.
Digital photomicrograph showing a typically prepared tooth slice (a) and a corresponding plot profile determined from the grayscale intensities of the image (b).
As an additional means of comparing the relative strengths and weaknesses of each treatment, Ld was nondimensionalized and the mineral content of each specimen was examined at 5 % increments along its depth. This allowed for the comparison of specimens with differing lesion depths.
Ion release from composite specimens
Calcium and phosphate ion release from the experimental ACP composite and fluoride ion release from the commercial orthodontic composite was examined at 23 °C, in continuously stirred saline solutions (0.13 mol/L sodium chloride; initial volume of saline solution per composite specimen: 100 mL). Five mL aliquots were taken at 7 d, 14 d, 21 d and 28 d immersion, respectively, and the kinetic changes in calcium and phosphate solution levels were determined by utilizing atomic emission spectroscopy (Prodigy High Dispersion ICP-OES, Teledyne Leeman Labs, Hudson, NH, USA). Solution fluoride content was determined by utilizing fluoride specific electrode (No. 100029; Thermo Fisher Scientific Inc., Waltham, MA, USA). Ion release kinetic experiments were run in triplicate.
Statistical analysis
The results were analyzed by the analysis of variance (ANOVA; α = 0.05). Statistical significance of change values was obtained from two-tailed P values using the Holm-Sidak test for paired data (before and after pH-cycling). Statistical calculations were done by means of SigmaStat software (version 3.5; SPSS Inc., Chicago, IL, USA). One standard deviation (SD) is identified in this paper for comparative purposes as the estimated uncertainty of the measurements.
Results
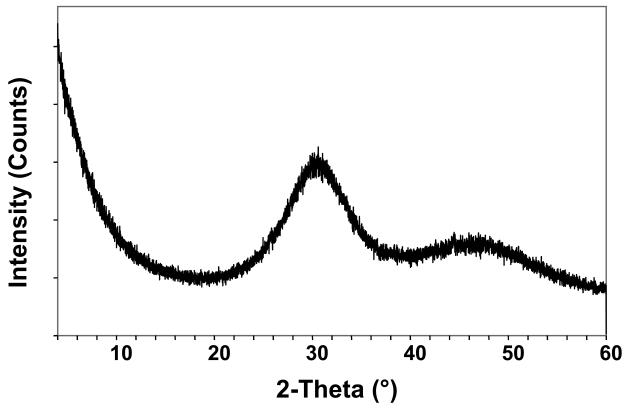
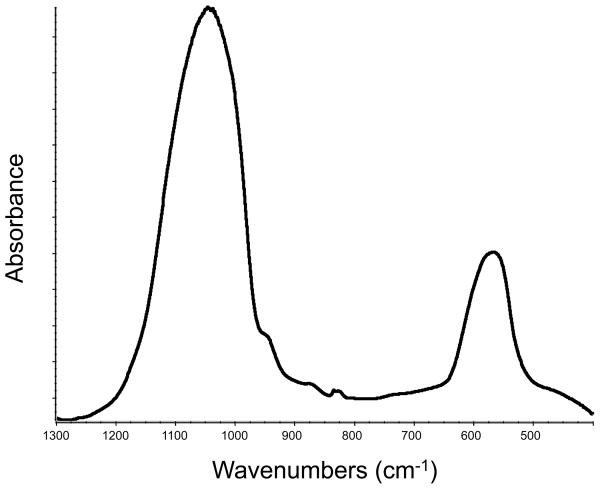
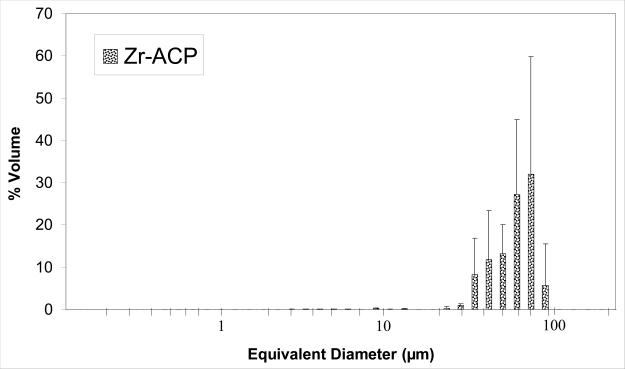
The amorphous character of the filler used to make the experimental ACP composites was confirmed by XRD (two diffuse broad bands in the 2θ = (4 to 60)° region) and FTIR (two wide phosphate absorbance bands at (1200 to 900) cm−1 and (630 to 550) cm−1) (Fig. 4a and 4b, respectively). Filler particle size varied from submicron to approximately 100 μm (Fig. 4c). The median diameter calculated from the volume distribution histogram was 55 μm. A typical SEM image (Fig. 4d) confirms the highly agglomerated nature and heterogeneous size distribution of Zr-ACP particles.
Fig. 4.
X-ray diffraction pattern (a), FTIR spectrum (b), volume particle size distribution (c) and the typical SEM image (d) and of Zr-ACP utilized in the experimental composite.
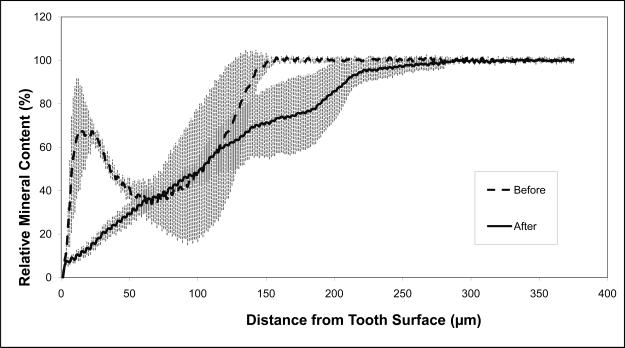
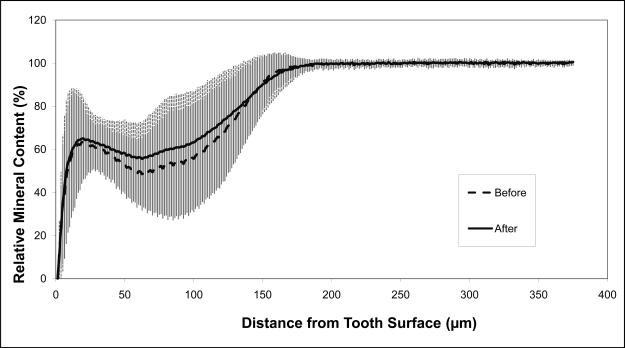
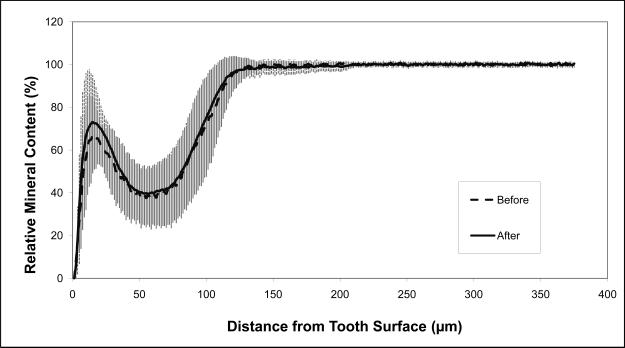
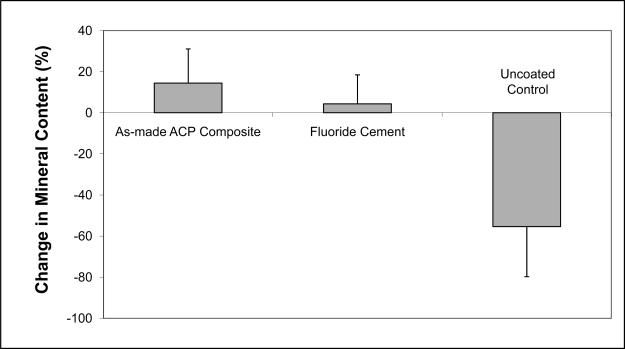
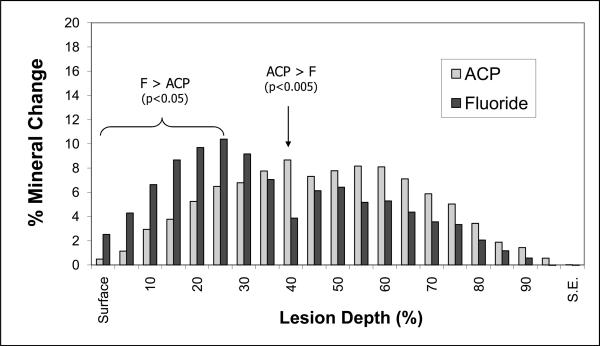
The average relative mineral profiles before and after pH-cycling are compared in Figs. 5a, b and c, respectively. A considerable image-to-image variability is indicated by the standard deviation values, shown here as vertical bars. Comparison of the summed Δ(ΔZ) values (Fig. 6; derived from the individual relative mineral content profiles) revealed that tooth sections coated with ACP composite recovered on average 14 % of the lost mineral [Δ(ΔZ) = + (14.4 ± 16.7) %], while identically treated, F-releasing composite-coated specimens regained only 4 % of the lost mineral [Δ(ΔZ) = + (4.4 ± 14.2) %]. Uncoated, control specimens lost on average an additional 55 % of the mineral [Δ(ΔZ) = − (55.4 ± 24.4) %]. The trends shown in Figs. 5 and 7 suggest that treatment from a F-releasing composites more efficiently regenerated mineral lost within the superficial portion of the lesion (the initial 30 % of its depth) while Ca- and PO4- releasing ACP composites were more effective within the deeper portions of the lesion and significantly more overall.
Fig. 5.
Mineral profiles (mean values ± standard deviation (indicated by vertical lines) of the uncoated control specimens (a), ACP composite-coated specimens (b), and Fluoride cement-coated specimens (c), before (blue) and after (pink) pH-cycling treatment. Number of specimens per group: 4 ≤ n ≤ 15; number of analyzed areas per group: 8 ≤ n ≤ 30.
Fig. 6.
Change in the relative mineral content (mean value + SD (indicated by bars)) as a function of the lesion depth for ACP composite and F-cement specimens. Data derived from the mean mineral profile values presented in Fig. 5.
Fig. 7.
Overall change in mineral content for each treatment (mean value + SD (indicated by bars)).
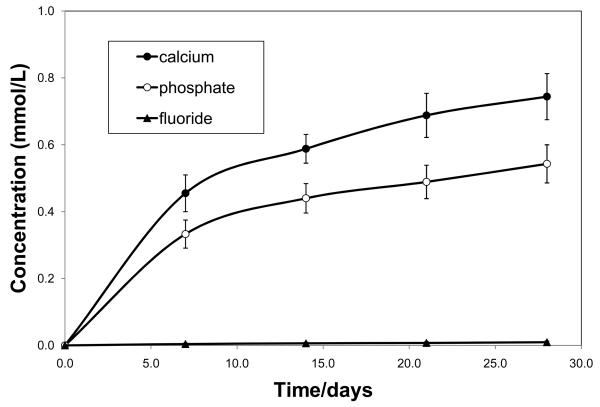
Kinetics of calcium and phosphate ions released from the experimental ACP composite and fluoride ions released from the commercial control composite are shown in Fig. 8.
Fig. 8.
Calcium and phosphate ion release from the experimental ACP composite and fluoride release from the commercial orthodontic cement. Indicated are mean values ± SD obtained from three repetitive measurements in each group.
The ion-release from the experimental ACP composite was much higher than the release of fluoride from the commercial control. After 4 weeks of saline immersion, the average solution calcium and phosphate concentrations reached (0.744±0.069) mmol/L and (0.543±0.057) mmol/L, respectively, compared to only (0.009±0.004) mmol/L fluoride released from the control specimens.
Discussion
The first evidence for the remineralization of tooth mineral was presented nearly a century ago in 1912, by J.A. Head [24]. In this study and many others over the next 70 years, diamond indenters and other metallurgical instruments were the primary tools for quantifying mineral change. In 1983, however, Featherstone et al. [25] found that a linear relationship existed between microhardness profiles of cross-sectioned enamel and microradiographic measurements of mineral loss. Since then, microradiographic examinations of thin tooth sections have become one of the preferred tools in studying the dynamic process of demineralization and remineralization.
Changes in ΔZ have been used in numerous studies as the main criterion for assessing the remineralization efficacy of various fluoride delivery systems [22, 23, 26, 27]. In our previous work on the in vitro recovery of caries-like lesions created in bovine enamel [17] comparing the overall ΔZ changes of differently treated tooth sections was also established as the most relevant parameter. A considerable variation in surface layer thickness and lesion depth of the bovine tooth specimens were noticeable, despite the identical, non-invasive treatment of the teeth prior to testing. Similar variations in lesion characteristics were encountered by Iijima et al. [28] and Ten Cate et al. [29], who attributed them to the uneven damage of the outer surfaces of the sections, including cracks and/or the loss of interprismatic material caused by polishing and cutting. Another important aspect of single section de/remineralization studies is the sealing off of the exposed section sides in order to protect these surfaces from external solution penetration. In the majority of studies, nail varnish is commonly used to protect cut surfaces of thin sections [28-34]. In this study, embedding the individual tooth slices in parafilm was seen as a sufficient means to isolate all but the designated enamel surfaces of the teeth.
In the literature, data on the extent of mineral recovery (Δ(ΔZ), expressed as percent increase of the net mineral content within caries-like lesions in human enamel) due to fluoride's remineralizing effect show significant variations with the type and duration of treatment. Single-solution fluoride rinses (12 mmol/L NaF, pH = 6.0) resulted in (46 ± 23) % mineral restoration compared to (94 ± 51) % recovery with two-solution rinses (Solution I: 20 mmol/L CaCl2, 25 mmol/L CH3COONa, pH = 7.1; Solution II: 4 mmol/L Na2SiF6, 5 mmol/LNa2HPO4, 5 mmol/L NaH2PO4, pH = 4.3) [27]. Applying Na2FPO3 solution at 1000 ppm recovered (58 ± 14) % mineral [32], while a combination of 0.05 % NaF and calcium phosphate mineralizing solution (pH adjusted to 6.0) restored only (15 ± 14) % of the mineral lost to acid attack [30]. Daily administration of a NaF dentifrice slurry to acid-etched enamel and exposure to a remineralizing solution containing 1.5 mmol/L CaCl2, 0.9 mmol/L KH2PO4 130 mmol/L KCl (pH = 7.0) repaired up to (55 ± 8) % under static remineralization conditions. Also under static pH conditions, casein phosphopeptide-containing calcium phosphate remineralizing solutions with a pH of 7.0 and 9.0 recovered (19 ± 12) % and (52 ± 21) % mineral, respectively [15]. Significantly, certain commercial dentifrices failed to show any mineral recovery under in vitro pH-cycling conditions regardless of the varying levels of fluoride claimed by manufacturers [9].
In this study, the average mineral recovery of tooth specimens coated with ACP composites was significantly higher than the recovery occurring in specimens treated with the F-releasing orthodontic adhesive (14.4 % vs. 4.4 %, respectively). This finding indicates that ACP-filled methacrylate-based composite formulated for orthodontic utility is indeed capable of releasing supersaturating levels of calcium and phosphate ions in proportions favorable for apatite formation and repairing teeth damaged by tooth decay.
The trends illustrated in Figs. 5 and 7 point to the relative strengths and weaknesses of each treatment. The fluoride-releasing cement deposits significantly more mineral than ACP composites in the initial 30 % of the lesion, but this may occlude surface pores and likely limits the extent to which the rest of the lesion may be repaired. While not providing significant advantage over fluoride in the superficial portion of the lesion, ACP composites are capable of delivering mineral deeper into the enamel, and deposited significantly more mineral overall than their fluoridated counterpart. In this case, the inability of fluoride to penetrate deeply into tooth structures puts it at a distinct disadvantage compared to ACP, especially given the evidence that saliva's natural remineralizing ability is only effective to a depth of 100 μm [35].
Ion release data (Fig. 8) suggest that the commercial fluoride-releasing composite simply does not have the ion-release capacity of the ACP material. It is, therefore, of no surprise that fluoride-containing commercial composite was a less effective material for remineralization beyond the top 30 % of the lesion compared to the experimental ACP composite.
The current formulation of ACP composites reflects their intended use as an orthodontic adhesive or a pit and fissure sealant. While the longevity of these particular materials may differ when placed in situ ((18 to 36) months for an orthodontic adhesive and, though undefined, sealants should reasonably be expected to last as long as a posterior resin composite restoration), both encompass significant periods of time. For this reason, a real-time experimental evaluation of their cumulative effects over their expected service life would be slightly unreasonable, both in the duration of the experiment and the overwhelming amount of data that would be generated. In this experiment, an aggressive demineralization attack was used to achieve the cumulative effects of years of service in a more realistic time-frame; this is clearly evidenced by the dissolution of the outer enamel in the uncoated control group (Fig. 5a).
Similar to many in vitro de/remineralization studies, the immersing solutions utilized here contain only inorganic ions and neglect the possible effects of salivary proteins, pellicle, and/or plaque, all of which may affect the in vivo mineralization. Therefore, although they should be interpreted with caution, the results reported in this study serve as proof of concept for this particular material and should be recognized as providing a necessary building block for future in vivo studies on the remineralizing effectiveness of ACP composites.
Conclusions
This study demonstrates that ACP polymeric composite formulated for orthodontic utility can effectively restore mineral lost due to acid attack. ACP composite can maintain a sufficient supply of calcium and phosphate ions and ion pairs into subsurface enamel lesions to provide a milieu favorable for remineralization. It can, therefore, be used as a useful adjuvant in caries prevention and/or repair.
Acknowledgements
This investigation was supported by USPHS Research grant DE13169 from the National Institute of Dental and Craniofacial Research, the National Institute of Standards and Technology and the American Dental Association Foundation. We acknowledge Esstech, Essington, PA, USA for generously providing the monomers used in this study. We also express gratitude to Mr. G.M. Flaim and Mr. S. Frukhtbeyn for their valuable technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclaimer
Certain commercial materials and equipment are identified in this work for adequate definition of the experimental procedures. In no instance does such identification imply recommendation or endorsement by the American Dental Association Foundation or the National Institute of Standards and Technology, or that the material and the equipment identified is necessarily the best available for the purpose.
References
- 1.ten Cate JM, Featherstone JDB. Mechanistic aspects of the interactions between fluoride and dental enamel. Crit Rev Oral Biol Med. 1991;2(2):283–296. doi: 10.1177/10454411910020030101. [DOI] [PubMed] [Google Scholar]
- 2.ten Cate JM. Current concepts on the theories of the mechanism of action of fluoride. Acta Odontol Scand. 1999;57:325–329. doi: 10.1080/000163599428562. [DOI] [PubMed] [Google Scholar]
- 3.Kashet S. Historical review of remineralization research. J Clin Dent. 1999;10:56–64. [Google Scholar]
- 4.ten Cate JM, Buijs MJ, Miller c.C., Exterkate RAM. Elevated fluoride products enhance remineralization of enamel. J Dent Res. 2008;87(10):943–947. doi: 10.1177/154405910808701019. [DOI] [PubMed] [Google Scholar]
- 5.Pessani JP, Al-Ibrahim NS, Busalap MAR, Toumba KJ. Slow-release fluoride devices: a literature review. J Appl Oral Sci. 2008;16(4):238–246. doi: 10.1590/S1678-77572008000400003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Toda S, Featherstone JD. Effects of fluoride dentifrices on enamel lesion formation. J Dent Res. 2008;87(3):224–227. doi: 10.1177/154405910808700303. [DOI] [PubMed] [Google Scholar]
- 7.Gonzales-Cabezas C, Fontana M, Dunipace AJ, Li Y, Fischer GM, Proskin HM, Stookey GK. Measurement of enamel remineralization using microradiography and confocal microscopy. Caries Res. 1998;32:385–392. doi: 10.1159/000016475. [DOI] [PubMed] [Google Scholar]
- 8.Schemerhorn BR, Orban JC, Wood GD, Fischer GM. Remineraliztion by fluoride enhanced with calcium and phosphate ingredients. J Clin Dent. 1999;10:13–16. [PubMed] [Google Scholar]
- 9.Itthagarun A, Wei SHY, Wefel JS. The effect of different commercial dentifrices on enamel lesion progression: an in vitro pH-cycling study. Int Dent J. 2000;50:21–28. doi: 10.1111/j.1875-595x.2000.tb00542.x. [DOI] [PubMed] [Google Scholar]
- 10.Chow LC, Vogel GL. Enhancing remineralization. Oper Dent. 2001;6:27–38. [Google Scholar]
- 11.ten Cate JM. Remineralization of caries lesions extending into dentin. J Dent Res. 2001;80(5):1407–1411. doi: 10.1177/00220345010800050401. [DOI] [PubMed] [Google Scholar]
- 12.Klont B, ten Cate JM. Remineralization of bovine incisor root lesions in vitro: the role of collageneous matrix. Caries Res. 1991;25:39–45. doi: 10.1159/000261340. [DOI] [PubMed] [Google Scholar]
- 13.Featherstone JD. Fluoride, remineralization and root caries. Am J Dent. 1994;7:271–274. [PubMed] [Google Scholar]
- 14.Lussi A, Linde A. Mineral induction in vivo by dentine proteins. Caries Res. 1993;27:241–248. doi: 10.1159/000261544. [DOI] [PubMed] [Google Scholar]
- 15.Reynolds EC, Cai F, Shen P, Walker GD. Retention in plaque and remineralization of enamel lesions by various forms of calcium in a mouthrinse or sugar-free chewing gum. J Dent Res. 2003;82(3):206–211. doi: 10.1177/154405910308200311. [DOI] [PubMed] [Google Scholar]
- 16.Reynolds EC, Cai F, Cochrane NJ, Shen P, Walker GD, Morgan MV, Reynolds C. Fluoride and casein phosphopeptide-amorphous calcium phosphate. J Dent Res. 2008;87(4):344–348. doi: 10.1177/154405910808700420. [DOI] [PubMed] [Google Scholar]
- 17.Skrtic D, Hailer AW, Takagi S, Antonucci JM, Eanes ED. Quantitative assessment of the efficacy of amorphous calcium phosphate/methacrylate composites in remineralizing caries-like lesions artificially produced in bovine enamel. J Dent Res. 1996;75(9):1679–1686. doi: 10.1177/00220345960750091001. [DOI] [PubMed] [Google Scholar]
- 18.Regnault WF, Icenogle TB, Antonucci JM, Skrtic D. Amorphous calcium phosphate/urethane methacrylate resin composites. I. Physicochemical characterization. J Mater Sci: Mater Med. 2008;19(2):507–515. doi: 10.1007/s10856-007-3178-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Antonucci JM, Skrtic D. Physicochemical properties of bioactive polymeric composites: Effects of resin matrix and the type of amorphous calcium phosphate filler. In: Shalaby SW, Salz U, editors. Polymers for dental and orthopedic applications. CRC Press; Boca Raton: 2007. pp. 217–242. [Google Scholar]
- 20.O'Donnell JNR, Langhorst SE, Fow MD, Antonucci JM, Skrtic D. Light-cured dimethacrylate based resins and their composites: Comparative study of mechanical strength, water sorption and ion release. J Bioact Compat Polym. 2008;23(3):207–226. doi: 10.1177/0883911508089932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chow LC, Takagi S, Tung W, Jordan TH. Digital image analysis assisted microradiography – measurement of mineral content of caries lesions in teeth. J Res Natl Inst Stand Technol. 1991;96:203–214. doi: 10.6028/jres.096.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dickens SH, Flaim GM, Takagi S. Mechanical properties and biochemical activity of remineralizing resin-based Ca-PO4 cements. Dent Mater. 2003;19:558–566. doi: 10.1016/s0109-5641(02)00105-7. [DOI] [PubMed] [Google Scholar]
- 23.Damato FA, Strang R, Stephen KW. Comparison of solution- and gel-prepared enamel lesions – an in vitro pH-cycling study. J Dent Res. 1988;67:1122–1125. doi: 10.1177/00220345880670081201. [DOI] [PubMed] [Google Scholar]
- 24.Head JA. A study of saliva and its action on tooth enamel in reference to its hardening and softening. J Amer Med Assoc. 1912;59:2118–2122. [PMC free article] [PubMed] [Google Scholar]
- 25.Featherstone JDB, ten Cate JM, Shariati M, Arends J. Comparison of artificial caries-like lesions by quantitative microradiography and microhardness profiles. Caries Res. 1983;17:385–391. doi: 10.1159/000260692. [DOI] [PubMed] [Google Scholar]
- 26.Featherstone JDB, Shariati M, Brugler S, Fu J, White DJ. Effect of an anticalculus dentifrice on lesion progression under pH-cycling in vitro. Caries Res. 1988;22:337–341. doi: 10.1159/000261133. [DOI] [PubMed] [Google Scholar]
- 27.Chow LC, Takagi S, Shih S. Effect of two-solution fluoride mouthrinse on remineralization of enamel lesions in vitro. J Dent Res. 1992;71:443–447. doi: 10.1177/00220345920710030401. [DOI] [PubMed] [Google Scholar]
- 28.Iijima Y, Takagi O, Duschner H, Ruben J, Arends J. Influence of nail varnish on the remineralization of enamel single sections assessed by microradiography and confocal laser scanning microscopy. Caries Res. 1998;32:393–400. doi: 10.1159/000016476. [DOI] [PubMed] [Google Scholar]
- 29.ten Cate JM, Extercate RA. Use of the single section technique in caries research. Caries Res. 1986;20:525–528. doi: 10.1159/000260984. [DOI] [PubMed] [Google Scholar]
- 30.Wefel JS, Maharry GJ, Jensen ME, Harless JD. Development of an intra-oral single-section remineralization model. J Dent Res. 1987;66:1485–1489. doi: 10.1177/00220345870660091401. [DOI] [PubMed] [Google Scholar]
- 31.Strang R, Damato FA, Stephen K.w. Comparison of in vitro demineralization of enamel sections and slabs. Caries Res. 1988;22:348–349. doi: 10.1159/000261135. [DOI] [PubMed] [Google Scholar]
- 32.Stephen KW, Damato FA, Strang R. An in situ enamel section model for assessment of enamel re/demineralization potential. J Dent Res. 1992;71:856–859. doi: 10.1177/002203459207100S13. [DOI] [PubMed] [Google Scholar]
- 33.Damato FA, Stephen KW. Demonstration of a fluoride dose response with an in situ single section dental caries model. Caries Res. 1994;28:277–283. doi: 10.1159/000261986. [DOI] [PubMed] [Google Scholar]
- 34.Al-Khateeb S, Extercate R, Angmar-Mansson B, ten Cate B. Effect of acid-etching on remineralization of enamel white spot lesions. Acta Odontol Scand. 2000;58:31–36. doi: 10.1080/000163500429406. [DOI] [PubMed] [Google Scholar]
- 35.Silverstone LM. Remineralization phenomena. Caries Res. 1977;11:59–84. doi: 10.1159/000260296. [DOI] [PubMed] [Google Scholar]