Abstract
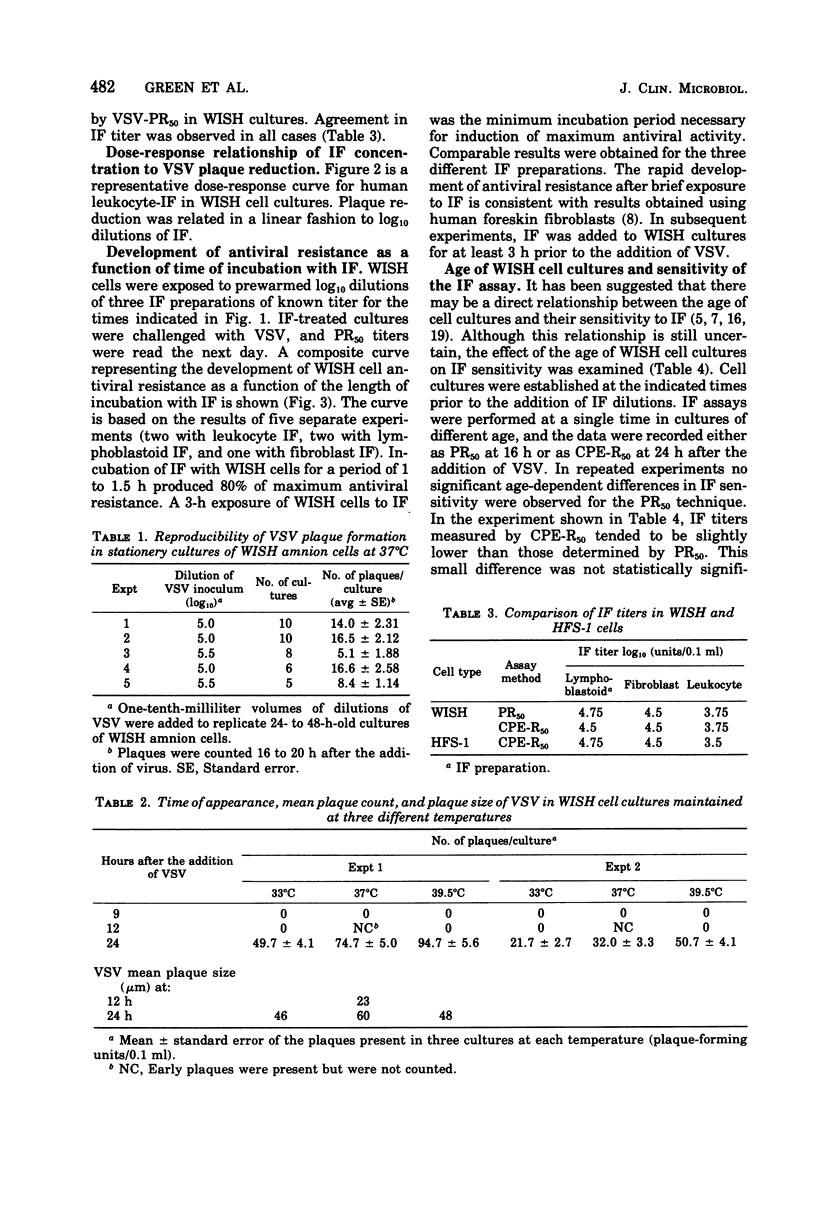
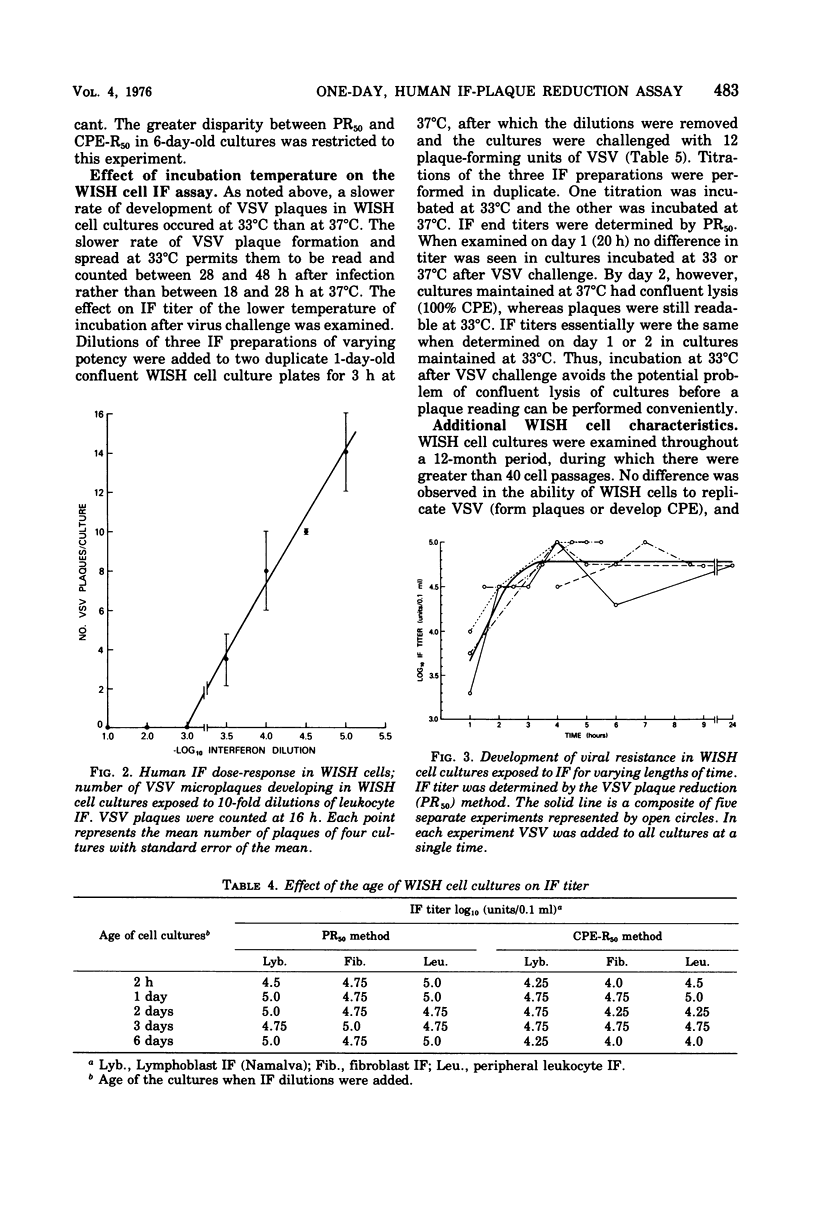
Vesicular stomatitis virus forms discrete, microscopic plaques in stationary cultures of the WISH amnion cell line. Microplaque formation is rapid, reproducible, and easily quantitated, occurs at temperatures ranging from 33 to 40 degrees C, and does not require a semisolid overlay. WISH cells, however, are less sensitive to vesicular stomatitis virus than are chicken embryo, 3T6, or Vero cells. WISH amnion cells also are highly sensitive to the antiviral effects of human interferon, and a quantitative human interferon assay, based on vesicular stomatitis virus plaque reduction in WISH cells, is described. This interferon assay can be performed within 1 day, uses a liquid overlay medium, does not require a vital stain, is as sensitive as other methods that use diploid cell strains, and is performed in a microtiter system.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen P. T., Giron D. J. Rapid sensitive assay for interferons based on the inhibition of MM virus nucleic acid synthesis. Appl Microbiol. 1970 Sep;20(3):317–322. doi: 10.1128/am.20.3.317-322.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong J. A. Semi-micro, dye-binding assay for rabbit interferon. Appl Microbiol. 1971 Apr;21(4):723–725. doi: 10.1128/am.21.4.723-725.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker D. A., Glasgow L. A. Rapid plaque assay for encephalomyocarditis virus. Appl Microbiol. 1969 Nov;18(5):932–934. doi: 10.1128/am.18.5.932-934.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass L. R., Turner W. Semi-micro XC cell assay technique for murine leukemia virus. Appl Microbiol. 1972 Jan;23(1):200–201. doi: 10.1128/am.23.1.200-201.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell J. B., Grunberger T., Kochman M. A., White S. L. A microplaque reduction assay for human and mouse interferon. Can J Microbiol. 1975 Aug;21(8):1247–1253. doi: 10.1139/m75-186. [DOI] [PubMed] [Google Scholar]
- Dianzani F., Baron S. Unexpectedly rapid action of human interferon in physiological conditions. Nature. 1975 Oct 23;257(5528):682–684. doi: 10.1038/257682a0. [DOI] [PubMed] [Google Scholar]
- Fujita N., Tamura M., Hotta S. Dengue virus plaque formation on microplate cultures and its application to virus neutralization (38564). Proc Soc Exp Biol Med. 1975 Feb;148(2):472–475. doi: 10.3181/00379727-148-38564. [DOI] [PubMed] [Google Scholar]
- HAYFLICK L. The establishment of a line (WISH) of human amnion cells in continuous cultivation. Exp Cell Res. 1961 Feb;23:14–20. doi: 10.1016/0014-4827(61)90059-3. [DOI] [PubMed] [Google Scholar]
- Hotchin J., Kinch W. Microplaque reduction: new assay for neutralizing antibody to lymphocytic choriomeningitis virus. J Infect Dis. 1975 Feb;131(2):186–188. doi: 10.1093/infdis/131.2.186. [DOI] [PubMed] [Google Scholar]
- McLaren C. Influence of cell age on production and assay of mouse interferon in L-cells. Arch Gesamte Virusforsch. 1970;32(1):13–18. doi: 10.1007/BF01241514. [DOI] [PubMed] [Google Scholar]
- McWilliams M., Finkelstein M. S., Allen P. T., Giron D. J. Assay of chick interferons by the inhibition of viral ribonucleic acid synthesis. Appl Microbiol. 1971 May;21(5):959–961. doi: 10.1128/am.21.5.959-961.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oie H. K., Buckler C. E., Uhlendorf C. P., Hill D. A., Baron S. Improved assays for a variety of interferons. 1. Proc Soc Exp Biol Med. 1972 Sep;140(4):1178–1181. doi: 10.3181/00379727-140-36636. [DOI] [PubMed] [Google Scholar]
- PARSONS R., TYRRELL D. A. A plaque method for assaying some viruses isolated from common colds. Nature. 1961 Feb 25;189:640–642. doi: 10.1038/189640a0. [DOI] [PubMed] [Google Scholar]
- Rossman T. G., Vilcek J. Influence of the rate of cell growth and cell density on interferon action in chick embryo cells. J Virol. 1969 Jul;4(1):7–11. doi: 10.1128/jvi.4.1.7-11.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedmak J. J., Grossberg S. E. Interferon bioassay: reduction in yield of myxovirus neuraminidases. J Gen Virol. 1973 Oct;21:1–7. doi: 10.1099/0022-1317-21-1-1. [DOI] [PubMed] [Google Scholar]
- Sukhavachana P., Yuill T. M., Russell P. K. Assay of arbovirus neutralizing antibody by micro methods. Trans R Soc Trop Med Hyg. 1969;63(4):446–455. doi: 10.1016/0035-9203(69)90031-5. [DOI] [PubMed] [Google Scholar]
- Tilles J. G., Finland M. Microassay for human and chick cell interferons. Appl Microbiol. 1968 Nov;16(11):1706–1707. doi: 10.1128/am.16.11.1706-1707.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassef A., Beaud G., Paucker K., Lengyel P. Interferon assay based on the inhibition of double-stranded reovirus RNA accumulation in mouse L cells. J Gen Virol. 1973 Apr;19(1):81–87. doi: 10.1099/0022-1317-19-1-81. [DOI] [PubMed] [Google Scholar]
- Yang J. P., Chiang W., Gale J. L., Chen N. S. A chick-embyo cell microtest for typing of Herpesvirus hominis (38531). Proc Soc Exp Biol Med. 1975 Feb;148(2):324–328. doi: 10.3181/00379727-148-38531. [DOI] [PubMed] [Google Scholar]



